Abstract
Objective
To describe the study population and estimate overall survival of women with a serous “borderline” ovarian tumor (SBT) in Denmark over 25 years relative to the general population.
Methods
The Danish Pathology Data Bank and the Danish Cancer Registry were used to identify 1487 women diagnosed with SBTs from 1978 to 2002. The histologic slides were collected from Danish pathology departments and reviewed by expert pathologists and classified as SBT/atypical proliferative serous tumor (APST) or noninvasive low-grade serous carcinoma (LGSC). Associated implants were classified as noninvasive or invasive. Medical records were collected from hospital departments and reviewed. Data were analyzed using Kaplan–Meier and relative survival was estimated with follow-up through September 2, 2013.
Results
A cohort of 1042 women with a confirmed SBT diagnosis was identified. Women with stage I had an overall survival similar to the overall survival expected from the general population (p = 0.3), whereas women with advanced stage disease had a poorer one (p < 0.0001). This was evident both in women with non-invasive (p < 0.0001) and invasive implants (p < 0.0001). Only among women with advanced stage, overall survival of women with SBT/APST (p < 0.0001) and noninvasive LGSC (p < 0.0001) was poorer than expected from the general population.
Conclusions
To date this is the largest nationwide cohort of SBTs where all tumors have been verified by expert pathologists. Only in women with advanced stage SBT, overall survival is poorer than in the general population which applies both to women with noninvasive and invasive implants as well as to women with SBT/APST and noninvasive LGSC.
Keywords: Serous “borderline” ovarian tumors, Population-based, Centralized pathology review, Long-term follow-up, Relative survival
Introduction
Ovarian cancer is the most lethal gynecologic cancer in the Western world and the sixth most common female cancer [1]. Serous tumors are the most common histologic type (75%) [2]. In 1973, the World Health Organization (WHO) proposed classifying a group of tumors that appeared to display behavior intermediate between a benign cystadenoma and the usual type of serous carcinoma as “tumors of borderline malignancy (carcinomas of low malignant potential)” [3]. This term eventually evolved into serous borderline tumor (SBT). Because many patients with widespread extraovarian disease did well even when not or inadequately treated, the extraovarian lesions were designated “implants” rather than metastases, and were eventually divided into noninvasive and invasive as the latter were more predictive of an adverse outcome [4]. Subsequent studies called attention to a particular growth pattern of SBTs, characterized by a micropapillary architecture which unlike the usual type of an SBT was associated with a significantly worse outcome [5]. It was proposed that the usual type of an SBT be designated “atypical proliferative serous tumor (APST)” and the micropapillary tumor “noninvasive micropapillary low-grade serous carcinoma (LGSC).” This proved to be controversial as some investigators acknowledged that although the micropapillary tumor was more often associated with advanced stage and a higher frequency of invasive implants, it was not significantly associated with an adverse outcome [6,7]. Accordingly, these investigators favor the term “SBT, micropapillary variant,” whereas others recognizing the similarity of the micropapillary tumors to LGSC prefer the designation “noninvasive (micropapillary) LGSC.” The terms are considered synonymous by the WHO in 2014 [8]. To allow comparison with previous studies, we will use the terms SBT/APST for the usual type of an SBT and noninvasive LGSC for the tumor displaying micropapillary architecture.
The nature of SBTs is still not fully understood, but recent data suggest that an SBT is a precursor of LGSC, whereas high-grade serous carcinoma (HGSC) develops independently of an SBT [9,10]. In contrast to serous ovarian cancer, SBTs affect younger women [11]. Bilateral salpingo-oophorectomy, hysterectomy and omentectomy with staging, is generally the treatment of choice in perimenopausal and menopausal women. However, since many women are of reproductive age, fertility-sparing surgery is an important consideration [11].
Based on two nationwide, population-based registries, we have an extensive registration of borderline ovarian tumors in Denmark, which gives us a unique opportunity to examine SBTs from the entire female population. We initiated a nationwide cohort of all women registered with an SBT in Denmark covering 1978–2002. We performed a centralized pathology review of all tumors and followed the women up to 36 years, making it the largest study of its kind. This is the first in a series of papers to be published based on data from the entire cohort. The aim of the present paper was to describe the study design and methodology, the characteristics of the study population and the overall survival of women with SBTs relative to that of the general female population of Denmark, stratified by stage, implant type and tumor type.
Materials and methods
Case identification and acquisition
Our cohort comprised all women registered in the Danish Pathology Data Bank (PDB) or the Danish Cancer Registry (CR) with an SBT diagnosis during 1978–2002. The nationwide PDB contains information about all cytologic and histologic diagnoses from pathology departments in Denmark since 1997. Each pathology department is mandated to report to the national PDB daily through an online system. In addition, the majority of pathology departments has transferred information to the national PDB from 1997 back to 1978, although data from this period are not entirely complete [12]. The nationwide CR has collected information on cancer cases in Denmark since 1943. In 1987, reporting of cancers to the registry became mandatory, although not for borderline ovarian tumors which are most likely underreported in the CR [12]. The inclusion of data from both registries increases completeness of data in our cohort. In the case of more than one recorded diagnosis, we used the first one. SBTs were identified in the PDB by the SNOMED topography codes starting with T87 and T86910, T86920, T86921 and T86922 and in the CR using the ICD-O3 topography code C569. We used the SNOMED/ICD-O3 morphology codes M84411, M84421, M84601, M84611, M84621, M84631 and M90141.
Pathology review
We retrieved all histologic slides from the primary ovarian tumor and extra-ovarian tissue from the various pathology departments in Denmark. The slides were reviewed by expert gynecologic pathologists (RV and RJK) who were blinded to all clinical parameters. The pathologists reviewed the cases in the same room and looked at several cases together in order to reach consensus about the diagnosis. The SBTs were classified according to the criteria defined at the National Cancer Institute’s Borderline Ovarian Tumor Workshop in 2003 [13] and as they are defined by the WHO in 2014 [8]. Briefly, an SBT was defined as a noninvasive low-grade serous tumor displaying greater architectural proliferation than a cystadenoma but lacking the stromal invasion of invasive carcinoma. In addition, a diagnosis of SBT required at least 10% epithelial proliferation of the tumor for the distinction from papillary serous cystadenoma/adenofibromas. SBTs were subclassified as typical SBT/APST or noninvasive (micropapillary) LGSC [9]. The micropapillary area had to measure at least 5 mm in confluent growth to be classified as noninvasive LGSC. Implants were classified as noninvasive (epithelial or desmoplastic) or invasive (LGSC) [14]. The latter are equivalent to LGSC. In addition, the expanded criteria of Bell et al. (infiltration of underlying tissue, micropapillary appearance similar to noninvasive LGSC, and/or solid nests within clear lacunar spaces) were also used to diagnose invasive implants [15]. We also reviewed cases diagnosed with well-differentiated serous carcinoma from 1997 to 2002 identified from the PDB since we surmised that some might have been SBTs that had been misclassified. We included cases, which fulfilled the criteria of either SBT/APST or noninvasive LGSC based on the centralized pathology review [16].
Clinical data retrieval
We linked our cohort with the National Patient Registry, a nationwide registry of virtually all somatic discharges from all Danish hospitals and outpatient clinics since, respectively, 1977 and 1995. All the hospitals in which the women had been admitted for surgery and subsequent treatment were identified and the medical records including a description of the surgical procedures, pathology reports and chemotherapy records were collected, reviewed and data abstracted. These data included type of surgery (salpingectomy, oophorectomy, hysterectomy, and other), tumor size, surface involvement, capsule rupture, ascites, and type of chemotherapy. All women were treated according to the Danish clinical guidelines for ovarian cancer which do not include staging and lymph node dissection. The tumor stage was determined using the International Federation of Gynecology and Obstetrics (FIGO) staging system [17], and it was based on information collected from the medical files and the review of the histologic slides from extra-ovarian sites, if any.
Study population
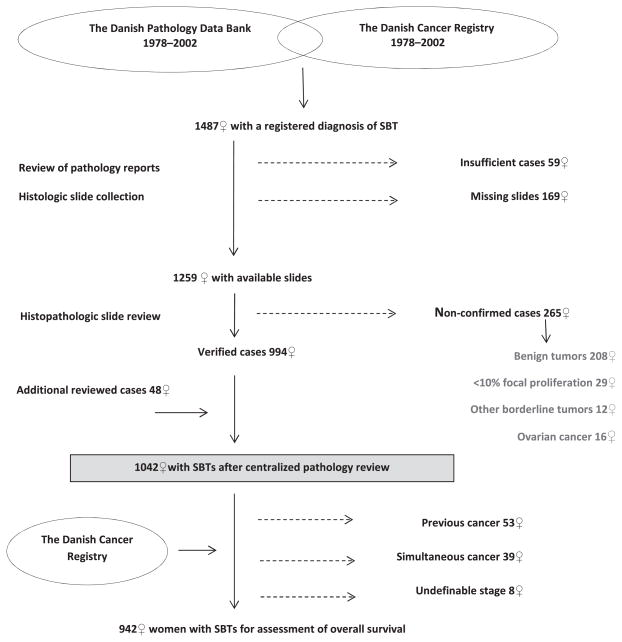
A flowchart of the study design is shown in Fig. 1. We identified 1487 women with a registration of an SBT in the PDB and/or CR during 1978–2002, based on the first recorded diagnosis. Based on the review of the pathology reports, we excluded 59 women (frozen section biopsy only, cyst fluid, needle biopsy, autopsy, diagnosis made outside of Denmark or prior to the study period, and coding errors). For the remaining 1428 women, we retrieved the microscopic slides from the pathology departments in Denmark except for 169 whose slides could not be located. All slides for the remaining 1259 women were reviewed by the pathology panel. The review process showed that 994 women (79%) fulfilled the study criteria of having an SBT, whereas 265 women (21%) did not. In addition, among 107 women with an initial diagnosis of well-differentiated serous carcinoma in 1997–2002, we included 48 women who upon the expert review were found to have an SBT (SBT/APST or noninvasive LGSC) [16], leaving 1042 women with an SBT for the present study.
Fig. 1.
Design of the study.
To assess overall survival, we linked our cohort with the CR. For all women who had a concomitant ovarian cancer, the ovarian cancer slides were also reviewed by the pathology panel. We excluded 53 women with a previous cancer and 39 women with a concomitant cancer diagnosis (except for non-melanoma skin cancer), and 8 women for whom we were unable to define the stage at diagnosis, leaving 942 women for the survival analysis.
Follow-up
To obtain information on vital status, we linked the cohort with the nationwide Danish Civil Registration System, which contains information on name, sex, addresses, migration and death. Since April 1, 1968 all residents in Denmark have been assigned a unique personal identification number (PIN). The PIN is included in all health registries and can be used as a key identifier to ensure accurate linkage of information between registries. All women were followed through linkage with the Civil Registration System and the vital status (alive, emigrated or dead) and date of event, if any, were determined for each woman through September 2, 2013. Follow-up was available on all women. The study was approved by the Danish Data Protection Agency and the Danish Scientific Ethical Committee.
Statistical analysis
Chi-square test was used to compare categorical variables, and t-test was used to compare continuous variables. The survival curve among women diagnosed with SBTs was estimated using the Kaplan–Meier estimator with survival times calculated from the date of diagnosis until the date of death, emigration or end of follow-up, whichever came first. Relative survival was calculated as the ratio of observed to expected survival with the latter estimated using standard Danish female mortality rates derived from Statistics Denmark stratified by age and calendar period in 1 year groups, calculated using the SAS-macro developed by Dickman [18]. Overall mortality for women diagnosed with SBTs was compared with the standard population mortality over the total follow-up period using the Score test. Statistical modeling was performed using the SAS/STAT version 9.2 (SAS Institute, Cary, NC, USA).
Results
The cohort comprised 1042 women with a confirmed SBT diagnosis during 1978–2002. The median age at diagnosis was 50 years (range: 16–97), and 27% of women were 40 years or younger. For women with SBT/APST, the median age was 50 years (range: 16–97), while it was 54 years (range: 24–91) for women with noninvasive LGSC. At end follow-up, a total of 333 women (32%) had died. The median time of follow-up was 15 years (range: 0–36) resulting in a total of 16 183 women-years of follow-up.
In 265 women who were registered with SBTs in Denmark from 1978 to 2002, the diagnosis was not confirmed by the pathology panel as seen in Fig. 1. These women were reclassified as having benign ovarian tumors (n = 208), SBTs with <10% epithelial proliferation (n = 29), other borderline ovarian tumors (n = 12) and primary or secondary ovarian carcinoma (n = 16). Characteristics of those women compared with the 1042 women in whom the SBT diagnosis was confirmed are shown in S1.
Clinical features
Clinical, pathologic and treatment features of the women in the SBT cohort (n = 1042), overall and according to stage at diagnosis, are shown in Table 1. Stage I was seen in 85% of women (n = 886), and 14% (n = 145) had advanced stage disease (II–IV). For 11 women, we were unable to define stage at diagnosis (1%). Women with stage I were older at diagnosis (median = 51 years) than were women with advanced stage disease (median = 45 years), although not significantly (p = 0.2). The median size of tumor was 9 cm (range: 1–35). Bilateral disease (p < 0.0001), surface involvement (p < 0.0001) and ascites (p < 0.0001) were more common in women with advanced stage.
Table 1.
Characteristics of 1042 women with a serous borderline ovarian tumor (SBT) in Denmark 1978–2002.
| Overall (n =1042)
|
Stage I (n=886)
|
Stages II–IV (n=145)
|
p-value
|
||||
|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | Stage I versus II–IV#,* | |
| Clinical features | |||||||
| FIGO stage | – | ||||||
| Stage I | 886 | (85.0) | – | – | – | – | |
| Stage II | 69 | (6.6) | – | – | – | – | |
| Stage III | 75 | (7.2) | – | – | – | – | |
| Stage IV | 1 | (0.1) | |||||
| Undefinable stage | 11 | (1.1) | – | – | – | – | |
| Median age (range) | 50 | (16–97) | 51 | (16–97) | 45 | (17–89) | 0.2 |
| Median tumor size (cm) (range)§ | 9 | (1–35) | 9 | (1–35) | 10 | (3–30) | 0.7 |
| Laterality | <0.0001 | ||||||
| Unilateral | 680 | (65.3) | 630 | (71.1) | 44 | (30.3) | |
| Bilateral | 362 | (34.7) | 256 | (28.9) | 101 | (69.7) | |
| Surface involvement | <0.0001* | ||||||
| No | 626 | (60.1) | 596 | (67.3) | 29 | (20.0) | |
| Yes | 387 | (37.1) | 266 | (30.0) | 112 | (77.2) | |
| Unknown | 29 | (2.8) | 24 | (2.7) | 4 | (2.8) | |
| Capsule rupture | 1.0* | ||||||
| No | 664 | (63.7) | 566 | (63.9) | 93 | (64.1) | |
| Yes | 250 | (24.0) | 213 | (24.0) | 33 | (22.8) | |
| Unknown | 128 | (12.3) | 107 | (12.1) | 19 | (13.1) | |
| Ascites | <0.0001* | ||||||
| No | 751 | (72.1) | 685 | (77.3) | 63 | (43.5) | |
| Yes | 252 | (24.2) | 168 | (19.0) | 77 | (53.1) | |
| Unknown | 39 | (3.7) | 33 | (3.7) | 5 | (3.4) | |
| Pathologic features | |||||||
| Tumor type | 0.0009 | ||||||
| SBT/APST | 961 | (92.2) | 828 | (93.4) | 124 | (85.5) | |
| Noninvasive LGSC (>5 mm) | 81 | (7.8) | 58 | (6.5) | 21 | (14.5) | |
| Implants | – | ||||||
| No | 886 | (85.0) | 886 | (100.0) | 0 | (0.0) | |
| Yes | 145 | (13.9) | 0 | (0.0) | 145 | (100.0) | |
| Noninvasive | 121 | (83.4) | 0 | (0.0) | 121 | (83.4) | |
| Invasive | 24 | (16.6) | 0 | (0.0) | 24 | (16.6) | |
| Undefinable stage | 11 | (1.1) | 0 | (0.0) | 0 | (0.0) | |
| Treatment features | |||||||
| Surgical treatment | – | ||||||
| USO | 143 | (14%) | 134 | (15%) | 8 | (6%) | |
| BSO | 74 | (7%) | 65 | (7%) | 7 | (5%) | |
| Other surgical treatment | 30 | (3%) | 26 | (3%) | 2 | (1%) | |
| USO/BSO + Hysterectomy | 795 | (76%) | 661 | (75%) | 128 | (88%) | |
| Chemotherapy | – | ||||||
| No | 833 | (80.0) | 770 | (86.9) | 62 | (42.7) | |
| Yes | 191 | (18.3) | 102 | (11.5) | 80 | (55.2) | |
| Unknown | 18 | (1.7) | 14 | (1.6) | 3 | (2.1) | |
Abbreviations: International Federation of Gynecology and Obstetrics (FIGO); atypical proliferative serous tumor (APST); low-grade serous carcinoma (LGSC); unilateral salpingo-oophorectomy (USO); bilateral salpingo-oophorectomy (BSO); number (n).
Based on biggest tumor size (cm) and 1014 women (97%) since 28 women did not have tumor size information (3%).
Excluding 11 women (1%) with undefinable stage.
Excluding women with unknown information.
Pathologic features
As shown in Table 1, the majority of women had SBT/APST (92%), whereas noninvasive LGSC was diagnosed in 81 women (8%). A total of 145 women had implants (14%) of which 121 (83%) had noninvasive and 24 (17%) had invasive implants. Noninvasive LGSC was significantly more common in women with advanced stage disease (p = 0.0009). Invasive implants were diagnosed significantly more frequently in women with noninvasive LGSC than in women with SBT/APST (p < 0.0001) (data not shown). The type of implant did not differ between women with stage II or stage III–IV (p = 0.1) (data not shown).
Treatment features
The majority of women had unilateral or bilateral salpingo-oophorectomy (USO/BSO) with hysterectomy (76%) (Table 1). A total of 191 women (18%) were treated with chemotherapy, comprising platinum mono-therapy (40%), platinum in combination with taxane therapy (27%) and other types (16%) (data not shown). For 17% of women who had chemotherapy, there was no available information on type because that part of the medical record was missing in the hospitals.
Overall survival
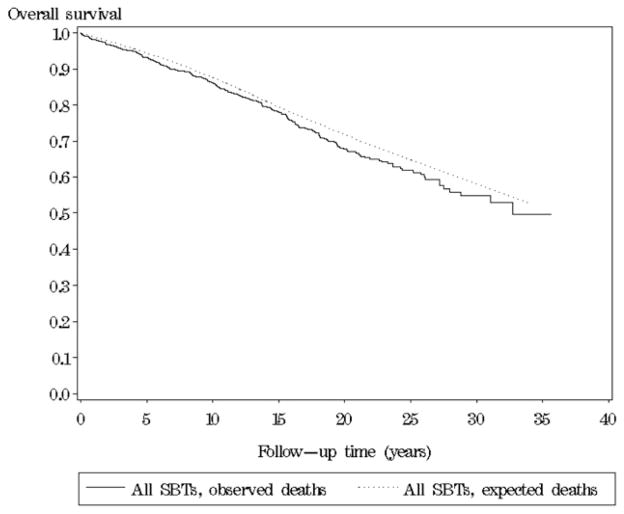
After excluding women with previous or concomitant cancers or undefinable stage at diagnosis, overall 5-, 10- and 15-year observed survival among the remaining 942 women was 93%, 86% and 77%, respectively. Fig. 2 shows that in the initial 15 years after diagnosis, women with SBTs had an overall observed survival comparable with the overall survival expected from the general female population of same age in the same calendar period; the relative 5-, 10- and 15-year overall survival was 99% (95% CI: 96.7–100.2), 98% (95% CI: 95.6–100.6) and 98% (95% CI: 94.6–101.6), respectively. Subsequently, women with SBT had a slightly poorer overall survival. During the entire follow-up period, a total of 272 deaths were observed among women with SBTs compared with 223.6 expected from the general female population of same age in the same calendar period (p = 0.001).
Fig. 2.
Overall survival of women with a serous borderline ovarian tumor (SBT) in Denmark 1978–2002 relative to the general population of same age in the same calendar year.
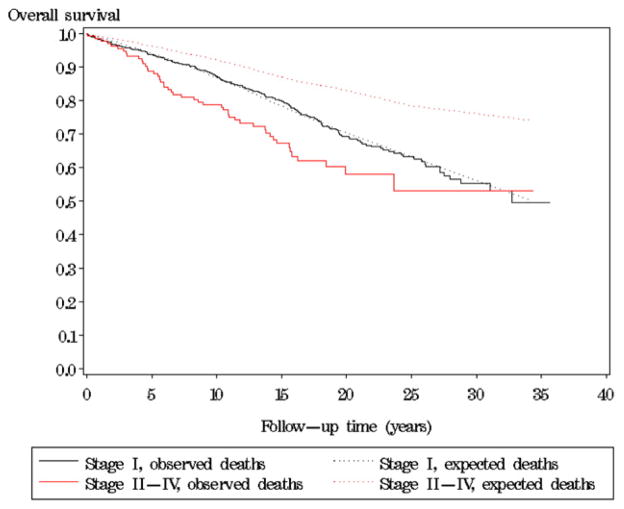
Fig. 3 shows overall survival for women with an SBT after stratifying by stage at diagnosis. The overall survival for women with stage I was similar to that of the general population (224 observed deaths versus 208.0 expected) (p = 0.3), whereas for women with advanced stage disease the overall survival was significantly poorer than expected from the general population mortality (48 observed deaths versus 15.6 expected) (p < 0.0001).
Fig. 3.
Overall survival of women with a serous borderline ovarian tumor (SBT) in Denmark 1978–2002 relative to the general population of same age in the same calendar year with regard to stage at diagnosis (I and II–IV).
Among women with advanced stage disease, the overall survival of both women with noninvasive and invasive implants, respectively, was poorer compared with the general population (p < 0.0001) as shown in Table 2. The relative 5- and 10-year overall survival was 95% (95% CI: 87.9–98.7) and 90% (95% CI: 80.7–95.7) for women with noninvasive implants and 75% (95% CI: 46.6–91.9) and 60% (95% CI: 32.7–82.6) for women with invasive implants.
Table 2.
Overall survival of women with an advanced stage (II–IV) serous borderline ovarian tumor (SBT) in Denmark 1978–2002 relative to the general female population of same age in the same calendar year.
| n | Relative survival
|
Total number of
|
p-value* | ||||
|---|---|---|---|---|---|---|---|
| 5-year (95% CI) | 10-year (95% CI) | 15-year (95% CI) | Observed deaths | Expected deaths | |||
| Implants | |||||||
| Noninvasive | 114 | 95% (87.9–98.7) | 90% (80.7–95.7) | 83% (72.1–91.6) | 35 | 13.6 | <0.0001 |
| Invasive | 19 | 75% (46.6–91.9) | 60% (32.7–82.6) | 37% (13.6–63.8) | 13 | 2.0 | <0.0001 |
| Tumor type | |||||||
| SBT/APST | 113 | 94% (86.2–98.0) | 85% (75.8–92.4) | 77% (65.0–86.0) | 40 | 13.4 | <0.0001 |
| Noninvasive LGSC | 20 | 83% (57.3–95.7) | 87% (59.7–99.6) | 80% (51.4–98.3) | 8 | 2.1 | <0.0001 |
Abbreviations: atypical proliferative serous tumor (APST); low-grade serous carcinoma (LGSC); number (n); confidence interval (CI).
Total number of observed deaths among women with advanced stage (II–IV) SBTs compared with the total number of expected deaths in the general female population of same age in the same calendar year over the total follow-up period using the Score test.
The survival of women with SBT/APST (248 observed deaths versus 209.1 expected; p = 0.007) and noninvasive LGSC (24 observed deaths versus 14.5 expected; p = 0.01), respectively, was slightly poorer compared with the general population (data not shown). When we looked at survival for the two different types of an SBT in relation to stage, we found no difference in overall survival for women with stage I SBT/APST (208 observed deaths versus 195.6 expected; p = 0.4) and noninvasive LGSC (16 observed deaths versus 12.4 expected; p = 0.3), respectively, compared with the general population (data not shown). In contrast as shown in Table 2, among women with advanced stage disease, women with SBT/APST and noninvasive LGSC, respectively, had a poorer overall survival compared with the general population (p < 0.0001). The relative 5- and 10-year overall survival was 94% (95% CI: 86.2–98.0) and 85% (95% CI: 75.8–92.4) for women with SBT/APST and 83% (95% CI: 57.3–95.7) and 87% (95% CI: 59.7–99.6) for women with noninvasive LGSC.
Discussion
We have established a nationwide cohort of 1042 women with SBTs diagnosed during 25 years in Denmark with follow-up time up to 36 years (median = 15 years) and with centralized pathology review of all histologic slides. A large proportion of previous studies have not had expert review [19–25], did not distinguish tumors by histologic subtypes [26], were based on relatively few cases or included cases from tertiary care centers potentially leading to selection bias as the most complex and difficult cases are sent to these institutions [6, 27–41]. Table 3 shows previous studies including more than 100 cases of SBTs, where the tumors had gone through a centralized pathology review. With the exception of du Bois et al. [42], the studies were based on relatively few cases (101–276). In addition, the majority of the studies had a relatively short follow-up (<7 years). Du Bois et al. [42] included 644 women diagnosed with SBTs during 11 years in 24 centers in Germany. The tumors were verified by centralized pathology review, but the women were only followed for a median of 3.4 years. Recurrences of SBT, subsequent development of extra-ovarian invasive LGSC, and death due to disease may occur many years after the initial diagnosis of an SBT. Therefore, to truly understand the behavior of SBTs, it has been previously demonstrated that long-term follow-up is necessary [43]. In our study, the median length of follow-up was 15 years, and we had a total of 16 183 women-years of follow-up, which makes this cohort by far the largest published to date, the other studies comprising 2500 women-years at risk or less (Table 3).
Table 3.
Other studies with centralized pathology review of women with a serous borderline ovarian tumor (SBT) including more than 100 women.
| Reference (year) | Country | Study period (total) | No. of cases | Age at diagnosis (years)
|
FIGO stage
|
Years of follow-up (years)
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median/mean | (Range) | I | II–IV | Unstaged | Median/mean | (Range) | Women-years** | ||||
| Hannibal et al. | Denmark | 1978–2002 (25 years) | 1042 | 50 | (15–96) | 886 (85%) | 145 (14%) | 11 (1%) | 15 | (0–36) | 16 183 |
| Du Bois et al. (2013) | Germany | 1998–2008 (11 years) | 644 | 48 | (14–91) | 489 (76%) | 155 (24%) | – | 3.4* | NA | 2200 |
| Park et al. (2011) | Korea | 1990–2010 (10 years) | 130 | 44 | (14–87) | 108 (83%) | 22 (17%) | – | 4.5 | (0.4–20) | 753 |
| Quddus et al. (2011) | USA | 1991–2005 (15 years) | 188 | NA | NA | 171 (91%) | 17 (9%) | – | 5.6 | (0.6–22) | 1058 |
| Shih et al. (2011) | USA | 1979–2008 (30 years) | 196 | 43* | (15–94) | 133 (68%) | 63 (32%) | – | 3.7* | (0–23) | 725 |
| Uzan et al. (2011) | France | 1969–2006 (38 years) | 154 | 36 | (14–74) | 0 (0%) | 168 (100%) | – | 4.4 | (1–36) | 643 |
| Kane et al. (2009) | France | 1973–2006 (34 years) | 168 | 36 | (14–77) | 41 (25%) | 127 (75%) | – | 4.8 | (0.1–36) | 760 |
| Akeseon et al. (2008) | Sweden | 1993–2004 (12 years) | 219 | 55* | (16–90) | 197 (82%) | 22 (10%) | – | 7.7* | (2–13) | 1687 |
| De laco et al. (2008) | Italy | 1985–2006 (22 years) | 102 | 46* | (14–85) | 72 (71%) | 30 (29%) | – | 5.0* | (0.3–20) | 514 |
| Ren et al. (2008) | China | 2001–2007 (7 years) | 101 | 40* | (14–80) | 66 (65%) | 35 (35%) | – | 3.3* | (0.7–7) | 337 |
| Cusido et al. (2007) | Spain | 1990–1997 (8 years) | 208 | 46* | NA | 177 (85%) | 21 (10%) | 10 (5%) | 7.3* | NA | 1525 |
| Longacre et al. (2005) | USA | 1958–1998 (41 years) | 276 | 51 | (12–89) | 163 (59%) | 113 (41%) | – | 9.2 | (0.7–48) | 2500 |
| Prat and Nictolis (2002) | Spain | 1980–1998 (19 years) | 137 | 39 | (14–84) | 92 (67%) | 45 (33%) | – | 7.2 | (1–18) | 762 |
| Zanetta et al. (2001) | Italy | 1982–1997 (16 years) | 205 | 39* | NA | 171 (83%) | 34 (17%) | – | 5.8* | (1–15) | 1155 |
| Rota et al. (1999) | Italy | 1982–1996 (15 years) | 188 | 46* | (16–74) | 125 (66%) | 18 (10%) | 45 (24%) | 6.6* | (0.8–14) | 1238 |
| Barnhill et al. (1995) | USA | 1983–1992 10 years) | 146 | 44 | (19–79) | 146 (100%) | 0 (0%) | – | 3.5 | (0.8–14) | 516 |
| Kaern et al. (1993) | Norway | 1970–1982 (13 years) | 174 | 53* | (15–83) | 148 (85%) | 26 (15%) | – | 12.4* | (0.4–21) | 2161 |
| Trope et al. (1993) | Norway | 1970–1988 (19 years) | 114 | 48* | (15–81) | 109 (96%) | 5 (4%) | – | 12.3* | (0.3–21) | 1397 |
| Leake et al. (1992) | USA | 1979–1984 (6 years) | 200 | 34 | (6–98) | 135 (67.5%) | 65 (32.5%) | – | 10 | (4–27) | 2240 |
Abbreviations: International Federation of Gynecology and Obstetrics (FIGO); number (No.); not available (NA). If a study population is published several times, only data from the newest paper is presented in the table.
Also based on women with other histologic subtypes of borderline ovarian tumors.
Calculated based on the number of women included in the study (N) and the number of median/mean years of follow-up (M) (N × M = women-years of follow-up).
We found that the survival of women with SBTs was closely correlated with stage. The overall survival of women with an SBT confined to the ovaries was similar to the overall survival expected from the general Danish female population (of same age in same calendar period), whereas for women with advanced stage disease (noninvasive and invasive implants) it was poorer. Overall survival compared with the one expected from the general population was poorer both for women with SBT/APST and noninvasive LGSC, but only for women with advanced stage disease. The analysis of women with noninvasive LGSC was, however, based on relatively few cases, so the statistical power in these analyses was limited.
Our centralized pathology review revealed that 21% of women, originally diagnosed with SBTs in Denmark in 1978–2002, were interpreted as not having an SBT. Most of these tumors were serous cystadenomas/fibromas that lacked sufficient proliferation to qualify as an SBT. This misclassification is somewhat higher than found by du Bois et al. [42] who found 9%. Similar to our study, du Bois et al. [42] judged the majority of misclassified SBTs as benign ovarian tumors. In addition, our pathology review of 107 cases with an original diagnosis of invasive well-differentiated serous carcinoma in 1997–2002 revealed that we included 48 women who were reclassified as SBTs (SBT/APST or noninvasive LGSC) highlighting the importance of a centralized pathology review for these types of large-scale epidemiologic studies. Unfortunately, we were unable to review well-differentiated serous carcinoma in the period 1978–1996. Most studies of SBTs have not reviewed well-differentiated serous carcinomas to uncover potentially misclassified SBTs; therefore, our inclusion of these cases identified after review of a subset is of importance.
Among the 265 women for whom the original diagnosis of an SBT was not confirmed by the pathology panel, 16 women (6%) were reclassified as having primary or secondary carcinoma involving the ovary. A significantly higher proportion of women in this group died during follow-up (56% died) compared with the women with a confirmed diagnosis of an SBT (32%). These findings strongly suggest that the malignant potential of SBTs in the prior population-based literature without centralized pathology review is likely overestimated because of inclusion of carcinoma misclassified as SBTs.
In summary, this study describes the largest population-based, nationwide SBT cohort to date with all diagnoses confirmed at a centralized pathology review. Overall survival was poorer than expected from the general female population among women with advanced stage disease only which applied both to women with noninvasive and invasive implants as well as to women with SBT/APST and noninvasive LGSC. To further assess the prognosis of women with SBTs (SBT/APST and noninvasive LGSC), we are currently analyzing disease-specific survival and investigating the role of different histopathologic features and biomarkers in women who have experienced recurrence or progression of disease. These studies will be the subject of forthcoming publications.
Supplementary Material
HIGHLIGHTS.
Only among women with an advanced stage serous borderline tumor (SBT), overall survival is poorer than the general population’s
The poorer survival applies both to women with noninvasive and invasive implants
The poorer survival applies both to women with SBT/atypical proliferative serous tumor (APST) and noninvasive low-grade serous carcinoma (LGSC)
Acknowledgments
We are grateful to Nick Martinussen for assistance with data management. The study has been funded by the Danish Cancer Society (22806056) and the National Cancer Institute (NCI) (RO1 CA116184).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ygyno.2014.06.002.
Conflict of interest statement
The authors declare no conflicts of interest.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Seidman JD, Cho KR, Ronnett BM, Kurman RJ. Surface Epithelial Tumors of the Ovary. In: Kurman RJ, Ellenson LH, Ronnett BM, editors. Blaustein’s Pathology of the Female Genital Tract. 6. New York: Springer; 2011. pp. 679–784. [Google Scholar]
- 3.Serov SF, Scully RE, Sobin LH. International histological classification and staging of tumors, histologic typing of ovarian tumors. Geneva: World Health Organization; 1973. [Google Scholar]
- 4.Seidman JD, Kurman RJ. Ovarian serous borderline tumors: a critical review of the literature with emphasis on prognostic indicators. Hum Pathol. 2000;31(5):539–57. doi: 10.1053/hp.2000.8048. [DOI] [PubMed] [Google Scholar]
- 5.Seidman JD, Kurman RJ. Subclassification of serous borderline tumors of the ovary into benign and malignant types. A clinicopathologic study of 65 advanced stage cases. Am J Surg Pathol. 1996;20(11):1331–45. doi: 10.1097/00000478-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Prat J, De NM. Serous borderline tumors of the ovary: a long-term follow-up study of 137 cases, including 18 with a micropapillary pattern and 20 with microinvasion. Am J Surg Pathol. 2002;26(9):1111–28. doi: 10.1097/00000478-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Deavers MT, Gershenson DM, Tortolero-Luna G, Malpica A, Lu KH, Silva EG. Micropapillary and cribriform patterns in ovarian serous tumors of low malignant potential: a study of 99 advanced stage cases. Am J Surg Pathol. 2002;26(9):1129–41. doi: 10.1097/00000478-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Kurman RJ, Carcangiu ML, Herrington S, Young RH, editors. World Health Organization Classification of Tumours of Female Reproductive Organs. 4. Lyon, France: IARC Press; 2014. pp. 18–21. [Google Scholar]
- 9.Vang R, Shih I, Kurman RJ. Ovarian low-grade and high-grade serous carcinoma: pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv Anat Pathol. 2009;16(5):267–82. doi: 10.1097/PAP.0b013e3181b4fffa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurman RJ, Shih I. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34(3):433–43. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morice P. Borderline tumours of the ovary and fertility. Eur J Cancer. 2006;42(2):149–58. doi: 10.1016/j.ejca.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 12.Kjaerbye-Thygesen A, Huusom LD, Frederiksen K, Kjaer SK. Primary ovarian cancer. A comparison of registrations in the Danish Cancer Registry and the Pathology Data Bank. Ugeskr Laeger. 2007;169(1):50–4. [PubMed] [Google Scholar]
- 13.Seidman JD, Soslow RA, Vang R, Berman JJ, Stoler MH, Sherman ME, et al. Borderline ovarian tumors: diverse contemporary viewpoints on terminology and diagnostic criteria with illustrative images. Hum Pathol. 2004;35(8):918–33. doi: 10.1016/j.humpath.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Bell DA, Weinstock MA, Scully RE. Peritoneal implants of ovarian serous borderline tumors. Histologic features and prognosis. Cancer. 1988;62(10):2212–22. doi: 10.1002/1097-0142(19881115)62:10<2212::aid-cncr2820621024>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 15.Bell KA, Smith Sehdev AE, Kurman RJ. Refined diagnostic criteria for implants associated with ovarian atypical proliferative serous tumors (borderline) and micropapillary serous carcinomas. Am J Surg Pathol. 2001;25(4):419–32. doi: 10.1097/00000478-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Hannibal CG, Vang R, Junge J, Kjaerbye-Thygesen A, Kurman RJ, Kjaer SK. A binary histologic grading system for ovarian serous carcinoma is an independent prognostic factor: a population-based study of 4317 women diagnosed in Denmark 1978–2006. Gynecol Oncol. 2012;125(3):655–60. doi: 10.1016/j.ygyno.2012.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.FIGO. Classification and staging of malignant tumours in the female pelvis. Acta Obstet Gynecol Scand. 1971;50(1):1–7. doi: 10.3109/00016347109157278. [DOI] [PubMed] [Google Scholar]
- 18.Paul Dickman. available in 2014 at http://www.pauldickman.com.
- 19.Morris CR, Liu L, Rodriguez AO, Cress RD, Snipes K. Epidemiologic features of borderline ovarian tumors in California: a population-based study. Cancer Causes Control. 2013;24(4):665–74. doi: 10.1007/s10552-013-0145-9. [DOI] [PubMed] [Google Scholar]
- 20.Levi F, Randimbison L, Blanc-Moya R, La VC. Second neoplasms after invasive and borderline ovarian cancer. Eur J Cancer Prev. 2009;18(3):216–9. doi: 10.1097/CEJ.0b013e3283240474. [DOI] [PubMed] [Google Scholar]
- 21.Mink PJ, Sherman ME, Devesa SS. Incidence patterns of invasive and borderline ovarian tumors among white women and black women in the United States. Results from the SEER Program, 1978–1998. Cancer. 2002;95(11):2380–9. doi: 10.1002/cncr.10935. [DOI] [PubMed] [Google Scholar]
- 22.Iscovich J, Shushan A, Schenker JG, Paltiel O. The incidence of borderline ovarian tumors in Israel: a population-based study. Cancer. 1998;82(1):147–51. doi: 10.1002/(sici)1097-0142(19980101)82:1<147::aid-cncr18>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 23.Bjorge T, Engeland A, Hansen S, Trope CG. Prognosis of patients with ovarian cancer and borderline tumours diagnosed in Norway between 1954 and 1993. Int J Cancer. 1998;75(5):663–70. doi: 10.1002/(sici)1097-0215(19980302)75:5<663::aid-ijc1>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 24.Harlow BL, Weiss NS, Lofton S. Epidemiology of borderline ovarian tumors. J Natl Cancer Inst. 1987;78(1):71–4. doi: 10.1093/jnci/78.1.71. [DOI] [PubMed] [Google Scholar]
- 25.Sherman ME, Mink PJ, Curtis R, Cote TR, Brooks S, Hartge P, et al. Survival among women with borderline ovarian tumors and ovarian carcinoma: a population-based analysis. Cancer. 2004;100(5):1045–52. doi: 10.1002/cncr.20080. [DOI] [PubMed] [Google Scholar]
- 26.Akeson M, Zetterqvist BM, Dahllof K, Jakobsen AM, Brannstrom M, Horvath G. Population-based cohort follow-up study of all patients operated for borderline ovarian tumor in western Sweden during an 11-year period. Int J Gynecol Cancer. 2008;18(3):453–9. doi: 10.1111/j.1525-1438.2007.01051.x. [DOI] [PubMed] [Google Scholar]
- 27.Barnhill DR, Kurman RJ, Brady MF, Omura GA, Yordan E, Given FT, et al. Preliminary analysis of the behavior of stage I ovarian serous tumors of low malignant potential: a Gynecologic Oncology Group study. J Clin Oncol. 1995;13(11):2752–6. doi: 10.1200/JCO.1995.13.11.2752. [DOI] [PubMed] [Google Scholar]
- 28.Shih KK, Zhou Q, Huh J, Morgan JC, Iasonos A, Aghajanian C, et al. Risk factors for recurrence of ovarian borderline tumors. Gynecol Oncol. 2011;120(3):480–4. doi: 10.1016/j.ygyno.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Longacre TA, McKenney JK, Tazelaar HD, Kempson RL, Hendrickson MR. Ovarian serous tumors of low malignant potential (borderline tumors): outcome-based study of 276 patients with long-term (>or =5-year) follow-up. Am J Surg Pathol. 2005;29(6):707–23. doi: 10.1097/01.pas.0000164030.82810.db. [DOI] [PubMed] [Google Scholar]
- 30.Kaern J, Trope CG, Abeler VM. A retrospective study of 370 borderline tumors of the ovary treated at the Norwegian Radium Hospital from 1970 to 1982. A review of clinicopathologic features and treatment modalities. Cancer. 1993;71(5):1810–20. doi: 10.1002/1097-0142(19930301)71:5<1810::aid-cncr2820710516>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 31.Leake JF, Currie JL, Rosenshein NB, Woodruff JD. Long-term follow-up of serous ovarian tumors of low malignant potential. Gynecol Oncol. 1992;47(2):150–8. doi: 10.1016/0090-8258(92)90099-5. [DOI] [PubMed] [Google Scholar]
- 32.Cusido M, Balaguero L, Hernandez G, Falcon O, Rodriguez-Escudero FJ, Vargas JA, et al. Results of the national survey of borderline ovarian tumors in Spain. Gynecol Oncol. 2007;104(3):617–22. doi: 10.1016/j.ygyno.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Zanetta G, Rota S, Chiari S, Bonazzi C, Bratina G, Mangioni C. Behavior of borderline tumors with particular interest to persistence, recurrence, and progression to invasive carcinoma: a prospective study. J Clin Oncol. 2001;19(10):2658–64. doi: 10.1200/JCO.2001.19.10.2658. [DOI] [PubMed] [Google Scholar]
- 34.Trope C, Kaern J, Vergote IB, Kristensen G, Abeler V. Are borderline tumors of the ovary overtreated both surgically and systemically? A review of four prospective randomized trials including 253 patients with borderline tumors. Gynecol Oncol. 1993;51(2):236–43. doi: 10.1006/gyno.1993.1279. [DOI] [PubMed] [Google Scholar]
- 35.Park JY, Kim DY, Kim JH, Kim YM, Kim KR, Kim YT, et al. Micropapillary pattern in serous borderline ovarian tumors: does it matter? Gynecol Oncol. 2011;123(3):511–6. doi: 10.1016/j.ygyno.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Quddus MR, Sung CJ, Zhang C, Moore RG, Ou JJ, Steinhoff MM, et al. The presence and location of epithelial implants and implants with epithelial proliferation may predict a higher risk of recurrence in serous borderline ovarian tumors: a clinicopathologic study of 188 cases. Hum Pathol. 2012;43(5):747–52. doi: 10.1016/j.humpath.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 37.Uzan C, Kane A, Rey A, Gouy S, Camatte S, Pautier P, et al. Prognosis and prognostic factors of the micropapillary pattern in patients treated for stage II and III serous borderline tumors of the ovary. Oncologist. 2011;16(2):189–96. doi: 10.1634/theoncologist.2009-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kane A, Uzan C, Rey A, Gouy S, Camatte S, Pautier P, et al. Prognostic factors in patients with ovarian serous low malignant potential (borderline) tumors with peritoneal implants. Oncologist. 2009;14(6):591–600. doi: 10.1634/theoncologist.2008-0263. [DOI] [PubMed] [Google Scholar]
- 39.De Iaco P, Ferrero A, Rosati F, Melpignano M, Biglia N, Rolla M, et al. Behaviour of ovarian tumors of low malignant potential treated with conservative surgery. Eur J Surg Oncol. 2009;35(6):643–8. doi: 10.1016/j.ejso.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 40.Ren J, Peng Z, Yang K. A clinicopathologic multivariate analysis affecting recurrence of borderline ovarian tumors. Gynecol Oncol. 2008;110(2):162–7. doi: 10.1016/j.ygyno.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 41.Rota SM, Zanetta G, Ieda N, Rossi R, Chiari S, Perego P, et al. Clinical relevance of retroperitoneal involvement from epithelial ovarian tumors of borderline malignancy. Int J Gynecol Cancer. 1999;9(6):477–80. doi: 10.1046/j.1525-1438.1999.99071.x. [DOI] [PubMed] [Google Scholar]
- 42.Du Bois A, Ewald-Riegler N, de GN, Reuss A, Mahner S, Fotopoulou C, et al. Borderline tumours of the ovary: a cohort study of the Arbeitsgmeinschaft Gynakologische Onkologie (AGO) Study Group. Eur J Cancer. 2013;49(8):1905–14. doi: 10.1016/j.ejca.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 43.Silva EG, Gershenson DM, Malpica A, Deavers M. The recurrence and the overall survival rates of ovarian serous borderline neoplasms with noninvasive implants is time dependent. Am J Surg Pathol. 2006;30(11):1367–71. doi: 10.1097/01.pas.0000213294.81154.95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.