Summary
Background
Intimate partner violence (IPV) is associated with HIV infection. We aimed to assess whether provision of a combination of IPV prevention and HIV services would reduce IPV and HIV incidence in individuals enrolled in the Rakai Community Cohort Study (RCCS), Rakai, Uganda.
Methods
We used pre-existing clusters of communities randomised as part of a previous family planning trial in this cohort. Four intervention group clusters from the previous trial were provided standard of care HIV services plus a community-level mobilisation intervention to change attitudes, social norms, and behaviours related to IPV, and a screening and brief intervention to promote safe HIV disclosure and risk reduction in women seeking HIV counselling and testing services (the Safe Homes and Respect for Everyone [SHARE] Project). Seven control group clusters (including two intervention groups from the original trial) received only standard of care HIV services. Investigators for the RCCS did a baseline survey between February, 2005, and June, 2006, and two follow-up surveys between August, 2006, and April, 2008, and June, 2008, and December, 2009. Our primary endpoints were self-reported experience and perpetration of past year IPV (emotional, physical, and sexual) and laboratory-based diagnosis of HIV incidence in the study population. We used Poisson multivariable regression to estimate adjusted prevalence risk ratios (aPRR) of IPV, and adjusted incidence rate ratios (aIRR) of HIV acquisition. This study was registered with ClinicalTrials.gov, number NCT02050763.
Findings
Between Feb 15, 2005, and June 30, 2006, we enrolled 11 448 individuals aged 15–49 years. 5337 individuals (in four intervention clusters) were allocated into the SHARE plus HIV services group and 6111 individuals (in seven control clusters) were allocated into the HIV services only group. Compared with control groups, individuals in the SHARE intervention groups had fewer self-reports of past-year physical IPV (346 [16%] of 2127 responders in control groups vs 217 [12%] of 1812 responders in intervention groups; aPRR 0·79, 95% CI 0·67–0·92) and sexual IPV (261 [13%] of 2038 vs 167 [10%] of 1737; 0·80, 0·67–0·97). Incidence of emotional IPV did not differ (409 [20%] of 2039 vs 311 [18%] of 1737; 0·91, 0·79–1·04). SHARE had no effect on male-reported IPV perpetration. At follow-up 2 (after about 35 months) the intervention was associated with a reduction in HIV incidence (1·15 cases per 100 person-years in control vs 0·87 cases per 100 person-years in intervention group; aIRR 0·67, 95% CI 0·46–0·97, p=0·0362).
Interpretation
SHARE could reduce some forms of IPV towards women and overall HIV incidence, possibly through a reduction in forced sex and increased disclosure of HIV results. Findings from this study should inform future work toward HIV prevention, treatment, and care, and SHARE's ecological approach could be adopted, at least partly, as a standard of care for other HIV programmes in sub-Saharan Africa.
Funding
Bill & Melinda Gates Foundation, US National Institutes of Health, WHO, President's Emergency Plan for AIDS Relief, Fogarty International Center.
Introduction
The relation between intimate partner violence (IPV), HIV, and other sexually transmitted infections1–3 is bidirectional.2,4 Several pathways might explain the links between IPV and HIV infection. Forced sex might increase risk of direct transmission of HIV.1,3,5 Gender inequalities are key drivers of both IPV and HIV, and they mediate the relation between abuse and HIV transmission.1–3,5 Social norms that give men power over women increase the risk of violence against women and reduce women and girls’ ability to negotiate safe and consensual sex and seek protection from abuse.1,5 In addition, women who experience IPV and men who perpetrate IPV have a clustering of factors that increase their risk of HIV acquisition. Compared with women who are not exposed to abuse, those who have experienced lifetime IPV are more likely to report concurrent sex partners, problematic alcohol and substance use, transactional sex, and low or inconsistent condom use.1,5,6–8 Norms related to masculinity often encourage men to practise more risky sex. Evidence suggests that male perpetrators of abuse are more likely to be infected with HIV or other sexually transmitted infections, engage the services of female sex workers, perpetrate non-IPV sexual assault, and (like women who have been abused) report concurrent sex partners, problematic alcohol and substance use, and low or inconsistent condom use.1,8–10 HIV-positive status and disclosure might increase risk of IPV, and fear of violence can prevent women from learning and sharing their HIV status and from accessing treatment.4,5,11
Several IPV and HIV prevention studies have been done in sub-Saharan Africa—the region most affected by HIV/ AIDS and with some of the highest rates of IPV.12,13 However, no intervention has successfully reduced both IPV and HIV.14 Two cluster-randomised trials in South Africa, where IPV has been found to increase risk of HIV infection,15 assessed the effect of interventions that target gender norms and gender-based violence on HIV incidence. The Stepping Stones intervention was associated with a 33% reduction in incidence of herpes simplex virus type 2, reduced violence perpetration, and lower frequency of some risky behaviours, but did not affect HIV incidence.16 The Intervention with Microfinance for AIDS and Gender Equity (IMAGE) study reduced IPV incidence by 55%, increased HIV testing uptake, and reduced HIV risk behaviours in young women,17 but did not affect the rate of unprotected sex with non-spousal partners or HIV incidence.18 Most recently, the SASA! study assessed the community-level effect of a gender-focused structured IPV and HIV prevention intervention in urban Uganda. Exposure to SASA! was associated with significant reductions in social acceptability of IPV in women and sexual concurrency in men. The programme was associated with decreases in physical and sexual IPV in women, but these reductions were not significant and the trial did not report an HIV outcome.19 Although these findings suggest that integrated gender equality and HIV training interventions offer potential synergies, they show the need for innovative strategies to reduce both HIV incidence and IPV.
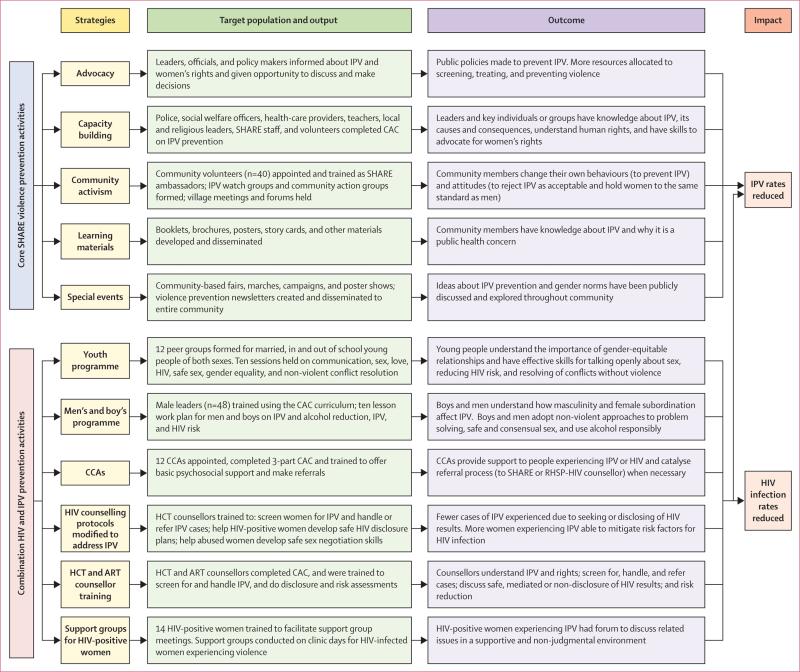
The Safe Homes and Respect for Everyone (SHARE) Project aimed to reduce physical and sexual IPV and HIV incidence. SHARE uses two main approaches: community-based mobilisation to change attitudes and social norms that contribute to IPV and HIV risk, and a screening and brief intervention to reduce HIV-disclosure-related violence and sexual risk in women seeking HIV counselling and testing (figure 1). We aimed to assess the effect on past-year IPV against women, HIV incidence, and certain sexual risk behaviours of adding SHARE into ongoing HIV treatment and prevention activities of the Rakai Health Sciences Programme (RHSP).
Figure 1. Logic model of the SHARE Project.
SHARE=Safe Homes and Respect for Everyone. IPV=intimate partner violence. CAC=community activism course. CCA=community counselling aides. RHSP=Rakai Health Sciences Program. HCT=HIV counselling and testing. ART=antiretroviral therapy.
Methods
Study design and participants
We did this comparative study in Rakai, Uganda, where IPV has been associated with HIV incidence.20 Rakai is a traditionally patriarchal society,21 with high HIV prevalence (12%) and incidence (1·2 per 100 person-years)22 and relatively high rates of IPV against women (29% in the past year).20 The study was nested in the Rakai Community Cohort Study (RCCS), described in detail elsewhere.23 RCCS is an open, community-based cohort study that takes a census, interviews, and serological surveys every 12–18 months in about 50 communities that are aggregated into 11 study clusters—originally selected to be relatively homogeneous.24 The 50 RCCS communities represent 7% of the 720 communities in the Rakai district. The 11 clusters are made up of two to eight communities each and the mean number of households per cluster is 809.22 Before the RCCS survey, investigators do a census in all residents (about 28 000 individuals) in roughly 9000 households. The investigators generate lists of eligible RCCS participants (ie, resident individuals aged 15–49 years) and contact each of the individuals and ask for their consent to enrol in RCCS. Surveys are done in person in the local language of Luganda with structured questionnaires to obtain sociodemographic, behavioural, health, and care-seeking data. Sexual network information is obtained for up to four partners in the previous year. About 90% of eligible participants have consented to an interview at each survey.22,23 Blood samples are taken for HIV detection. About 98% of participants provide a sample for HIV testing, and are offered free HIV counselling and testing and risk reduction education; about 80% request their results and counselling.25 Those individuals who test positive are referred for care and treatment,25,26 including with HAART.
Our inclusion criteria were individuals aged 15–49 years who completed an RCCS interview and provided blood for HIV testing at baseline (Feb 15, 2005, to June 30, 2006) and at two follow-up visits in Aug 30, 2006, to April 24, 2008, and June 6, 2008, to Dec 7, 2009. The baseline survey was done before the intervention was given, and follow-up appointments were staggered so that each intervention cluster had roughly the same duration of exposure to the SHARE intervention. The first follow-up began about 16 months after the baseline and spanned the intervention scale-up and full implementation phases. The final follow-up began about 35 months after baseline. The SHARE intervention was planned to last about 4 years, 1 year for each phase (after the community assessment): raising awareness, building networks, integrating action, and consolidating efforts.
Surveys were done in private by same-sex interviewers who were trained by certified Rakai Health Science Program ethics and clinical practice trainers in research ethics and good clinical practice. All interviewers were trained using WHO's guidelines for safe and ethical research on domestic violence,27 including IPV sensitisation, and how to minimise possible distress related to research on violence or sensitive topics. All women who reported IPV and requested assistance were actively referred to an RHSP counsellor trained to provide basic psychosocial support and risk reduction skills to victims. No services were made available to perpetrators of violence. The study was approved by the Western Institutional Review Board (Olympia, WA, USA), WHO's Ethics Review Committee, the Uganda Virus Research Institute's Scientific and Ethics Committee, and the Ugandan National Council of Science and Technology. All individuals gave written consent.
Cluster selection
Our study was built on a cluster-randomised trial done between April 6, 1999, and Aug 1, 2003, to assess the effect of enhanced family planning outreach in Rakai.28 In that trial, five clusters were randomly assigned to receive standard family planning services (control) and six clusters were randomly assigned to receive a family planning intervention. We used these clusters in our trial. Because of funding limitations for our trial, only four intervention clusters were randomly chosen (with a computer-generated randomisation programme) from the original six family planning intervention clusters to take advantage of existing infrastructure for community health workers. All five control clusters from the original family planning study plus the remaining two family planning intervention clusters were included in the seven control clusters for our trial. Investigators were not masked to study group assignment.
Procedures
We offered all individuals (control and intervention clusters) routine HIV prevention and treatment services, including free HIV testing, pretest and post-test counselling through community-based counsellors, and referral for care25 (panel 1). Participants in the intervention group were exposed to the SHARE violence-reduction intervention and screening and a brief intervention to reduce IPV related to HIV disclosure and risk behaviours in women seeking HIV counselling and testing. SHARE29 is an ecological framework to intervene against drivers of IPV and HIV transmission at the individual, relationship, and societal levels (figure 1). SHARE was based on the stages of change theory,30 which is most commonly used at the individual-level but adapted for use in Rakai at the community-level by use of the Raising Voices Resource Guide for Organizations in East and Southern Africa.31 SHARE involved five distinct phases and used five main strategies (panel 2).29
SHARE staff included three women and two men with tertiary education, training in HIV and psychosocial counselling, and fluency in English and Luganda language, who received 4 weeks of training on IPV awareness and prevention, provision of violence-related support, and ethical protection of participants. SHARE also appointed and trained 40 community volunteers and 12 community counselling aides to work with core SHARE staff (appendix). Additionally, SHARE provided focused programmes for men, boys, and young people and integrated violence prevention into RHSP's existing HIV counselling and testing and HAART programmes (appendix).
Outcomes
Our primary endpoints were self-reported experience and perpetration of past-year IPV (physical, emotional, and sexual) and laboratory-based diagnosis of HIV incidence in the study population. Sexual IPV was defined as penetrative and non-penetrative sex, and other unwanted sexual acts. Abused women commonly experience more than one type of violence in Rakai,20 but we measured physical and sexual IPV separately to assess whether SHARE had a differential effect on these two outcomes. Our secondary endpoints were forced sex (defined as unwanted, physically forced penetrative sex) and HIV risk behaviour and disclosure (respondent's and primary partner's) in the last year of the intervention. We used an adaptation of the conflict tactics scales32 to measure each type of IPV (appendix). HIV risk behaviours were total number of sex partners, number of non-marital sex partners, alcohol use with last sex, and condom use, and disclosure of their HIV status in the past year (appendix).
HIV status was established from venous blood samples with two enzyme immunoassays (Vironostika HV-1 [Organon Teknika, Charlotte, NC, USA] and Cambridge Biotech [Worcester, MA, USA]), with Western blot (bioMérieux VITEK, St Louis, MO, USA) confirmation of discordant enzyme immunoassay results and all seroconversions. We analysed HIV incidence data during the SHARE trial to assess intervention efficacy before the SHARE baseline (2004–05), pre-baseline similarity between study groups, and after the trial ended (2010–11) to assess postintervention sustainability of any effects on HIV incidence.
We also obtained data for social and demographic characteristics, HAART use, and male circumcision. The SHARE trial coincided with the introduction of HAART in June, 2004, by the Government of Uganda, and the last year of follow-up of a male circumcision for HIV prevention trial33 (stopped in December, 2006) and provision of circumcision services thereafter. Circumcision status was assessed by questions asking men, “are you circumcised?” and women were asked “is/ was your husband/recent sexual partner circumcised?” Female reported circumcision status of male partners has been validated in this population.34 HAART uptake in both groups of the trial was measured at baseline and follow-up with a combination of RHSP HAART clinic data and self-reported data from the RCCS survey.
Statistical analysis
We postulated that exposure to SHARE would lead to a minimum 10% relative reduction of each type of violence in the intervention group, assuming an initial physical IPV prevalence of about 20% and sexual IPV prevalence of about 14·5%. Because the study was nested within the RCCS, the effective sample size was established by enrolment into the cohort. For IPV outcomes, the study was powered on the basis of an approximate intervention group population of about 3500 individuals and a control population of roughly 11 100 people, giving the study 80% power to detect the hypothesised IPV reductions (two sided α=0·05). For the HIV incidence outcome (about 1·2 per 100 person-years), we expected about 4753 person-years of exposure in the intervention group and about 16 969 person-years of exposure in the control group, and estimated 80% power (two sided α=0·05) to detect 36% lower HIV incidence in the intervention group compared with the control group. After selection of intervention clusters, sample sizes were higher for the intervention groups and lower for the control groups than we had initially anticipated, which had little effect on the power of the study.
Social and demographic characteristics, circumcision status, IPV, risk behaviours, HIV prevalence, and HAART use were measured at baseline to assess comparability between study groups. Baseline differences between groups were estimated using Pearson's χ2 and Fisher's exact tests for differences in proportions, and t-tests and Wilcoxon rank-sum tests for continuous variables. These outcomes and HIV incidence were assessed at follow-up to show differences between study groups with an intention-to-treat approach in which all eligible participants with complete data were included in the analysis by study group, irrespective of crossovers or direct exposure to SHARE activities. Differences in loss-to-follow-up between the two groups were assessed by χ2 tests. We established study retention on the basis of the number of participants interviewed at either of the two follow-up visits. We used two-tailed p values (p<0·05) for statistical inference.
We analysed all outcomes separately for men and women and for the first and second follow-up visits. Our analysis included respondents who attended both the first and second follow-up visits plus respondents who were not available in follow-up 1 but were then available in follow-up 2. We accounted for the cluster effect by fitting a random-intercept model at the cluster level to test for potential within-cluster correlation. We analysed IPV and secondary HIV risk behaviour outcomes as dichotomous variables. In view of the low correlation within clusters and difficulty of verification of the random effects assumptions because of the small number of clusters (n=11), we fit generalised linear models for the main analysis. We used a modified Poisson multivariate regression that combines a log Poisson regression model with number of people as the offset, and robust variance estimation, allowing for an approximation to the log binomial model to estimate adjusted prevalence risk ratios (aPRRs) for the IPV and HIV risk behaviour outcomes.35 We fitted a generalised linear model using a log link with the observed population as an offset and an assumed underlying Poisson distribution.
For analysis of HIV incidence, we calculated person-years of exposure from baseline to the last negative HIV result when the person remained negative, or to the midpoint of the interval between the last negative and first positive tests for seroconverters. HIV incidence was estimated per 100 person-years. We used Poisson multivariate regression models with robust variance estimation to estimate the adjusted incidence rate ratios (aIRR) of HIV acquisition between intervention to control groups. Our analysis was preplanned and did not adjust for multiple comparisons, but we do account for them in the interpretation.36 We used Stata/SE version 12 for data analysis. This trial is registered with ClinicalTrials.gov, number NCT02050763.
Role of the funding source
The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data and had final responsibility for the decision to submit for publication.
Results
Between Jan 17, 2005, and May 24, 2006, 21 636 individuals from 11 clusters were assessed for eligibility by the RCCS census team. We enrolled 11 448 men and women aged 15–49 years: 6111 in the control group clusters and 5337 in the intervention group clusters (figure 2). 8565 (75%) attended either round of follow-up (4499 [74%] in the control vs 4068 [76%] in the intervention; p=0·0012), consistent with average RCCS follow-up rates of about 75% between successive visits.22,23 Across the entire trial, 7842 (69%) participants were assessed at follow-up 1 and 6526 (57%) participants at follow-up 2. Cumulative retention rates (ie, at follow-up 2) were higher in the control group (3564 [58%]) than in the intervention group (2962 [56%]; p=0·0031), and in women (3939 [59%]) than in men (2587 [55%]; p=0·0001). Because of the open nature of the cohort, loss to follow-up was mainly because participants could not be located. The study lasted 4 years and 7 months.
Figure 2. Study profile.
*Four intervention clusters from the Rakai Community Cohort Study (RCCS) randomly chosen; two of these and five RCCS control clusters became the control populations. †People who could not be re-contacted were those who could not be contacted at the first follow-up but available at follow-up 2; those lost to follow-up were individuals who were not available at first follow-up and second follow-up.
Baseline characteristics of the two study groups were broadly similar (table 1). There were more younger (age 15–19 years) and older (age 35 years and older) individuals in the intervention group than in the control group, and control group participants had higher levels of secondary (or more) education than had the intervention group. Physical and sexual IPV in women, forced sex, and HIV results disclosure were more common in the control group than in the intervention group at baseline. Baseline HIV prevalence was higher in the control (12%) than in the intervention group (9%) for both women and men (table 1).
Table 1.
Baseline characteristics and risk behaviours in past year
| Women (n=6702) |
Men (n=4746) |
|||||
|---|---|---|---|---|---|---|
| Control (n=3544; 53%) | Intervention (n=3158; 47%) | p value | Control (n=2567; 54%) | Intervention (n=2179; 46%) | p value | |
| Age, years | 0·0012 | 0·0343 | ||||
| 15-19 | 374 (11%) | 369 (12%) | .. | 199 (8%) | 225 (10%) | .. |
| 20-24 | 887 (25%) | 766 (24%) | .. | 484 (19%) | 397 (18%) | .. |
| 25-29 | 956 (27%) | 755 (24%) | .. | 590 (23%) | 474 (22%) | .. |
| 30-34 | 629 (18%) | 538 (17%) | .. | 503 (20%) | 437 (20%) | .. |
| ≥35 | 698 (20%) | 730 (23%) | .. | 791 (31%) | 646 (30%) | .. |
| Religion | 0·0949 | 0·4802 | ||||
| Christian | 2948 (83%) | 2621 (84%) | .. | 2126 (83%) | 1778 (82%) | .. |
| Muslim | 566 (16%) | 481 (15%) | .. | 421 (16%) | 371 (17%) | .. |
| Other or no religion | 22 (1%) | 34 (1%) | .. | 19 (1%) | 22 (1%) | .. |
| Education level | 0·0425 | 0·0110 | ||||
| None | 523 (15%) | 526 (17%) | .. | 262 (10%) | 222 (10%) | .. |
| Primary | 1981 (56%) | 1769 (56%) | .. | 1435 (57%) | 1304 (60%) | .. |
| Secondary or higher | 1040 (30%) | 863 (27%) | .. | 870 (34%) | 653 (30%) | .. |
| Marital status | 0·5109 | 0·0491 | ||||
| Never married | 476 (13%) | 456 (14%) | .. | 575 (22%) | 528 (24%) | .. |
| Currently married or in union | 2991 (84%) | 2635 (83%) | .. | 1949 (76%) | 1596 (73%) | .. |
| Previously married | 77 (2%) | 67 (2%) | .. | 46 (2%) | 55 (3%) | .. |
| Circumcised participants (if men) or partners (if women) | 1191 (34%) | 1017 (32%) | 0·2612 | 381 (20%) | 376 (22%) | 0·2501 |
| Past-year IPV | ||||||
| Emotional | 878 (25%) | 772 (25%) | 0·7574 | 771 (30%) | 626 (29%) | 0·2163 |
| Physical | 653 (18%) | 523 (17%) | 0·0451 | 316 (12%) | 242 (11%) | 0·1853 |
| Sexual | 580 (16%) | 415 (13%) | 0·0005 | 147 (6%) | 92 (4%) | 0·0153 |
| Past-year forced sex | 500 (14%) | 357 (12%) | 0·0001 | 101 (4%) | 58 (3%) | 0·0192 |
| >1 sexual partners in past year | 214 (6%) | 157 (5%) | 0·0621 | 1208 (47%) | 914 (42%) | 0·0001 |
| Alcohol with sex (past year) | 887 (25%) | 821 (26%) | 0·3654 | 943 (39%) | 876 (40%) | 0·2019 |
| Number non-marital sex partners in past year | 0·0561 | 0·0001 | ||||
| 0 | 2516 (82%) | 2268 (84%) | .. | 1378 (54%) | 1280 (59%) | .. |
| 1 | 483 (16%) | 392 (15%) | .. | 800 (31%) | 608 (28%) | .. |
| ≥2 | 69 (2%) | 42 (2%) | .. | 389 (15%) | 291 (13%) | .. |
| Condom use in past year | 0·7330 | 0·0911 | ||||
| Yes, always | 316 (9%) | 274 (9%) | .. | 398 (16%) | 378 (17%) | .. |
| None or inconsistent | 3228 (91%) | 2884 (91%) | .. | 2169 (85%) | 1801 (83%) | .. |
| Partner's disclosure of HIV status (past year) | 669 (19%) | 481 (15%) | 0·0001 | 544 (22%) | 365 (17%) | 0·0001 |
| Self-disclosure of HIV status to partner (past year) | 949 (27%) | 764 (24%) | 0·0210 | 647 (26%) | 411 (19%) | 0·0001 |
| HIV prevalence* | 448/3175 (14%) | 343/2814 (12%) | 0·0026 | 253/2896 (11%) | 184/2789 (9%) | 0·0288 |
| HAART uptake* | 49/448 (11%) | 46/343 (13%) | 0·2888 | 17/253 (7%) | 19/184 (10%) | 0·1758 |
Data are n (%), unless otherwise indicated.
Population size differs due to availability of blood samples.
Table 2 shows IPV outcomes by group. The proportions of women who experienced physical IPV, sexual IPV, and forced sex were significantly lower in the SHARE intervention group at the second follow-up than in the control group after adjustment for baseline IPV, age, education, and marital status (table 2). The intervention did not significantly reduce women's experiences of emotional IPV (table 2). Men's reports of emotional and physical IPV perpetration decreased over the course of the trial in both groups, but reported final IPV rates at follow-up did not differ significantly (table 2).
Table 2.
Effect of intervention on IPV outcomes
| Control | Intervention | PRR (95% CI) | aPRR*(95% CI) | |
|---|---|---|---|---|
| Women | ||||
| Experience of past-year emotional (verbal) abuse | ||||
| Follow-up 1 | 498/2338 (21%) | 495/2257 (22%) | 1·03 (0·92-1·15) | 1·02 (0·92-1·14) |
| Follow-up 2 | 409/2039 (20%) | 311/1737 (18%) | 0·89 (0·78-1·02) | 0·91 (0·79-1·04) |
| Experience of past-year physical IPV | ||||
| Follow-up 1 | 397/2426 (16%) | 353/2342 (15%) | 0·92 (0·81-1·05) | 0·97 (0·85-1·11) |
| Follow-up 2 | 346/2127 (16%) | 217/1812 (12%) | 0·74 (0·63-0·86) | 0·79 (0·67-0·92) |
| Experience of past-year sexual IPV | ||||
| Follow-up 1 | 292/2337 (13%) | 296/2257 (13%) | 1·05 (0·90-1·22) | 1·12 (0·96-1·31) |
| Follow-up 2 | 261/2038 (13%) | 167/1737 (10%) | 0·75 (0·62-0·90) | 0·80 (0·67-0·97) |
| Experience of past-year forced sex | ||||
| Follow-up 1 | 261/2337 (11%) | 262/2257 (12%) | 1·04 (0·88-1·22) | 1·12 (0·95-1·32) |
| Follow-up 2 | 232/2038 (11%) | 145/1737 (8%) | 0·73 (0·60-0·89) | 0·79 (0·65-0·96) |
| Men | ||||
| Perpetration of past-year emotional (verbal) IPV | ||||
| Follow-up 1 | 520/1586 (33%) | 401/1367 (29%) | 0·92 (0·80-0·99) | 0·88 (0·78-0·98) |
| Follow-up 2 | 315/1407 (22%) | 251/1104 (23%) | 1·02 (0·88-1·18) | 0·99 (0·85-1·16) |
| Perpetration of past-year physical IPV | ||||
| Follow-up 1 | 185/1641 (11%) | 132/1433 (9%) | 0·82 (0·66-1·01) | 0·80 (0·64-1·00) |
| Follow-up 2 | 124/1437 (9%) | 99/1150 (9%) | 1·00 (0·77-1·28) | 1·00 (0·77-1·30) |
| Perpetration of past-year sexual IPV | ||||
| Follow-up 1 | 73/1586 (5%) | 56/1367 (4%) | 0·89 (0·63-1·25) | 0·90 (0·63-1·28) |
| Follow-up 2 | 53/1407 (4%) | 30/1104 (3%) | 0·72 (0·46-1·12) | 0·81 (0·52-1·26) |
| Perpetration of past-year forced sex | ||||
| Follow-up 1 | 47/1586 (3%) | 38/1367 (3%) | 0·94 (0·61-1·43) | 1·00 (0·65-1·55) |
| Follow-up 2 | 39/1407 (3%) | 24/1104 (2%) | 0·78 (0·47-1·30) | 0·85 (0·50-1·42) |
Data are n/number who responded (%) unless otherwise stated. IPV=intimate partner violence. PRR=prevalence rate ratio. aPRR=adjusted prevalence rate ratio.
Effects of intervention adjusted for baseline age, baseline education, baseline marital status, and baseline experience of IPV victimisation (women) or perpetration (men), according to type measured.
Women's reports of their own disclosure of HIV status and their partner's disclosure of his HIV status were higher in the intervention than in the control group at second follow-up (table 3). Men in the intervention group more frequently reported their primary female partner's disclosure of her HIV status, and their own disclosure of HIV status (table 3). HIV incidence for both sexes combined was lower in the intervention group than in the control group after adjustment (table 4). The intervention was associated with a significantly lower HIV incidence in men (p=0·0304) but did not differ in women (p=0·1020; table 4).
Table 3.
Effect of intervention on risk behaviours and HIV disclosure
| Control | Intervention | PRR (95% CI) | aPRR* (95% CI) | |
|---|---|---|---|---|
| Women | ||||
| >1 sexual partners in past year | ||||
| Follow-up 1 | 214/2426 (9%) | 201/2341 (9%) | 1·00 (0·98-1·01) | 1·00 (0·98-1·01) |
| Follow-up 2 | 207/2127 (10%) | 157/1812 (9%) | 0·99 (0·97-1·01) | 0·98 (0·97-1·00) |
| Non-marital sex partners in past year | ||||
| Follow-up 1 | 377/2224 (17%) | 367/2127 (17%) | 1·02 (0·89-1·16) | 1·01 (0·90-1·15) |
| Follow-up 2 | 396/2006 (20%) | 306/1693 (18%) | 0·92 (0·80-1·05) | 0·89 (0·78-1·01) |
| Alcohol use with sex in past year | ||||
| Follow-up 1 | 647/2426 (27%) | 620/2342 (27%) | 0·99 (0·90-1·09) | 0·99 (0·91-1·09) |
| Follow-up 2 | 435/2127 (21%) | 361/1812 (20%) | 0·97 (0·86-1·10) | 0·96 (0·85-1·09) |
| Condom use in past year | ||||
| Follow-up 1 | 201/2362 (9%) | 216/2282 (10%) | 1·11 (0·93-1·34) | 1·12 (0·95-1·33) |
| Follow-up 2 | 192/1170 (16%) | 157/931 (17%) | 1·03 (0·85-1·25) | 1·01 (0·84-1·21) |
| Partner's disclosure of HIV status in past year | ||||
| Follow-up 1 | 497/2340 (21%) | 492/2257 (22%) | 1·03 (0·92-1·15) | 1·03 (0·92-1·15) |
| Follow-up 2 | 455/2037 (22%) | 457/1740 (26%) | 1·18 (1·05-1·32) | 1·18 (1·06-1·32) |
| Self-disclosure of HIV status to partner in past year | ||||
| Follow-up 1 | 874/2339 (37%) | 880/2257 (39%) | 1·04 (0·97-1·12) | 1·05 (0·97-1·12) |
| Follow-up 2 | 752/2036 (37%) | 731/1740 (42%) | 1·14 (1·05-1·23) | 1·15 (1·06-1·24) |
| Men | ||||
| >1 sexual partners in past year | ||||
| Follow-up 1 | 829/1612 (51%) | 668/1406 (48%) | 0·92 (0·90-0·99) | 0·96 (0·90-1·03) |
| Follow-up 2 | 676/1437 (47%) | 538/1150 (47%) | 0·99 (0·91-1·08) | 1·02 (0·94-1·10) |
| Non-marital sex partners in past year | ||||
| Follow-up 1 | 804/1641 (49%) | 642/1433 (45%) | 0·91 (0·85-0·99) | 0·95 (0·89-1·02) |
| Follow-up 2 | 645/1437 (45%) | 495/1150 (43%) | 0·96 (0·88-1·05) | 0·98 (0·90-1·06) |
| Alcohol use with sex (past year) | ||||
| Follow-up 1 | 787/1612 (49%) | 697/1406 (50%) | 1·02 (0·94-1·09) | 1·03 (0·97-1·09) |
| Follow-up 2 | 670/1437 (47%) | 525/1150 (46%) | 0·98 (0·90-1·06) | 0·98 (0·91-1·05) |
| Condom use in past year | ||||
| Follow-up 1 | 217/1613 (14%) | 216/1408 (15%) | 1·14 (0·96-1·36) | 1·06 (0·90-1·24) |
| Follow-up 2 | 188/806 (23%) | 153/601 (26%) | 1·09 (0·91-1·31) | 1·04 (0·87-1·24) |
| Partner's disclosure of HIV status in past year | ||||
| Follow-up 1 | 425/1607 (27%) | 372/1374 (27%) | 1·02 (0·91-1·15) | 1·09 (0·97-1·22) |
| Follow-up 2 | 401/1409 (29%) | 385/1107 (35%) | 1·22 (1·09-1·37) | 1·24 (1·11-1·39) |
| Self-disclosure of HIV status to partner in past year | ||||
| Follow-up 1 | 549/1607 (34%) | 494/1374 (36%) | 1·05 (0·95-1·16) | 1·10 (1·00-1·21) |
| Follow-up 2 | 444/1409 (32%) | 404/1107 (37%) | 1·16 (1·04-1·29) | 1·17 (1·05-1·31) |
All data are n/number who responded (%), unless otherwise indicated. PRR=prevalence rate ratio. aPRR=adjusted prevalence rate ratio.
Effects of intervention adjusted for baseline age, baseline education, baseline marital status and baseline number of non-marital sex partners. Each outcome was also adjusted for its baseline measure.
Table 4.
Incidence of HIV by study group
| Control |
Intervention |
Comparisons |
||||||
|---|---|---|---|---|---|---|---|---|
| Incident cases (person-years) |
Participants* | Incident cases per 100 person-years |
Incident cases (person-years) |
Participants* | Incident cases per 100 person-years |
IRR (95% CI); p value |
aIRR† (95% CI); p value |
|
| Women | 71 (6154) | 2038 | 1·15 | 56 (5649) | 1925 | 0·99 | 0·86 (0·61-1·22); p=0·396 | 0·72 (0·49-1·07); p=0·1020 |
| Men | 48 (4237) | 1435 | 1·13 | 27 (3861) | 1326 | 0·70 | 0·62 (0·39-0·99); p=0·045 | 0·59 (0·35-0·95); p=0·0304 |
| Overall | 119 (10 390) | 3473 | 1·15 | 83 (9510) | 3251 | 0·87 | 0·76 (0·58-1·01); p=0·057 | 0·67 (0·46-0·97); p=0·0362 |
IRR=incidence rate ratio. aIRR=adjusted incidence rate ratio.
Participants who contributed to the person-year calculation.
Adjusted for baseline HIV prevalence by trial group, baseline age, baseline education, baseline marital status, and circumcision status of men or primary male partner of female respondents.
The appendix contains a table to show secular trends in HIV incidence rates by groups for the RCCS population during five consecutive RCCS survey intervals between 2004 and 2011. The first interval (2004–05) was before study initiation. At baseline (2005–06), HIV incidence did not significantly differ between groups (1·05 per 100 person-years). HIV incidence was lower in the intervention than in the control group during the intervention roll-out phase (2006–08), at the first follow-up interval (aIRR 0·60, 95% CI 0·42–0·84), and full implementation phase (2008–09) at the second follow-up interval (0·66, 0·49–0·90). During the scale-up and full implementation phases of the intervention (2006–09), HIV incidence decreased in the intervention group, compared with pre-baseline, and at follow-up intervals one and two. By contrast, incidence increased in the control group during these two follow-up intervals. However, during the post-SHARE follow-up (2010–11) incidence was similar between study groups, suggesting that the difference in HIV incidence between groups was not sustained post-intervention.
Although baseline uptake of HAART was similar between groups (table 1), more women than men infected with HIV were taking HAART in both the control (49 [11%] vs 17 [7%]) and intervention (46 [13%] vs 19 [10%]) clusters, which might have contributed to the larger decrease in HIV incidence in men at second follow-up. Men and women's use of HAART increased over the course of the trial and remained similar at final follow-up for women in the control group at 34% (111 of 328) versus the intervention group at 32% (72 of 227; p=0·6009). In men, final follow-up HAART use in the control group was 24% (46 of 193) versus 33% (39 of 118, p=0·0768) in the intervention group. We noted no significant correlation within clusters for all IPV outcomes (r=0, p=1·0) and previous research established low within-cluster correlation for HIV outcomes.24
Discussion
SHARE significantly reduced reports of women's physical IPV, sexual IPV, and forced sex. However, male-reported perpetration of IPV was not significantly affected by the intervention and differences in emotional IPV were not significant. SHARE was also associated with significant increases in disclosure of HIV status in men and women. Finally, SHARE was associated with a lower HIV incidence during the intervention period, but this reduction was not maintained after SHARE ended, suggesting that continued exposure to the intervention might be need to achieve a sustained effect.
Reports of physical and sexual IPV victimisation from women were much more common than were reports of perpetration from men, as seen in other studies37 and consistent with findings from the SASA! trial in Uganda (panel 3).40 Although some of these differences might be because of less effective programming with men or difficulty involving men in SHARE activities,29 we believe that there was substantial under-reporting of IPV perpetration. Male under-reporting could be due to a social desirability bias and stigma associated with disclosing abuse37 or gender differences in patterns of reporting abusive behaviours.21 As in the SASA! trial,40 the discrepancy between reports of sexual violence between men and women were larger than were those of physical violence, possibly due to social norms condoning physical male disciplinary violence in Uganda.21,41 Marital rape (ie, forced sex) is not illegal in Uganda and choices about when to have sex are considered to be a man's prerogative.42 Therefore, men might have under-reported sexual violence because they did not perceive it to be abuse. For these reasons, we considered women's accounts of IPV to be the more reliable outcomes.
The intervention was associated with a lower HIV incidence in the intervention group (aIRR 0·67 [95% CI 0·46–0·97]), which was more pronounced in men (0·59 [0·35–0·95]) than in women (0·72 [0·49–1·07]). Although we cannot identify the specific cause of these reductions, decreases in forced sex and increases in HIV disclosure might have contributed to reductions in HIV incidence in the intervention group.
There is a plausible biological explanation for a causal association between decreases in forced sex and falls in HIV infection in women. Forced, penetrative sex might induce a traumatic inflammatory response that recruits target cells to the vaginal or cervical site of injury3,43 and disrupts the integrity of the epithelial barrier, increasing susceptibility to HIV.3 However, the small number of women reporting forced sex in our study suggests that the reductions in forced sex would have only had a slight effect on female HIV incidence. Women who experience IPV might also have heightened susceptibility to HIV infection due to a clustering of their own risk behaviours and those of their violent male partner. Unmeasured behaviours of women (eg, anal sex, commercial sex work) and their male partners (eg, substance use, undisclosed sexual assault on partners and non-partners) might account for the decreases of HIV incidence in our study. Thus, although interpretation of the mechanisms by which reductions in IPV might have contributed to differences in HIV incidence is problematic, our results are supported by an observational study20 from Rakai that estimated that the adjusted population fraction of HIV attributable to IPV (emotional, physical, and sexual) was 22%.
The intervention was also associated with significantly increased rates of self-disclosure and partner-disclosure of HIV results, which might have contributed to differences in HIV incidence. Increased disclosure suggests a rise in communication between partners and improved discussion and implementation of HIV risk reduction. Disclosure might have motivated more men and women, particularly in the intervention group, to seek HIV testing or access medical care, including HAART.
This trial coincided with the introduction of HAART and a HIV prevention trial that circumcised men.33 The use of HAART was higher in women than in men at baseline, which might have contributed to lower rates of new infections in men, but HAART was equitably provided to all participants and so availability of treatment should not have biased incidence between study groups. Although circumcision reduces male HIV acquisition,33,44 it is unlikely to account for differences in rates for HIV acquisition in men between study groups since circumcision prevalence was similar at baseline and we controlled for circumcision status at follow-up.
Our trial has several limitations. Cluster randomisation was based on a randomisation scheme designed for a previous family planning cluster-randomised trial and due to financial constraints, we could only introduce the intervention into four of the 11 clusters, leading to an imbalance in numbers and poor comparability for some variables at baseline. However, adjustment for the covariates that differed at baseline probably minimised confounding. Additionally, the previous family planning intervention increased hormonal contraceptive use and decreased the pregnancy rate, but had no effect on condom use,28 and thus probably had little to no effect on the results of our study. The retention rate (75% at either follow-up visit) was low and differed between groups and so it is possible that selective losses to follow-up might have affected trial results; higher-risk individuals were more likely to discontinue than were those at a lower risk (data not shown). Additionally, retention rates were somewhat higher in the control than in the intervention group (figure 2) so selective loss to follow-up of higher-risk individuals in the intervention group might have differentially reduced HIV incidence.
Intervention group participants might have been more motivated to report lower IPV than were those in the control group due to a social desirability bias. However, a meta-analysis suggested low to moderate correlation between self-reports of IPV and validated scores of social desirability.45 Although contamination between groups could have occurred (eg, by police or community services whose jurisdictions covered both areas), we believe it is unlikely because RCCS clusters are geographically separated and any contamination would bias results towards the null.
As noted in other studies in Africa,46 reports of self-disclosure of HIV status were higher than were reports of partner-disclosure in both men and women, which might be due to a social desirability bias. Alternatively, respondents who were HIV-infected and in violent relationships might have feared to share their results with a partner, but falsely claim that they did so.46 Self-reports of HIV status disclosure might be potentially of questionable reliability, but reports of partner disclosure significantly increased in both men and women with exposure to SHARE, and might have contributed to differences in HIV incidence.
We did not obtain data for the frequency or severity of IPV. Thus, we were not able to distinguish repeated abuse from isolated events, and severe and moderate forms of violence from minor abuse. We recommend that future research assess the severity and frequency of violence. As noted, we also did not measure all sexual risk behaviours that are potential pathways between IPV and HIV infection and thus we do not know how they might have contributed to decreases in HIV incidence.
In conclusion, exposure to SHARE was associated with significant decreases in both intimate partner violence and HIV incidence in Rakai. These findings hold great potential for HIV programmes and should inform future work toward universal targets for HIV prevention, treatment, and care. We believe our findings can be extended to other settings in sub-Saharan Africa and as countries in the region are scaling up combination HIV prevention and interventions to eliminate mother-to-child transmission of HIV, stakeholders should consider the potential use of investigating IPV prevention into HIV counselling and testing, treatment, and care services. The SHARE community mobilisation approach is a potential model that countries can incorporate into their national programmes and we recommend that it be replicated and rigorously assessed through longitudinal research to investigate its effect in other settings. It is also important to establish which risk behaviours mediate the relation between IPV and HIV infection. Finally, as international initiatives emphasise the importance of health strategies that promote gender equality and prioritise the needs of women and girls, this study's findings suggest that the SHARE model is a promising, gender-responsive intervention to reduce both IPV against women and infection with HIV.
Supplementary Material
Panel 1: Rakai Health Sciences Program's routine HIV prevention and treatment services.
Both groups were given Rakai Health Sciences Program's routine HIV prevention and treatment services, which included the following items:
Provision of free condoms
Syndromic sexual transmitted infection treatment
General medical care
Prevention of mother-to-child HIV transmission
HIV prevention and general health education
HIV monitoring and treatment: people with HIV who accepted voluntary counselling and testing were referred for free CD4 cell count assessment and HIV care, including co-trimoxazole prophylaxis for opportunistic diseases, bednets for malaria prevention, clean water containers and hypochlorite for prevention of diarrhoea, and positive living education
HAART: individuals were started on standard first-line ART when they reached WHO stage IV disease or had a CD4 cell count of lower than 250 cells per μL; individuals taking HAART were monitored via CD4 cell counts and HIV viral lo ads
Panel 2: Safe Homes and Respect for Everyone (SHARE) Intervention Structure and Strategy.
Phase 1: Community assessment (2000–04)
This pre-intervention phase aimed to assess the magnitude, determinants, correlates, and outcomes of intimate partner violence (IPV), and to develop relationships with the community, hire and train SHARE staff, and prepare for intervention rollout.
Phase 2: Raising awareness (2005–06)
Intervention rollout: aimed to introduce SHARE and stimulate dialogue on IPV, help people to define and understand it, and raise awareness about IPV's negative consequences.
Phase 3: Building networks (2006–07)
Intervention scale-up aimed to prepare the community to change their attitudes about IPV and reduce their own violent behaviours.
Phase 4: Integrating action (2007–08)
Full intervention implementation; goal was to make actions against IPV part of everyday life and institutional policies and practices.
Phase 5: Consolidating efforts (2008–09)
This final, intervention wind-down phase aimed to transition SHARE staffmembers away from routine work in the intervention clusters while community members assumed the day-to-day tasks of the project. The goal was to ensure that the community could sustain the reduced levels of IPV through development of long-term action plans and local bylaws that continue prevention efforts.
Panel 3: Research in context.
Systematic review
In January, 2004, we searched PubMed, Scopus, and PsycINFO for English-language systematic reviews and randomised controlled trials of community-based interventions to reduce intimate partner violence (IPV) and HIV infection. We used a combination of text terms and subject headings, and open-ended search dates, but did not identify any reviews or trials on integrated IPV and HIV reduction efforts. A 2000 review paper recommended that HIV prevention programmes integrate multidisciplinary approaches to account for the role that violence has in women's lives.5 In response, we used a public health process to integrate the Safe Homes and Respect for Everyone (SHARE) violence reduction intervention into Rakai Health Sciences Program's (RHSP) existing HIV services in Uganda.29 With data from the Rakai Community Cohort Study, we estimated the prevalence, determinants, and correlates of IPV and HIV infection. Next, we chose two prevention approaches adapted to meet the needs of the population. We chose the Resource Guide for Mobilising Communities to Prevent Domestic Violence31 and Stepping Stones38 because both were developed for use in rural Africa and had been implemented in Uganda; both were community-based, developed for sustainability, and focused on change at multiple levels; and together they included strategies to address IPV, gender norms and inequalities, HIV, communication and relationship skills among adults and adolescents. We also used recommendations5 to add procedures to RHSP's HIV counselling and testing programmes to identify and assist women at risk of IPV. Reviews done in 201014 and 201339 examined interventions addressing HIV and IPV globally and in sub-Saharan Africa. Both highlighted the promise multifaceted community-based interventions hold for improving women's health and empowerment, but noted that although some previous interventions have reduced IPV, none have shown a decrease in HIV infection.14,39
Interpretation
SHARE is the first study of behavioural interventions to show significant decreases in both IPV and HIV incidence. As for Stepping Stones,16 IMAGE,18 and SASA!,19 SHARE was multifaceted and done at the population-level. Uniquely, SHARE was nested within the infrastructure of an existing HIV research and service provision organisation. The SHARE intervention model could inform other HIV programmes’ efforts to address IPV and HIV simultaneously, and its approach could be adopted, at least partly, as a standard of care for other HIV programmes in sub-Saharan Africa. HIV counselling and testing provides an opportunity to screen for and address IPV and counsellors could mitigate important contextual risks factors for HIV transmission that are associated with experiences of violence. We also recommend5 that HIV counselling and testing services consider the needs of violence survivors and offer risk reduction counselling and disclosure support in the context of women's risk of abuse.
Acknowledgments
The Rakai Community Cohort Study (RCCS) was funded by the Bill & Melinda Gates Foundation (22006.02) and the US National Institutes of Health (U1AI51171). The Fogarty International Center (5D43TW001508 and 2D43TW000010-19-AITRP) contributed to training RHSP's junior investigators. Measurement of the effect of the intervention on intimate partner violence was funded through a grant from the WHO's Department of Reproductive Health and Research (A55085). The SHARE intervention was funded by the President's Emergency Plan for AIDS Relief (CoAg GH000817). Analysis of the research reported in this publication was supported by a Ruth L Kirschstein National Research Service Award from the National Institute of Mental Health (F31MH095649) and a training grant (T32DA023356). Publication of this article was funded in part by Open Access Promotion Funds from the Johns Hopkins University Libraries and the University of California, San Diego. The authors thank the Rakai Health Sciences Program for their efforts in study design, implementation, data collection and management; the SHARE intervention team for their dedication to successful roll out of the violence prevention project; Lawrence Moulton for helpful comments on the dissertation from which this work is drawn; and David Celentano for extensive reviews and feedback on the manuscript. We especially thank the study participants for providing extensive information for this research.
Footnotes
Contributors
JAW, DS, RHG, and HB oversaw the design and conduct of the trial, and participated in all data analyses and in writing the report. JK was the trial coordinator and did the Rakai Community Cohort Study in the field. FN, GN, and JK participated in study implementation and data analysis and interpretation. JCC provided technical assistance with violence measures, advised on the design and analysis of the trial, and contributed to interpretation of results. MT, AN, and JS participated in statistical analysis and data management. All authors contributed to the preparation of the paper and approved the final version.
Declaration of interests
We declare no competing interests.
References
- 1.Campbell JC, Baty ML, Ghandour R, Stockman J, Francisco L, Wagman J. The intersection of violence against women and HIV/AIDS: a review. Int J Inj Contr Saf Promot. 2008;15:221–31. doi: 10.1080/17457300802423224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kouyoumdjian FG, Findlay N, Schwandt M, Calzavara LM. A systematic review of the relationships between intimate partner violence and HIV/AIDS. PLoS One. 2013;8:11. doi: 10.1371/journal.pone.0081044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell JC, Lucea MB, Stockman JK, Draughon JE. Forced sex and HIV risk in violent relationships. Am J Reprod Immunol. 2013;69(suppl 1):41–44. doi: 10.1111/aji.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maman S, Mbwambo J, Hogan N, Kilonzo G, Campbell JC, Weiss E. HIV-positive women report more lifetime partner violence: findings from a voluntary counseling and testing clinic in Dar es Salaam, Tanzania. Am J Public Health. 2002;92:1331–37. doi: 10.2105/ajph.92.8.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maman S, Campbell J, Sweat MD, Gielen AC. The intersections of HIV and violence: directions for future research and interventions. Soc Sci Med. 2000;50:459–78. doi: 10.1016/s0277-9536(99)00270-1. [DOI] [PubMed] [Google Scholar]
- 6.Bensley L, Van Eenwyk J, Wynkoop Simmons K. Childhood family violence history and women's risk for intimate partner violence and poor health. Am J Prev Med. 2003;25:38–44. doi: 10.1016/s0749-3797(03)00094-1. [DOI] [PubMed] [Google Scholar]
- 7.Coid J, Petruckevitch A, Feder G, Chung W, Richardson J, Moorey S. Relation between childhood sexual and physical abuse and risk of revictimisation in women: a cross-sectional survey. Lancet. 2001;358:450–54. doi: 10.1016/s0140-6736(01)05622-7. [DOI] [PubMed] [Google Scholar]
- 8.Dunkle KL, Jewkes RK, Brown HC, Gray GE, McIntryre JA, Harlow SD. Gender-based violence, relationship power, and risk of HIV infection in women attending antenatal clinics in South Africa. Lancet. 2004;363:1415–21. doi: 10.1016/S0140-6736(04)16098-4. [DOI] [PubMed] [Google Scholar]
- 9.Decker MR, Seage GR, 3rd, Hemenway D, et al. Intimate partner violence functions as both a risk marker and risk factor for women's HIV infection: findings from Indian husband-wife dyads. J Acquir Immune Defic Syndr. 2009;51:593–600. doi: 10.1097/QAI.0b013e3181a255d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunkle KL, Jewkes RK, Nduna M, et al. Perpetration of partner violence and HIV risk behavior among young men in the rural Eastern Cape, South Africa. AIDS. 2006;20:2107–14. doi: 10.1097/01.aids.0000247582.00826.52. [DOI] [PubMed] [Google Scholar]
- 11.Fonck K, Leye E, Kidula N, Ndinya-Achola J, Temmerman M. Increased risk of HIV in women experiencing physical partner violence in Nairobi, Kenya. AIDS Behav. 2005;9:335–39. doi: 10.1007/s10461-005-9007-0. [DOI] [PubMed] [Google Scholar]
- 12.UNAIDS, WHO [April 30, 2014];AIDS epidemic update. http://www.who.int/hiv/pub/epidemiology/epidemic/en/
- 13.Garcia-Moreno C, Jansen HAFM, Ellsberg M, Heise L, Watts CH. on behalf of the WHO Multi-country Study on Women's Health and Domestic Violence against Women Study Team. Prevalence of intimate partner violence: findings from the WHO multi-country study on women's health and domestic violence. Lancet. 2006;368:1260–69. doi: 10.1016/S0140-6736(06)69523-8. [DOI] [PubMed] [Google Scholar]
- 14.WHO . Addressing violence against women and HIV/AIDS: what works? World Health Organization; Geneva, Switzerland: 2010. [Google Scholar]
- 15.Jewkes RK, Dunkle K, Nduna M, Shai N. Intimate partner violence, relationship power inequity, and incidence of HIV infection in young women in South Africa: a cohort study. Lancet. 2010;376:41–48. doi: 10.1016/S0140-6736(10)60548-X. [DOI] [PubMed] [Google Scholar]
- 16.Jewkes R, Nduna M, Levin J, et al. Impact of stepping stones on incidence of HIV and HSV-2 and sexual behavior in rural South Africa: a cluster randomised controlled trial. BMJ. 2008;337:a506. doi: 10.1136/bmj.a506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pronyk PM, Kim JC, Abramsky T, et al. A combined microfinance and training intervention can reduce HIV risk behaviour in young female participants. AIDS. 2008;22:1659–65. doi: 10.1097/QAD.0b013e328307a040. [DOI] [PubMed] [Google Scholar]
- 18.Pronyk PM, Hargreaves JR, Kim JC, et al. Effect of a structural intervention for the prevention of intimate-partner violence and HIV in rural South Africa: a cluster randomised trial. Lancet. 2006;368:1973–83. doi: 10.1016/S0140-6736(06)69744-4. [DOI] [PubMed] [Google Scholar]
- 19.Abramsky T, Devries K, Kiss L, et al. Findings from the SASA! Study: a cluster randomized controlled trial to assess the impact of a community mobilization intervention to prevent violence against women and reduce HIV risk in Kampala, Uganda. BMC Med. 2014;12:122. doi: 10.1186/s12916-014-0122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kouyoumdjian FG, Calzavara LM, Bondy SJ, et al. Intimate partner violence is associated with incident HIV infection in women in Uganda. AIDS. 2013;27:1331–38. doi: 10.1097/QAD.0b013e32835fd851. [DOI] [PubMed] [Google Scholar]
- 21.Ellis A, Manuel C, Blackden CM. [Dec 31, 2011];Gender and economic growth in Uganda: unleashing the power of women. http://siteresources. worldbank.org/INTAFRREGTOPGENDER/Resources/gender_econ_growth_ug.pdf.
- 22.Grabowski MK, Lessler J, Redd AD, et al. The role of viral introductions in sustaining community-based HIV epidemics in rural Uganda: evidence from spatial clustering, phylogenetics, and egocentric transmission models. PLoS Med. 2014;11:e1001610. doi: 10.1371/journal.pmed.1001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wawer MJ, Gray RH, Sewankambo NK, et al. A randomized community trial of intensive sexually transmitted disease control for AIDS prevention. AIDS. 1998;12:1211–25. doi: 10.1097/00002030-199810000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Todd J, Carpenter L, Li X, Nakiyingi J, Gray R, Hayes R. The effects of alternative study designs on the power of community randomized trials: evidence from three studies of human immunodeficiency virus prevention in East Africa. Int J Epidemiol. 2003;32:755–62. doi: 10.1093/ije/dyg150. [DOI] [PubMed] [Google Scholar]
- 25.Matovu JK, Kigozi G, Nalugoda F, Wabwire-Mangen F, Gray RH. The Rakai Project counselling programme experience. Trop Med Int Health. 2002;7:1064–67. doi: 10.1046/j.1365-3156.2002.00964.x. [DOI] [PubMed] [Google Scholar]
- 26.Matovu JK, Gray RH, Kiwanuka N, et al. Repeat voluntary HIV counseling and testing (VCT), sexual risk behavior and HIV incidence in Rakai, Uganda. AIDS Behav. 2007;11:71–78.. doi: 10.1007/s10461-006-9170-y. [DOI] [PubMed] [Google Scholar]
- 27.WHO Department of Gender, Women and Health [May 27, 2013];Putting women first: ethical and safety recommendations for research on domestic violence against women. http://whqlibdoc.who.int/hq/2001/WHO_FCH_GWH_01.1.pdf.
- 28.Lutalo T, Kigozi G, Kimera E, et al. A randomized community trial of enhanced family planning outreach in Rakai, Uganda. Stud Fam Plann. 2010;41:55–60. doi: 10.1111/j.1728-4465.2010.00224.x. [DOI] [PubMed] [Google Scholar]
- 29.Wagman JA, Namatovu F, Nalugoda F, et al. A public health approach to intimate partner violence prevention in Uganda: the SHARE Project. Violence Against Women. 2012;18:1390–412. doi: 10.1177/1077801212474874. [DOI] [PubMed] [Google Scholar]
- 30.Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12:38–48. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- 31.Michau L, Naker D. Mobilizing communities to prevent domestic violence: a resource guide for organizations in east and southern Africa. Raising Voices; Kampala, Uganda: 2003. [Google Scholar]
- 32.Straus MA. Measuring intra family conflict and violence: The Conflict Tactics Scale. J Marriage Fam. 1979;41:75–88. [Google Scholar]
- 33.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–66. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 34.Kong X, Ndyanabo A, Nalugoda F, et al. The accuracy of women's reports of their partner's male circumcision status in Rakai, Uganda. AIDS. 2013;27:662–64. doi: 10.1097/QAD.0b013e32835c557c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–06. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 36.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 37.Hamby S. The gender debate about intimate partner violence: solutions and dead ends. Psychol Trauma. 2009;1:24–34. [Google Scholar]
- 38.Welbourn A. Strategies for hope training series no 1. ActionAid London; London, UK: 1995. Stepping stones. A training package on HIV/AIDS, communication and relationship skills. [Google Scholar]
- 39.Anderson JC, Campbell JC, Farley JE. Interventions to address HIV and intimate partner violence in Sub-Saharan Africa: a review of the literature. J Assoc Nurses AIDS Care. 2013;24:383–90. doi: 10.1016/j.jana.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Francisco L, Abramsky T, Kiss L, et al. Violence against women and HIV risk behaviors in Kampala, Uganda: baseline findings from the SASA! Study. Violence Against Women. 19:814–32. doi: 10.1177/1077801213497557. [DOI] [PubMed] [Google Scholar]
- 41.Koenig MA, Lutalo T, Zhao F, et al. Domestic violence in Rakai, Uganda: evidence from a community-based survey. Bull World Health Org. 2003;81:53–60.. [PMC free article] [PubMed] [Google Scholar]
- 42.Wagman J, Baumgartner JN, Waszak CG, et al. Experiences of sexual coercion among adolescent women: qualitative findings from Rakai district, Uganda. J Interpers Violence. 2009;24:2073–95. doi: 10.1177/0886260508327707. [DOI] [PubMed] [Google Scholar]
- 43.Prakash M, Patterson S, Gotch F, Kapembwa MS. Recruitment of CD4+ T lymphocytes and macrophages into the cervical epithelium of women after coitus. Obstet Gynecol. 2003;188:376–81. doi: 10.1067/mob.2003.16. [DOI] [PubMed] [Google Scholar]
- 44.Gray R, Kigozi G, Kong X, et al. The effectiveness of male circumcision for HIV prevention and effects on risk behaviors in a posttrial follow-up study. AIDS. 2012;26:609–15. doi: 10.1097/QAD.0b013e3283504a3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugarman DB, Hotaling GT. Intimate violence and social desirability: a meta-analytic review. J Interpers Violence. 1997;12:275–90. [Google Scholar]
- 46.Anglewicz P, Chintsanya J. Disclosure of HIV status between spouses in rural Malawi. AIDS Care. 2011;23:998–1005. doi: 10.1080/09540121.2010.542130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.