Abstract
Previous reports suggest that plasminogen activator inhibitor-1 (PAI-1) promotes airway remodeling and that human and mouse mast cells (MCs) are an important source of PAI-1. In the present study we investigated MC–epithelial cell (EC) interactions in the production of PAI-1. We stimulated the human MC line LAD2 with IgE-receptor cross-linking and collected the supernatants. We incubated the human bronchial EC line BEAS-2B with the LAD2 supernatants and measured the level of PAI-1. When the supernatants from IgE-stimulated LAD2 were added to BEAS-2B, there was a significant enhancement of PAI-1 production by BEAS-2B. When we treated the MC supernatants with a transforming growth factor (TGF)-β1 neutralizing antibody, the MC-derived induction of PAI-1 from BEAS-2B was completely abrogated. Although TGF-β1 mRNA was constitutively expressed in resting LAD2, it was not highly induced by IgE-mediated stimulation. Nonetheless, active TGF-β1 protein was significantly increased in LAD2 after IgE-mediated stimulation. Active TGF-β1 produced by primary cultured human MCs was significantly reduced in the presence of a chymase inhibitor, suggesting a role of MC chymase as an activator of latent TGF-β1. This study indicates that stimulation of human MCs by IgE receptor cross-linking triggers activation of TGF-β1, at least in part via chymase, which in turn induces the production of PAI-1 by bronchial ECs. Our data suggest that human MCs may play an important role in airway remodeling in asthma as a direct source of PAI-1 and by activating bronchial ECs to produce further PAI-1 via a TGF-β1–mediated activation pathway.
Keywords: plasminogen activator inhibitor-1, mast cells, epithelial cells, transforming growth factor-β1, chymase
Clinical Relevance
This study indicates that stimulation of human mast cells by IgE receptor cross-linking triggers activation of TGF-β1, at least in part via chymase, which in turn induces the production of plasminogen activator inhibitor-1 (PAI-1) by bronchial epithelial cells. Our data suggest that human mast cells may play an important role in airway remodeling in asthma both as a direct source of PAI-1 and also by activating bronchial epithelial cells to produce further PAI-1 via a TGF-β1 mediated activation pathway.
Asthma is widely viewed as a disorder with reversible loss of lung function, characterized by airway eosinophilic inflammation and airway hyperreactivity (1). However, recent studies demonstrate that antiinflammatory therapy is not effective in some patients with asthma, and a nonreversible component of lung function loss has been recognized (2). As a consequence, increased attention has been paid to structural changes that occur in asthma, sometimes referred to as “airway remodeling.” Airway remodeling is characterized by subepithelial deposition of extracellular matrix (ECM), goblet cell hyperplasia, and increased airway smooth muscle cell mass (3). Subepithelial fibrosis, a key feature of asthma, appears during early stages of the disease and is generally unresponsive to steroid therapy. Recent attention has been paid to this thickening of the lamina reticularis and epidermal mesenchymal trophism, in which fibromyocytes or myofibroblasts are formed and activated (1). Remodeling may contribute to the irreversible component of airflow limitation in asthma (4) and has been used as a good histopathologic marker of disease activity in patients with severe asthma (5).
Tissue remodeling usually involves two distinct processes: (1) physiologic remodeling or regeneration, which is the replacement of injured tissue by parenchymal cells of the same type, and (2) pathologic remodeling, which is the replacement of injured tissue by ECM. Pathologic remodeling eventually leads to impaired restitution of airways structures, such as occurs in subepithelial fibrosis (3). In spite of their importance in tissue remodeling, ECM abnormalities in asthma are poorly understood (3).
The plasmin system is comprised of plasminogen that can be converted to the active enzyme plasmin by tissue-type plasminogen activator or urokinase-type PA (u-PA). Tissue-PA and u-PA are involved in the dissolution of fibrin and in the degradation of ECM components (6). Plasminogen activator inhibitor (PAI)-1 is a major inhibitor of tissue-type plasminogen activator and u-PA and can therefore indirectly contribute to matrix deposition by preventing matrix dissolution. PAI-1 is consistently and dramatically up-regulated in a variety of fibrotic diseases (7–9). Furthermore, PAI-1–deficient mice are protected against ECM deposition and fibrosis in the lung after bleomycin challenge (10, 11), whereas PAI-1 overexpressing mice suffer from extensive fibrotic changes (10). This suggests that PAI-1 plays a key role in deposition of ECM in the airways.
Mast cells (MCs) in the airways of patients with asthma are important in initiating allergic inflammation after allergen challenge and maintain inflammation via interaction with resident cells and other inflammatory cells in the airways. Increased MC numbers in the airway epithelial layer (12), in bronchial brushings (13), and in bronchoalveolar lavage fluid (14) have been reported in asthma. Evidence has been published demonstrating MCs with morphologic characteristics of activation in bronchial biopsies of patients with asthma (15), and ongoing MC activation in well-controlled asthmatic patients may produce elevated levels of histamine, leukotrienes, and PGD2 in bronchoalveolar lavage fluid or sputum (16, 17). MCs have been associated with fibrosis in the skin and other organs for many years (18, 19). Recently, it has been found that MCs may play a role in tissue fibrosis in asthma. Yu and colleagues (20) demonstrated that MC-deficient mice had reduced deposition of collagen in the airways in a murine model of chronic asthma and that the deposition of collagen was restored by MC reconstitution. However, the mediators that play a major role in this process are not known.
MCs have been reported to be a major source of PAI-1, and MC-derived PAI-1 is associated with human fatal asthma (21). The 4G allele of the PAI-1 gene, which is associated with an elevated plasma PAI-1 level, is associated with asthma (22). Furthermore, deletion of PAI-1 prevents ECM deposition in a murine model of chronic asthma (23), and the level of PAI-1 was reduced in the airways of ovalbumin-challenged, MC-deficient mice compared with wild-type controls, suggesting an important contribution of MCs in the production of PAI-1 (S. Cho and colleagues, unpublished observation). Bronchial epithelial cells (ECs) are another major source of PAI-1 in the airways. The interactions between epithelium and inflammatory cells play a key role in maintaining persistent inflammation and structural changes in asthma (24). The production of PAI-1 was enhanced by mechanical stimulation in normal human bronchial ECs (NHBECs) (25), and the expression of PAI-1 was induced in bronchial epithelium after chronic ovalbumin challenge in a murine model of asthma (26). MCs reside in physical proximity to bronchial epithelium and adhere to bronchial ECs, and MC mediators stimulate ECs to produce IL-8 and mucin genes (27). The role of the interaction between ECs and MCs in tissue fibrosis remains unclear.
In this study, we investigated the cross-talk between MCs and ECs in the production of PAI-1, a mediator that plays a major role in the fibrogenic process. We found that supernatants from MCs after stimulation via the IgE receptor contained active transforming growth factor (TGF)-β1 and induced bronchial ECs to produce a large amount of PAI-1, suggesting that MC activation in vivo may lead to epithelial activation and PAI-1 production and may promote fibrosis in the airways.
Materials and Methods
Cell Culture, Reagents, and Treatments
The LAD2 human MC line was a generous gift of Dr. Kirshenbaum. LAD2 cells were cultured in StemPro-34 SFM (Life Technologies, Grand Island, NY) as previously described (28). Primary cultured human MCs (PCHMCs) were obtained as described previously (28). Briefly, lineage-negative mononuclear cells were separated from human peripheral blood mononuclear cells by using an autoMACS system (Miltenyi Biotec, Auburn, CA) according to the manufacturer’s instructions. The cells were suspended in Iscove’s methylcellulose medium (StemCell Technologies, Vancouver, BC, Canada) containing 200 ng/ml stem cell factor, IL-6, 5 ng/ml IL-3, 100 U/ml penicillin, and 100 μg/ml streptomycin and then incubated at 37°C in 5% CO2. At 6 weeks, all cells were retrieved after dissolving the methylcellulose medium with PBS. The cells were then suspended and cultured in Iscove’s modified Dulbecco’s medium supplemented with 100 ng/ml stem cell factor, 50 ng/ml IL-6, 0.1% BSA, insulin-transferrin-selenium, 55 μM 2-ME, 100 U/ml penicillin, and 100 μg/ml streptomycin, and the culture medium was changed weekly thereafter and incubated for an additional 5 to 7 weeks. The final purity of the MCs always exceeded 98%. LAD2 and primary MCs were then sensitized with 1 μg/ml human myeloma IgE (Calbiochem, Billerica, MA) at 37°C for 48 hours and were stimulated with 1.5 μg/ml anti-IgE Ab (Dako, Carpinteria, CA) with or without 1-hour treatment of 100 μg/ml soybean trypsin inhibitor (SBTI) (chymase inhibitor) or Antipain (tryptase inhibitor) (Sigma, St. Louis, MO). The human bronchial EC line BEAS-2B was cultured in DMEM/F12 (Life Technologies) as previously described (29). NHBECs were obtained from Cambrex (East Rutherford, NJ) and plated in 24-well culture plates coated with collagen (Vitrogen; Cohesion Technologies, Palo Alto, CA). Supernatants were collected from resting and stimulated LAD2 cells or primary MCs and then incubated with BEAS-2B or NHBECs for 16 or 24 hours. Human recombinant TGF-β1 was obtained from R&D Systems (Minneapolis, MN). Neutralizing antibodies for TGF-β1 and TNF-α and their isotype control (rabbit IgG) were from Abcam (Cambridge, MA).
ELISA
The concentrations of PAI-1 and TGF-β1 proteins in cell-free supernatants were measured using specific ELISA kits according to the manufacturer’s instructions (Diapharma, West Chest, OH and Promega, Madison, WI, respectively). The minimal detection limits for these kits are 0.5 ng/ml and 32 pg/ml, respectively.
Real-Time RT-PCR
Real-time RT-PCR was performed with a TaqMan method using a 7500 Sequence Detection System (Applied Biosystems, Foster City, CA) in 20-μl reactions (2x TaqMan Master mix [Applied Biosystems], 400 nM each primer, and 200 nM TaqMan probe plus cDNA). Primer and probe sets for the following five genes were synthesized by Applied Biosystems: TGF-β1 (sense, 5′-TGACAAGTTCAAGCAGAGTACACACA-3′; antisense, 5′-GGAGAGCAACACGGGTTCA-3′), TGF-β2 (sense, 5′-GATGGCACCTCCACATATACCA-3′; antisense, 5′-TTTCCACCCTAGATCCCTCTTG-3′), TGF-βR1 (sense, 5′-CATCACCTGGCCTTGGTCC-3′; antisense, 5′-CGATGGTGAATGACAGTGCG-3′), GAPDH (sense, 5′-GAAGGTGAAGGTCGGAGTC-3′; antisense, 5′-GAAGATGGTGATGGGATTTC-3′), and EEF1A1 (sense, 5′-TGCTAACATGCCTTGGTTCAAG-3′; antisense, 5′-TTGGACGAGTTGGTGGTAGGAT-3′). Messenger RNA levels were expressed as relative gene copy numbers normalized to the two stable housekeeping genes (GAPDH and EEF1A1) (30).
Statistical Analysis
All data are presented as mean ± SEM. Differences between groups were analyzed using the Student’s t test and considered to be significant at P < 0.05.
Results
Stimulated MCs Enhance the Production of PAI-1 by Bronchial ECs
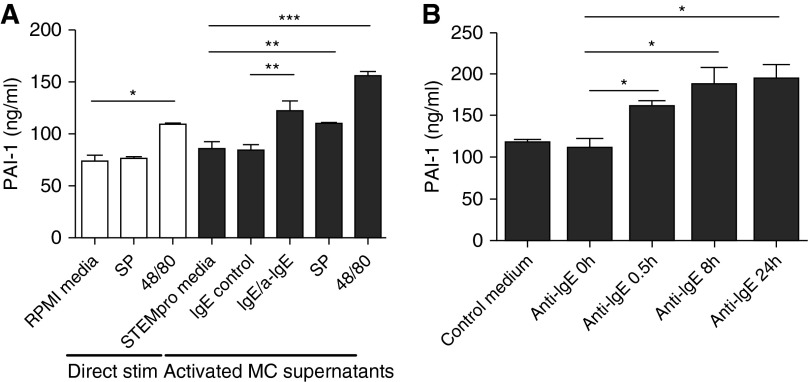
A human MC line, LAD2, is useful because it displays consistent degranulation in response to IgE-dependent activation. We used cultured LAD2 cells in the present studies and stimulated the cells with IgE receptor cross-linking, substance P (SP), and compound 48/80 for 24 hours. We measured baseline levels of PAI-1 from LAD2 cells using ELISA as described (21). Production of PAI-1 was not detectable in the supernatants from resting or stimulated LAD2 (data not shown), which is a surprising result because HMC-1 and PCHMCs have been reported to produce significant amounts of PAI-1 (21). In addition, primary human lung MCs and skin MCs produce PAI-1 (31). Although the reason that LAD2 do not release PAI-1 is unknown, we took advantage of this fact in our experimental design to test the ability of MC supernatants to activate PAI-1 production in ECs because there was no background PAI-1 in the LAD2 supernatants. We tested whether SP or compound 48/80 alone can stimulate BEAS-2B to produce PAI-1 and found that compound 48/80, but not SP, induced PAI-1 production from BEAS-2B cells (108.3 ± 1.2 ng/ml versus RMPI media control of 73.2 ± 6.2 [n = 4]; P < 0.01) (Figure 1A). We collected the supernatants from the resting and stimulated LAD2 MCs and then cultured BEAS-2B with MC supernatants for 16 hours. There was significant enhancement of PAI-1 production by the ECs when they were exposed to supernatants from MCs stimulated with IgE receptor cross-linking (121.5 ± 10.2 ng/ml versus IgE control of 82.9 ± 6.4 [n = 4]; P < 0.05), SP (108.4 ± 1.9 ng/ml versus 84.1 ± 8.2 of StemPro media control [n = 4]; P < 0.05), and compound 48/80 (154.3 ± 5.0 ng/ml versus 84.1 ± 8.2 of StemPro media control [n = 4]; P < 0.005) (Figure 1A). PAI-1 production from BEAS-2B cells after incubation with compound 48/80-conditioned LAD2 media was significantly higher than levels after direct stimulation with compound 48/80 (154.3 ± 5.0 ng/ml versus 108.3 ± 1.2 [n = 4]; P < 0.001), suggesting that the BEAS-2B–derived PAI production by compound 48/80-conditioned LAD2 supernatant was not just due to the presence of compound 48/80 in the conditioned LAD2 media.
Figure 1.
Production of plasminogen activator inhibitor-1 (PAI-1) from BEAS-2B cells after incubation with stimulated LAD2 supernatants. (A) BEAS-2B cells were stimulated with substance P (SP) and compound 48/80 for 16 hours, and PAI-1 levels were measured by ELISA (direct stimulation). LAD2 cells were stimulated with SP, compound 48/80 (48/80), and human IgE receptor cross-linking for 24 hours, and supernatants were collected. BEAS-2B cells were incubated with the LAD2 supernatants for 16 hours (activated mast cell [MC] supernatants). Data represent means ± SE of four independent experiments performed in duplicate. *P < 0.01, **P < 0.05, and ***P < 0.005 by Student’s t test. (B) LAD2 cells were stimulated with IgE receptor cross-linking for 0.5, 8, or 24 hours, and supernatants were collected. BEAS-2B cells were incubated with the LAD2 supernatants for 16 hours. Data represent means ± SE of four independent experiments performed in duplicate. *P < 0.05 by Student’s t test.
Time Course of PAI-1 Production from Bronchial ECs after Incubation with IgE-Stimulated MC Supernatants
IgE crosslinking is a physiologic stimulus and the most important mechanism of MC activation in allergic diseases, including asthma. Thus, we further investigated the time course of PAI-1 production induced in BEAS-2B by MC mediators derived by IgE stimulation. LAD2 cells were sensitized with IgE and then stimulated with IgE receptor cross-linking using anti-IgE for 30 minutes, 8 hours, or 24 hours. Supernatants were collected from the resting and stimulated MCs. BEAS-2B cells were incubated with MC supernatants for 16 hours. When supernatants from MCs stimulated with IgE/anti-IgE for 30 minutes, 8 hours, or 24 hours were added to BEAS-2B, there was significant enhancement of PAI-1 production (110.9 ± 11.6 ng/ml baseline versus 160.0 ± 8.0, 188.0 ± 19.9, and 193.2 ± 17.9, respectively [n = 4]; P < 0.05 in all cases) (Figure 1B).
MC-Derived TGF-β1 Mediates the Enhancement of PAI-1 Production from ECs
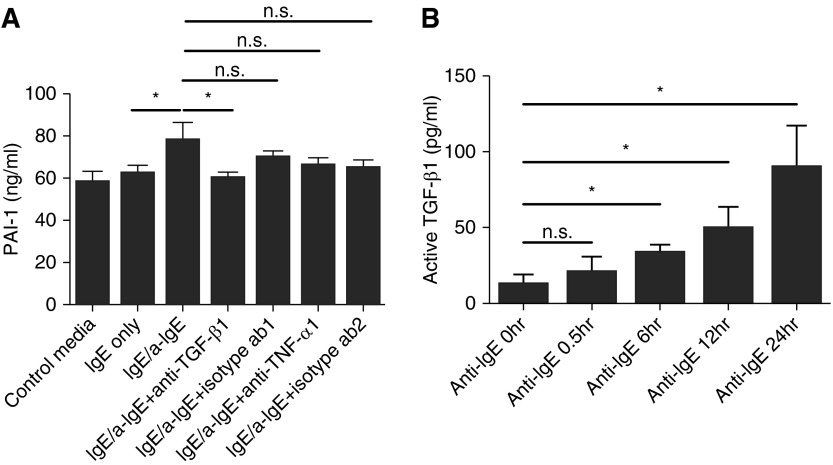
TGF-β1 has been known to stimulate bronchial ECs to produce PAI-1 (32, 33), although the cellular sources of TGF-β1 and the mechanism of the activation in the airways of patients with asthma are unclear. Together with TGF-β1, TNF-α is an important regulatory cytokine of PAI-1 production (34) and is released from human MCs (28). To investigate the role of TGF-β1 and TNF-α in inducing PAI-1 from ECs, we preincubated the supernatants from activated LAD2 with neutralizing antibodies against TGF-β1 and TNF-α for 1 hour and then added the supernatants to BEAS-2B. We found that a TGF-β1 neutralizing antibody completely abrogated the IgE-stimulated, MC-derived induction of PAI-1 by BEAS-2B (59.9 ± 1.7 ng/ml versus 78.0 ± 4.9 of IgE-mediated stimulation without antibody treatment [n = 3]; P < 0.05) (Figure 2A). On the other hand, a TNF-α antibody did not significantly block PAI-1 production from ECs. This result suggested that TGF-β1 is an important mediator in the induction of PAI-1 production from BEAS-2B, although this TGF-β1 could be derived from either LAD2 or BEAS-2B.
Figure 2.
LAD2-derived TGF-β1 and TNF-α on the production of PAI-1. (A) The supernatants from LAD2 activated with IgE receptor cross-linking were incubated with the neutralizing antibodies (both rabbit polyclonal) for TGF-β1 (5 μg/ml) or TNF-α (100 ng/ml) for 1 hour. Isotype antibody 1 was 5 μg/ml of rabbit IgG; isotype antibody 2 was 100 ng/ml of rabbit IgG. The supernatants from IgE control or IgE-stimulated LAD2 with/without antibodies were added to BEAS-2B and incubated for 16 hours. Data represent means ± SE of three independent experiments performed in duplicate. n.s., not significant. *P < 0.05 by Student’s t test. (B) LAD2 cells were stimulated with IgE receptor cross-linking for 0, 0.5, 6, 12, and 24 hours, and the level of active TGF-β1 was measured by ELISA. Data represent means ± SE of four independent experiments performed in duplicate. *P < 0.05 by Student’s t test.
IgE-Stimulated MCs Produce Significant Quantities of Active TGF-β1
TGF-β1 is a well-known cytokine that generates tissue fibrosis, including the airway remodeling in asthma. Kanbe and colleagues (35) previously reported that human MCs produce TGF-β1. However, the concentration of TGF-β1 was too low to be detectable by ELISA in their experiments, and, to our knowledge, there are no other reports of the production of active TGF-β1 in human MCs other than reports of immunohistochemical staining of TGF-β1 in MCs (36). TGF-β1 is secreted from cells as a latent form and must be activated by cleavage by proteases, exposure to low pH, or exposure to ionizing radiation (37). We measured the production of active TGF-β1 in supernatants of LAD2 to determine whether the cells produce active TGF-β1 and to attempt to explain the blocking effect of anti–TGF-β1 (Figure 2A). There was a slightly detectable level of active TGF-β1 in the MC supernatants at the 0-hour time point immediately before challenge (12.6 ± 11.1 pg/ml), and the production of active TGF-β1 in supernatants of IgE-stimulated LAD2 subsequently increased in a time-dependent manner from 6 hours after stimulation (6 h, 30.3 ± 5.6 pg/ml; 12 h, 49.4 ± 14.4 pg/ml; 24 h, 89.4 ± 27.7 pg/ml [n = 3]; P < 0.05) (Figure 2B). This result indicates that latent TGF-β1 slowly produced by IgE-stimulated MCs is activated by other products of MCs, possibly preformed MC mediator(s). We also measured active TGF-β1 in the supernatants from LAD2 cells after stimulation with SP or compound 48/80 and found that there was a significant increase of active TGF-β1 production with these two stimuli to a degree similar to of that induced by IgE receptor cross-linking (data not shown), suggesting that TGF-β1 may also play a role in SP-induced and compound 48/80–induced PAI-1 production by MC-EC cross-talk (Figure 1A).
Time Course of TGF-β1, TGF-β2, and TGF-β1R Gene Expression in IgE-Stimulated LAD2 Cells
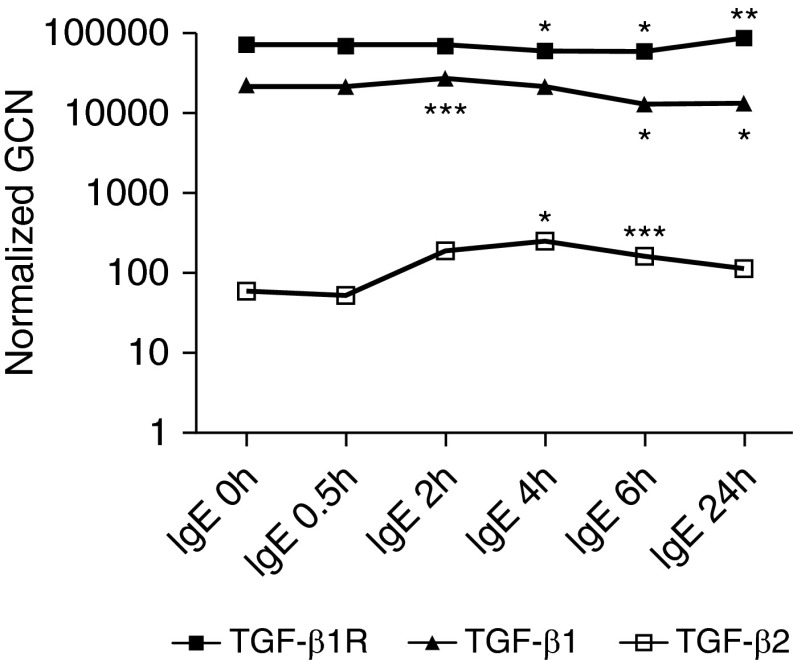
To investigate whether or not the expression of TGF-β1 is regulated at the gene transcription level, the time course of changes in TGF-β1 mRNA in LAD2 MCs was examined by real-time RT-PCR. We also tested the expression of TGF-β2 and TGF-β1R. TGF-β1 mRNA was constitutively expressed, slightly elevated 2 hours after IgE-receptor cross-linking, and then slightly down-regulated at the 6- and 24-hour time points (Figure 3). TGF-β1R mRNA was also constitutively expressed, slightly decreased 4 and 6 hours after IgE-receptor cross-linking, and then slightly up-regulated at the 24-hour time points. However, the biologic significance of these small alterations is questionable. The expression of TGF-β2 mRNA was minimally detectable and very low compared with the expression of TGF-β1 mRNA, although it was up-regulated from 4 hours after IgE stimulation. Kanbe and colleagues (35) were also not able to detect the expression of TGF-β2 mRNA in primary human MCs by conventional RT-PCR, which is consistent with our finding. In light of the data in Figure 2B, the data in Figure 3 suggest that TGF-β1 mRNA in LAD2 cells is likely regulated at the translational or posttranslational level.
Figure 3.
Time course of TGF-β1, TGF-β2, and TGF-β1R gene expression in IgE-stimulated LAD2 cells. LAD2 cells were stimulated with IgE receptor cross-linking for 0, 0.5, 2, 4, 6, and 24 hours, and the gene expression of TGF-β1, TGF-β2, and TGF-β1R was measured by real-time RT-PCR. Data represent three independent experiments. *P < 0.005, **P < 0.01, and ***P < 0.05 compared with 0–h IgE by Student’s t test. GCN, gene copy number; normalized GCN, relative GCNs normalized to the two most stable housekeeping genes, GAPDH and EEF1A1.
IgE-Stimulated PCHMCs Produce Significant Quantities of Active TGF-β1
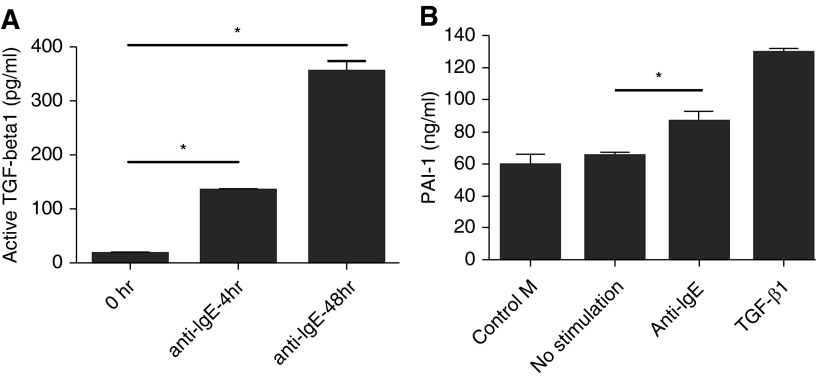
To confirm the production of active TGF-β1 in a primary cultured cell system, we used human peripheral blood–derived MCs. PCHMCs were stimulated with IgE receptor cross-linking for 0, 4, and 48 hours, and the level of active TGF-β1 was measured by ELISA (Figure 4A). There was a barely detectable level of active TGF-β1 at the 0-hour time point immediately before challenge (16.3 ± 4.4 pg/ml), and the production of active TGF-β1 in supernatants of IgE-stimulated PCHMCs subsequently increased in a time-dependent manner, exceeding the quantities made by LAD2, a MC line (4 h, 132.7 ± 5.3 pg/ml; 48 h, 352.4 ± 20.2 pg/ml [n = 2–4]; P < 0.0001).
Figure 4.
Production of active TGF-β1 in primary cultured human MCs (PCHMCs) or PAI-1 in human bronchial epithelial cells. (A) PCHMCs were stimulated with IgE receptor cross-linking for 0, 4, and 48 hours, and the level of active TGF-β1 was measured by ELISA. Data represent means ± SE of two, three, or four independent experiments performed in duplicate. *P < 0.0001 by Student’s t test. (B) LAD2 cells were stimulated with human IgE receptor cross-linking for 24 hours. The supernatants from resting and stimulated LAD2 were collected. Primary cultured human bronchial epithelial cells were incubated with the LAD2 supernatants for 16 hours. Data represent means ± SE of three independent experiments performed in duplicate. *P < 0.05 by Student’s t test.
Stimulated MCs Enhanced the Production of PAI-1 from NHBECs
To confirm the MC-induced enhancement of PAI-1 production from ECs in a primary cultured cell system, we used NHBECs. LAD2 cells were stimulated by IgE receptor cross-linking for 24 hours. Supernatants were collected from the resting and stimulated MCs and cultured NHBECs with the MC supernatants for 16 hours. There was a significant enhancement of levels of PAI-1 in the EC supernatants after stimulation with supernatants from LAD2 cells activated by IgE receptor cross-linking (86.0 ± 6.2 ng/ml [n = 3]; P < 0.05) compared with medium control (58.9 ± 6.7 ng/ml [n = 3]) (Figure 4B). Human recombinant TGF-β1 (8 ng/ml) (R&D Systems) was used as a positive control for the production of PAI-1 from NHBECs and induced a robust response.
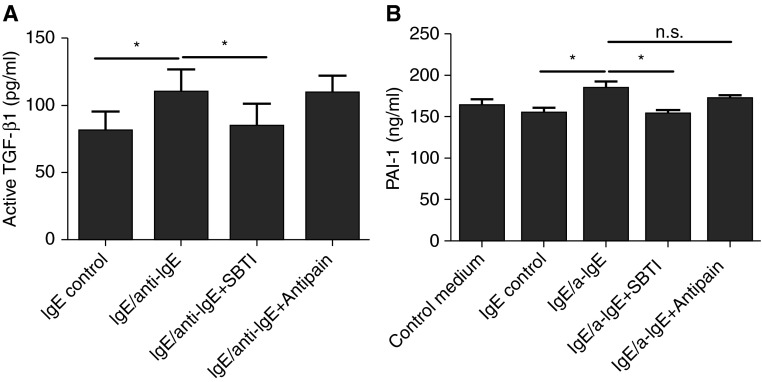
A Chymase Inhibitor, Not a Tryptase Inhibitor, Inhibited the Production of Active TGF-β1 from PCHMCs
TGF-β1 is secreted from cells in a latent form and must be activated by cleavage by proteases, exposure to low pH, or exposure to ionizing radiation (37). Human MCs produce proteases, including chymase and trypase. To test the hypothesis that TGF-β1 activation in human MCs was mediated by these MC enzymes, we used a chymase inhibitor and a tryptase inhibitor (SBTI and Antipain, respectively). SBTI is a human MC chymase-specific inhibitor without antitryptase activity, and Antipain is a human MC tryptase specific inhibitor with minimal antichymase activity (38). PCHMCs were stimulated with IgE receptor cross-linking for 24 hours with or without 1-hour pretreatment with SBTI or Antipain, and the level of active TGF-β1 was measured by ELISA (Figure 5A). There was baseline production of active TGF-β1 in the resting state with IgE only for 24 hours (80.7 ± 14.9 pg/ml), and the levels of active TGF-β1 were significantly increased with IgE receptor cross-linking (109.8 ± 17.1 pg/ml [n = 3]; P < 0.05 versus IgE only). SBTI, but not Antipain, significantly reduced the production of active TGF-β1 (109.8 ± 17.1 versus 84.3 ± 17.0 pg/ml [n = 3]; P < 0.05), suggesting a potential role of chymase in the activation of MC-derived TGF-β1. We further studied the effects of chymase and tryptase inhibitors on PAI-1 production from ECs using SBTI and Antipain. The supernatants from LAD2 cells activated with IgE receptor cross-linking for 24 hours were incubated with SBTI or Antipain for 1 hour. The supernatants from IgE control or IgE-stimulated LAD2 with/without SBTI or Antipain were added to BEAS-2B cells and incubated for 16 hours. There was significant reduction of EC-derived PAI-1 production in the SBTI treatment group compared with the IgE receptor cross-linking group without SBTI treatment (152.9 ± 5.4 versus 183.3 ± 9.6 ng/ml [n = 3]; P < 0.05) (Figure 5B), indicating a role for MC-derived chymase in the production of PAI-1 from ECs. However, there was no significant effect of Antipain treatment on EC-derived PAI-1 production.
Figure 5.
Production of active TGF-β1 by Ig-E–stimulated human MCs with or without a tryptase or chymase inhibitor and its effect on PAI-1 production from human airway epithelial cells. (A) PCHMCs were stimulated with IgE receptor cross-linking for 24 hours with or without 1-hour pretreatment of soybean trypsin inhibitor (SBTI) (chymase inhibitor) or Antipain (tryptase inhibitor), and the level of active TGF-β1 was measured by ELISA. Data represent means ± SE of three independent experiments performed in duplicate. *P < 0.05 by Student’s t test. (B) The supernatants from LAD2 cells activated with IgE receptor cross-linking for 24 hours were incubated with SBTI or Antipain for 1 hour. The supernatants from IgE control or IgE-stimulated LAD2 cells with/without SBTI or Antipain were added to BEAS-2B and incubated for 16 hours. Data represent means ± SE of three independent experiments performed in duplicate. *P < 0.05 by Student’s t test.
Discussion
Published reports have demonstrated that human MCs are a major source of PAI-1 and that there is an association between MC-derived PAI-1 and human asthma (21). Accordingly, Yu and colleagues (20) demonstrated that MCs play a critical role in collagen deposition in the airways in a murine model of asthma. Although MCs are well known to play an important role in initiating allergic inflammation in the airways, there are other cellular sources of PAI-1 in the airways, and the number of MCs in the airways is limited compared with other structural cells. In the present study, we have investigated the influence of MCs on PAI-1 production by bronchial ECs because ECs are another major source of PAI-1 and because MCs and ECs reside in close physical proximity in the airways.
We found that supernatants from MCs activated by IgE receptor cross-linking, or stimulation with SP or compound 48/80, enhanced the production of PAI-1 by airway ECs. IgE receptor–mediated activation of MCs enhanced the production of PAI-1 from the immortalized BEAS-2B cell line via active TGF-β1. TGF-β1 may cause different responses between BEAS-2B cells and NHBECs. For example, NHBECs are sensitive to TGF-β1 in terminal squamous differentiation, but BEAS-2B cells are not (32). Therefore, we confirmed the observation in BEAS-2B cells that IgE receptor–mediated activation of MCs induced PAI-1 in NHBECs and also found that TGF-β1 increased production of PAI-1 in these cells. We did not observe any significant morphologic changes in BEAS-2B or NHBE cells during the 16-hour incubation period with MCs or TGF-β1. Because IgE-mediated activation of MCs is a major mechanism of triggering allergic inflammation in the airways, our study suggests that persistent IgE-mediated activation of MCs may lead to fibrotic changes in the airways by enhancing the production of PAI-1 from bronchial epithelium. Indeed, chronic antigen challenge models in mice suggest that fibrosis can result from antigen exposure and that MCs play a role in this response (20). SP is a member of the tachykinin family of neuropeptides that bind three neurokinin receptors (NK1R, NK2R, and NK3R) (39). Although SP is widely known as a neuronally derived peptide, rodent studies have shown that eosinophils, lymphocytes, macrophages, and dendritic cells may also produce SP (40–43). The content of SP in human airways is increased in asthma, suggesting that SP may be involved in MC activation in asthma (44). SP has recently been shown to be a potent activator and induces production of nanogram quantities of chemokines and TNF from human MCs (28). The present study showed that SP is an important FcεRI-independent activator of human MCs and induces production of a factor that activates the production of PAI-1 by bronchial ECs. We speculate that SP-mediated activation of MCs may also play a role in airway remodeling in asthma via such a mechanism.
TGF-β1 is a key molecule in the initiation and maintenance of tissue fibrosis in the lung by promoting fibroblast proliferation and the production of ECM components (45, 46). Of the three forms of TGF-β, TGF-β1 likely has the most important role in fibrosis. The inactive form of TGF-β1 has a C-terminal TGF-β1 sequence and an N-terminal prodomain called “latency-associated peptide.” TGF-β1 and latency-associated peptide are secreted as a complex, and this latent form is unable to bind to its cognate receptor until it is converted to the active form by cleavage by proteases or by exposure to low pH or ionizing radiation (37). TGF-β1 is known to induce PAI-1 production from human bronchial ECs (32, 33, 47), and this may be one of the mechanisms by which TGF-β1 promotes tissue fibrosis in the airways. Published studies have demonstrated that activated murine or rat MCs produce significant amounts of active TGF-β1 (48, 49). Kanbe and colleagues (35) also reported that PCHMCs constitutively expressed TGF-β1 mRNA and that IgE-stimulated human MCs produce functional TGF-β1, although the quantity detected was low. The present study demonstrated that TGF-β1 is a mediator found in the supernatants of activated MCs that enhances the production of PAI-1 from bronchial ECs. This conclusion is supported by the nearly complete reduction of stimulated PAI-1 production from BEAS-2B cells when a neutralizing antibody of TGF-β1 was added to the LAD2 MC supernatants. In further experiments, LAD2 and PCHMCs were shown to slowly but steadily produce significant quantities of active TGF-β1 after activation via IgE receptor cross-linking. This finding suggests that the latent form of TGF-β1 from resting MCs or ECs becomes activated by unknown mediator(s) released from IgE-stimulated MCs. Together with MCs, human bronchial ECs can be a source of TGF-β1 in the airways (50). Therefore, our data indicate that TGF-β1 from MCs and ECs can be activated by allergen-IgE receptor–mediated stimulation of MCs.
Our gene expression data confirmed the previous report by Kanbe and colleagues (35), demonstrating that human MCs constitutively express TGF-β1 and that the levels of TGF-β1 mRNA are not dramatically altered by stimulation. The real-time RT-PCR data also indicate that TGF-β1 is probably the main form of TGF-β produced by human MCs compared with TGF-β2. In a time-course experiment, active TGF-β1 from LAD2 cells was slightly elevated in 30 minutes after IgE stimulation (statistically not significant), significantly produced 6 hours after the stimulation, and then accumulated gradually in a time-dependent manner. Human MC–derived TGF-β1 has not been well characterized and the mechanism of its activation has not been well elucidated. As mentioned above, TGF-β1 requires extracellular modification by heat, acid, proteases, or integrin αvβ6 to be functionally active (37). MCs are known to be a rich source of proteases such as chymase and tryptase, and both of these proteases have been reported to activate TGF-β1 (48, 51, 52). LAD2 cells express tryptase and chymase and are therefore most similar to double-positive connective tissue type MCs. We speculate that tryptase or chymase may play a role in the activation process that we have observed. CD34+ cell–derived primary MCs are mainly trypase/chymase double-positive MCs. On the other hand, human MCs from normal lung tissue are mainly (80–90%) tryptase single-positive (mucosal type). Tryptase-positive MCs seem to be important in asthma because of their large numbers in lung and close proximity to bronchial epithelium. However, Balzar and colleagues (53) showed that the number of tryptase/chymase double-positive MCs increased in small airway regions and that only tryptase/chymase-positive MCs among inflammatory cells significantly and positively correlated with lung function in severe asthma. Therefore, it would be important to determine whether tryptase or chymase or both proteases play a role in activating MC-derived TGF-β1, establishing its further ability to stimulate ECs to produce PAI-1. Such information could provide a further rationale to target MC proteases for the prevention or treatment of airway remodeling in asthma. In our experiments, we found that a chymase inhibitor, but not a tryptase inhibitor, reduced the production of active TGF-β1. The role of purified rat MC chymase as an activator of latent TGF-β1 was previously reported by another group (48). In this study, we confirmed that human MC chymase can also activate TGF-β1. However, we failed to show that human MC tryptase activates TGF-β1. Woodman and colleagues (51) used human lung MCs in their tryptase inhibition assay and these MCs are mainly tryptase single-positive MCs. On the other hand, we used tryptase-chymase double-positive MCs in our experiments. We speculate that chymase may play a dominant role in the presence of both MC proteases in activating TGF-β1, although this should be carefully tested. Our study is limited to in vitro findings. Therefore, the role of MC-derived chymase/TGF-β1 and EC-derived PAI-1 in asthma must at present be viewed as speculation based the in vitro results. We plan to test the relevance of these in vitro data in further studies using a murine model of asthma or human asthma samples.
In this study, we found that human MC–derived active TGF-β1 induced by IgE receptor cross-linking enhances the production of PAI-1 from bronchial ECs. IgE-mediated allergic inflammation is relevant to the pathogenesis of asthma, and this indirect IgE-mediated induction of PAI-1 from ECs via active TGF-β1 may play an important role in the development of the fibrosis that occurs immediately adjacent to the epithelium. This study suggests a potentially important interaction of MCs and epithelium in PAI-1–induced airway remodeling in asthma. Although the number of MCs in the airways is not large, the number of ECs is substantial, such that ECs might serve as secondary PAI-1–producing cells after MC activation and thereby leverage the antigen-specific activation of these sentinel cells. These results suggest a potential role of inhibition of MC activation by anti-IgE or other targeted drugs in preventing or treating airway remodeling in asthma.
Footnotes
This study was supported by National Institutes of Health grants R37HL0546, R01HL078860, and AI106683; by AHA grant 11SDG7590063, and by the Ernest S. Bazley Trust.
Author Contributions: Conception and design: S.H.C., S.H.L., and R.P.S. Analysis and interpretation: S.H.C., S.H.L., A.K., T.T., M.K., S.C.S., and R.P.S. Drafting the manuscript for important intellectual content: S.H.C., S.H.L., A.K., and R.P.S.
Originally Published in Press as DOI: 10.1165/rcmb.2013-0399OC on July 2, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Holgate ST. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol Rev. 2011;242:205–219. doi: 10.1111/j.1600-065X.2011.01030.x. [DOI] [PubMed] [Google Scholar]
- 2.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma: from bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 3.Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. J Clin Invest. 1999;104:1001–1006. doi: 10.1172/JCI8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 5.Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, Dziura JD, Reed J, Coyle AJ, Kiener P, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007;357:2016–2027. doi: 10.1056/NEJMoa073600. [DOI] [PubMed] [Google Scholar]
- 6.Rijken DC, Sakharov DV. Basic principles in thrombolysis: regulatory role of plasminogen. Thromb Res. 2001;103:S41–S49. doi: 10.1016/s0049-3848(01)00296-1. [DOI] [PubMed] [Google Scholar]
- 7.Tuan TL, Zhu JY, Sun B, Nichter LS, Nimni ME, Laug WE. Elevated levels of plasminogen activator inhibitor-1 may account for the altered fibrinolysis by keloid fibroblasts. J Invest Dermatol. 1996;106:1007–1011. doi: 10.1111/1523-1747.ep12338552. [DOI] [PubMed] [Google Scholar]
- 8.Kotani I, Sato A, Hayakawa H, Urano T, Takada Y, Takada A. Increased procoagulant and antifibrinolytic activities in the lungs with idiopathic pulmonary fibrosis. Thromb Res. 1995;77:493–504. doi: 10.1016/0049-3848(95)00025-9. [DOI] [PubMed] [Google Scholar]
- 9.Inuzuka S, Ueno T, Torimura T, Tamaki S, Sugawara H, Sakata R, Kusaba N, Sata M, Tanikawa K. The significance of colocalization of plasminogen activator inhibitor-1 and vitronectin in hepatic fibrosis. Scand J Gastroenterol. 1997;32:1052–1060. doi: 10.3109/00365529709011224. [DOI] [PubMed] [Google Scholar]
- 10.Hattori N, Degen JL, Sisson TH, Liu H, Moore BB, Pandrangi RG, Simon RH, Drew AF. Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J Clin Invest. 2000;106:1341–1350. doi: 10.1172/JCI10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eitzman DT, McCoy RD, Zheng X, Fay WP, Shen T, Ginsburg D, Simon RH. Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor-1 gene. J Clin Invest. 1996;97:232–237. doi: 10.1172/JCI118396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pesci A, Foresi A, Bertorelli G, Chetta A, Olivieri D. Histochemical characteristics and degranulation of mast cells in epithelium and lamina propria of bronchial biopsies from asthmatic and normal subjects. Am Rev Respir Dis. 1993;147:684–689. doi: 10.1164/ajrccm/147.3.684. [DOI] [PubMed] [Google Scholar]
- 13.Gibson PG, Allen CJ, Yang JP, Wong BJ, Dolovich J, Denburg J, Hargreave FE. Intraepithelial mast cells in allergic and nonallergic asthma: assessment using bronchial brushings. Am Rev Respir Dis. 1993;148:80–86. doi: 10.1164/ajrccm/148.1.80. [DOI] [PubMed] [Google Scholar]
- 14.Tomioka M, Ida S, Shindoh Y, Ishihara T, Takishima T. Mast cells in bronchoalveolar lumen of patients with bronchial asthma. Am Rev Respir Dis. 1984;129:1000–1005. doi: 10.1164/arrd.1984.129.6.1000. [DOI] [PubMed] [Google Scholar]
- 15.Djukanovic R, Wilson JW, Britten KM, Wilson SJ, Walls AF, Roche WR, Howarth PH, Holgate ST. Quantitation of mast cells and eosinophils in the bronchial mucosa of symptomatic atopic asthmatics and healthy control subjects using immunohistochemistry. Am Rev Respir Dis. 1990;142:863–871. doi: 10.1164/ajrccm/142.4.863. [DOI] [PubMed] [Google Scholar]
- 16.Papadaki G, Bakakos P, Kostikas K, Hillas G, Tsilogianni Z, Koulouris NG, Papiris S, Loukides S. Vascular endothelial growth factor and cysteinyl leukotrienes in sputum supernatant of patients with asthma. Respir Med. 2013;107:1339–1345. doi: 10.1016/j.rmed.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Liu MC, Bleecker ER, Lichtenstein LM, Kagey-Sobotka A, Niv Y, McLemore TL, Permutt S, Proud D, Hubbard WC. Evidence for elevated levels of histamine, prostaglandin D2, and other bronchoconstricting prostaglandins in the airways of subjects with mild asthma. Am Rev Respir Dis. 1990;142:126–132. doi: 10.1164/ajrccm/142.1.126. [DOI] [PubMed] [Google Scholar]
- 18.Abe M, Yokoyama Y, Amano H, Matsushima Y, Kan C, Ishikawa O. Effect of activated human mast cells and mast cell-derived mediators on proliferation, type I collagen production and glycosaminoglycans synthesis by human dermal fibroblasts. Eur J Dermatol. 2002;12:340–346. [PubMed] [Google Scholar]
- 19.Holdsworth SR, Summers SA. Role of mast cells in progressive renal diseases. J Am Soc Nephrol. 2008;19:2254–2261. doi: 10.1681/ASN.2008010015. [DOI] [PubMed] [Google Scholar]
- 20.Yu M, Tsai M, Tam SY, Jones C, Zehnder J, Galli SJ. Mast cells can promote the development of multiple features of chronic asthma in mice. J Clin Invest. 2006;116:1633–1641. doi: 10.1172/JCI25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho SH, Tam SW, Demissie-Sanders S, Filler SA, Oh CK. Production of plasminogen activator inhibitor-1 by human mast cells and its possible role in asthma. J Immunol. 2000;165:3154–3161. doi: 10.4049/jimmunol.165.6.3154. [DOI] [PubMed] [Google Scholar]
- 22.Cho SH, Hall IP, Wheatley A, Dewar J, Abraha D, Del Mundo J, Lee H, Oh CK. Possible role of the 4G/5G polymorphism of the plasminogen activator inhibitor 1 gene in the development of asthma. J Allergy Clin Immunol. 2001;108:212–214. doi: 10.1067/mai.2001.117260. [DOI] [PubMed] [Google Scholar]
- 23.Oh CK, Ariue B, Alban RF, Shaw B, Cho SH. PAI-1 promotes extracellular matrix deposition in the airways of a murine asthma model. Biochem Biophys Res Commun. 2002;294:1155–1160. doi: 10.1016/S0006-291X(02)00577-6. [DOI] [PubMed] [Google Scholar]
- 24.Schleimer RP, Kato A, Kern R, Kuperman D, Avila PC. Epithelium: at the interface of innate and adaptive immune responses. J Allergy Clin Immunol. 2007;120:1279–1284. doi: 10.1016/j.jaci.2007.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu EK, Cheng J, Foley JS, Mecham BH, Owen CA, Haley KJ, Mariani TJ, Kohane IS, Tschumperlin DJ, Drazen JM. Induction of the plasminogen activator system by mechanical stimulation of human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2006;35:628–638. doi: 10.1165/rcmb.2006-0040OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly MM, Leigh R, Bonniaud P, Ellis R, Wattie J, Smith MJ, Martin G, Panju M, Inman MD, Gauldie J. Epithelial expression of profibrotic mediators in a model of allergen-induced airway remodeling. Am J Respir Cell Mol Biol. 2005;32:99–107. doi: 10.1165/rcmb.2004-0190OC. [DOI] [PubMed] [Google Scholar]
- 27.Okumura S, Sagara H, Fukuda T, Saito H, Okayama Y. FcepsilonRI-mediated amphiregulin production by human mast cells increases mucin gene expression in epithelial cells. J Allergy Clin Immunol. 2005;115:272–279. doi: 10.1016/j.jaci.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008;123:398–410. doi: 10.1111/j.1365-2567.2007.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kato A, Truong-Tran AQ, Scott AL, Matsumoto K, Schleimer RP. Airway epithelial cells produce B cell-activating factor of TNF family by an IFN-beta-dependent mechanism. J Immunol. 2006;177:7164–7172. doi: 10.4049/jimmunol.177.10.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F.Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes Genome Biol 20023RESEARCH0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wojta J, Kaun C, Zorn G, Ghannadan M, Hauswirth AW, Sperr WR, Fritsch G, Printz D, Binder BR, Schatzl G, et al. C5a stimulates production of plasminogen activator inhibitor-1 in human mast cells and basophils. Blood. 2002;100:517–523. doi: 10.1182/blood.v100.2.517. [DOI] [PubMed] [Google Scholar]
- 32.Gerwin BI, Keski-Oja J, Seddon M, Lechner JF, Harris CC. TGF-beta 1 modulation of urokinase and PAI-1 expression in human bronchial epithelial cells. Am J Physiol. 1990;259:L262–L269. doi: 10.1152/ajplung.1990.259.4.L262. [DOI] [PubMed] [Google Scholar]
- 33.Jakowlew SB, Mariano JM, You L, Mathias A. Differential regulation of protease and extracellular matrix protein expression by transforming growth factor-beta 1 in non-small cell lung cancer cells and normal human bronchial epithelial cells. Biochim Biophys Acta. 1997;1353:157–170. doi: 10.1016/s0167-4781(97)00068-7. [DOI] [PubMed] [Google Scholar]
- 34.Hou B, Eren M, Painter CA, Covington JW, Dixon JD, Schoenhard JA, Vaughan DE. Tumor necrosis factor alpha activates the human plasminogen activator inhibitor-1 gene through a distal nuclear factor kappaB site. J Biol Chem. 2004;279:18127–18136. doi: 10.1074/jbc.M310438200. [DOI] [PubMed] [Google Scholar]
- 35.Kanbe N, Kurosawa M, Nagata H, Saitoh H, Miyachi Y. Cord blood-derived human cultured mast cells produce transforming growth factor beta1. Clin Exp Allergy. 1999;29:105–113. doi: 10.1046/j.1365-2222.1999.00459.x. [DOI] [PubMed] [Google Scholar]
- 36.Aceves SS, Chen D, Newbury RO, Dohil R, Bastian JF, Broide DH.Mast cells infiltrate the esophageal smooth muscle in patients with eosinophilic esophagitis, express TGF-beta1, and increase esophageal smooth muscle contraction J Allergy Clin Immunol 20101261198–1204.e1194 [DOI] [PubMed] [Google Scholar]
- 37.Gleizes PE, Munger JS, Nunes I, Harpel JG, Mazzieri R, Noguera I, Rifkin DB. TGF-beta latency: biological significance and mechanisms of activation. Stem Cells. 1997;15:190–197. doi: 10.1002/stem.150190. [DOI] [PubMed] [Google Scholar]
- 38.He SH, Chen P, Chen HQ. Modulation of enzymatic activity of human mast cell tryptase and chymase by protease inhibitors. Acta Pharmacol Sin. 2003;24:923–929. [PubMed] [Google Scholar]
- 39.O'Connor TM, O'Connell J, O'Brien DI, Goode T, Bredin CP, Shanahan F. The role of substance P in inflammatory disease. J Cell Physiol. 2004;201:167–180. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- 40.Weinstock JV, Blum A, Walder J, Walder R. Eosinophils from granulomas in murine schistosomiasis mansoni produce substance P. J Immunol. 1988;141:961–966. [PubMed] [Google Scholar]
- 41.Lambrecht BN, Germonpre PR, Everaert EG, Carro-Muino I, De Veerman M, de Felipe C, Hunt SP, Thielemans K, Joos GF, Pauwels RA. Endogenously produced substance P contributes to lymphocyte proliferation induced by dendritic cells and direct TCR ligation. Eur J Immunol. 1999;29:3815–3825. doi: 10.1002/(SICI)1521-4141(199912)29:12<3815::AID-IMMU3815>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 42.Lambert RW, Granstein RD. Neuropeptides and Langerhans cells. Exp Dermatol. 1998;7:73–80. doi: 10.1111/j.1600-0625.1998.tb00306.x. [DOI] [PubMed] [Google Scholar]
- 43.Marriott I, Bost KL. Expression of authentic substance P receptors in murine and human dendritic cells. J Neuroimmunol. 2001;114:131–141. doi: 10.1016/s0165-5728(00)00466-5. [DOI] [PubMed] [Google Scholar]
- 44.Bediwy AS, Elkholy MG, Al-Biltagi M, Amer HG, Farid E.Induced sputum substance P in children with difficult-to-treat bronchial asthma and gastroesophageal reflux: effect of esomeprazole therapy Int J Pediatr 20112011967460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonniaud P, Kolb M, Galt T, Robertson J, Robbins C, Stampfli M, Lavery C, Margetts PJ, Roberts AB, Gauldie J. Smad3 null mice develop airspace enlargement and are resistant to TGF-beta-mediated pulmonary fibrosis. J Immunol. 2004;173:2099–2108. doi: 10.4049/jimmunol.173.3.2099. [DOI] [PubMed] [Google Scholar]
- 46.Broekelmann TJ, Limper AH, Colby TV, McDonald JA. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci USA. 1991;88:6642–6646. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakao A, Sagara H, Setoguchi Y, Okada T, Okumura K, Ogawa H, Fukuda T. Expression of Smad7 in bronchial epithelial cells is inversely correlated to basement membrane thickness and airway hyperresponsiveness in patients with asthma. J Allergy Clin Immunol. 2002;110:873–878. doi: 10.1067/mai.2002.129236. [DOI] [PubMed] [Google Scholar]
- 48.Lindstedt KA, Wang Y, Shiota N, Saarinen J, Hyytiainen M, Kokkonen JO, Keski-Oja J, Kovanen PT. Activation of paracrine TGF-beta1 signaling upon stimulation and degranulation of rat serosal mast cells: a novel function for chymase. FASEB J. 2001;15:1377–1388. doi: 10.1096/fj.00-0273com. [DOI] [PubMed] [Google Scholar]
- 49.Yang FC, Chen S, Clegg T, Li X, Morgan T, Estwick SA, Yuan J, Khalaf W, Burgin S, Travers J, et al. Nf1+/− mast cells induce neurofibroma like phenotypes through secreted TGF-beta signaling. Hum Mol Genet. 2006;15:2421–2437. doi: 10.1093/hmg/ddl165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Milara J, Peiro T, Serrano A, Cortijo J. Epithelial to mesenchymal transition is increased in patients with COPD and induced by cigarette smoke. Thorax. 2013;68:410–420. doi: 10.1136/thoraxjnl-2012-201761. [DOI] [PubMed] [Google Scholar]
- 51.Woodman L, Siddiqui S, Cruse G, Sutcliffe A, Saunders R, Kaur D, Bradding P, Brightling C. Mast cells promote airway smooth muscle cell differentiation via autocrine up-regulation of TGF-beta 1. J Immunol. 2008;181:5001–5007. doi: 10.4049/jimmunol.181.7.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tatler AL, Porte J, Knox A, Jenkins G, Pang L. Tryptase activates TGFbeta in human airway smooth muscle cells via direct proteolysis. Biochem Biophys Res Commun. 2008;370:239–242. doi: 10.1016/j.bbrc.2008.03.064. [DOI] [PubMed] [Google Scholar]
- 53.Balzar S, Chu HW, Strand M, Wenzel S. Relationship of small airway chymase-positive mast cells and lung function in severe asthma. Am J Respir Crit Care Med. 2005;171:431–439. doi: 10.1164/rccm.200407-949OC. [DOI] [PubMed] [Google Scholar]