Abstract
Purpose
In recent years, colorectal cancer (CRC) screening rates have increased steadily in the USA, though racial and ethnic disparities persist. In a community-based randomized controlled trial, we investigated the effect of patient navigation on increasing CRC screening adherence among older African Americans.
Methods
Participants in the Cancer Prevention and Treatment Demonstration were randomized to either the control group, receiving only printed educational materials (PEM), or the intervention arm where they were assigned a patient navigator in addition to PEM. Navigators assisted participants with identifying and overcoming screening barriers. Logistic regression analyses were used to assess the effect of patient navigation on CRC screening adherence. Up-to-date with screening was defined as self-reported receipt of colonoscopy/sigmoidoscopy in the previous 10 years or fecal occult blood testing (FOBT) in the year prior to the exit interview.
Results
Compared with controls, the intervention group was more likely to report being up-to-date with CRC screening at the exit interview (OR 1.55, 95 % CI 1.07–2.23), after adjusting for select demographics. When examining the screening modalities separately, the patient navigator increased screening for colonoscopy/sigmoidoscopy (OR 1.53, 95 % CI 1.07–2.19), but not FOBT screening. Analyses of moderation revealed stronger effects of navigation among participants 65–69 years and those with an adequate health literacy level.
Conclusions
In a population of older African Americans adults, patient navigation was effective in increasing the likelihood of CRC screening. However, more intensive navigation may be necessary for adults over 70 years and individuals with low literacy levels.
Keywords: Colorectal cancer, Patient navigator, Health disparities, Randomized controlled trial
Introduction
Colorectal cancer (CRC) is the third leading cause of cancer mortality in the USA with an estimated 50,830 deaths in 2013 [1]. Compared with other racial/ethnic groups, African Americans have the highest rates of colorectal cancer incidence and mortality [2, 3]. Several factors are thought to contribute to CRC disparities including greater exposure to risk factors [4, 5], lower rates of health insurance [6], lower screening rates [7], delayed follow-up after a screen-detected abnormality [8], and higher stage and grade at diagnosis [9, 10]. The large and persistent CRC disparities among African American men and women highlight the need for evidence-based strategies and targeted interventions.
Patient navigation is one method shown to be effective in increasing screening adherence for colorectal and other cancers. Patient navigators, or health coordinators, are individuals who are trained to assist patients with navigating the health care system and ensure the removal of barriers encountered by patients while seeking screening, diagnosis, or treatment [11]. Studies examining the effects of patient navigation on adherence to CRC screening have observed an increase in screening rates among individuals receiving patient navigator services [12–16]. Much of the existing literature consists of pilot studies [13, 15] and/or participants recruited from a single hospital or community clinic [12, 14, 16]. Moreover, none of the reported studies examined moderators of the patient navigation intervention effectiveness. Analysis of potential moderators of the intervention’s effectiveness is relevant in that it may aid in tailoring existing interventions to relevant populations and assist in identifying groups that require more intensive interventions.
Our current study examines the efficacy of a patient navigation intervention in increasing CRC screening, specifically among older African American adults in an urban setting. Further, in secondary analysis we examined the role of age, gender, and participant’s level of health literacy as possible moderators of patient navigation’s effectiveness.
Methods
Study setting, design, and participants
The data presented in this study were obtained from the Baltimore City, Maryland site of the CPTD Screening Trial, a 4-year, 6-site national demonstration project of patient navigation. Participants were recruited between April 2006 and June 2010 through population-based and convenience-based sampling from the Medicare enrollment database, as well as in clinical settings and community-based venues (i.e., senior centers). Participants were deemed eligible for the study if they were a Baltimore City resident, aged 65 and older, and enrolled in Medicare Parts A and B. Exclusion criteria for the CPTD Screening Trial included individuals enrolled in Medicare Part C (managed care), residing in a chronic care facility or otherwise institutionalized, unable or unwilling to give informed consent. Also, individuals diagnosed with cancer within 5 years of enrollment or those having a cancer diagnosis more than 5 years ago but in remission for <5 years were excluded from the study. Eligible participants responded to interviewer-administered questionnaires at baseline, annually for up to 4 years, and at the conclusion of the trial. This analysis includes data from the baseline, annual follow-up, and exit in-person interviews. The maximum follow-up time from baseline to exit screening for the CPTD study participants aged 60–75 years was 46 months [mean (range), 17 months (2–46 months)]. The Institutional Review Board of the Johns Hopkins Bloomberg School of Public Health reviewed and approved this study.
Following the administration of the baseline questionnaire, participants were randomly allocated in a 1:1 assignment to either an education-only arm (control) versus a patient navigation arm using a health coordinator. Randomization was completed using a website designed and maintained by Thomson Reuters, a third party contractor for the Centers for Medicare and Medicaid Services (CMS), and was not based on any participant characteristic. In the printed educational materials (PEM) only arm (control group), participants received educational materials from CMS and the American Cancer Society containing general information about cancer and Medicare-covered services. In the patient navigation intervention arm, participants received PEM plus the addition of a patient navigator. The Johns Hopkins trained and certified patient navigator (health coordinator) offered to assist participants with identifying and overcoming barriers to cancer screening thereby facilitating adherence to care.
For this analysis, additional exclusion criteria were applied (outlined in Fig. 1). Our analysis focused on participants aged 65–75 years at baseline, given that the US Preventive Services Task Force recommends CRC screening until age 75 for average risk individuals [17]. We also excluded participants with a self-reported history of irritable bowel disease and those lost to follow-up. Participants who were lost to follow-up were more likely to be male, less educated, lower income, overweight, current smokers, and not screening compliant for CRC at baseline, compared with those who completed the exit interview; however, similar factors were associated with loss to follow-up in each study arm (Supplementary Table 1). The final study population consisted of 1,220 adults.
Fig. 1.
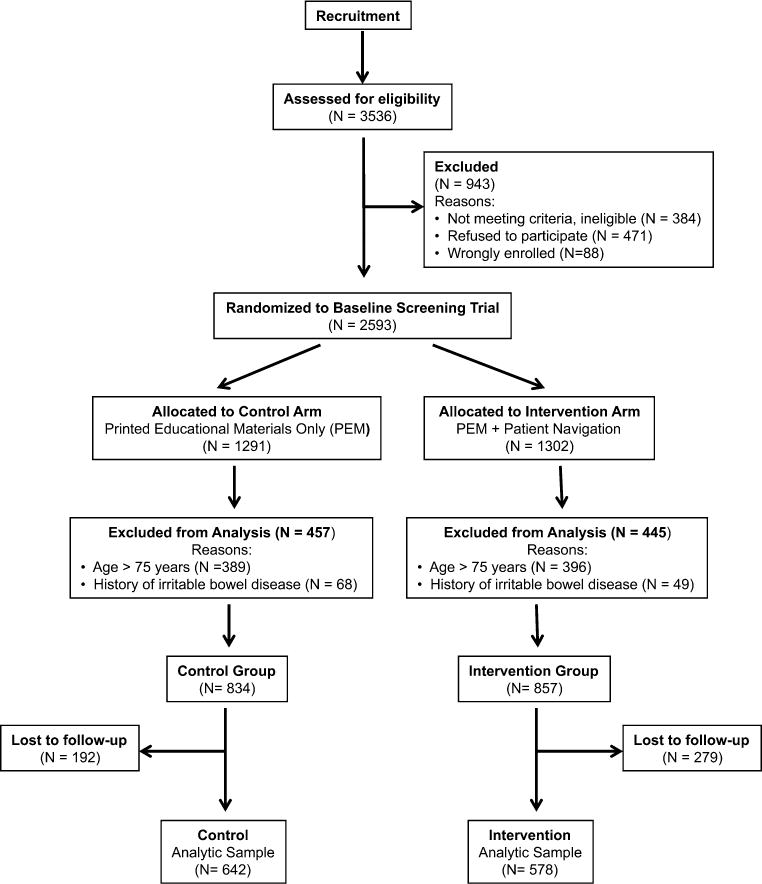
Study participant flow chart
Study variables and outcome measures
Outcome measures
At the time of the exit interviews, individuals were considered up-to-date with CRC screening if they reported having either colonoscopy or sigmoidoscopy in the 10 years prior to the exit interview or an FOBT in the year prior to the exit screening interview. Survey questionnaires did not differentiate between colonoscopy and sigmoidoscopy but grouped the two screening modalities together when assessing adherence.
Study variables
Participant age, gender, race/ethnicity, income, and education were determined from the baseline survey. Self-rated health (health perception) was dichotomized as poor/fair versus good/very good/excellent. The participant’s comorbidities were assessed by tallying the number of self-reported medical conditions from the following list: hypertension, diabetes, chronic lung disease such as asthma, coronary heart disease or other heart problems, stroke, gastrointestinal problems, psychiatric disorders, arthritis, and memory-related disease. With participants averaging three comorbidities, this variable was dichotomized as <3 or ≥3. The Rapid Estimate of Adult Literacy in Medicine-Revised (REALM-R) screening instrument [18] was used to assess the study participants’ risk of low health literacy skills. The resulting REALM-R score was examined as a dichotomous variable with participants receiving a score of six or less classified as having low health literacy and those receiving a score greater than six classified as having adequate health literacy. Family history of colon cancer was assessed by the question, “Have your father, mother, sister, brother, son, or daughter ever been diagnosed with colon cancer or had polyps removed from colon?” Possible responses were “yes,” “no,” and “don’t know.”
Statistical analyses
To examine the effect of the patient navigation intervention on CRC screening, we used weighted multivariable logistic regression models to compare study groups on the outcome measure after adjusting for potentially confounding variables. Weighted regression analyses using the inverse probability method helped account for differential loss to follow-up between the control and intervention groups [19]. The inclusion of confounding variables was determined by assessing the literature for predictors of CRC screening in addition to utilizing Pearson’s χ2 and simple logistic regression to determine the crude effect of the variable on the outcome measure. Variables that were well documented in the literature (literacy [20–22], comorbidities [23–25], and gender [26, 27]) and those with statistically significant or borderline significant bivariate or crude associations (age and health perception) were included in the final multivariate models. Self-reported CRC screening status at baseline was also included in all adjusted models. Overall rates of missingness, for the study variables included in the multivariable models, ranged from 0.10 to 15.6 %. To account for missing values, the nearest neighbor hotdeck method was used to impute responses for covariates [28].
Research suggests that communication may vary by age [29], gender [30, 31], and literacy skills [32]. Since interaction with the patient navigator involved various levels of communication, we hypothesized that age, gender, and health literacy at baseline may act as moderators of the patient navigation intervention. Analysis of moderation effects was performed by including a two-way interaction term (i.e., intervention group by age). Model comparison with and without the interaction term was assessed using the likelihood ratio test (LRT) statistic to determine the significance of the interaction term in the model. All analyses were performed using Stata version 11.2 [33].
Results
Baseline characteristics
Table 1 outlines the baseline characteristics of the study participants in the intervention and control groups. We did not observe any significant differences between the two groups when examining multiple demographic characteristics. At enrollment, 81 % of participants reported being up-to-date with CRC screening. Among those, 21 % had an FOBT within the previous year and 78 % had a colonoscopy or sigmoidoscopy within 10 years of randomization.
Table 1.
Baseline characteristics of study participants by intervention group
| Variable | Control group printed education materials only (n = 642) n (%) |
Intervention group patient navigation + PEM (n = 578) n (%) | p valuea |
|---|---|---|---|
| Age group | |||
| 65–69 | 323 (50.3) | 288 (49.8) | 0.87 |
| 70–75 | 319 (49.7) | 290 (50.2) | |
| Gender | |||
| Male | 178 (27.7) | 157 (27.2) | 0.83 |
| Female | 464 (72.3) | 421 (72.8) | |
| Education | |||
| ≤High school diploma | 374 (58.3) | 313 (54.3) | 0.15 |
| >High school diploma | 267 (41.7) | 264 (45.7) | |
| Income | |||
| <$9,999 | 109 (19.8) | 88 (18.4) | 0.63 |
| $10,000–$19,999 | 151 (27.4) | 122 (25.5) | |
| $20,000–$29,000 | 116 (21.0) | 99 (20.7) | |
| >$30,000 | 175 (31.8) | 170 (35.5) | |
| Health perception | |||
| Fair/poor | 140 (21.8) | 128 (22.2) | 0.89 |
| Good/very good/excellent | 502 (78.2) | 450 (77.8) | |
| Family history of CRC | |||
| No | 528 (85.3) | 478 (85.0) | 0.57 |
| Yes | 91 (14.7) | 85 (15.0) | |
| Comorbidities | |||
| <3 | 337 (52.5) | 308 (53.3) | 0.78 |
| ≥3 | 305 (47.5) | 270 (46.7) | |
| Body mass index [BMI (kg/m2)] | |||
| Normal (<25) | 96 (15.8) | 96 (17.5) | 0.39 |
| Overweight (25 –<30) | 216 (35.6) | 172 (31.3) | |
| Obese class I (30–<35) | 144 (23.8) | 146 (26.6) | |
| Obese class II (≥35) | 150 (24.7) | 135 (24.6) | |
| Smoking status | |||
| Never | 243 (40.4) | 235 (43.3) | 0.41 |
| Former | 265 (44.0) | 218 (40.1) | |
| Current | 94 (15.6) | 90 (16.6) | |
| Level of health literacy | |||
| Low | 276 (46.0) | 268 (49.4) | 0.26 |
| Adequate | 324 (54.0) | 275 (50.6) | |
| Up-to-date with CRC screening at baselineb | |||
| Yes | 527 (82.1) | 476 (82.5) | 0.85 |
| No | 115 (17.9) | 101 (19.1) | |
Column percentages that do not equal 100 % are due to missing data
PEM printed educational materials, CRC colorectal cancer
p value calculated using Pearson’s Chi-squared test
Up-to-date with CRC screening defined as self-reported FOBT within a year of date of randomization or colonoscopy/sigmoidoscopy within 10 years of date of randomization
Primary outcome
At the exit interview, a greater proportion of participants receiving the patient navigation intervention reported being screened for CRC cancer compared with the control group receiving only PEM (94 vs. 91 %; p = 0.04). Among participants who were not up-to-date at baseline, 72.5 % in the intervention group reported being up-to-date at the exit interview, compared with 58.6 % in the control arm (p = 0.008).
Table 2 shows the adjusted association between the intervention and being up-to-date with CRC screening at the exit interview. Patient navigation remained significantly associated with screening (OR 1.56, 95 % CI 1.08–2.25), after adjusting for age, gender, health perception, number of comorbidities, and level of health literacy. When stratifying by screening modality, we observed that the intervention was significantly associated with being screened by colonoscopy/sigmoidoscopy and not FOBT. Also, compared with younger participants (65–69 years), individuals aged 70–74 years were less likely to report being screened by colonoscopy/sigmoidoscopy [(OR 0.68, 95 % CI 0.47–0.98); Table 2]. Assessment of the intervention effectiveness at the first annual follow-up survey demonstrated that the patient navigation intervention was also associated with being screened by colonoscopy/sigmoidoscopy in the 12 months prior to the survey [(OR 1.47, 95 % CI 1.17–1.84); Supplementary Table 2].
Table 2.
Adjusted odds ratios (OR) for being up-to-date with colorectal cancer screening at the exit interview among study participants
| Predictor variable | Any CRC screening
|
Colonoscopy or sigmoidoscopy
|
FOBT
|
|||
|---|---|---|---|---|---|---|
| ORa (95 % CI) | p | ORa (95 % CI) | p | ORa (95 % CI) | p | |
| Intervention | ||||||
| Control, PEM (ref) | 1.00 | 0.02 | 1.00 | 0.02 | 1.00 | 0.68 |
| Patient navigation + PEM | 1.56 (1.08–2.25) | 1.54 (1.08–2.20) | 1.09 (0.72–1.64) | |||
| Gender | ||||||
| Male (ref) | 1.00 | 0.42 | 1.00 | 0.22 | 1.00 | 0.50 |
| Female | 0.85 (0.57–1.26) | 1.27 (0.87–1.87) | 1.18 (0.73–1.89) | |||
| Age | ||||||
| 65–69 (ref) | 1.00 | 0.90 | 1.00 | 0.04 | 1.00 | 0.82 |
| 70–74 | 0.98 (0.67–1.42) | 0.68 (0.47–0.98) | 1.05 (0.69–1.58) | |||
| Health perception | ||||||
| Fair/poor (ref) | 1.00 | 0.10 | 1.00 | 0.14 | 1.00 | 0.27 |
| Good/very good/excellent | 0.68 (0.43–1.10) | 0.71 (0.45–1.12) | 1.35 (0.79–2.32) | |||
| Comorbidities | ||||||
| <3 (ref) | 1.00 | 0.90 | 1.00 | 0.44 | 1.00 | 0.44 |
| ≥3 | 0.99 (0.67–1.42) | 0.86 (0.59–1.25) | 1.18 (0.77–1.80) | |||
| Level of health literacy | ||||||
| Low (ref) | 1.00 | 0.57 | 1.00 | 0.57 | 1.00 | |
| Adequate | 1.11 (0.76–1.63) | 1.11 (0.77–1.60) | 0.82 (0.54–1.24) | 0.34 | ||
Bolded p-value indicates statistical significance at p<0.05
CI confidence interval, PEM printed educational materials only, CRC colorectal cancer, Ref reference group
Odds ratios (OR) and 95 % CIs were calculated using weighted multiple logistic regression models adjusted for variables in the table and baseline screening status
Moderation effects
We next examined potential moderators of the patient navigation intervention, including baseline age, gender, and health literacy level, to determine whether there were sub-groups within our population in which the intervention would be most effective. Significant interaction effects were found between age (p = 0.03) and health literacy (p = 0.04) and the intervention, but not gender (p = 0.61). The intervention was significantly associated with screening status for younger participants aged 65–69 years (OR 2.27, 95 % CI 1.08–4.76) but not for older adults (aged ≥70 years). Patient navigation was also effective among those with adequate health literacy (OR 2.17, 95 % CI 1.03–4.56), but not among participants with low health literacy.
Discussion
Among urban African American Medicare beneficiaries, the use of a patient navigator significantly increased the likelihood of reporting being screened for colorectal cancer. We were also able to determine that baseline age and health literacy were moderators of the intervention effect with stronger effects in younger participants (aged 65–69 years) and those with an adequate health literacy level. The significance of these results are twofold: They demonstrate the potential benefits of patient navigation in increasing CRC screening and identify subgroups within the population for whom additional interventions may be necessary to improve screening compliance.
Although the patient navigation intervention was shown to be effective for increasing CRC screening by any method, analyses of the screening modalities separately revealed a significant impact only for screening by colonoscopy/sigmoidoscopy. Our findings are similar to what was reported by Jandorf and colleagues [14] who observed a significant increase in the incidence of endoscopic examination with the use of a patient navigator, but not in FOBT. The lack of effect on screening by FOBT observed in our study may represent the relatively low rate of provider utilization of this screening modality in the target population. Behavioral Risk Factor Surveillance System data from 2010 showed that 60.3 % of respondents reported colonoscopy as their most recent CRC screening test, while only 11.7 % had FOBT and 1.3 % had sigmoidoscopy in combination with FOBT [34]. Further, a study examining primary care physician (PCP) patterns for CRC screening recommendations using a nationally representative sample of PCPs revealed that 43.3 % recommend colonoscopy over other tests when discussing multiple options [35]. These data highlight the importance of the PCP in facilitating screening adherence and their role in patient decision-making.
We observed that the patient navigation intervention was most effective among participants with adequate levels of health literacy. The lack of effect among participants with low health literacy suggests that low literacy levels may have hindered the participants from absorbing and/or translating the navigator’s suggestions into actionable items. Based on these results, it is conceivable that patient navigators may need to employ a more intensive strategy for those who are less familiar with the health system. A recent systematic review examining CRC screening interventions among racial and ethnic minorities concluded that in addition to patient navigation services, physician training in communicating with patients of low health literacy modestly improved CRC screening adherence [36]. As such, patient navigation services paired with physicians who are trained to assist patients with low health literacy may provide a more significant impact than either intervention alone. We similarly found that the navigation intervention had stronger effects among younger adults (aged 60– 69 years). This result could possibly be due to literacy differences within the age groups, or perhaps point to a stronger intrinsic motivation for screening among the younger participants. Similar to those with low heath literacy, more intensive interventions may be required for adults in the oldest age groups.
This study has several limitations. First, though we attempted to account for loss to follow-up using weighted logistic regression models, loss to follow-up may have biased our results. Second, the statistical power of our study was limited by the high percentage of participants reporting being screened at baseline. The disparity in CRC screening seen in older African Americans may have been attenuated in our study population due to uniform Medicare coverage among our participants, resulting in higher screening rates. Similarly, Burgess and colleagues found that CRC screening disparities between African Americans and whites were attenuated in the Veterans Affairs (VA) system [7]. Third, given that screening was assessed by self-report, it is possible that our results are prone to reporting bias. Fourth, the CPTD did not collect information on whether participants had a primary care physician (PCP) at baseline or on subsequent health care utilization. Given appropriate randomization across a range of covariates, we would not expect participants in the intervention and control arms to have different rates of PCP utilization at baseline. Patient navigation is designed to help overcome barriers to seeking and obtaining health care. Future research should assess whether patients who receive patient navigation have a higher number of health care visits at follow-up and whether this is a mechanism of increased screening. Finally, though the CPTD collected data on comorbidities and adjusted for these measures using disease counts, we did not have sufficient information to construct many commonly used comorbidity indices for use in subsequent analyses [37, 38].
The strengths of the study include the randomized controlled design, collection of multiple sociodemographic characteristics allowing for adjusted models, and assessment of moderators of the interventions effectiveness on CRC screening. Importantly, we conducted our study in a minority population that has been shown to face a greater number of barriers to cancer care compared with non-minority patients [25], and is at increased risk of developing and subsequently dying from colorectal cancer.
Screening for colorectal cancer is a proven and effective strategy for reducing incidence of, and mortality from the disease. The Centers for Disease Control and Prevention (CDC) estimates that if everyone 50 years and older followed the recommended CRC screening guidelines, at least 60 % of deaths from this cancer could be avoided. Consequently, methods for increasing CRC screening will have a substantial public health impact. The results presented here support the use of a patient navigation intervention to increase colorectal cancer screening among older African American adults in an urban setting, while recognizing that more intensive interventions and/or modifications of patient navigation may be necessary for adults over 70 and those with low levels of health literacy.
Supplementary Material
Acknowledgments
The authors would like to thank the Cancer Prevention and Treatment Demonstration participants, staff, and Community Advisory Committee. This work was supported by the Cancer Prevention and Treatment Demonstration for Ethnic and Racial Minorities of the Centers for Medicare and Medicaid Services (Cooperative Agreement #1A0CMS300066), the Community Networks Program (Grant U54CA153710) of the National Cancer Institute, the Maryland Cigarette Restitution Fund. In addition, Dr. M. A. Garza was supported, in part, through her Mentored Research Scientist Development Award to Promote Diversity (K01CA140358).
Footnotes
Conflict of interest J.G. Ford is a consultant/advisory board member of GSK. The remaining authors declare that they have no conflict of interest.
Electronic supplementary material The online version of this article (doi:10.1007/s10552-014-0505-0) contains supplementary material, which is available to authorized users.
Contributor Information
Hisani N. Horne, Email: hisani.horne@nih.gov, Cancer Prevention Fellowship Program, Division of Cancer Prevention, National Cancer Institute, National Institutes of Health, Rockville, MD, USA National Institutes of Health/NCI/DCEG/HREB, 9609 Medical Center Drive, Rm 7E234, MSC 7234, Bethesda, MD 20892-7234, USA.
Darcy F. Phelan-Emrick, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA
Craig E. Pollack, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA Department of General Internal Medicine, Johns Hopkins School of Medicine, Baltimore, MD, USA.
Diane Markakis, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Jennifer Wenzel, Department of Oncology, Department of Acute and Chronic Care, Johns Hopkins School of Medicine, Baltimore, MD, USA; Johns Hopkins University School of Nursing, Baltimore, MD, USA.
Saifuddin Ahmed, Department of Population, Family and Reproductive Health and Department of Biostatistics, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Mary A. Garza, Department of Behavioral and Community Health, School of Public Health, University of Maryland College Park, College Park, MD, USA
Gary R. Shapiro, Health Partners Cancer Program and Institute for Education and Research, Minneapolis, MN, USA
Lee R. Bone, Department of Health, Behavior and Society, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA
Lawrence B. Johnson, Park West Health Systems, Baltimore, MD, USA
Jean G. Ford, The Brooklyn Hospital Center, Brooklyn, NY, USA
References
- 1.Society AC. Cancer facts and figures 2013. American Cancer Society; Atlanta: 2013. [Google Scholar]
- 2.Dimou A, Syrigos KN, Saif MW. Disparities in colorectal cancer in African-Americans vs Whites: before and after diagnosis. World J Gastroenterol. 2009;15:3734–3743. doi: 10.3748/wjg.15.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallace K, Hill EG, Lewin DN, et al. Racial disparities in advanced-stage colorectal cancer survival. Cancer Causes Control. 2013;24:463–471. doi: 10.1007/s10552-012-0133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavicchia PP, Adams SA, Steck SE, et al. Racial disparities in colorectal cancer incidence by type 2 diabetes mellitus status. Cancer Causes Control. 2013;24:277–285. doi: 10.1007/s10552-012-0095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doubeni CA, Major JM, Laiyemo AO, et al. Contribution of behavioral risk factors and obesity to socioeconomic differences in colorectal cancer incidence. J Natl Cancer Inst. 2012;104:1353–1362. doi: 10.1093/jnci/djs346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niu X, Roche LM, Pawlish KS, Henry KA. Cancer survival disparities by health insurance status. Cancer Med. 2013;2:403–411. doi: 10.1002/cam4.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgess DJ, van Ryn M, Grill J, et al. Presence and correlates of racial disparities in adherence to colorectal cancer screening guidelines. J Gen Intern Med. 2011;26:251–258. doi: 10.1007/s11606-010-1575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas JB, Silverman DT, Pollak MN, Tao Y, Soliman AS, Stolzenberg-Solomon RZ. Serum IGF-I, IGF-II, IGFBP-3, and IGF-I/IGFBP-3 molar ratio and risk of pancreatic cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2010;19:2298–2306. doi: 10.1158/1055-9965.EPI-10-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroy PC, 3rd, Coe A, Chen CA, O’Brien MJ, Heeren TC. Prevalence of advanced colorectal neoplasia in white and black patients undergoing screening colonoscopy in a safety-net hospital. Ann Intern Med. 2013;159:13–20. doi: 10.7326/0003-4819-159-1-201307020-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henley SJ, King JB, German RR, Richardson LC, Plescia M. Surveillance of screening-detected cancers (colon and rectum, breast, and cervix)—United States, 2004–2006. MMWR Surveill Summ. 2010;59:1–25. [PubMed] [Google Scholar]
- 11.Freeman HP. Patient navigation: a community based strategy to reduce cancer disparities. J Urban Health. 2006;83:139–141. doi: 10.1007/s11524-006-9030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen LA, Santos S, Jandorf L, et al. A program to enhance completion of screening colonoscopy among urban minorities. Clin Gastroenterol Hepatol. 2008;6:443–450. doi: 10.1016/j.cgh.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Christie J, Itzkowitz S, Lihau-Nkanza I, Castillo A, Redd W, Jandorf L. A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. J Natl Med Assoc. 2008;100:278–284. doi: 10.1016/s0027-9684(15)31240-2. [DOI] [PubMed] [Google Scholar]
- 14.Jandorf L, Gutierrez Y, Lopez J, Christie J, Itzkowitz SH. Use of a patient navigator to increase colorectal cancer screening in an urban neighborhood health clinic. J Urban Health. 2005;82:216–224. doi: 10.1093/jurban/jti046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lasser KE, Murillo J, Medlin E, et al. A multilevel intervention to promote colorectal cancer screening among community health center patients: results of a pilot study. BMC Fam Pract. 2009;10:37. doi: 10.1186/1471-2296-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Percac-Lima S, Grant RW, Green AR, et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. J Gen Intern Med. 2009;24:211–217. doi: 10.1007/s11606-008-0864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitlock EP, Lin JS, Liles E, Bell TL, Fu R. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 18.Bass PF, 3rd, Wilson JF, Griffith CH. A shortened instrument for literacy screening. J Gen Intern Med. 2003;18:1036–1038. doi: 10.1111/j.1525-1497.2003.10651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lippman SA, Shade SB, Hubbard AE. Inverse probability weighting in sexually transmitted infection/human immunodeficiency virus prevention research: methods for evaluating social and community interventions. Sex Transm Dis. 2010;37:512–518. doi: 10.1097/OLQ.0b013e3181d73feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi LC, Wardle J, von Wagner C. Limited health literacy is a barrier to colorectal cancer screening in England: evidence from the English Longitudinal Study of Ageing. Prev Med. 2014;61:100–105. doi: 10.1016/j.ypmed.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez-Donate AP, Halverson J, Simon NJ, et al. Identifying health literacy and health system navigation needs among rural cancer patients: findings from the Rural Oncology Literacy Enhancement Study (ROLES) J Cancer Educ. 2013;28:573–581. doi: 10.1007/s13187-013-0505-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson EA, Wolf MS, Curtis LM, et al. Literacy, cognitive ability, and the retention of health-related information about colorectal cancer screening. J Health Commun. 2010;15(Suppl 2):116–125. doi: 10.1080/10810730.2010.499984. [DOI] [PubMed] [Google Scholar]
- 23.Dinh TA, Alperin P, Walter LC, Smith R. Impact of comorbidity on colorectal cancer screening cost-effectiveness study in diabetic populations. J Gen Intern Med. 2012;27:730–738. doi: 10.1007/s11606-011-1972-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrante JM, Ohman-Strickland P, Hudson SV, Hahn KA, Scott JG, Crabtree BF. Colorectal cancer screening among obese versus non-obese patients in primary care practices. Cancer Detect Prev. 2006;30:459–465. doi: 10.1016/j.cdp.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hendren S, Chin N, Fisher S, et al. Patients’ barriers to receipt of cancer care, and factors associated with needing more assistance from a patient navigator. J Natl Med Assoc. 2011;103:701–710. doi: 10.1016/s0027-9684(15)30409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez KA, Pollack CE, Phelan DF, et al. Gender differences in correlates of colorectal cancer screening among black medicare beneficiaries in Baltimore. Cancer Epidemiol Biomarkers Prev. 2013;22:1037–1042. doi: 10.1158/1055-9965.EPI-12-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong RK, Wong ML, Chan YH, Feng Z, Wai CT, Yeoh KG. Gender differences in predictors of colorectal cancer screening uptake: a national cross sectional study based on the health belief model. BMC Public Health. 2013;13:677. doi: 10.1186/1471-2458-13-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, Shao J. Nearest neighbor imputation for survey data. J Off Stat. 2000;16:113–131. [Google Scholar]
- 29.Horton WS, Spieler DH. Age-related differences in communication and audience design. Psychol Aging. 2007;22:281–290. doi: 10.1037/0882-7974.22.2.281. [DOI] [PubMed] [Google Scholar]
- 30.Jefferson L, Bloor K, Birks Y, Hewitt C, Bland M. Effect of physicians’ gender on communication and consultation length: a systematic review and meta-analysis. J Health Serv Res Policy. 2013;18:242–248. doi: 10.1177/1355819613486465. [DOI] [PubMed] [Google Scholar]
- 31.Law J, Rush R, Parsons S, Schoon I. The relationship between gender, receptive vocabulary, and literacy from school entry through to adulthood. Int J Speech Lang Pathol. 2013;15:407–415. doi: 10.3109/17549507.2012.721897. [DOI] [PubMed] [Google Scholar]
- 32.Weiss BD, Reed RL, Kligman EW. Literacy skills and communication methods of low-income older persons. Patient Educ Couns. 1995;25:109–119. doi: 10.1016/0738-3991(95)00710-h. [DOI] [PubMed] [Google Scholar]
- 33.StataCorp. Stata Statistical Software: Release 11. StataCorp LP: College Station; 2009. [Google Scholar]
- 34.Joseph D, King J, Miller J, Richardson L. Prevalence of colorectal cancer screening among adults—behavioral risk factor surveillance system, United States, 2010. Morb Mortal Wkly Rep Suppl. 2010;61:51–56. [PubMed] [Google Scholar]
- 35.Zapka JM, Klabunde CN, Arora NK, Yuan G, Smith JL, Kobrin SC. Physicians’ colorectal cancer screening discussion and recommendation patterns. Cancer Epidemiol Biomarkers Prev. 2011;20:509–521. doi: 10.1158/1055-9965.EPI-10-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naylor K, Ward J, Polite BN. Interventions to improve care related to colorectal cancer among racial and ethnic minorities: a systematic review. J Gen Intern Med. 2012;27:1033–1046. doi: 10.1007/s11606-012-2044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 38.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


