Abstract
Implantation of the embryo into the uterus triggers the initiation of hemochorial placentation. The hemochorial placenta facilitates the acquisition of maternal resources required for embryo/fetal growth. Uterine spiral arteries form the nutrient supply line for the placenta and fetus. This vascular conduit undergoes gestation stage-specific remodeling directed by maternal natural killer cells and embryo-derived invasive trophoblast lineages. The placentation site, including remodeling of the uterine spiral arteries, is shaped by environmental challenges. In this review, we discuss the cellular participants controlling pregnancy-dependent uterine spiral artery remodeling and mechanisms responsible for their development and function.
Keywords: hemochorial placentation, natural killer cells, trophoblast, PI3K/AKT, FOSL1, hypoxia, uterine spiral artery, NOTCH, STOX1
Introduction
The embryo implants into the uterus to gain access to maternal nutrients. Delivery of nutrients is facilitated by the placenta, which develops in association with the embryo/fetus. Trophoblast cells are the functional units of the placenta and key contributors to establishing the maternal-fetal interface. Hemochorial is the categorization of placentation displaying the closest connections between maternal and fetal tissues (Amoroso 1959; Mossman 1987; Wooding and Burton 2008). Maternal blood directly bathes trophoblast, which requires restructuring of the uterine spiral arterial tree. Distal segments of the uterine spiral arterial tree are targeted and structurally modified to create conduits with altered vasoregulation properties, maximizing the flow of maternal resources to the placenta (Osol and Mandala 2009; Leonard et al. 2013; Osol and Moore 2014). This represents an orchestrated process involving the activities of specialized cell types derived from trophectoderm (outer layer of the blastocyst-stage embryo) and the modulatory influences of cells situated within the uterine stromal compartment (Pijnenborg et al. 2006; Wallace et al. 2012). The extent of pregnancy-dependent uterine spiral artery remodeling differs among species (Amoroso 1959; Mossman 1987; Wooding and Burton 2008) and aberrations in uterine vascular modifications are associated with pregnancy-related diseases (Pijnenborg et al. 2006; Wallace et al. 2012).
In this review, we discuss the cellular participants controlling pregnancy-dependent uterine spiral artery remodeling and mechanisms responsible for their development and function.
Uterine spiral artery remodeling
Uterine spiral arteries are the conduits for delivering maternal nutrients to the fetus. These blood vessels undergo restructuring during the establishment of pregnancy (Pijnenborg et al. 2006; Osol and Mandala 2009; Harris 2010). The maternal uterine spiral arteries undergoes fundamental changes of their cellular (endothelial and smooth muscle cell) and extracellular constituents, including hyperplasia, hypertrophy, apoptosis, dedifferentiation, migration, and extracellular matrix remodeling (Osol and Mandala 2009; Harris 2011). We focus on two key architects of uterine spiral artery remodeling are maternal natural killer (NK) cells and invasive trophoblast cells of extraembryonic origin (Smith et al. 2009; Harris 2011; Wallace et al. 2012). Maternal uterine macrophages also contribute to the remodeling process (Smith et al. 2009). There is a temporal dependence to their contributions during pregnancy with NK cells arriving and acting first followed by invasive trophoblast cells. Each cell type exhibits similarities and differences in their tasks. Both NK cells and trophoblast cells target the extracellular matrix and smooth muscle cells surrounding the spiral arteries leading to an alteration of vasoregulation and the facilitation of nutrient delivery (Wallace et al. 2012). Trophoblast cells go even further and supplant the endothelium of distal arterial segments. Subsequently, they assume a phenotype resembling the endothelium termed pseudo-vascularization (Damsky and Fisher 1998; Rai and Cross 2014). The actions and effectiveness of these specialized cell populations are guided through signals impinging on the intrauterine environment. They react and adapt to appropriately direct formation of the maternal-fetal interface, balancing the needs of the mother and fetus. Aberrations in NK cell and/or invasive trophoblast cell performance lead to disruptions in nutrient delivery to the placenta and subsequently to the embryo.
Models for studying mechanisms controlling uterine spiral artery remodeling
Control systems regulating any aspect of the vascular network can be complex and difficult to analyze. Uterine spiral artery remodeling is no exception. Human placentation exhibits extensive uterine spiral artery remodeling guided through the actions of NK cells and invasive trophoblast. Effective dissection of mechanisms controlling uterine spiral artery remodeling requires a combined in vivo and in vitro effort, which presents limitations for investigations of the in vivo pregnant human intrauterine environment. There is an assortment of mammalian species adapted to laboratory research that possess hemochorial placentation and could potentially be used to model uterine spiral artery remodeling; however, all are not suitable. Although, hemochorial placentation exhibits elements of conservation across mammalian species, it also shows striking differences, including a dearth of trophoblast involvement in uterine spiral artery remodeling in some species, notably the mouse (Adamson et al. 2002; Ain et al. 2003). In contrast, placentation in other common laboratory species, including the rat, hamster, and guinea pig possess deep trophoblast invasion not unlike that seen in human placentation (Pijnenborg and Vercruysse 2010). The rat is an effective model for examining regulatory roles of both NK cells and invasive trophoblast in uterine spiral artery remodeling (Soares et al. 2012; Fig. 1). The rat is especially attractive because excellent in vitro and in vivo approaches have been established for dissecting regulatory pathways controlling placentation. Trophoblast stem (TS) cells can be isolated from early embryos and readily propagated and manipulated for experimentation on invasive trophoblast lineage differentiation (Asanoma et al. 2011; Chakraborty et al. 2011; Kent et al. 2011; Konno et al. 2011). The size of the rat presents advantages for surgical preparations and repeated tissue sampling, and the rat can be genetically modified using newly developed genome editing strategies (Jacob et al. 2010; Rumi et al. 2014). These approaches coupled to complementary experimentation with ex vivo human tissues obtained from normal and diseased placentation sites (Aplin 2006; Hunkapiller and Fisher 2008; Hazan et al. 2010; Robson et al. 2012), a collection of immortalized human extravillous trophoblast cell lines (HTR-8/SVneo, SGHPL-4/5, Swan 71; Graham et al. 1993; Whitley 2006; Straszewski-Chavez et al. 2009), and BMP4-treated human embryonic or induced pluripotent stem cells (Xu et al. 2002; Ezashi et al. 2011; Amita et al. 2013; Li et al. 2013) provide a robust toolset for dissecting conserved mechanisms regulating the maternal-fetal interface.
Fig. 1. Hemochorial placentation.
Schematic diagram showing homologous structures within human and rat hemochorial placentation sites. In the human, trophoblast cells destined for the maternal compartment are referred to as extravillous trophoblast cells, whereas in the rat these cells are referred to as invasive trophoblast cells. Invasive trophoblast cells are specialized into interstitial and endovascular subtypes. Collectively, invasive trophoblast cells and natural killer cells direct uterine spiral artery restructuring. Adapted from Soares et al. 2012.
NK cells
NK cells are constituents of the innate immune system possessing unique trafficking characteristics during pregnancy. Unlike other immune cell lineages, NK cells accumulate at the implantation site. These NK cells possess a unique phenotype and are distinct from circulating NK cells (Koopman et al. 2003; Cerdeira et al. 2013). They are embedded in the decidua and establish conspicuous relationships with uterine spiral arteries and invasive trophoblast cells, a process conserved among rodents and primates (Chakraborty et al. 2011; Zhang et al. 2011; Dambaeva et al. 2012). NK cell deficient animal models have been used to demonstrate the involvement of NK cells in uterine spiral artery remodeling (Guimond et al. 1997; Barber and Pollard 2003; Chakraborty et al. 2011). This assessment has been supported by co-culture experiments involving NK cells and uterine spiral artery segments (Robson et al. 2012). NK cells contribute to pregnancy-dependent restructuring of the uterine spiral arteries, especially loss of tunica media integrity, which facilitates fetal nutrient delivery. Infiltration, differentiation, and maintenance of NK cells within the uterus during the establishment of pregnancy are influenced by interleukin 15, interleukin 11, Hoxa-10, transforming growth factor-β (TGFB), bone morphogenetic protein (BMP), and adrenomedullin signaling pathways (Barber and Pollard 2003; Ain et al. 2004; Rahman et al. 2006; Keskin et al. 2007; Li et al. 2013; Nagashima et al. 2013). NK cell effects on uterine spiral arteries may be mediated by interferon γ (Ashkar and Croy, 2001), nitric oxide (Hunt et al. 1997), and an assortment of angiogenic growth factors and extracellular matrix modifying enzymes (Li et al. 2001; Wang et al. 2000, 2003; Hanna et al. 2006; Lash et al. 2006b; Kopcow and Karumanchi 2007; Wallace et al. 2012).
NK cells have also been implicated in the regulation of trophoblast cell invasion into the uterus and trophoblast cell interactions with uterine spiral arteries. A range of direct actions of NK cells on trophoblast cells has been postulated based primarily on in vitro analyses. The outcomes of these experiments are not entirely consistent and are likely influenced by the gestational age of the specimens and culture conditions. Some studies provide evidence for NK cell promotion of trophoblast migration and invasiveness (Hanna et al. 2006; Lash et al. 2010; Wallace et al. 2013), whereas other reports show that NK cells and their secretory products inhibit these vital functions (Ain et al. 2003; Hu et al. 2006; Lash et al. 2006a; Eastabrook et al. 2008). Furthermore some researchers have advocated for the importance of physical interactions between NK cells and invasive trophoblast cells. Specific patterns of polymorphic trophoblast cell surface ligands consisting of histocompatibility antigen isoforms and their cognate polymorphic NK cell receptor isoforms can define successful versus compromised pregnancies (Hiby et al. 2004; Parham and Moffett 2013; Xiong et al. 2013; Kieckbusch et al. 2014). These NK cell receptor-trophoblast cell histocompatibility antigen interactions may be best developed in primates (Parham and Moffett 2013). Direct signaling between NK cells and trophoblast cells is intriguing; however, the indirect actions of NK cells on trophoblast development via their effects on the vasculature may be as compelling.
Pivotal insights into the roles of NK cells in the regulation of hemochorial placentation were achieved using an in vivo rat model (Chakraborty et al. 2011). An immunodepletion strategy was employed to remove uterine NK cells during the establishment of the hemochorial placenta. The resulting phenotype confirmed a role for NK cells in the development and restructuring of uterine spiral arteries and unexpected functions in modulating the invasive trophoblast lineage. Surprisingly, trophoblast-directed uterine spiral artery remodeling was accelerated and much more pronounced in the absence of NK cells. The presence of NK cells also influenced the trophoblast pseudovascular phenotype. Based on these observations it was proposed that any actions of NK cells in promoting trophoblast endovascular invasion must be subtle and secondary to an overall restraining function (Chakraborty et al. 2011). Furthermore, the NK cell inhibitory action was viewed as indirect through NK cell regulation of uterine spiral artery development. Depletion of NK cells limited uterine mesometrial vascular development, lowering oxygen tension at the placentation site, and triggering trophoblast lineage decisions favoring differentiation of the invasive trophoblast lineage. Thus NK cell modulation of oxygen delivery is viewed as a key signal impacting trophoblast invasiveness and trophoblast-directed uterine spiral artery remodeling. These insights required an in vivo test using the rat, a species with deep trophoblast invasion.
Overview
NK cells have two key higher order functions regarding hemochorial placentation. They collectively act as a pacemaker, determining the timing of key developmental events and they fine-tune placental morphogenesis, defining the allocation of trophoblast lineages and thus the structure/function features of the placenta. The operative NK cell functions are to delay and restrict the invasive trophoblast program. In performing these tasks they effectively protect the mother and prevent precocious and excessive trophoblast invasion and restructuring of the uterine spiral arteries.
Trophoblast lineage development
Placentation is characterized by temporally and spatially relevant differentiation of trophoblast cells. The first cellular specification event during development occurs as totipotent cells of the embryo are allocated to an outer position (trophectoderm) versus an inner position (inner cell mass; Cockburn and Rossant 2010). Trophectoderm is destined for expansion as a multi-potential trophoblast stem (TS) cell population and further differentiation into specialized trophoblast cell types (e.g. invasive trophoblast, syncytiotrophoblast, etc), whereas cells of the inner cell mass retain a broader developmental potential, including the ability to form embryonic and extraembryonic structures. Some regulatory factors controlling trophoblast lineage determination, expansion, and differentiation have been discerned (Roberts and Fisher 2011; Pfeffer and Pearton 2012).
Extracellular signal control
Fibroblast growth factor (FGF)- and BMP-mediated signaling pathways are key regulators of the trophoblast lineage. The FGF4-FGFR2 signaling pathway promotes self-renewal in rodent TS cells (Tanaka et al. 1998; Abell et al. 2009; Murohashi et al. 2010; Asanoma et al. 2011). These cells can be maintained in a proliferative stem state or they can be induced to differentiate into specialized trophoblast lineages (Tanaka et al. 1998; Asanoma et al. 2011). A cocktail containing FGF4 and either TGFB or activin is sufficient to maintain TS cells ex vivo in a proliferative and undifferentiated state (Erlebacher et al. 2004; Kubaczka et al. 2014). An in vivo corollary to the TS cell has been identified in both rodent and human placentation sites (Rielland et al. 2008; Hemberger et al. 2010; Roberts and Fisher 2011; Pfeffer and Pearton 2012). However, the presumptive human TS cell population has not been successfully propagated ex vivo. There have been some attempts at isolating and culturing TS/trophoblast progenitor cell populations (Genbacev et al. 2011; Takao et al. 2011). These cells self renew and possess some capacity to differentiate into specialized trophoblast cell types; however, they exhibit a very different gene expression profile than do rodent TS cells or the putative TS cells identified in the human placenta (Hemberger et al. 2010). BMP4 is a downstream target of the FGF4-FGFR2 pathway in rodent TS cells (Murohashi et al. 2010) and is also an inducer of the trophoblast lineage when presented to pluripotent stem cells (Xu et al. 2002; Ezashi et al. 2012; Amita et al. 2013; Li et al. 2013). Following BMP4 treatment, pluripotent stem cells exhibit features of specialized differentiated trophoblast lineages. Presumably a multipotent TS cell population arises as the pluripotent stem cells commit to the trophoblast lineage; however, these cells have not yet been captured and propagated ex vivo. Expansion of such a cell population may require supplementation with special TS cell sustaining factors that are yet to be identified. The power of this pluripotent stem cell system for studying development of the trophoblast lineage is twofold: i) trophoblast development of the pluripotent stem cells is activated by BMP4, a known physiological regulator of trophoblast lineage determination (Hayashi et al. 2010; Amita et al. 2013; Home et al. 2013; Li et al. 2013) and ii) the model system is a window into the development of human trophoblast (Ezashi et al. 2012).
Transcriptional/epigenetic control
During trophoblast lineage determination, key factors sustaining the totipotent state are repressed and/or downregulated, while other key factors supporting the trophoblast lineage are activated and/or upregulated. These factors include transcription factors, histone modifiers, and chromatin organizers. POU5F1 (also called OCT4) is an essential transcription factor promoting totipotency and inhibiting trophoblast lineage development, whereas transcription factors such as TEAD4, CDX2, and EOMES are critical for development of the trophoblast lineage (Roberts and Fisher 2011; Pfeffer and Pearton 2012). Ectopic expression of any of these three transcription factors can convert pluripotent mouse embryonic stem cells into TS cells (Niwa et al. 2005; Tolkunova et al. 2006; Nishioka et al. 2009). TEAD4 is an upstream regulator of CDX2 and CDX2 is an upstream regulator of EOMES. SOX2, ESRRB, and TCFAP2C are essential for TS cell self-renewal (Adachi et al. 2013). These transcription factors and others, including GATA3, ELF5, and ETS2 contribute to development of the trophoblast lineage but their positions in the gene regulatory network are not yet precisely defined (Tremblay et al. 2001; Wen et al. 2007; Ng et al. 2008; Home et al. 2009, 2012; Keramari et al. 2010; Kidder and Palmer 2010; Kuckenberg et al. 2010; Ralston et al. 2010; Adachi et al. 2013; Choi et al. 2013). The actions of these transcription factors are presumably mediated through the delivery of enzymatic machinery that post-translationally modifies histones creating chromatin states that are more favorable or less favorable for transcription. Modulation of histone post-translational modifications has been implicated in the regulation of TS cell stemness and differentiation (Yeap et al. 2009; Yuan et al. 2009; Alder et al. 2010; Rugg-Gunn et al. 2010; Santos et al. 2010; Abell et al. 2011; Chuong et al. 2013; Saha et al. 2013). Collectively, each of these transcription factor and histone modifier activities is coordinated by a higher order of regulation controlled by chromatin organizers. SATB1 and SATB2 are prototypical genome organizers and have been implicated in controlling the TS cell stem state (Asanoma et al. 2012). Trophoblast lineage specific differentiation requires the downregulation of factors that maintain the TS cell stem state and activation of regulatory factors that promote lineage-specific differentiation. For example, CDX2, EOMES, SOX2, ID1/2, ESRRB, ELF5, and SATB1/2 sustain the TS cell stem state and are downregulated during differentiation (Tanaka et al. 1998; Janatpour et al. 2000; Ralston et al. 2010; Asanoma et al. 2011; Adachi et al. 2013), whereas transcription factors such as GCM1, GATA2, ASCL2, FOSL1, JUNB, BHLHE40, and OVOL2 are activated and promote differentiation into mature sub-lineages (Tanaka et al. 1998; Janatpour et al. 1999; Schorpp-Kistner et al. 1999; Schreiber et al. 2000; Hughes et al. 2004; Ray et al. 2009; Asanoma et al. 2011; Kent et al. 2011; Ueno et al. 2013; Renaud et al. 2014; Zhu et al. 2014). A schematic of the progression from a totipotent stem cell to a multipotent TS cell to a specialized differentiated trophoblast cell is shown in Figure 2. In summary, transcription factors, histone modifiers, and chromatin organizers cooperate to control totipotent stem cells, multipotent TS cells, and specialized trophoblast cells (Hemberger 2010; Maltepe et al. 2010; Wang et al. 2010; Rugg-Gunn 2012; Latos and Hemberger 2014; Paul and Knott 2014).
Fig. 2. Overview of the developmental progression from a totipotent stem cell to a multipotent trophoblast stem (TS) cell to a specialized differentiated trophoblast cell.
Each step along the developmental progression is controlled by positive and negative modulatory factors. These factors can be characterized as transcription factors, histone modifiers, and chromatin organizers (lists are provided in boxes below each cell type). See text for additional information.
Invasive trophoblast program
The development of trophoblast cells is multi-directional. Trophoblast can specialize into cells with transport, hormone secreting, and invasive features. Cells exhibiting invasive capabilities are termed invasive trophoblast or in the case of the human as extravillous trophoblast. Invasive trophoblast cells possess gene expression profiles distinguishing themselves from other trophoblast cell lineages (Bilban et al. 2010; Apps et al. 2011). Two phenotypically distinct types of invasive trophoblast can be identified: i) cells moving between uterine stromal cells termed interstitial invasive trophoblast cells and ii) cells moving within uterine spiral arteries termed endovascular invasive trophoblast cells (Pijnenborg et al. 2006). In rodents, interstitial invasive trophoblast cells are characterized by their accumulation of glycogen (Teesalu et al. 1998; Vercruysse et al. 2006). Interstitial invasion is severely restricted in some rat strains, including the Brown Norway rat, which may be due to uterine progesterone resistance and attenuated decidua development (Konno et al. 2007, 2010, 2011). Both interstitial and endovascular invasive trophoblast cells contribute to uterine spiral artery modifications; however, based on their positioning they each have some unique targets and modes of action. Interstitial invasive trophoblast cells via their distribution throughout the uterine stroma likely possess a broad set of actions supportive of the maternal-fetal interface. The activities of endovascular invasive trophoblast cells are focused on the vasculature. They breach the spiral artery wall and propel themselves within the lumen of the vessel, where they replace the endothelium and can mimic components of an endothelial cell phenotype (Damsky and Fisher 1998; Rai and Cross 2014). There are species differences in this process and evidence that trophoblast replacement of the endothelium is incomplete, transitory, and that endothelial cells can repopulate the lining of vessel wall (Pijnenborg et al. 2006; Ockleford 2010). Migratory features of invasive trophoblast are associated with their expression of proteins facilitating movement, including those proteins capable of modifying extracellular matrices. The mode of movement of interstitial versus endovascular trophoblast differs. In interstitial invasion – trophoblast cells dissociate and display elements of an epithelial to mesenchymal transformation as they penetrate the decidual stroma (Vicovac and Aplin 1996), whereas in endovascular invasion – cells maintain connectivity and exhibit an epithelial to endothelial-like transformation (Damsky and Fisher 1998). Aspects of the invasive trophoblast cell phenotype can be modeled in vitro; however, distinctions between the types of invasive trophoblast cells are more difficult to discern and are generally associated with acquisition of pseudovascular characteristics, which defines the endovascular invasive trophoblast cell population.
Regulation of invasive trophoblast differentiation
Considerable attention has been directed to elucidating pathways controlling the invasive trophoblast lineage. In this section, our discussion will be restricted to examples of conserved regulation demonstrated in vitro and in vivo using animal model systems and if available complemented with experimental findings showing relevance to human implantation/placentation.
Phosphatidylinositol 3-kinase/AKT/FOSL1
Several years ago, a linkage between Src family nonreceptor tyrosine kinases and phosphatidylinositol 3-kinase (PI3K) was established in differentiating rat TS cells (Kamei et al. 1997, 2002). PI3K is an intracellular enzyme responsible for the phosphorylation of phosphatidylinositol, which serves as a downstream activator of a signaling cascade controlling cell proliferation, differentiation, motility, metabolism, and survival (Cantley 2002). The LYN nonreceptor tyrosine kinase associates with PI3K and both proteins exhibit increases in enzymatic activity during trophoblast differentiation (Kamei et al. 1997, 2002; Kent et al. 2010). Small molecule inhibition of PI3K inhibits both endocrine and invasive activities of differentiating rat TS cells (Kamei et al. 2002; Kent et al. 2010). PI3K is an upstream regulator of the serine/threonine kinase, AKT. Inhibition of AKT also interferes with activation of the invasive trophoblast cell phenotype (Kent et al. 2011). AKT consists of three isoforms (AKT1, AKT2, AKT3). Each isoform is expressed in differentiating trophoblast cells and contributes to regulating the invasive trophoblast lineage (Kent et al. 2011). Both shared and isoform-specific actions characterize AKT signaling in differentiating trophoblast leading to complexities not yet fully appreciated (Kent et al. 2011; Haslinger et al. 2013).
There are numerous potential upstream activators of the PI3K/AKT signaling cascade in trophoblast cells, consisting of growth factors, cytokines, and extracellular matrix constituents (Polheimer and Knöfler 2005; Knöfler 2010). EGF, HGF, IGF2, chorionic gonadotropin, and wingless (WNT) family ligands have been shown to stimulate invasive properties of human trophoblast or trophoblast cell lines, at least in part, through activation of the PI3K/AKT signaling pathway (Cartwright et al. 2002; Qiu et al. 2004; Prast et al. 2008; Sonderegger et al. 2010; Pollheimer et al. 2011; Haslinger et al. 2013). However, demonstration of the involvement of any of these activators of PI3K/AKT in the regulation of in vivo intrauterine trophoblast invasion is lacking.
One intriguing downstream mediator of PI3K/AKT regulation of trophoblast invasiveness is the activator protein 1 (AP1) transcription factor component, FOSL1. PI3K/AKT signaling stabilizes the nuclear localization of FOSL1 (Kent et al. 2011). Trophoblast gene expression and invasion are regulated by FOSL1. In vitro knockdown of FOSL1 using specific short hairpin RNAs (shRNAs) disrupts the expression of key genes encoding proteins associated with dissolution of extracellular matrices, cell migration, and vascular remodeling and inhibits trophoblast cell migration through extracellular matrices (Kent et al. 2011). Furthermore, in vivo knockdown of FOSL1 in the rat using trophoblast-specific lentiviral delivery of specific FOSL1 shRNAs significantly reduced the depth of trophoblast invasion into the uterus (Kent et al. 2011). Such an action of FOSL1 was not evident from mouse mutagenesis experiments (Schreiber et al. 2000). FOSL1 was shown to regulate mouse placentation but a clue to its actions on the invasive trophoblast lineage was not forthcoming. These results do not diminish FOSL1 as a regulator of the invasive trophoblast phenotype but instead highlight the limitations of using the mouse as a model system for investigating the invasive trophoblast lineage. In fact, the pro-invasive action of FOSL1 is conserved in human trophoblast (Renaud et al. 2014). FOSL1 is localized to extravillous trophoblast at the leading edge of trophoblast columns penetrating the uterine decidua. Interestingly, two other FOS family transcription factors, FOS and FOSB, restrain the actions of FOSL1 and instead are prominently expressed in proliferating trophoblast cells embedded within the core of the trophoblast columns (Renaud et al. 2014).
Collectively, the data suggest the participation of a conserved PI3K/AKT/FOSL1 pathway in the regulation of invasive trophoblast. Upstream activation of PI3K/AKT signaling and downstream events, including FOSL1 partners and specific transcriptional targets, mediating invasive trophoblast differentiation remain to be elucidated.
Notch
The Notch signaling pathway is a highly conserved cell-cell communication system directing embryonic development. Notch signaling components include a family of receptors (NOTCH1-4) and membrane-associated ligands of the DLL and JAG families (Hori et al. 2013). Signal transduction is activated when a ligand-expressing cell apposes a NOTCH-expressing cell impacting potentially a broad spectrum of cellular processes (e.g. survival, proliferation, differentiation, motility, and vascular specification). Mouse and human trophoblast cells express components of the Notch signaling pathway (Nakayama et al. 1997; Hunkapiller et al. 2011; Haider et al. 2014). Selective genetic inactivation of Notch2 in mouse trophoblast lineages results in disruptions in placentation, including failed trophoblast cell invasion of uterine spiral arteries and impaired perfusion of the placenta (Hunkapiller et al. 2011). In vitro experimentation has demonstrated the importance of Notch signaling in human trophoblast cell biology but led to differing conclusions. In one report, disruption of Notch signaling with a small molecule inhibitor interfered with trophoblast invasive properties and impaired acquisition of a pseudo-vascular phenotype directly supporting the Notch2 mutant mouse phenotype (Hunkapiller et al. 2011), whereas another report highlighted the importance of Notch signaling in maintaining trophoblast proliferation and its antagonism of trophoblast motility and invasive properties (Haider et al. 2014). These in vitro experimental outcomes point to the importance of Notch signaling in trophoblast cells and also its dynamic nature. Multiple NOTCH receptors and ligands expressed by several placentation site-associated cell types each possessing gestational stage-specific expression profiles creates complexities for planning in vitro experiments designed to recapitulate aspects of in vivo trophoblast cell development.
Oxygen
Cells require oxygen and possess intricate and highly conserved mechanisms for adapting to oxygen deprivation (Semenza 2010). Central to cellular adaptations to low oxygen is a transcriptional complex referred to as hypoxia-inducible factor (HIF). HIF is composed of an oxygen labile alpha subunit (HIF1A or HIF2A) and a constitutive partner referred to as HIF1 beta (HIF1B, also called aryl hydrocarbon nuclear translocator, ARNT). The HIF alpha subunit is vulnerable to degradation at oxygen replete conditions. In contrast, at conditions of oxygen scarcity the HIF transcriptional complex is stabilized and activates target genes encoding proteins essential for cellular adaptation to low oxygen. Some definitions of a couple of terms associated with oxygen homeostasis are required before we proceed. Normoxia represents a condition of “normal” oxygen availability. Hypoxia is a condition associated with low oxygen tension, especially one that evokes the HIF-mediated cellular adaptive response. Importantly, a specific oxygen tension cannot be used to define hypoxia or normoxia. These are relative terms and are absolutely dependent upon cell type and physiological or pathological setting. It should be appreciated that under physiological conditions hypoxia is a transient homeostatic process corrected by an assortment of cellular and systemic adaptations. Chronic hypoxia is a pathological event associated with failures in adaptation. These fundamental principles need to be considered in designing experiments to investigate the impact of oxygen tension on trophoblast cell biology. Unfortunately, attempts to model hypoxia in vitro have been fraught with numerous inaccurate assumptions and misleading interpretations (see Tuuli et al. 2011 for additional discussion).
Oxygen tensions at the placentation site change during the course of gestation (Zamudio 2003). Establishment of the hemochorial interface is the pivotal event determining trophoblast cell oxygen exposure. Oxygen increases once trophoblast-vascular connectivity is established. It has also become evident that oxygen is an orchestrator of placental morphogenesis (Dunwoodie 2009). Such insight has been gained from mouse mutagenesis of several key regulators of oxygen homeostasis, including HIF1A, HIF2A, HIF1B, EGLN1, VHL, and CITED2 (Gnarra et al. 1997; Kozak et al. 1997; Adelman et al. 2000; Cowden Dahl et al. 2005a; Maltepe et al. 2005; Takeda et al. 2006; Withington et al. 2006). Additional understanding has been achieved from using oxygen tension as an in vivo experimental tool to investigate placentation site-associated adaptations in the rat (Rosario et al. 2008). Exposure of pregnant rats to 10-11% oxygen from the onset of embryo implantation until midgestation results in profound effects on the maternal-fetal interface (Ho-Chen et al. 2007; Rosario et al. 2008). The hypoxic conditions drive increases in uterine mesometrial vascularity, uterine spiral arterial remodeling, and dramatic increases in the depth of intrauterine endovascular invasive trophoblast cell penetration (Fig. 3). This environmental challenge accelerated and exaggerated changes at the placentation site that would not normally occur until the latter stages of pregnancy. Activation of the invasive trophoblast lineage required exposure to hypoxia between gestation days 8.5 and 9.5. This critical window of in vivo sensitivity to oxygen correlates with the initiation of essential trophoblast cell differentiation leading to formation of the bi-compartmental rat placenta. Hypoxia results in preferential expansion of the junctional zone and its resident invasive trophoblast cell population, which is situated at the maternal interface, and a proportional reduction in the size of the labyrinth zone (Rosario et al. 2008; Chakraborty et al. 2011). This is a conserved adaptive response. In vivo hypoxia activation of the invasive trophoblast lineage has also been observed in primates (Zhou et al. 1993; Kadyrov et al. 2003). Trophoblast cells migrate toward oxygen in the human placental bed (Jauniaux et al. 2001). Alternatively, others have used in vivo chronic hypoxia to overwhelm adaptive responses and create placental injury (Tomlinson et al. 2010; Lai et al. 2011). Thus, oxygen availability can serve as context-dependent instructive or pathological signals affecting placentation.
Fig. 3. Maternal hypoxia stimulates trophoblast invasion into uterine spiral arteries of the rat.

Wild-type female rats were mated to homozygous chβA-enhanced green fluorescent protein (EGFP) transgenic male rats and exposed to an atmospheric oxygen tension (21 percent at sea level; panel A) or hypoxia (10-11%; gestation day 6.5 to day 13.5; panel B). Placentation sites were examined on gestation day 13.5 and uterine mesometrial compartments inspected for EGFP positive cells. Note that maternal hypoxia stimulated the invasion of extraembryonic-derived EGFP positive cells deep into maternal uterine spiral arteries, representing a potentially effective placental-associated adaptation to an environmental challenge. Scale bar = 0.5 mm. Adapted from Rosario et al. 2008.
Oxygen tension is also a potent regulator of in vitro trophoblast cell behavior, including development of the invasive trophoblast lineage. Fisher and colleagues first showed that low oxygen tension (2 percent) promoted first trimester human trophoblast proliferation, whereas atmospheric oxygen (21 percent at sea level) stimulated differentiation (Genbacev et al. 1996, 1997). Since then an assortment of observations have been made on trophoblast cell responses to low oxygen, including seemingly contradictory findings. It is now apparent that oxygen concentration and duration of exposure can have fundamentally different effects on trophoblast cell behavior (Tuuli et al. 2010; Zhou et al. 2011). Additionally, the origin and characteristics of the trophoblast cell (e.g. primary trophoblast, gestational age, immortalized trophoblast cell line, trophoblast cancer cell) influences its responses to oxygen availability. Rodent TS cells have proven to be a robust in vitro model system for studying trophoblast cell adaptations to low oxygen (Cowden Dahl et al. 2005a,b; Chakraborty et al. 2011; Zhou et al. 2011). Gradients in oxygen tension can differentially stimulate trophoblast cell proliferation or trophoblast cell differentiation (Zhou et al. 2011). Very low oxygen concentrations (0.5-1.5%) activate development of the invasive trophoblast lineage (Cowden Dahl et al. 2005b; Chakraborty et al. 2011). This differentiation event is associated with decreased cell-cell adhesion, upregulation of matrix metalloproteinases, and increased cellular movement through extracellular matrices; and is dependent upon activation of the HIF signaling pathway. The targets downstream of HIF transcriptional regulation in hypoxia-exposed TS cells have not been identified but should include genes controlling key stages in the differentiation of the invasive trophoblast lineage and also the activation of homeostatic regulatory processes designed to promote trophoblast cell survival and function. Activation of endothelial nitric oxide synthase and production of nitric oxide is an adaptation used in the mouse placenta to prevent local hypoxia (Schaffer et al. 2006). There is also evidence that non-canonical HIF signaling contributes to the regulation of trophoblast differentiation (Choi et al. 2013) and that hypoxia can affect trophoblast differentiation independent of HIF (Tache et al. 2013). Links between hypoxia and epigenetic regulation, including histone acetylation and DNA methylation, have been investigated in trophoblast cells (Maltepe et al. 2005; Yuen et al. 2013). Interestingly, in human trophoblast cells hypoxia-sensitive DNA methylation regions are enriched for AP1 binding motifs (Yuen et al. 2013), thus potentially connecting AP1, including FOSL1, and hypoxia/HIF regulatory pathways in trophoblast cell adaptive mechanisms.
In summary, TS cells present during the formative stages of placentation are characterized by their plasticity. They can differentiate into trophoblast cells targeted to the uterine vasculature or alternatively into trophoblast cells dedicated to nutrient transport, effectively establishing the functional limits for placental performance and the milieu for fetal growth and development. Oxygen delivery to the TS cell niche contributes to decision-making governing trophoblast cell differentiation. Low oxygen activates HIF signaling and favors differentiation directed toward the invasive trophoblast cell lineage. Thus factors controlling oxygen delivery to the TS cell niche, especially NK cells (see above), have a major influence on the organization of the maternal-fetal interface.
Disease processes associated with disruptions in uterine spiral artery remodeling
Failures in trophoblast-directed uterine spiral artery remodeling have been reported in diseased human pregnancies diagnosed with preeclampsia, Hemolysis, Elevated Liver enzymes, Low Platelets (HELLP) syndrome, and intrauterine growth restriction (Kaufmann et al. 2003; Pijnenborg et al. 2006). These pathologies have been a major driver of placental research. There are exquisite descriptions of the phenomenology surrounding the diseases but much less insight into their actual etiologies. The lack of progress is certainly associated with the multifactorial nature of any pregnancy related event. The embryo, its ability to give rise to multiple trophoblast lineages, and the host maternal environment in which the embryo develops each contribute to the manifestation of the disease. Two routes of inquiry are apparent: i) identify potential sensitive junctures in normal developmental processes, which may impact susceptibility to disease; ii) directly study the disease and determine how the disruption of candidate regulators leads to disease. Strategies for studying trophoblast-directed uterine spiral artery remodeling have been provided above. Investigations on mechanisms underlying pregnancy-related diseases are inherently confounded because key events associated with the ontogeny of the disease pre-date diagnosis. Diseased tissue specimens are affected by a failed placentation (e.g. hypoxia, inflammation) and thus compromise acquisition of mechanistic insights into etiology from any phenotypic analysis. Genetic analyses have offered an alternative approach (van Dijk and Oudejans 2013).
Several genetic screens have been performed to establish linkages between pregnancy-related diseases associated with impairments in trophoblast cell invasion and uterine spiral artery remodeling, including preeclampsia and the HELLP syndrome (van Dijk and Oudejans 2013). Among these efforts, a couple of genes have been pursued and complemented with functional analyses leading to the identification of previously unappreciated regulatory pathways.
Stox1
A polymorphism at the STOX1 locus was identified in genetic screens of families with severe preeclampsia and intrauterine growth restriction (van Dijk et al. 2005). The STOX1 protein possesses a winged helix domain present in FOX family transcription factors, suggesting that STOX1 may contribute to the regulation of gene expression. The polymorphism affects the amino acid structure of the STOX1 DNA binding domain (Y153H). The H isoform is associated with the diseased state, expressed in extravillous trophoblast, and specifically targets and promotes transcription of the CTNNA3 gene. CTNNA3 encodes a cell-cell adhesion molecule, α-T-catenin, and restrains the invasive properties of trophoblast cells (van Dijk et al. 2010). These observations are further supported by experiments using a transgenic mouse model. Pregnant female mice possessing conceptuses overexpressing STOX1 exhibit preeclampsia-like symptoms (Doridot et al. 2013). Although placentation was altered in the STOX1 overexpressing conceptuses, a description of invasive trophoblast cells and uterine spiral artery remodeling was not reported. Most interestingly, nuclear localization of the STOX1 protein is regulated by AKT (van Dijk et al. 2010). AKT-mediated STOX1 phosphorylation prevents entry of STOX1 into the nucleus, consistent with the pro-invasive role of AKT signaling in trophoblast cells.
HELLP long non-coding RNA
A genetic screen of familial HELLP syndrome led to the identification of mutations in a gene encoding a long non-coding RNA specifically expressed in extravillous trophoblast (van Dijk et al. 2012). Initial evidence indicates that the HELLP long non-coding RNA promotes proliferation and inhibits differentiation into the extravillous invasive trophoblast phenotype. This finding opens an avenue for future exploration into a potentially novel mechanism controlling trophoblast differentiation.
Other candidate genes
A candidate gene approach has also been used to elucidate mechanisms associated with disruptions in trophoblast cell invasion and preeclampsia. Two examples will be discussed. Experimental evidence has accumulated suggesting that catechol-O-methyltransferase (COMT) and corin serine peptidase (CORIN) are modulators of trophoblast cell invasion and disrupted in preeclampsia (Kanasaki et al. 2008; Lee et al. 2010; Cui et al. 2012). Their potential involvement in the etiology of the pregnancy-associated disease surfaced from examination of tissues from preeclamptic patients and has been bolstered through phenotypic investigations of genetically modified rodent models. COMT is expressed in the placenta and methylates several circulating hormones, including catechol estrogens, and is responsible for the generation of 2-methoxyestradiol (2-ME). 2-ME synergizes with hypoxia to promote trophoblast cell invasive properties (Lee et al. 2010). Preeclamptic patients exhibit decreased placental COMT activities and disruption of the Comt gene in the mouse is associated with a preeclampsia-like phenotype in pregnant mice (Kanasaki et al. 2008). Polymorphisms in the COMT gene affecting expression or enzymatic activity may contribute to the development of preeclampsia (Shenoy et al. 2010). In contrast, the CORIN-associated preeclamptic phenotype reflects disruptions in the maternal environment. CORIN is expressed in the uterus and acts to generate biologically active atrial natriuretic peptide (ANP). ANP stimulates in vitro trophoblast cell invasion through extracellular matrices and either CORIN or ANP deficiencies in the mouse result in deficits in both trophoblast invasion and uterine spiral artery remodeling and are characterized by preeclamptic-like symptoms (Cui et al. 2012). Uteri from preeclamptic patients express less CORIN and some preeclamptic patients possess polymorphisms negatively affecting CORIN enzymatic activity (Cui et al. 2012), further supporting a role for CORIN in the etiology of preeclampsia.
Overview
Diseases affecting trophoblast invasion and uterine spiral artery remodeling are examples of classic positive feedback paradigms. Early failures in the process of uterine spiral artery remodeling lead to hypoxia and inflammation that perpetuate and exacerbate the deficits (Renaud et al. 2011; Cotechini et al. 2014) and are not remedied until the pregnancy is terminated and the protagonist placenta removed. It is expected that clarity will be achieved as we gain insights into both the physiology and pathophysiology of uterine spiral artery remodeling.
Final thoughts on adaptations at the maternal-fetal interface
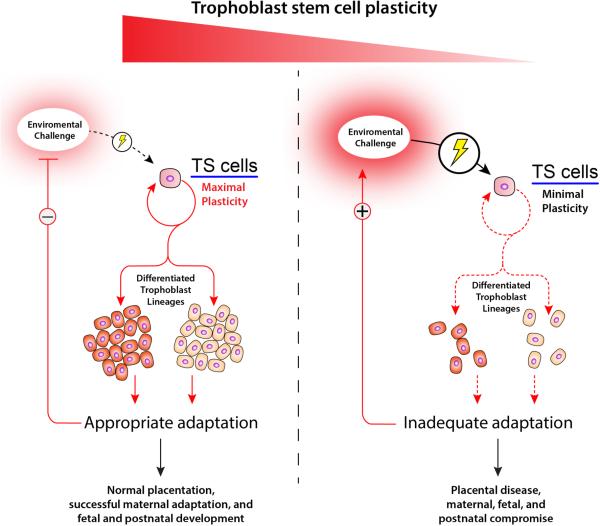
Plasticity and the ability to adapt to environmental challenges are favorable attributes for pregnancy success (see Fig. 4). Intrinsic to any adaptation affecting pregnancy is the control of the decision-making process directing multi-lineage differentiation of trophoblast stem cell populations. These stem cells can self-renew, die, become quiescent, or differentiate and assume one of several specialized functions within the placenta. This flexibility is gestation-stage dependent with greater capacity for adaptation during early versus later phases of pregnancy and it ensures that the placentation site is optimally organized to facilitate fetal growth and development. Failures in early organizational decisions yield rigidity and inability to effectively adapt to later challenges. The entry points for understanding placentation site-associated plasticity are homeostatic mechanisms that all cells utilize to optimize their survival when exposed to environmental stressors. Some elements of this fundamental adaptive process can be modeled in a dish and are most meaningful when complemented with investigations using animal models (Bonney 2013; Clark 2014). Logically, placental disease results when adaptive responses at the implantation/placentation site fail or are inappropriate, thus sustaining or intensifying the environmental challenge and leading to maternal, fetal, and postnatal compromise. Determining whether a placenta is destined for success or failure, especially considering the importance of the placenta to the growth and development of the fetus and its future postnatal health, is inherently complicated but fundamental to improving human health.
Fig. 4. Trophoblast stem (TS) cell plasticity and placental disease.
Environmental challenges, including nutrient availability, at the maternal-fetal interface direct the decision-making of TS cells. TS cells possess the capacity to self-renew, die, become quiescent, or differentiate into specialized trophoblast lineages. A successful pregnancy is associated with robust and gestation stage-appropriate homeostatic responses to environmental challenges resulting in effective organization of the placentation site, maternal adaptations and normal progression of fetal development and healthy offspring. In contrast, we propose that placental disease is associated with a TS cell that lacks plasticity and the ability to effectively adapt to environmental challenges, which activates a positive feedback loop sustaining and potentially intensifying the environmental challenge, resulting in failures in placentation and maternal, fetal, and postnatal compromise.
Acknowledgements
We would like to thank past and current members of our laboratory for contributing to our research effort. This work was supported by grants from the National Institutes of Health (HD020676, MJS; HD072100, MAKR) and fellowships from the American Heart Association (DC, KK), the Japan Society for the Promotion of Science (KK), and the Canadian Institutes of Health Research (SJR).
References
- ABELL AN, GRANGER DA, JOHNSON NL, VINCENT-JORDAN N, DIBBLE CF, JOHNSON GL. Trophoblast stem cell maintenance by fibroblast growth factor 4 requires MEKK4 activation of Jun N-terminal kinase. Mol Cell Biol. 2009;29:2748–2761. doi: 10.1128/MCB.01391-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ABELL AN, JORDAN NV, HUANG W, PRAT A, MIDLAND AA, JOHNSON NL, GRANGER DA, MIECZKOWSKI PA, PEROU CM, GOMEZ SM, LI L, JOHNSON GL. MAP3K4/CBP-regulated H2B acetylation controls epithelial-mesenchymal transition in trophoblast stem cells. Cell Stem Cell. 2011;8:525–537. doi: 10.1016/j.stem.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADACHI K, NIKAIDO I, OHTA H, OHTSUKA S, URA H, KADOTA M, WAKAYAMA T, UEDA H, NIWA H. Context-dependent wiring of Sox2 regulatory networks for self-renewal of embryonic and trophoblast stem cells. Mol Cell. 2013;52:380–392. doi: 10.1016/j.molcel.2013.09.002. [DOI] [PubMed] [Google Scholar]
- ADAMSON SL, LU Y, WHITELEY KJ, HOLMYARD D, HEMBERGER M, PFARRER C, CROSS JC. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol. 2002;250:358–373. doi: 10.1016/s0012-1606(02)90773-6. [DOI] [PubMed] [Google Scholar]
- ADELMAN DM, GERTSENSTEIN M, NAGY A, SIMON MC, MALTEPE E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 2000;14:3191–3203. doi: 10.1101/gad.853700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AIN R, CANHAM LN, SOARES MJ. Gestational stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: novel endocrine phenotype and regulation. Dev Biol. 2003;260:176–190. doi: 10.1016/s0012-1606(03)00210-0. [DOI] [PubMed] [Google Scholar]
- AIN R, CANHAM LN, SOARES MJ. Dexamethasone-induced intrauterine growth restriction impacts the placental prolactin family and the insulin-like growth factor-II/Akt signaling pathway. J Endocrinol. 2005;185:253–263. doi: 10.1677/joe.1.06039. [DOI] [PubMed] [Google Scholar]
- AIN R, TRINH M-L, SOARES MJ. Interleukin-11 signaling is required for the differentiation of natural killer cells at the maternal-fetal interface. Dev Dyn. 2004;231:700–708. doi: 10.1002/dvdy.20183. [DOI] [PubMed] [Google Scholar]
- ALDER O, LAVIAL F, HELNESS A, BROOKES E, PINHO S, CHANDRASHEKRAN A, ARNAUD P, POMBO A, O'NEILL L, AZUARA V. Ring1B and Suv39h1 delineate distinct chromatin states at bivalent genes during early mouse lineage commitment. Development. 2010;137:2483–2492. doi: 10.1242/dev.048363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMITA M, ADACHI K, ALEXENKO AP, SINHA S, SCHUST DJ, SCHULZ LC, ROBERTS RM, EZASHI T. Complete and unidirectional conversion of human embryonic stem cells to trophoblast by BMP4. Proc Natl Acad Sci U S A. 2013;110:E1212–E1221. doi: 10.1073/pnas.1303094110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMOROSO EC. Comparative anatomy of the placenta. Ann N Y Acad Sci. 1959;75:855–872. doi: 10.1111/j.1749-6632.1959.tb44596.x. [DOI] [PubMed] [Google Scholar]
- APLIN JD. In vitro analysis of trophoblast invasion. Methods Mol Med. 2006;122:45–57. doi: 10.1385/1-59259-989-3:45. [DOI] [PubMed] [Google Scholar]
- APPS R, SHARKEY A, GARDNER L, MALE V, TROTTER M, MILLER N, NORTH R, FOUNDS S, MOFFETT A. Genome-wide expression profile of first trimester villous and extravillous human trophoblast cells. Placenta. 2011;32:33–43. doi: 10.1016/j.placenta.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASANOMA K, KUBOTA K, CHAKRABORTY D, RENAUD SJ, WAKE N, FUKUSHIMA K, SOARES MJ, RUMI MAK. SATB homeobox proteins regulate trophoblast stem cell renewal and differentiation. J Biol Chem. 2012;287:2257–2268. doi: 10.1074/jbc.M111.287128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASANOMA K, RUMI MAK, KENT LN, CHAKRABORTY D, RENAUD SJ, WAKE N, LEE D-S, KUBOTA K, SOARES MJ. FGF4-dependent stem cells derived from rat blastocysts differentiate along the trophoblast lineage. Dev Biol. 2011;351:110–119. doi: 10.1016/j.ydbio.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASHKAR AA, CROY BA. Functions of uterine natural killer cells are mediated by interferon gamma production during murine pregnancy. Semin Immunol. 2001;13:235–241. doi: 10.1006/smim.2000.0319. [DOI] [PubMed] [Google Scholar]
- BARBER EM, POLLARD JW. The uterine NK cell population requires IL-15 but these cells are not required for pregnancy nor the resolution of a Listeria monocytogenes infection. J Immunol. 2003;171:37–46. doi: 10.4049/jimmunol.171.1.37. [DOI] [PubMed] [Google Scholar]
- BILBAN M, TAUBER S, HASLINGER P, POLLHEIMER J, SALEH L, PEHAMBERGER H, WAGNER O, KNÖFLER M. Trophoblast invasion: assessment of cellular models using gene expression signatures. Placenta. 2010;31:989–996. doi: 10.1016/j.placenta.2010.08.011. [DOI] [PubMed] [Google Scholar]
- BONNEY EA. Demystifying animal models of adverse pregnancy outcomes: touching bench and bedside. Am J Reprod Immunol. 2013;69:567–584. doi: 10.1111/aji.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANTLEY LC. The phosphoinositides 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- CARTWRIGHT JE, TSE WK, WHITLEY GS. Hepatocyte growth factor induced human trophoblast motility involves phosphatidylinositol-3-kinase, mitogen-activated protein kinase, and inducible nitric oxide synthase. Exp Cell Res. 2002;279:219–226. doi: 10.1006/excr.2002.5616. [DOI] [PubMed] [Google Scholar]
- CERDEIRA AS, RAJAKUMAR A, ROYLE CM, LO A, HUSAIN Z, THADHANI RI, SUKHATME VP, KARUMANCHI SA, KOPCOW HD. Conversion of peripheral blood NK cells to a decidual NK-like phenotype by a cocktail of defined factors. J Immunol. 2013;190:3939–3948. doi: 10.4049/jimmunol.1202582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAKRABORTY D, RUMI MAK, KONNO T, SOARES MJ. Natural killer cells direct hemochorial placentation by regulating HIF-dependent trophoblast lineage decisions. Proc Natl Acad Sci U S A. 2011;108:16295–16300. doi: 10.1073/pnas.1109478108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOI HJ, SANDERS TA, TORMOS KV, AERI K, TSAI JD, PARK AM, GONZALEZ J, RAJAH AM, LIU X, QUINONEZ DM, RINAUDO PF, MALTEPE E. ECM-dependent HIF induction directs trophoblast stem cell fate via LIMK1-mediated cytoskeletal rearrangement. PLoS One. 2013;8:e56949. doi: 10.1371/journal.pone.0056949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOI I, CAREY TS, WILSON CA, KNOTT JG. Evidence that transcription factor AP-2γ is not required for Oct4 repression in mouse blastocysts. PLoS One. 2013;8:e65771. doi: 10.1371/journal.pone.0065771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUONG EB, RUMI MA, SOARES MJ, BAKER JC. Endogenous retroviruses function as species-specific enhancer elements in the placenta. Nat Genet. 2013;45:325–329. doi: 10.1038/ng.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK DA. The use and misuse of animal analog models of human pregnancy disorders. J Reprod Immunol. 2014 doi: 10.1016/j.jri.2014.02.006. http://www.jrijournal.org/article/S0165-0378%2814%2900028-X/abstract. [DOI] [PubMed]
- COCKBURN K, ROSSANT J. Making the blastocyst: lessons from the mouse. J Clin Invest. 2010;120:995–1003. doi: 10.1172/JCI41229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTECHINI T, KOMISARENKO M, SPEROU A, MACDONALD-GOODFELLOW S, ADAMS MA, GRAHAM CH. Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J Exp Med. 2014;211:165–179. doi: 10.1084/jem.20130295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COWDEN DAHL KD, FRYER BH, MACK FA, COMPERNOLLE V, MALTEPE E, ADELMAN DM, CARMELIET P, SIMON MC. Hypoxia-inducible factors 1α and 2α regulate trophoblast differentiation. Mol Cell Biol. 2005a;25:10479–10491. doi: 10.1128/MCB.25.23.10479-10491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COWDEN DAHL KD, ROBERTSON SE, WEAVER VM, SIMON MC. Hypoxia-inducible factor regulates αvβ3 integrin cell surface expression. Mol Biol Cell. 2005b;16:1901–1912. doi: 10.1091/mbc.E04-12-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUI Y, WANG W, DONG N, LOU J, SRINIVASAN DK, CHENG W, HUANG X, LIU M, FANG C, PENG J, CHEN S, WU S, LIU Z, DONG L, ZHOU Y, WU Q. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature. 2012;484:246–250. doi: 10.1038/nature10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAMBAEVA SV, DURNING M, ROZNER AE, GOLOS TG. Immunophenotype and cytokine profiles of rhesus monkey CD56bright and CD56dim decidual natural killer cells. Biol Reprod. 2012;86:1–10. doi: 10.1095/biolreprod.111.094383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAMSKY CH, FISHER SJ. Trophoblast pseudo-vasculogenesis: faking it with endothelial adhesion receptors. Curr Opin Cell Biol. 1998;10:660–666. doi: 10.1016/s0955-0674(98)80043-4. [DOI] [PubMed] [Google Scholar]
- DORIDOT L, PASSET B, MÉHATS C, RIGOURD V, BARBAUX S, DUCAT A, MONDON F, VILOTTE M, CASTILLE J, BREUILLER-FOUCHÉ M, DANIEL N, LE PROVOST F, BAUCHET AL, BAUDRIE V, HERTIG A, BUFFAT C, SIMEONI U, GERMAIN G, VILOTTE JL, VAIMAN D. Preeclampsia-like symptoms induced in mice by fetoplacental expression of STOX1 are reversed by aspirin treatment. Hypertension. 2013;61:662–668. doi: 10.1161/HYPERTENSIONAHA.111.202994. [DOI] [PubMed] [Google Scholar]
- DUNWOODIE SL. The role of hypoxia in development of the mammalian embryo. Dev Cell. 2009;17:755–773. doi: 10.1016/j.devcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- EASTABROOK G, HU Y, VON DADELSZEN P. The role of decidual natural killer cells in normal placentation and in the pathogenesis of preeclampsia. J Obstet Gynaecol Can. 2008;30:467–476. doi: 10.1016/S1701-2163(16)32862-6. [DOI] [PubMed] [Google Scholar]
- ERLEBACHER A, PRICE KA, GLIMCHER LH. Maintenance of mouse trophoblast stem cell proliferation by TGF-beta/activin. Dev Biol. 2004;275:158–169. doi: 10.1016/j.ydbio.2004.07.032. [DOI] [PubMed] [Google Scholar]
- EZASHI T, TELUGU BP, ROBERTS RM. Model systems for studying trophoblast differentiation from human pluripotent stem cells. Cell Tissue Res. 2012;349:809–824. doi: 10.1007/s00441-012-1371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENBACEV O, DONNE M, KAPIDZIC M, GORMLEY M, LAMB J, GILMORE J, LAROCQUE N, GOLDFIEN G, ZDRAVKOVIC T, MCMASTER MT, FISHER SJ. Establishment of human trophoblast progenitor cell lines from the chorion. Stem Cells. 2011;29:1427–36. doi: 10.1002/stem.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENBACEV O, JOSLIN R, DAMSKY CH, POLLIOTTI BM, FISHER SJ. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest. 1996;97:540–550. doi: 10.1172/JCI118447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENBACEV O, ZHOU Y, LUDLOW JW, FISHER SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- GNARRA JR, WARD JM, PORTER FD, WAGNER JR, DEVOR DE, GRINBERG A, EMMERT-BUCK MR, WESTPHAL H, KLAUSNER RD, LINEHAN WM. Defective placental vasculo-genesis causes embryonic lethality in VHL-deficient mice. Proc Natl Acad Sci USA. 1997;94:9102–9107. doi: 10.1073/pnas.94.17.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAHAM CH, HAWLEY TS, HAWLEY RG, MACDOUGALL JR, KERBEL RS, KHOO N, LALA PK. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206:204–211. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- GUIMOND MJ, LUROSS JA, WANG B, TERHORST C, DANIAL S, CROY BA. Absence of natural killer cells during murine pregnancy is associated with reproductive compromise in TgE26 mice. Biol Reprod. 1997;56:169–179. doi: 10.1095/biolreprod56.1.169. [DOI] [PubMed] [Google Scholar]
- HAIDER S, MEINHARDT G, VELICKY P, OTTI GR, WHITLEY G, FIALA C, POLLHEIMER J, KNÖFLER M. Notch signaling plays a critical role in motility and differentiation of human first trimester cytotrophoblasts. Endocrinology. 2014;155:263–274. doi: 10.1210/en.2013-1455. [DOI] [PubMed] [Google Scholar]
- HANNA J, GOLDMAN-WOHL D, HAMANI Y, AVRAHAM I, GREENFIELD C, NATANSON-YARON S, PRUS D, COHEN-DANIEL L, ARNON TI, MANASTER I, GAZIT R, YUTKIN V, BENHARROCH D, PORGADOR A, KESHET E, YAGEL S, MANDELBOIM O. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- HARRIS LK. Trophoblast-vascular cell interactions in early pregnancy: how to remodel a vessel. Placenta. 2010;31(Suppl):S93–S98. doi: 10.1016/j.placenta.2009.12.012. [DOI] [PubMed] [Google Scholar]
- HARRIS LK. Transformation of the spiral arteries in human pregnancy: key events in the remodelling timeline. Placenta 32 Suppl. 2011;2:S154–S158. doi: 10.1016/j.placenta.2010.11.018. [DOI] [PubMed] [Google Scholar]
- HASLINGER P, HAIDER S, SONDEREGGER S, OTTEN JV, POLLHEIMER J, WHITLEY G, KNÖFLER M. AKT isoforms 1 and 3 regulate basal and epidermal growth factor-stimulated SGHPL-5 trophoblast cell migration in humans. Biol Reprod. 2013;88:54. doi: 10.1095/biolreprod.112.104778. [DOI] [PubMed] [Google Scholar]
- HAYASHI Y, FURUE MK, TANAKA S, HIROSE M, WAKISAKA N, DANNO H, OHNUMA K, OEDA S, AIHARA Y, SHIOTA K, OGURA A, ISHIURA S, ASASHIMA M. BMP4 induction of trophoblast from mouse embryonic stem cells in defined culture conditions on laminin. In Vitro Cell Dev Biol Anim. 2010;46:416–430. doi: 10.1007/s11626-009-9266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAZAN AD, SMITH SD, JONES RL, WHITTLE W, LYE SJ, DUNK CE. Vascular-leukocyte interactions: mechanisms of human decidual spiral artery remodeling in vitro. Am J Pathol. 2010;177:1017–1030. doi: 10.2353/ajpath.2010.091105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEMBERGER M. Genetic-epigenetic intersection in trophoblast differentiation: implications for extraembryonic tissue function. Epigenetics. 2010;5:24–29. doi: 10.4161/epi.5.1.10589. [DOI] [PubMed] [Google Scholar]
- HEMBERGER M, UDAYASHANKAR R, TESAR P, MOORE H, BURTON GJ. ELF5-enforced transcriptional networks define an epigenetically regulated trophoblast stem cell compartment in the human placenta. Hum Mol Genet. 2010;19:2456–2467. doi: 10.1093/hmg/ddq128. [DOI] [PubMed] [Google Scholar]
- HIBY SE, WALKER JJ, O'SHAUGHNESSY KM, REDMAN CW, CARRINGTON M, TROWSDALE J, MOFFETT A. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200:957–965. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HO-CHEN JK, BUSTAMANTE JJ, SOARES MJ. Prolactin-like protein-f subfamily of placental hormones/cytokines: responsiveness to maternal hypoxia. Endocrinology. 2007;148:559–565. doi: 10.1210/en.2006-1146. [DOI] [PubMed] [Google Scholar]
- HOME P, RAY S, DUTTA D, BRONSHTEYN I, LARSON M, PAUL S. GATA3 is selectively expressed in the trophectoderm of peri-implantation embryo and directly regulates Cdx2 gene expression. J Biol Chem. 2009;284:28729–28737. doi: 10.1074/jbc.M109.016840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOME P, SAHA B, RAY S, DUTTA D, GUNEWARDENA S, YOO B, PAL A, VIVIAN JL, LARSON M, PETROFF M, GALLAGHER PG, SCHULZ VP, WHITE KL, GOLOS TG, BEHR B, PAUL S. Altered subcellular localization of transcription factor TEAD4 regulates first mammalian cell lineage commitment. Proc Natl Acad Sci USA. 2012;109:7362–7367. doi: 10.1073/pnas.1201595109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORI K, SEN A, ARTAVANIS-TSAKONAS S. Notch signaling at a glance. J Cell Sci. 2013;126:2135–2140. doi: 10.1242/jcs.127308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HU Y, DUTZ JP, MACCALMAN CD, YONG P, TAN R, VON DADELSZEN P. Decidual NK cells alter in vitro first trimester extravillous cytotrophoblast migration: a role for IFN-gamma. J Immunol. 2006;177:8522–8530. doi: 10.4049/jimmunol.177.12.8522. [DOI] [PubMed] [Google Scholar]
- HUGHES M, DOBRIC N, SCOTT IC, SU L, STAROVIC M, ST-PIERRE B, EGAN SE, KINGDOM JC, CROSS JC. The Hand1, Stra13 and Gcm1 transcription factors override FGF signaling to promote terminal differentiation of trophoblast stem cells. Dev Biol. 2004;271:26–37. doi: 10.1016/j.ydbio.2004.03.029. [DOI] [PubMed] [Google Scholar]
- HUNKAPILLER NM, FISHER SJ. Placental remodeling of the uterine vasculature. Methods Enzymol. 2008;445:281–302. doi: 10.1016/S0076-6879(08)03012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNKAPILLER NM, GASPEROWICZ M, KAPIDZIC M, PLAKS V, MALTEPE E, KITAJEWSKI J, CROSS JC, FISHER SJ. A role for Notch signaling in trophoblast endovascular invasion and in the pathogenesis of pre-eclampsia. Development. 2011;138:2987–2998. doi: 10.1242/dev.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT JS, MILLER L, VASSMER D, CROY BA. Expression of the inducible nitric oxide synthase gene in mouse uterine leukocytes and potential relationships with uterine function during pregnancy. Biol Reprod. 1997;57:827–836. doi: 10.1095/biolreprod57.4.827. [DOI] [PubMed] [Google Scholar]
- JACOB HJ, LAZAR J, DWINELL MR, MORENO C, GEURTS AM. Gene targeting in the rat: advances and opportunities. Trends Genet. 2010;26:510–518. doi: 10.1016/j.tig.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANATPOUR MJ, MCMASTER MT, GENBACEV O, ZHOU Y, DONG J, CROSS JC, ISRAEL MA, FISHER SJ. Id-2 regulates critical aspects of human cytotrophoblast differentiation, invasion and migration. Development. 2000;127:549–558. doi: 10.1242/dev.127.3.549. [DOI] [PubMed] [Google Scholar]
- JANATPOUR MJ, UTSET MF, CROSS JC, ROSSANT J, DONG J, ISRAEL MA, FISHER SJ. A repertoire of differentially expressed transcription factors that offers insight into mechanisms of human cytotrophoblast differentiation. Dev Genet. 1999;25:146–157. doi: 10.1002/(SICI)1520-6408(1999)25:2<146::AID-DVG9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- JAUNIAUX E, WATSON A, BURTON G. Evaluation of respiratory gases and acid-base gradients in human fetal fluids and uteroplacental tissue between 7 and 16 weeks' gestation. Am J Obstet Gynecol. 2001;184:998–1003. doi: 10.1067/mob.2001.111935. [DOI] [PubMed] [Google Scholar]
- KADYROV M, SCHMITZ C, BLACK S, KAUFMANN P, HUPPERTZ B. Pre-eclampsia and maternal anaemia display reduced apoptosis and opposite invasive phenotypes of extravillous trophoblast. Placenta. 2003;24:540–548. doi: 10.1053/plac.2002.0946. [DOI] [PubMed] [Google Scholar]
- KAMEI T, HAMLIN GP, CHAPMAN BM, BURKHARDT AL, BOLEN JB, SOARES MJ. Signaling pathways controlling trophoblast cell differentiation: Src family protein tyrosine kinases in the rat. Biol Reprod. 1997;57:1202–1311. doi: 10.1095/biolreprod57.6.1302. [DOI] [PubMed] [Google Scholar]
- KAMEI T, JONES SR, CHAPMAN BM, MCGONIGLE K, DAI G, SOARES MJ. Activation and involvement of the phosphatidylinositol 3-kinase/akt-signaling pathway in the endocrine differentiation of trophoblast cells. Mol Endocrinol. 2002;16:1469–1481. doi: 10.1210/mend.16.7.0878. [DOI] [PubMed] [Google Scholar]
- KANASAKI K, PALMSTEN K, SUGIMOTO H, AHMAD S, HAMANO Y, XIE L, PARRY S, AUGUSTIN HG, GATTONE VH, FOLKMAN J, STRAUSS JF, KALLURI R. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. 2008;453:1117–1121. doi: 10.1038/nature06951. [DOI] [PubMed] [Google Scholar]
- KAUFMANN P, BLACK S, HUPPERTZ B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69:1–7. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- KENT LN, KONNO T, SOARES MJ. Phosphatidylinositol 3-kinase modulation of trophoblast cell differentiation. BMC Dev Biol. 2010;10:97. doi: 10.1186/1471-213X-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENT LN, RUMI MAK, KUBOTA K, LEE D-S, SOARES MJ. FOSL1 is integral to establishing the maternal-fetal interface. Mol Cell Biol. 2011;31:4801–4813. doi: 10.1128/MCB.05780-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERAMARI M, RAZAVI J, INGMAN KA, PATSCH C, EDENHOFER F, WARD CM, KIMBER SJ. Sox2 is essential for formation of trophectoderm in the preimplantation embryo. PLoS One. 2010;5:e13952. doi: 10.1371/journal.pone.0013952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESKIN DB, ALLAN DS, RYBALOV B, ANDZELM MM, STERN JN, KOPCOW HD, KOOPMAN LA, STROMINGER JL. TGFbeta promotes conversion of CD16+ peripheral blood NK cells into CD16- NK cells with similarities to decidual NK cells. Proc Natl Acad Sci USA. 2007;104:3378–3383. doi: 10.1073/pnas.0611098104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIDDER BL, PALMER S. Examination of transcriptional networks reveals an important role for TCFAP2C, SMARCA4, and EOMES in trophoblast stem cell maintenance. Genome Res. 2010;20:458–472. doi: 10.1101/gr.101469.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIECKBUSCH J, GAYNOR LM, MOFFETT A, COLUCCI F. MHC-dependent inhibition of uterine NK cells impedes fetal growth and decidual vascular remodeling. Nat Commun. 2014;5:3359. doi: 10.1038/ncomms4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNÖFLER M. Critical growth factors and signaling pathways controlling human trophoblast invasion. Int J Dev Biol. 2010;54:269–280. doi: 10.1387/ijdb.082769mk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONNO T, GRAHAM AR, REMPEL LA, HO-CHEN JK, ALAM SMK, BU P, RUMI MAK, SOARES MJ. Subfertility linked to combined luteal insufficiency and uterine progesterone resistance. Endocrinology. 2010;151:4537–4550. doi: 10.1210/en.2010-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONNO T, REMPEL LA, ARROYO JA, SOARES MJ. Pregnancy in the Brown Norway rat: a model for investigating the genetics of placentation. Biol Reprod. 2007;76:709–718. doi: 10.1095/biolreprod.106.056481. [DOI] [PubMed] [Google Scholar]
- KONNO T, REMPEL LA, RUMI MAK, GRAHAM AR, ASANOMA K, RENAUD SJ, SOARES MJ. Chromosome-substituted rat strains provide insights into the genetics of placentation. Physiol Genomics. 2011;43:930–941. doi: 10.1152/physiolgenomics.00069.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOOPMAN LA, KOPCOW HD, RYBALOV B, BOYSON JE, ORANGE JS, SCHATZ F, MASCH R, LOCKWOOD CJ, SCHACHTER AD, PARK PJ, STROMINGER JL. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOPCOW HD, KARUMANCHI SA. Angiogenic factors and natural killer (NK) cells in the pathogenesis of preeclampsia. J Reprod Immunol. 2007;76:23–29. doi: 10.1016/j.jri.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOZAK KR, ABBOTT B, HANKINSON O. ARNT-deficient mice and placental differentiation. Dev Biol. 1997;191:297–305. doi: 10.1006/dbio.1997.8758. [DOI] [PubMed] [Google Scholar]
- KUBACZKA C, SENNER C, ARAÚZO-BRAVO MJ, SHARMA N, KUCKENBERG P, BECKER A, ZIMMER A, BRÜSTLE O, PEITZ M, HEMBERGER M, SCHORLE H. Derivation and maintenance of murine trophoblast stem cells under defined conditions. Stem Cell Reports. 2014;2:232–242. doi: 10.1016/j.stemcr.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUCKENBERG P, BUHL S, WOYNECKI T, VAN FÜRDEN B, TOLKUNOVA E, SEIFFE F, MOSER M, TOMILIN A, WINTERHAGER E, SCHORLE H. The transcription factor TCFAP2C/AP-2gamma cooperates with CDX2 to maintain trophectoderm formation. Mol Cell Biol. 2010;30:3310–3320. doi: 10.1128/MCB.01215-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAI Z, KALKUNTE S, SHARMA S. A critical role of interleukin-10 in modulating hypoxia-induced preeclampsia-like disease in mice. Hypertension. 2011;57:505–514. doi: 10.1161/HYPERTENSIONAHA.110.163329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASH GE, OTUN HA, INNES BA, KIRKLEY M, DE OLIVEIRA L, SEARLE RF, ROBSON SC, BULMER JN. Interferon-gamma inhibits extravillous trophoblast cell invasion by a mechanism that involves both changes in apoptosis and protease levels. FASEB J. 2006a;20:2512–8. doi: 10.1096/fj.06-6616com. [DOI] [PubMed] [Google Scholar]
- LASH GE, OTUN HA, INNES BA, PERCIVAL K, SEARLE RF, ROBSON SC, BULMER JN. Regulation of extravillous trophoblast invasion by uterine natural killer cells is dependent on gestational age. Hum Reprod. 2010;25:1137–1145. doi: 10.1093/humrep/deq050. [DOI] [PubMed] [Google Scholar]
- LASH GE, SCHIESSL B, KIRKLEY M, INNES BA, COOPER A, SEARLE RF, ROBSON, BULMER JN. Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy. J Leukocyte Biol. 2006b;80:1–9. doi: 10.1189/jlb.0406250. [DOI] [PubMed] [Google Scholar]
- LATOS PA, HEMBERGER M. The transcriptional and signaling networks of mouse trophoblast stem cells. Placenta. 2014;35(Suppl A):S81–S85. doi: 10.1016/j.placenta.2013.10.013. [DOI] [PubMed] [Google Scholar]
- LEE SB, WONG AP, KANASAKI K, XU Y, SHENOY VK, MCELRATH TF, WHITESIDES GM, KALLURI R. Preeclampsia: 2-methoxyestradiol induces cytotrophoblast invasion and vascular development specifically under hypoxic conditions. Am J Pathol. 2010;176:710–720. doi: 10.2353/ajpath.2010.090513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEONARD S, LIMA PD, CROY BA, MURRANT CL. Gestational modification of murine spiral arteries does not reduce their drug-induced vasoconstrictive responses in vivo. Biol Reprod. 2013;89:139. doi: 10.1095/biolreprod.113.113688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI M, SCHWERBROCK NM, LENHART PM, FRITZ-SIX KL, KADMIEL M, CHRISTINE KS, KRAUS DM, ESPENSCHIED ST, WILLCOCKSON HH, MACK CP, CARON KM. Fetal-derived adrenomedullin mediates the innate immune milieu of the placenta. J Clin Invest. 2013;123:2408–2420. doi: 10.1172/JCI67039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI XF, CHARNOCK-JONES DS, ZHANG E, HIBY S, MALIK S, DAY K, LICENCE D, BOWEN JM, GARDNER L, KING A, LOKE YW, SMITH SK. Angiogenic growth factor mRNAs in uterine natural killer cells. J Clin Endocrinol Metab. 2001;86:1823–1834. doi: 10.1210/jcem.86.4.7418. [DOI] [PubMed] [Google Scholar]
- LI Y, MORETTO-ZITA M, SONCIN F, WAKELAND A, WOLFE L, LEON-GARCIA S, PANDIAN R, PIZZO D, CUI L, NAZOR K, LORING JF, CRUM CP, LAURENT LC, PARAST MM. BMP4-directed trophoblast differentiation of human embryonic stem cells is mediated through a ΔNp63+ cytotrophoblast stem cell state. Development. 2013;140:3965–3976. doi: 10.1242/dev.092155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALTEPE E, BAKARDJIEV AI, FISHER SJ. The placenta: transcriptional, epigenetic, and physiological integration during development. J Clin Invest. 2010;120:1016–1025. doi: 10.1172/JCI41211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALTEPE E, KRAMPITZ GW, OKAZAKI KM, RED-HORSE K, MAK W, SIMON MC, FISHER SJ. Hypoxia-inducible factor-dependent histone deacetylase activity determines stem cell fate in the placenta. Development. 2005;132:3393–3403. doi: 10.1242/dev.01923. [DOI] [PubMed] [Google Scholar]
- MOSSMAN HW. Vertebrates Fetal Membranes. Rutgers University Press; New Brunswick, NJ: 1987. [Google Scholar]
- MUROHASHI M, NAKAMURA T, TANAKA S, ICHISE T, YOSHIDA N, YAMAMOTO T, SHIBUYA M, SCHLESSINGER J, GOTOH N. An FGF4-FRS2alpha-Cdx2 axis in trophoblast stem cells induces Bmp4 to regulate proper growth of early mouse embryos. Stem Cells. 2010;28:113–121. doi: 10.1002/stem.247. [DOI] [PubMed] [Google Scholar]
- NAGASHIMA T, LI Q, CLEMENTI C, LYDON JP, DEMAYO FJ, MATZUK MM. BMPR2 is required for postimplantation uterine function and pregnancy maintenance. J Clin Invest. 2013;123:2539–2550. doi: 10.1172/JCI65710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAYAMA H, LIU Y, STIFANI S, CROSS JC. Developmental restriction of Mash-2 expression in trophoblast correlates with potential activation of the Notch-2 pathway. Dev Genet. 1997;21:21–30. doi: 10.1002/(SICI)1520-6408(1997)21:1<21::AID-DVG3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- NG RK, DEAN W, DAWSON C, LUCIFERO D, MADEJA Z, REIK W, HEMBERGER M. Epigenetic restriction of embryonic cell lineage fate by methylation of Elf5. Nat Cell Biol. 2008;10:1280–1290. doi: 10.1038/ncb1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIOKA N, INOUE K, ADACHI K, KIYONARI H, OTA M, RALSTON A, YABUTA N, HIRAHARA S, STEPHENSON RO, OGONUKI N, MAKITA R, KURIHARA H, MORIN-KENSICKI EM, NOJIMA H, ROSSANT J, NAKAO K, NIWA H, SASAKI H. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell. 2009;16:398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- NIWA H, TOYOOKA Y, SHIMOSATO D, STRUMPF D, TAKAHASHI K, YAGI R, ROSSANT J. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell. 2005;123:917–929. doi: 10.1016/j.cell.2005.08.040. [DOI] [PubMed] [Google Scholar]
- OCKLEFORD CD. The allo-epi-endothelial lining of the intervillous space. Placenta. 2010;31:1035–1042. doi: 10.1016/j.placenta.2010.09.007. [DOI] [PubMed] [Google Scholar]
- OSOL G, MANDALA M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 2009;24:58–71. doi: 10.1152/physiol.00033.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSOL G, MOORE LG. Maternal uterine vascular remodeling during pregnancy. Microcirculation. 2014;21:38–47. doi: 10.1111/micc.12080. [DOI] [PubMed] [Google Scholar]
- PARHAM P, MOFFETT A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat Rev Immunol. 2013;13:133–144. doi: 10.1038/nri3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAUL S, KNOTT JG. Epigenetic control of cell fate in mouse blastocysts: The role of covalent histone modifications and chromatin remodeling. Mol Reprod Dev. 2014;81:171–182. doi: 10.1002/mrd.22219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFEFFER PL, PEARTON DJ. Trophoblast development. Reproduction. 2012;143:231–246. doi: 10.1530/REP-11-0374. [DOI] [PubMed] [Google Scholar]
- PIJNENBORG R, VERCRUYSSE L. Animal models of deep trophoblast invasion. In: Pijnenborg R, Brosens I, Romero R, editors. Placental bed disorders: basic science and its translation to obstetrics. Cambridge University Press; Cambridge: 2010. pp. 127–139. [Google Scholar]
- PIJNENBORG R, VERCRUYSSE L, HANSSENS M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- POLLHEIMER J, HASLINGER P, FOCK V, PRAST J, SALEH L, BIADASIEWICZ K, JETNE-EDELMANN R, HARALDSEN G, HAIDER S, HIRTENLEHNER-FERBER K, KNÖFLER M. Endostatin suppresses IGF-II-mediated signaling and invasion of human extravillous trophoblasts. Endocrinology. 2011;152:4431–4442. doi: 10.1210/en.2011-1196. [DOI] [PubMed] [Google Scholar]
- POLLHEIMER J, KNÖFLER M. Signalling pathways regulating the invasive differentiation of human trophoblasts: a review. Placenta. 2005;26(Suppl A):S21–S30. doi: 10.1016/j.placenta.2004.11.013. [DOI] [PubMed] [Google Scholar]
- PRAST J, SALEH L, HUSSLEIN H, SONDEREGGER S, HELMER H, KNÖFLER M. Human chorionic gonadotropin stimulates trophoblast invasion through extracellularly regulated kinase and AKT signaling. Endocrinology. 2008;149:979–987. doi: 10.1210/en.2007-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QIU Q, YANG M, TSANG BK, GRUSLIN A. EGF-induced trophoblast secretion of MMP-9 and TIMP-1 involves activation of both PI3K and MAPK signalling pathways. Reproduction. 2004;128:355–363. doi: 10.1530/rep.1.00234. [DOI] [PubMed] [Google Scholar]
- RAHMAN MA, LI M, LI P, WANG H, DEY SK, DAS SK. Hoxa-10 deficiency alters region-specific gene expression and perturbs differentiation of natural killer cells during decidualization. Dev Biol. 2006;290:105–117. doi: 10.1016/j.ydbio.2005.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAI A, CROSS JC. Development of the hemochorial maternal vascular spaces in the placenta through endothelial and vasculogenic mimicry. Dev Biol. 2014;387:131–141. doi: 10.1016/j.ydbio.2014.01.015. [DOI] [PubMed] [Google Scholar]
- RALSTON A, COX BJ, NISHIOKA N, SASAKI H, CHEA E, RUGG-GUNN P, GUO G, ROBSON P, DRAPER JS, ROSSANT J. Gata3 regulates trophoblast development downstream of Tead4 and in parallel to Cdx2. Development. 2010;137:395–3403. doi: 10.1242/dev.038828. [DOI] [PubMed] [Google Scholar]
- RAY S, DUTTA D, RUMI MA, KENT LN, SOARES MJ, PAUL S. Context-dependent function of regulatory elements and a switch in chromatin occupancy between GATA3 and GATA2 regulate Gata2 transcription during trophoblast differentiation. J Biol Chem. 2009;284:4978–4988. doi: 10.1074/jbc.M807329200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RENAUD SJ, COTECHINI T, QUIRT JS, MACDONALD-GOODFELLOW SK, OTHMAN M, GRAHAM CH. Spontaneous pregnancy loss mediated by abnormal maternal inflammation in rats is linked to deficient uteroplacental perfusion. J Immunol. 2011;186:1799–1808. doi: 10.4049/jimmunol.1002679. [DOI] [PubMed] [Google Scholar]
- RENAUD SJ, KUBOTA K, RUMI MAK, SOARES MJ. The FOS transcription factor family differentially controls trophoblast migration and invasion. J Biol Chem. 2014;289:5025–5039. doi: 10.1074/jbc.M113.523746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIELLAND M, HUE I, RENARD JP, JOUNEAU A. Trophoblast stem cell derivation, cross-species comparison and use of nuclear transfer: new tools to study trophoblast growth and differentiation. Dev Biol. 2008;322:1–10. doi: 10.1016/j.ydbio.2008.07.017. [DOI] [PubMed] [Google Scholar]
- ROBERTS RM, FISHER SJ. Trophoblast stem cells. Biol Reprod. 2011;84:412–421. doi: 10.1095/biolreprod.110.088724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBSON A, HARRIS LK, INNES BA, LASH GE, ALJUNAIDY MM, APLIN JD, BAKER PN, ROBSON SC, BULMER JN. Uterine natural killer cells initiate spiral artery remodeling in human pregnancy. FASEB J. 2012;26:4876–4885. doi: 10.1096/fj.12-210310. [DOI] [PubMed] [Google Scholar]
- ROSARIO GX, KONNO T, SOARES MJ. Maternal hypoxia-activated endovascular trophoblast cell invasion. Dev Biol. 2008;314:362–375. doi: 10.1016/j.ydbio.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUGG-GUNN PJ, COX BJ, RALSTON A, ROSSANT J. Distinct modifications in stem cell lines and tissue lineages from the early mouse embryo. Proc Natl Acad Sci USA. 2010;107:10783–10790. doi: 10.1073/pnas.0914507107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUGG-GUNN PJ. Epigenetic features of the mouse trophoblast. Reprod BioMed Online. 2012;25:21–30. doi: 10.1016/j.rbmo.2012.01.012. [DOI] [PubMed] [Google Scholar]
- RUMI MAK, DHAKAL P, KUBOTA K, CHAKRABORTY D, LEI T, LARSON MA, WOLFE MW, ROBY KF, VIVIAN JL, SOARES MJ. Generation of Esr1 knockout rats using zinc finger nuclease-mediated genome editing. Endocrinology. 2013;155:1991–1999. doi: 10.1210/en.2013-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAHA B, HOME P, RAY S, LARSON M, PAUL A, RAJENDRAN G, BEHR B, PAUL S. EED and KDM6B coordinate the first mammalian cell lineage commitment to ensure embryo implantation. Mol Cell Biol. 2013;33:2691–2705. doi: 10.1128/MCB.00069-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANTOS J, PEREIRA CF, DI-GREGORIO A, SPRUCE T, ALDER O, RODRIQUEZ T, AZUARA V, MERKENSCHLAGER M, FISHER AG. Differences in the epigenetic and reprogramming properties of pluripotent and extra-embryonic stem cells implicate chromatin remodelling as an important early event in the developing mouse embryo. Epigenetics Chromatin. 2010;3:1. doi: 10.1186/1756-8935-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHÄFFER L, VOGEL J, BREYMANN C, GASSMANN M, MARTI HH. Preserved placental oxygenation and development during severe systemic hypoxia. Am J Physiol Regul Integr Comp Physiol. 2006;290:R844–R851. doi: 10.1152/ajpregu.00237.2005. [DOI] [PubMed] [Google Scholar]
- SCHORPP-KISTNER M, WANG ZQ, ANGEL P, WAGNER EF. JunB is essential for mammalian placentation. EMBO J. 1999;18:934–948. doi: 10.1093/emboj/18.4.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHREIBER M, WANG ZQ, JOCHUM W, FETKA I, ELLIOTT C, WAGNER EF. Placental vascularisation requires the AP-1 component fra1. Development. 2000;127:4937–4948. doi: 10.1242/dev.127.22.4937. [DOI] [PubMed] [Google Scholar]
- SEMENZA GL. Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med. 2010;2:336–361. doi: 10.1002/wsbm.69. [DOI] [PubMed] [Google Scholar]
- SHENOY V, KANASAKI K, KALLURI R. Pre-eclampsia: connecting angiogenic and metabolic pathways. Trends Endocrinol Metab. 2010;21:529–536. doi: 10.1016/j.tem.2010.05.002. [DOI] [PubMed] [Google Scholar]
- SMITH SD, DUNK CE, APLIN JD, HARRIS LK, JONES RL. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am J Pathol. 2009;174:1959–1971. doi: 10.2353/ajpath.2009.080995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONDEREGGER S, HASLINGER P, SABRI A, LEISSER C, OTTEN JV, FIALA C, KNÖFLER M. Wingless (Wnt)-3A induces trophoblast migration and matrix metalloproteinase-2 secretion through canonical Wnt signaling and protein kinase B/AKT activation. Endocrinology. 2010;151:211–220. doi: 10.1210/en.2009-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOARES MJ, CHAKRABORTY D, RUMI MAK, KONNO T, RENAUD SJ. Rat placentation: an experimental model for investigating the hemochorial maternal-fetal interface. Placenta. 2012;33:233–243. doi: 10.1016/j.placenta.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRASZEWSKI-CHAVEZ SL, ABRAHAMS VM, ALVERO AB, ALDO PB, MA Y, GULLER S, ROMERO R, MOR G. The isolation and characterization of a novel telomerase immortalized first trimester trophoblast cell line, Swan 71. Placenta. 2009;30:939–948. doi: 10.1016/j.placenta.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TACHE V, CIRIC A, MORETTO-ZITA M, LI Y, PENG J, MALTEPE E, MILSTONE DS, PARAST MM. Hypoxia and trophoblast differentiation: a key role for PPARγ. Stem Cells Dev. 2013;22:2815–2824. doi: 10.1089/scd.2012.0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAO T, ASANOMA K, KATO K, FUKUSHIMA K, TSUNEMATSU R, HIRAKAWA T, MATSUMURA S, SEKI H, TAKEDA S, WAKE N. Isolation and characterization of human trophoblast side-population (SP) cells in primary villous cytotrophoblasts and HTR-8/SVneo cell line. PLoS One. 2011;6:e21990. doi: 10.1371/journal.pone.0021990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEDA K, HO VC, TAKEDA H, DUAN LJ, NAGY A, FONG GH. Placental but not heart defects are associated with elevated hypoxia-inducible factor alpha levels in mice lacking prolyl hydroxylase domain protein 2. Mol Cell Biol. 2006;26:8336–8346. doi: 10.1128/MCB.00425-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANAKA S, KUNATH T, HADJANTONAKIS AK, NAGY A, ROSSANT J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- TEESALU T, BLASI F, TALARICO D. Expression and function of the urokinase type plasminogen activator during mouse hemochorial placental development. Dev Dyn. 1998;213:27–38. doi: 10.1002/(SICI)1097-0177(199809)213:1<27::AID-AJA3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- TOLKUNOVA E, CAVALERI F, ECKARDT S, REINBOLD R, CHRISTENSON LK, SCHÖLER HR, TOMILIN A. The caudal-related protein Cdx2 promotes trophoblast differentiation of mouse embryonic stem cells. Stem Cells. 2006;24:139–144. doi: 10.1634/stemcells.2005-0240. [DOI] [PubMed] [Google Scholar]
- TOMLINSON TM, GARBOW JR, ANDERSON JR, ENGELBACH JA, NELSON DM, SADOVSKY Y. Magnetic resonance imaging of hypoxic injury to the murine placenta. Am J Physiol Regul Integr Comp Physiol. 2010;298:R312–R319. doi: 10.1152/ajpregu.00425.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREMBLAY GB, KUNATH T, BERGERON D, LAPOINTE L, CHAMPIGNY C, BADER JA, ROSSANT J, GIGUÈRE V. Diethylstilbestrol regulates trophoblast stem cell differentiation as a ligand of orphan nuclear receptor ERR beta. Genes Dev. 2001;15:833–838. doi: 10.1101/gad.873401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUULI MG, LONGTINE MS, NELSON DM. Oxygen and trophoblast biology – a source of controversy. Placenta 32 Suppl. 2011;2:S109–S118. doi: 10.1016/j.placenta.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UENO M, LEE LK, CHHABRA A, KIM YJ, SASIDHARAN R, VAN HANDEL B, WANG Y, KAMATA M, KAMRAN P, SERETI KI, ARDEHALI R, JIANG M, MIKKOLA HK. c-Met-dependent multipotent labyrinth trophoblast progenitors establish placental exchange interface. Dev Cell. 2013;27:373–386. doi: 10.1016/j.devcel.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DIJK M, MULDERS J, POUTSMA A, KÖNST AA, LACHMEIJER AM, DEKKER GA, BLANKENSTEIN MA, OUDEJANS CB. Maternal segregation of the Dutch preeclampsia locus at 10q22 with a new member of the winged helix gene family. Nat Genet. 2005;37:514–519. doi: 10.1038/ng1541. [DOI] [PubMed] [Google Scholar]
- VAN DIJK M, OUDEJANS C. (Epi)genetics of pregnancy-associated diseases. Front Genet. 2013;4:180. doi: 10.3389/fgene.2013.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DIJK M, THULLURU HK, MULDERS J, MICHEL OJ, POUTSMA A, WINDHORST S, KLEIVERDA G, SIE D, LACHMEIJER AM, OUDEJANS CB. HELLP babies link a novel lincRNA to the trophoblast cell cycle. J Clin Invest. 2012;122:4003–4011. doi: 10.1172/JCI65171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DIJK M, VAN BEZU J, VAN ABEL D, DUNK C, BLANKENSTEIN MA, OUDEJANS CB, LYE SJ. The STOX1 genotype associated with pre-eclampsia leads to a reduction of trophoblast invasion by alpha-T-catenin upregulation. Hum Mol Genet. 2010;19:2658–2667. doi: 10.1093/hmg/ddq152. [DOI] [PubMed] [Google Scholar]
- VAN DIJK M, THULLURU HK, MULDERS J, MICHEL OJ, POUTSMA A, WINDHORST S, KLEIVERDA G, SIE D, LACHMEIJER AM, OUDEJANS CB. HELLP babies link a novel lincRNA to the trophoblast cell cycle. J Clin Invest. 2012;122:4003–4011. doi: 10.1172/JCI65171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERCRUYSSE L, CALUWAERTS S, LUYTEN C, PIJNENBORG R. Interstitial trophoblast invasion in the decidua and mesometrial triangle during the last third of pregnancy in the rat. Placenta. 2006;27:22–33. doi: 10.1016/j.placenta.2004.11.004. [DOI] [PubMed] [Google Scholar]
- VIĆOVAC L, APLIN JD. Epithelial-mesenchymal transition during trophoblast differentiation. Acta Anat (Basel) 1996;156:202–216. doi: 10.1159/000147847. [DOI] [PubMed] [Google Scholar]
- WALLACE AE, FRASER R, CARTWRIGHT JE. Extravillous trophoblast and decidual natural killer cells: a remodelling partnership. Hum Reprod Update. 2012;18:458–471. doi: 10.1093/humupd/dms015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLACE AE, HOST AJ, WHITLEY GS, CARTWRIGHT JE. Decidual natural killer cell interactions with trophoblasts are impaired in pregnancies at increased risk of preeclampsia. Am J Pathol. 2013;183:1853–1861. doi: 10.1016/j.ajpath.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG C, TANAKA T, NAKAMURA H, UMESAKI N, HIRAI K, ISHIKO O, OGITA S, KANEDA K. Granulated metrial gland cells in the murine uterus: localization, kinetics, and the functional role in angiogenesis during pregnancy. Micros Res Tech. 2003;60:420–429. doi: 10.1002/jemt.10280. [DOI] [PubMed] [Google Scholar]
- WANG C, UMESAKI N, NAKAMURA H, TANAKA T, NAKATANI K, SAKAGUCHI I, OGITA S, KANEDA K. Expression of vascular endothelial growth factor by granulated metrial gland cells in pregnant murine uteri. Cell Tissue Res. 2000;300:285–293. doi: 10.1007/s004410000198. [DOI] [PubMed] [Google Scholar]
- WANG K, SENGUPTA S, MAGNANI L, WILSON CA, HENRY RW, KNOTT JG. Brg1 is required for Cdx2-mediated repression of Oct4 expression in mouse blastocysts. PLoS One. 2010;5:e10622. doi: 10.1371/journal.pone.0010622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEN F, TYNAN JA, CECENA G, WILLIAMS R, MÚNERA J, MAVROTHALASSITIS G, OSHIMA RG. Ets2 is required for trophoblast stem cell self-renewal. Dev Biol. 2007;312:284–299. doi: 10.1016/j.ydbio.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITLEY GS. Production of human trophoblast cell lines. Methods Mol Med. 2006;121:219–228. doi: 10.1385/1-59259-983-4:217. [DOI] [PubMed] [Google Scholar]
- WIEMERS DO, AIN R, OHBOSHI S, SOARES MJ. Migratory trophoblast cells express a newly identified member of the prolactin gene family. J Endocrinol. 2003;179:335–346. doi: 10.1677/joe.0.1790335. [DOI] [PubMed] [Google Scholar]
- WITHINGTON SL, SCOTT AN, SAUNDERS DN, LOPES FLORO K, PREIS JI, MICHALICEK J, MACLEAN K, SPARROW DB, MARTINEZ BARBERA JP, DUNWOODIE SL. Loss of Cited2 affects trophoblast formation and vascularization of the mouse placenta. Dev Biol. 2006;294:67–82. doi: 10.1016/j.ydbio.2006.02.025. [DOI] [PubMed] [Google Scholar]
- WOODING FBP, BURTON GJ. Comparative Placentation – Structures, Functions, and Evolution. Springer-Verlag; Berlin, Heidelberg: 2008. [Google Scholar]
- XIONG S, SHARKEY AM, KENNEDY PR, GARDNER L, FARRELL LE, CHAZARA O, BAUER J, HIBY SE, COLUCCI F, MOFFETT A. Maternal uterine NK cell-activating receptor KIR2DS1 enhances placentation. J Clin Invest. 2013;123:4264–4272. doi: 10.1172/JCI68991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU RH, CHEN X, LI DS, LI R, ADDICKS GC, GLENNON C, ZWAKA TP, THOMSON JA. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- YEAP L-S, HAYASHI K, SURANI MA. ERG-associated protein with SET domain (ESET)-Oct 4 interaction regulates pluripotency and represses the trophectoderm lineage. Epigenetics Chromatin. 2009;2:12. doi: 10.1186/1756-8935-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUAN P, HAN J, GUO G, ORLOV YL, HUSS M, LOH Y-H, YAW L-P, ROBSON P, LIM B, NG H-H. Eset partners with Oct4 to restrict extraembryonic trophoblast lineage potential in embryonic stem cells. Genes Dev. 2009;23:2507–2520. doi: 10.1101/gad.1831909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUEN RKC, CHEN B, BLAIR JD, ROBINSON WP, NELSON DM. Hypoxia alters the epigenetic profile in cultured human placental trophoblasts. Epigenetics. 2013;8:192–202. doi: 10.4161/epi.23400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAMUDIO S. The placenta at high altitude. High Alt Med Biol. 2003;4:171–191. doi: 10.1089/152702903322022785. [DOI] [PubMed] [Google Scholar]
- ZHANG J, CHEN Z, SMITH GN, CROY BA. Natural killer cell-triggered vascular transformation: maternal care before birth? Cell Mol Immunol. 2011;8:1–11. doi: 10.1038/cmi.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU S, XIE Y, PUSCHECK EE, RAPPOLEE DA. Oxygen levels that optimize TSC culture are identified by maximizing growth rates and minimizing stress. Placenta. 2011;32:475–481. doi: 10.1016/j.placenta.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU Y, CHIU K, BRESCIA RJ, COMBS A, KATZ MA, KITZMILLER JL, HEILBRON DC, FISHER SJ. Increased depth of trophoblast invasion after chronic constriction of the lower aorta in rhesus monkeys. Am J Obstet Gynecol. 1993;169:224–229. doi: 10.1016/0002-9378(93)90172-f. [DOI] [PubMed] [Google Scholar]
- ZHU D, HÖLZ S, METZGER E, PAVLOVIC M, JANDAUSCH A, JILG C, GALGOCZY P, HERZ C, MOSER M, METZGER D, GÜNTHER T, ARNOLD SJ, SCHÜLE R. Lysine-specific demethylase 1 regulates differentiation onset and migration of trophoblast stem cells. Nat Commun. 2014;5:3174. doi: 10.1038/ncomms4174. [DOI] [PubMed] [Google Scholar]