Abstract
Design
We introduced a long-term care facility (LTCF) Infectious Disease (ID) consult service (LID) that provides on-site consultations to residents of a VA LTCF. We determined the impact of the LID service on antimicrobial use and Clostridium difficile infections at the LTCF.
Setting
A 160-bed Veterans Affairs (VA) LTCF.
Methods
Systemic antimicrobial use and the rate of positive C. difficile tests at the LTCF were compared for 36 months before and 18 months after the initiation of the ID consultation service using segmented regression analysis of an interrupted time-series.
Results
In contrast to the pre-intervention period, total systemic antibiotic administration decreased by 30% (P <.001) with a significant reduction in both oral (32%; P<.001) and intravenous agents (25%; P =.008). The greatest reductions were seen for tetracyclines (64%, P <.001), clindamycin (61%; P <.001), sulfamethoxazole/trimethoprim (38%; P <.001), fluoroquinolones (38%; P <.001) and beta-lactam/beta-lactamase inhibitor combinations (28%; P <.001). Rates of change for positive C. difficile tests at the LTCF declined in the post- vs. preintervention periods (P = .04).
Conclusions
Implementation of a LTCF ID service led to a significant reduction in total antimicrobial use. Bringing providers with infectious disease expertise to the LTCF represents a new and effective means to achieve antimicrobial stewardship.
Introduction
Long-term care facilities (LTCFs) hold an increasingly important role in the nation’s healthcare system. Among people ≥ 65 years of age, 3.6% are residents of LTCFs.1 In 2008, there were 1.7 million nursing home beds in 15,730 facilities compared to only 0.95 million beds in 5,815 hospitals.2 Between 2000 and 2050, the US Census Bureau anticipates that the number of adults ≥ 65 years of age will double, increasing the need for long-term care beds.1,3
LTCF residents acquire an estimated 1.6-3.8 million infections each year and are especially vulnerable to healthcare-associated infections due to immune senescence, functional impairments and the care environment.4 With aging comes a decline in innate and adaptive immunity. Age-related primary immunosenescence is often exacerbated by secondary immune dysfunction related to poor nutrition, multiple co-morbid and degenerative conditions and medications with immunosuppressive effects.5,6 According to the 2004 National Nursing Home Survey, over 75% of LTCF residents required assistance with at least 4 of the 5 activities of daily living (ADLs): bathing, dressing, toileting, transferring and eating.1 Furthermore, shared dining, recreational, therapeutic, bathing and bathroom facilities increase the risk for pathogen transmission among LTCF residents, many of whom may be asymptomatic carriers of multi-drug resistant organisms and Clostridium difficile.7,8
Concern for increased risks of infection may account for the frequency of unnecessary antimicrobial use at LTCFs. Nationally, 25-75% of antibiotic prescriptions for long-term care residents have been found to be inappropriate.9 In our own LTCF, 42% of antibiotic courses were determined to be unnecessary and an additional 22% of courses were too long.10 Unnecessary antibiotic exposure increases the risk of selection for multi-drug resistant organisms, C. difficile infection (CDI), drug-drug interactions and other adverse events.9,11 We instituted an on-site LTCF Infectious Disease (LID) consult service as a multifaceted intervention to improve the utilization of antimicrobials at our LTCF. We report the impact of the LID service on antimicrobial use and on rates of change in positive C. difficile tests at the LTCF.
Methods
Setting and Intervention
In July 2009, we formed an Infectious Disease service for the 160-bed LTCF affiliated with a large urban VA Medical Center. Three of the LTCF 4 wards provide skilled nursing, rehabilitation, restorative, respite and continuing care. The 4th ward focuses primarily on dementia care. A nurse practitioner or physician assistant staffs each ward; two physicians staff two wards each. The LTCF Infectious Disease consult (LID) team consisted of an infectious disease physician and nurse practitioner that examined residents at the LTCF once each week and were available for remote consultation the remainder of the week. LTCF residents seen in consultation were identified by the LTCF staff or referred by the hospital-based ID consult service. Urgent questions received the LID team’s immediate attention, usually both over the phone and via the electronic medical record, while patients with more routine matters (i.e. parenteral antimicrobials, concerns for chronic osteomyelitis) were seen during weekly rounds. Whenever possible, the LID team saw patients prior to making recommendations. Some concerns, such as adjusting vancomycin doses or tailoring antimicrobials to culture results, were addressed over the phone or through the electronic medical record prior to seeing the patient. When antimicrobial treatment was indicated, the LID team based their recommendations on cultures when possible and favored narrow-spectrum agents.
Outcomes
The primary outcome was the use of antimicrobials at the LTCF, measured in Days of Therapy (DOT) per 1,000 patient Days of Care (DOC) per month. Secondary outcomes included comparison of antimicrobial classes and specific agents as well as positive C. difficile tests.
We used structured query language (SQL; Microsoft SQL 2005, Redmond, WA) to obtain data regarding systemic antibiotic administration and positive C. difficile tests at the LTCF and affiliated VA hospital. Using a database containing Bar Code Medication Administration (BCMA) data, we obtained systemic antibiotic doses given on the LTCF wards for 36 months before (July 2006 – June 2009) and 18 months after (July 2009 – December 2010) initiation of the LID service. Agents that were not on the formulary throughout the study period (i.e. levofloxacin, fidaxomicin, doripenem, ceftaroline) were included. Topical, ophthalmic and otic agents were excluded as were all antifungal and antiviral medications. A potential source of error relates to doses that were held, missing or refused, these represented <5% of the total antibiotic doses given. To determine DOT, duplicate doses of antibiotics given on the same day to the same patient were eliminated. DOT/1000 DOC was determined for the form of medication administered (intravenous/intramuscular or oral), the antimicrobial class and individual agents.
Other studies have used both International Classification of Diseases, Ninth Revision (ICD-9) codes and positive toxin assays to measure rates of CDI in hospital settings.12 Illnesses that develop after admission to our LTCF rarely receive ICD-9 codes and thus, these data severely underestimated the rate of CDI. Accordingly we used the number of positive C. difficile tests/1000 DOC to measure the CDI rate. We evaluated C. difficile tests 21 months before (October 2007 –June 2009) and 18 months after (July 2009 – December 2010) initiation of the LID service and then calculated the rates of positive C. difficile tests/1000 DOC. The data pertaining to C. difficile test results prior to October 2007 were erratic and thus excluded.
Analysis
The mean DOT/1000 DOC for total, oral and intravenous antibiotics administered at the LTCF before and after initiation of the LID service was compared using Student’s t test. We then used segmented regression analysis of an interrupted time series to control for antibiotic use and adjust for potential serial correlation of the observations.13 The mean number of admissions and mean number of transfers from the LTCF to the affiliated VA hospital per month were included in the model as covariates. Within this regression framework we tested the significance of changes in level and slope of the regression lines before and after the LID service began. We checked for autocorrelation using the Durbin-Watson statistic (range 1.7 to 2.0; values close to 2 indicate no serial correlation) followed by a generalized least squared estimation using the Prais-Winsten method.14 Seasonal variation (by months) was tested by fitting a model for each outcome without month terms and then plotting the residuals of that model against the month. We did not identify any patterns suggestive of seasonality. For a control, we applied the same model (absent the covariate of transfers from the LTCF to the hospital) and analysis to systemic antimicrobials used at the hospital.
To compare the use of antibiotic classes and individual agents before and after initiation of the LID service for, we used a Student’s t test with a Bonferroni adjustment to account for multiple comparisons. To determine the estimated rates of change for positive C. difficile tests, we again used segmented regression analysis of an interrupted time series to assess the significance of changes in level and slope of the regression lines before and after the introduction of the LID service, using the data obtained from the hospital as a control. No covariates were included this model. All statistical analyses were performed using Stata (Stata Statistical Software: Release 12. College Station, TX).
Results
LTCF Infectious Disease (LID) consultation service
The LID service saw an average of 7 patients and fielded 5-10 phone calls each week. During the study period, the LID service saw 250 individuals with a total of 291 consults. Nearly 1/3 of the consults required only 1 visit; the remaining patients required an average of 3.6 visits (range 2 – 20). More than 95% of recommendations made by the LID team were followed. The most common reason for the LTCF staff to consult the LID service was for CDI, which is endemic in our region.15 The LID service reduced the time from symptom onset to symptom recognition and diagnostic testing for CDI, promoting more rapid initiation of treatment and isolation precautions.16 The second most common reason for consultation was for conditions that were ultimately deemed non-infectious including asymptomatic bacteriuria, colonized wounds and heart failure exacerbations.
Antimicrobial use at the LTCF
After initiation of the LID consult service, total antimicrobial use decreased by 30.1% (P <.001), with a greater reduction in oral agents (31.6%; P <.001) compared to intravenous agents (25.0%; P=.008; Table 1). Antimicrobial use at the affiliated VA hospital did not change over the same period. We also analyzed the change in antibiotic use over time using segmented linear regression of an interrupted time series (Figure 1). In the preintervention period, the rate of total antibiotic use at the LTCF did not change significantly (P=.55) while it increased at the hospital (P=.03). The slight decline observed in rate of oral antibiotic use at the LTCF in the preintervention period (P=.18) amplified significantly in the post-intervention period (P=.04). In contrast, the rate of oral antibiotic use at the hospital increased significant in the preintervention period (P=.03) and did not change in post intervention period (P=.44). Rates of intravenous antibiotic use at the LTCF and hospital did not change in the pre- vs. postintervention periods (P >.05 for all intravenous antibiotic rates). Comparison of the change in intercepts in the time series analysis revealed no immediate effect of the LID service on antimicrobial use (Table 2).
Table 1.
Comparison of the LTCF vs. Hospital before and after the Intervention
| Variable | Preinterventiona | Postinterventionb | % Reduction | P value |
|---|---|---|---|---|
| Antibiotics (DOT/1000 DOC) as mean (± SD)c | ||||
| Total in LTCF | 175.1 ± 28.0 | 122.3 ± 26.9 | 30.1% | <.001 |
| Total in Hospital | 631.8 ± 44.9 | 649.0 ± 38.4 | -2.7% | .15 |
| Oral in LTCF | 136.1 ± 25.6 | 93.1 ± 22.0 | 31.6% | <.001 |
| Oral in Hospital | 185.3 ± 18.8 | 182.3 ± 19.4 | -4.5% | .59 |
| Intravenous in LTCF | 39.0 ± 14.5 | 29.3 ± 10.6 | 25.0% | .01 |
| Intravenous in Hospital | 446.7 ± 39.0 | 466.7 ± 42.5 | 1.6% | .10 |
| Admissions per month | ||||
| LTCF | 58.6 ± 11.3 | 48.1 ± 7.7 | 18.0% | <.001 |
| Hospital | 671.6 ± 36.1 | 720.4 ± 39.9 | -7.3% | <.001 |
| Transfers to Hospital per month | ||||
| LTCF | 19.0 ± 4.6 | 17.6 ± 4.5 | 7.6% | .27 |
| Hospital | (not applicable) | (not applicable) | ||
July, 2006 – June, 2009 (36 months)
July 2009 – December, 2010 (18 months)
DOT/1000 DOC, Days of Therapy/1000 Days of Care; SD, Standard Deviation
Figure 1.
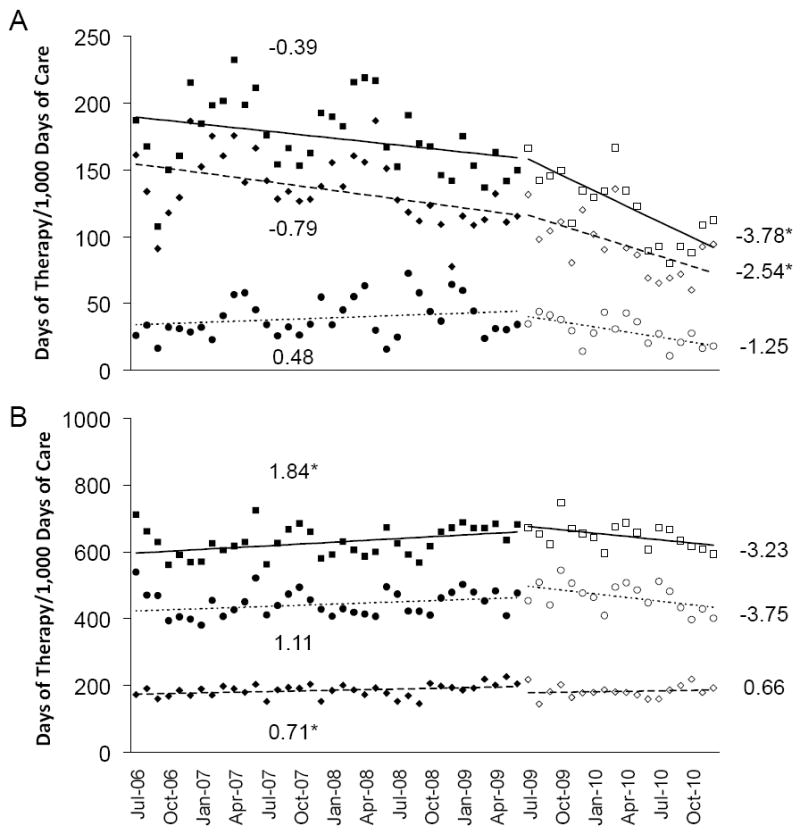
Observed rates of antibiotic use before and after initiation of the LID Consult Service, shown as black and white symbols, respectively, in the (A) LTCF and the (B) hospital. The corresponding lines and their slopes (indicated on the graph) represent the estimated rates of change in antimicrobial use for total antimicrobials (squares), oral agents (diamonds) and intravenous agents (circles), determined using segmented regression analysis of an interrupted time series. * P ≤ .05.
Table 2.
Change in the Rate of Antibiotic Use and of Positive C. difficile Tests in the LTCF vs. Hospital
| Change in Intercept | Change in Slope | |||||||
|---|---|---|---|---|---|---|---|---|
| Rate of Antibiotic Use | mean | LCIa | UCIb | P value | mean | LCIa | UCIb | P value |
| Total, LTCF | 7.8 | -26.2 | 41.8 | .65 | 3.4 | 0.3 | 6.5 | .03 |
| Total, hospital | -14.9 | -67.8 | 37.9 | .57 | 5.1 | 0.4 | 9.7 | .03 |
| Oral, LTCF | 2.5 | -27.5 | 32.4 | .87 | 1.8 | -1.0 | 4.5 | .21 |
| Oral, hospital | 18.7 | -2.4 | 39.7 | .08 | 0.0 | -1.8 | 1.8 | .96 |
| Intravenous, LTCF | 6.3 | -11.6 | 24.1 | .48 | 1.7 | 0.1 | 3.4 | .04 |
| Intravenous, hospital | -32.8 | -80.1 | 14.5 | .17 | 4.9 | 0.76 | 9.0 | .02 |
| Rates of Change of Positive C. difficile test/1000 DOC | ||||||||
| LTCF | -0.20 | -0.79 | 0.38 | .48 | 0.1 | 0.00 | 0.1 | .04 |
| Hospital | 0.79 | -0.11 | 1.69 | .09 | 0.0 | -0.79 | -0.1 | .93 |
Lower Confidence Interval
Upper Confidence Interval
Figure 2 compares DOT/1000 DOC before and after starting the LID consult service for several antimicrobial classes and some individual agents. Fluoroquinolone use decreased 38% (P<.0001). Ciprofloxacin accounted for the majority of this change (41% decrease; P<.0001). Sulfamethoxazole/trimethoprim use decreased 38% (P<.0001) while nitrofurantoin use remained unchanged. Frequently employed as a first line agent for CDI, metronidazole administration decreased 22% (P=.005). In contrast, oral vancomycin, for which the only indication is CDI, increased 89% (P=.005). The LID consult service also led to statistically significant reductions in tetracyclines (64%; P=.0001) and clindamycin (61%; P=.0015), both of which are used infrequently.
Figure 2.
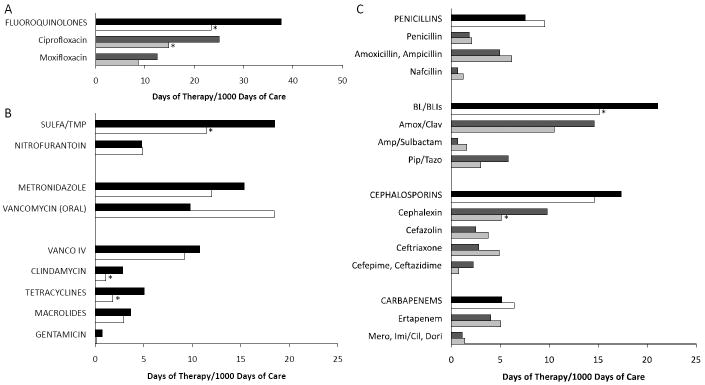
Comparison of mean antimicrobial use for (A) fluoroquinolones, (B) other non-beta-lactams and (C) beta-lactam antimicrobials. Bars depict the mean days of therapy/1000 days of care for drug classes before and after the LID consult service for drug classes (black and white bars, respectively) and for individual antibiotics (dark and light grey bars, respectively). *, with Bonferroni correction, significant P-value ≤ .0017.
The LID consult service also influenced administration of beta-lactams. Use of beta-lactam/beta-lactamase inhibitor combinations dropped significantly (28%; P=.0005). The greatest reduction was in piperacillin/tazobactam use (48%; P=.005) followed by amoxicillin/clavulanate (28%; P=0.003) although these changes were not statistically significant. Total cephalosporin use declined 16% (P=.01) with a significant decrease only for cephalexin (48%; P<.0001). In contrast, cefazolin administration increased 51% (P=0.30). Among extended-spectrum beta-lactams, administration of the anti-pseudomonal agents cefepime and ceftazidime decreased 65% while ceftriaxone use increased 75%. Interestingly, the use of carbapenems increased 37% (P=.50) after initiating the ID consult service. None of the changes among these broad-spectrum antibiotics reached statistical significance.
Rates of positive C. difficile tests
At the LTCF, the rate of change of positive C. difficile tests in the preintervention period showed a trend toward increasing (P=.09) whereas in the postintervention period the trend is reversed (P=.21; Figure 3). The difference between the slopes in pre- vs. postintervention period is significant (P=.04; Table 2). While the rate of change in positive C. difficile tests did not change significantly over time for the two individual time periods, the difference in the rates of change between the two time periods was significantly different, indicating an improvement in the postintervention period. In contrast, the rate of change of positive C. difficile tests at the hospital presented a nearly identical increase in the pre- and postintervention period (P=.02 and .05, respectively) without a significant difference in the slopes between the two periods (P=.93).
Figure 3.
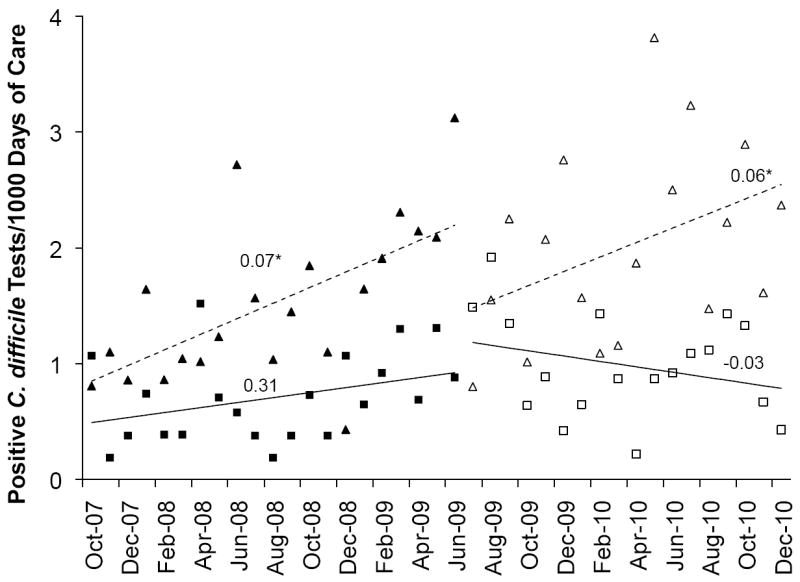
Observed rates of change in positive C. difficile tests at the LTCF (squares) and hospital (triangles) before (black symbols) and after (white symbols) initiation of the LID Consult Service. The corresponding lines and their slopes (noted on graph) represent the estimated rates of change for positive C. difficile tests at the LTCF (solid lines) and the hospital (dashed lines), determined using segmented regression analysis of an interrupted time series. * P ≤ .05.
Discussion
Most LTCF antibiotic stewardship strategies have focused on education using booklets, teaching sessions, formulary guidelines, physician feedback and diagnosis and treatment algorithms.17-19 In our facility, Zabarsky et al. used an educational intervention directed at LTCF nurses and successfully decreased inappropriate submission of urine cultures, antibiotic prescriptions for asymptomatic bacteriuria and total days of antibiotic therapy.20 Building on these efforts, we developed an alternative approach to achieve antibiotic stewardship, specifically a program through which we brought subspecialty expertise to the LTCF. This intervention reduced total antibiotic use and the rate of change for positive C. difficile tests at the LTCF. Although total antimicrobial use at the LTCF showed a slight decline preintervention period, following initiation of the LID service it declined significantly.
Fluoroquinolones account for 30 to 44% of antimicrobial prescriptions in LTCFs, likely a reflection of their broad spectrum of activity, oral bioavailability and ease of dosing.21,22 In our LTCF, ciprofloxacin was being used, often empirically, for suspected urinary tract infections (UTIs). While the LTCF staff was familiar with using sulfamethoxazole/trimethoprim and nitrofurantoin for UTIs, they were also quick to adopt the LID recommendations for other narrow-spectrum drugs like amoxicillin and cephalexin. The LID service led to a reduction in the use of piperacillin/tazobactam, cefepime and cetazidime, all of which have anti-pseudomonal activity. The corresponding increase in ampicillin/sulbactam, ceftriaxone and penicillins suggests that the LID service successfully directed de-escalation of antimicrobial therapy. The overall use of carbapenems, however, showed a non-significant increase following implementation of the LID service. As a result of these data, we have implemented further efforts to reduce total carbapenem use at the LTCF.
The decline in the rate of change of positive C. difficile tests at the LTCF following implementation of the LID consult service is multifactorial. In addition to decreasing total antimicrobial use, the LID team promoted narrow-spectrum agents whenever possible. Furthermore, the LID consult service actively discouraged use of clindamycin and fluoroquinolones. Clindamycin has a long association with increased CDI risk23; fluoroquinolone exposure may select for the fluoroquinolone-resistant epidemic C. difficile strain.24 Beyond supporting the measures implemented by infection control practitioners, the LID team also provided direct education and feedback to the LTCF staff and improved treatment of CDI through use of guideline-driven recommendations regarding the antimicrobial agents selected, the dose administered and the length of treatment.11 Finally, suggestions to avoid “test of cure” in asymptomatic patients and to forego testing among those in whom recurrent disease was clinically evident may have also contributed to a decline in positive C. difficile tests.
Our study has several limitations. We only measured total days of therapy but did not consider the average length of therapy or the number of antimicrobial courses initiated.22,25 We did not focus on antimicrobials applied to specific syndromes, such as suspected UTIs or pneumonia, which also limits our ability to comment upon the appropriateness of therapy. Additionally, while the use of positive C. difficile tests to determine the rate of CDI has not been validated in a LTCF setting, Dubberke et al. report equivalent results regarding CDI incidence in acute care settings using either C. difficile toxin assays or ICD-9 codes.12 Furthermore, our intervention occurred at a single VA LTCF in which all of the prescribers (2 physicians, 3 nurse practitioners and 1 physician assistant) are full-time staff. This is atypical for most LTCFs. Less than 20% of nursing homes employ full-time staff physicians and most LTCF medical directors typically provide primary care at 4 facilities, spending 8-12 hours/week in nursing homes seeing residents while also serving as the medical director for two facilities.1,26 Finally the ID specialists that initiated the LID service at the LTCF are full-time VA employees and thus there were no concerns about reimbursement or insurance claims, limiting the applicability of the LID service to non-VA LTCFs.
The increasing prevalence of nosocomial pathogens and lack of new antimicrobials under development have prompted many hospitals to implement stewardship programs to improve the use of antimicrobial agents, minimize selection for organisms with antimicrobial resistance and reduce the incidence of CDI.11,27 LTCFs have been slower to adopt stewardship measures. The reasons for this are multi-factorial and include the lack of necessary personnel, funding and electronic resources as well as a paucity of well-validated strategies specific to LTCFs.27-30 A survey of LTCFs in Nebraska found that the greatest perceived barrier to effective antimicrobial stewardship was physician practice; specifically a treat-first attitude, lack of response to input from other healthcare workers, non-participation in LTCF initiatives and lack of knowledge regarding appropriate use of antimicrobials.28 The scarcity of infectious disease clinical expertise is perhaps the most significant challenge faced by LTCFs in conducting concurrent review and adjustment of antibiotic therapy.27,29,30
To our knowledge, this is the first description of effective antimicrobial stewardship at a LTCF achieved by bringing subspecialist expertise directly to the residents.30 While labor intensive, our intervention reduced not only total antimicrobial use, but also the rate of change for positive C. difficile tests, providing a functional measure of the influence of the LID service. It is our hope that this success within the VA system may support measures to implement similar programs in other LTCFs.
Acknowledgments
RLPJ gratefully acknowledges the T. Franklin Williams Scholarship with funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, the Association of Specialty Professors, the Infectious Diseases Society of America and the National Foundation for Infectious Diseases.
Funding
This work was supported by the National Institutes of Health (R03-AG040722 to RLPJ, K23-DK087919 to PED, R01-AI063517 to RAB), by the Veterans Affairs Merit Review Program (RAB, CJD) and the Veterans Integrated Service Network 10 Geriatric Research Education and Clinical Center (VISN 10 GRECC; RLPJ, NS, GK, LAJ, RAB, CJD).
Footnotes
Potential Conflicts of Interest
RLPJ reports having consulted for GOJO and Pfizer and has received grant support from Steris, Merck and ViroPharma. RAB reports having consulted for AstraZeneca and having received grant support from AstraZeneca, Ribx, Pfizer and Steris. CJD reports having consulted for BioK, Optimer and GOJO and has received grant support from ViroPharma, Merck and Pfizer. All remaining authors report no conflicts of interest relevant to this article. All authors submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest, and the conflicts that the editors consider relevant to this article are disclosed here.
Presented in Part: Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago; 2011.
References
- 1.Jones AL, Dwyer LL, Bercovitz AR, Strahan GW. The National Nursing Home Survey: 2004 overview. Vital Health Stat. 2009;13(167):1–155. [PubMed] [Google Scholar]
- 2.Anon. Health, United States, 2010: With Special Feature on Death and Dying. Hyattsville, MD: National Center for Heatlh Statistics; 2011. [PubMed] [Google Scholar]
- 3.US Census Bureau Public Information Office. [March 7th, 2012];US Census Bureau Newsroom: Population: An Older and More Diverse Nation by Midcentury. 2008 http://www.census.gov/newsroom/releases/archives/population/cb08-123.html.
- 4.Strausbaugh LJ, Joseph CL. The burden of infection in long-term care. Infect Control Hosp Epidemiol. 2000;21(10):674–679. doi: 10.1086/501712. [DOI] [PubMed] [Google Scholar]
- 5.Strausbaugh LJ. Emerging health care-associated infections in the geriatric population. Emerging Infect Dis. 2001;7(2):268–271. doi: 10.3201/eid0702.010224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juthani-Mehta M, Quagliarello VJ. Infectious diseases in the nursing home setting: challenges and opportunities for clinical investigation. Clin Infect Dis. 2010;51(8):931–936. doi: 10.1086/656411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trick WE, Weinstein RA, DeMarais PL, et al. Colonization of skilled-care facility residents with antimicrobial-resistant pathogens. J Am Geriatr Soc. 2001;49(3):270–276. doi: 10.1046/j.1532-5415.2001.4930270.x. [DOI] [PubMed] [Google Scholar]
- 8.Riggs MM, Sethi AK, Zabarsky TF, Eckstein EC, Jump RLP, Donskey CJ. Asymptomatic Carriers Are a Potential Source for Transmission of Epidemic and Nonepidemic Clostridium difficile Strains among Long-Term Care Facility Residents. Clinical Infectious Diseases. 2007;45(8):992–998. doi: 10.1086/521854. [DOI] [PubMed] [Google Scholar]
- 9.Nicolle LE, Bentley DW, Garibaldi R, Neuhaus EG, Smith PW. Antimicrobial Use in Long-Term–Care Facilities. Infection Control and Hospital Epidemiology. 2000;21(8):537–545. doi: 10.1086/501798. [DOI] [PubMed] [Google Scholar]
- 10.Peron EP, Hirsch AA, Jump RLP, Donskey CJ. Don’t Let the Steward Sail Without Us: High Rate of Unncessary Antimicrobial Use in Long-Term Care Facility; Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); Boston, MA. 2010. [Google Scholar]
- 11.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31(5):431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 12.Dubberke E, Reske K, McDonald LC, Fraser V. ICD-9 Codes and Surveillance for Clostridium difficile–associated Disease. Emerging Infectious Diseases. 2006;12(10):1576–1579. doi: 10.3201/eid1210.060016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 14.Judge GG. The Theory and practice of econometrics. Wiley; 1980. [Google Scholar]
- 15.Jump RLP, Riggs MM, Sethi AK, et al. Multihospital Outbreak of Clostridium difficile Infection, Cleveland, Ohio, USA. Emerg Infect Dis. 2010 doi: 10.3201/eid1605.071606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jury LA, Jump RLP, Sitzlar B, Guerrero DM, Donskey CJ. Improved Management of Clostridium difficile Infection in a Veterans Affairs Long-term Care Facility After the Initiation of On-Site Infectious Disease Consultation Service. Society for Healthcare Epidemiology of America (SHEA); Dallas, Texas: 2011. [Google Scholar]
- 17.Schwartz DN, Abiad H, DeMarais PL, et al. An Educational Intervention to Improve Antimicrobial Use in a Hospital-Based Long-Term Care Facility. Journal of the American Geriatrics Society. 2007;55(8):1236–1242. doi: 10.1111/j.1532-5415.2007.01251.x. [DOI] [PubMed] [Google Scholar]
- 18.Monette J, Miller MA, Monette M, et al. Effect of an Educational Intervention on Optimizing Antibiotic Prescribing in Long-Term Care Facilities. Journal of the American Geriatrics Society. 2007;55(8):1231–1235. doi: 10.1111/j.1532-5415.2007.01250.x. [DOI] [PubMed] [Google Scholar]
- 19.Loeb M. Effect of a multifaceted intervention on number of antimicrobial prescriptions for suspected urinary tract infections in residents of nursing homes: cluster randomised controlled trial. BMJ. 2005;331(7518):669–0. doi: 10.1136/bmj.38602.586343.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zabarsky TF, Sethi AK, Donskey CJ. Sustained reduction in inappropriate treatment of asymptomatic bacteriuria in a long-term care facility through an educational intervention. Am J Infect Control. 2008;36(7):476–480. doi: 10.1016/j.ajic.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Benoit SR, Nsa W, Richards CL, et al. Factors Associated with Antimicrobial Use in Nursing Homes: A Multilevel Model. Journal of the American Geriatrics Society. 2008;56(11):2039–2044. doi: 10.1111/j.1532-5415.2008.01967.x. [DOI] [PubMed] [Google Scholar]
- 22.Mylotte JM. Measuring antibiotic use in a long-term care facility. Am J Infect Control. 1996;24(3):174–179. doi: 10.1016/s0196-6553(96)90009-7. [DOI] [PubMed] [Google Scholar]
- 23.Johnson S, Samore MH, Farrow KA, et al. Epidemics of diarrhea caused by a clindamycin-resistant strain of Clostridium difficile in four hospitals. N Engl J Med. 1999;341(22):1645–1651. doi: 10.1056/NEJM199911253412203. [DOI] [PubMed] [Google Scholar]
- 24.Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353(23):2442–2449. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 25.Polk RE, Hohmann SF, Medvedev S, Ibrahim O. Benchmarking Risk-Adjusted Adult Antibacterial Drug Use in 70 US Academic Medical Center Hospitals. Clinical Infectious Diseases. 2011;53(11):1100–1110. doi: 10.1093/cid/cir672. [DOI] [PubMed] [Google Scholar]
- 26.Caprio TV, Karuza J, Katz PR. Profile of physicians in the nursing home: time perception and barriers to optimal medical practice. J Am Med Dir Assoc. 2009;10(2):93–97. doi: 10.1016/j.jamda.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Dellit TH, Owens RC, McGowan JE, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America Guidelines for Developing an Institutional Program to Enhance Antimicrobial Stewardship. Clinical Infectious Diseases. 2007;44(2):159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 28.VanSchooneveld TV, Miller H, Sayles H, Watkins K, Smith PW. Survey of Antimicrobial Stewardship Practices in Nebraska Long-Term Care Facilities. Infection Control and Hospital Epidemiology. 2011;32(7):732–734. doi: 10.1086/660855. [DOI] [PubMed] [Google Scholar]
- 29.Smith PW, Bennett G, Bradley S, et al. SHEA/APIC Guideline: Infection prevention and control in the long-term care facility. Am J Infect Control. 2008;36(7):504–535. doi: 10.1016/j.ajic.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith PW, Watkins K, Miller H, VanSchooneveld T. Antibiotic Stewardship Programs in Long-Term Care Facilities. Annals of Long-Term Care: Clinical Care and Aging. 2011;19(4):20–25. [Google Scholar]


