Abstract
Cinnamomum osmophloeum Kanehira belongs to the Lauraceae family of Taiwan's endemic plants. In this study, C. osmophloeum Kanehira extract has shown inhibition of tyrosinase activity on B16-F10 cellular system first. Whether extracts inhibited mushroom tyrosinase activity was tested, and a considerable inhibition of mushroom tyrosinase activity by in vitro assays was presented. Animal experiments of C. osmophloeum Kanehira were carried out by observing animal wound repair, and the extracts had greater wound healing power than the vehicle control group (petroleum jelly with 8% DMSO, w/v). In addition, the antioxidant capacity of C. osmophloeum Kanehira extracts in vitro was evaluated. We measured C. osmophloeum Kanehira extract's free radical scavenging capability, metal chelating, and reduction power, such as biochemical activity analysis. The results showed that a high concentration of C. osmophloeum Kanehira extract had a significant scavenging capability of free radical, a minor effect of chelating ability, and moderate reducing power. Further exploration of the possible physiological mechanisms and the ingredient components of skincare product for skin-whitening, wound repair, or antioxidative agents are to be done.
1. Introduction
Skin, made up of three layer cells including, epidermis, dermis, and hypodermis, is the largest vertebrates organ in the human body. Human skin is commonly exposed to oxidative stresses from solar ultraviolet (UV) radiation and free radicals as well as its induced cellular reactive oxygen species (ROS) [1, 2], which are the common reasons for tumor genesis or skin aging. To protect skin from UV radiation, skin operates complex defense system including skin thickening, pigment synthesis, and a network of nonenzymatic and enzymatic antioxidative mechanisms [2]. In addition to a significant responsibility, in the prevention of human skin UV-caused damage, which increases melanocytes transfer of melanosomes to keratinocytes, melanin determines skin color [3]. Hyperpigmentation is commonly cared with therapeutic drugs or cosmetics of pigment-reducing or skin-whiten abilities. During the melanin synthesis processes, tyrosinase is classified to be the rate-limiting oxidase at first two steps [4]. It catalyzes the pigments production such as eumelanin and phenomelanin. Two types of pigments production were reported, including the L-tyrosine hydroxylation to 3,4-dihydroxy-L-phenylalanine (L-DOPA) and then the L-DOPA oxidation to dopaquinone (a biochemical precursor to pigments) [5]. In the active site for tyrosinase, two copper ions are essential to catalyze colorful pigments or melanin by oxidative stress. To antagonize tyrosinase activity can reduce the syndrome of hyperpigmentation and dermatological disorders.
Skin as the first immune defense line of human plays a noteworthy role in avoiding various biological, chemical, mechanical, and physical damages [1, 2]. Chronic or acute severe injuries on the skin, such as abrasions, burns, leg ulcers, or lesions, in consequence considerable losses of dermal tissues pose huge challenges to the therapeutic processes. Keratinocytes in epidermis and fibroblasts in dermis are the first stop for body protection against external stimulus or for the skin wound healing [6]. In terms of wound healing, wound closure is known to be initiated by fibroblast migration from its margins. Based on the migratory force, resistance from the regenerated tissue may lead to fibroblast differentiation [7], which is featured by the local expression profiles of skin cells, such as several growth factors and the extracellular matrix. Skin wound healing is a cutting edge study for many medicine fields [8].
Smoking factors, salted food, or environmental toxicants bring about various oxidative stresses to human being [9]. The level of excessive free radicals produces a high oxidative stress which is a negative effect against the normal skin and results in aging or some diseases. Through biochemical processes, the intracellular physiological oxidants are engendered from nonenzymatic systems such as those involving enzymatic catalysis, transition metals, various oxidases that transformed them into the reactive nitrogen species, or reactive oxygen species [1]. If antioxidants are invigorated, they can significantly prevent or reduce the oxidative pressure damages [10]. There are several important components constructed to cellular membrane lipids from the phospholipids, membrane proteins, polyunsaturated fatty acids, cholesterol, and nucleic acids [11]. Excessive free radicals and ROS cause oxidative pressure injury on lipids, proteins, and DNA, and the damage eventually induced cellular damage, aging, neural disorders, diabetes, atherosclerosis, inflammatory, cancer, and cardiovascular disease, especially unwanted pigment accumulation [12].
Cinnamomum osmophloeum Kanehira is commonly recognized as indigenous cinnamon or pseudocinnamomum. The natural plant is native to broad-leaved forests of Taiwan's endemic plants (Figure 1) and lots of exercises as a Chinese herbal medicine, including tannin, resin, mucilage, sugar, and essential oil, among which essential oils had excellent inhibitions on bacterial pharmacological characteristics [13]. This plant contains many nutrients such as manganese, dietary fiber, iron, and calcium, commonly used as a spice and flavoring agent for many foods [14]. Various biofunctional applications have been found that C. osmophloeum Kanehira has high anti-inflammatory and antioxidative properties which plays a key role in tissue repair of traditional medicine [15]. Moreover, C. osmophloeum Kanehira could be used as a xanthine oxidase inhibitor for management of hyperuricemia and related medicinal situations including gout, hence a potential drug [16].
Figure 1.

The photos of Cinnamomum osmophloeum Kanehira. (a) Leaves. (b) A whole plant.
2. Materials and Methods
2.1. Reagents and Materials
All the reagents were purchased from Sigma Chemical (St. Louis, MO), including dimethyl sulfoxide (DMSO), 1,1-diphenyl-2-picrylhydrazyl (DPPH), 3-(4,5-dimetylthiazol-2-yl)-2,5-diphenyl, tetrazolium bromide (MTT), 3-tert-butyl-4-hydroxyanisole (BHA), ethylenediaminetetraacetic acid (EDTA), FeCl3, FeCl2·4H2O, kojic acid, L-tyrosine, mushroom tyrosinase, potassium ferricyanide [K3Fe(CN)6], trichloroacetic acid and vitamin C, and other highest purity chemical buffers and reagents. Cell culture reagents were purchased from GIBCO BRL (Gaithersburg, MD), including fetal bovine serum (FBS) and Dulbecco's modified Eagle medium (DMEM).
2.2. Extraction of C. osmophloeum Kanehira Leaves
The plant specimen was authenticated by Ladies Biotech Co., LTD, where voucher specimens were kept. Dry leaves of C. osmophloeum Kanehira (0.6 kg) were sliced and soaked in 3 L ethyl alcohol for one day before further three ethyl alcohol extractions. After filtration, the extracts were evaporated to final weight of 8.49 g.
2.3. B16-F10 Melanoma Cell Cultures
The melanoma B16-F10 cells (BCRC 60031 in ATCC) were maintained at 37°C under 5% CO2 atmosphere by feeding the medium (10 mM HEPES, 13.4 mg/mL DMEM, 100 μg/mL streptomycin sulfate, 143 U/mL benzylpenicillin potassium, and 24 mM NaHCO3, pH 7.1) with 10% FBS [17].
2.4. B16-F10 Cell Viability
MTT assay was used to evaluate the effects of cell viabilities for the treatments of C. osmophloeum Kanehira extracts [17]. Briefly, cells (6 × 103 cells/well) were plated in 96-well plates for overnight. Cells were treated with either vehicle (DMSO) or indicated concentrations of eachsample for 24 h. Subsequently, 0.5 mg/mL MTT in 100 μL of fresh medium was used to replace the medium and the reaction was performed in a 37°C cell culture incubator for 2 h. The generating crystals were dissolved in 100 μL of DMSO with smooth shaking for 10 min in darkness. Finally, the absorbance (A) value of this reaction was detected at 595 nm by multiplate reader (UV-vis, BioTek, Winooski, VT). Cell viability (%) was formulated as follows:
| (1) |
2.5. B16-F10 Cellular Tyrosinase Activity
The tyrosinase activity was dependent on the dopachrome formation rate as previously described [17]. Melanoma B16-F10 cells (105 cells/well) were seeded in a 12-well plate with 1,000 μL of medium, and they were treated with indicated concentrations of extracts for 48 h. After PBS washing, B16-F10 cells lysed with 1% triton X-100/PBS and 50 μL of 2 mM L-tyrosine were added. Standing for 3 h at 37°C in darkness, its absorbance at 490 nm was examined spectrophotometrically, where the tyrosinase activity evaluation formula was similar to 2.
2.6. B16-F10 Cellular Melanin Contents
The cellular melanin contents were measured with minor modifications as previously described [17]. Briefly, B16-F10 melanoma cells (2.5 × 105 cells/well/1500 μL of medium) plated in 6-well plates were treated with extracts and incubated for 48 h. After dissolving in 10% DMSO with 50 μL of 2.0 N NaOH for 1 h at 90°C, cell lysates were centrifuged at 10,000 ×g for 10 min to collect the supernatants for melanin determination using spectrophotometer at 475 nm with similar formula to 2.
2.7. Mushroom Tyrosinase Activity
The mushroom tyrosinase activity was measured with minor modifications as previously described [18, 19]. The various concentrations of extracts were added: 2 μL with 68 μL of 50 mM phosphate buffer (pH 6.8), 10 μL of 0.5 units/mL of mushroom tyrosinase, and 10 μL of the mixture. The absorbance of the mushroom tyrosinase inhibition assay at 490 nm was determined at 5 min per interval until 30 minutes with a 96-well plate spectrophotometer, where kojic acid was regarded as a positive control. Mushroom tyrosinase activity (%) was formulated as follows:
| (2) |
where A is the OD value under no sample; B is the OD value under no sample and tyrosinase; C is the OD value under sample; and D is the OD value under sample but no tyrosinase.
2.8. Animal Experiments
Six-week-old male Wistar rats are used. These rats are kept on standard rat chow and water ad libitum for 1 week before challenging. The animal studies were performed under authorization from the Animal Use Committee of Kaohsiung Medical University. The experimental rats were housed on a 12/12-hour light-dark cycle with the air conditioner and adequate supply of food and water. Twelve rats were grouped into two sets, that is, petroleum jelly and C. osmophloeum Kanehira extracts (experimental groups). The wound healing of skin was measured with minor modifications as previously described [20]. After rats were anesthetized, its dorsal hair was shaved and the wounds of 1 cm in diameter were generated. After a back skin excising, the wounds of all experimental rats were quickly covered with the petroleum jelly (8% DMSO, w/v) or 0.8 mg extracts, where the petroleum jelly was used as a reference.
2.9. Measurement of the Wound Area
Digital camera (Coolpix P6000, Nikon, Japan) was used to record the progression of skin wound after 0, 1, 3, and 5 days with protocol parameters (aperture: F/7.2, shutter speed: 1/60). The wound dressing was not removed under healing period unless the substance was easy to detach manually. The area of each skin wound was determined by SPOT software (Diagnostic Instruments, Inc., Sterling Heights, MI, USA). Some random Sani-Chips were visualized on wound sites; however, wound size measurements were not interfered. The wound healing index was formulated as follows:
| (3) |
2.10. Determination Antioxidation Ability by DPPH∙ Radical Scavenging
The principle of antioxidation determination is based on the color change of DPPH to light yellow if free radicals are scavenged [21]. The more the light color rendered, the higher the antioxidant capacity from the component. Suitable concentration doses of C. osmophloeum Kanehira extracts were added to 1 μL and with 99 μL of DPPH solution. When DPPH reacted with antioxidants or vitamin C (positive control), it changed to reduced form and led to a lower absorbance at 517 nm. The scavenging activity (%) of DPPH radical was formulated as follows:
| (4) |
2.11. Metal Chelating Activity
Metal chelating activity was measured with slight modifications as previously described [5]. Briefly, C. osmophloeum Kanehira extracts dissolved in DMSO were mixed with a reagent containing 10 μL of 2 mM FeCl2·4H2O. To initiate the reaction with the addition of 20 μL of 5 mM ferrozine, the solution was vigorously shaken and then it was stood for 10 min at room temperature. EDTA was regarded to be a positive control. Its absorbance at 562 nm was calculated as the chelating activity (%) and its method was alike to 4.
2.12. Reducing Power
The reducing power of C. osmophloeum Kanehira extracts was determined according to the previous method [21]. The extracts were incubated with 85 μL of 67 mM phosphate buffer (pH 6.8) and 2.5 μL of 20% K3Fe(CN)6 at 50°C for 20 min. After the addition of 160 μL of trichloroacetic acid (10%), it was centrifuged at 3,000 ×g for 10 min to collect the supernatant (75 μL) for reacting with 2% FeCl3 (25 μL). BHA was regarded to be a positive control. Finally, its absorbance at 700 nm was measured by spectrophotometer.
2.13. Statistics
Data are indicated as a mean and standard deviation in triplicate at least. Its difference significance was evaluated by Student's t-test.
3. Results and Discussion
3.1. B16-F10 Cytotoxicity of C. osmophloeum Kanehira Extracts
Melanoma B16-F10 cells were cultured in the indicated doses of tested extracts (10, 25, 50, 100, and 200 μg/mL). In Figure 2, the cell viability was determined by MTT assay. The proliferation of B16-F10 cell was inhibited by extracts in a dose-responsive manner ranging from 10 to 200 μg/mL. When the mouse melanoma cells were incubated in a higher assay surrounding 100 μg/mL, the viabilities of extract-treated B16-F10 cells were more than 50% at 48 h treatment, suggesting that extracts had discernable cytotoxic effect on mouse melanoma cells.
Figure 2.
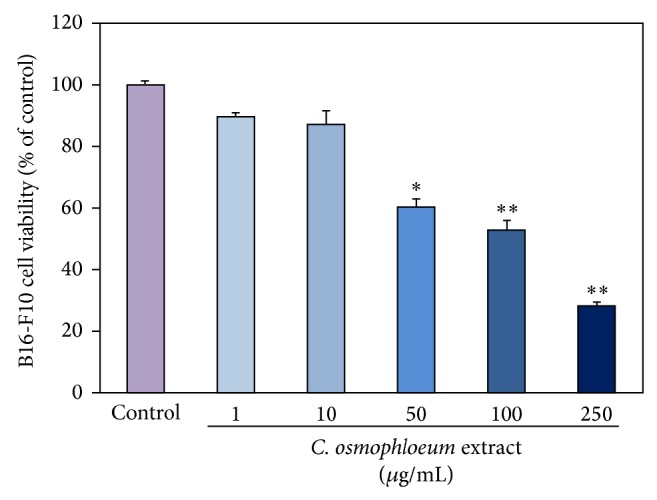
The cytotoxicity of C. osmophloeum Kanehira extracts on marine melanoma B16-F10 cells. Data: the mean value ± SD (triplicate values for three independent experiments); ∗ < 0.01, ∗∗ < 0.001.
3.2. C. osmophloeum Kanehira Extracts on B16-F10 Cellular Tyrosinase Activity and Melanin Content
We further investigated the in situ cellular tyrosinase and melanin suppressions of extracts. The melanin generation mechanisms contain the L-tyrosine hydroxylation and the L-DOPA oxidation to its corresponding dopaquinone to form pigment by additional multiple biosynthesis steps through the enzymatic tyrosinase. We verified that the extracts had the tyrosinase-inhibiting ability and melanin content effectiveness in mouse melanoma cell, B16-F10. In Figure 3(a), the extracts had revealed superior obvious suppressions even at a moderate quantity concentration to both tyrosinase activity and melanin content. Additionally, Figure 3(b) shows that melanin contents and tyrosinase activities were highly correlated under the same dose-responsive manner upon C. osmophloeum Kanehira treatments. Both tyrosinase activity and melanin content decreased in a similar dose-dependent tendency, when we increased dosages of extracts, indicating that the inhibition of cellular tyrosinase activity might induce the epidermal melanin reduction. But interestingly, extracts at the concentration 100 μg/mL, the cell viability remained 52% (Figure 2), and the tyrosinase activity was 18% lower than cell viability (Figure 3(a)). Melanin content did not show evident reduction. On the contrary, with highest concentration of C. osmophloeum Kanehira extracts at 50 μg/mL, the melanin content was decreased for low cell viability. As the tendency of tyrosinase activity was lower than cell viability, the melanin content was a bit higher than cell viability.
Figure 3.
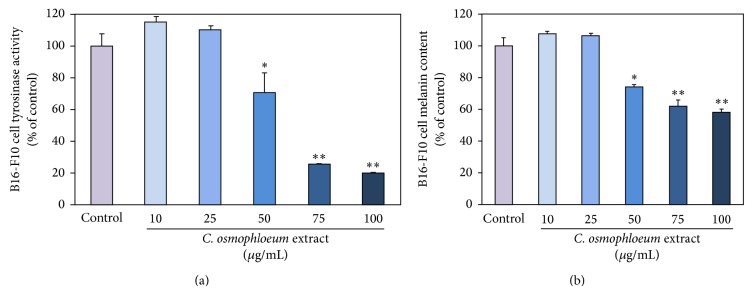
The inhibitory effects of various concentrations of C. osmophloeum extracts on B16-F10 cells. (a) The tyrosinase activity and (b) the melanin content of B16-F10 cells were incubated with indicated concentrations of C. osmophloeum extracts. Data: the mean value ± SD (triplicate values for three independent experiments); ∗ < 0.01, ∗∗ < 0.001.
3.3. Measurement of C. osmophloeum Kanehira Extracts on Mushroom Tyrosinase Activity
We previously reported that UV exposure may induce the oxidative stress which is prone to be skin darkening and ROS generation for tumor progression [22, 23]. For the prevention of skin darkening and the hyperpigmentation, we evaluated the inhibitory effects of C. osmophloeum Kanehira extracts using in vitro mushroom tyrosinase inhibitory assay. The inhibition effectiveness of C. osmophloeum Kanehira extracts demonstrates moderate suppression to the activity of mushroom tyrosinase at 200 μM (Figure 4). Accordingly, these compounds have the potential use for supplements in industry of cosmetics and pharmaceuticals.
Figure 4.
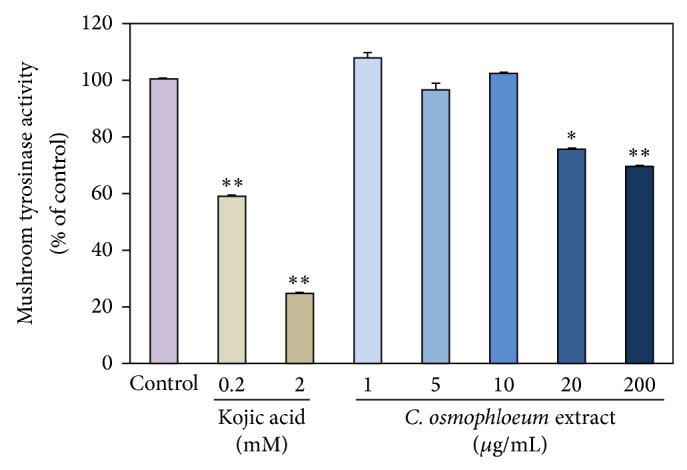
The inhibitory effects of various concentrations of C. osmophloeum extracts and kojic acid on mushroom tyrosinase for 5 minutes. Data: the mean value ± SD (triplicate values in three independent experiments); ∗ < 0.01, ∗∗ < 0.001.
3.4. Evaluation of the In Vivo Wound Size Assay
In order for C. osmophloeum Kanehira extracts to be utilized as a dermal agent, they should exhibit minimal toxicity towards normal tissues. The wound-healing performance of the C. osmophloeum Kanehira extracts was measured by an animal model in terms of the full thickness wound assay and monitored by image analysis of excision wound area. We discovered that the mice displayed no obvious evidences of gross toxicity during the course of the treatment period. The body weight of the mice was also monitored at an interval of every other day over the course of this study. The results showed that the body weight of mice in the treatment and the control groups was not significantly different over the duration of the experiment (data not shown). Additionally, the mean values of the heart, liver, and kidney weights after sacrifice between these two groups of mice were not significantly changed. The wound areas of both petroleum jelly (8% DMSO, w/v) and 0.8 mg extract treatment groups were decreased in a time-dependent manner (Figure 5). Wound areas of extract-treatment groups at days 0, 1, 3, and 5 were 100 ± 0.1%, 86.7 ± 5.8%, 63.0 ± 6.1%, and 42.7 ± 6.4%, respectively, which were smaller than those of petroleum jelly group (100 ± 0.4%, 96.7 ± 5.8%, 83.3 ± 11.5%, and 73.0 ± 11.3%) (Figure 5(a)). In the beginning of the wound creation, the experimental group displayed smaller area than that of petroleum jelly group which showed a constant repair trend after 3 days. The C. osmophloeum Kanehira-treated wound healing displayed over 30% and 50% wound area closure after 3 and 5 days, respectively (Figure 5(b)). Therefore, the repairing ability and the wound shrinking ratio of the extract experimental group were higher and more effective.
Figure 5.
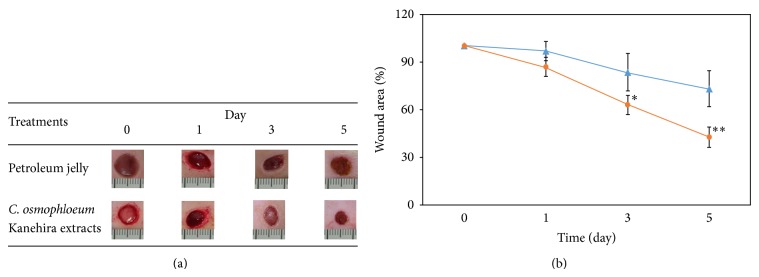
The wound healing of C. osmophloeum extracts on animal model. (a) Wound healing after 0, 1, 3, and 5 days after injury. After the full thickness excisions of 1 cm in diameter were made, the petroleum jelly (8% DMSO, w/v) or 0.8 mg C. osmophloeum extracts covered on the hurt. For injury group, wounds were not covered for control. At the first day, the wound healing area from petroleum jelly (8% DMSO) group was smaller than that of C. osmophloeum group until 5 days. (b) The differences between wounds of petroleum jelly (8% DMSO, w/v, blue solid triangle) and 0.8 mg C. osmophloeum group (orange solid circle) were statistically significant at day 5. Data: the mean value ± SD (triplicate values in two independent experiments); ∗ < 0.01, ∗∗ < 0.001.
3.5. Antioxidative Properties of C. osmophloeum Kanehira Extracts
Accumulating evidence shows that free radical increases can lead to skin melanin overexpression and speed up the variety of oxidations of lipids in manufactured food and cosmetics [1]. Natural antioxidants, particularly free radical eliminating abilities, are essential to skin caring. In Table 1, the inhibitory data for extracts to DPPH from 0 to 250 μg/mL were found. Based on this information, extracts showed a dose-dependent manner in our testing conditions and might function as chain-breaking agents or radical ion neutralizers inhibiting the generation of excess free radicals in reference to control of 100 μM vitamin C.
Table 1.
Measurement of C. osmophloeum extracts on antioxidant experiments. The data were expressed as a mean value in three independent experiments.
|
C. osmophloeum extract (μg/mL) |
DPPH scavenging (%) | Metal chelating ability (%) | Reducing power (OD 700) |
|---|---|---|---|
| 1 | ≤10.0 | ≤10.0 | 0.09 ± 0.00 |
| 10 | ≤10.0 | ≤10.0 | 0.10 ± 0.00 |
| 50 | ≤10.01 | ≤10.0 | 0.18 ± 0.02 |
| 100 | 13.23 ± 0.01 | ≤10.0 | 0.27 ± 0.03 |
| 250 | 38.97 ± 0.02 | ≤10.0 | 0.48 ± 0.02 |
|
| |||
| Vitamin Ca | 80.82 ± 0.00 | — | — |
| EDTAb | — | 80.76 ± 0.01 | — |
| BHAc | — | — | 0.56 ± 0.03 |
“—” is no testing.
aVitamin C was used as a positive control on DPPH assay at 100 μM.
bEDTA was used as a positive control on metal chelating ability at 100 μM.
cBHA was used as a positive control on reducing power at 100 μM.
Several literatures reported that reducing power and metal ions-chelating ability from a reagent are commonly displayed through antioxidative effect [18, 19]. In Table 1, the ferrous ion chelating data of C. osmophloeum Kanehira extracts were found. When the complex formation of ferrozine and Fe(II) is interfered, it was detectable spectrophotometrically. Therefore, C. osmophloeum Kanehira extracts were demonstrated to have few Fe(II) chelating belongings.
The measurement of reducing power is one of the easy and effective methods by reacting with a Fe(III) ferricyanide complex [18, 19]. The solution color changes from light yellow to dark green and blue depended on the level of antioxidants within samples. As shown in Table 1, C. osmophloeum Kanehira extracts reducing powers were compared to similar antioxidative power with 3-tert-butyl-4-hydroxyanisole (BHA) at 100 μM.
4. Conclusion
Various biochemical characterization properties of C. osmophloeum Kanehira extracts were explored within this study. In the cellular experiments, extracts had tyrosinase inhibition effect and melanin content diminution but were cytotoxic. The extracts might have lower cell viabilities to contribute to the results in both inhibited tyrosinase activity and reduced melanin content. In the animal model, we found that the extracts could repair the wound. The antioxidative assays suggested that high concentration of extracts had moderate antioxidative abilities in terms of DPPH scavenging capacity and reducing power ability except metal chelating. In the future, we will analyze purification from C. osmophloeum Kanehira extracts to find an effective antioxidant and tyrosinase inhibitor.
Acknowledgments
This study was supported by grants from the National Science Council, Taiwan (NSC 99-2221-E-037-006-MY3 and NSC 102-2221-E-037-005). The authors also thank Ladies Biotech Co., LTD, for providing the testing sample plant materials, C. osmophloeum Kanehira extracts. They also thank the support from Center for Stem Cell Research, Kaohsiung Medical University, Kaohsiung, Taiwan (KMU-TP103G00 and KMU-TP103G02-05).
Conflict of Interests
The authors had no conflict of interests.
References
- 1.Malireddy S., Kotha S. R., Secor J. D., et al. Phytochemical antioxidants modulate mammalian cellular epigenome: implications in health and disease. Antioxidants & Redox Signaling. 2012;17(2):327–339. doi: 10.1089/ars.2012.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silveira J. P. S., Seito L. N., Eberlin S., et al. Photoprotective and antioxidant effects of Rhubarb: inhibitory action on tyrosinase and tyrosine kinase activities and TNF-α, IL-1α and α-MSH production in human melanocytes. BMC Complementary and Alternative Medicine. 2013;13, article 49 doi: 10.1186/1472-6882-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato K., Toriyama M. The inhibitory effect of non-steroidal anti-inflammatory drugs (NSAIDs) on the monophenolase and diphenolase activities of mushroom tyrosinase. International Journal of Molecular Sciences. 2011;12(6):3998–4008. doi: 10.3390/ijms12063998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ko H.-H., Tsai Y.-T., Yen M.-H., et al. Norartocarpetin from a folk medicine Artocarpus communis plays a melanogenesis inhibitor without cytotoxicity in B16F10 cell and skin irritation in mice. BMC Complementary and Alternative Medicine. 2013;13, article 348 doi: 10.1186/1472-6882-13-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee C.-C., Chiu C.-C., Liao W.-T., et al. Alpinia oxyphylla Miq. bioactive extracts from supercritical fluid carbon dioxide extraction. Biochemical Engineering Journal. 2013;78:101–107. doi: 10.1016/j.bej.2013.03.009. [DOI] [Google Scholar]
- 6.Wang H.-M., Chou Y.-T., Wen Z.-H., Wang Z.-R., Chen C.-H., Ho M.-L. Novel biodegradable porous scaffold applied to skin regeneration. PLoS ONE. 2013;8(6) doi: 10.1371/journal.pone.0056330.e56330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H.-M., Chou Y.-T., Wu C.-S., Yeh J.-T. Polyester/cellulose acetate composites: preparation, characterization and biocompatible. Journal of Applied Polymer Science. 2012;126(2):E242–E251. doi: 10.1002/app.36965. [DOI] [Google Scholar]
- 8.Wang H.-M. D., Chen C.-Y., Wu P.-F. Isophilippinolide a arrests cell cycle progression and induces apoptosis for anticancer inhibitory agents in human melanoma cells. Journal of Agricultural and Food Chemistry. 2014;62(5):1057–1065. doi: 10.1021/jf403730z. [DOI] [PubMed] [Google Scholar]
- 9.Kulkarni A. C., Kuppusamy P., Parinandi N. Oxygen, the lead actor in the pathophysiologic drama: enactment of the trinity of normoxia, hypoxia, and hyperoxia in disease and therapy. Antioxidants & Redox Signaling. 2007;9(10):1717–1730. doi: 10.1089/ars.2007.1724. [DOI] [PubMed] [Google Scholar]
- 10.Temneanu O. R., Zamfir C., Eloaie Zugun F., Cojocaru E., Tocan L. Oxidants and antioxidants relevance in rats' pulmonary induced oxidative stress. Journal of Medicine and Life. 2011;4(3):244–249. [PMC free article] [PubMed] [Google Scholar]
- 11.Cheeseman K. H. Mechanisms and effects of lipid peroxidation. Molecular Aspects of Medicine. 1993;14(3):191–197. doi: 10.1016/0098-2997(93)90005-X. [DOI] [PubMed] [Google Scholar]
- 12.Gul M. Z., Bhakshu L. M., Ahmad F., Kondapi A. K., Qureshi I. A., Ghazi I. A. Evaluation of Abelmoschus moschatus extracts for antioxidant, free radical scavenging, antimicrobial and antiproliferative activities using in vitro assays. BMC Complementary and Alternative Medicine. 2011;11, article 64 doi: 10.1186/1472-6882-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nuryastuti T., van der Mei H. C., Busscher H. J., Iravati S., Aman A. T., Krom B. P. Effect of cinnamon oil on icaA expression and biofilm formation by Staphylococcus epidermidis . Applied and Environmental Microbiology. 2009;75(21):6850–6855. doi: 10.1128/AEM.00875-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mashhadi NS G. R., Askari G., Feizi A., et al. Influence of ginger and cinnamon intake on inflammation and muscle soreness endued by exercise in Iranian female athletes. International Journal of Preventive Medicine. 2013;4:S11–S15. [PMC free article] [PubMed] [Google Scholar]
- 15.Molania T., Moghadamnia A. A., Pouramir M., et al. The effect of Cinnamaldehyde on mucositis and salivary antioxidant capacity in gamma-irradiated rats (a preliminary study) DARU Journal of Pharmaceutical Sciences. 2012;20(1, article 89) doi: 10.1186/2008-2231-20-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S. Y., Yang C. W., Liao J. W., Zhen W. W., Chu F. H., Chang S. T. Essential oil from leaves of Cinnamomum osmophloeum acts as a xanthine oxidase inhibitor and reduces the serum uric acid levels in oxonate-induced mice. Phytomedicine. 2008;15(11):940–945. doi: 10.1016/j.phymed.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Halaban R., Patton R. S., Cheng E., et al. Abnormal acidification of melanoma cells induces tyrosinase retention in the early secretory pathway. The Journal of Biological Chemistry. 2002;277(17):14821–14828. doi: 10.1074/jbc.M111497200. [DOI] [PubMed] [Google Scholar]
- 18.Wang H.-M., Chen C.-Y., Wen Z.-H. Identifying melanogenesis inhibitors from Cinnamomum subavenium with in vitro and in vivo screening systems by targeting the human tyrosinase. Experimental Dermatology. 2011;20(3):242–248. doi: 10.1111/j.1600-0625.2010.01161.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang H.-M., Chou Y.-T., Hong Z.-L., et al. Bioconstituents from stems of Synsepalum dulcificum Daniell (Sapotaceae) inhibit human melanoma proliferation, reduce mushroom tyrosinase activity and have antioxidant properties. Journal of the Taiwan Institute of Chemical Engineers. 2011;42(2):204–211. doi: 10.1016/j.jtice.2010.05.008. [DOI] [Google Scholar]
- 20.Huang M.-H., Yang M.-C. Evaluation of glucan/poly(vinyl alcohol) blend wound dressing using rat models. International Journal of Pharmaceutics. 2008;346(1-2):38–46. doi: 10.1016/j.ijpharm.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 21.Wang H.-M., Chen C.-Y., Ho M.-L., et al. (-)-N-formylanonaine from Michelia alba as a human tyrosinase inhibitor and antioxidant. Bioorganic & Medicinal Chemistry. 2010;18(14):5241–5247. doi: 10.1016/j.bmc.2010.05.045. [DOI] [PubMed] [Google Scholar]
- 22.Wicks N. L., Chan J. W., Najera J. A., Ciriello J. M., Oancea E. UVA phototransduction drives early melanin synthesis in human melanocytes. Current Biology. 2011;21(22):1906–1911. doi: 10.1016/j.cub.2011.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eller M. S., Ostrom K., Gilchrest B. A. DNA damage enhances melanogenesis. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(3):1087–1092. doi: 10.1073/pnas.93.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]


