Abstract
Thalamocortical axons must cross a complex cellular terrain through the developing forebrain and this terrain has to be understood for us to learn how thalamocortical axons reach their destinations. Selective fasciculation, guidepost cells, various diencephalic and telencephalic gradients have been implicated in thalamocortical guidance. As our understanding of the relevant forebrain patterns has increased, so has out knowledge of the guidance mechanisms. Our aim here is to review recent observations of cellular and molecular mechanisms relating to: the growth of thalamofugal projections to the ventral telencephalon, thalamic axon avoidance of the hypothalamus and extension into the telencephalon to form the internal capsule, the crossing of the pallial-subpallial boundary and the growth towards the cerebral cortex. We shall review current theories for the explanation of the maintenance and alteration of topographic order in the thalamocortical projections to the cortex. It is now increasingly clear that several mechanisms are involved at different stages of thalamocortical development and each contributes substantially to the eventual outcome. Revealing the molecular and cellular mechanisms can help to link specific genes to details of actual developmental mechanisms.
Keywords: thalamocortical projections, ventral thalamus, corridor cells, perireticular thalamic nucleus, handshake hypothesis, subplate neurons
1. General Introduction
In 2011 the “handshake hypothesis” celebrated its 21st birthday. This hypothesis was formulated by Blakemore and Molnár (1990) as a way to explain how ascending thalamic axons navigate to their appropriate cortical targets with help from reciprocal descending cortical axons (Molnár and Blakemore, 1995). It was based on in vivo observations demonstrating an intimate anatomical relationship between developing thalamic and early cortical axons (Molnár et al., 1998a,b) and on in vitro findings that cortical explants from different regions to accept innervations from any region of the thalamus (Molnár and Blakemore, 1991). It has been suggested that a mechanism such as guidance from descending axons that is present in vivo, but disrupted in explant culture, might be necessary to achieve specific patterns of thalamocortical connectivity (Molnár and Blakemore, 1991; Molnár and Blakemore, 1999). The original formulation of the hypothesis stated that ‘…the descending and ascending axons each pioneer the pathway through their own segment of the brain and, after a “handshake” near the internal capsule, each may guide the growth of the other over the distal part of its trajectory…’ (Molnár and Blakemore, 1991).
However, this hypothesis was not supported by several observations. Some of the major objections are related the separate route of the thalamic and corticofugal projections observed in adult. Indeed, the layer 6 and layer 5 projections take separate routes between any one thalamic nucleus and its cortical areas, and each component involves complex crossing that appear to occur at different sites for the thalamocortical and the corticothalamic components (Adams et al., 1997). We have very limited information about these points in the adult, and need more tracing studies on single cell level to resolve them (Lozsádi et al., 1996; Grant et al., 2012). Furthermore, there is great deal of difference between layer 5 and layer 6 cell axons that target the core and the matrix thalamic neurons in the adult (Jones, 2001; Jones, 2007; Sherman and Guillery, 2005; Sherman, 2007). The handshake hypothesis only accounted for the earliest corticofugal projections and the thalamic projections and their encounter in the internal capsule at the time of crossing the pallial subpallial boundaries. However, even these early interactions were questioned in several tracing studies (Bicknese et al., 1994; Miller et al., 1993) and in dissociated cultures thalamic and cortical growth cones often extended along their kind and after contacts between cortical and thalamic fibers, in most cases growth cones collapsed and retracted from the axons (Bagnard et al., 2001). However, the co-fasciculation of the early thalamic and early corticofugal projections have been demonstrated in mouse and rat (Molnár et al., 1998a,b) and the close association of these fibres were apparent in the fascicles crossing the embryonic cortical plate in the reeler mutant (Molnár et al., 1998b). The relationship between thalamic and early corticofugal projections has not been observed in organotypic cultures in their natural environment.
Over the last 21 years, the handshake hypothesis has remained influential because of its attractive simplicity, whilst being challenging to test experimentally. Since the thalamocortical projections traverse the entire telencephalon, a better understanding of patterning within the diencephalon and telencaphalon was required to reveal possible guidance mechanisms. We now know that thalamic and early corticofugal projections are not pioneering their own growth towards the internal capsule, they are aided by other cells with projections (in pre thalamus, internal capsule) or with migratory paths defined by corridor cells. Nevertheless, the interactions between early corticofugal projections and thalamic fibres at the pallial subpallial boundary are still postulated to explain various phenotypes in mouse knockouts (Hevner et al., 2002; López-Bendito and Molnár, 2002). Many of these questions were not readily testable experimentally 21 years ago. However, with the generation of mouse lines that express reporter genes in selected cell groups (Jacobs et al., 2007; Piñon et al., 2009), and the increased understanding of selective gene expression patterns of subplate and other cell populations (Ayoub et al., 2009; McKellar and Shatz, 2009; Osheroff and Hatten, 2009; Hoerder-Suabedissen et al., 2009; Oeschger et al., 2012) we now have the chance to revisit this issue. Moreover, our understanding of the molecular and cellular aspects of the telencephalic development is also increasing rapidly and with this background, we can refine our questions on axon guidance. We have come a long way since the handshake hypothesis was first suggested but, despite new knowledge of many additional mechanisms, the idea still retains considerable importance for a particular stage and segment of thalamocortical development (Fig. 1A and B).
Figure 1.
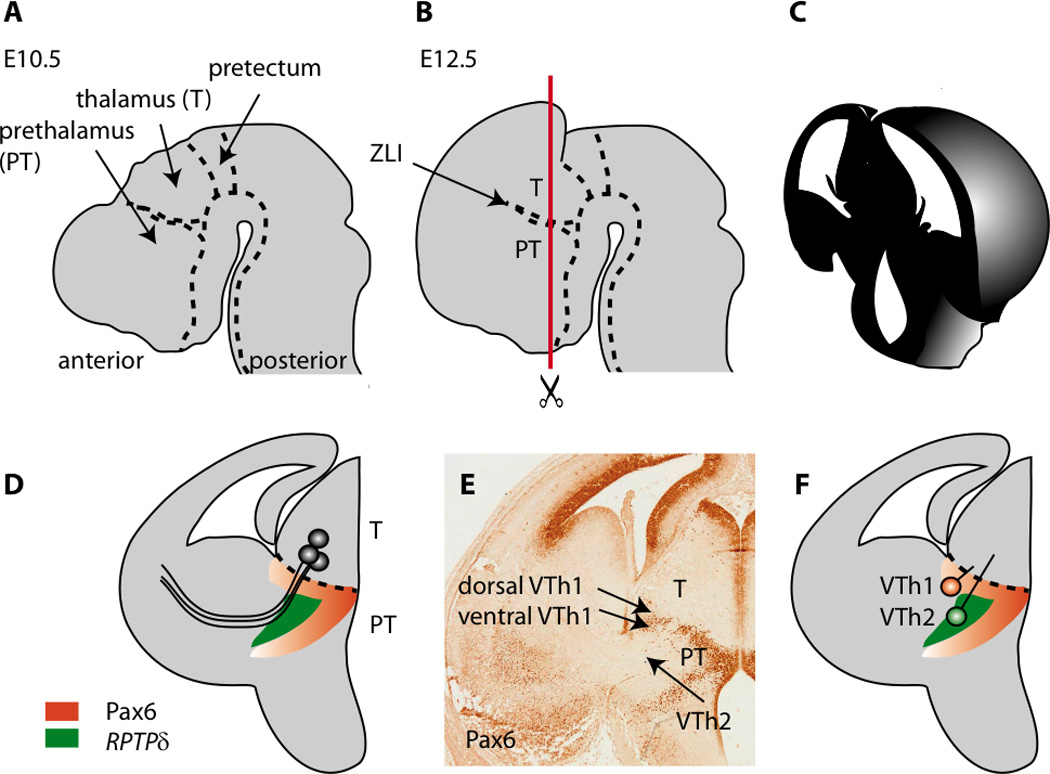
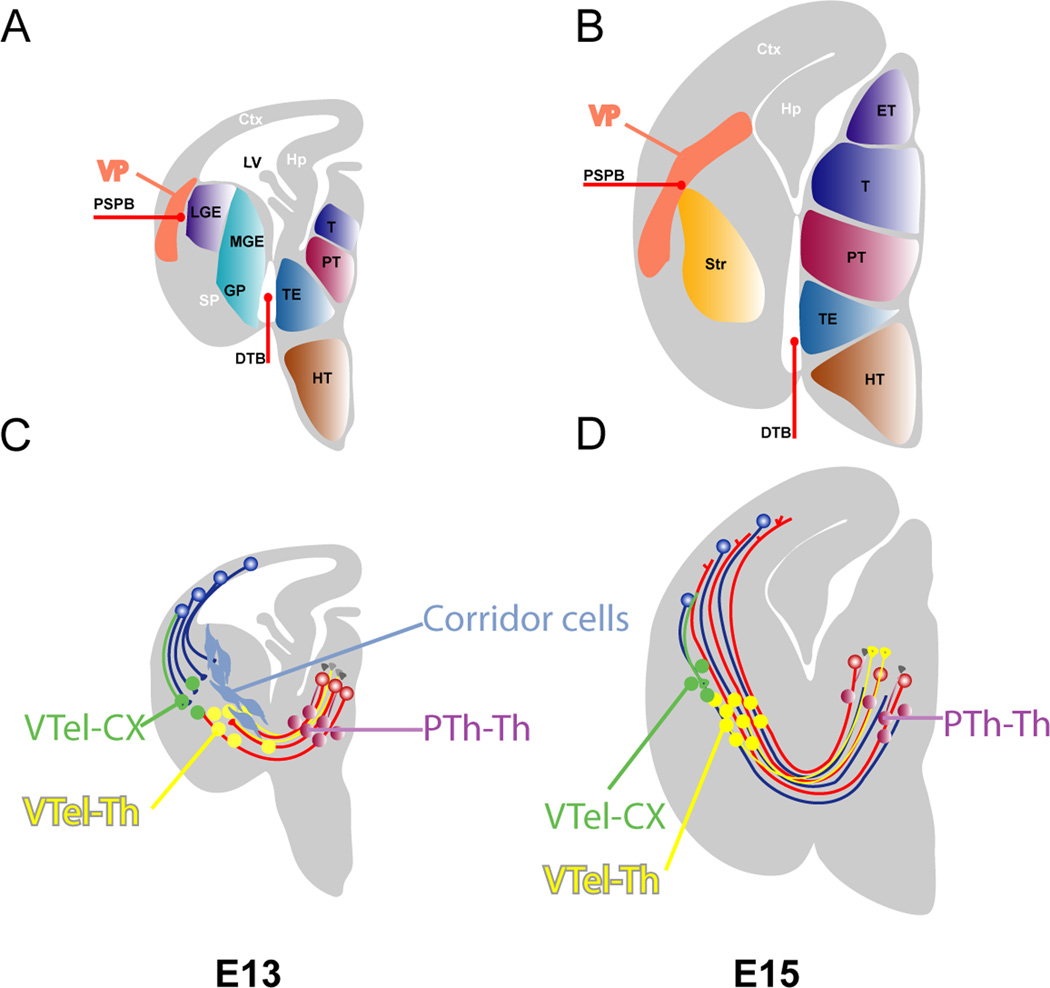
The anatomy of the developing forebrain and the location of prethalamic cell groups providing guidance for thalamocortical axons in embryonic mouse brain. (A) A sagittal view of the brain around E10.5 showing the pretectal, thalamic and prethalamic anlagen. (B) By E12.5 the telencephalic vesicles expand over the diencephalon; note that the prethalamus (PT) lies anterior to the thalamus (T). These two structures are separated by the zona limitans intrathalamica (ZLI). (C) The appearance of the forebrain when cut as shown by the red line in B at E14 (D) Thalamocortical axons grow from the thalamus, through the prethalamus and into the telencephalon. The prethalamus contains cells that express the markers Pax6 and RPTPδ (Tuttle et al., 1999). (E) An example of a section stained with an antibody for the Pax6 transcription factor (E14). The positions of prethalamic groups of cells proposed by Tuttle et al. (1999) to project to the thalamus and provide guidance to thalamocortical axons are shown. These groups were originally called VTh1 and VTh2, with VTh1 split into a dorsal and a ventral domain. The dorsal domain of VTh1 expresses a low level of Pax6, whereas the ventral domain of VTh1 expresses a high level of Pax6. (F) VTh2 does not express Pax6 but does express RPTPδ.
2. Molecular patterning of the early thalamus
The thalamic region of the diencephalon comprises three functionally distinct zones, the prethalamus, the thalamus proper and the pretectum that extends in a rostro-caudal fashion (Puelles and Rubenstein, 2003; Larsen et al., 2001). Traditionally, the thalamus was described as having two major components: a ventral thalamus and a dorsal thalamus, with the latter being the component that processes and relays most sensory information from the periphery to the cerebral cortex. This nomenclature is confusing since the ventral thalamus in fact lies rostral to the dorsal thalamus along the curved axis of the neural tube. In recent years, in developmental studies it has become increasingly common to use the terms prethalamus and thalamus to describe the ventral thalamus and dorsal thalamus respectively (Puelles and Rubenstein, 2003). A major advantage of this prethalamus/thalamus nomenclature is that the regional descriptors ‘ventral’ and ‘dorsal’ can then be applied in their descriptive meaning without confusion. As such we adopt this nomenclature here.
The mammalian thalamus is composed of dozens of morphologically and functionally distinct nuclei (Jones, 2007). Some of these nuclei project topographically to specific areas of the cortex relaying sensory input from the periphery and playing a critical role in sensory functions (Jones, 2001; Sherman and Guillery, 2011; Clascá et al., 2009). In contrast, the prethalamus, comprising of the zona incerta, reticular nucleus and ventral lateral geniculate nucleus (vLGN), does not project to the cortex (Jones, 2007). The Zona Limitans Intrathalamica (ZLI) separates the prethalamus and the thalamus.
The thalamus develops from neural progenitor cells located within the p2 domain of the alar plate of the caudal diencephalon between embryonic day (E) 10.5 and E16.5 (Angevine, 1970; Puelles and Rubenstein, 1993; Puelles and Rubenstein, 2003). Although recent studies have identified molecules that may influence the patterning of the diencephalon, it has remained largely unknown how the distinct, postmitotic thalamic nuclei emerge from discrete developmental units (Scholpp and Lumsden 2010). As occurs in the neocortex and other brain regions, molecules secreted by signalling centres between tissue compartments organise the patterning and growth of specific tissues. The ZLI expresses members of the Sonic hedgehog (Shh) signal molecule family, together with other secreated factors such as Wnts and fibroblast growth factors (FGFs), and has been demonstrated to act as a local organizer for thalamic development. Although Wnt signalling is important for setting up the initial antero-posterior regionalization (Salinas and Nusse, 1992; Murray et al., 2007; Quinlan et al., 2009), it remains unknown whether this is directly required for thalamic specification. FGF signalling has also been implicated in organizing diencephalic development. Fgf15 and Fgf19 have been shown to function downstream of Shh in the thalamus and are therefore implicated in some aspects of thalamic development (Miyake et al., 2005; Gimeno and Martinez 2007). On the basis of elegant in utero manipulations in the thalamus, recent reports have added Fgf8 activity to this scenario and have shown that Fgf8 activity controls the patterning of thalamic nuclei (Kataoka and Shimogori, 2008).
Nevertheless, several studies have shown that Shh is the principal requirement for cell fate specification during thalamic development. Indeed, there are at least three Shh-dependent steps in patterning of the thalamic Anlage. These include the induction of specific sets of transcription factors, through which Shh determines cell specification during thalamic development (Scholpp and Lumsden 2010). Moreover, elimination of Shh activity in both chick and zebrafish results in the loss of genetic fate determinants and cell identity in both the prethalamus and the thalamus (Scholpp et al., 2006; Kiecker and Lumsden 2004). A recent study has determined that ectopic activation of the Shh signalling pathway induces the expression of thalamic markers such as Gbx2, Olig2, Neurog2 and Olig3 in the mouse pretectum demonstrating that Shh plays a crucial role in patterning thalamic progenitor domains (Vue et al., 2009).
3. Transcriptional control of thalamocortical axon (TCA) guidance
Several transcription factors (TFs) are expressed in distinct yet often overlapping patterns in the thalamus, suggesting that they cooperate to control the specification and differentiation of thalamic nuclei and cell types. One of the first attempts at looking at the cell-autonomous role of TFs in thalamocortical pathfinding was the work by Pratt and colleagues (Pratt et al., 2000; Pratt et al., 2002). They showed that the development of the thalamus is compromised in Pax6−/− embryos and that the thalamus exhibits abnormalities of differentiation and of the projection of axons (also see Jones et al., 2002). Gbx2 is expressed broadly early in the thalamus (Bulfone et al., 1993) and later it is required for the differentiation of a subset of nuclei and the development of TCA projections (Miyashita-Lin et al., 1999; Jones et al., 2002). A recent study has demonstrated that Gbx2 plays a cell-nonautonomous role in controlling the segregation of postmitotic thalamic neurons from the neighboring brain structures that do not express Gbx2 (Chen et al., 2009). Another key piece of work on the transcriptional control of TCA pathfinding came from the study by Seibt and colleagues demonstrating that the bHLH transcription factor Neurogenin2 cell autonomously specifies the projection of thalamic neurons to frontal cortical areas (Seibt et al., 2003). Ngn2-knockout mice are characterized by a targeting shift in the TCA projections that occurs initially in the ventral telencephalon (VTel) (Seibt, Schuurmans et al. 2003), suggesting that Ngn2-regulates the guidance receptors in these axons that read ventral telencephalic cues. However, to date no downstream targets of Ngn2 have been identified.
It remains unclear how distinct pools of thalamocortical projecting neurons are topographically specified, and which transcription factors regulate the growth of their axons (Shimogori and Grove, 2005; López-Bendito and Molnár, 2003; Price et al., 2006). Transcription factors expressed in post-mitotic neurons are responsible for specifying neuronal identity and for activating specific axon guidance programs in other neuronal pathways. Genetic studies in mice have demonstrated the role of the LIM homeodomain (LIM-HD) proteins in determining the identity of motor neurons (Jurata, Thomas et al. 2000; Kania, Johnson et al. 2000; Lee and Pfaff 2001; Kania and Jessell 2003). Moreover, a specific combination of transcription factors from the LIM homeodomain family regulates the topographic targeting of distinct pools of axons to specific muscles in the limb mesenchyma (Sharma, Sheng et al. 1998; Kania, Johnson et al. 2000). In recent years, several candidate genes have been identified as potential downstream effectors of these transcription factors. For example, Lim1 expression in lateral motor column neurons in the spinal cord regulate the expression of the tyrosine kinase EphA4, a protein essential for the final targeting of axons to the limb (Kania and Jessell 2003). Similarly, the transcription factor Zic2 regulate midline crossing by retinal axons in conjunction with another member of the Eph family, EphB1 (Lee, Petros et al. 2008; García-Frigola and Herrera 2010). These molecular pathways may be important for thalamocortical pathfinding too.
In the thalamus, the Lhx2 transcription factor is a member of the LIM-HD family of proteins, and is strongly expressed during development (Retaux, Rogard et al. 1999; Nakagawa and O’Leary 2001). Severe thalamocortical pathfinding defects have been described in Lhx2 null mice (Lakhina, Falnikar et al. 2007), implicating this transcription factor in the guidance of these axons. However, the death of these mice at early embryonic stages precludes in vivo studies of the role of Lhx2 in later aspects of TCA connectivity. As Lhx2 is also expressed in other forebrain areas, such as the neocortex, it is essential to restrict the loss of Lhx2 to thalamic neurons in order to determine the role of this transcription factor in thalamocortical development precisely. Disrupting Lhx2 regulatory activity only in thalamic neurons leads to axonal pathfinding defects in TCAs, with fewer axons ultimately reaching their cortical targets (Marcos-Mondejar et al., 2012).
Mice deficient in Robo1, Robo2 or both, display prominent defects in TCA guidance during development, including abnormal axonal invasion of the hypothalamus (Andrews, Liapi et al. 2006; López-Bendito, Flames et al. 2007). Overexpression of Lhx2 in rostral and intermediate thalamic neurons by in utero electroporation results in the abnormal invasion of the hypothalamus by electroporated axons (Marcos-Mondejar et al., 2012). Moreover, this study demonstrates that Lhx2 is a direct repressor of Robo1 and Robo2 receptors, since their thalamic expression is altered in the absence of this transcription factor. The list of transcription factor pathways involved in the early differentiation of the thalamus and the early guidance of TCAs is impressive, but it is most probably far from complete.
4. Guidance from the thalamus to the subpallium
4.1 The role of prethalamic and ventral telencephalic projections to the thalamus in the early guidance of TCAs
The molecular mechanisms that guide the first axons from the thalamus and into the prethalamus, which they must cross to access the border between the diencephalon and the telencephalon, are poorly understood. Coordinated control of the polarity of newly differentiating thalamic neurons might ensure that the first axonal extensions grow towards the boundary of the thalamus and prethalamus, but it is also likely that projections from the prethalamus to the thalamus (abbreviated here as PTh-Th projections; Box 1 and Table 1) and projections from the ventral telencephalon to the thalamus (abbreviated here as VTel-Th projections) provide guidance (Fig. 2). A study by Métin and Godement (1996) in hamster showed that as axons grow from the thalamus and intermingle with reciprocal projections from the prethalamus (PTh-Th) and ventral telencephalon (VTel-Th) to the thalamus. Equivalent prethalamic neurons were subsequently discovered in rat embryos (Molnár et al. 1998a; Molnár and Cordery, 1999), in a region described by Mitrofanis (1992) as the perireticular nucleus (Fig. 2). Braisted et al (1999) also examined this region in embryonic mice, suggesting that VTel-Th neurons project axons into the thalamus at around the time at which the first thalamocortical axons reach the ventral telencephalon (E13–14). These authors suggested that the VTel-Th neurons probably belong to the globus pallidus rather than being perireticular cells. This suggestion stemmed from the fact that perireticular cells were retrogradely labelled from the thalamus in postnatal but not embryonic rats (Mitrofanis and Baker, 1993) and ferrets (Mitrofanis, 1994a,b). Despite these and other differences in the details of the various studies, the spatial and temporal features of the axonal projection of the VTel-Th neurons are consistent with the idea that this axonal projection, and possibly the cell bodies themselves, may act as a scaffold to guiding thalamocortical axons through the developing prethalamus and towards the diencephalic-telencephalic border or other axons in the opposite direction or perhaps both. Consistent with this hypothesis, in Ascl1−/− and Pax6−/− embryos this population of ventral telencephalic VTel-Th cells appears to be missing and thalamocortical axons fail to extend into the ventral telencephalon (Tuttle et al., 1999; Pratt et al., 2002; Figure 3). There is a decrease in number and a displacement of these cells in Lhx2−/− and Emx2−/− mutants respectively and this is associated with guidance defects of thalamocortical axons (Tuttle, Nakagawa et al. 1999; Bishop, Goudreau et al. 2000; López-Bendito, Chan et al. 2002; Bishop, Garel et al. 2003; Lakhina, Falnikar et al. 2007).
Box 1. Complexities of the Current Nomenclature.
Neurons that cross the early diencephalic and telencephalic subdivisions
The thalamocortical and corticofugal projections start to develop while the various sectors of the telencephalon and diencephalon are being generated. Numerous cell groups migrate tangentially (perpendicular to the orientation of the radial glia) from the pallidum, from LGE, MGE, CGE (De Carlos et al., 1996; Parnavelas, 2000; Marín and Rubenstein, 2002); there are streams of cells leaving the LGE and tangentially migrating along the ventral pallium to enter the diencephalon (López-Bendito et al., 2006).
In addition to these tangentially migrating neuronal populations, there are several precocious groups of neurons in the diencephalon and the telencephalon whose axons and possibly cell bodies provide guidance for thalamic axons. These cell groups have mostly been identified by their connectivity (Métin and Godement, 1996; Mitrofanis and Guillery; 1993; Molnár et al., 1998a,b; López-Bendito et al., 2006). Unfortunately they have not been named consistently in the literature. We shall propose a simple nomenclature based on abbreviations for the site of the cell bodies and their target tissue: for example, a transient axonal projection from the ventral telencephalon to the thalamus is called VTel-Th, one from the ventral telencephalon to the cortex is called VTel-Cx and one from the prethalamus to the thalamus is called PTh-Th (see Table 1; Fig. 2). In this way we are not confined by insufficient knowledge of their origin, gene expression or other little known factors.
VTel-Th: Ventral telencephalon to the thalamus
VTel-Cx: Ventral telencephalon to the cortex
PTh-Th: Prethalamus to the thalamus
Table 1.
Suggested nomenclature for
| CELL GROUP | SYNONYMOUS NAME |
REFERNCES |
|---|---|---|
| Neurons with projections from prethalamus to the thalamus (PTh-Th) |
TRN Ventral thalamus |
Mitrofanis and Guillery (1993) Métin and Godement (1996) Molnár et al., 1998a,b; Molnár and Cordery, (1999) Tuttle et al., (1999) Mitrofanis (1992) as the perireticular nucleus. Braisted et al (1999) |
| Neurons with projections from ventral telencephalon to the thalamus (VTel-Th) |
Perireticular cell Perireticular nucleus Internal capsule guidepost cells |
Mitrofanis and Guillery (1993) Métin and Godement (1996) Molnár et al., (1998a,b); Molnár and Cordery, (1999) Tuttle et al., (1999) Mitrofanis (1992) as the perireticular nucleus. Braisted et al (1999) |
| Neurons with projections from ventral telencephalon to cortex (VTel-Cx) |
Nucleus basalis Métin and Godement, (1996) and Adams and Baker, 1995 described numerous cells from the pallidum with projections to the cortex, they associated these labelled cells with the perireticular nucleus (Admas and Baker, 1995). |
Adams and Baker, (1995) Coleman and Mitrofanis, (1999) Métin and Godement (1996) |
| Corridor cells | The adult equivalent of these cells is not clear. We do not know the proportions of the surviving cells. The distinctions between VTel-Cx from the corridor cells is not resolved. |
López-Bendito et al., (2006); Bielle et al., (2011a) |
Figure 2.
The position of various cell populations guide thalamic axons. A and B depict the various subdivisions in the diencephalon (ET - epithalamus, T-thalamus, PT-prethalamus, TE-thalamic eminence, HT-hypothalamus) and telencephalon (MGE-medial ganglionic eminence, GP-globus pallidus, LGE-lateral ganglionic eminence, PSPB-pallial subpallial boundary, VP-ventral pallium or SP-subpallium, CTX-cerebral cortex, Str-striatum) and their bounadary (DTB-diencephalic and telencephalic boundary).
C and D issultrates the early connectivity in the telencephalon and diencephalon. Prethalamic (PTh-Th) and ventral telencephalic (internal capsule, VThel-Th) cells with thalamic projections (purple and yellow respectively) are instrumental in the early thalamic axon guidance. Panel in C illustrates the migration of the corridor cells and their interactions with the thalamocortical projections. Corridor cells (light blue) originate from the lateral ganglionic eminence (LGE) at embryonic day 12 (E12) and migrate tangentially toward the diencephalon, where they form a permissive “corridor” for the thalamic projections (red) to navigate them through the internal capsule. Modified based on López-Bendito and Molnár (2003) and Hanashima et al., (2006).
Figure 3.
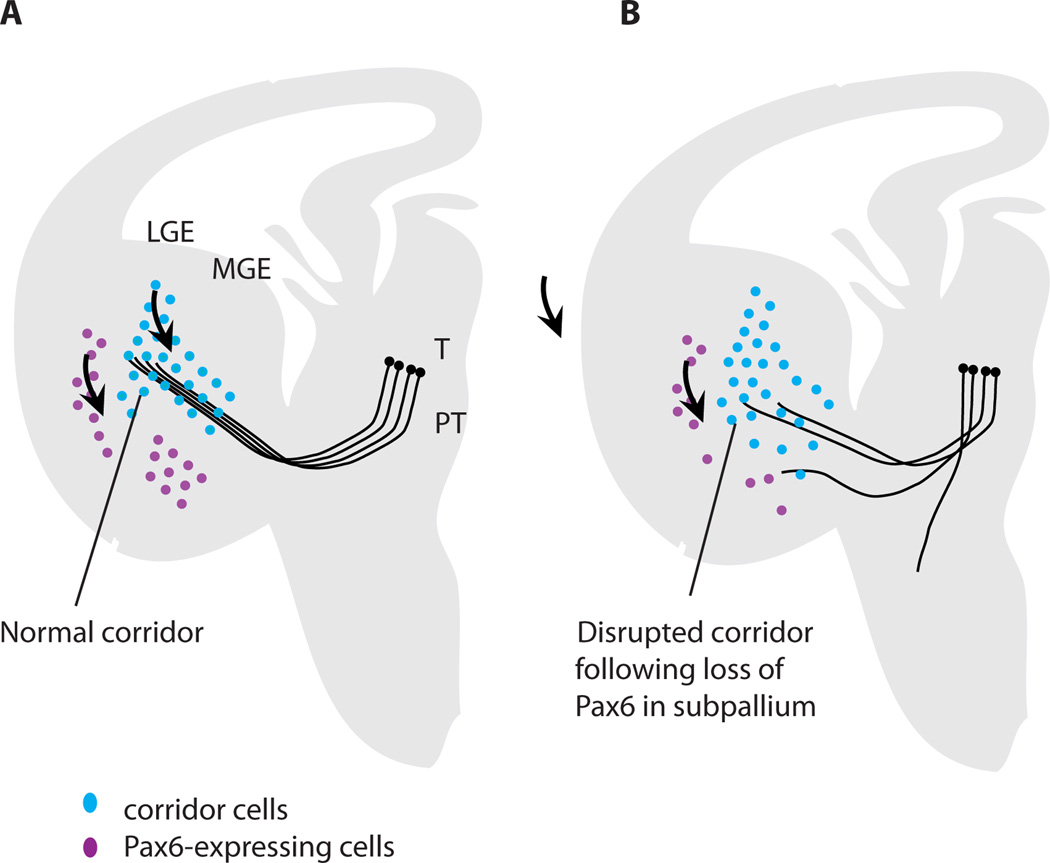
Evidence that Pax6 plays a role in corridor formation. (A) Normally, Pax6-expressing cells (purple) are located ventral to the corridor/ developing internal capsule in the medial ganglionic eminence (MGE); those located laterally comprise the lateral cortical stream migrating from the pallial-subpallial border (arrow). Islet1-expressing cells (green) migrate from the progenitor layer of the lateral ganglionic eminence (LGE; arrow) to form the corridor through which thalamocortical axons grow (arrow). (B) In conditional mutant embryos with selective reduction of Pax6 in specifically the ventral telencephalon but neither thalamus not cortex, there are fewer Pax6-expressing cells ventral to the corridor than normal (other populations of Pax6-expressing cells outside the region of Pax6 deletion are not shown since they are not affected). Cells from the lateral ganglionic eminence migrate to form a corridor that is abnormally broad with a lower peak density of Islet1-expressing cells; many Islet1-expressing cells stray into the area depleted of Pax6 expression. Many thalamic axons fail to enter this abnormal corridor or exit it along its length. Data are taken from Simpson et al. (2009).
A study by Tuttle et al. (1999) further subdivided the prethalamus to thalamus (PTh-Th) projections into two groups. Tuttle et al. (1999) named these VTh1 and VTh2, with VTh1 split into a dorsal and a ventral domain. The location of these groups is shown in Figure 1E and F. Their nomenclature is, of course, now confusing since VTh stands for ventral thalamus, and since “prethalamus” is preferred to “ventral thalamus” they might be better renamed PTh-Th1 and PTh-Th2. The dorsal domain of VTh1/ PTh-Th1 expresses a low level of the transcription factor Pax6, whereas the ventral domain of VTh1/ PTh-Th1 expresses a high level of Pax6; VTh2/ PTh-Th2 does not express Pax6 (Figure 1E). There is much less functional information on the possible roles of the PTh-TH groups in thalamocortical axonal guidance than on the VTel-Th cells.
In the case of both the PTh-Th and VTel-Th groups of axons, association between the loss of these cells and TCA pathfinding defects in mutants cannot be taken to imply causation. At present, we know very little about these projections: their embryological origins, molecular identities, fates and potential roles in TCA guidance remain to be determined. We do not have the tools to interfere selectively with their function since molecular markers that distinguish them have not been identified.
4.2 Repulsive activity from the hypothalamus
As thalamic axons traverse the prethalamus at E11–13 in mouse or E12–14 in rat, they grow in the direction of the hypothalamus before they turn laterally towards the internal capsule. Tuttle et al. (1999) showed cells in the hypothalamus with projections to the thalamus, but clearly these projections do not succeed in drawing the thalamic axons to the hypothalamus. On the contrary, thalamic axons turn very sharply away from the hypothalamus into the internal capsule in the direction of the diencephalic-telencephalic border. Several studies have shown that: (i) the hypothalamus expresses high levels of Slits, which are generally chemorepellent for growing axons: (ii) hypothalamic explants repel thalamic axons in explant cultures; (iii) in both Slit2−/− and Slit1−/−;Slit2−/− mutants, a large number of thalamic projections fail to enter the telencephalon and instead descend into the hypothalamus (Bagri et al., 2002; Braisted et al., 1999, 2009; López-Bendito et al., 2007; Bielle et al., 2011b). These findings provide quite compelling evidence that thalamic axons, which express Robo receptors through which Slits signal, deviate away from the hypothalamus and across the diencephalic-telencephalic boundary due to Slit-mediated repulsion.
5. The role of tangentially migrating “corridor” cells in delineating the internal path of TCAs
More recently, a distinct population of guidepost cells has been identified that controls the precise pathfinding of TCAs along an internal trajectory within the subpallium (Lopez-Bendito, Cautinat et al. 2006). These cells are GABAergic neurons that migrate tangentially from the lateral ganglionic eminence (LGE ) into the medial ganglionic eminence (MGE) and form a cellular “corridor” between the proliferative zones of the MGE and the globus pallidus (GP)(Figure 2; Box 1; Table 1; López-Bendito, Cautinat et al. 2006). Accordingly, they are located in the MGE, but express molecular markers of LGE-derived neurons, such as Islet1, Ebf1 and Meis2 and do not express MGE-molecular markers such as Nkx2.1. These neurons, named “corridor cells” migrate from E11.5 to E14 in the superficial mantle of the subpallium in a ventral direction, superficially to the large stream of MGE-derived interneurons that migrate towards the cerebral cortex. In vitro analysis in embryonic brain slices have shown that corridor cells constitute a permissive territory for the internal growth of TCAs through MGE-derived cell groups, which are otherwise non-permissive for TCAs. While the factors controlling the non-permissive activities of MGE-derived territories remain to be determined, corridor cells were shown to express a membrane-bound isoform of Neuregulin1. TCAs express the Neuregulin1 receptor ErbB4 and gain-of-function experiments in embryonic slices as well as telencephalic conditional Neuregulin1 mutants and constitutive ErbB4 mutants indicate that this signaling pathway regulates the pathfinding of TCAs throughout the corridor. These findings show that the migration of corridor neurons generates a Neuregulin1 permissive domain essential for the internal pathfinding of TCAs within the subpallium. As such, corridor cells are immature neurons that act via contact or a short-range activity to position an axonal tract and constitute bona fide guidepost cells. Interestingly, these corridor cells are conserved in diverse species and show distinct positioning that could underly evolutionary changes in the positioning of TCA in the subpallium (Bielle et al., 2011a).
What is the relationship between corridor cells and perireticular/internal capsule cells? These two proposed guidepost cell populations are not located in exactly similar regions as some perireticular cells are in the prethalamus or in its vicinity, while corridor cells are in the MGE (Table 1; Fig. 2). Accordingly, perireticular cells have been proposed to regulate the entrance of TCAs into the subpallium (Métin and Godement, 1996; Molnár et al., 1998a,b; Molnár and Cordery, 1999; Tuttle et al., 1999) whereas corridor cells orient the internal pathfinding of TCAs inside the MGE (López-Bendito, Cautinat et al. 2006). However, some back-labeled neurons from thalamus are also found in the corridor and LGE, raising the possibility that perireticular and corridor cells may be related to some extent (Fig. 2). Further analyses is needed for the determination of the molecular identity of back-labeled cells in the internal capsule, and thereby reveal whether some corridor cells may settle in that region and act by axon-mediated contact.
6. Guidance of TCAs across the ventral telencephalon
6.1 The subpallium is a main intermediate target for TCAs
In contrast to the hypothalamus, the subpallium exerts an attraction for TCAs and corticofugal axons and constitutes a main intermediate target for these projections (Métin and Godement 1996; Braisted, Tuttle et al. 1999; Garel and Rubenstein, 2004). Analyses of mice in which mouse mutations the regionalization and development of the subpallium has been affected (Marín and Baker, 2002) have started an assessment of the relative importance of the LGE and MGE in corticofugal and TCA pathfinding. In particular, mutatations affecting the development of the LGE, such as Ebf1 or Gsh1−/−;Gsh2−/− double mutants, severely impair TCA navigation, in contrast to mutations that perturb MGE development, such as Nkx2.1 mutants (Garel, Marin et al. 1999; Sussel, Marin et al. 1999; Marin, Baker et al. 2002; Yun, Garel et al. 2003). As previously mentioned, in vitro experiments in embryonic brain slices have revealed that the globus pallidus and the MGE proliferative zones exert repulsive activities that are likely to channel TCAs along an internal route (López-Bendito, Cautinat et al. 2006).
In parallel to the identification of the structures regulating TCA pathfinding through the subpallium, several studies have been conducted to determine the molecular nature of the guidance mechanisms involved. In particular, analyses of mice carrying mutations for guidance cues or their receptors have implicated Netrin-1, Slit1 and Slit2 and their receptors Robo1 and Robo2 as well as Sema6A in the general pathfinding of TCAs in the subpallium (Braisted, Catalano et al. 2000; Leighton, Mitchell et al. 2001; Bagri, Marin et al. 2002; Bonnin, Torii et al. 2007; Lopez-Bendito, Flames et al. 2007; Powell, Sassa et al. 2008; Braisted, Ringstedt et al. 2009; Little, Lopez-Bendito et al. 2009). In addition, members of the protocadherin family were shown to play essential roles in TCA guidance and internal capsule formation (Tissir, Bar et al. 2005; Uemura, Nakao et al. 2007; Zhou, Bar et al. 2008; Zhou, Qu et al. 2009). In particular, Celsr3 is a seven-pass cadherin ortholog to drosophila flamingo, which acts both in the planar cell polarity pathway and in relation to neurite outgrowth and is widely expressed in the mantle of the telencephalon and forebrain. Its specific experimental inactivation in the subpallium and prethalamus severely impairs the formation of the thalamocortical connections: TCAs stall in the ventral subpallium just after crossing the telencephalic/diencephalic boundary, whereas corticofugal axons stall after crossing the pallial subpallial boundary in the proximal part of the LGE (Zhou, Bar et al. 2008; Zhou, Qu et al. 2009). These studies revealed an absolute requirement of Celsr3 expression by an intermediate target that acts at short-range, and also demonstrated in vivo a function of these intermediate targets. Constitutive mutants in the Frizzled3 gene that in Drosophila participates in the planar cell polarity pathway with flamingo, has a very similar phenotype in the pathfinding of the internal capsule, suggesting that the two genes also cooperate in mice during this major axonal wiring event (Wang, Thekdi et al. 2002; Wang, Zhang et al. 2006).
Collectively, these experiments show that the subpallium is a major intermediate target for TCAs, and that the LGE is particularly involved in their guidance. At the molecular level, a series of secreted and transmembrane molecules expressed in the subpallium contribute to TCA pathfinding and their inactivation in mice has provided definitive evidence for the in vivo requirement of this intermediate target.
6.2 Molecular determinants in the subpallium specify intermediate sorting of TCAs
As TCAs travel internally through the subpallium, they diverge rostro-caudally along a fan-like structure, allowing distinct thalamocortical // thalamofugal axons, already segregated inside the tract, to navigate toward different cortical areas. Analyses of mutant in which the development of the subpallium or thalamus has been affected have revealed that this initial topography is largely independent of cortical regionalization; instead it is chiefly controlled by information contained within the subpallium (Garel et al., 2002; Dufour et al., 2003; Garel et al., 2003; Seibt et al., 2003; Shimogori et al., 2004). At the molecular level, initial topographic sorting of pre-segregated TCAs inside the internal capsule is mediated by countergradients of distinct ligand/receptor systems expressed by TCAs and cells in the ventral telencephalon (Fig. 4). Key determinants of initial TCA divergence include EphrinAs/EphAs, Netrin-1/DCC/Unc5a–c, class III Semaphorins/Neuropilins, and the L1 family of cell adhesion molecules (L1-CAMs), which specify sorting of distinct TCA contingents (Vanderhaeghen and Polleux, 2004).
Figure 4.
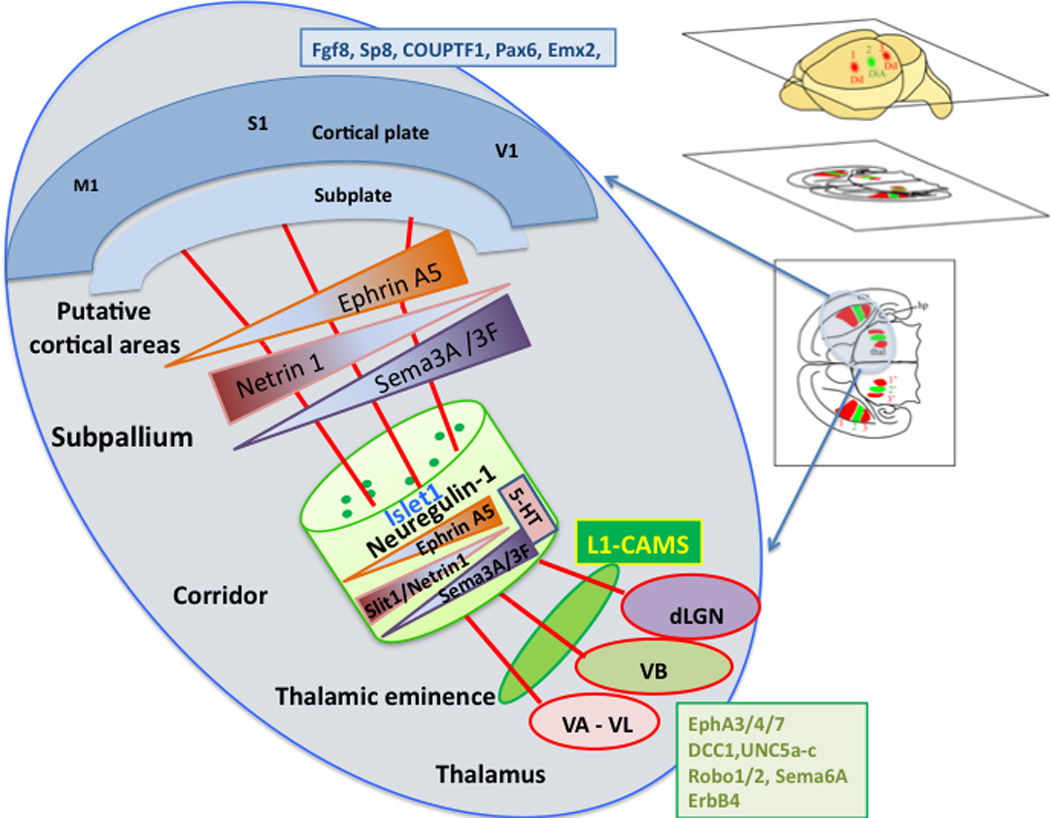
Scheme of thalamocortical axon trajectories from thalamic nuclei through the subpallium/ventral telencephalon to arrive to distinct neocortical areas.
Upper right panles: Schematic diagram illustrating multiple carbocyanine dye placements in the cerebral cortex positioned along an anterioposterior axis revealed the arrangements of backfilled dorsal thalamic neurons in a mediolateral fashion. The schematic panels indicate the appropriate sections with labelling. The right hemisphere is enlarged to illustrate some of the moleculas mechanisms that are involved in the guidance fo the thalamic axons across the thalamic eminence, corridor and subpallium to reach the appropriate regions in the cortex.
TCAs from different nuclei in the thalamus (VA/VL: ventroanterior/ventrolateral nuclei, VB: ventrobasal complex, dLGN: dorsal lateral geniculate nucleus), emerge at the thalamic eminence en route to the neocortex, and are sorted within a corridor of Islet1-positive cells in the subpallium/ventral telencephalon along the rostral to caudal axis (E13.5-E15.5 in the mouse). Within the corridor, TCAs expressing different combinations of axon guidance cue receptors (listed in the box within the dorsal thalamus) are guided by gradients of repellent and attractant cues (EphrinA5, Netrin1, Semaphorin3A, 3F, Slit1), influenced by Neuregulin-1 and serotonin (5-HT, 5-hydroxy tryptamine). In the ventral pallium, with the exception of Slit1, similar gradients are present. The thalamic axons target cortical areas that will contribute putative primary motor cortex (M1), somatosensory cortex (S1), and visual cortex (V1), but their entry to the cortex is regulated by subplate. The some of the various grandients in subplate and cortical plate are listed (Fgf8, Sp8, COUPTF1, Pax6, Emx2). Within the neocortex additional molecular cues and activity- dependent mechanisms promote the final synaptic targeting of TCAs. This simple initial topography can be considerably rearranged at the time of entry to the cortical plate.
Appropriate targeting of motor thalamic axons from the ventrolateral (VL) nucleus of the dorsal thalamus to the primary motor cortex (M1) is enabled by repellent TCA guidance in the subpallium mediated by countergradients of ephrinA5/EphAs. TCAs in the rostral dorsal thalamus express high levels of EphA4 and EphA7 receptors and are repelled from a high-caudolateral to low-rostromedial gradient of EphrinA5 expressed in the subpallium (Dufour, Seibt et al. 2003; Egea, Nissen et al. 2005; Fig. 4). In mice deficient in EphA4, EphA7, or both EphrinA5 and EphA4, contingents of VL axons become shifted caudally in the subpallium and misproject to S1 (Dufour, Seibt et al. 2003; Dufour et al., 2006). EphrinA5 knockout mice also show a caudal misprojection of a portion of afferents from the laterodorsal thalamic nucleus to S1 (Uziel et al., 2002). In vitro studies indicate that ephrinA5 can act as a repellent (Gao et al., 1998) or attractant cue for different populations of thalamic and cortical axons (Castellani et al., 1998; Mann et al., 2002). Within the cortex, deletion of EphrinA5 decreases the arborization of thalamic axons (Uziel et al., 2008), and may promote compensatory dendritic branching of thalamocortical recipient cells, as shown for spiny stellate cells in layer 4 of S1 (Guellmar et al., 2009). Netrin-1 provides a counterforce to EphrinA5-induced TCA repulsion, playing a dual role in attracting rostral TCAs and repelling caudal TCAs (Braisted, Catalano et al. 2000; Bonnin, Torii and Levitt, 2005; Powell, Sassa et al. 2008; Fig. 4). The opposing responses are mediated by different expression levels of Netrin-1 receptors DCC (deleted in colorectal carcinoma) and Unc5a,c on TCAs, and modulated by serotonin (5-hydroxytryptamine; 5HT)(Bonnin, Torii et al. 2007).
L1-CAMs (L1, Close Homolog of L1 (CHL1), and Neuron-glial related CAM (NrCAM)) are immunoglobulin-class axon guidance molecules that regulate pathfinding of TCAs by mediating repellent responses to gradients of EphrinAs and class III Semaphorins (Sema3A–G) (Maness and Schachner 2007). Sema3s are secreted ligands that promote axon repulsion or attraction by binding Neuropilin-1/2 receptors (Npn-1/2). Npn-1/2 receptors recruit PlexinA subunits (PlexA1–4) and activate Rac1-GTPase intrinsic to PlexinAs (Tran, Kolodkin et al. 2007; Pasterkamp and Giger 2009). In turn, Rac1 capable of inducing repellant responses in growthcones by promoting rearrangements of actin filaments. Deletion of CHL1 or Npn-1 in mice causes a caudal shift of axon contingents from ventrobasal complex (VB) within the subpallium, resulting in mistargeting to primary visual cortex (V1) (Gu, Rodriguez et al. 2003; Wright, Demyanenko et al. 2007). CHL1 normally binds Npn-1 to enable repellent guidance from the caudal-high gradient of Sema3A in the ventral telencephalon so that TCAs correctly target S1 (Wright, Demyanenko et al. 2007). Sema3A–induced growth cone collapse depends on binding of ezrin-radixin-myosin cytoskeletal adaptors to the CHL1 cytoplasmic domain (Mintz, Carcea et al. 2008; Schlatter, Buhusi et al. 2008). In an analogous mechanism, NrCAM and Npn-2 direct TCA contingents from more rostral thalamic nuclei (VA/VL) to M1(Demyanenko, Riday et al. 2011a). NrCAM associates with Npn-2, but not Npn-1, to mediate growth cone collapse to Sema3F, which is expressed in a caudal-high gradient in the subpallium. Sema6A, also functions in the TC projection, enabling dLGN axons to turn within the ventral telecephalon to enter the neocortex (Leighton, Mitchell et al. 2001). Functional consequences of mistargeting to incorrect cortical areas have been revealed in NrCAM null mice, which display impaired visual acuity and imparied binocular interactions due to impaired V1 cortical responses (Demyanenko, Riday et al. 2011a).
L1, like CHL1 (Wright, Demyanenko et al. 2007), binds Npn-1 required for growth cone collapse to Sema3A (Castellani, Chedotal et al. 2000). Unlike CHL1, deletion of L1 in mice does not alter area-specific topographic targeting of TCAs. However, when both L1 and CHL1 are deleted in mice, a more severe phenotype is observed (Demyanenko, Siesser et al. 2011b) in which TCAs from rostral (VA/VL) as well as VB nuclei mistarget to V1. The double mutant phenotype suggests a cooperative role for L1 and CHL1 in mediating repellent responses to Sema3A or to EphrinA5 (Demyanenko, Siesser et al. 2011b). L1 and CHL1 coimmunoprecipitate with the principal EphrinA5 receptors in the dorsal thalamus (EphA3, EphA4, and EphA7) and mediate ephrinA5-induced growth cone collapse (Demyanenko, Siesser et al. 2011). Why does genetic deletion of CHL1 (Wright, Demyanenko et al. 2007), NrCAM (Demyanenko, Riday et al. 2011a), L1/CHL1 (Demyanenko, Siesser et al. 2011b), or of their interacting partners Npn-1/2 and Sema3A/3F result in caudal misprojection of TCAs? Caudal misprojection of TCAs also occurs in mouse mutants deficient in EphA4/EphrinA5 (Dufour, Seibt et al. 2003), Netrin-1 (Powell, Sassa et al. 2008), and Sema6A (Little, Lopez-Bendito et al. 2009). One hypothesis is that caudal mistargeting of TCAs in the absence of caudal repellent cues may result from gradients of unidentified rostral repellents or caudal attractants in the ventral telencephalon.
The complex patterns of expression of Semaphorins, Ephrins, Netrins, their receptors, and L1-CAMs may serve to precisely direct TCA subpopulations to cortical targets (Wright, Demyanenko et al. 2007; Demyanenko, Riday et al. 2011a; Demyanenko, Siesser et al. 2011b). These ligand/receptor complexes may be localized in distinct subdomains of the growth cone membrane. Within these growth cone subdomains, downstream signaling from activated receptors may impinge asymmetrically on actin filaments (Zhang, Schaefer et al. 2003; Marquardt, Shirasaki et al. 2005; Burnette, Ji et al. 2008), resulting in localized retraction of filopodia and lamellipodia (Schaefer, Schoonderwoert et al. 2008), thus specifying directional navigation. An important goal for the future will be to identify the intracellular signaling pathways activated by each guidance receptor system at the crucial choice points along the thalamocortical pathway. Furthermore, it is likely that many more axon guidance cues and receptors will cooperate to guide TCAs at various decision points en route to the cortex.
While these guidance cues were initially proposed to act mainly in the LGE-derived striatum, in vitro experiments in slices have revealed that corridor neurons likely act proximal to the striatum in orienting TCAs along the rostrocaudal axis, where many of the guidance cues are also expressed (Bielle et al., in revision). In vivo and in vitro analyses of Slit1 and Robo1/2 mutant mice have confirmed that localized cues in the corridor act to orient pathfinding of intermediate and rostral axons, indicating that in this system, guidepost corridor neurons participate not only in the internal navigation of TCAs, but also to the formation of their fan-shaped topographic arrangement within the intermediate target (Bielle et al., 2011a; Bielle et al., 2011b).
Further studies investigating potential crosstalk among the different guidance signaling pathways, as well as the molecular mechanisms involved, may provide decisive information for understanding how TCAs are initially topographically ordered. Furthermore, how this intermediate subpallial topography interacts with positional information located in the neocortex to control the final spatial arrangement of TCAs remains to be explored. In the adult, the topographic order is not based on a single principle (outlined in Fig. 4), many thalamocortical interconnections involve mirror reversals between thalamus and cortex (Adams et al., 1997) suggesting substantial rearrangements at a later stage, closer to the termination sites. There are examples for such rearrangements in the adult (see Nelson and Levay, 1985; reviewed by Grant et al., 2012).
7. How thalamocortical axons enter the cerebral cortex
The boundary between the pallium and subpallium (the PSPB), which thalamocortical axons must cross to reach the cortex, is first established as a gene expression boundary. By the age at which thalamic axons approach the pallium (E13 for mouse, see Fig. 2C), the PSPB has developed a striking radial glial fascicle that runs across the trajectory of thalamocortical axons and it has a high density of cells, including those of the lateral cortical stream (LCS), which migrate across the path of thalamocortical axons (Carney et al., 2006; Carney et al., 2009; Chapouton et al., 2001). These features have been suggested to make this region relatively hostile to the passage of thalamic axons and that descending corticofugal axons from the cortex interact with ascending thalamic axons and assist them across this region (Molnár et al., 1998 a,b; Molnár and Butler, 2002). The pioneer corticofugal axons arrive to PSPB prior the thalamic axons and tracing studies suggest that at least the earliest cohort of these fibres can cross the PSPB without thalamic axons. It is possible that a breakdown of this interaction explains thalamocortical axon defects in some strains of mutant mice (Hevner et al., 2002; Jones et al., 2002; López-Bendito et al., 2002; López-Bendito and Molnár, 2003; Dwyer et al., 2011).
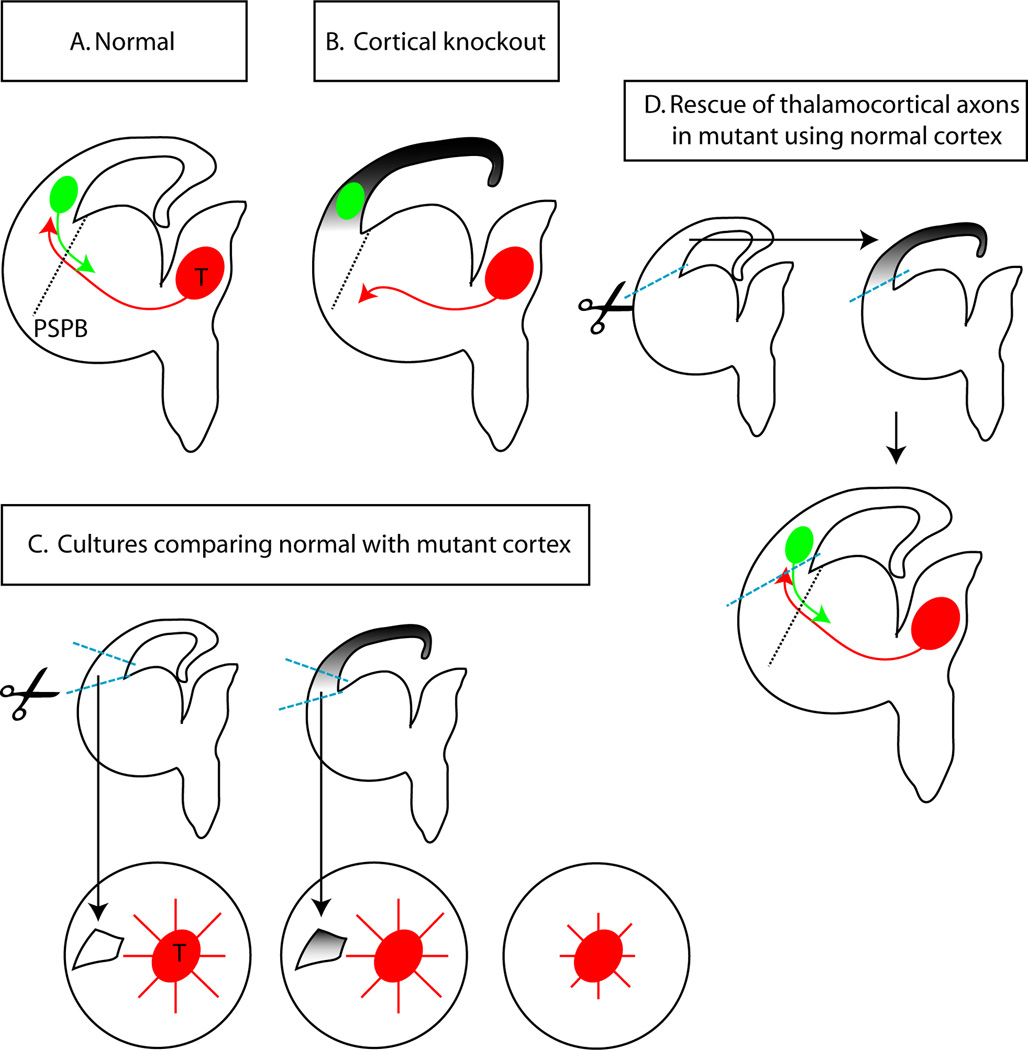
Recently, this hypothesis has been tested using conditional mutagenesis to assess the effects of blocking corticofugal axonal development without disrupting thalamus, subpallium or the PSPB in the Emx1Cre; APCloxP/loxP mutants (Chen et al., 2012). It was found that, while thalamic axons still traversed the subpallium in topographic order, they did not cross the PSPB (Figure 5B). Normal cortex and mutant cortex stimulate the growth of axons from the thalamus by equal amounts in culture experiments (Fig. 5C). This suggests that the inability of thalamic axons to cross the PSPB in Emx1Cre; APCloxP/loxP mutants is unlikely to be explained by long-range chemorepulsion by mutant cortex. By providing evidence against alternative explanations and by showing that replacement of mutant cortex with control cortex restored corticofugal efferents and allowed thalamic axons from conditional mutants to cross the PSPB (Fig. 4D), this work provided the most compelling evidence to date that cortical efferents are required to guide thalamocortical axons across the PSPB. The molecular mechanisms involved require further investigation. These studies are aided by our better understanding of embryonic subplate and thalamus gene expression patterns (Osheroff and Hatten, 2009; Oeschger et al., 2012).
Figure 5.
A summary of recent experiments testing the importance of corticofugal axons for thalamic axonal crossing of the PSPB carried out by Chen et al. (2012). (A) Normally, axons from the thalamus (T; red) cross the PSPB in close association with descending axons from the cortex (green). (B) In conditional Emx1Cre; APCloxP/loxP mutants, the development of cortical neurons and hence of corticofugal axons is blocked but, although the thalamus and ventral telencephalon are unaffected, thalamic axons do not cross the PSPB. (C) Culture experiments showed that both normal cortex and mutant cortex stimulate the growth of axons from the thalamus by equal amounts. This suggests that the inability of thalamic axons to cross the PSPB in Emx1Cre; APCloxP/loxP mutants is unlikely to be explained by long-range chemorepulsion by mutant cortex. (D) When normal cortex was substituted for mutant cortex in slice cultures from the brains of Emx1Cre; APCloxP/loxP embryos, corticofugal axons were restored and thalamic axons were able to cross the PSPB. These results provide evidence for the importance of corticofugal axons in allowing thalamic axons to cross the PSPB.
8. Guidance of TCAs within the cortex
Early topography during accumulation below the cortical plate
Thalamocortical projections arrive at the cerebral cortex prior to the birth of the majority of cortical neurons and prior to their migration is complete (Rakic, 1976; Shatz and Luskin, 1986). At this stage, the peak of cerebral cortical neurogenesis and neural migration, the cortical germinal- and intermediate-zones undergo highly dynamic changes. Meanwhile the cortical plate is increasing in thickness and new cells are added to it in an inside-first and outside-last fashion. The subplate zone, generated earliest, can be considered a relatively stable platform in the developing cortex during this period (Marin-Padilla, 1971; Lund and Mustari, 1977). The subplate zone contains postmigratory, mature neurons that are the first to express neuronal markers and develop functional synapses (Kostovic and Rakic, 1990; Molliver and Van der Loos, 1970; Friauf and Shatz, 1991; Higashi et al., 2001). The ingrowing thalamocortical projections start to accumulate in this zone for considerable periods depending on the species (Rakic, 1976; Shatz and Luskin, 1986; Catalano et al., 1991; Molnár et al., 1998a,b). Thalamic afferents overshoot their targets and develop transient side-branches on more proximal segments of their path through delayed branching (Naegele et al., 1988). These “side branches” within the intermediate zone and subplate extend over considerable distances and have been considered as the anatomical substrate for the rearrangements of cortical maps both during experimentally induced and normal development (Molnár et al., 2000; Shimogori and Grove, 2005). However, the mechanisms that deliver the thalamic projections and initiate their accumulation below the cortical plate are largely considered autonomous (Price et al., 2006). Studies in mice with the SNARE complex knocked out suggests that the early ingrowth of the thalamic axons does not depend on early neuronal communication transmitted through regulated or spontaneous vesicular release mechanisms (Molnár et al., 2002; López-Bendito and Molnár, 2003; Blakey et al., in this issue), but after this initial entry an activity dependent mechanism may start to dominate (Catalano and Shatz, 1998; Uesaka et al., 2006; Uesaka et al., 2007; Yamada et al., 2010).
Areal differences in the topographic organisation after thalamic fibre entrance to the cortex
Thalamic organisation is changing as the process of normal development; and it can be altered through the (i) manipulations of the early guidance mechanisms in the subpallium, (ii) through the manipulations of the early cortical regionalisation or (iii) changing the flow of sensory input from the sense organs.
While the subpallium controls the early guidance of TCA, cortical regionalization, which is controlled by the morphogen Fgf8 and gradients of transcription factors (Pax6, COUP-TFI, Emx2, Sp8) is sufficient to reorient the thalamocortical map within the neocortex (Rash and Grove, 2006; O’Leary and Sahara, 2008; Garel et al., 2003). The deployment and initial entry of thalamocortical projections to the subplate zone is considerably modified as the TCAs enter the cortical plate in cortical areas, such as the primary visual cortex of rodents (Krug et al., 1998; Naegele et al., 1988; Ravary et al., 2003). There are areal differences in the density, topographic precision and maturity of thalamocortical projections. Whereas thalamocortical axons undergo significant rearrangements in the cortex after entry into the primary visual cortex of rodents, in the rodent primary somatosensory cortex the topography is essentially established immediately after entry (Agmon et al., 1993; 1995). The period during which the thalamocortical projections can be re-arranged after sensory manipulations shows considerable variations. Altering early cortical gene expression patterns of Fgf8 imposes shifts or even two opposing cortical gradients with corresponding shifts and duplications of thalamocortical projections (Shimogori and Grove, 2005), whereas changes in Pax6 gradients failed to elicit substantial changes in thalamocortical topography (Piñon et al., 2008). Fgf8 gradient alterations can lead to duplication of the thalamic input from the same VB nucleus into multiple areas. The thalamocortical projections develop additional branches within the white matter, a region that corresponds with the location of subplate neurons (Shimogori and Grove, 2005). The earliest thalamocortical interactions and the eventual thalamocortical entry into the cortical plate is orchestrated by subplate (Allendoerfer and Shatz, 1994; Kanold and Luhmann, 2010). The recognition of the ultimate target neurons within layer 4 of the cerebral cortex and the maturation of these connections relies on multiple cellular and molecular mechanisms (see Blakey et al., 2012 and Yamamoto and López-Bendito, 2012, both in this issue of EJN). The transient circuits between subplate neurons, thalamic afferents, and layer 4 neurons is now widely recognised as a key mechanism for the early circuit formation (Kanold and Luhmann, 2010). The subplate neurons integrate into the cortical circuits in an age- and area-specific dynamic fashion (Piñon et al., 2009; Hoerder-Suabedissen and Molnár, 2012; Tolner et al., 2012; Viswanathan et al., 2012).
9. Concluding Remarks
The development of thalamocortical connections relies on multiple mechanisms. Early connectivity or migrating cell populations shape the trajectory of this axonal tract as well as assist the crossing of several boundaries. The diencephalic-telencephalic and the pallial subpallial boundaries are considered as the most vulnerable sectors of the pathway, with various guidance defects and several default pathways reported in mutants with transcription factor or axon guidance molecule defects. The initial topography is also guided by several factors, some establishing gradients in the ventral telencephalon. The initial deployment of thalamic projections and their accumulation below the cortex is orchestrated by gradients in the subplate. There are several examples for substantial rearrangements in this region before the final ingrowth into the cortical plate either through the manipulations of the early cortical regionalisation or changing the flow of sensory input from sense organs.
Acknowledgements
The authors are grateful to Ray Guillery, Elanor Grant and Anna Hoerder-Suabedissen for their thoughtful comments on the manuscript and to Daniel Blakey drawing upper left panels of Fig. 4. The work of the authors laboratories is supported by: MRC, The Wellcome Trust, BBSRC UK (DJP and ZM); Avenir Program (Inserm), European Young Investigator Award (EURYI)(SG); Spanish MICINN BFU2009-08261 and an ERC Grant ERC-2009-StG_20081210 (GL-B); National Science Foundation (0822969), Autism Speaks (1847), and NIH funding of the University of North Carolina Silvio O. Conte Center for the Neuroscience of Mental Disorders (MH064065) (PM).
References
- Adams NC, Baker GE. Cells of the perireticular nucleus project to the developing neocortex of the rat. J Comp Neurol. 1995 Sep 4;359(4):613–626. doi: 10.1002/cne.903590408. 1995. [DOI] [PubMed] [Google Scholar]
- Adams NC, Lozsádi DA, Guillery RW. Complexities in the thalamocortical and corticothalamic pathways. Eur J Neurosci. 1997;9(2):204–209. doi: 10.1111/j.1460-9568.1997.tb01391.x. [DOI] [PubMed] [Google Scholar]
- Agmon A, Yang LT, Jones EG, O’Dowd DK. Topological precision in the thalamic projection to neonatal mouse barrel cortex. J Neurosci. 1995;15(1 Pt 2):549–561. doi: 10.1523/JNEUROSCI.15-01-00549.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agmon A, Yang LT, O’Dowd DK, Jones EG. Organized growth of thalamocortical axons from the deep tier of terminations into layer IV of developing mouse barrel cortex. J Neurosci. 1993;13(12):5365–5382. doi: 10.1523/JNEUROSCI.13-12-05365.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu Rev Neurosci. 1994;17:185–218. doi: 10.1146/annurev.ne.17.030194.001153. [DOI] [PubMed] [Google Scholar]
- Andrews W, Liapi A, Plachez C, Camurri L, Zhang J, Mori S, Murakami F, Parnavelas JG, Sundaresan V, Richards LJ. Robo1 regulates the development of major axon tracts and interneuron migration in the forebrain. Development. 2006;133:2243–2252. doi: 10.1242/dev.02379. [DOI] [PubMed] [Google Scholar]
- Angevine JB., Jr Time of neuron origin in the diencephalon of the mouse. An autoradiographic study. J Comp Neurol. 1970;139(2):129–187. doi: 10.1002/cne.901390202. [DOI] [PubMed] [Google Scholar]
- Ayoub AE, Kostovic I. New horizons for the subplate zone and its pioneering neurons. Cereb Cortex. 2009;19(8):1705–1707. doi: 10.1093/cercor/bhp025. [DOI] [PubMed] [Google Scholar]
- Bagri A, Marín O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, Tessier-Lavigne M. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248. doi: 10.1016/s0896-6273(02)00561-5. [DOI] [PubMed] [Google Scholar]
- Bagnard D, Chounlamountri N, Püschel AW, Bolz J. Axonal surface molecules act in combination with semaphorin 3a during the establishment of corticothalamic projections. Cereb Cortex. 2001;11(3):278–285. doi: 10.1093/cercor/11.3.278. Erratum in: Cereb Cortex 2001 Sep;11(9):891. [DOI] [PubMed] [Google Scholar]
- Bicknese AR, Sheppard AM, O’Leary DD, Pearlman AL. Thalamocortical axons extend along a chondroitin sulfate proteoglycan-enriched pathway coincident with the neocortical subplate and distinct from the efferent path. J Neurosci. 1994 Jun 14;(6):3500–3510. doi: 10.1523/JNEUROSCI.14-06-03500.1994. 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielle F, Marcos-Mondejar P, Keita M, Mailhes C, Verney C, Nguyen Ba-Charvet K, Tessier-Lavigne M, Lopez-Bendito G, Garel S. Slit2 activity in the migration of guidepost neurons shapes thalamic projections during development and evolution. Neuron. 2011a;69:1085–1098. doi: 10.1016/j.neuron.2011.02.026. [DOI] [PubMed] [Google Scholar]
- Bielle F, Marcos-Mondéjar P, Leyva-Díaz E, Lokmane L, Mire E, Mailhes C, Keita M, García N, Tessier-Lavigne M, Garel S, López-Bendito G. Emergent growth cone responses to combinations of slit1 and netrin 1 in thalamocortical axon topography. Curr Biol. 2011b;21:1748–1755. doi: 10.1016/j.cub.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Bielle, et al. 2012 (IN REVISION) [Google Scholar]
- Bishop KM, Goudreau G, et al. “Regulation of area identity in the mammalian neocortex by Emx2 and Pax6”. Science. 2000;288(5464):344–349. doi: 10.1126/science.288.5464.344. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Garel S, et al. “Emx1 and Emx2 cooperate to regulate cortical size, lamination, neuronal differentiation, development of cortical efferents, and thalamocortical pathfinding”. J Comp Neurol. 2003;457(4):345–360. doi: 10.1002/cne.10549. [DOI] [PubMed] [Google Scholar]
- Blakemore C, Molnár Z. Factors involved in the establishment of specific interconnections between thalamus and cerebral cortex. Cold Spring Harb Symp Quant Biol. 1990;55:491–504. doi: 10.1101/sqb.1990.055.01.048. [DOI] [PubMed] [Google Scholar]
- Blakey D, Wilson MC, Molnár Z. Termination and initial branch formation of SNAP-25 deficient thalamocortical fibres in heterochronic organotypic co-cultures. European Journal of NeuroScience. 2012 doi: 10.1111/j.1460-9568.2012.08120.x. (in press, same issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin A, Torii M, Wang L, Rakic P, Levitt P. Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nat Neurosci. 2007;10:588–597. doi: 10.1038/nn1896. [DOI] [PubMed] [Google Scholar]
- Braisted JE, Catalano SM, Stimac R, Kennedy TE, Tessier-Lavigne M, Shatz CJ, O’Leary DD. Netrin-1 promotes thalamic axon growth and is required for proper development of the thalamocortical projection. J Neurosci. 2000;20:5792–5801. doi: 10.1523/JNEUROSCI.20-15-05792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braisted JE, Ringstedt T, O’Leary DD. Slits are chemorepellents endogenous to hypothalamus and steer thalamocortical axons into ventral telencephalon. Cereb Cortex. 2009;(Suppl 1):144–151. doi: 10.1093/cercor/bhp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braisted JE, Tuttle R, O’leary DD. Thalamocortical axons are influenced by chemorepellent and chemoattractant activities localized to decision points along their path. Dev Biol. 1999;208:430–440. doi: 10.1006/dbio.1999.9216. [DOI] [PubMed] [Google Scholar]
- Braisted JE, Catalano SM, et al. “Netrin-1 promotes thalamic axon growth and is required for proper development of the thalamocortical projection”. J Neurosci. 2000;20(15):5792–5801. doi: 10.1523/JNEUROSCI.20-15-05792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braisted JE, Ringstedt T, et al. “Slits are chemorepellents endogenous to hypothalamus and steer thalamocortical axons into ventral telencephalon”. Cereb Cortex. 2009;19(Suppl 1):144–151. doi: 10.1093/cercor/bhp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulfone A, Puelles L, Porteus MH, Frohman MA, Martin GR, Rubenstein JL. Spatially restricted expression of Dlx-1, Dlx-2 (Tes-1), Gbx-2, and Wnt-3 in the embryonic day 12.5 mouse forebrain defines potential transverse and longitudinal segmental boundaries. J Neurosci. 1993;13(7):3155–3172. doi: 10.1523/JNEUROSCI.13-07-03155.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette DT, Ji L, et al. “Myosin II activity facilitates microtubule bundling in the neuronal growth cone neck”. Dev Cell. 2008;15(1):163–169. doi: 10.1016/j.devcel.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney RS, Alfonso TB, Cohen D, Dai H, Nery S, et al. Cell migration along the lateral cortical stream to the developing basal telencephalic limbic system.. J Neurosci. 2006;26:11562–11574. doi: 10.1523/JNEUROSCI.3092-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney RS, Cocas LA, Hirata T, Mansfield K, Corbin JG. Differential regulation of telencephalic pallial-subpallial boundary patterning by Pax6 and Gsh2. Cereb Cortex. 2009;19:745–759. doi: 10.1093/cercor/bhn123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani V, Yue Y, Gao PP, Zhou R, Bolz J. Dual action of a ligand for Eph receptor tyrosine kinases on specific populations of axons during the development of cortical circuits. J Neurosci. 1998;18:4663–4672. doi: 10.1523/JNEUROSCI.18-12-04663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani V, Chedotal A, et al. “Analysis of the L1-deficient mouse phenotype reveals cross-talk between Sema3A and L1 signaling pathways in axonal guidance [see comments]”. Neuron. 2000;27(2):237–249. doi: 10.1016/s0896-6273(00)00033-7. [DOI] [PubMed] [Google Scholar]
- Catalano SM, Robertson RT, Killackey HP. Early ingrowth of thalamocortical afferents to the neocortex of the prenatal rat. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):2999–3003. doi: 10.1073/pnas.88.8.2999. 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano SM, Shatz CJ. Activity-dependent cortical target selection by thalamic axons. Science. 1998;281(5376):559–562. doi: 10.1126/science.281.5376.559. [DOI] [PubMed] [Google Scholar]
- Chapouton P, Schuurmans C, Guillemot F, Gotz M. The transcription factor neurogenin 2 restricts cell migration from the cortex to the striatum. Development. 2001;128:5149–5159. doi: 10.1242/dev.128.24.5149. [DOI] [PubMed] [Google Scholar]
- Chen Y, Magnani D, Theil T, Pratt T, Price DJ. Evidence that descending cortical axons are essential for thalamocortical axons to cross the pallial-subpallial boundary in the embryonic forebrain. PLOS One. 2012 doi: 10.1371/journal.pone.0033105. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clascá F, Rubio-Garrido P, Galazo MJ, Porrero C. Diversity in thalamic relay neurons: evidence for “bottom-up” and “top-down” information flow in thalamocortical pathways] An R Acad Nac Med (Madr) 2009;126(3):357–372. [PubMed] [Google Scholar]
- Coleman KA, Mitrofanis J. Does the perireticular thalamic nucleus project to the neocortex? Anat Embryol (Berl) 1999 Nov;200(5):521–531. doi: 10.1007/s004290050300. 1999. [DOI] [PubMed] [Google Scholar]
- de Carlos JA, López-Mascaraque L, Valverde F. Dynamics of cell migration from the lateral ganglionic eminence in the rat. J Neurosci. 1996;16(19):6146–6156. doi: 10.1523/JNEUROSCI.16-19-06146.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carlos JA, O’Leary DD. Growth and targeting of subplate axons and establishment of major cortical pathways. J Neurosci. 1992;12(4):1194–1211. doi: 10.1523/JNEUROSCI.12-04-01194.1992. Erratum in: J Neurosci 1993 Mar;13(3):followi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyanenko GP, Siesser PF, et al. “L1 and CHL1 Cooperate in Thalamocortical Axon Targeting”. Cereb Cortex. 2011b;21(2):401–412. doi: 10.1093/cercor/bhq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyanenko GP, Riday TT, et al. “NrCAM deletion causes topographic mistargeting of thalamocortical axons to the visual cortex and disrupts visual acuity”. J Neurosci. 2011a;31(4):1545–1558. doi: 10.1523/JNEUROSCI.4467-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour A, Egea J, Kullander K, Klein R, Vanderhaeghen P. Genetic analysis of EphA-dependent signaling mechanisms controlling topographic mapping in vivo. Development. 2006;133:4415–4420. doi: 10.1242/dev.02623. [DOI] [PubMed] [Google Scholar]
- Dufour A, Seibt J, Passante L, Depaepe V, Ciossek T, Frisén J, Kullander K, Flanagan JG, Polleux F, Vanderhaeghen P. Area specificity and topography of thalamocortical projections are controlled by ephrin/Eph genes. Neuron. 2003;39:453–465. doi: 10.1016/s0896-6273(03)00440-9. [DOI] [PubMed] [Google Scholar]
- Dwyer ND, Manning DK, Moran JL, Mudbhary R, Fleming MS, Favero CB, Vock VM, O’Leary DD, Walsh CA, Beier DR. A forward genetic screen with a thalamocortical axon reporter mouse yields novel neurodevelopment mutants and a distinct emx2 mutant phenotype. Neural Dev. 2011;6:3. doi: 10.1186/1749-8104-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egea J, Nissen UV, et al. “Regulation of EphA 4 kinase activity is required for a subset of axon guidance decisions suggesting a key role for receptor clustering in Eph function”. Neuron. 2005;47(4):515–528. doi: 10.1016/j.neuron.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Friauf E, Shatz CJ. Changing patterns of synaptic input to subplate and cortical plate during development of visual cortex. J Neurophysiol. 1991 Dec;66(6):2059–2071. doi: 10.1152/jn.1991.66.6.2059. 1991. [DOI] [PubMed] [Google Scholar]
- Gao PP, Yue Y, Zhang JH, Cerretti DP, Levitt P, et al. Regulation of thalamic neurite outgrowth by the Eph ligand ephrin-A5: implications in the development of thalamocortical projections. Proc Natl Acad Sci U S A. 1998;95:5329–5334. doi: 10.1073/pnas.95.9.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Frigola C, Herrera E. “Zic2 regulates the expression of Sert to modulate eye-specific refinement at the visual targets”. EMBO J. 2010;29(18):3170–3183. doi: 10.1038/emboj.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel S, Rubenstein JL. Intermediate targets in formation of topographic projections: inputs from the thalamocortical system. Trends Neurosci. 2004;27:533–539. doi: 10.1016/j.tins.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Garel S, Marin F, et al. “Ebf1 controls early cell differentiation in the embryonic striatum”. Development. 1999;126(23):5285–5294. doi: 10.1242/dev.126.23.5285. [DOI] [PubMed] [Google Scholar]
- Garel S, Yun K, Grosschedl R, Rubenstein JL. The early topography of thalamocortical projections is shifted in Ebf1 and Dlx1/2 mutant mice. Development. 2002;129:5621–5634. doi: 10.1242/dev.00166. [DOI] [PubMed] [Google Scholar]
- Garel S, Huffman KJ, Rubenstein JL. Molecular regionalization of the neocortex is disrupted in Fgf8 hypomorphic mutants. Development. 2003;130(9):1903–1914. doi: 10.1242/dev.00416. [DOI] [PubMed] [Google Scholar]
- Gimeno L, Martinez S. Expression of chick Fgf19 and mouse Fgf15 orthologs is regulated in the developing brain by Fgf8 and Shh. Dev Dyn. 2007;236(8):2285–2297. doi: 10.1002/dvdy.21237. [DOI] [PubMed] [Google Scholar]
- Gu C, Rodriguez ER, et al. “Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development”. Dev Cell. 2003;5(1):45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guellmar A, Rudolph J, Bolz J. Structural alterations of spiny stellate cells in the somatosensory cortex in ephrin-A5-deficient mice. J Comp Neurol. 2009;517:645–654. doi: 10.1002/cne.22198. [DOI] [PubMed] [Google Scholar]
- Hanashima C, Molnár Z, Fishell G. Building bridges to the cortex. Cell. 2006;125(1):24–27. doi: 10.1016/j.cell.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Higashi S, Molnár Z, Kurotani T, Toyama K. Prenatal development of neural excitation in rat thalamocortical projections studied by optical recording. NeuroScience. 2001;115(4):1231–1246. doi: 10.1016/s0306-4522(02)00418-9. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Miyashita-Lin E, Rubenstein JL. Cortical and thalamic axon pathfinding defects in Tbr1, Gbx2, and Pax6 mutant mice: evidence that cortical and thalamic axons interact and guide each other. J Comp Neurol. 2002 May 20;447(1):8–17. doi: 10.1002/cne.10219. 2002. [DOI] [PubMed] [Google Scholar]
- Hoerder-Suabedissen A, Wang WZ, Lee S, Davies KE, Goffinet AM, Rakić S, Parnavelas J, Reim K, Nicolić M, Paulsen O, Molnár Z. Novel markers reveal subpopulations of subplate neurons in the murine cerebral cortex. Cereb Cortex. 2009;19(8):1738–1750. doi: 10.1093/cercor/bhn195. [DOI] [PubMed] [Google Scholar]
- Hoerder-Suabedissen A, Molnár Z. Morphology of mouse subplate cells with identified projection targets changes with age. J Comp Neurol. 2012;520(1):174–185. doi: 10.1002/cne.22725. [DOI] [PubMed] [Google Scholar]
- Jacobs EC, Campagnoni C, Kampf K, Reyes SD, Kalra V, Handley V, Xie YY, Hong-Hu Y, Spreur V, Fisher RS, Campagnoni AT. Visualization of corticofugal projections during early cortical development in a tau-GFP-transgenic mouse. Eur J Neurosci. 2007;25(1):17–30. doi: 10.1111/j.1460-9568.2006.05258.x. [DOI] [PubMed] [Google Scholar]
- Jones EG. The Thalamus. Cambridge University Press; 2007. [Google Scholar]
- Jones EG. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 2001;24(10):595–601. doi: 10.1016/s0166-2236(00)01922-6. [DOI] [PubMed] [Google Scholar]
- Jones L, López-Bendito G, Gruss P, Stoykova A, Molnár Z. Pax6 is required for the normal development of the forebrain axonal connections. Development. 2002;129:5041–5052. doi: 10.1242/dev.129.21.5041. [DOI] [PubMed] [Google Scholar]
- Jurata LW, Thomas JB, et al. “Transcriptional mechanisms in the development of motor control”. Curr Opin Neurobiol. 2000;10(1):72–79. doi: 10.1016/s0959-4388(99)00053-7. [DOI] [PubMed] [Google Scholar]
- Kania A, Jessell T. “Topographic motor projections in the limb imposed by LIM homeodomain protein regulation of ephrin-A:EphA interactions”. Neuron. 2003;38(4):581–596. doi: 10.1016/s0896-6273(03)00292-7. [DOI] [PubMed] [Google Scholar]
- Kania A, Johnson RL, et al. “Coordinate roles for LIM homeobox genes in directing the dorsoventral trajectory of motor axons in the vertebrate limb”. Cell. 2000;102(2):161–173. doi: 10.1016/s0092-8674(00)00022-2. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Luhmann HJ. The subplate and early cortical circuits. Annu Rev Neurosci. 2010;33:23–48. doi: 10.1146/annurev-neuro-060909-153244. [DOI] [PubMed] [Google Scholar]
- Kataoka A, Shimogori T. Fgf8 controls regional identity in the developing thalamus. Development. 2008;135(17):2873–2881. doi: 10.1242/dev.021618. [DOI] [PubMed] [Google Scholar]
- Kiecker C, Lumsden A. Hedgehog signaling from the ZLI regulates diencephalic regional identity. Nat Neurosci. 2004;7(11):1242–1249. doi: 10.1038/nn1338. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 1990;297(3):441–470. doi: 10.1002/cne.902970309. [DOI] [PubMed] [Google Scholar]
- Krug K, Smith AL, Thompson ID. The development of topography in the hamster geniculo-cortical projection. J Neurosci. 1998;18(15):5766–5776. doi: 10.1523/JNEUROSCI.18-15-05766.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhina V, Falnikar A, Bhatnagar L, Tole S. Early thalamocortical tract guidance and topographic sorting of thalamic projections requires LIM-homeodomain gene Lhx2. Dev Biol. 2007;306:703–713. doi: 10.1016/j.ydbio.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Larsen CW, Zeltser LM, Lumsden A. Boundary formation and compartition in the avian diencephalon. J Neurosci. 2001 Jul 1;21(13):4699–4711. doi: 10.1523/JNEUROSCI.21-13-04699.2001. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Petros TJ, et al. “Zic2 regulates retinal ganglion cell axon avoidance of ephrinB2 through inducing expression of the guidance receptor EphB1”. J Neurosci. 2008;28(23):5910–5919. doi: 10.1523/JNEUROSCI.0632-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Pfaff SL. “Transcriptional networks regulating neuronal identity in the developing spinal cord”. Nat Neurosci. 2001;4(Suppl):1183–1191. doi: 10.1038/nn750. [DOI] [PubMed] [Google Scholar]
- Leighton PA, Mitchell KJ, et al. “Defining brain wiring patterns and mechanisms through gene trapping in mice”. Nature. 2001;410(6825):174–179. doi: 10.1038/35065539. [DOI] [PubMed] [Google Scholar]
- Little GE, Lopez-Bendito G, et al. “Specificity and plasticity of thalamocortical connections in Sema6A mutant mice”. PLoS Biol. 2009;7(4):e98. doi: 10.1371/journal.pbio.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bendito G, Cautinat A, Sánchez JA, Bielle F, Flames N, Garratt AN, Talmage DA, Role LW, Charnay P, Marín O, Garel S. Tangential neuronal migration controls axon guidance: a role for neuregulin-1 in thalamocortical axon navigation.. Cell. 2006;125:127–142. doi: 10.1016/j.cell.2006.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bendito G, Flames N, Ma L, Fouquet C, Di Meglio T, Chedotal A, Tessier-Lavigne M, Marín O. Robo1 and Robo2 cooperate to control the guidance of major axonal tracts in the mammalian forebrain. J Neurosci. 2007;27:3395–3407. doi: 10.1523/JNEUROSCI.4605-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bendito G, Molnár Z. Thalamocortical development: how are we going to get there? Nat Rev Neurosci. 2003;4(4):276–289. doi: 10.1038/nrn1075. [DOI] [PubMed] [Google Scholar]
- López-Bendito G, Chan C-H, Mallamaci A, Parnavelas J, Molnár Z. The role of Emx2 in the development of the reciprocal connectivity between cortex and thalamus. J. Comp. Neurol. 2002;451:153–169. doi: 10.1002/cne.10345. [DOI] [PubMed] [Google Scholar]
- López-Bendito G, Flames N, et al. “Robo1 and Robo2 cooperate to control the guidance of major axonal tracts in the mammalian forebrain”. J Neurosci. 2007;27(13):3395–3407. doi: 10.1523/JNEUROSCI.4605-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozsádi DA, Gonzalez-Soriano J, Guillery RW. The course and termination of corticothalamic fibres arising in the visual cortex of the rat. Eur J Neurosci. 1996;8(11):2416–2427. doi: 10.1111/j.1460-9568.1996.tb01205.x. [DOI] [PubMed] [Google Scholar]
- Lund RD, Mustari MJ. Development of the geniculocortical pathway in rats. J Comp Neurol. 1977;173(2):289–306. doi: 10.1002/cne.901730206. [DOI] [PubMed] [Google Scholar]
- Maness PF, Schachner M. “Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration.”. Nat Neurosci. 2007;10(1):19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- Mann F, Peuckert C, Dehner F, Zhou R, Bolz J. Ephrins regulate the formation of terminal axonal arbors during the development of thalamocortical projections. Development. 2002;129:3945–3955. doi: 10.1242/dev.129.16.3945. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Early prenatal ontogenesis of the cerebral cortex (neocortex) of the cat (Felis domestica). A Golgi study. I. The primordial neocortical organization. Z Anat Entwicklungsgesch. 1971;134(2):117–145. doi: 10.1007/BF00519296. [DOI] [PubMed] [Google Scholar]
- Marin O, Baker J, et al. “Patterning of the basal telencephalon and hypothalamus is essential for guidance of cortical projections”. Development. 2002;129(3):761–773. doi: 10.1242/dev.129.3.761. [DOI] [PubMed] [Google Scholar]
- Marin O, Rubenstein JLR. “Patterning, regionalization and cell differentiation in the forebrain. In: Rossant J, Tam P, editors. Mouse development.”. San Diego: Academic Press; 2002. pp. 75–106. [Google Scholar]
- Marquardt T, Shirasaki R, et al. “Coexpressed EphA receptors and ephrin-A ligands mediate opposing actions on growth cone navigation from distinct membrane domains”. Cell. 2005;121(1):127–139. doi: 10.1016/j.cell.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Marcos-Mondéjar P, Peregrin S, Li JY, Carlsson L, Tole S, López-Bendito G. The Lhx2 transcription factor controls thalamocortical axonal guidance by specific regulation of Robo1 and Robo2 receptors. Journal of NeuroScience. 2012 doi: 10.1523/JNEUROSCI.5851-11.2012. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKellar CE, Shatz CJ. Synaptogenesis in purified cortical subplate neurons. Cereb Cortex. 2009;19(8):1723–1737. doi: 10.1093/cercor/bhn194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métin C, Godement P. “The ganglionic eminence may be an intermediate target for corticofugal and thalamocortical axons”. J Neurosci. 1996;16(10):3219–3235. doi: 10.1523/JNEUROSCI.16-10-03219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz CD, Carcea I, et al. “ERM proteins regulate growth cone responses to Sema3A”. J Comp Neurol. 2008;510(4):351–366. doi: 10.1002/cne.21799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B, Chou L, Finlay BL. The early development of thalamocortical and corticothalamic projections. J Comp Neurol. 1993;335(1):16–41. doi: 10.1002/cne.903350103. [DOI] [PubMed] [Google Scholar]
- Mitrofanis J, Guillery RW. New views of the thalamic reticular nucleus in the adult and the developing brain. Trends Neurosci. 1993;16:240–245. doi: 10.1016/0166-2236(93)90163-g. [DOI] [PubMed] [Google Scholar]
- Mitrofanis J, Baker GE. Development of the thalamic reticular and perireticular nuclei in rats and their relationship to the course of growing corticofugal and corticopetal axons. J Comp Neurol. 1993;338:575–587. doi: 10.1002/cne.903380407. [DOI] [PubMed] [Google Scholar]
- Mitrofanis J. Patterns of antigenic expression in the thalamic reticular nucleus of developing rats. J Comp Neurol. 1992;320(2):161–181. doi: 10.1002/cne.903200203. [DOI] [PubMed] [Google Scholar]
- Mitrofanis J. Development of the pathway from the reticular and perireticular nuclei to the thalamus in ferrets: a Dil study. Eur J Neurosci. 1994;6(12):1864–1882. doi: 10.1111/j.1460-9568.1994.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Mitrofanis J. Development of the thalamic reticular nucleus in ferrets with special reference to the perigeniculate and perireticular cell groups. Eur J Neurosci. 1994;6(2):253–263. doi: 10.1111/j.1460-9568.1994.tb00268.x. [DOI] [PubMed] [Google Scholar]
- Miyake A, Nakayama Y, Konishi M, Itoh N. Fgf19 regulated by Hh signaling is required for zebrafish forebrain development. Dev Biol. 2005 Oct 25;288(1):259–275. doi: 10.1016/j.ydbio.2005.09.042. Epub 2005. [DOI] [PubMed] [Google Scholar]
- Miyashita-Lin EM, Hevner R, Wassarman KM, Martinez S, Rubenstein JL. Early neocortical regionalization in the absence of thalamic innervation. Science. 1999;285:906–909. doi: 10.1126/science.285.5429.906. [DOI] [PubMed] [Google Scholar]
- Molliver ME, Van der Loos H. The ontogenesis of cortical circuitry: the spatial distribution of synapses in somesthetic cortex of newborn dog. Ergeb Anat Entwicklungsgesch. 1970;42(4):5–53. 1970. [PubMed] [Google Scholar]
- Molnár Z, Blakemore C. How do thalamic axons find their way to the cortex? Trends Neurosci. 1995;18(9):389–397. doi: 10.1016/0166-2236(95)93935-q. [DOI] [PubMed] [Google Scholar]
- Molnár Z, Blakemore C. Lack of regional specificity for connections formed between thalamus and cortex in coculture. Nature. 1991;351(6326):475–477. doi: 10.1038/351475a0. [DOI] [PubMed] [Google Scholar]
- Molnár Z, Blakemore C. Development of signals influencing the growth and termination of thalamocortical axons in organotypic culture.. Exp Neurol. 1999;156(2):363–393. doi: 10.1006/exnr.1999.7032. [DOI] [PubMed] [Google Scholar]
- Molnár Z, Adams R, Blakemore C. Mechanisms underlying the early establishment of thalamocortical connections in the rat. J Neurosci. 1998a Aug 1;18(15):5723–5745. doi: 10.1523/JNEUROSCI.18-15-05723.1998. 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár Z, Adams R, Goffinet AM, Blakemore C. The role of the first postmitotic cortical cells in the development of thalamocortical innervation in the reeler mouse. J Neurosci. 1998b;18(15):5746–5765. doi: 10.1523/JNEUROSCI.18-15-05746.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár Z, Cordery P. Connections between cells of the internal capsule, thalamus, and cerebral cortex in embryonic rat. J Comp Neurol. 1999;413(1):1–25. doi: 10.1002/(sici)1096-9861(19991011)413:1<1::aid-cne1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Molnár Z, Butler AB. The corticostriatal junction: a crucial region for forebrain development and evolution. Bioessays. 2002;24(6):530–541. doi: 10.1002/bies.10100. [DOI] [PubMed] [Google Scholar]
- Molnár Z, López-Bendito G, Small J, Partridge LD, Blakemore C, Wilson MC. Normal development of embryonic thalamocortical connectivity in the absence of evoked synaptic activity. J Neurosci. 2002;22(23):10313–10323. doi: 10.1523/JNEUROSCI.22-23-10313.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár Z, Adams R, Blakemore C. Mechanisms underlying the establishment of topographically ordered early thalamocortical connections in the rat. J Neurosci. 1998;18:5723–5745. doi: 10.1523/JNEUROSCI.18-15-05723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár Z, Higashi S, López-Bendito G. Choreography of early thalamocortical development. Cereb Cortex. 2003;13:661–669. doi: 10.1093/cercor/13.6.661. [DOI] [PubMed] [Google Scholar]
- Molnár Z, Higashi S, Adams R, Toyama K. Earliest thalamocortical interactions. In: Kossut FP, editor. Plasticity of Adult Barrel Cortex. Johnson City, TN: Graham Publishing Co; 2000. pp. 45–77. [Google Scholar]
- Naegele JR, Jhaveri S, Schneider GE. Sharpening of topographical projections and maturation of geniculocortical axon arbors in the hamster. J Comp Neurol. 1988;277(4):593–607. doi: 10.1002/cne.902770411. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, O’Leary DD. “Combinatorial expression patterns of LIM-homeodomain and other regulatory genes parcellate developing thalamus”. J Neurosci. 2001;21(8):2711–2725. doi: 10.1523/JNEUROSCI.21-08-02711.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SB, LeVay S. Topographic organization of the optic radiation of the cat. J Comp Neurol. 1985;240(3):322–330. doi: 10.1002/cne.902400308. [DOI] [PubMed] [Google Scholar]
- Osheroff H, Hatten ME. Gene expression profiling of preplate neurons destined for the subplate: genes involved in transcription, axon extension, neurotransmitter regulation, steroid hormone signaling, and neuronal survival. Cereb Cortex. 2009;19(Suppl 1):126–134. doi: 10.1093/cercor/bhp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeschger FM, Wang WZ, Lee S, García-Moreno F, Goffinet AM, Arbonés ML, Rakic S, Molnár Z. Gene Expression Analysis of the Embryonic Subplate. Cereb Cortex. 2012 Aug 22; doi: 10.1093/cercor/bhr197. 2011 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary DD, Sahara S. Genetic regulation of arealization of the neocortex. Curr Opin Neurobiol. 2008;18(1):90–100. doi: 10.1016/j.conb.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnavelas JG. The origin and migration of cortical neurones: new vistas. Trends Neurosci. 2002;23(3):126–131. doi: 10.1016/s0166-2236(00)01553-8. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Giger RJ. “Semaphorin function in neural plasticity and disease”. Curr Opin Neurobiol. 2009;19(3):263–274. doi: 10.1016/j.conb.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñon MC, Tuoc TC, Ashery-Padan R, Molnár Z, Stoykova A. Altered molecular regionalization and normal thalamocortical connections in cortex-specific Pax6 knock-out mice. J Neurosci. 2008;28(35):8724–8734. doi: 10.1523/JNEUROSCI.2565-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñon MC, Jethwa A, Jacobs E, Campagnoni A, Molnár Z. Dynamic integration of subplate neurons into the cortical barrel field circuitry during postnatal development in the Golli-tau-eGFP (GTE) mouse. J Physiol. 2009;587(Pt 9):1903–1915. doi: 10.1113/jphysiol.2008.167767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell AW, Sassa T, et al. “Topography of thalamic projections requires attractive and repulsive functions of Netrin-1 in the ventral telencephalon”. PLoS Biol. 2008;6(5):e116. doi: 10.1371/journal.pbio.0060116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt T, Quinn JC, Simpson TI, West JD, Mason JO, Price DJ. Disruption of early events in thalamocortical tract formation in mice lacking the transcription factors Pax6 or Foxg1. J Neurosci. 2002;22:8523–8531. doi: 10.1523/JNEUROSCI.22-19-08523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt T, Vitalis T, Warren N, Edgar JM, Mason JO, Price DJ. A role of Pax6 in the normal development of dorsal thalamus and its cortical connections. Development. 2000;127:5167–5178. doi: 10.1242/dev.127.23.5167. [DOI] [PubMed] [Google Scholar]
- Price DJ, Kennedy H, Dehay C, Zhou L, Mercier M, Jossin Y, Goffinet AM, Tissir F, Blakey D, Molnár Z. The development of cortical connections. Eur J Neurosci. 2006;23(4):910–920. doi: 10.1111/j.1460-9568.2006.04620.x. [DOI] [PubMed] [Google Scholar]
- Puelles L, Rubenstein JL. Expression patterns of homeobox and other putative regulatory genes in the embryonic mouse forebrain suggest a neuromeric organization. Trends Neurosci. 1993 Nov;16(11):472–479. doi: 10.1016/0166-2236(93)90080-6. 1993. [DOI] [PubMed] [Google Scholar]
- Puelles L, Rubenstein JL. Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci. 2003 Sep;26(9):469–476. doi: 10.1016/S0166-2236(03)00234-0. 2003. [DOI] [PubMed] [Google Scholar]
- Quinlan R, Graf M, Mason I, Lumsden A, Kiecker C. Complex and dynamic patterns of Wnt pathway gene expression in the developing chick forebrain. Neural Dev. 2009;4:35. doi: 10.1186/1749-8104-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature. 1976;261(5560):467–471. doi: 10.1038/261467a0. [DOI] [PubMed] [Google Scholar]
- Ravary A, Muzerelle A, Hervé D, Pascoli V, Ba-Charvet KN, Girault JA, Welker E, Gaspar P. Adenylate cyclase 1 as a key actor in the refinement of retinal projection maps. J Neurosci. 2003 Mar 15;23(6):2228–2238. doi: 10.1523/JNEUROSCI.23-06-02228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retaux S, Rogard M, et al. “Lhx9: a novel LIM-homeodomain gene expressed in the developing forebrain”. J Neurosci. 1999;19(2):783–793. doi: 10.1523/JNEUROSCI.19-02-00783.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash BG, Grove EA. Area and layer patterning in the developing cerebral cortex. Curr Opin Neurobiol. 2006;16(1):25–34. doi: 10.1016/j.conb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Scholpp S, Lumsden A. Building a bridal chamber: development of the thalamus. Trends Neurosci. 2010;33(8):373–380. doi: 10.1016/j.tins.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholpp S, Wolf O, Brand M, Lumsden A. Hedgehog signalling from the zona limitans intrathalamica orchestrates patterning of the zebrafish diencephalon. Development. 2006;133(5):855–864. doi: 10.1242/dev.02248. [DOI] [PubMed] [Google Scholar]
- Salinas PC, Nusse R. Regional expression of the Wnt-3 gene in the developing mouse forebrain in relationship to diencephalic neuromeres. Mech Dev. 1992;39(3):151–160. doi: 10.1016/0925-4773(92)90042-i. [DOI] [PubMed] [Google Scholar]
- Schaefer AW, Schoonderwoert VT, Ji L, Mederios N, Danuser G, Forscher P. Coordination of actin filament and microtubule dynamics during neurite outgrowth. Dev Cell. 2008;15(1):146–162. doi: 10.1016/j.devcel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlatter MC, Buhusi M, et al. “CHL1 promotes Sema3A–induced growth cone collapse and neurite elaboration through a motif required for recruitment of ERM proteins to the plasma membrane”. J Neurochem. 2008;104(3):731–744. doi: 10.1111/j.1471-4159.2007.05013.x. [DOI] [PubMed] [Google Scholar]
- Seibt J, Schuurmans C, Gradwhol G, Dehay C, Vanderhaeghen P, Guillemot F, Polleux F. Neurogenin2 specifies the connectivity of thalamic neurons by controlling axon responsiveness to intermediate target cues. Neuron. 2003;39:439–452. doi: 10.1016/s0896-6273(03)00435-5. [DOI] [PubMed] [Google Scholar]
- Sharma K, Sheng HZ, et al. “LIM homeodomain factors Lhx3 and Lhx4 assign subtype identities for motor neurons”. Cell. 1998;95(6):817–828. doi: 10.1016/s0092-8674(00)81704-3. [DOI] [PubMed] [Google Scholar]
- Shatz CJ, Luskin MB. The relationship between the geniculocortical afferents and their cortical target cells during development of the cat’s primary visual cortex. J Neurosci. 1986 Dec;6(12):3655–3668. doi: 10.1523/JNEUROSCI.06-12-03655.1986. 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Distinct functions for direct and transthalamic corticocortical connections. J Neurophysiol. 2011;106(3):1068–1077. doi: 10.1152/jn.00429.2011. [DOI] [PubMed] [Google Scholar]
- Sherman SM. The thalamus is more than just a relay. Curr Opin Neurobiol. 2007;17:417–422. doi: 10.1016/j.conb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Exploring the Thalamus and Its Role in Cortical Function. The MIT Press; 2005. [Google Scholar]
- Shimogori T, Banuchi V, Ng HY, Strauss JB, Grove EA. Embryonic signaling centers expressing BMP, WNT and FGF proteins interact to pattern the cerebral cortex. Development. 2004;131(22):5639–5647. doi: 10.1242/dev.01428. [DOI] [PubMed] [Google Scholar]
- Shimogori T, Grove EA. Fibroblast growth factor 8 regulates neocortical guidance of area-specific thalamic innervation. J Neurosci. 2005;25(28):6550–6560. doi: 10.1523/JNEUROSCI.0453-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TI, Pratt T, Mason JO, Price DJ. Normal ventral telencephalic expression of Pax6 is required for normal development of thalamocortical axons in embryonic mice. Neural Dev. 2009;4:19. doi: 10.1186/1749-8104-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussel L, Marin O, et al. “Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum”. Development. 1999;126(15):3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- Tissir F, Bar I, et al. “Protocadherin Celsr3 is crucial in axonal tract development”. Nat Neurosci. 2005;8(4):451–457. doi: 10.1038/nn1428. [DOI] [PubMed] [Google Scholar]
- Tolner EA, Sheikh A, Yukin AY, Kaila K, Kanold PO. Subplate neurons promote spindle bursts and thalamocortical patterning in the neonatal rat somatosensory cortex. J Neurosci. 2012;32(2):692–702. doi: 10.1523/JNEUROSCI.1538-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii M, Levitt P. Dissociation of corticothalamic and thalamocortical axon targeting by an EphA7-mediated mechanism. Neuron. 2005;48:563–575. doi: 10.1016/j.neuron.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Tran TS, Kolodkin AL, et al. “Semaphorin regulation of cellular morphology”. Annu Rev Cell Dev Biol. 2007;23:263–292. doi: 10.1146/annurev.cellbio.22.010605.093554. [DOI] [PubMed] [Google Scholar]
- Tuttle R, Nakagawa Y, Johnson JE, O’Leary DD. Defects in thalamocortical axon pathfinding correlate with altered cell domains in Mash-1-deficient mice. Development. 1999;126:1903–1916. doi: 10.1242/dev.126.9.1903. [DOI] [PubMed] [Google Scholar]
- Uemura M, Nakao S, et al. “OL-Protocadherin is essential for growth of striatal axons and thalamocortical projections”. Nat Neurosci. 2007;10(9):1151–1159. doi: 10.1038/nn1960. [DOI] [PubMed] [Google Scholar]
- Uesaka N, Hayano Y, Yamada A, Yamamoto N. Interplay between laminar specificity and activity-dependent mechanisms of thalamocortical axon branching. J Neurosci. 2007;27(19):5215–5223. doi: 10.1523/JNEUROSCI.4685-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesaka N, Ruthazer ES, Yamamoto N. The role of neural activity in cortical axon branching. Neuroscientist. 2006;12(2):102–106. doi: 10.1177/1073858405281673. [DOI] [PubMed] [Google Scholar]
- Uziel D, Muhlfriedel S, Bolz J. Ephrin-A5 promotes the formation of terminal thalamocortical arbors. Neuroreport. 2008;19:877–881. doi: 10.1097/WNR.0b013e3282ffdeec. [DOI] [PubMed] [Google Scholar]
- Uziel D, Muhlfriedel S, Zarbalis K, Wurst W, Levitt P, et al. Miswiring of limbic thalamocortical projections in the absence of ephrin-A5. J Neurosci. 2002;22:9352–9357. doi: 10.1523/JNEUROSCI.22-21-09352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhaeghen P, Polleux F. Developmental mechanisms patterning thalamocortical projections: intrinsic, extrinsic and in between. Trends Neurosci. 2004 Jul;27(7):384–391. doi: 10.1016/j.tins.2004.05.009. 2004. [DOI] [PubMed] [Google Scholar]
- Viswanathan S, Bandyopadhyay S, Kao JP, Kanold PO. Changing microcircuits in the subplate of the developing cortex. J Neurosci. 2012;32(5):1589–1601. doi: 10.1523/JNEUROSCI.4748-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vue TY, Bluske K, Alishahi A, Yang LL, Koyano-Nakagawa N, Novitch B, Nakagawa Y. Sonic hedgehog signaling controls thalamic progenitor identity and nuclei specification in mice. J Neurosci. 2009;29(14):4484–4497. doi: 10.1523/JNEUROSCI.0656-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang J, et al. “Axonal growth and guidance defects in Frizzled3 knock-out mice: a comparison of diffusion tensor magnetic resonance imaging, neurofilament staining, and genetically directed cell labeling”. J Neurosci. 2006;26(2):355–364. doi: 10.1523/JNEUROSCI.3221-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Thekdi N, et al. “Frizzled-3 is required for the development of major fiber tracts in the rostral CNS”. J Neurosci. 2002;22(19):8563–8573. doi: 10.1523/JNEUROSCI.22-19-08563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AG, Demyanenko GP, et al. “Close homolog of L1 and neuropilin 1 mediate guidance of thalamocortical axons at the ventral telencephalon”. J Neurosci. 2007;27(50):13667–13679. doi: 10.1523/JNEUROSCI.2888-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada A, Uesaka N, Hayano Y, Tabata T, Kano M, Yamamoto N. Role of pre- and postsynaptic activity in thalamocortical axon branching. Proc Natl Acad Sci U S A. 2010;107(16):7562–7567. doi: 10.1073/pnas.0900613107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, López-Bendito G. Shaping Brain Connections through Spontaneous Neural Activity. 2012 doi: 10.1111/j.1460-9568.2012.08101.x. (review to appear in the same special issue on Thalamocortical Systems) [DOI] [PubMed] [Google Scholar]
- Yun K, Garel S, et al. “Patterning of the lateral ganglionic eminence by the Gsh1 and Gsh2 homeobox genes regulates striatal and olfactory bulb histogenesis and the growth of axons through the basal ganglia”. J Comp Neurol. 2003;461(2):151–165. doi: 10.1002/cne.10685. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Schaefer AW, et al. “Rho-dependent contractile responses in the neuronal growth cone are independent of classical peripheral retrograde actin flow”. Neuron. 2003;40(5):931–944. doi: 10.1016/s0896-6273(03)00754-2. [DOI] [PubMed] [Google Scholar]
- Zhou L, Bar I, et al. “Early forebrain wiring: genetic dissection using conditional Celsr3 mutant mice”. Science. 2008;320(5878):946–949. doi: 10.1126/science.1155244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Qu Y, et al. “Role of the atypical cadherin Celsr3 during development of the internal capsule”. Cereb Cortex. 2009;19(Suppl 1):114–119. doi: 10.1093/cercor/bhp032. [DOI] [PubMed] [Google Scholar]