Abstract
Renal cell carcinomas (RCCs) harboring the t(6;11)(p21;q12) translocation were first described in 2001 and recently recognized by the 2013 International Society of Uro-logical Pathology Vancouver Classification of Renal Neoplasia. Although these RCCs are known to label for melanocytic markers HMB45 and Melan A and the cysteine protease cath-epsin K by immunohistochemistry (IHC), a comprehensive IHC profile has not been reported. We report 10 new t(6;11) RCCs, all confirmed by break-apart TFEB fluorescence in situ hybridization. A tissue microarray containing 6 of these cases and 7 other previously reported t(6;11) RCCs was constructed and immunolabeled for 21 different antigens. Additional whole sections of t(6;11) RCC were labeled with selected IHC markers. t(6;11) RCC labeled diffusely and consistently for cathepsin K and Melan A (13 of 13 cases) and almost always at least focally for HMB45 (12 of 13 cases). They labeled frequently for PAX8 (14 of 23 cases), CD117 (10 of 14 cases), and vimentin (9 of 13 cases). A majority of cases labeled at least focally for cytokeratin Cam5.2 (8 of 13 cases) and CD10 and RCC marker antigen (10 of 14 cases each). In contrast to a prior study's findings, only a minority of cases labeled for Ksp-cadherin (3 of 19 cases). The median H score (product of intensity score and percentage labeling) for phosphorylated S6, a marker of mTOR pathway activation, was 101, which is high relative to most other RCC subtypes. In summary, IHC labeling for PAX8, Cam5.2, CD10, and RCC marker antigen supports classification of the t(6;11) RCC as carcinomas despite frequent negativity for broad-spectrum cytokeratins and EMA. Labeling for PAX8 distinguishes the t(6;11) RCC from epithelioid angiomyolipoma, which otherwise shares a similar immunoprofile. CD117 labeling is more frequent in the t(6;11) RCC compared with the related Xp11 translocation RCC. Increased pS6 expression suggests a possible molecular target for the uncommon t(6;11) RCCs that metastasize.
Keywords: renal cell carcinoma, biomarkers, TFEB
Renal cell carcinomas (RCCs) harboring the t(6;11)-(p21;ql2) translocation were first described in 2001.1 Although their lineage was initially unclear, the t(6;11) RCCs have now been accepted by the 2013 International Society of Urological Pathology Vancouver Classification of Renal Neoplasia as a subtype of the MiT family of translocation RCC, which includes the more common Xp11 translocation RCC.2 The t(6;11) translocation fuses the transcription factor EB (TFEB), a transcription factor related to microphthalmia transcription factor (MITF), with Alpha, an untranslated gene of unknown function, resulting in overexpression of native TFEB.3,4 TFEB, MITF, TFE3, and TFEC belong to the MITF subfamily of transcription factors, all of which have overlapping transcriptional activities.5 Overexpression of TFEB in the t(6;11) RCC is thought to result in expression of proteins normally driven by MITF in other cell types. For example, unlike non-MiT family translocation RCCs, the t(6;11) RCCs underexpress cytokeratins and consistently label by immunohistochemistry (IHC) for the melanocytic markers HMB45 and Melan A.1,6–10 In addition, the t(6;11) RCCs label for cathepsin K, a protease that is expressed in osteoclasts but that is not present in non–MiT family translocation RCCs.7,11,12
Histologically, t(6;11) RCC typically displays a solid or alveolar architecture and features larger epithelioid cells surrounding distinctive foci of smaller cells with dense chromatin, which are clustered around hyaline basement membrane material. Cases with atypical morphology, including absence of small cells and cases resembling clear cell RCC, papillary RCC, Xp11 translocation RCC, and epithelioid angiomyolipoma, have been reported.1,6,8–10,13–16 Overall, only approximately 40 cases have previously been described in the literature (reviewed in Argani et al16). Given the potential for misclassification, it is possible that the t(6;11) RCC is more common than currently thought.
By IHC, the most sensitive and specific marker of the t(6;11) RCC is TFEB protein, which is overexpressed as a result of the Alpha-TFEB gene fusion. However, this marker is difficult to optimize using standard incubation protocols on automated IHC stainers, and few laboratories see enough cases to justify adding it to their repertoire. Only a small number of studies have assessed the IHC phenotype of t(6;11) RCC for other more commonly used renal tumor markers, such as PAX8, carbonic anhydrase IX (CA IX), kidney-specific Cadherin (Ksp-cadherin), and α-methylacyl-CoA racemase. The studies are generally characterized by small sample sizes and a limited number of IHC markers.6,7 Moreover, therapeutic targets have not been identified. In this study, we provide a more complete IHC characterization of t(6;11) RCC with a diverse range of markers.
Materials And Methods
Institutional Review Board Approval
This study was approved by the Institutional Review Board of The Johns Hopkins Hospital.
Tissue Microarray Design
A tissue microarray containing 6 of the newly reported cases and 7 other previously reported cases from our archives (13 total genetically confirmed cases) of t(6;11) RCC was constructed and immunolabeled for cathepsin K, Melan A, HMB45, cytokeratins Cam5.2 and AE1/3, CD117, PAX8, RCC marker antigen, vimentin, CD 10, α-methylacyl-CoA racemase, PAX2, Ksp-cadherin, EMA, CA IX, inhibin, SOX10, estrogen receptor (ER), epithelial cell adhesion molecule (EpCAM), phosphory-lated S6 (pS6), and MITF. Whole sections of a recent additional genetically confirmed case were immunolabeled for PAX8, CD117, RCC marker antigen, and CD10. For PAX8 and Ksp-cadherin, whole sections of additional t(6;11) RCC cases were also studied to address the possibility of heterogeneity of intratumoral labeling (see the Results section). For purposes of comparison, 19 cases of epithelioid angiomyolipoma were labeled for PAX8 and pS6. For all markers except pS6, labeling in > 10% of cells was considered a positive result, whereas labeling in 1 % to 10% of cells was considered focally positive. For pS6, the product of the percentage of neoplastic cells labeling for pS6 and the intensity of labeling (0 = negative, 1 = weak, 2 = moderate, 3 = strong) was multiplied to give an H score (0 to 300).
IHC Methods
IHC labeling was performed on the Benchmark XT autostainer (Ventana Medical Systems Inc., Tucson, AZ) using the I-View detection kit. The standard antibodies used, vendors, pretreatments, and dilutions were as follows: cathepsin K (Abcam; steam, 1:800), HMB45 (Novacastra; catalog#ncl-hmb45, steam, 1:100), Melan A (Cell Marque; catalog 281 M-8, clone A103, steam, 1:500), Cam5.2 (Cell Marque; steam, prediluted), AE1/3 (Chemicon; steam, 1:4000), MITF (Dako; steam, 1:50), PAX2 (Zymed; catalog#71 to 6000, steam, 1:100), RCC marker antigen (Leica; steam, 1:50), vimentin (Ventana, 790-2917; prediluted), CD10 (Leica; org-8941, steam, prediluted), racemase (Zeta; P504S, steam, 1:100), SOX10 (Santa; SC-17,342, steam, 1:100), EMA (Ventana; 760-4259, stream, prediluted), inhibin (Serotec; steam, 1:25), PAX8 (ProteinTech Group, Chicago, IL; steam, 1:100), CA IX (Novacastra NCL-L-CA IX; steam, 1:100), EpCAM (Santa Cruz; sc-25,308, steam, 1:200), Ksp-cadherin (Invitrogen, San Francisco, CA; steam, 1:100), CD117 (Cell Marque CMA768; steam, prediluted), and ER (Novacastra; 6F11, 1 μg/mL). For pS6, after a 50-minute steam pretreatment in EDTA buffer, we used the antibody from Cell Signaling (#2215) at 1:200 dilution overnight at 4°C, followed by the Dako Polyclonal Envision + secondary for 30 minutes.
Fluorescence In Situ Hybridization Methods
Fluorescence in situ hybridization (FISH) was performed as previously described with a probe consisting of 2 contigs that flank the TFEB gene on 6p21. The distal contig consists of 3 BAC/PAC clones (RP11-298J23, RP5-973N23, and RP11-533020) labeled with Spectrum Orange, and the proximal contig consists of 2 BAC/PAC clones (RP1-149M18 and RP11-328M4) labeled with Spectrum Green, from BacPac Resources at CHORI (Children's Hospital Oakland Research Institution), designed for research use. Sections of 5 μm thickness were deparaffinized, dehydrated, and pretreated in a VP 2000 processor (Abbott Molecular), followed by subsequent treatment with pepsin. The probe mixture was applied, and the slides were incubated in a humidified atmosphere (Thermobrite; Abbott Molecular) at 80°C for 8 minutes to denature the probe and target DNA simultaneously, followed by hybridization overnight at 37°C. The slides were then washed in 2 × SSC/0.3% NP-40 for 2 minutes at 72°C. The nuclei were counterstained with 4,6-diamidino-2-phenylindole. After hybridization, all slides were maintained at 4°C in the dark. FISH signals were assessed using a Zeiss Axioskop microscope. The presence of split signals in > 15.8% of a total of 120 nuclei was considered a positive result.16
Results
Additional Genetically Confirmed Cases of t(6;11) RCC
Clinical
The mean and median age of the additional genetically confirmed cases of t(6;11) RCC were 33 and 34 years, respectively (range, 3 to 68 y). One of these cases (case 5) was previously reported as a TFEB IHC-positive case but without genetic confirmation.18 It is noteworthy that the 68-year-old patient (case 8) is as old as any other patient reported with t(6;11) RCC. One patient (case 9) developed a rib metastasis 8 years after resection of a localized renal tumor. The cases were found in 7 male and 2 female patients and in 1 whose sex was unknown. Additional clinical data are found in Table 1.
Table 1. Summary of New Genetically Confirmed Cases of t(6;11) RCC in This Report.
| Case# | Age/Sex | Clinical/Gross Appearance | IHC | TFEB FISH (% Split Signals) |
|---|---|---|---|---|
| 1 | Unknown | NA | TFEB +, Cathepsin K +, HMB45 +, Melan A+, PAX8 +; TFE3− | 86 |
| 2 | 44/M | Chronic renal insufficiency; 7.8 cm cyst with a 3.5cm solid nodule | TFEB + | 62 |
| 3 | 9/M | 4 cm | TFEB +, HMB45 focal+ | 58 |
| 4 | 3/F | Pigmented grossly | HMB45 +; TFE3−, TFEB−, MITF− | 56 |
| 5* | 23/M | 10 cm | HMB45 +, TFEB focal+ | 78 |
| 6 | 9/F | 9 cm | Melan A+, HMB45 focal+; TFE3−, TFEB− | 38 |
| 7 | 46/M | 15 cm | PAX8 +, Cathepsin K+, Melan A+ | 52.5 |
| 8 | 68/M | 2.8 cm | Melan A+, PAX8 +; TFE3− | 43.3 |
| 9 | 34/M | 3 cm | PAX8 +, Melan A +, CA IX− | 80 |
| 10 | 62/M | 3 cm | Melan A+, Cathepsin K+, CA IX focal+ | 69 |
Case reported in 2011 without FISH confirmation.
NA indicates not available.
Radiology
Radiology data were available for 1 of the cases (case 8, Table 1). A computed tomography (CT) scan with intravenous contrast revealed a 2.8 cm hetero-genously enhancing renal mass involving the left upper pole. The tumor had higher attenuation than normal renal parenchyma on noncontrast imaging and demonstrated enhancement with intravenous contrast administration (Fig. 1A). Coronal images from the delayed phase of an intravenous contrast-enhanced CT scan showed a 2.8 cm left upper pole renal mass extending deep into the kidney. The tumor appeared to involve the collecting system and abutted the sinus fat of the kidney (Fig. 1B).
Figure 1.
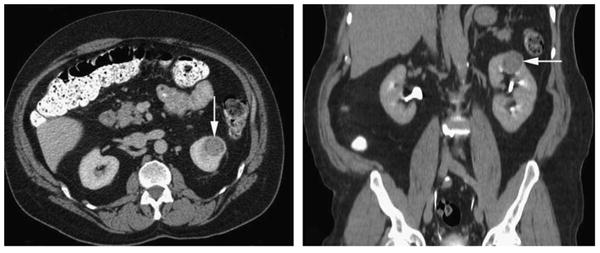
Radiologic findings for case 8 (Table 1). A, A 2.8 cm heterogenously enhancing left upper pole renal mass (arrow) was seen on CT with intravenous contrast. The tumor had higher attenuation than normal renal parenchyma on noncontrast imaging and demonstrated enhancement with intravenous contrast administration. B, Coronal images from the delayed phase of an intravenous contrast-enhanced CT scan showed a 2.8 cm left upper pole renal mass (arrow) extending deep into the kidney.
Histologic Appearance
All but 1 of the new t(6;11) cases showed a solid or alveolar architecture featuring larger epithelioid cells surrounding clusters of smaller cells (Figs. 2A, B). Case 9 lacked a small cell component. Several cases showed previously unreported histologic features. Case 2 was a predominantly cystic 7.8 cm mass radiologically classified as a Bosniak class IV cyst, which contained a 3.5 cm solid tumor nodule. Microscopically, the cystic areas consisted of thin septa containing clear cells simulating the appearance of multilocular cystic RCC (Fig. 2C). The solid areas corresponded to a nested clear cell neoplasm similar to clear cell RCC, which contained prominent entrapped and distorted native renal tubules simulating a biphasic renal neoplasm (Figs. 2D-F). Case 10 was an angioin-vasive, nested, eosinophilic polygonal cell neoplasm with high-grade nuclei throughout, which suggested the diagnosis of high-grade clear cell RCC or unclassified high-grade RCC. Only 1 focus demonstrated a cluster of smaller cells, which suggested the possibility of t(6;11) RCC (Figs. 2G, H).
Figure 2.
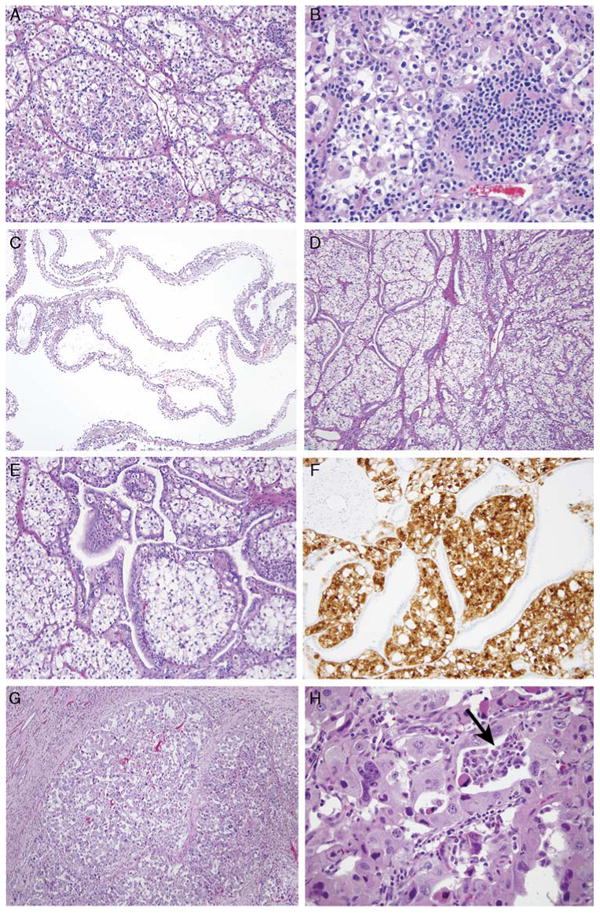
Typical and unusual morphology of t(6;11) RCC in this study. A and B, The typical morphology of t(6;11) RCC is that of a nested epithelioid cell neoplasm with a second population of smaller epithelioid cells associated with hyaline basement membrane material. C, Case 2 was extensively cystic radiologically and grossly, and microscopically this corresponded to thin cysts lined by clear cells simulating multilocular cystic RCC. D and E, The solid areas of this neoplasm demonstrated nests of clear cells associated with a florid proliferation of entrapped native renal tubules. F, The distinction between the t(6;11) RCC and the entrapped tubules is highlighted on a Melan A stain, which labels the neoplasm but not the entrapped tubules. G, Case 10 was a rather nondescript high-grade nested eosinophilic RCC. H, Very focally, a smaller cell population was present (arrow), which suggested the t(6;11) RCC.
Immunohistochemistry
Established Markers: Cytokeratins Cam5.2 and AE1/3; Melanocytic Markers Melan A and HMB45; Cathepsin K
Previous reports have shown that the t(6; 11) RCCs underexpress cytokeratins and overexpress the cysteine protease cathepsin K and melanocytic markers Melan A and HMB45.1,10,12 All 13 cases in this study labeled diffusely for cathepsin K (83% mean labeling) and Melan A (92% mean labeling). Twelve of 13 cases labeled for HMB45 but the labeling was much less extensive; the mean labeling of the positive cases was 7%, and 7 of the 12 positive cases labeled focally (Figs. 3A, B). Eight of 13 cases (62%) labeled for cytokeratin Cam5.2, which detects low–molecular weight cytokeratins 8 and 18. Labeling was focal in 3 of the 8 cases, and the mean percentage of tumor cells labeling in the positive cases was only 40%. Six of 13 cases (46%) labeled for broad-spectrum cytokeratin AE1/3, which detects cytokeratins 1 to 8, 10, 14 to 16, and 19. Three of these cases labeled focally, and the mean labeling among the positive cases was only 16%.
Figure 3.
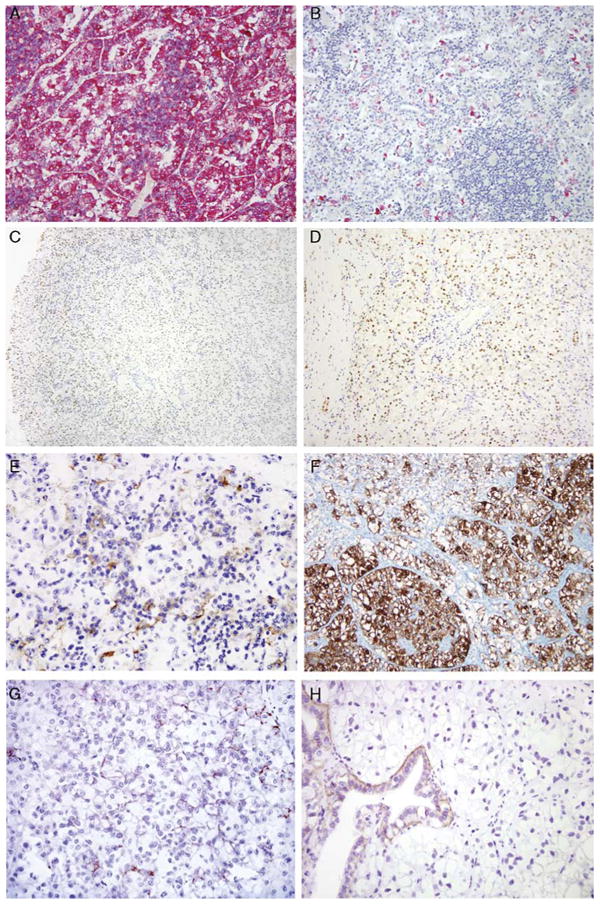
IHC markers evaluated in the t(6;11) RCC. Melan A labeling was consistently diffuse (A), in contrast to the focal, individual cell labeling consistently seen with HMB45 (B). C, PAX8 immunoreactivity was characteristically stronger at the edge of the sections than in the center, likely representing a fixation artifact. D, At the edge of the tumor distinctive PAX8 nuclear labeling of the neoplasm in the absence of labeling of the intermixed blood vessels was apparent. E, RCC marker labeling was characteristically membranous and distinctly labeled the larger epithelioid cells of a majority of cases. F, The majority of the tumors labeled for CD11 7, including several cases that labeled diffusely. G, A minority of cases labeled for Ksp-cadherin. H, However, the majority of cases were negative, including cases with entrapped native renal tubules, which served as internal controls.
Transcription Factors PAX8, PAX2, MITF, and SOX10
PAX2 and PAX8 are lineage-restricted transcription factors, which are known to be expressed in the renal and Müllerian systems, and have been found to be expressed in most clear cell RCCs, papillary RCCs, and Xp11 translocation RCCs.13,19–24 The t(6;11) RCC were positive for PAX8 in 14 of 23 cases (59%). PAX8 labeling in the cases of t(6;11) RCC was stronger toward the edge of whole sections of 10 t(6;11) RCC cases studied (Figs. 3C, D). In contrast, 18 of 19 epithelioid angiomyolipomas were completely negative for PAX8. The one case that did label was overtly malignant and labeled focally in anaplastic areas. PAX2 labeling was seen in only 2 of 13 t(6;11) RCC cases.
MITF, a transcription factor that is commonly expressed in melanocytes and their corresponding neoplasms, belongs to the same subfamily of transcription factors as TFE3 and TFEB and forms heterodimers with them. SOX 10 is a transcription factor involved in neural crest and melanocytic differentiation.25 The t(6;11) RCC showed no labeling for MITF or SOX 10.
Clear Cell and Papillary RCC Markers: RCC Marker Antigen, CD10, CA IX EMA, EpCAM, and Vimentin
RCC marker antigen is a proximal brush border antigen, which is fairly specific and sensitive for RCC.26 RCC marker antigen is positive in most clear cell, papillary, and Xp11 translocation RCCs but negative in chromophobe RCC. CD10 is a cell membrane metal-lopeptidase whose expression is also localized to the proximal tubular brush border. Similar to the RCC marker antigen, expression is found in clear cell, papillary, and Xp11 translocation RCC, whereas chromophobe RCC and oncocytoma rarely express the antigen.26 RCC marker antigen and CD10 each labeled 10 of 14 cases of t(6;11) RCC (Fig. 3E). RCC marker antigen showed only focal labeling (< 10% cells) in 7 of the positive cases, whereas CD10 showed focal labeling in 8 of the positive cases.
EMA is a glycoprotein, which is found on the surface of the distal tubule and collecting duct epithelium and is generally highly expressed in all RCCs including clear cell, papillary, and chromophobe.27 Only a minority of Xp11 translocation RCCs label for EMA.28 Similarly, only 1 of 13 cases of t(6;11) RCC showed focal labeling for EMA.
The HIF 1-α/CA IX pathway is constitutively activated in clear cell RCC. In clear cell RCC, inactivation of the VHL gene allows HIF 1-α to escape degradation, which leads to activation of downstream targets such as vascular endothelial growth factor, GLUT-1, and CA IX.30 Expression of HIF 1-a and CA IX is typically diffuse in clear cell RCC and focal in other subtypes of RCC including Xp11 translocation RCC. In the latter, expression is mainly associated with areas of necrosis and thus is likely secondary to focal hypoxia.29 Only 1 of the cases of t(6;11) RCC showed necrosis, and this was the only case that showed focal CA IX labeling; all other cases were completely negative.
EpCAM is overexpressed in a variety of carcinomas and thus has drawn interest as a potential therapeutic target, as humanized anti-EpCAM antibodies are now in clinical trials.30 EpCAM has been shown to be more frequently expressed in papillary and chromophobe RCCs than in clear cell RCC.31 t(6;11) RCC failed to show any EpCAM labeling.
Vimentin is an intermediate filament protein found in mesenchymal cells. Clear cell and papillary RCCs are typically positive for vimentin, chromophobe RCCs are negative, and Xp11 translocation RCC are typically either negative or only focally positive.28,32–34 Nine of 13 t(6;11) RCCs (69%) labeled for vimentin. a-methylacyl-CoA racemase is frequently used as a marker for papillary RCC, although it also labels a minority of clear cell and Xp11 translocation RCCs.35 t(6;11) RCC labeled for racemase in 3 of 13 cases.
Chromophobe RCC Markers: CD117 and Ksp-Cadherin
CD117 expression has been shown to distinguish chromophobe RCC from clear cell RCC.36,37 Strong membranous labeling for CD 117 was seen in 10 of 14 cases (71%) of t(6;11) RCC (Fig. 3F). Ksp-cadherin is a renal-specific Cadherin involved in cell-cell adhesion localized to the distal tubules.38 Ksp-cadherin has been found to label a majority of chromophobe RCCs.39,40 Clear cell and papillary RCCs, however, are less likely to stain with the marker.13,41 A recent study has suggested that Ksp-cadherin is a sensitive marker of the t(6;11) RCC.7 However, we found focal labeling for Ksp-cadherin in only 3 of 19 t(6;11) RCCs (Fig. 3G) with entrapped distal tubules labeling appropriately as internal controls (Fig. 3H). These cases included whole sections of 6 additional cases not on the tissue microarray.
pS6
Immunoreactivity for pS6 represents a measure of activation of the mammalian target of rapamycin (mTOR) pathway, which promotes cell growth, is highly active in many RCCs, and can be targeted for therapy.42 We have previously shown that Xp11 translocation RCCs express pS6 at slightly higher levels (mean H score 88) compared with clear cell RCC (mean H score 54) and papillary RCC (mean H score 44).13 The median H score for pS6 in the t(6;11) RCC and epithelioid angiomyolipoma were 101 and 245, respectively. pS6 immunolabel ing in these renal neoplasms is shown in Figure 4.
Figure 4.
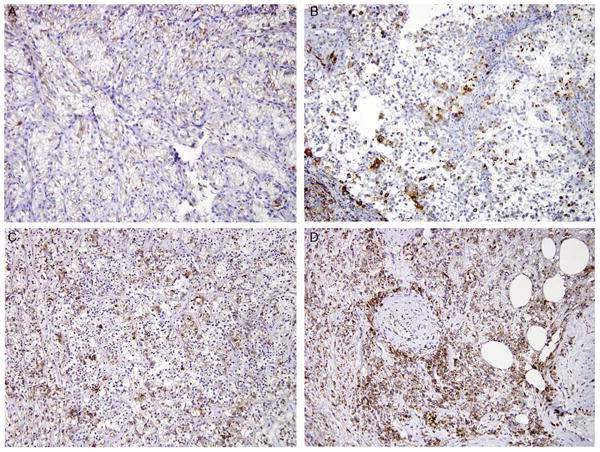
IHC labeling for pS6 in renal neoplasms. All these images are taken at ×20 magnification. Progressively increased labeling is seen in clear cell RCC (A), Xp11 translocation RCC (B), and t(6;11) RCC (C). The most diffuse and intense labeling is seen in epithelioid angiomyolipoma (D).
Gynecologic Tract Markers: ER and Inhibin
ER is expressed in a variety of gynecologic tract tumors. Inhibin is a glycoprotein produced by the granulosa cells of the ovary to inhibit the release of follicle-stimulating hormone and is useful in the diagnosis of sex cord stromal tumors.43 The small cell pseudorosettes of the t(6;11) RCC resemble the Call-Exner rosettes of granulosa cell tumor. No labeling was identified for ER or inhibin in any of the cases of t(6;11) RCC.
The IHC results are summarized in Table 2.
Table 2. Immunohistochemical Results in t(6; 11) RCC.
| IHC Marker | Proportion of t(6;11) RCC Positive (%) |
Mean %Labeling Among Positive Cases |
|---|---|---|
| Cathepsin K | 13/13 (100) | 83 |
| Melan A | 13/13 (100) | 92 |
| HMB45 | 12/13 (91)* | 7 |
| CD117 (C-kit) | 10/14 (71) | 64 |
| RCC marker antigen | 10/14 (71)† | 13 |
| CD10 | 10/14 (71)‡ | 11 |
| Vimentin | 9/13 (69) | 66 |
| Cytokeratin Cam5.2 | 8/13 (62)§ | 40 |
| PAX8 | 14/23 (59) | 63 |
| Cytokeratin AE1/3 | 6/13 (46)∥ | 16 |
| Racemase | 3/13 (23) | 65 |
| PAX2 | 2/13 (15)¶ | 7 |
| Ksp-cadherin | 3/19 (15)¶ | 7 |
| EMA | 1/13 (8)¶ | 20 |
| CA IX | 1/14 (7) | 3 |
| Inhibin, SOX10, ER, | 0/13 (0) | NA |
| EpCAM, MITF |
7 of the 12 positive cases labeled focally.
7 of the 10 positive cases labeled focally.
8 of the 10 positive cases labeled focally.
3 of the 8 positive cases labeled focally.
3 of the 6 positive cases labeled focally.
Focal labeling only.
Discussion
Our study adds 10 genetically confirmed t(6;11) RCC to the literature and expands the clinicopathologic spectrum of this neoplasm. The 68-year-old reported herein is as old as any other reported patient with t(6;11) RCC; one other 68-year-old patient has been reported in the literature (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/PAS/A197). The 8-year interval between resection of the primary tumor and metastasis in case 9 of this series equals the interval between resection and metastasis of a case reported by Inamura et al,6 and highlights the potential for these neoplasms to recur late, similar to the reported behavior of the Xp11 translocation RCC.33 The imaging findings of these neoplasms have not previously been reported. Moreover, the predominant multilocular cystic appearance with prominent dilated entrapped tubules in case 2 has not previously been documented. The largely nondescript high-grade morphology of case 10 puts t(6;11) RCC in the differential diagnosis of high-grade unclassified RCC. An updated table of all genetically confirmed reported t(6;11) RCC cases is presented as Supplemental Table 1 (Supplemental Digital Content 1, http://links.lww.com/PAS/A197).
Our study has greatly expanded the reported IHC profile of the t(6;11) RCC. We confirm the consistent expression of melanocytic markers Melan A and HMB45, but document that the Melan A is typically diffusely positive in the t(6;11) RCC, whereas HMB45 is usually only focally positive, labeling individual neoplastic cells in a background of cells that do not get labeled. We confirm that these RCCs underexpress cytokeratin AE1/3 and overexpress cathepsin K. It should be noted that the lineage of the t(6;11) RCC was not initially clear: we initially described these lesions as “neoplasms,” not carcinomas, given their unusual morphology, consistent labeling for melanocytic markers, and minimal immunoreactivity for epithelial markers.1 We were not certain whether these lesions represented unusual epithelioid angiomyolipomas or RCCs. IHC labeling for PAX8, RCC marker antigen, CD 10, and often Cam5.2 as reported herein supports classification of the t(6;11) RCC as carcinomas of renal tubular differentiation despite frequent negativity for broad-spectrum cytokeratins and EMA. This pattern of labeling (positive for renal tubular markers, often negative for epithelial markers) is similar to that previously reported for the Xp11 translocation RCC. We previously mentioned PAX8 labeling in several test cases of t(6;11) RCC studied during validation of our TFEB FISH assay (mainly as a demonstration that these cases would label for a nuclear IHC marker despite occasionally being negative for TFEB by IHC)16; however, PAX8 labeling has not previously been formally studied in t(6;11) RCC. In this study, we noted frequent absence of PAX8 labeling at the center of whole sections of the t(6;11) RCC, with intact labeling at the periphery, and suspect that this represents variable fixation with the antigen being better preserved at the periphery of the tissue blocks. The minimal PAX2 labeling we saw in our cases is more difficult to explain but may represent a more exaggerated effect of fixation on this marker, which we and others have found to be less sensitive than PAX8.22 Although PAX8 and RCC marker antigen have not been studied in the t(6;11) RCC by other groups, we note that Rao et al7 did not find CD 10 labeling in their small series of t(6;11) RCC, which differs from our results.
We found that the t(6;11) RCC labeled frequently for CD117 and vimentin. These findings highlight the potential for the t(6;11) RCC to be misdiagnosed as epithelioid angiomyolipoma. The t(6;11) RCC and epithelioid angiomyolipoma show marked IHC overlap, as both also consistently label for melanocytic markers and cathepsin K, often label for CD117 and vimentin, and are typically cytokeratin AE1/3 negative. Moreover, although TFEB IHC should distinguish the 2, it has potential for false negatives and false positives. Not all genetically confirmed t(6;11) RCCs have been shown to label for TFEB by IHC, likely because of fixation issues.16 Along these lines, in the current study 2 of the new t(6;l 1) RCCs reported did not label for TFEB. Moreover, 1 group44 has reported TFEB IHC labeling in PEComa family lesions, although this has not been our experience, and we have subsequently found that all PEComas tested so far lack TFEB gene rearrangements. Illustrating the diagnostic difficulty, the provided images of a distinctive renal neoplasm reported as “oncocytic angiomyolipoma” in 200345 appear to be quite consistent with a t(6;11) RCC. Moreover, there are also at least 2 t(6;l 1) RCCs reported in the literature in which epithelioid angiomyolipoma was the initial favored diagnosis.10,15 These cases highlight the importance of PAX8 IHC in distinguishing the t(6;11) RCC from epithelioid angiomyolipoma. PAX8 is a very specific (95%), although not a very sensitive (59%), marker in distinguishing the t(6;l 1) RCC from epithelioid angiomyolipoma. Clearly, not all t(6;11) RCCs will label for PAX8. In such cases, TFEB FISH may be the most useful test in making this distinction.
In contrast to a prior report,7 we find that Ksp-cadherin is not a consistent marker of the t(6;11) RCC. Only 3 of 19 cases (15%) of t(6;11) showed focal labeling for this marker. Appropriate labeling of the distal convoluted tubules as an internal control for Ksp-cadherin supports the validity of our findings. The labeling for CD 10 and RCC marker antigen and typical negativity for Ksp-cadherin that we found argue against this prior report's outright assignment of distal nephron origin to the t(6;11)RCC.
Histologic and clinical overlap between t(6;l 1) RCC and Xp11 translocation RCC may also confound diagnosis. CD117 was identified in a majority of t(6;11) RCCs in the current series (71%) but labeled none of the Xp11 translocation RCCs in our prior report. However, we have recently seen in consultation an Xp11 translocation RCC that mimicked the t(6;11) RCC morphologically and also labeled for CD 117 (P. Argani, unpublished data, 2013).13 Thus, although CD117 is a fairly specific and relatively sensitive marker in distinguishing these related neoplasms, it is clearly not perfect. Although the morphologic overlap between t(6;11) RCC and clear cell RCC typically presents less of a diagnostic dilemma, CD117 labeling also helps distinguish the t(6;11) RCC from clear cell RCC. CA IX negativity also favors the t(6;11) RCCs over Xp11 translocation RCCs, which were focally positive in over half of the studied cases.13 However, the labeling seen in the Xp11 translocation RCC was focal and likely reflected the effects of focal hypoxia associated with necrosis. As most of the t(6;11) RCCs in this study (and in our experience overall) did not showed necrosis, their absence of CA IX labeling is not unexpected. Notably, the one t(6;11) RCC in this study with necrosis did label focally for CA IX, suggesting that CA IX labeling in translocation RCC reflects a physiological response to local hypoxia rather than a distinctive intrinsic biological feature.
The mTOR pathway is upregulated in many human neoplasms and represents a potential therapeutic target for inhibition.42 Derangement of the mTOR pathway has been identified in clear cell RCC and renal angiomyolipoma, especially metastatic disease and more aggressive disease.42,46 A large multicenter trial in 2007 showed that temsirolimus, an analog of rapamycin, increased survival of patients with metastatic clear cell RCC prompting FDA approval for the drug.47 Renal angiomyolipomas are commonly associated with a tuberous sclerosis complex, which results from knockout of the TSC1 and TSC2 genes. The products of the TSC1 and TSC2 genes act as negative regulators of the mTOR pathway.48 mTOR inhibitors have been shown effective in both reducing the angiomyolipoma size49,50 and treating subependymal giant cell astrocytomas found in tuberous sclerosis patients.51 pS6 is a downstream target and surrogate marker of increased mTOR pathway activity. In our current series, pS6 expression appeared higher in t(6;11) RCC than what we previously reported in clear cell RCC, although not as high as in epithelioid angiomyolipoma (H scores of 101, 50, and 245, respectively). The higher H score of t(6;11) RCC compared with that of clear cell RCC suggests the possibility that mTOR inhibitors would also be effective when used against the rare t(6;11) RCCs that metastasize.
In summary, our results show that a small panel of routine immunochemical markers may be useful in suggesting the t(6;11) RCC when presented with an initially unclassifiable renal neoplasm (Table 3). A panel of PAX8, Melan A, cytokeratin AE1/3, and cathepsin K can be helpful in distinguishing t(6;11) RCC from the more common clear cell and papillary RCC and epithelioid angiomyolipoma. IHC labeling for PAX8, cathepsin K, and Melan A in the absence of cytokeratin AE1/3 labeling is suggestive of a t(6;11) RCC. CD 117 labeling favors the t(6;11) RCC over Xp11 RCC. Nonetheless, we believe that the diagnosis of these neoplasms should be confirmed definitively by TFEB FISH studies. In addition, as recognition of t(6;11) RCC increases, recently approved mTOR pathway inhibitors may prove useful in treatment of the rare cases that are inoperable or metastasize.
Table 3. Routine IHC Markers Useful in Differential Diagnosis of Renal Translocation Carcinomas.
| PAX8 | Vimentin | CD117 | HMB45 | Melan A | CA IX | Cathepsin K | Cytokeratin | |
|---|---|---|---|---|---|---|---|---|
| Clear cell RCC | + | + | − | − | − | Diffuse | − | + |
| Papillary RCC | + | + | − | − | − | Focal | − | + |
| Chromophobe RCC | + | − | + | − | − | Focal | − | + |
| Xp11 RCC | + | −/+ | −/+ | −/Focal | −/Focal | Focal | +/− | −/+ |
| t(6;11)RCC | + | + | +/− | Focal | + | − | + | −/+ |
| Epithelioid angiomyolipoma | − | + | +/− | + | + | Not known | + | − |
Supplementary Material
Footnotes
Conflicts of Interest and Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.ajsp.com.
References
- 1.Argani P, Hawkins A, Griffin CA, et al. A distinctive pediatric renal neoplasm characterized by epithelioid morphology, basement membrane production, focal HMB45 immunoreactivity, and t(6;11)(p21.1;ql2) chromosome translocation. Am J Pathol. 2001;158:2089–2096. doi: 10.1016/S0002-9440(10)64680-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srigley JR, Delahunt B, Eble JN, et al. The International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia. Am J Surg Pathol. 2013;37:1469–1489. doi: 10.1097/PAS.0b013e318299f2d1. [DOI] [PubMed] [Google Scholar]
- 3.Davis IJ, Hsi BL, Arroyo JD, et al. Cloning of an Alpha-TFEB fusion in renal tumors harboring the t(6;11)(p21;ql3) chromosome translocation. Proc Natl Acad Sci USA. 2003;100:6051–6056. doi: 10.1073/pnas.0931430100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuiper RP, Schepens M, Thijssen J, et al. Upregulation of the transcription factor TFEB in t(6;11)(p21;ql3)-positive renal cell carcinomas due to promoter substitution. Hum Mol Genet. 2003;12:1661–1669. doi: 10.1093/hmg/ddg178. [DOI] [PubMed] [Google Scholar]
- 5.Argani P, Lui MY, Couturier J, et al. A novel CLTC-TFE3 gene fusion in pediatric renal adenocarcinoma with t(X;17)(p11.2;q23) Oncogene. 2003;22:5374–5378. doi: 10.1038/sj.onc.1206686. [DOI] [PubMed] [Google Scholar]
- 6.Inamura K, Fujiwara M, Togashi Y, et al. Diverse fusion patterns and heterogeneous clinicopathologic features of renal cell carcinoma with t(6;11) translocation. Am J Surg Pathol. 2012;36:35–42. doi: 10.1097/PAS.0b013e3182293ec3. [DOI] [PubMed] [Google Scholar]
- 7.Rao Q, Liu B, Cheng L, et al. Renal cell carcinomas with t(6;11)(p21;ql2): a clinicopathologic study emphasizing unusual morphology, novel Alpha-TFEB gene fusion point, immunobio-markers, and ultrastructural features, as well as detection of the gene fusion by fluorescence in situ hybridization. Am J Surg Pathol. 2012;36:1327–1338. doi: 10.1097/PAS.0b013e31825aafb5. [DOI] [PubMed] [Google Scholar]
- 8.Hora M, Hes O, Urge T, et al. A distinctive translocation carcinoma of the kidney [“rosette-like forming,” t(6;11), HMB45-positive renal tumor] Int Urol Nephrol. 2009;41:553–557. doi: 10.1007/s11255-008-9495-8. [DOI] [PubMed] [Google Scholar]
- 9.Pecciarini L, Cangi MG, Lo Cunsolo C, et al. Characterization of t(6;11)(p21;ql2) in a renal-cell carcinoma of an adult patient. Genes Chromosomes Cancer. 2007;46:419–426. doi: 10.1002/gcc.20422. [DOI] [PubMed] [Google Scholar]
- 10.Argani P, Lae M, Hutchinson B, et al. Renal carcinomas with the t(6;11)(p21;ql2): clinicopathologic features and demonstration of the specific Alpha-TFEB gene fusion by immunohisto-chemistry, RT-PCR, and DNA PCR. Am J Surg Pathol. 2005;29:230–240. doi: 10.1097/01.pas.0000146007.54092.37. [DOI] [PubMed] [Google Scholar]
- 11.Martignoni G, Gobbo S, Camparo P, et al. Differential expression of cathepsin K in neoplasms harboring TFE3 gene fusions. Mod Pathol. 2011;24:1313–1319. doi: 10.1038/modpathol.2011.93. [DOI] [PubMed] [Google Scholar]
- 12.Martignoni G, Pea M, Gobbo S, et al. Cathepsin-K immunor-eactivity distinguishes MiTF/TFE family renal translocation carcinomas from other renal carcinomas. Mod Pathol. 2009;22:1016–1022. doi: 10.1038/modpathol.2009.58. [DOI] [PubMed] [Google Scholar]
- 13.Argani P, Hicks J, De Marzo AM, et al. Xp11 translocation renal cell carcinoma (RCC): extended immunohistochemical profile emphasizing novel RCC markers. Am J Surg Pathol. 2010;34:1295–1303. doi: 10.1097/PAS.0b013e3181e8ce5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishihara A, Yamashita Y, Takamori H, et al. Renal carcinoma with (6;11)(p21;ql2) translocation: report of an adult case. Pathol Int. 2011;61:539–545. doi: 10.1111/j.1440-1827.2011.02711.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhan HQ, Wang CF, Zhu XZ, et al. Renal cell carcinoma with t(6;11) translocation: a patient case with a novel Alpha-TFEB fusion point. J Clin Oncol. 2010;28:e709–e713. doi: 10.1200/JCO.2010.30.3172. [DOI] [PubMed] [Google Scholar]
- 16.Argani P, Yonescu R, Morsberger L, et al. Molecular confirmation of t(6;11)(p21;ql2) renal cell carcinoma in archival paraffin-embedded material using a break-apart TFEB FISH assay expands its clinicopathologic spectrum. Am J Surg Pathol. 2012;36:1516–1526. doi: 10.1097/PAS.0b013e3182613d8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersson F, Michal M, Vanecek T, et al. Bilateral renal tumors; conventional clear cell carcinoma and contralateral t(6;11)/t(X;17)-like tumor histomorphologic, immunohistochemical, ultrastructural and molecular genetic studies including the report of a novel mutation in the VHL gene. Ann Diagn Pathol. 2011;15:362–369. doi: 10.1016/j.anndiagpath.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Suarez-Vilela D, Izquierdo-Garcia F, Mendez-Alvarez JR, et al. Renal translocation carcinoma with expression of TFEB: presentation of a case with distinctive histological and immunohistochemical features. Int J Surg Pathol. 2011;19:506–509. doi: 10.1177/1066896909340531. [DOI] [PubMed] [Google Scholar]
- 19.Daniel L, Lechevallier E, Giorgi R, et al. Pax-2 expression in adult renal tumors. Hum Pathol. 2001;32:282–287. doi: 10.1053/hupa.2001.22753. [DOI] [PubMed] [Google Scholar]
- 20.Mazal PR, Stichenwirth M, Koller A, et al. Expression of aquaporins and PAX-2 compared to CD10 and cytokeratin 7 in renal neoplasms: a tissue microarray study. Mod Pathol. 2005;18:535–540. doi: 10.1038/modpathol.3800320. [DOI] [PubMed] [Google Scholar]
- 21.Nonaka D, Chiriboga L, Soslow RA. Expression of PAX8 as a useful marker in distinguishing ovarian carcinomas from mammary carcinomas. Am J Surg Pathol. 2008;32:1566–1571. doi: 10.1097/PAS.0b013e31816d71ad. [DOI] [PubMed] [Google Scholar]
- 22.Ozcan A, Zhai J, Hamilton C, et al. PAX-2 in the diagnosis of primary renal tumors: immunohistochemical comparison with renal cell carcinoma marker antigen and kidney-specific Cadherin. Am J Clin Pathol. 2009;131:393–404. doi: 10.1309/AJCPM7DW0XFHDHNY. [DOI] [PubMed] [Google Scholar]
- 23.Tong GX, Weeden EM, Hamele-Bena D, et al. Expression of PAX8 in nephrogenic adenoma and clear cell adenocarcinoma of the lower urinary tract: evidence of related histogenesis? Am J Surg Pathol. 2008;32:1380–1387. doi: 10.1097/PAS.0b013e31816b1020. [DOI] [PubMed] [Google Scholar]
- 24.Gupta R, Balzer B, Picken M, et al. Diagnostic implications of transcription factor Pax 2 protein and transmembrane enzyme complex carbonic anhydrase IX immunoreactivity in adult renal epithelial neoplasms. Am J Surg Pathol. 2009;33:241–247. doi: 10.1097/PAS.0b013e318181b828. [DOI] [PubMed] [Google Scholar]
- 25.Kelsh RN. Sorting out Sox 10 functions in neural crest development. Bioessays. 2006;28:788–798. doi: 10.1002/bies.20445. [DOI] [PubMed] [Google Scholar]
- 26.Pan CC, Chen PC, Ho DM. The diagnostic utility of MOC31, BerEP4, RCC marker and CD 10 in the classification of renal cell carcinoma and renal oncocytoma: an immunohistochemical analysis of 328 cases. Histopathology. 2004;45:452–459. doi: 10.1111/j.1365-2559.2004.01962.x. [DOI] [PubMed] [Google Scholar]
- 27.Langner C, Ratschek M, Rehak P, et al. Expression of MUC1 (EMA) and E-cadherin in renal cell carcinoma: a systematic immunohistochemical analysis of 188 cases. Mod Pathol. 2004;17:180–188. doi: 10.1038/modpathol.3800032. [DOI] [PubMed] [Google Scholar]
- 28.Argani P, Ladanyi M. Translocation carcinomas of the kidney. Clin Lab Med. 2005;25:363–378. doi: 10.1016/j.cll.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Tickoo SK, Alden D, Olgac S, et al. Immunohistochemical expression of hypoxia inducible factor-lalpha and its downstream molecules in sarcomatoid renal cell carcinoma. J Urol. 2007;177:1258–1263. doi: 10.1016/j.juro.2006.11.100. [DOI] [PubMed] [Google Scholar]
- 30.Trzpis M, McLaughlin PM, de Leij LM, et al. Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. Am J Pathol. 2007;171:386–395. doi: 10.2353/ajpath.2007.070152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Went P, Dirnhofer S, Salvisberg T, et al. Expression of epithelial cell adhesion molecule (EpCam) in renal epithelial tumors. Am J Surg Pathol. 2005;29:83–88. doi: 10.1097/01.pas.0000.146028.70868.7a. [DOI] [PubMed] [Google Scholar]
- 32.Skinnider BF, Folpe AL, Hennigar RA, et al. Distribution of cytokeratins and vimentin in adult renal neoplasms and normal renal tissue: potential utility of a cytokeratin antibody panel in the differential diagnosis of renal tumors. Am J Surg Pathol. 2005;29:747–754. doi: 10.1097/01.pas.0000163362.78475.63. [DOI] [PubMed] [Google Scholar]
- 33.Argani P, Antonescu CR, Couturier J, et al. PRCC-TFE3 renal carcinomas: morphologic, immunohistochemical, ultrastructural, and molecular analysis of an entity associated with the t(X;l)(p11.2;q21) Am J Surg Pathol. 2002;26:1553–1566. doi: 10.1097/00000478-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Argani P, Antonescu CR, Illei PB, et al. Primary renal neoplasms with the ASPL-TFE3 gene fusion of alveolar soft part sarcoma: a distinctive tumor entity previously included among renal cell carcinomas of children and adolescents. Am J Pathol. 2001;159:179–192. doi: 10.1016/S0002-9440(10)61684-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tretiakova MS, Sahoo S, Takahashi M, et al. Expression of alpha-methylacyl-CoA racemase in papillary renal cell carcinoma. Am J Surg Pathol. 2004;28:69–76. doi: 10.1097/00000478-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Wang HY, Mills SE. KIT and RCC are useful in distinguishing chromophobe renal cell carcinoma from the granular variant of clear cell renal cell carcinoma. Am J Surg Pathol. 2005;29:640–646. doi: 10.1097/01.pas.0000157943.33903.92. [DOI] [PubMed] [Google Scholar]
- 37.Petit A, Castillo M, Santos M, et al. KIT expression in chromophobe renal cell carcinoma: comparative immunohistochemical analysis of KIT expression in different renal cell neoplasms. Am J Surg Pathol. 2004;28:676–678. doi: 10.1097/00000478-200405000-00017. [DOI] [PubMed] [Google Scholar]
- 38.Thedieck C, Kuczyk M, Klingel K, et al. Expression of Ksp-cadherin during kidney development and in renal cell carcinoma. Br J Cancer. 2005;92:2010–2017. doi: 10.1038/sj.bjc.6602597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adley BP, Gupta A, Lin F, et al. Expression of kidney-specific Cadherin in chromophobe renal cell carcinoma and renal oncocytoma. Am J Clin Pathol. 2006;126:79–85. doi: 10.1309/JFE2-B57Y-QFPW-PL10. [DOI] [PubMed] [Google Scholar]
- 40.Kuehn A, Paner GP, Skinnider BF, et al. Expression analysis of kidney-specific Cadherin in a wide spectrum of traditional and newly recognized renal epithelial neoplasms: diagnostic and histogenetic implications. Am J Surg Pathol. 2007;31:1528–1533. doi: 10.1097/PAS.0b013e318058818c. [DOI] [PubMed] [Google Scholar]
- 41.Horstmann M, Geiger LM, Vogel U, et al. Kidney-specific Cadherin correlates with the ontogenetic origin of renal cell carcinoma subtypes: an indicator of a malignant potential? World J Urol. 2012;30:525–531. doi: 10.1007/s00345-011-0763-3. [DOI] [PubMed] [Google Scholar]
- 42.Pantuck AJ, Seligson DB, Klatte T, et al. Prognostic relevance of the mTOR pathway in renal cell carcinoma: implications for molecular patient selection for targeted therapy. Cancer. 2007;109:2257–2267. doi: 10.1002/cncr.22677. [DOI] [PubMed] [Google Scholar]
- 43.Deavers MT, Malpica A, Liu J, et al. Ovarian sex cord-stromal tumors: an immunohistochemical study including a comparison of calretinin and inhibin. Mod Pathol. 2003;16:584–590. doi: 10.1097/01.MP.0000073133.79591.A1. [DOI] [PubMed] [Google Scholar]
- 44.Antic T, Meyer P, Pins M, et al. TFEB immunopositivity in renal angiomyolipomas. Mod Pathol. 2007;20(S2):135A. Abstract #608. [Google Scholar]
- 45.Sironi M, Spinelli M. Oncocytic angiomyolipoma of the kidney: a case report. Int J Surg Pathol. 2003;11:229–234. doi: 10.1177/106689690301100314. [DOI] [PubMed] [Google Scholar]
- 46.El-Hashemite N, Zhang H, Henske EP, et al. Mutation in TSC2 and activation of mammalian target of rapamycin signalling pathway in renal angiomyolipoma. Lancet. 2003;361:1348–1349. doi: 10.1016/S0140-6736(03)13044-9. [DOI] [PubMed] [Google Scholar]
- 47.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 48.Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- 49.Wienecke R, Fackler I, Linsenmaier U, et al. Antitumoral activity of rapamycin in renal angiomyolipoma associated with tuberous sclerosis complex. Am J Kidney Dis. 2006;48:e27–e29. doi: 10.1053/j.ajkd.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 50.Peces R, Peces C, Cuesta-Lopez E, et al. Low-dose rapamycin reduces kidney volume angiomyolipomas and prevents the loss of renal function in a patient with tuberous sclerosis complex. Nephrol Dial Transplant. 2010;25:3787–3791. doi: 10.1093/ndt/gfq456. [DOI] [PubMed] [Google Scholar]
- 51.Curran MP. Everolimus: in patients with subependymal giant cell astrocytoma associated with tuberous sclerosis complex. Paediatr Drugs. 2012;14:51–60. doi: 10.2165/11207730-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 52.Geller JI, Argani P, Adeniran A, et al. Translocation renal cell carcinoma: lack of negative impact due to lymph node spread. Cancer. 2008;112:1607–1616. doi: 10.1002/cncr.23331. [DOI] [PubMed] [Google Scholar]
- 53.Martignoni G, Tardanico R, Pea M, et al. t(6;11) renal cell tumor: a clinicopathological study of two cases in adults [abstract 715] Mod Pathol. 2005;18:155A. [Google Scholar]
- 54.Petersson F, Vanecek T, Michal M, et al. A distinctive translocation carcinoma of the kidney: “rosette forming,” t(6;11), HMB45-positive renal tumor: a histomorphologic, immunohistochemical, ultrastructural, and molecular genetic study of 4 cases. Hum Pathol. 2012;43:726–736. doi: 10.1016/j.humpath.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 55.Argani P, Laé M, Ballard ET, et al. Translocation carcinomas of the kidney after chemotherapy in childhood. J Clin Oncol. 2006;24:1529–1534. doi: 10.1200/JCO.2005.04.4693. [DOI] [PubMed] [Google Scholar]
- 56.Camparo P, Vasiliu V, Molinie V, et al. Renal translocation carcinomas: clinicopathologic, immunohistochemical, and gene expression profiling analysis of 31 cases with a review of the literature. Am J Surg Pathol. 2008;32:656–670. doi: 10.1097/PAS.0b013e3181609914. [DOI] [PubMed] [Google Scholar]
- 57.Zhong M, De Angelo P, Osborne L, et al. Dual-color, break-apart FISH assay on paraffin-embedded tissues as an adjunct to diagnosis of Xp11 translocation renal cell carcinoma and alveolar soft part sarcoma. Am J Surg Pathol. 2010;34:757–766. doi: 10.1097/PAS.0b013e3181dd577e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rao Q, Zhang XM, Tu P, et al. Renal cell carcinomas with t(6;l1)(p21;ql2) presenting with tubulocystic-like features. Int J Clin Exp Pathol. 2013;6:1452–1457. [PMC free article] [PubMed] [Google Scholar]
- 59.Matsuura K, Inoue T, Kai T, et al. Molecular analysis of a case of renal cell carcinoma with t(6;11)(p21;ql2) reveals a link to a lysosome-like structure. Histopathology. doi: 10.1111/his.12238. In press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


