Abstract
A strong placebo response in psychiatric disorders has been noted for the past 50 years and various attempts have been made to identify predictors of it, by use of meta-analyses of randomised controlled trials and laboratory studies. We reviewed 31 meta-analyses and systematic reviews of more than 500 randomised placebo-controlled trials across psychiatry (depression, schizophrenia, mania, attention-deficit hyperactivity disorder, autism, psychosis, binge-eating disorder, and addiction) for factors identified to be associated with increased placebo response. Of 20 factors discussed, only three were often linked to high placebo responses: low baseline severity of symptoms, more recent trials, and unbalanced randomisation (more patients randomly assigned to drug than placebo). Randomised controlled trials in non-drug therapy have not added further predictors, and laboratory studies with psychological, brain, and genetic approaches have not been successful in identifying predictors of placebo responses. This comprehensive Review suggests that predictors of the placebo response are still to be discovered, the response probably has more than one mediator, and that different and distinct moderators are probably what cause the placebo response within psychiatry and beyond.
Introduction
Although placebos have been used in general medicine for almost 200 years,1 their use in psychiatry is less well documented (panel).3 Systematic use of placebos in drug trials and beyond is, however, restricted to the past 60 years. Systematic exploration of the effects and efficacy of placebo application is even more recent, and restricted to the past two decades.
Are placebos powerful or powerless?
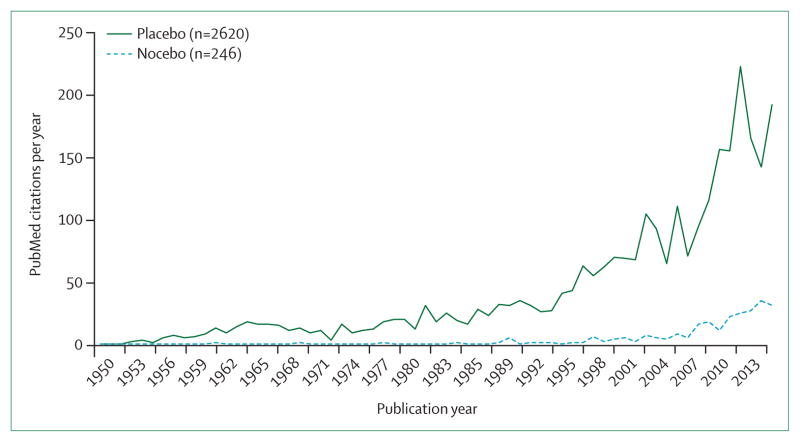
In the past two decades, the number of publications devoted to the placebo effect itself—ie, not on the effect of drugs (or other therapies) in comparison with a placebo treatment—has steadily increased, with an exponential rise since the early 1990s (figure 1). About 10% of all these publications were related to psychiatric disorders, predominantly depression.
Figure 1.
Number of genuine placebo and nocebo publications in PubMed per year between 1950 and 2014
Two questions have driven the scientific discussion in these papers: what is the effect size of the placebo response in clinical trials and in clinical practice, and what are its mediators (ie, factors generating the placebo response, such as personality) and moderators (factors modulating the placebo response, such as age, sex, and disease characteristics)?
In 2001, the Nordic Cochrane Centre published one of the first meta-analyses5 with respect to placebo effect sizes, which questioned The Powerful Placebo report by Henry Beecher6 that for years had dominated the discussion. Beecher, in a review of the scientific literature of his time, had shown that placebos could have notable analgesic effects, leading him to conclude that use of placebos can be a powerful technique in the hands of doctors when treating patients, especially those with pain syndromes.
A meta-analysis of 130 randomised clinical trials (RCTs) by Hróbjartsson and colleagues,5 in which patients were randomly allocated to either a placebo group (eg, drug placebo, sham manipulation, or sham psychotherapy) or to a no treatment group, in 40 different medical disorders, challenged Beecher’s conclusion, asking “Is the placebo powerless?”. Although consistent placebo effects were reported in most investigated disorders (pain, depression, nausea, insomnia, smoking, hypertension, anxiety, asthma, and obesity),5 their effect size was shown to be minor in comparison with the standards of effective medical treatment,5 as defined by clinical experience and regulatory authorities (the US Food and Drug Administration and the European Medicines Agency). For example, Hróbjartsson and colleagues5 noted that a decrease of 6 mm on a 100 mm visual analogue scale in pain would not be regarded as an effective clinical outcome. The placebo effect in depression (three trials included) was not significant. Later follow-up with another 52 RCTs (including two more depression trials) substantiated these findings.7
The two consensuses, that a placebo is a powerful instrument in the hands of a doctor, but quite ineffective in RCTs, have remained stable but rather incompatible throughout the years.
In their 2001 meta-analysis, Hróbjartsson and colleagues5 noted that the small but consistent placebo effects seen in RCTs include spontaneous variation in symptoms that have to be controlled for (and distinguished from placebo). However, no-treatment control groups in RCTs are difficult to achieve and ethically questionable. In 2009, the Nordic Cochrane Centre published another meta-analysis,8 in which they included 37 three-arm trials (drug, placebo, and no treatment), of diseases at levels of minor severity (depression, acute and chronic pain, nausea, phobia, smoking, obesity, and insomnia). When the placebo effects in RCTs were compared with the no-treatment controls, the data suggested that about half of the placebo effects could be attributed to spontaneous symptom variation and recovery, with some variation between clinical disorders. For minor depression, this effect was shown to be as high as 81%.
Early findings on the placebo effect in psychiatry
Psychiatry researchers were quick to critically assess the placebo response in RCTs, long before a similar discussion started in other medical subspecialties.
From as early as 1961, placebo effects in psychiatric drug research have been systematically assessed. Loranger and colleagues9 showed, using an approach that would be deemed ethically questionable nowadays (providing patients with false information about new energising or tranquillising drugs, without informed consent), the need for double-blinding and randomisation. In 1969, Rickels and colleagues10 reported that in crossover studies, patients with previous positive drug experience had lower responses to placebo than did those who were previously untreated. Researchers noted a higher response to placebo treatment when they gave the placebo before any drug treatment rather than after.
Panel: History of placebo use in psychiatry.
During the 19th century, the use of the term placebo varied substantially in medical literature. A quantitative, semantic analysis2 from the British Medical Journal issues between 1840 and 1899 contained definitions, including: to permit unfolding of the natural history of a disease, to satisfy patient needs, and to fulfil the physician’s performance role, or to buy time. Only one report implied that the placebo might have clinical effectiveness. The 20th century saw a more rigorous definition than the 19th, and the term placebo was used to denote the pharmacologically inert, sham simulator of an active drug that serves as a control in clinical trials designed to find out the clinical efficacy of that particular drug.
In psychiatry, as in any other medical subspecialty, placebos have been crucial in the validation of new therapies.3
In 1922, Nicholas Kopeloff, a bacteriologist, and Clarence O’Cheney, a psychiatrist, ran the first non-randomised clinical trial in psychiatry that included a control group to test the common view that infections of the teeth and tonsils would cause major psychiatric illness (eg, manic-depressive illness and dementia praecox). The authors divided 60 patients into a group receiving an operation for removing infections of the teeth or tonsils and a control group who received no operation. The study revealed no clinically significant difference between the two groups.
In 1935, Myron Prinzmetal, an internist, and Wilfred Bloomberg, a neuropsychiatrist, performed a crossover non-randomised clinical trial to test the effect of amfetamine versus ephedrine and sodium chloride placebo for the relief of narcolepsy. All the treatments tasted similar and patients served as their own control, receiving placebo first, then amfetamine, ephedrine, and finally, amfetamine again. The study showed that ephedrine and placebo were equally ineffective, and a net improvement induced by amfetamine, thus providing a clear and meaningful result even in the absence of randomisation.
In 1938, Leonard Dub and Louis Lurie also tested the effect of amfetamine in a double-blinded crossover study, alternating amfetamine with a lactose placebo in women with depression. Amfetamine produced an improvement as compared with placebo.
Although crossover study designs became common in medicine along with the masking of patients, in the early 1900s randomisation was absent. The first randomised placebo-controlled trials were not done until 1952, in Denmark and the USA.
In Risskov, Denmark, Mogens Schou, a psychiatrist at the Psychiatric Research Institute of Aarhus University performed a randomised clinical trial in patients with mania treated with lithium. Flipping a coin every 2 weeks of treatment, Schou alternated treatment in a random way from lithium to placebo and vice versa. Lithium showed higher benefit than placebo, paving the way for the use of lithium in bipolar disorder. During the same year, Louis Lasagna in the USA, introduced a randomised controlled trial to compare the hypnotic agents, chloral hydrate and pentobarbital sodium with the agents, methylpentynol and placebo.
These two studies were followed by additional pioneering randomised clinical trials.
In 1954, various nucleotides (eg, adenylic acid) were tested at the Saskatchewan Hospital in Weyburn, Canada for the treatment of schizophrenia in a randomised, controlled-clinical trial, with negative results.
In 1955, in the UK, David L Davies and Michael Shepherd performed the first randomised clinical trial of reserpine for the treatment of patients with anxiety or depression, in which both patients and clinicians were masked to treatment allocation.
In 1954, John Hampson, David Rosenthal, and Jerome Frank designed a double blind, randomised, placebo-controlled study4 to test the effect of a new potential tranquilliser, mephenesin in outpatient psychotherapy at the Department of Psychiatry at Johns Hopkins University in Baltimore, MD, USA. The study reported that placebo and mephenesin produced similar effects. The authors stated that “The high value which our culture places on pills and medicines may be involved in this phenomenon whereby even inert substances become endowed with physiologic potency when they are presented to the patient as therapeutic agents”.4 This intriguing intuition led neuroscientists to investigate the mechanisms underpinning behavioural and brain placebo responses.
Two landmark papers11,12 published in 2002 dealing specifically with the placebo response in psychiatry (with a focus on depression) inspired a range of other placebo studies in psychiatry.
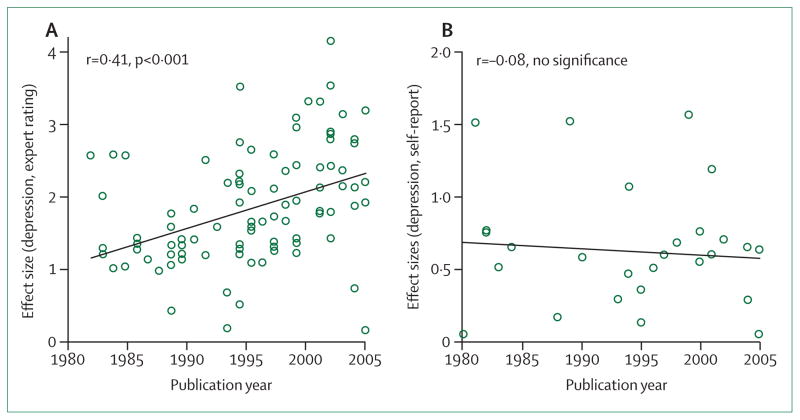
One paper was a systematic review11 of 75 RCTs published between 1960 and 1990, reviewed with respect to the variability and change of the placebo response in treatment of depression. Walsh and colleagues11 noted that the placebo response grew by about 7% per decade between 1981 and 2000, irrespective of the antidepressant comparator, including tricyclic antidepressants or (since 1983) SSRIs and SNRIs.
The paper11 started a long-lasting discussion on what drives the change in placebo response with time. One of the factors suggested to be driving the change was whether the prescribing doctor or the treated patient was assessing treatment efficacy.13 In a similar analysis13 of nearly the same dataset that examined patient assessments of treatment outcome, the trend was no longer visible (figure 2). This assessor bias has led some commentators to conclude that design factors (and the pressure of the pharmaceutical industry on designs) could have driven the higher placebo responses reported in more recent studies compared with earlier studies done between 1960 and 1980. This increase in placebo response over time was looked for in other medical subspecialties, where it was found in some but not others, leading to similar conclusions. The time trend was shown in RCTs of schizophrenia and bipolar mania,14 but not in somatisation disorders.15
Figure 2. Correlation between placebo effect size and year of publication.
The graphs show the mean placebo effect size as given by observer ratings (A) and self-reported ratings (B) in antidepressant trials. Reproduced from Rief and colleagues.13
The second line of research on placebo effect derived from the development of brain imaging technology in the 1990s, specifically, but not exclusively, in neurology and psychiatry. The technology gave rise to the first papers on the neurobiology of the placebo response in Parkinson’s disease,16 placebo analgesia,17 and eventually depression.12
Mayberg and colleagues12 described changes in brain metabolism (as measured by PET) in response to a 6 week double-blind treatment with fluoxetine or placebo in patients with unipolar depression. Placebo responses were associated with regional metabolic increases in some areas of the brain and decreases in others that mimicked some of the effects of fluoxetine. However, the fluoxetine response was associated with additional subcortical and limbic changes in the brainstem, striatum, anterior insula, and hippocampus. Although true placebo effects were difficult to quantify because of the absence of a natural history group, Mayberg and colleagues12 proposed a bottom-up inhibitory action by fluoxetine on paralimbic and subcortical regions via the brainstem and hippocampus, and a top-down activation by placebo of the same brain regions via the cingulate cortex. A later review18 discussing unpublished data from the study12 noted early changes in the ventral striatum in groups scanned 1 week after treatment, suggesting an anticipatory, non-specific drug-expectation effect.18
Placebo responders in pharmacological RCTs
In an early attempt (1992) to predict the placebo response, Brown and colleagues19 re-analysed the individual data of 241 patients being treated for depression who had received placebo in a multicentre RCT. Brown and colleagues19 subdivided these patient into responders (105 patients), partial responders (48), and non-responders (88) on the basis of several clinical assessment methods, such as the Hamilton-Depression Score, the Symptom-Checklist-90, and strict responder definitions. By analysing the data, Brown and colleagues19 identified major determinants of increased placebo response, such as short duration of the actual depression episode, low percentage of previous and effective treatments for depression, and low overall symptom severity.
The papers by Hróbjartsson and colleagues5 and Walsh and colleagues11 were soon followed by a series of systematic reviews and meta-analyses of RCTs in depression, schizophrenia, attention-deficit hyperactivity disorder, addiction, and other psychiatric disorders (tables 1, 2). We found 31 systematic reviews and meta-analyses related to psychiatric diseases, nine analyses for neurological diseases, 13 for pain, 12 for gastrointestinal disorders, and ten for other general medical disorders in our placebo literature database. We recently evaluated these 75 analyses to explore the role of sex and age for the placebo response across different subspecialties in medicine49 and found three analyses that supported a role of sex and 15 of age.
Table 1.
Systematic reviews and meta-analyses of randomised controlled trials in psychiatric disorders
| Number of studies analysed | Diagnosis | Patient-centred factors and their association with placebo response (positive or negative) | Study design-based factors and their association with placebo response (positive or negative) | |
|---|---|---|---|---|
| Woods et al (2005)20 | 32 | Schizophrenia | ·· | Unbalanced randomisation (+) |
| Kemp et al (2010)21 | 28 | Schizophrenia | Shorter disease duration (+) | More recent of studies published between 1993 and 2006 (+); US trials (+) |
| Mallinckrodt et al (2010)22 | 27 | Schizophrenia | Women (+) | More study sites (−); shorter trial duration (+); unbalanced randomisation (+); US trials (−) |
| Chen et al (2010)23 | 31 | Schizophrenia | Younger patients (+); lower symptom severity (−) | More recent of studies with data submitted to the FDA between 1993 and 2005 (+) |
| Potkin et al (2011)24 | 3* | Schizophrenia | Previously untreated patients (−); lower symptom severity (+) | ·· |
| Agid et al (2013)25 | 50 | Psychosis | Younger patients (+); lower symptom severity (−); shorter disease duration (+) | More study sites (+); shorter trial duration (+); unbalanced randomisation (+) |
| Rutherford et al (2014)26 | 39 | Psychosis | Lower symptom severity (+) | More recent of studies published between 1960 and 2013 (+); shorter trial duration (+) |
| King et al (2013)27 | 1* | Autism (children) | Lower symptom severity (+) | ·· |
| Sysko et al (2007)28 | 20 | Bipolar mania | ·· | More recent of studies published between 1980 and 2005 (+) |
| Yildiz et al (2011)29 | 38 | Bipolar mania | Younger patients (−); women (+) | More recent of studies published between 1991 and 2010 (+); more study sites (+) |
| Cohen et al (2010)30 | 40 | Obsessive compulsive disorder; anxiety (children) | Younger patients (+); non-white people (+); lower symptom severity (+); shorter disease duration (+) | ·· |
| Newcorn et al (2009)31 | 10 | Attention-deficit hyperactivity disorder (children) | Younger patients (−); non-white people (+); previously untreated patients (+) | ·· |
| Waxmonsky et al (2011)32 | 2* | Attention-deficit hyperactivity disorder | Lower symptom severity (+)† | ·· |
| Buitelaar et al (2012)33 | 2* | Attention-deficit hyperactivity disorder | Younger patients (+); lower symptom severity (−); shorter disease duration (+); lower education (+) | ·· |
| Blom et al (2014)34 | 10* | Binge eating disorder | Lower symptom severity (+); higher body-mass index (+) | ·· |
| Greene et al (2010)35 | 107 | Smoking | ·· | Lower industry support (+) |
| Litten et al (2013)36 | 48 | Alcohol | Younger patients (+) | More recent of studies published between 1966 and 2011 (+) |
+ positive association between factor and placebo effect.
− negative association between factor and placebo effect.
FDA=US Food and Drug Administration.
Individual patient data were available.
In adults only.
Table 2.
Systematic reviews and meta-analyses of randomised controlled trials in depression
| Number of studies analysed | Diagnosis | Patient-centred factors and their association with placebo response (positive or negative) | Study design-based factors and their association with placebo response (positive or negative) | |
|---|---|---|---|---|
| Brown et al (1992)19 | 1* | Depression | Previously untreated patients (+); lower symptom severity (+); shorter disease duration (+); family history (+) | ·· |
| Walsh et al (2002)11 | 75 | Depression | ·· | More recent of studies published between 1981 and 2000 (+) |
| Khan et al (2002)37 | 45 | Depression | Lower symptom severity (+) | ·· |
| Evans et al (2004)38 | 4 | Depression | Worsening of symptoms (+) | ·· |
| Stein et al (2006)39 | 12 | Anxiety; major depression disorder | Lower symptom severity† (+) | US trials (−) |
| Kirsch et al (2008)40 | 35 | Depression | Lower symptom severity (+) | ·· |
| Papakostas et al (2009)41 | 182 | Depression | Younger patients (+); lower symptom severity (+) | More recent of studies published between 1980 and 2007 (+); unbalanced randomisation (+) |
| Bridge et al (2009)42 | 12 | Depression (children) | Younger patients (+); lower symptom severity (+) | More recent of studies published between 1997 and 2007 (+); more study sites (+) |
| Brunoni et al (2009)43 | 41 | Depression (drug treatment only) | Lower symptom severity (+) | Lower number of patients (+) |
| Sinyor et al (2010)44 | 91 | Depression | ·· | Unbalanced randomisation (+) |
| Hunter et al (2010)45 | 1* | Depression | Previously untreated patients (+) | ·· |
| Rutherford et al (2011)46 | 11 | Depression (children) | ·· | Higher number of visits‡ (+) |
| Khin et al (2011)47 | 81 | Depression | Lower symptom severity (+) | More recent of studies published between 1983 and 2008 (+); US trials (+) |
| Mancini et al (2014)48 | 14* | Depression | Lower symptom severity (+) | Shorter trial duration (+); unbalanced randomisation (+); higher number of visits (+) |
+ positive association between factor and placebo effect.
− negative association between factor and placebo effect.
Individual patient data were available.
Only for anxiety and US patients.
In older children only.
Based on this first review,49 we grouped putative factors that might drive the placebo response in RCTs for our Review into either patient-centred factors that relate to individual characteristics or study design-based factors that relate to RCT characteristics.
Notably, the findings in these systematic reviews and meta-analyses of RCTs of psychiatric disorders are not wholly independent. The meta-analyses used similar literature search terms and (allowing for the addition of new RCTs in the scientific literature throughout the years) extracted, at least partly, the same studies from the body of published trials. However, the meta-analyses did use different statistical approaches to identify relevant factors that drive the placebo response and therefore were able to support or oppose the factors identified in previous analyses or identify new factors not previously reported. This Review is qualitative and does not attempt to quantify the meta-analyses.
Patient-centred factors
This section will discuss factors that are either patient or disease specific and have been reported to modulate the placebo response across different RCTs.
From our analysis of the data, we could not firmly conclude that age has an effect on the placebo response in psychiatry or other medical subspecialties.49 Seven studies23,25,30,33,36,41,42 reported a higher placebo response with younger age, whereas two studies29,31 reported the opposite.
Again, we could not firmly conclude that sex has an effect on the placebo response in psychiatry. Only two22,29 of 31 analyses we reviewed state that women are more likely to have higher placebo responses than men.
Just two analyses,30,31 and only in children with anxiety or attention-deficit hyperactivity disorder, reported an association between ethnic origin and placebo response; an increased response in non-white people. Whether this association is caused by differences in parental care or a differential genetic contribution remains uncertain.
A successful previous therapy seems to predict an increased placebo response in schizophrenia,24 but contrary to the early findings by Rickels and colleagues,10 previously untreated patients overall had increased responses in attention-deficit hyperactivity disorder (in children)31 and in depression;19,45 each finding reported in one study only.
The most consistent finding is that a low symptom severity at baseline is a strong predictor of the placebo response in schizophrenia,24 psychosis,26 children with autism,27 obsessive-compulsive disorder,30 attention-deficit hyperactivity disorder,32 binge-eating disorder,34 and depression,19,37,39–43,47,48 despite the fact that in three studies the opposite was reported for schizophrenia,23 psychosis,25 and attention-deficit hyperactivity disorder.33
Another factor reported to be associated with high placebo responses was a short disease history,19,21,25,30,33 which might represent lower symptom severity as well. Associations between a positive family history of depression,19 symptom worsening between screening and baseline assessment,38 lower education,33 and a higher body-mass index34 with increased placebo responses were presumably random findings in individual analyses only and across all diseases.
Patient-centred factors that could be of relevance but are not usually addressed in RCTs and, consequently, cannot appear in meta-analyses are placebo-by-proxy effects.50 Patients are usually embedded in a social network that contributes to wellbeing and therapy. A patient’s experience of a therapy might be influenced by another patient’s similar or divergent experiences, of the same or other therapies, for the same or other clinical disorders. The effects of placebo-by-proxy have only been shown in children,51 and parents’ attitudes towards treatment have been proven to mediate the placebo response of children, whereas direct interaction between children and their treating physician seems to be of lesser relevance.46
In summary, the most predictive individual factor for a high placebo response is a low symptom severity at baseline. One of the methodological implications of this consistent finding is the assumption that patients recruited in trials done within the past 20 years are only a subpopulation of all that are affected and need treatment, which has cast doubts on the usefulness of recent drug development (especially SSRI and SNRI) for the treatment of depression in real world settings.52 However, the controversy about the clinical usefulness of SSRIs has other aspects, including ineffective masking and increased risk of suicidal attempts.53,54 Similar findings of increased placebo response over time to those in depression were noted in some21 but not all55 other clinical disorders.
Study design-based factors
The following section will discuss features of the designs of trials that have been identified to modulate the placebo response across different RCTs.
Following the paper by Walsh and colleagues,11 other meta-analyses have proven that the placebo response is higher in more recently published studies in depression,41,42,47 schizophrenia,21,23 psychosis,26 bipolar mania,28,29 and alcohol addiction therapy.36
More recent trials are frequently also trials with the most study sites, which could explain high placebo responses,25,29,42 but contrary evidence exists.22 Trials in the USA had high placebo responses in two analyses in depression47 and schizophrenia,21 but contrary evidence is also reported for depression39 and schizophrenia,22 suggesting that these findings could be random.
Short trial duration was reported to be predictive of high placebo responses,22,25,26 but this factor could be linked to small study samples.43
Unbalanced randomisation is any deviation from a 1:1 randomisation schedule, and can happen for different reasons, such as researchers assigning more patients to the drug group in a two arm design or adding more groups with equal numbers of patients (eg, for different drug dosing) to the RCT. An unbalanced randomisation and therefore (usually) an increased chance of receiving active medication was the most frequent finding for increased placebo responses across all disorders, including schizo phrenia,20,22 psychosis,25 and depression.41,44,48 Unbalanced randomisation does not seem to affect the placebo effect in children.46
Other factors identified only in some analyses to be associated with higher placebo effect were a high number of study visits46,48 (but only in older children and adults) and a low level of industry support.35
As with patient-centred factors, only one mediator (unbalanced randomisation) of the many tested mediators of high placebo responses seems to be consistent across disorders. In psychiatric20,22,25,41,44,48 and non-psychiatric disorders (eg, migraine56), an increased chance of being randomly assigned to drug increased the efficacy of both drug and placebo, whereas best sensitivity (and drug–placebo discrimination) is achieved with a 1:1 allocation—this finding is also lent support by neurobiological evidence in animals57 and human beings.58 However, unbalanced randomisation as predictor of the placebo response is not a consistent finding across all medical disorders, such as in functional (bowel) disorders.15
Placebo responders in non-pharmacological trials
Non-drug therapy in psychiatry includes psychotherapy interventions (eg, hypnosis) and technical interventions (eg, transcranial direct-current or magnetic stimulation, electroconvulsive therapy, acupuncture, and bright-light therapy). Both approaches share a common pitfall: the difficulty, if not inability, to provide the interventions in a blinded or even double-blinded way. However, they are distinctly different in other aspects.
The placebo effect in RCTs consists of biases and the regression to the mean, and the individual placebo response occurs because of mechanisms, such as learning and expectations, which are influenced by traits of patients and practitioners;59 the same applies to psychotherapy. By contrast, psychotherapy research questions whether (unspecific) common factors or (specific) techniques unique to different kinds of psychotherapy are crucial factors that act in psychotherapy.60 Whereas learning and expectations are both deemed to be unspecific factors in medical treatments, they are regarded as specific and unspecific but important parts in psychotherapy.
Therefore, to apply the model of RCTs in which the pharmacological ingredient of a treatment should be replaced by a placebo intervention (eg, by a sugar pill or saline infusion), knowledge of which part of psychotherapy is effective is important. However, as long as researchers cannot reach a consensus on what the so-called active ingredient of psychotherapy is, no consent can be reached of what should be controlled for. In view of this inconsistency many studies have examined the efficacy of some psychotherapies in comparison with different kinds of control conditions. A meta-analysis by Baskin and colleagues61 examined whether the structure of placebo control conditions has an effect on the placebo effect in RCTs of psychotherapies. Criteria for acceptable placebo control conditions were that treatments were face-to-face (in which the supposed active ingredient was omitted) and were provided by a trained therapist. Therapies meeting the criteria were supportive therapy, credible attention placebo, relaxation training control, discussion group, aware ness through movement, befriending, non-prescriptive treatment, cognitive analytical treatment, and modest contact. The comparison of the structural equivalence—ie, the number, length, and format of sessions or the training of the therapist—of 21 RCTs of psychotherapy revealed that “placebo controls that were structurally equivalent to the treatment produced effects that were nearly equal to those produced by active treatments, whereas placebo controls that were structurally inferior produced demonstrably poorer outcomes than active treatments”.61 Therefore, “well-designed placebos are nearly as beneficial as active treatments”.
However, Baskin and colleagues61 discuss that one methodological problem is especially limiting for researchers assessing the placebo effects in and the efficacy of psychotherapy: double-blindness cannot be achieved with a therapist who provides the therapy. This fact will always corrupt results, with the behaviour of the therapist tending to increase effects in the treatment group and decrease effects in the placebo group. Furthermore, to keep patients masked when treatments are too different or seem to be illogical is difficult—eg, when patients are not allowed to talk about their specific problems in the control group.62
Although placebo effects in psychotherapy research can be as high as the treatment effect itself, psychotherapy should not be thought of as a placebo. Furthermore, researchers need to consider whether medical treatments should be adapted to use the so-called common factors in psychotherapy to enhance specific treatments. Brain imaging studies showed that in patients with depression, placebo effects in RCTs of fluoxetine and the effects of psychotherapy differ even on the neurophysiological level, and placebo effects are more similar to the treatment effect with fluoxetine.61 This finding could lead to the assumption that they are complementary effects in the treatment of patients that use different pathways. Wampold and Budge60 proposed a model of common and specific factors that work in psychotherapy. After the establishment of a therapeutic bond through perceived empathy, trustworthiness, and clinical expertise of the therapist, the so-called real relationship between therapist and patient can develop and expectations can be created.60 These milestones are important parts of the treatment and are used as a fundament on which specific techniques (participation in healthy actions according to the form of psychotherapy) can deploy. In medical treatments, those common factors are deemed to be unimportant and the specific treatment is the only so-called true treatment. However, study results have shown that unspecific factors, such as empathy, can also have a relevant effect on typical medical disorders (eg, in patients who rated their physician as very empathic, the common cold lasted 1 day less than it did in patients who rated their physician as less empathic).63
Technical sham therapies in psychiatry
That the invasiveness of interventions codetermines the response rates to treatments and that invasive sham conditions generate larger placebo responses than drug placebos for some disorders is well established.64–66
In a meta-analysis comparing drug (escitalopram) placebos with sham repetitive transcranial magnetic stimulation in depression, Brunoni and colleagues43 reported similar effect sizes for both sham interventions, and that sham responses to repetitive transcranial magnetic stimulation are associated with refractoriness and with the use of repetitive transcranial magnetic stimulation as an add-on therapy, but are not associated with age, sex, and sham method used.
The clinical efficacy of electroconvulsive therapy in depression with or without psychosis and in other psychiatric disorders has been both supported67 and questioned68 in meta-analyses and systematic reviews, but the efficacy of attempted control conditions (sham or simulated electroconvulsive therapy)69 are quite high and close to the efficacy of electroconvulsive therapy itself.70 Researchers still debate the mode of operation of electroconvulsive therapy (something not unique to electroconvulsive therapy), leading some people to conclude that most of the effects noted in the hospital might be due to placebo responses71 and that the continuation of this practice might no longer be justified beyond thoroughly planned RCTs, especially because patients cannot be given a rational explanation of the efficacy of electroconvulsive therapy beyond placebo effects. Researchers have not yet identified any specific predictors of placebo efficacy with electroconvulsive therapy because of the small number of studies on the subject and their ethical delicacy.
Direct current stimulation, which has a much lower invasiveness than electroconvulsive therapy, has also been investigated to increase food intake72 in anorexia nervosa (but without adequate sham control) or to reduce food intake in healthy volunteers,73 but otherwise mostly in neurological (motor) disorders.
By contrast with direct current stimulation, electro-convulsive therapy, and repetitive transcranial magnetic stimulation, sham procedures have been used widely in acupuncture trials; several sham techniques are available.74 Except for the meta-analyses by Linde and colleagues,65,66 only individual RCTs have been reported in the scientific literature with variable sham response rates on disorders, such as irritable bowel syndrome,75 premenstrual syndrome,76 and post-chemotherapy chronic fatigue.77 This predominant efficacy in somatoform disorders is not surprising, in view of the strong action of acupuncture on peripheral autonomic functions.78 The same could hold true for bright-light therapy—eg, in (antenatal or postpartal) depression and seasonal affective disorders that would be easy to control for sham therapeutic effects—but a systematic assessment of factors driving the placebo response in such trials has not yet been done.79
The search for predictors of experimental placebo responses
Beyond RCTs, researchers in all specialties of medicine have attempted to identify predictors of the placebo response in experimental settings, predominantly by provoking placebo responses with experimental stimuli—eg, somatic and visceral pain, nausea, immune stimulation or inhibition, cardiac functions, or sleep induction.59
Personality
Whereas the scientific literature before 1990 often referred to placebo responders as neurotic, anxious, introvert, or extrovert (see references in Kaptchuk and colleagues80), notably, even psychometric results from psychiatric RCTs are rarely reported to be associated with high placebo responses, despite the fact that in psychiatric trials, the assessment of personality traits and states would seem normal by comparison with most other medical sub specialties. In non-psychiatric specialties, inclusion of psychometric tests into pivotal trials can put the entire study at risk by potentially limiting the scope of indications the drug receives and is usually avoided by pharmaceutical companies. Under the assumption that at least some psychometric tests were included in all trials in patients with depression, anxiety, and other psychiatric disorders, the scarcity of reports that found (or missed) associations between the results of those tests and the placebo response (with one exception19) could suggest that no definitive association of personality profiles with the placebo response exists or that the psychometric tests implemented (but not reported) were ineffective. Kaptchuk and colleagues80 have concluded that stable individual characteristics of a placebo responder do not exist.
Investigators have instead mostly looked for personality profiles in experimental studies eliciting placebo responses (eg, placebo analgesia), predominantly in healthy volunteers.81 These studies only identified a few individual characteristics that can be found more frequently in patients responding with symptom improvement after a placebo intervention than in non-responders (eg, optimism,82 neuroticism,83 and an external locus of control84).
In 2014, we published a review85 screening the respective literature on personality and placebo response and found 21 studies, most with pain as the dependent variable. Most predictors of the placebo response were psychological constructs related to actions, expected outcomes, and the emotional valence attached to these events (eg, goal seeking, self-efficacy or self-esteem, locus of control, and optimism). Other predictors included behavioural control (eg, desire for control and eating restraint), and personality variables (eg, fun or sensation seeking, and neuroticism). Finally, suggestibility and belief in expectation biases, body consciousness, and baseline symptom severity were identified as be predictive. Overall however, the picture is inconsistent because replication studies of single findings are absent. We concluded that the placebo response seems to be moderated by patient expectations of how the symptom might change after treatment or how symptom repetition can be coped with. Most standard psychometric tests do not screen for these variables.
Feltner and colleagues86 developed a highly specific psychometric screening scale for placebo susceptibility and tested it in more than 200 patients with general anxiety disorder. A score of more than 50 (of 100) points on the Placebo Response Screening Scale86 was able to improve sensitivity. Exclusion of patients scoring more than 50 points from the sample resulted in substantially better separation of active treatment from placebo in general anxiety disorder. However, an independent validation of the scale in general anxiety disorder and other disorders is still needed.
Brain networks
Since the first studies imaging the placebo response in the brain published in 2001 and 2002, investigators have searched for common neurobiological mechanisms that underlie placebo responses in various medical disorders. However, the fact that the placebo response has not one but many mechanisms was already evident from the paper by Mayberg and colleagues.12 Placebos seem to use the same pathways as the respective drugs that they are tested against, and mimic drug action in a similar though less effective way.59 One reason why placebo effects in RCTs seem less effective than in experimental settings87 is because in many experiments, placebo responses are enforced verbally, are practised by an unmasked investigator, and do not control for so-called spontaneous variation of symptoms (habituation and sensitisation) and thereby can confound response biases with placebo responses.88
A common neural substrate of placebo effects that has been identified across studies and disorders is the dopaminergic reward system, specifically the nucleus accumbens and areas of the brainstem and prefrontal cortex. Additionally, distinct brain circuitries and pathways have been reported for different disorders. For placebo analgesia, brain activation during a placebo response involves pain-processing areas, including the amygdala, anterior cingulate cortex, and prefrontal cortex, with subsequent descending inhibitory noxious control down to the level of the spinal cord.89 For placebo effects in motor dysfunctions, such as in Parkinson’s disease, activation of the dopaminergic system in the striatum90,91 has been reported. For placebo effects in immune suppressive functions, the control of the peripheral release of cytokines,92 and for depression, metabolic changes involving the prefrontal cortex, subgenual cingulate, parahippocampus, and thalamus have been reported.12,59 Many neural mechanisms in other diseases still need investigation, especially in patients with psychiatric disorders that are difficult, if not impossible, to investigate with an experimental model in healthy volunteers.
Genes
Evidence for genetic variants associated with placebo responses is best documented for anxiety disorders and depression. Serotonin-related gene polymorphisms have been reported to affect the individual placebo response in social anxiety, both at the behavioural and neural level.93 Additionally, genetic polymorphisms modulating monoaminergic tone have been related to the degree of placebo responsiveness in major depressive disorder and in somatisation disorder.94,95 In view of genetic studies in non-psychiatric disorders pointing towards a wholly different set of genes regulating brain anatomy or function, or both, from those associated with the placebo response in psychiatric disorders, the present database of papers on placebo we have gathered seems too small to reliably identify genetic mediators of the placebo response in psychiatric disorders and beyond.
Ethics of placebo use in psychiatry
All placebo-controlled trials are increasingly questioned because they provide less-than-maximum therapy to patients, and the Declaration of Helsinki allows them only when inadequate or ineffective routine treatment options are available.96 Deception of patients is even more prominent in experimental research, where the focus is on the mechanisms of the placebo effect rather than on drug–placebo differences. Presumed methodological alternatives, such as unbalanced randomisation or comparator trials do not provide a consistent solution towards this dilemma, but rather increase it: these alternatives tend to increase the placebo response and therefore need to include more patients into RCTs for the test of superiority or non-inferiority, thereby contradicting their own ethical justification. The Declaration of Helsinki rules are even more restrictive with the inclusion of children or patients with restricted intellectual abilities to consent; this can frequently be the case in psychiatry.
Search strategy and selection criteria.
On Jan 1, 2004, we searched PubMed for all available manuscripts published in English using the search term “placebo” both retrospectively and prospectively to select manuscripts dealing with the placebo effect.
We retrieved about 100 000 citations in 2004. We (KW, PE) screened their titles and abstracts retrospectively and excluded manuscripts describing placebo-controlled trials of individual drugs and other medical interventions that only assessed differences between drug and placebo for evaluation of therapeutic benefits of the therapy. We also excluded meta-analyses of placebo-controlled trials and respective reviews. After exclusion of letters and editorials, we were left with about 1000 manuscripts that discussed different aspects of the placebo response or placebo effects, or both in different medical and psychological subspecialties. These manuscripts were predominantly experimental data (exploring the different mechanisms of the placebo response) and reviews, systematic reviews, re-analyses, and meta-analyses of data from randomised controlled trials. PDFs of these manuscripts were retrieved and stored into an EndNote database.
Since 2004, we have prospectively screened all manuscripts published on a weekly basis (176 301 manuscripts in total, as of Nov 17, 2014) using the same search term “placebo”. In 2010, we added the search term “nocebo” (269 citations, as of Nov 17, 2014). We occasionally added manuscripts that explored and discussed psychosocial contributions to placebo-like effects, even without using the term placebo.
The database (as of Jan 29, 2015) contains 2672 manuscripts of various aspects of the placebo and nocebo response in medicine and beyond. The distribution of these manuscripts on the genuine placebo and nocebo effects between 1960 and 2014 is depicted in figure 1, and shows an exponential increase, similar to the increase seen in the remaining placebo literature.
This database was hand-searched by all authors for systematic reviews, meta-analyses, and meta-regression of the placebo effects and its determinants (mediators and moderators) in psychiatry. We then sourced new manuscripts for the database from the references of the manuscripts found in the search. Altogether, we identified 31 systematic reviews, meta-analyses, and meta-regressions in this way that were used for this Review.
Each of these manuscripts was then screened for patient-centred and study design-based factors that were identified as moderators of the placebo response in the placebo arm of respective trials, irrespective of the type of statistical analysis (ANOVA, regression, multiple regression, and meta-regression) that was used to identify the factor. We listed the factors in their order of appearance and categorised whether or not each of the factors was identified (yes or no) in each manuscript, and whether it had a positive or negative association with the placebo response.
Some aspects of the ethical discussion of placebo use are specifically relevant in psychiatry and related disorders. One is the requirement of the Declaration of Helsinki to provide informed consent when exposed (or potentially exposed) to placebo, which can often be compromised in patients with diseases of the central nervous system. In these cases, even putative alternatives (authorised deception or concealment) can be inappropriate or conflict with the mental disorder of patients (eg, patients with paranoia). Authorised deception97 has been shown to have little effect on outcome in experiments with healthy volunteers,98 but could generate rather than reduce suspicious concerns of patients and jeopardise clinical settings and therapeutic goals. A second requirement of informed consent that is specifically relevant to psychiatry is the ability of the patient to understand the true risks of interventions—eg, the side-effects during drug trials. A patient with depression in an acute state might not be able to understand that “in very seldom cases (eg, one out of 1 million)” means an overall statistical risk (even experts might not consent to this statement99), but rather conclude that “as always, I will attract this” in agreement with his or her overall depressive thinking.
A paper100 published in 2013 posed a provocative question: which placebo to cure depression? Similarly, another paper101 questioned whether the elderly would be better off if they were given more placebos? If placebos are overall almost as effective as the drugs in treatment of psychiatric diseases (particularly depression), would prescription of a placebo be as helpful as prescription of an (ineffective) drug? Evans and Hungin102 put forward the same arguments. They argue that in a fictional but representative general practice consultation of a patient with irritable bowel syndrome, if a drug fails to outperform the placebo and the disorder in question is a functional illness with no demonstrable underlying pathology, then the action of the drug is not only no better than placebo, but is also not different from it. They suggest that under these circumstances “it is striking that current governance deems it ethical for a practitioner to prescribe either a drug or a placebo, both of which appear to rely for their effectiveness on a measure of concealment on the part of the doctor, yet deems it unethical for a practitioner openly to prescribe a harmless and enjoyable substance which (in equivalent conditions of transparency and information) is likely to be no less effective than either drug or placebo and is also likely to be better-tolerated and cheaper than the drug”.102
Whereas the common use of placebos in daily medical practice has been acknowledged across subspecialties and cultures,103 open label placebo application is still regarded as ineffective. By contrast, open-label placebo application has been shown experimentally to maintain, at least in part, its efficacy to improve clinical symptoms in neuroticism,104 depression,105 and functional bowel disorders.106 However, the degree and determinants of such elicited placebo responses still need future assessment.
Limitations
Our analysis seems to downplay potential differences in moderators and mediators of the placebo response in specific diseases and disorders, and focuses on factors that occur across these disorders. This focus across the disorders does not imply that we believe that the placebo response is the same across all clinical disorders; instead, experimental research (eg, brain imaging and neurobiological approaches) has shown that different mechanisms of the placebo response are associated with different diseases and disorders.59
This Review is restricted to mediator and moderator analyses of the placebo response in drug and non-drug trials, but withholds from discussing traditional and novel trial designs to enhance, explore, or restrict the placebo response in drug development and clinical investigations: namely, the advantages and pitfalls of waiting list controls and its alternatives, comparator studies, enrichment designs, and the use of registries and historic controls to overcome the limitations of conventional placebo-controlled trials.107 We have also excluded some meta-analyses108 of the placebo response in diseases with high affinity to psychiatry but not in its core.
Conclusions
Our Review of present knowledge of placebo responses in psychiatry across different clinical disorders in children and adults shows that although the placebo response is evident and effective in all disorders—both in RCTs and in laboratory testing—its predictors are still widely unknown. Of the many potential moderators that have been investigated, only some have shown persistent relevance across different diseases. The moderators shown to be most strongly associated with increased placebo effect include low symptom severity at baseline and modern trial design (which both might be linked). A further, reproducible predictor of the placebo response in psychiatry, unbalanced randomisation, creates a similar paradox: more patients are needed to show superiority of drug over placebo, increasing the economic and ethical burden of the trial, contrary to the intentions of the approach. This vicious cycle has to be interrupted in the interest of the patients that need effective therapy.
Acknowledgments
This work was supported by a grant from Deutsche Forschungsgemeinschaft (WE 5658/2-1).
Footnotes
Contributors
PE had the idea for the Review. KW and PE conceptualised the Review. KW, LC, and PE wrote different parts of the Review and all authors read and edited all parts of the Review.
Declaration of interests
We declare no competing interests.
References
- 1.Stolberg M. Inventing the randomized double-blind trial: the Nuremberg salt test of 1835. J R Soc Med. 2006;99:642–43. doi: 10.1258/jrsm.99.12.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raicek JE, Stone BH, Kaptchuk TJ. Placebos in 19th century medicine: a quantitative analysis of the BMJ. BMJ. 2012;345:e8326. doi: 10.1136/bmj.e8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shorter E. A brief history of placebos and clinical trials in psychiatry. Can J Psychiatry. 2011;56:193–97. doi: 10.1177/070674371105600402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hampson JL, Rosenthal D, Frank JD. A comparative study of the effect of mephenesin and placebo on the symptomatology of a mixed group of psychiatric outpatients. Bull Johns Hopkins Hosp. 1954;95:170–77. [PubMed] [Google Scholar]
- 5.Hróbjartsson A, Gøtzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344:1594–602. doi: 10.1056/NEJM200105243442106. [DOI] [PubMed] [Google Scholar]
- 6.Beecher HK. The powerful placebo. JAMA. 1955;159:1602–06. doi: 10.1001/jama.1955.02960340022006. [DOI] [PubMed] [Google Scholar]
- 7.Hróbjartsson A, Gøtzsche PC. Is the placebo powerless? Update of a systematic review with 52 new randomized trials comparing placebo with no treatment. J Intern Med. 2004;256:91–100. doi: 10.1111/j.1365-2796.2004.01355.x. [DOI] [PubMed] [Google Scholar]
- 8.Krogsbøll LT, Hróbjartsson A, Gøtzsche PC. Spontaneous improvement in randomised clinical trials: meta-analysis of three-armed trials comparing no treatment, placebo and active intervention. BMC Med Res Methodol. 2009;9:1. doi: 10.1186/1471-2288-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loranger AW, Prout CT, White MA. The placebo effect in psychiatric drug research. JAMA. 1961;176:920–25. doi: 10.1001/jama.1961.03040240026010. [DOI] [PubMed] [Google Scholar]
- 10.Rickels K, Lipman R, Raab E. Previous medication, duration of illness and placebo response. J Nerv Ment Dis. 1966;142:548–54. doi: 10.1097/00005053-196606000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287:1840–47. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
- 12.Mayberg HS, Silva JA, Brannan SK, et al. The functional neuroanatomy of the placebo effect. Am J Psychiatry. 2002;159:728–37. doi: 10.1176/appi.ajp.159.5.728. [DOI] [PubMed] [Google Scholar]
- 13.Rief W, Nestoriuc Y, Weiss S, Welzel E, Barsky AJ, Hofmann SG. Meta-analysis of the placebo response in antidepressant trials. J Affect Disord. 2009;118:1–8. doi: 10.1016/j.jad.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 14.Alphs L, Benedetti F, Fleischhacker WW, Kane JM. Placebo-related effects in clinical trials in schizophrenia: what is driving this phenomenon and what can be done to minimize it? Int J Neuropsychopharmacol. 2012;15:1003–14. doi: 10.1017/S1461145711001738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enck P, Horing B, Weimer K, Klosterhalfen S. Placebo responses and placebo effects in functional bowel disorders. Eur J Gastroenterol Hepatol. 2012;24:1–8. doi: 10.1097/MEG.0b013e32834bb951. [DOI] [PubMed] [Google Scholar]
- 16.de la Fuente-Fernández R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ. Expectation and dopamine release: mechanism of the placebo effect in Parkinson’s disease. Science. 2001;293:1164–66. doi: 10.1126/science.1060937. [DOI] [PubMed] [Google Scholar]
- 17.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia—imaging a shared neuronal network. Science. 2002;295:1737–40. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 18.Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta J-K. Neurobiological mechanisms of the placebo effect. J Neurosci. 2005;25:10390–402. doi: 10.1523/JNEUROSCI.3458-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown WA, Johnson MF, Chen MG. Clinical features of depressed patients who do and do not improve with placebo. Psychiatry Res. 1992;41:203–14. doi: 10.1016/0165-1781(92)90002-k. [DOI] [PubMed] [Google Scholar]
- 20.Woods SW, Gueorguieva RV, Baker CB, Makuch RW. Control group bias in randomized atypical antipsychotic medication trials for schizophrenia. Arch Gen Psychiatry. 2005;62:961–70. doi: 10.1001/archpsyc.62.9.961. [DOI] [PubMed] [Google Scholar]
- 21.Kemp AS, Schooler NR, Kalali AH, et al. What is causing the reduced drug-placebo difference in recent schizophrenia clinical trials and what can be done about it? Schizophr Bull. 2010;36:504–09. doi: 10.1093/schbul/sbn110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mallinckrodt CH, Zhang L, Prucka WR, Millen BA. Signal detection and placebo response in schizophrenia: parallels with depression. Psychopharmacol Bull. 2010;43:53–72. [PubMed] [Google Scholar]
- 23.Chen YF, Wang SJ, Khin NA, Hung HM, Laughren TP. Trial design issues and treatment effect modeling in multi-regional schizophrenia trials. Pharm Stat. 2010;9:217–29. doi: 10.1002/pst.439. [DOI] [PubMed] [Google Scholar]
- 24.Potkin S, Agid O, Siu C, Watsky E, Vanderburg D, Remington G. Placebo response trajectories in short-term and long-term antipsychotic trials in schizophrenia. Schizophr Res. 2011;132:108–13. doi: 10.1016/j.schres.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 25.Agid O, Siu CO, Potkin SG, et al. Meta-regression analysis of placebo response in antipsychotic trials, 1970–2010. Am J Psychiatry. 2013;170:1335–44. doi: 10.1176/appi.ajp.2013.12030315. [DOI] [PubMed] [Google Scholar]
- 26.Rutherford BR, Pott E, Tandler JM, Wall MM, Roose SP, Lieberman JA. Placebo response in antipsychotic clinical trials: a meta-analysis. JAMA Psychiatry. 2014;71:1409–21. doi: 10.1001/jamapsychiatry.2014.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King BH, Dukes K, Donnelly CL, et al. Baseline factors predicting placebo response to treatment in children and adolescents with autism spectrum disorders: a multisite randomized clinical trial. JAMA Pediatr. 2013;167:1045–52. doi: 10.1001/jamapediatrics.2013.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sysko R, Walsh BT. A systematic review of placebo response in studies of bipolar mania. J Clin Psychiatry. 2007;68:1213–17. doi: 10.4088/jcp.v68n0807. [DOI] [PubMed] [Google Scholar]
- 29.Yildiz A, Vieta E, Tohen M, Baldessarini RJ. Factors modifying drug and placebo responses in randomized trials for bipolar mania. Int J Neuropsychopharmacol. 2011;14:863–75. doi: 10.1017/S1461145710001641. [DOI] [PubMed] [Google Scholar]
- 30.Cohen D, Consoli A, Bodeau N, et al. Predictors of placebo response in randomized controlled trials of psychotropic drugs for children and adolescents with internalizing disorders. J Child Adolesc Psychopharmacol. 2010;20:39–47. doi: 10.1089/cap.2009.0047. [DOI] [PubMed] [Google Scholar]
- 31.Newcorn JH, Sutton VK, Zhang S, et al. Characteristics of placebo responders in pediatric clinical trials of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:1165–72. doi: 10.1097/CHI.0b013e3181bc730d. [DOI] [PubMed] [Google Scholar]
- 32.Waxmonsky JG, Waschbusch DA, Glatt SJ, Faraone SV. Prediction of placebo response in 2 clinical trials of lisdexamfetamine dimesylate for the treatment of ADHD. J Clin Psychiatry. 2011;72:1366–75. doi: 10.4088/JCP.10m05979pur. [DOI] [PubMed] [Google Scholar]
- 33.Buitelaar JK, Sobanski E, Stieglitz RD, Dejonckheere J, Waechter S, Schäuble B. Predictors of placebo response in adults with attention-deficit/hyperactivity disorder: data from 2 randomized trials of osmotic-release oral system methylphenidate. J Clin Psychiatry. 2012;73:1097–102. doi: 10.4088/JCP.11m07528. [DOI] [PubMed] [Google Scholar]
- 34.Blom TJ, Mingione CJ, Guerdjikova AI, Keck PE, Jr, Welge JA, McElroy SL. Placebo response in binge eating disorder: a pooled analysis of 10 clinical trials from one research group. Eur Eat Disord Rev. 2014;22:140–46. doi: 10.1002/erv.2277. [DOI] [PubMed] [Google Scholar]
- 35.Greene NM, Taylor EM, Gage SH, Munafò MR. Industry funding and placebo quit rate in clinical trials of nicotine replacement therapy: a commentary on Etter et al. (2007) Addiction. 2010;105:2217–18. doi: 10.1111/j.1360-0443.2010.03155.x. [DOI] [PubMed] [Google Scholar]
- 36.Litten RZ, Castle IJ, Falk D, et al. The placebo effect in clinical trials for alcohol dependence: an exploratory analysis of 51 naltrexone and acamprosate studies. Alcohol Clin Exp Res. 2013;37:2128–37. doi: 10.1111/acer.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan A, Leventhal RM, Khan SR, Brown WA. Severity of depression and response to antidepressants and placebo: an analysis of the Food and Drug Administration database. J Clin Psychopharmacol. 2002;22:40–45. doi: 10.1097/00004714-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Evans KR, Sills T, Wunderlich GR, McDonald HP. Worsening of depressive symptoms prior to randomization in clinical trials: a possible screen for placebo responders? J Psychiatr Res. 2004;38:437–44. doi: 10.1016/j.jpsychires.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Stein DJ, Baldwin DS, Dolberg OT, Despiegel N, Bandelow B. Which factors predict placebo response in anxiety disorders and major depression? An analysis of placebo-controlled studies of escitalopram. J Clin Psychiatry. 2006;67:1741–46. doi: 10.4088/jcp.v67n1111. [DOI] [PubMed] [Google Scholar]
- 40.Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5:e45. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Papakostas GI, Fava M. Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. Eur Neuropsychopharmacol. 2009;19:34–40. doi: 10.1016/j.euroneuro.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Bridge JA, Birmaher B, Iyengar S, Barbe RP, Brent DA. Placebo response in randomized controlled trials of antidepressants for pediatric major depressive disorder. Am J Psychiatry. 2009;166:42–49. doi: 10.1176/appi.ajp.2008.08020247. [DOI] [PubMed] [Google Scholar]
- 43.Brunoni AR, Lopes M, Kaptchuk TJ, Fregni F. Placebo response of non-pharmacological and pharmacological trials in major depression: a systematic review and meta-analysis. PLoS One. 2009;4:e4824. doi: 10.1371/journal.pone.0004824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sinyor M, Levitt AJ, Cheung AH, et al. Does inclusion of a placebo arm influence response to active antidepressant treatment in randomized controlled trials? Results from pooled and meta-analyses. J Clin Psychiatry. 2010;71:270–79. doi: 10.4088/JCP.08r04516blu. [DOI] [PubMed] [Google Scholar]
- 45.Hunter AM, Cook IA, Leuchter AF. Impact of antidepressant treatment history on clinical outcomes in placebo and medication treatment of major depression. J Clin Psychopharmacol. 2010;30:748–51. doi: 10.1097/JCP.0b013e3181faa474. [DOI] [PubMed] [Google Scholar]
- 46.Rutherford BR, Sneed JR, Tandler JM, Rindskopf D, Peterson BS, Roose SP. Deconstructing pediatric depression trials: an analysis of the effects of expectancy and therapeutic contact. J Am Acad Child Adolesc Psychiatry. 2011;50:782–95. doi: 10.1016/j.jaac.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khin NA, Chen YF, Yang Y, Yang P, Laughren TP. Exploratory analyses of efficacy data from major depressive disorder trials submitted to the US Food and Drug Administration in support of new drug applications. J Clin Psychiatry. 2011;72:464–72. doi: 10.4088/JCP.10m06191. [DOI] [PubMed] [Google Scholar]
- 48.Mancini M, Wade AG, Perugi G, Lenox-Smith A, Schacht A. Impact of patient selection and study characteristics on signal detection in placebo-controlled trials with antidepressants. J Psychiatr Res. 2014;51:21–29. doi: 10.1016/j.jpsychires.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Weimer K, Colloca L, Enck P. Age and sex as moderators of the placebo response: an evaluation of systematic reviews and metaanalyses across medicine. Gerontology. 2014 doi: 10.1159/000365248. published online Nov 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grelotti DJ, Kaptchuk TJ. Placebo by proxy. BMJ. 2011;343:d4345. doi: 10.1136/bmj.d4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whalley B, Hyland ME. Placebo by proxy: the effect of parents’ beliefs on therapy for children’s temper tantrums. J Behav Med. 2013;36:341–46. doi: 10.1007/s10865-012-9429-x. [DOI] [PubMed] [Google Scholar]
- 52.Kirsch I. The emperor’s new drugs: medication and placebo in the treatment of depression. Handb Exp Pharmacol. 2014;225:291–303. doi: 10.1007/978-3-662-44519-8_16. [DOI] [PubMed] [Google Scholar]
- 53.Nutt DJ, Goodwin GM, Bhugra D, Fazel S, Lawrie S. Attacks on antidepressants: signs of deep-seated stigma? Lancet Psychiatry. 2014;1:102–04. doi: 10.1016/S2215-0366(14)70232-9. [DOI] [PubMed] [Google Scholar]
- 54.Gøtzsche PC. Why I think antidepressants cause more harm than good. Lancet Psychiatry. 2014;1:104–06. doi: 10.1016/S2215-0366(14)70280-9. [DOI] [PubMed] [Google Scholar]
- 55.Enck P, Vinson B, Malfertheiner P, Zipfel S, Klosterhalfen S. The placebo response in functional dyspepsia—reanalysis of trial data. Neurogastroenterol Motil. 2009;21:370–77. doi: 10.1111/j.1365-2982.2008.01241.x. [DOI] [PubMed] [Google Scholar]
- 56.Diener HC, Dowson AJ, Ferrari M, Nappi G, Tfelt-Hansen P. Unbalanced randomization influences placebo response: scientific versus ethical issues around the use of placebo in migraine trials. Cephalalgia. 1999;19:699–700. doi: 10.1046/j.1468-2982.1999.019008699.x. [DOI] [PubMed] [Google Scholar]
- 57.Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- 58.Lidstone SC, Schulzer M, Dinelle K, et al. Effects of expectation on placebo-induced dopamine release in Parkinson Disease. Arch Gen Psychiatry. 2010;67:857–65. doi: 10.1001/archgenpsychiatry.2010.88. [DOI] [PubMed] [Google Scholar]
- 59.Enck P, Bingel U, Schedlowski M, Rief W. The placebo response in medicine: minimize, maximize or personalize? Nat Rev Drug Discov. 2013;12:191–204. doi: 10.1038/nrd3923. [DOI] [PubMed] [Google Scholar]
- 60.Wampold BE, Budge SL. The 2011 Leona Tyler Award Address: the relationship—and its relationship to the common and specific factors of psychotherapy. Couns Psychol. 2012;40:601–23. [Google Scholar]
- 61.Baskin TW, Tierney SC, Minami T, Wampold BE. Establishing specificity in psychotherapy: a meta-analysis of structural equivalence of placebo controls. J Consult Clin Psychol. 2003;71:973–79. doi: 10.1037/0022-006X.71.6.973. [DOI] [PubMed] [Google Scholar]
- 62.Tschacher W, Junghan UM, Pfammatter M. Towards a taxonomy of common factors in psychotherapy—results of an expert survey. Clin Psychol Psychother. 2014;21:82–96. doi: 10.1002/cpp.1822. [DOI] [PubMed] [Google Scholar]
- 63.Rakel DP, Hoeft TJ, Barrett BP, Chewning BA, Craig BM, Niu M. Practitioner empathy and the duration of the common cold. Fam Med. 2009;41:494–501. [PMC free article] [PubMed] [Google Scholar]
- 64.Kaptchuk TJ, Stason WB, Davis RB, et al. Sham device v inert pill: randomised controlled trial of two placebo treatments. BMJ. 2006;332:391–97. doi: 10.1136/bmj.38726.603310.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meissner K, Fässler M, Rücker G, et al. Differential effectiveness of placebo treatments: a systematic review of migraine prophylaxis. JAMA Intern Med. 2013;173:1941–51. doi: 10.1001/jamainternmed.2013.10391. [DOI] [PubMed] [Google Scholar]
- 66.Linde K, Niemann K, Meissner K. Are sham acupuncture interventions more effective than (other) placebos? A re-analysis of data from the Cochrane review on placebo effects. Forsch Komplementmed. 2010;17:259–64. doi: 10.1159/000320374. [DOI] [PubMed] [Google Scholar]
- 67.Pagnin D, de Queiroz V, Pini S, Cassano GB. Efficacy of ECT in depression: a meta-analytic review. J ECT. 2004;20:13–20. doi: 10.1097/00124509-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 68.Read J, Bentall R. The effectiveness of electroconvulsive therapy: a literature review. Epidemiol Psichiatr Soc. 2010;19:333–47. doi: 10.1017/s1121189x00000671. [DOI] [PubMed] [Google Scholar]
- 69.Ross CA. The sham ECT literature: implications for consent to ECT. Ethical Hum Psychol Psychiatry. 2006;8:17–28. doi: 10.1891/ehpp.8.1.17. [DOI] [PubMed] [Google Scholar]
- 70.Rasmussen KG. Sham electroconvulsive therapy studies in depressive illness: a review of the literature and consideration of the placebo phenomenon in electroconvulsive therapy practice. J ECT. 2009;25:54–59. doi: 10.1097/YCT.0b013e3181719b23. [DOI] [PubMed] [Google Scholar]
- 71.Blease CR. Electroconvulsive therapy, the placebo effect and informed consent. J Med Ethics. 2013;39:166–70. doi: 10.1136/medethics-2012-100955. [DOI] [PubMed] [Google Scholar]
- 72.Khedr EM, Elfetoh NA, Ali AM, Noamany M. Anodal transcranial direct current stimulation over the dorsolateral prefrontal cortex improves anorexia nervosa: a pilot study. Res Restor Neurol Neurosci. 2014;32:789–97. doi: 10.3233/RNN-140392. [DOI] [PubMed] [Google Scholar]
- 73.Jauch-Chara K, Kistenmacher A, Herzog N, Schwarz M, Schweiger U, Oltmanns KM. Repetitive electric brain stimulation reduces food intake in humans. Am J Clin Nutr. 2014;100:1003–09. doi: 10.3945/ajcn.113.075481. [DOI] [PubMed] [Google Scholar]
- 74.Enck P, Klosterhalfen S, Zipfel S. Acupuncture, psyche and the placebo response. Auton Neurosci. 2010;157:68–73. doi: 10.1016/j.autneu.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 75.Schneider A, Enck P, Streitberger K, et al. Acupuncture treatment in irritable bowel syndrome. Gut. 2006;55:649–54. doi: 10.1136/gut.2005.074518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carvalho F, Weires K, Ebling M, Padilha MdSR, Ferrão YA, Vercelino R. Effects of acupuncture on the symptoms of anxiety and depression caused by premenstrual dysphoric disorder. Acupunct Med. 2013;31:358–63. doi: 10.1136/acupmed-2013-010394. [DOI] [PubMed] [Google Scholar]
- 77.Deng G, Chan Y, Sjoberg D, et al. Acupuncture for the treatment of post-chemotherapy chronic fatigue: a randomized, blinded, sham-controlled trial. Support Care Cancer. 2013;21:1735–41. doi: 10.1007/s00520-013-1720-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schneider A, Weiland C, Enck P, et al. Neuroendocrinological effects of acupuncture treatment in patients with irritable bowel syndrome. Complement Ther Med. 2007;15:255–63. doi: 10.1016/j.ctim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 79.Dennis CL, Dowswell T. Interventions (other than pharmacological, psychosocial or psychological) for treating antenatal depression. Cochrane Database Syst Rev. 2013;7:CD006795. doi: 10.1002/14651858.CD006795.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaptchuk TJ, Kelley JM, Deykin A, et al. Do “placebo responders” exist? Contemp Clin Trials. 2008;29:587–95. doi: 10.1016/j.cct.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 81.Colloca L, Grillon C. Understanding placebo and nocebo responses for pain management. Curr Pain Headache Rep. 2014;18:419. doi: 10.1007/s11916-014-0419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Geers AL, Wellman JA, Fowler SL, Helfer SG, France CR. Dispositional optimism predicts placebo analgesia. J Pain. 2010;11:1165–71. doi: 10.1016/j.jpain.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pecina M, Azhar H, Love TM, et al. Personality trait predictors of placebo analgesia and neurobiological correlates. Neuropsychopharmacology. 2013;38:639–46. doi: 10.1038/npp.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Darragh M, Booth RJ, Consedine NS. Investigating the “placebo personality” outside the pain paradigm. J Psychosom Res. 2013;76:414–21. doi: 10.1016/j.jpsychores.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 85.Horing B, Weimer W, Muth ER, Enck P. Prediction of the placebo response: review of the literature. Front Psychol. 2014;5:1079. doi: 10.3389/fpsyg.2014.01079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feltner D, Hill C, Lenderking W, Williams V, Morlock R. Development of a patient-reported assessment to identify placebo responders in a generalized anxiety disorder trial. J Psychiatr Res. 2009;43:1224–30. doi: 10.1016/j.jpsychires.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 87.Vase L, Riley JL, 3rd, Price DD. A comparison of placebo effects in clinical analgesic trials versus studies of placebo analgesia. Pain. 2002;99:443–52. doi: 10.1016/S0304-3959(02)00205-1. [DOI] [PubMed] [Google Scholar]
- 88.Horing B. PhD Thesis. University of Tübingen; 2014. Placebo effects and their prediction across multiple experimentally induced symptoms: motion sickness, cutaneous heat and cold pain, and rectal distension. [Google Scholar]
- 89.Eippert F, Finsterbusch J, Bingel U, Büchel C. Direct evidence for spinal cord involvement in placebo analgesia. Science. 2009;326:404. doi: 10.1126/science.1180142. [DOI] [PubMed] [Google Scholar]
- 90.Keitel A, Wojtecki L, Hirschmann J, et al. Motor and cognitive placebo-/nocebo-responses in Parkinson’s disease patients with deep brain stimulation. Behav Brain Res. 2013;250:199–205. doi: 10.1016/j.bbr.2013.04.051. [DOI] [PubMed] [Google Scholar]
- 91.Frisaldi E, Carlino E, Lanotte M, Lopiano L, Benedetti F. Characterization of the thalamic-subthalamic circuit involved in the placebo response through single-neuron recording in Parkinson patients. Cortex. 2013;60:3–9. doi: 10.1016/j.cortex.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 92.Goebel MU, Meykadeh N, Kou W, Schedlowski M, Hengge UR. Behavioral conditioning of antihistamine effects in patients with allergic rhinitis. Psychother Psychosom. 2008;77:227–34. doi: 10.1159/000126074. [DOI] [PubMed] [Google Scholar]
- 93.Furmark T, Appel L, Henningsson S, et al. A link between serotonin-related gene polymorphisms, amygdala activity, and placebo-induced relief from social anxiety. J Neurosci. 2008;28:13066–74. doi: 10.1523/JNEUROSCI.2534-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leuchter AF, McCracken JT, Hunter AM, Cook IA, Alpert JE. Monoamine oxidase a and catechol-o-methyltransferase functional polymorphisms and the placebo response in major depressive disorder. J Clin Psychopharmacol. 2009;29:372–77. doi: 10.1097/JCP.0b013e3181ac4aaf. [DOI] [PubMed] [Google Scholar]
- 95.Hall KT, Lembo AJ, Kirsch I, et al. Catechol-O-methyltransferase val158met polymorphism predicts placebo effect in irritable bowel syndrome. PLoS One. 2012;7:e48135. doi: 10.1371/journal.pone.0048135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.WHO. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–94. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 97.Miller FG, Colloca L. The legitimacy of placebo treatments in clinical practice: evidence and ethics. Am J Bioeth. 2009;9:39–47. doi: 10.1080/15265160903316263. [DOI] [PubMed] [Google Scholar]
- 98.Martin AL, Katz J. Inclusion of authorized deception in the informed consent process does not affect the magnitude of the placebo effect for experimentally induced pain. Pain. 2010;149:208–15. doi: 10.1016/j.pain.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 99.Ziegler A, Hadlak A, Mehlbeer S, König IR. Comprehension of the description of side effects in drug information leaflets: a survey of doctors, pharmacists and lawyers. Dtsch Arztebl Int. 2013;110:669–73. doi: 10.3238/arztebl.2013.0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Naudet F, Millet B, Charlier P, Reymann JM, Maria AS, Falissard B. Which placebo to cure depression? A thought-provoking network meta-analysis. BMC Med. 2013;11:230. doi: 10.1186/1741-7015-11-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cherniack EP. Would the elderly be better off if they were given more placebos? Geriatr Gerontol Int. 2010;10:131–37. doi: 10.1111/j.1447-0594.2009.00580.x. [DOI] [PubMed] [Google Scholar]
- 102.Evans HM, Hungin AP. Uncomfortable implications: placebo equivalence in drug management of a functional illness. J Med Ethics. 2007;33:635–38. doi: 10.1136/jme.2006.019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fässler M, Meissner K, Schneider A, Linde K. Frequency and circumstances of placebo use in clinical practice—a systematic review of empirical studies. BMC Med. 2010;8:15. doi: 10.1186/1741-7015-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Park LC, Covi L. Nonblind placebo trial: an exploration of neurotic patients’ responses to placebo when its inert content is disclosed. Arch Gen Psychiatry. 1965;12:36–45. [PubMed] [Google Scholar]
- 105.Brown WA. Placebo as a treatment for depression. Neuropsychopharmacology. 1994;10:265–69. doi: 10.1038/npp.1994.53. [DOI] [PubMed] [Google Scholar]
- 106.Kelley JM, Kaptchuk TJ, Cusin C, Lipkin S, Fava M. Open-label placebo for major depressive disorder: a pilot randomized controlled trial. Psychother Psychosom. 2012;81:312–14. doi: 10.1159/000337053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Weimer K, Enck P. Traditional and innovative experimental and clinical trial designs—advantages and pitfalls. Handb Exp Pharmacol. 2014;225:237–72. doi: 10.1007/978-3-662-44519-8_14. [DOI] [PubMed] [Google Scholar]
- 108.Cho HJ, Hotopf M, Wessely S. The placebo response in the treatment of chronic fatigue syndrome: a systematic review and meta-analysis. Psychosom Med. 2005;67:301–13. doi: 10.1097/01.psy.0000156969.76986.e0. [DOI] [PubMed] [Google Scholar]