Abstract
Some members of the transient receptor potential (TRP) family of cation channels mediate sensory responses to irritant substances. Although it is well known that TRPA1 channels are activated by pungent compounds found in garlic, onion, mustard and cinnamon extracts, activation of TRPV1 by these extracts remains controversial. Here we establish that TRPV1 is activated by pungent extracts from onion and garlic, as well as by allicin, the active compound in these preparations, and participates together with TRPA1 in the pain-related behavior induced by this compound. We found that in TRPV1 these agents act by covalent modification of cysteine residues. In contrast to TRPA1 channels, modification of a single cysteine located in the N-terminal region of TRPV1 was necessary and sufficient for all the effects we observed. Our findings point to a conserved mechanism of activation in TRP channels, which provides new insights into the molecular basis of noxious stimuli detection.
The family of TRP channels is composed of a large variety of cation-permeable channels and shows a great diversity of activation mechanisms. Members of the TRPV subfamily are expressed in sensory neurons and other cell types, and mediate a large number of sensory responses1. For example, TRPV1 responds to a variety of stimuli, such as high temperatures, tissue damage and exposure to pungent compounds such as capsaicin2,3, all of which converge on these channels and underlie the common perceptual experience of pain.
Recently, pungent or irritant compounds other than capsaicin have been found to activate TRPV1 channels4. Some of these compounds are present in plants from the Allium genus (that is, onion and garlic)5–8. Notably, the same compounds found in onion and garlic extracts are also capable of activating TRPA1 (refs. 4,9,10), another member of the TRP family. TRPA1 and TRPV1 are expressed in the same subset of dorsal root ganglia (DRG) neurons, and to date there is controversy over whether both or only one of these molecules is the target for pungent compounds.
In the case of TRPA1 channels, activation by some pungent agents occurs through their direct covalent modification of several cysteines in the N-terminal domain9.
The crystal structure of the N-terminal region of the TRPV1 channel was recently solved and shown to be formed by ankyrin repeats11. The N-terminus of TRPA1 has also been proposed to be formed by ankyrin repeats. The role of the N-terminal ankyrin repeats in the function of TRP channels has remained obscure; however, recent studies have demonstrated that this region forms a multi-ligand binding domain that mediates the response to ATP, PIP2 and calmodulin in TRPV1 (ref. 11).
In this study, we directly addressed the participation of TRPV1 in the response to cysteine-modifying agents, including those compounds found in onion and garlic extracts, and examine the mechanism by which they activate this channel. Using a combination of electrophysiological and behavioral assays in wild-type, TRPV1- and TRPA1-null mice, we established that TRPV1 also participates in the response to allicin in vivo. We extended this analysis to activation of heterologously expressed TRPV1 by allicin and other cysteine-modifying compounds. We identified a single cysteine for the interaction of these compounds in the N-terminus of TRPV1 and have produced a functional cysteineless TRPV1 channel, which should prove to be a valuable tool for future structure-function studies. The results reported here provide us with new insights into the regulatory functions of the N-terminal region in the TRPV1 channel.
RESULTS
Both TRPA1 and TRPV1 are activated by allicin
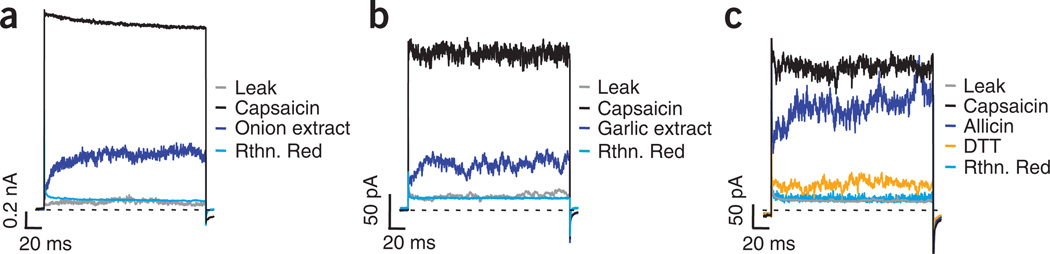
To determine whether TRPV1 could be activated by compounds found in onion and garlic, we explored the effects of these extracts in membrane patches from dissociated mouse DRG neurons. We first applied a saturating concentration of capsaicin (4 µM) to inside-out patches to test for the presence of TRPV1 channels in these cells. After removal of capsaicin, subsequent application of either onion or garlic extract elicited smaller, but considerable, currents. Finally, the currents activated by capsaicin, onion or garlic were all blocked by ruthenium red, a nonspecific blocker of TRP channels (Fig. 1a,b). These results suggest that TRPV1 may be a target of garlic and onion compounds.
Figure 1.
DRG neurons respond to onion and garlic extracts and to allicin. (a–c) Onion (a) and garlic (b) extracts, as well as allicin (200 µM) (c), activate currents in inside-out patches from DRG neurons. Traces shown are representative of five equal experiments obtained by stepping the voltage from 0 mV to 110 mV for 200 ms. Leak currents were obtained before any treatment was applied. Membrane patches from DRG neurons were first exposed to 4 µM capsaicin to identify TRPV1-expressing cells. Extracts or allicin were applied to capsaicin-responsive membrane patches after capsaicin had been washed from the bath. All currents were blocked by ruthenium red (Rthn. Red, 10 µM) applied together with either of the extracts or with allicin. Application of DTT (10 mM) to patches reversed allicin effects.
We next tested whether the effects of onion and garlic extracts are due to allicin, the same compound responsible for activation of TRPA1. Allicin is a cysteine-reactive species and a major component of onion and garlic5–8,12. Application of allicin to inside-out patches from DRG neurons activated a similar outward current as the one activated by the extracts (Fig. 1c). The mechanism underlying this effect appeared to arise from covalent modification, as currents did not spontaneously reverse on removal of allicin from the bath. Consistent with this mechanism, application of 10 mM dithiothreitol (DTT), a reducing agent, produced a near-complete reversal of activation by allicin (Fig. 1c).
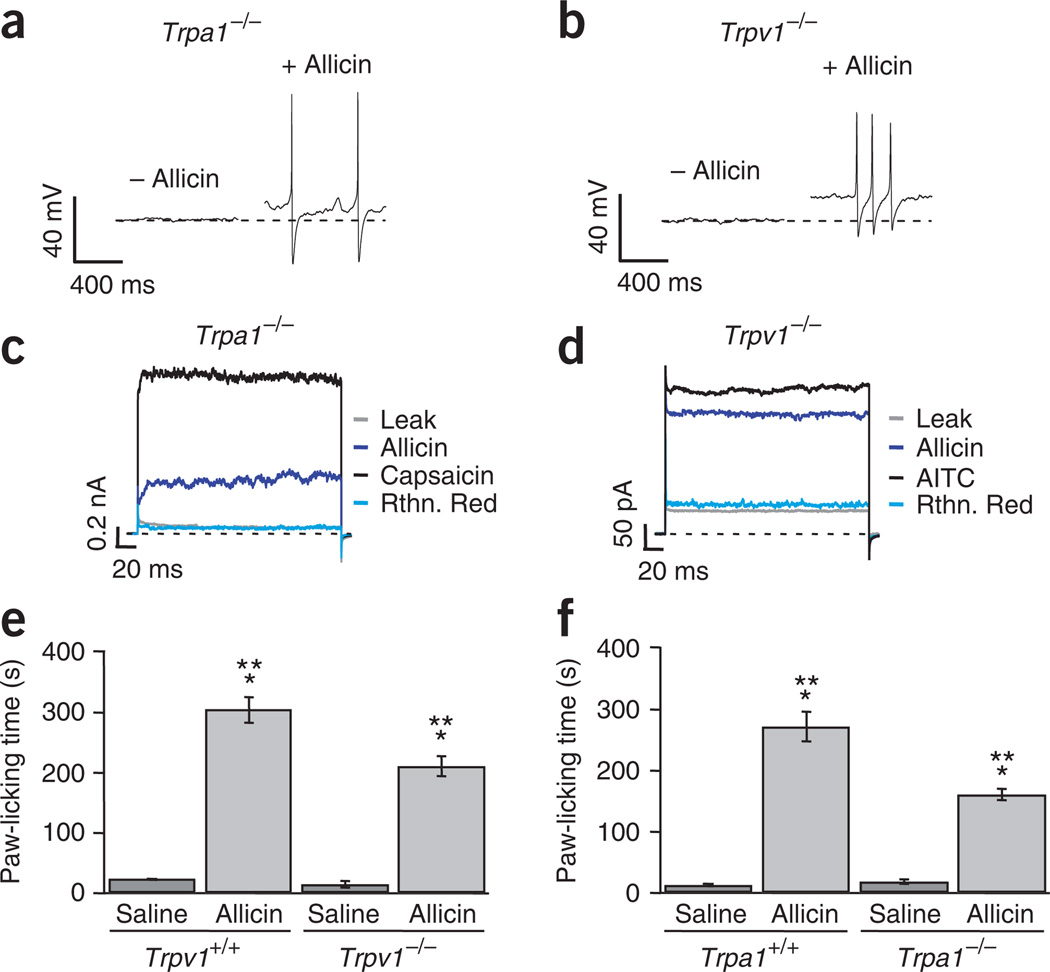
Given the present controversy regarding whether allicin’s effects are mediated by TRPA1 or TRPV1 (refs. 4,9,10), we carried out electrophysiological recordings in DRG neurons from Trpv1−/− and Trpa1−/− mice to establish the physiological response of each of these channels to allicin and to onion and garlic extracts. Application of 200 µM allicin to DRG neurons isolated from these mice elicited a depolarization in the range of 7–20 mV and subsequent action potential firing (Fig. 2a,b). This result clearly indicates that TRPV1 and TRPA1 in DRG neurons are both involved in the response to allicin.
Figure 2.
Allicin induces TRPA1- and TRPV1-mediated action potentials and a behavioral pain-like response. (a,b) Allicin-induced action potentials recorded from mice DRG neurons from Trpa1−/− (a) and Trpv1−/− (b) mice. Membrane potential before (left) and after (right) application of 200 µM allicin. The dotted line denotes resting membrane potential (n = 6 per genotype). (c,d) Current activation by allicin. Representative recordings obtained from Trpa1−/− (c) and Trpv1−/− (d) DRG neurons (n = 10 per genotype). (e,f) Pain-related behavior, as determined by the time spent licking the injected paw. Allicin-injected animals spent more time licking the affected paw (210 ± 17 s for Trpv1−/− and 161 ± 10 s for Trpa1−/− mice) than did those injected with saline (14.5 ± 5 s for Trpv1−/− and 18.5 ± 4 s for Trpa1−/− mice). The response of Trpv1−/− and Trpa1−/− mice to allicin was also smaller than the response of their wild-type (WT) littermates (304 ± 21 s for TRPV1-WT background and 271 ± 25 s for TRPA1-WT background). Saline injection into wild-type animals produced a short licking response (22.5 ± 0.25 s for TRPV1-WT background and 13 ± 2 s for TRPA1-WT background). * indicates a significant difference between the saline- and allicin-injected animals (P < 0.01). ** indicates a significant difference (P < 0.01) on comparison of wild-type and knockout animals injected with allicin (Student’s t-test, n = 8 per genotype for all experiments). Data are mean ± s.e.m.
A cationic current could be activated in response to allicin in DRG neurons from Trpv1−/− and Trpa1−/− mice (Fig. 2c,d). Allicin activation of these currents was characterized by the fractional activation, the ratio of current activated by allicin to current activated by each of the well documented agonists for each of the TRP channels addressed in this study, namely capsaicin (4 µM) or allyl isothiocyanate (AITC, 200 µM). This fractional activation was 0.4 ± 0.06 (n = 10) for the Trpa1−/− cells and 0.85 ± 0.05 (n = 10) for Trpv1−/− cells (data not shown).
Moreover, in the Trpa1−/− mice, where only TRPV1 is present, we found that those cells that did not respond to capsaicin did not respond to allicin either (19 out of 19 cells), whereas in the TRPV1-null mice, cells that did not respond to allicin did not respond to AITC (18 out of 18). These data show that the presence of TRPV1 or TRPA1 channels is a requirement for the response to allicin and that TRPV1 is a target for allicin.
To assess the physiological role of TRPV1 in pain-related behavior, we injected either saline solution or allicin-containing solution (2 µg) into the left front paw of Trpv1−/−, Trpa1−/− and their littermate wild-type mice, using a protocol similar to one previously reported13, and measured the total time that the animals spent licking the affected paw during a 30-min period. Trpv1−/− and Trpa1−/− mice showed a significant increase in paw-licking time when injected with allicin as compared with the animals injected with only saline solution (P < 0.01; Fig. 2e,f). We found it interesting that the Trpv1−/− mice showed a more pronounced reaction to the treatment when compared with the Trpa1−/− animals, but the reaction in both types of mice was significantly less intense than that observed for their corresponding wild-type littermates (P < 0.01; Fig. 2e,f). These results strongly indicate that allicin is capable of inducing a painful stimulus that is mediated not only through activation of TRPA1, in Trpv1−/− mice, but also through the activation of TRPV1, as evidenced by the behavioral response observed in Trpa1−/− mice.
Activation of native and heterologously expressed TRPV1
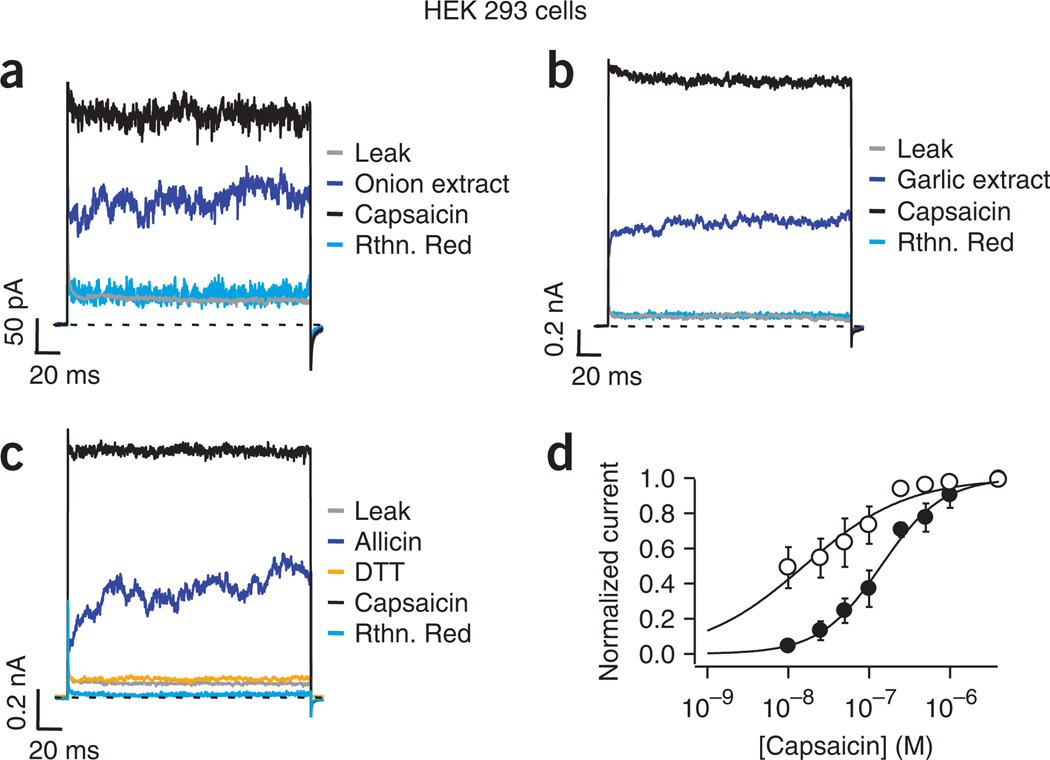
The subset of DRG neurons that express TRPV1 also express TRPA1 (ref. 14), so the pungent compound-induced currents we observed in wild-type cells (Fig. 1) were likely the result of activation of either channel type. To determine whether isolated TRPV1 can be activated by onion and garlic extracts, we examined the responses of TRPV1 heterologously expressed in human embryonic kidney (HEK293) cells. When onion or garlic extracts or allicin were applied to inside-out patches from TRPV1-transfected cells, we observed robust channel activation (Fig. 3a–c). TRPV1-mediated currents in HEK293 cells activated by these compounds were blocked by ruthenium red and required DTT to reverse activation. DTT did not affect either capsaicin- or temperature-mediated activation of TRPV1 (Supplementary Fig. 1 online). Patches from HEK293 cells expressing only green fluorescent protein (GFP) did not respond to capsaicin, or to any of the extracts or cysteine-modifying agents used (Supplementary Fig. 2 online). From these experimental data, we concluded that TRPV1 can be robustly activated by onion and garlic extracts (Fig. 3a,b) and by purified allicin (Fig. 3c).
Figure 3.
Heterologously expressed TRPV1 channels respond to pungent compounds. (a–c) Currents from inside-out patches were obtained as in Figure 1. Onion (a) and garlic (b) extracts and allicin (c) activate currents in HEK293 cells expressing TRPV1. (d) Dose response for activation by capsaicin before (filled symbols) and after (empty symbols) exposure of patches from HEK293 cells to allicin. Smooth curves are fits with the Hill equation. KD values before (141 ± 39 nM) and after (24 ± 14 nM) allicin were statistically different (Student’s t-test, P < 0.05, n = 5). Data are mean ± s.e.m.
To determine whether allicin is a channel activator or an allosteric regulator of capsaicin activation, we examined whether allicin could modify the response of TRPV1 to capsaicin. We measured the capsaicin dose-response by applying various concentrations of this agonist to the cytoplasmic surface of inside-out patches from HEK293 cells expressing TRPV1. After carrying out the initial measurements (Fig. 3d, filled symbols), we exposed the patches for 2 min to allicin and subsequently re-measured the capsaicin-activated currents. We found a decrease in the apparent dissociation constant (KD), determined from fits to the Hill equation15 (Fig. 3d, empty symbols). A possible interpretation of these results is that allicin is capable of making TRPV1 channels more sensitive to capsaicin, presumably through an allosteric coupling mechanism. On the other hand, it is also possible that allicin acts as an independent activator. We sought to explore these possibilities first by establishing if other cysteine-modifying reagents were capable of supporting channel activation and then by exploring the mechanism of allicin activation.
Cysteine-modifying reagents induce TRPV1 activation
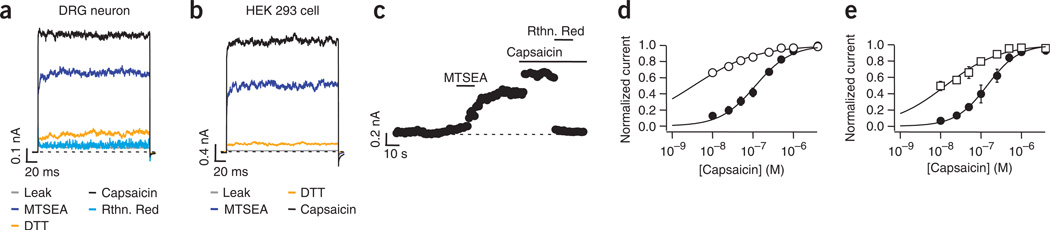
Activation of TRPA1 by pungent compounds is known to be the result of covalent modification of three cysteine residues in the amino-terminal region. The ability of DTT to reverse TRPV1 activation by onion and garlic extracts and by allicin suggested that cysteine modification has a role in TRPV1 activation as well. To explore this possibility, we tested whether cysteine-modifying reagents other than allicin could act as activators of TRPV1. Using inside-out patches from DRG neurons and TRPV1-transfected HEK293 cells, we were able to activate channels by applying 2 mM 2-aminoethyl methanethiosulfonate (MTSEA). This activation was reversed only after application of DTT, and the currents could be blocked by ruthenium red (Fig. 4a,b). The time course of activation by MTSEA occurred with a time constant of 13.1 ± 2 s (n = 3; Fig. 4c). As with allicin, treatment with MTSEA altered the channel’s response to capsaicin, reflected in a leftward shift in the KD (Fig. 4d). When [2-(trimethylammonium) ethyl] methane-thiosulfonate chloride (MTSET) was used, the KD for capsaicin activation was also decreased (Fig. 4e).
Figure 4.
Cysteine-modifying reagents act as activators of TRPV1 channels. (a,b) MTSEA promotes activation of currents in inside-out patches from DRG neurons (a) and HEK293 cells (b). The voltage protocol was the same as in Figure 1. Leak currents and currents after MTSEA (2 mM) were measured and then DTT (10 mM) was applied, followed by capsaicin (4 µM). Ruthenium red (10 µM), applied together with MTSEA, blocked currents (representative traces of five experiments). (c) Activation of TRPV1 by MTSEA. Bars indicate the application of 2 mM MTSEA, 4 µM capsaicin and 10 µM Rthn Red. Voltage was stepped from a holding potential of 0 mV to 100 mV at 1 Hz. A 10-s application of MTSEA to membrane patches expressing TRPV1 channels was enough to induce current activation equivalent to 60–70% of the current activated by 4 µM capsaicin. Ruthenium red blocks the currents to initial leak levels. (d,e) Dose responses for activation of TRPV1 channels by capsaicin before (filled symbols) and after (empty symbols) MTSEA (d) and MTSET (e). Smooth curves are fits with the Hill equation. KD values before (124 ± 25 nM) and after (4 ± 1 nM) MTSEA or before (158 ± 38 nM) and after (8 ± 3 nM) MTSET were statistically different for both cases (Student’s t-test, P < 0.002; n = 5 and n = 4, respectively). Data are mean ± s.e.m.
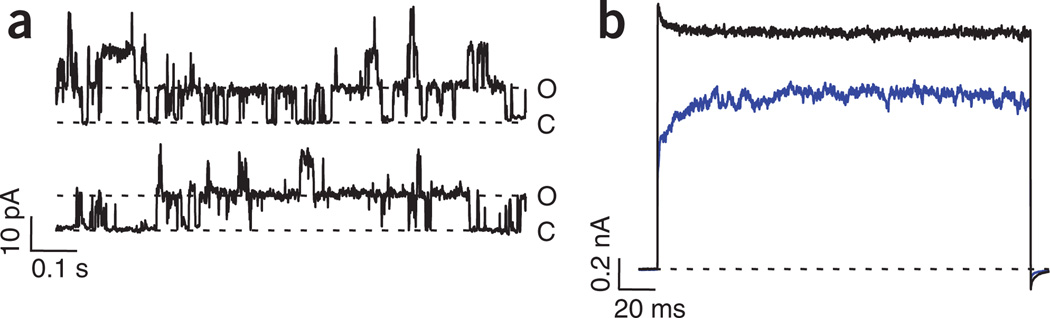
Because the application of these cysteine-modifying reagents was sufficient to produce TRPV1 current increases, we sought to determine whether these compounds promoted activation of the channel by measuring the open probability in the absence of capsaicin. Comparison of the capsaicin-independent opening probability before MTSEA and after cysteine modification resulted in significantly increased open probability (from 0.0016 ± 0.0009 to 0.45 ± 0.08 after exposure to MTSEA, P < 0.001, n = 5, Student’s t-test; Figure 5a,b and data not shown). The single-channel conductance (gi) was not affected, as determined by non-stationary noise analysis of macroscopic currents (gi = 12.3 ± 5 pS and 14.1 ± 3 pS before and after MTSEA modification, n = 3; data not shown).
Figure 5.
MTSEA increases open probability in TRPV1 in the absence of capsaicin. (a) Channel openings in the absence of agonist in an inside-out membrane patch. (b) Macroscopic currents induced by MTSEA (blue trace) and capsaicin (4 µM) after MTSEA (black trace) in the same patch as in a. The number of channels in the patch was ~133.
This result indicated that cysteine modification alone was sufficient to produce channel activation, although allosteric interactions between different activation mechanisms are still possible, as is the case for activation by capsaicin and voltage15, and voltage and temperature-dependent activation16. It is therefore clear that cysteine modifiers are activators of the channel, as their action did not depend on the presence of capsaicin. Other cysteine-modifying reagents known to activate TRPA1 channels, such as 200 µM AITC, 100 µM cinnamaldehyde and 2 mM N-ethyl maleimide, did not activate TRPV1 channels, neither in inside-out patches (Supplementary Fig. 3 online) nor in cell-attached patches (data not shown), in accordance with previous reports9,17.
Residues responsible for TRPV1 activation by allicin
Some of the cysteines in the pore region of TRPV1 are modifiable from the extracellular side18,19. To assess the localization of any of the modifiable cysteines responsible for the effects seen here, we carried out experiments including free cysteine in the patch pipette to act as a scavenger of membrane-permeable reactive compounds, both for inside-out and outside-out patches.
The presence of free cysteine in the opposite side to which MTSEA was applied insured that any molecules that permeated the membrane were quickly reacted, so that modification was only possible on the side of application. When channels in inside-out patches were activated with a subsaturating capsaicin concentration (50 nM) and free cysteine (20 mM) was present in the extracellular side of the channels, currents could be potentiated by the application of MTSEA to the intracellular side (Supplementary Fig. 4 online). In contrast, in outside-out patches with the excess of free cysteine located at the intracellular face of the channels, MTSEA applied to the extracellular region did not produce current potentiation (Supplementary Fig. 4). These results lead us to conclude that the cysteine or cysteines responsible for the activation by modifying agents are located in an intracellular region of the channel.
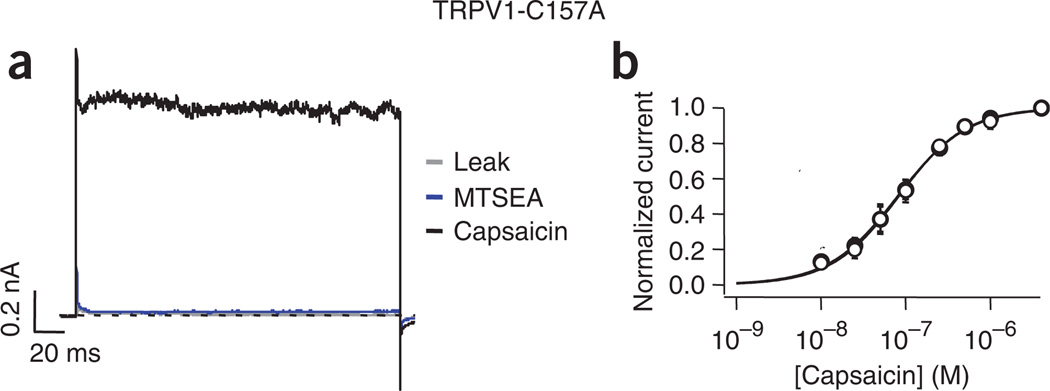
The rTRPV1 channel contains 18 cysteines in its primary sequence. To identify the cysteine(s) responsible for activation of TRPV1 by cysteine-modifying compounds, we individually mutated each of the 18 cysteines to a conserved or equivalent residue, as determined by the sequence similarity between TRP family members. We found that substitution of the majority of the cysteines produced functional channels that retained their response to MTSEA. The main effect of cysteine substitution was a reduced expression level, but these channels could still be activated by voltage and capsaicin. At the same time, we identified a single residue responsible for this cysteine modification–dependent activation. Mutation of a single cysteine at position 157 in the N-terminus to alanine (C157A) rendered the channel insensitive to MTSEA (Fig. 6a), and the response to capsaicin was unchanged after MTSEA treatment (Fig. 6b). C157A channels were also insensitive to activation by onion or garlic extracts (Supplementary Fig. 5 online). The localization of C157 in the N-terminus was consistent with the intracellular location of the cysteine responsible for activation, (Supplementary Fig. 4). These data demonstrate that C157 is necessary for activation of TRPV1 by the cysteine-modifying compounds tested.
Figure 6.
A single cysteine is necessary for activation of the TRPV1 channel by cysteine-modifying reagents. The mutation C157A renders the TRPV1 channel insensitive to MTSEA. (a) Representative traces obtained as in Figure 1. The gray trace represents leak current obtained from an inside-out patch from HEK293 cells expressing TRPV1-C157A channels. The blue trace was obtained after applying 2 mM MTSEA to the intracellular face of the membrane patch for 2 min (longer applications of up to 8 min also failed to induce current activation), and the black trace represents currents activated by 4 µM capsaicin. (b) Dose response for activation by capsaicin before (filled symbols) and after (empty symbols) exposure of the inside-out patch to MTSEA. Smooth curves represent fits with the Hill equation. KD values before (118 ± 24 nM) and after (133 ± 32 nM) MTSEA for TRPV1-C157A were not statistically different (Student’s t-test, P > 0.5, n = 5). Data are mean ± s.e.m.
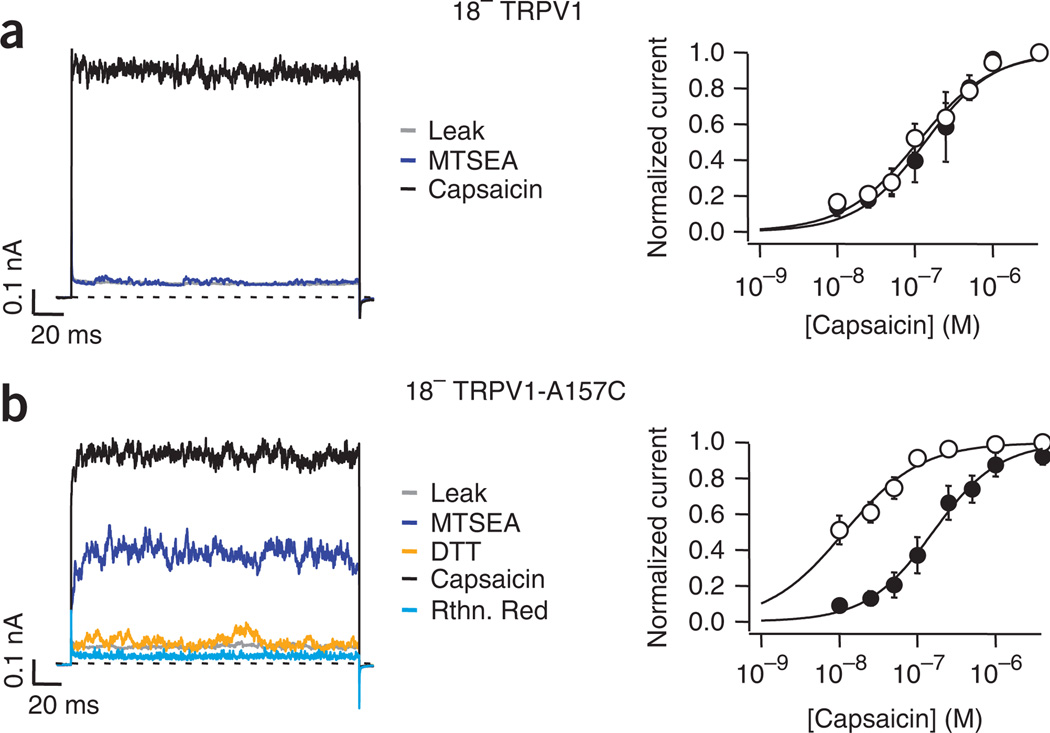
To demonstrate that these effects were indeed mediated by a single cysteine, we generated a channel in which all 18 cysteines were substituted and called it 18− TRPV1. This channel remained functional and retained sensitivity to capsaicin. As expected, 18− TRPV1 channels were not activated by application of MTSEA (Fig. 7a) or by onion or garlic extracts (Supplementary Fig. 5) and the dose response for activation by capsaicin was not altered (Fig. 7a, right).
Figure 7.
The presence of C157 is sufficient to promote MTSEA effects on TRPV1. (a,b) Traces on the left panels were obtained as in Figure 1. The right panels depict dose responses for activation by capsaicin before (filled symbols) and after (empty symbols) exposure of the inside-out patches to MTSEA in the different mutant channels. Smooth curves represent fits with the Hill equation. The cysteine-less TRPV1 channel (18− TRPV1) was functional and resulted in a lack of current activation by MTSEA, whereas application of capsaicin induced current activation (a). KD values before (106 ± 17 nM, filled symbols) and after MTSEA (129 ± 16nM, empty symbols) for 18− TRPV1 channels were not statistically different. (Student’s t-test, P > 0.5, n = 5). Reintroduction of C157 into the cysteine-less TRPV1 channel promoted recovery of sensitivity to MTSEA (b). The traces in the left show that activation by MTSEA could now be attained and reversal of MTSEA effects could be achieved in the presence of DTT. Current activation can be re-attained by application of 4 µM capsaicin. Finally, application of 10 µM ruthenium red resulted in current block. KD values before (128 ± 37 nM) and after (10 ± 2 nM) MTSEA (filled and empty symbols, respectively) for 18− TRPV1-A157C channels were statistically different (Student’s t-test, P < 0.002, n = 5). Data are mean ± s.e.m.
We next determined whether modification of C157 was sufficient to promote activation by reinserting it into the 18− TRPV1 background. These channels, 18− TRPV1–C157, responded to capsaicin similarly to the wild type, were activated by application of MTSEA and this activation was reversed by application of DTT (Fig. 7b, left). Reinsertion of C157 into the 18− TRPV1 also promoted a reduction in the KD for activation by capsaicin after treatment with MTSEA (Fig. 7b, right). In addition, 18− TRPV1–C157 channels recovered activation by onion and garlic extracts (Supplementary Fig. 5).
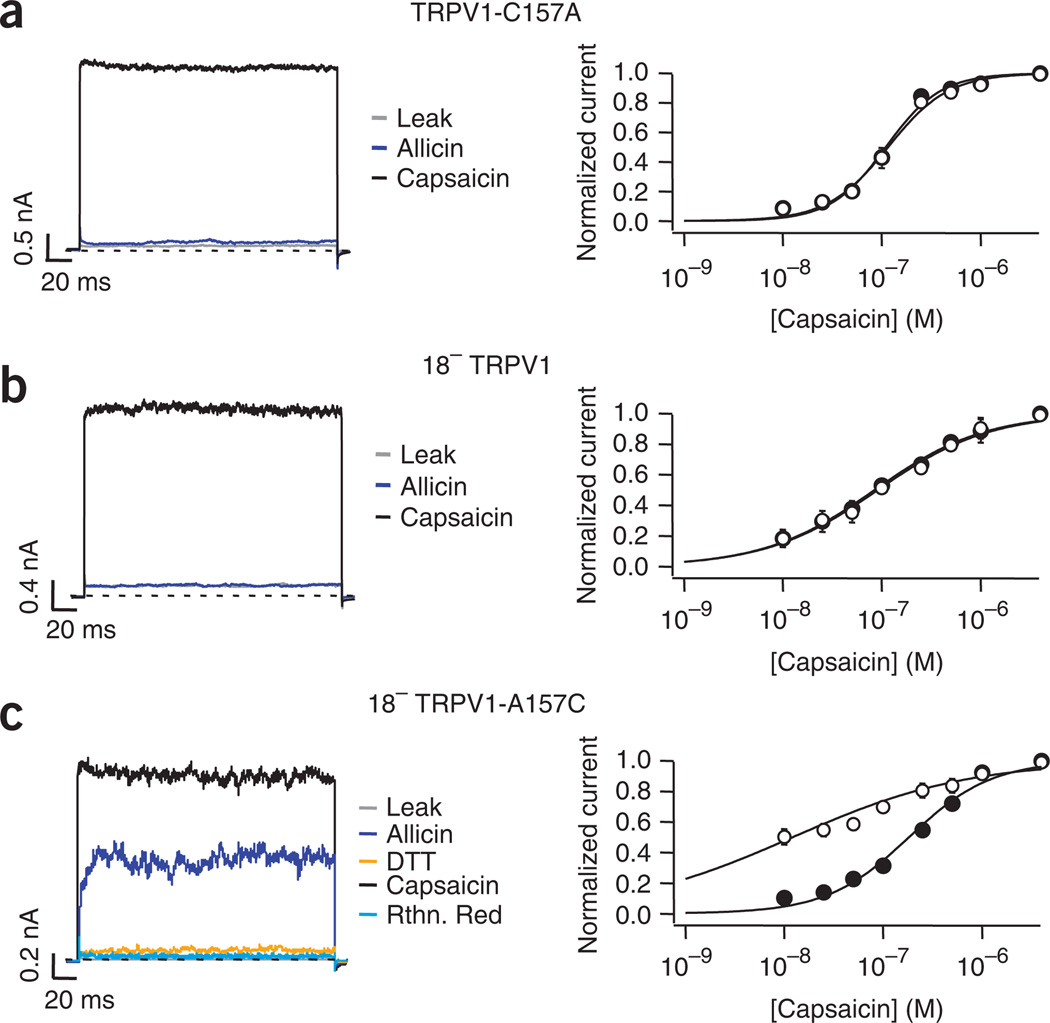
To firmly establish the mechanism of garlic and onion activation of TRPV1, we examined the effects of allicin on TRPV1–C157A, 18− TRPV1 and 18− TRPV1–C157. The substitution C157A alone produced a construct that did not show changes in KD induced by allicin application (Fig. 8a). This was also true of 18− TRPV1 channels, where allicin did not elicit current activation and the KD values for activation by capsaicin were unchanged (Fig. 8b). On the other hand, reinsertion of C157 into the cysteine-less channel recovered sensitivity to allicin (Fig. 8c). The data show that modification of the single cysteine C157 is necessary and sufficient to produce activation of TRPV1 by allicin.
Figure 8.
Cysteine C157 is also responsible for the activation of the TRPV1 channel by allicin. (a–c) Representative traces shown in the left panel were obtained as in Figure 1.The right panel depicts dose responses for activation by capsaicin before (filled symbols) and after (empty symbols) exposure of the inside-out patches to allicin. The mutation of C157 (a) and the substitution of all cysteines in the channel sequence (b) produced an allicin-insensitive TRPV1 channel (KD = 111 ± 14 nM and 117 ± 20 nM before and after allicin for TRPV1-C157, and KD = 90 ± 32 nM and 96 ± 33 nM before and after allicin for 18− TRPV1; n = 5). Reinsertion of C157 into 18− TRPV1 restored channel sensitivity to allicin (c; KD = 177 ± 12 nM and 15 ± 5 nM before and after allicin, Student’s t-test, P < 0.05, n = 5). Data are mean ± s.e.m.
DISCUSSION
TRP channels are fundamentally involved in the perception of environmental stimuli and some show sensitivity to cinnamaldehyde, mustard oil and allicin, substances that cause pain-like sensations20,21. To date, there is still controversy over whether TRPA1 is the only target for these compounds and the sole mediator of their effects10,22. Although it seems clear that cinnamaldehyde and mustard oil only activate TRPA1 channels, different reports have had opposite conclusions on whether compounds found in onion and garlic also activate TRPV1 (refs. 10,22,23).
Our data show that TRPV1 is a target for garlic actions and that onion extracts are also capable of activating it. Moreover, we found that TRPV1 activation by allicin is of physiological significance, with TRPV1 mediating part of the responses to this compound in isolated DRG neurons and in the in vivo model. Both our electrophysiological and behavioral data from TRPV1- and TRPA1-null mice, which allowed the assessment of the role of each of these ion channels separately, indicated that TRPV1 is important in the response to allicin. In fact, DRG neurons from each of the null mice were able to respond to allicin with increased excitability, and this result, combined with the responses obtained from the behavioral assays, clearly indicates that both TRPV1 and TRPA1 channels contribute to the perception of pain induced by allicin.
Cysteine residues are implicated in the modulation of the activity of several types of channels, including TRP channels10,18,19,22,24–26. To explore the mechanism by which allicin activates the TRPV1 channel, we constructed a functional cysteine-less TRPV1 that will be a most valuable tool for future structure-function studies. In this work, we have described a straightforward mechanism by which onion and garlic extracts, as well as allicin, the major active compound found in these extracts, activate TRPV1 through modification of a single cysteine, C157, in the N-terminus of the protein.
The crystal structure of the N-terminal regions of TRPV1 and TRPV2 channels were recently solved and shown to be formed by six ankyrin repeats11,27. It is also established that the N-terminal region of TRPV1 forms a multi-ligand binding domain that mediates the response to ATP, PIP2 and calmodulin11. Examination of the published structure of the N-terminus of TRPV111 reveals that C157 localizes to inner helix 2 of the ANK-2 repeat (Supplementary Fig. 6 online). Perhaps not surprisingly, this is also the region in which multiple other regulatory ligands bind. For example, the nearby sites K155, K160 and L163 in the inner helix 2 of the N-terminus are known to form the ATP-binding site in the TRPV1 channel. Mutation of these sites results in impaired tachyphylaxis in the absence of ATP11. These results and our data suggest that this region may undergo a substantial conformational change that is coupled to channel gating and constitutes an important regulatory region of TRP channel function.
The importance of the N-terminus in TRP-channel function seems to be conserved, as a cluster of three cysteines in this region of TRPA1 (C619, C639 and C663) has been identified as the region that mediates activation by cinnamaldehyde and mustard oil9. Moreover, this region in TRPA1, which also contains a stretch of ankyrin repeats, is proposed to be important in TRPA1 activation in response to changes in tension at the cell surface28–30. Other cysteines in the TRPV1 sequence have been described to be partly responsible for activation of TRPV1 by nitric oxide and H2O2. It is possible then that modification of C157 mediates part of the effects of these agents26.
Although activation by cysteine modifiers may be a conserved mechanism in TRP channels, the lack of activation of TRPV1 by cinnamaldehyde, N-ethyl maleimide, aldehydes31 and particularly by AITC, which is structurally similar to allicin, poses a conundrum in regard to agonist specificity in TRP channels. The fact that both charged and uncharged MTS reagents promoted channel activation strongly suggests that these effects are the result of steric, rather than electrostatic interactions. Also, the fact that capsaicin sensitivity remained intact in C157A and in 18− TRPV1 channels suggests that these two activation mechanisms are separated.
TRPV1 and TRPA1 show only 13% sequence identity (22% conservation). Nonetheless, our results indicate that they share a conserved mechanism of activation. The understanding of the nature of this mechanism should provide us with new insights into the molecular basis underlying the detection of noxious stimuli. Future studies will determine the molecular basis of these distinctions.
METHODS
Preparation of DRG neurons
We isolated DRG neurons by manual dissection from C57BL/6, Trpa1−/− and Trpv1−/− mice as previously described32, and carried out electrophysiological experiments 6–36 h after plating.
HEK293 cell culture and recording
HEK293 cells expressing large T antigen were transfected with wild-type and mutant rTRPV1-pCDNA3 and pIRES-GFP (BD Biosciences) by lipofectamine (Invitrogen) as previously described15. Recordings were carried out using symmetrical solutions consisting of 130 mM NaCl, 3 mM HEPES (pH 7.2) and 1 mM EDTA for Ca2+-free conditions. Solutions were changed with a RSC-200 rapid solution changer (Molecular Kinetics). Unless otherwise indicated, all chemicals were purchased from Sigma.
Dose-response curves were measured at 100 mV and 24 °C. Dose-response relations were fitted with the Hill equation15. Electrophysiological experiments were carried out using an EPC 10 amplifier (HEKA Elektronik GMBH).
Data were acquired and analyzed with PULSE software (HEKA Elektronik) and were plotted and analyzed with programs written using Igor Pro (Wave-metrics). Single-channel recording and non-stationary noise analysis were carried out as previously described15,33.
The unliganded open probability, pul, was determined from the ensemble average of 60 traces at 100 mV, and the number of channels, N, was estimated from the formula I = piN, where I is the macroscopic current induced by capsaicin, i = 10.5 pA, and P = 0.92 is the open probability at 4 µM capsaicin. i and p were determined from single-channel recordings.
For action potential recordings, we used the whole-cell configuration to record from acutely dissociated DRG neurons from WT, Trpv1−/− or Trpa1−/− mice. For these experiments, the pipette solution contained 140 mM KCl, 0.5 mM EGTA, 5 mM HEPES, 3 mM Mg-ATP and 5 mM glucose (pH 7.2), and the bath solution contained 140 mM NaCl, 3 mM KCl, 2 mM MgCl2, 2 mM CaCl2 and 10 mM HEPES. The resting membrane potential was recorded continuously and allicin was applied directly to the bath solution.
MTSEA and MTSET were purchased from Toronto Research Chemicals. Onion and garlic extracts were prepared by crushing one white onion or 10 g of fresh garlic into 300 ml and 30 ml of recording solution with a kitchen blender. Extracts were centrifuged for 20 min at 1,500g (5 °C). The supernatants were collected and further diluted in recording solution (1:1,000).
Site-directed mutagenesis
Constructs were generated by introducing mutations into the wild-type and rTRPV1–cysteine-less channel (18− TRPV1). We constructed 18− TRPV1 by introducing the following mutations into the rTRPV1 channel: C21S, C31L, C63L, C73S, C126A, C157A, C257A, C362L, C386S, C390S, C442L, C578L, C616A, C621A, C634A, C715F, C741S and C766N. Mutations were constructed by a PCR method as previously described34.
Pain-related behavioral assays
We obtained mice from strains C57BL/6J, Trpv1−/− (B6.129X1-Trpv1tm1Jul/J), Trpa1−/− and Trpa1+/– (B6;129PTrpa1tm1Kykw/J) from the Jackson Laboratory. Mating and genotyping by PCR reactions using gDNA from tail fragments were carried out as previously described13,35.
To perform the behavioral assays, we acclimatized animals for 30 min before experiments. A total volume of 10 µl of either saline solution (0.9% NaCl) or allicin (2 µg) was injected intraplantar using a 30G needle and paw-licking behavior was quantified for 30 min. Animals were handled according to institutional standards from the US National Institutes of Health.
Statistical analysis
Group data are presented as mean ± s.e.m. Statistical comparisons were made with a Student’s t-test. P < 0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank D. Julius for providing the TRPV1 cDNA and S. Simon and M. Rosenbaum for thoughtful discussion of this manuscript. We also thank L. Ongay, A. Aguilera Jiménez, J. Barbosa, F. Sierra, C. Rivera and H. Malagón for expert technical support and R. Argüello García for his most kind gift of allicin. This work was supported by grants from Dirección General de Asuntos del Personal Académico-Universidad Nacional Autónoma de México (DGAPA-UNAM) IN201705 and IN200308 to T.R. and IN202006–39 to L.D.I. and Consejo Nacional de Ciencia y Tecnología (CONACyT) No. 58038 to T.R., No. 48990/24777 to L.D.I., and No. 43128 to R.G.-V.; and by a grant from the National Eye Institute to S.E.G.
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
AUTHOR CONTRIBUTIONS
I.L. and T.R. carried out all site-directed mutagenesis experiments. H.S., I.L., A.J.O. and L.D.I. performed electrophysiological recordings. R.G.V. and T.R. carried out DRG neuron and behavioral experiments. L.D.I. and T.R. analyzed the data and wrote the manuscript. S.E.G. and M.M. discussed the results and commented on the manuscript.
References
- 1.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 2.Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- 3.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu. Rev. Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 4.Macpherson LJ, et al. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr. Biol. 2005;15:929–934. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 5.Block E. The chemistry of garlic and onions. Sci. Am. 1985;252:114–119. doi: 10.1038/scientificamerican0385-114. [DOI] [PubMed] [Google Scholar]
- 6.Block E. Recent results in the organosulfur and organoselenium chemistry of genus Allium and Brassica plants. Relevance for cancer prevention. Adv. Exp. Med. Biol. 1996;401:155–169. doi: 10.1007/978-1-4613-0399-2_13. [DOI] [PubMed] [Google Scholar]
- 7.Calvey EM, Roach JA, Block E. Supercritical fluid chromatography of garlic (Allium sativum) extracts with mass spectrometric identification of allicin. J. Chromatogr. Sci. 1994;32:93–96. doi: 10.1093/chromsci/32.3.93. [DOI] [PubMed] [Google Scholar]
- 8.Calvey EM, White KD, Matusik JE, Sha D, Block E. Allium chemistry: identification of organosulfur compounds in ramp (Allium tricoccum) homogenates. Phytochemistry. 1998;49:359–364. doi: 10.1016/s0031-9422(98)00191-5. [DOI] [PubMed] [Google Scholar]
- 9.Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc. Natl. Acad. Sci. USA. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macpherson LJ, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 11.Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron. 2007;54:905–918. doi: 10.1016/j.neuron.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 12.Jones MG, et al. Biosynthesis of the flavor precursors of onion and garlic. J. Exp. Bot. 2004;55:1903–1918. doi: 10.1093/jxb/erh138. [DOI] [PubMed] [Google Scholar]
- 13.Caterina MJ, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 14.Story GM, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 15.Jara A, Islas LD, Garcia-Villegas R, Rosenbaum T. On the mechanism of TBA block of the TRPV1 channel. Biophys J. 2007;92:3901–3914. doi: 10.1529/biophysj.106.102400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nilius B, et al. Gating of TRP channels: a voltage connection? J. Physiol. (Lond.) 2005;567:35–44. doi: 10.1113/jphysiol.2005.088377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordt SE, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 18.Vyklicky L, Lyfenko A, Susankova K, Teisinger J, Vlachova V. Reducing agent dithiothreitol facilitates activity of the capsaicin receptor VR-1. Neuroscience. 2002;111:435–441. doi: 10.1016/s0306-4522(02)00051-9. [DOI] [PubMed] [Google Scholar]
- 19.Susankova K, Tousova K, Vyklicky L, Teisinger J, Vlachova V. Reducing and oxidizing agents sensitize heat-activated vanilloid receptor (TRPV1) current. Mol. Pharmacol. 2006;70:383–394. doi: 10.1124/mol.106.023069. [DOI] [PubMed] [Google Scholar]
- 20.Cortright DN, Krause JE, Broom DC. TRP channels and pain. Biochim. Biophys. Acta. 2007;1772:978–988. doi: 10.1016/j.bbadis.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Venkatachalam K, Montell C. TRP channels. Annu. Rev. Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bautista DM, et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc. Natl. Acad. Sci. USA. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bautista DM, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 24.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat. Rev. Mol. Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 25.Matalon S, et al. Regulation of ion channel structure and function by reactive oxygen-nitrogen species. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;285:L1184–L1189. doi: 10.1152/ajplung.00281.2003. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida T, et al. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat. Chem. Biol. 2006;2:596–607. doi: 10.1038/nchembio821. [DOI] [PubMed] [Google Scholar]
- 27.Jin X, Touhey J, Gaudet R. Structure of the N-terminal ankyrin repeat domain of the TRPV2 ion channel. J. Biol. Chem. 2006;281:25006–25010. doi: 10.1074/jbc.C600153200. [DOI] [PubMed] [Google Scholar]
- 28.Corey DP, et al. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature. 2004;432:723–730. doi: 10.1038/nature03066. [DOI] [PubMed] [Google Scholar]
- 29.Howard J, Bechstedt S. Hypothesis: a helix of ankyrin repeats of the NOMPC-TRP ion channel is the gating spring of mechanoreceptors. Curr. Biol. 2004;14:R224–R226. doi: 10.1016/j.cub.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 30.Michaely P, Tomchick DR, Machius M, Anderson RG. Crystal structure of a 12 ANK repeat stack from human ankyrinR. EMBO J. 2002;21:6387–6396. doi: 10.1093/emboj/cdf651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macpherson LJ, et al. An ion channel essential for sensing chemical damage. J. Neurosci. 2007;27:11412–11415. doi: 10.1523/JNEUROSCI.3600-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3–kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J. Gen. Physiol. 2006;128:509–522. doi: 10.1085/jgp.200609576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sigworth FJ. The variance of sodium current fluctuations at the node of Ranvier. J. Physiol. (Lond.) 1980;307:97–129. doi: 10.1113/jphysiol.1980.sp013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenbaum T, Gordon SE. Dissecting intersubunit contacts in cyclic nucleotide-gated ion channels. Neuron. 2002;33:703–713. doi: 10.1016/s0896-6273(02)00599-8. [DOI] [PubMed] [Google Scholar]
- 35.Kwan KY, et al. TRPA1 contributes to cold, mechanical, and chemical nociception, but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.