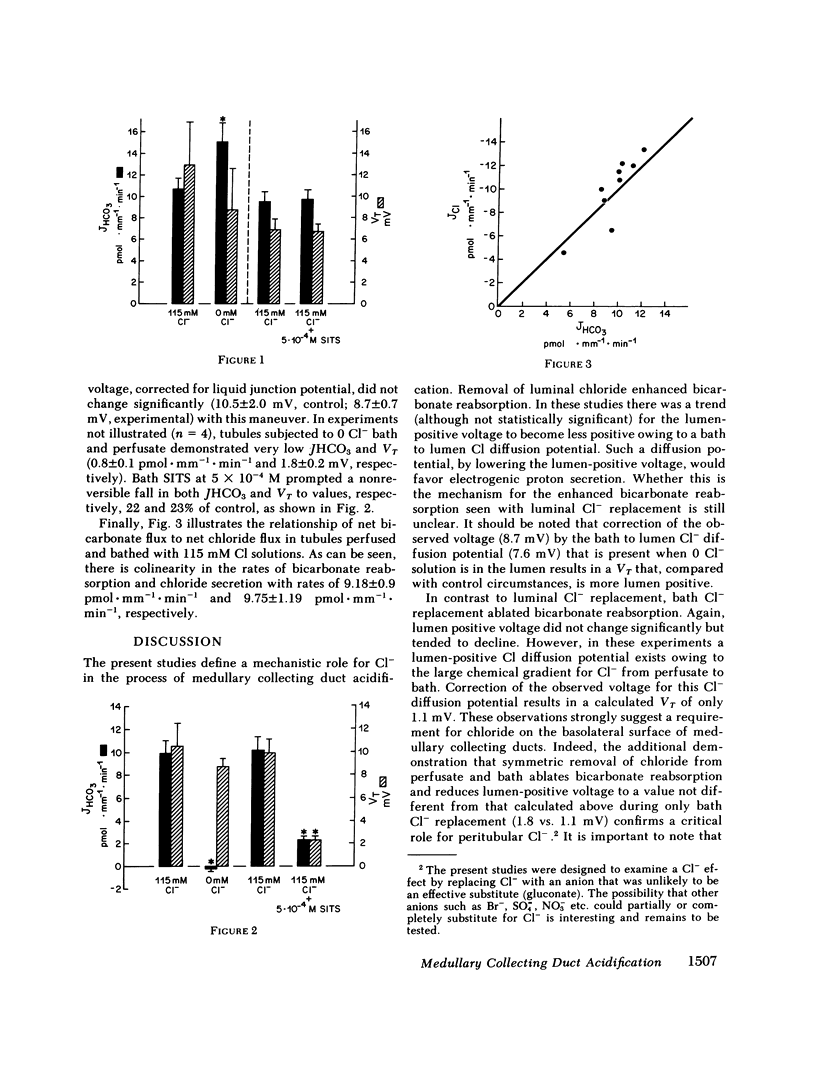
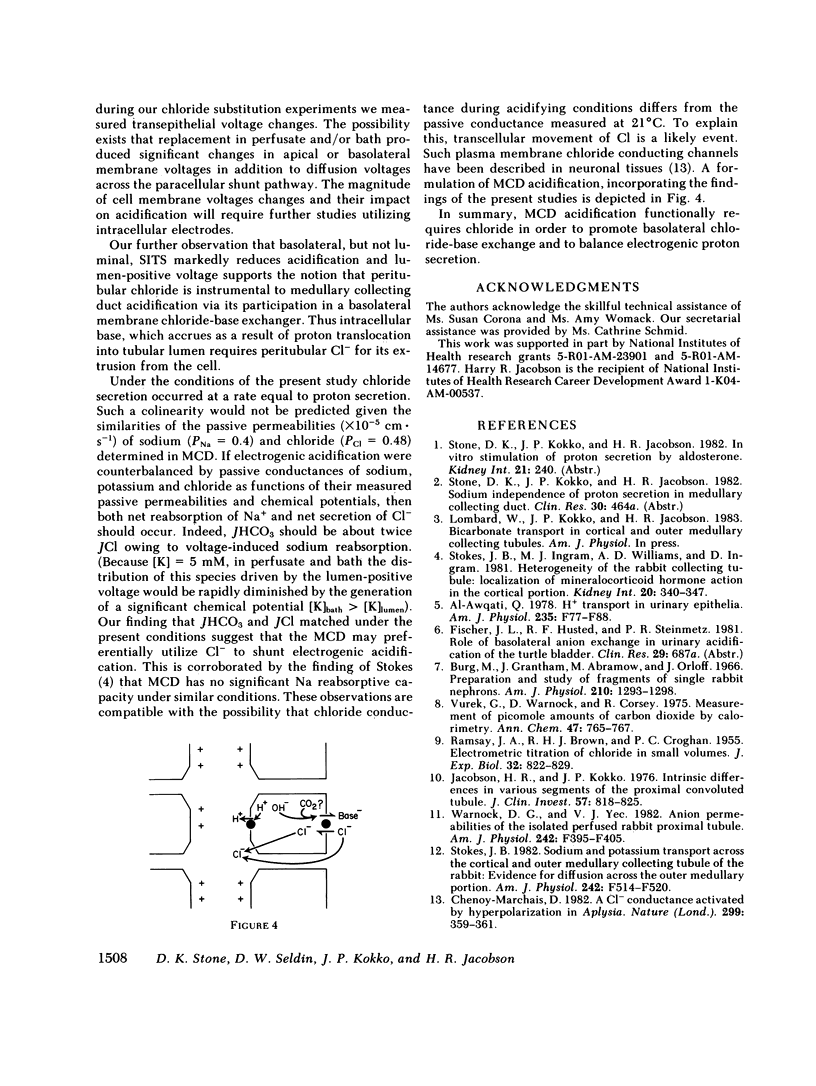
Abstract
Rabbit medullary collecting duct (MCD) acidification has been demonstrated to occur by means of a sodium-independent, aldosterone-stimulated mechanism. We have examined the anionic dependence of this process by means of the isolated perfused tubule technique. Total replacement of perfusate chloride with gluconate enhanced tubular bicarbonate reabsorption (JHCO3), from a basal rate of 10.7 +/- 1.0 pmol X mm-1 X min-1 to a rate of 15.01 +/- 1.0 pmol X mm-1 X min-1. Removal of bath chloride, with and without removal of perfusate chloride completely abolished acidification. Bath, but not luminal 4-acetamido-4' isothiocyano-2,2'-disulfonic stilbene provoked a marked decrease in JHCO3 from 10.1 +/- 1.2 pmol X mm-1 X min-1 to 2.3 +/- 0.3 pmol X mm-1 X min-1. Measurement of chloride reabsorptive rate (JCl) revealed colinearity between JHCO3 (9.18 +/- 0.9 pmol X mm-1 X min-1) and JCl (9.75 +/- 1.18 pmol X mm-1 X min-1). We propose a model of mammalian distal nephron acidification in which (a) cellular base exit is effected by means of a basolateral membrane Cl-base exchanger and (b) net electroneutrality of electrogenic proton secretion is maintained by the parallel movement of an anionic species, functionally chloride.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Awqati Q. H + transport in urinary epithelia. Am J Physiol. 1978 Aug;235(2):F77–F88. doi: 10.1152/ajprenal.1978.235.2.F77. [DOI] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- Chenoy-Marchais D. A Cl- conductance activated by hyperpolarization in Aplysia neurones. Nature. 1982 Sep 23;299(5881):359–361. doi: 10.1038/299359a0. [DOI] [PubMed] [Google Scholar]
- Jacobson H. R., Kokko J. P. Intrinsic differences in various segments of the proximal convoluted tubule. J Clin Invest. 1976 Apr;57(4):818–825. doi: 10.1172/JCI108357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes J. B., Ingram M. J., Williams A. D., Ingram D. Heterogeneity of the rabbit collecting tubule: localization of mineralocorticoid hormone action to the cortical portion. Kidney Int. 1981 Sep;20(3):340–347. doi: 10.1038/ki.1981.144. [DOI] [PubMed] [Google Scholar]
- Stokes J. B. Na and K transport across the cortical and outer medullary collecting tubule of the rabbit: evidence for diffusion across the outer medullary portion. Am J Physiol. 1982 May;242(5):F514–F520. doi: 10.1152/ajprenal.1982.242.5.F514. [DOI] [PubMed] [Google Scholar]
- Vurek G. G., Warnock D. G., Corsey R. Measurement of picomole amounts of carbon dioxide by calorimetry. Anal Chem. 1975 Apr;47(4):765–767. doi: 10.1021/ac60354a024. [DOI] [PubMed] [Google Scholar]
- Warnock D. G., Yee V. J. Anion permeabilities of the isolated perfused rabbit proximal tubule. Am J Physiol. 1982 Apr;242(4):F395–F405. doi: 10.1152/ajprenal.1982.242.4.F395. [DOI] [PubMed] [Google Scholar]



