Abstract
Control of airway inflammation is critical in asthma treatment. Soluble epoxide hydrolase (sEH) has recently been demonstrated as a novel therapeutic target for treating inflammation, including lung inflammation. We hypothesized that pharmacological inhibition of sEH can modulate the inflammatory response in a murine ovalbumin (OVA) model of asthma. BALB/c mice were sensitized and exposed to OVA over 6 weeks. A sEH inhibitor (sEHI) was administered for 2 weeks. Respiratory system compliance, resistance, and forced exhaled nitric oxide were measured. Lung lavage cell counts were performed, and selected cytokines and chemokines in the lung lavage fluid were measured. A LC/MS/MS method was used to measure 87 regulatory lipids mediators in plasma, lung tissue homogenates, and lung lavage fluid. The pharmacological inhibition of sEH increased concentrations of the antiinflammatory epoxy eicosatrienoic acids and simultaneously decreased the concentrations of the proinflammatory dihydroxyeicosatrienoic acids and dihydroxyoctadecenoic acids. All monitored inflammatory markers, including FeNO levels, and total cell and eosinophil numbers in the lung lavage of OVA-exposed mice were reduced by sEHI. The type 2 T helper cell (Th2) cytokines (IL-4, IL-5) and chemokines (Eotaxin and RANTES) were dramatically reduced after sEHI administration. Resistance and dynamic lung compliance were also improved by sEHI. We demonstrated that sEHI administration attenuates allergic airway inflammation and airway responsiveness in a murine model. sEHI may have potential as a novel therapeutic strategy for allergic asthma.
Keywords: soluble epoxide hydrolase, asthma, inflammation, lipid mediators, type 2 T helper cell cytokines
Clinical Relevance
We demonstrated that inhibition of soluble epoxide hydrolase attenuates allergic airway inflammation and airway responsiveness in a murine model. Therefore, sEH inhibitors may have potential as a novel therapeutic strategy for allergic asthma.
Three hundred million people worldwide suffer from episodic or persistent asthma (1). The cornerstones of treatment for persistent asthma are inhaled corticosteroids and β-agonist bronchodilators; however, a significant minority of patients with asthma does not respond well to these therapies (2). Thus, there are ongoing efforts to develop novel treatment strategies (3), such as specific antagonists of type 2 T helper cell (Th2) cytokines and mediators.
Epoxyeicosatrienoic acids (EETs, or EpETrEs according to LIPIDMAPS nomenclature) are a class of important lipid mediators with critical physiological functions that include vasodilation, antiinflammation, antihypertension, organ protection, and analgesic effects (4). Specifically in lung health and lung disease, EETs are reported to affect lung epithelial ion transport (5–7), relax precontracted bronchi (8), reduce inflammation (9, 10), regulate endothelial permeability in the lung (11), and regulate pulmonary vascular pressures (12, 13). Thus, modulation of endogenous EETs is an attractive approach to potentially control the symptoms of asthma, which include chronic airway inflammation and airway hyperresponsiveness (AHR).
The soluble epoxide hydrolase (sEH) hydrolyzes these bioactive EETs to their corresponding diols, which are less beneficial and may be toxic. Using potent inhibitors of sEH to stabilize endogenous EETs (14–17), sEH has been recently demonstrated in animal models as a novel therapeutic target (4) for treating cardiovascular diseases (18–20), inflammation (21, 22), pain (23–25), and pulmonary diseases such as pulmonary hypertension (26, 27) and tobacco smoke–induced chronic obstructive pulmonary disease (9, 10).
In the present study, we hypothesized that pharmacological inhibition of sEH can modulate the inflammatory response in a well-established murine ovalbumin (OVA)-exposure asthma model. We found that administration of an sEH inhibitor (sEHI; t-TUCB) reduced total inflammatory cell infiltration into the airway and lung and inhibited OVA-induced influx of eosinophils. The profiling of regulatory lipid mediators shows that t-TUCB administration not only increased the antiinflammatory lipid mediators (EETs) but also increased other antiinflammatory mediators, such as 17-hydroxy docosahexaenoic acid, and decreased proinflammatory lipid mediators, including dihydroxyoctadecenoic acids (DHOMEs) and LTB4 in plasma, lung tissue, and lavage. Stabilization of the antiinflammatory epoxide lipid mediators through pharmacological inhibition of sEH decreased production of Th2 cytokines at the protein and mRNA levels after OVA induction. Furthermore, compliance and resistance of the respiratory system were improved after sEHI administration. These findings support the hypothesis that sEH is a potential target to treat asthma.
Materials and Methods
Animals
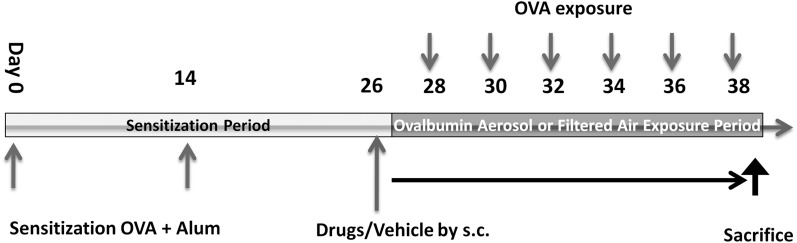
Pathogen-free male BALB/c mice, aged 8 to 10 weeks, were purchased from Charles River Laboratory (Wilmington, MA). All mice were maintained in a HEPA-filtered laminar flow cage rack with a 12-hour light/dark cycle and allowed free access to food and water. Figure 1A shows the animal protocol. All procedures with mice were performed in accordance with an IACUC-approved protocol.
Figure 1.
The protocol for exposure and treatments. BALB/c mice were sensitized by intraperitoneal injection of ovalbumin (OVA) and alum adjuvant solution for 4 weeks then exposed to OVA aerosol six times. Depending on the treatment groups, mice were injected subcutaneously with vehicle solution, 1 mg/kg t-TUCB, or 3 mg/kg t-TUCB every other day from Day 26 to Day 38. Previous pharmacokinetic studies with t-TUCB in mice indicated that this injection schedule maintains a sufficient plasma concentration to inhibit target activity.
Drug Solutions and Exposure of Mice to OVA Aerosol
The sEHI trans-4-{4-[3-(4-trifluoromethoxyphenyl)-ureido]-cyclohexyloxy}-benzoic acid (t-TUCB) was synthesized as previously described (17). t-TUCB was dissolved in 0.05% (v/v) Tween-80 water solution. This solution (1 or 3 mg/kg) was administered subcutaneously to the mice every day for 14 days. On the last day, the drugs were administered 30 minutes before OVA aerosol exposure. The exposure procedures of OVA have been described in detail previously (28).
Lung Compliance and Resistance Measurements
Dynamic lung compliance and respiratory system resistance were simultaneously measured with a whole body plethysmograph for restrained animals (Buxco Inc., Troy, NY) 1 to 3 hours after termination of the final OVA exposure. Further details are provided in the online supplement.
Measurement of Exhaled NO
A 5-minute sample of exhaled gases was collected from the cannulated mice through the ventilator exhalation port immediately after insertion of the mouse into a plethysmograph as previously described (29).
Cytokine and Chemokine Assays
The concentrations of selected cytokines and chemokines (Eotaxin, IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, RANTES, and TNF-α) from the bronchoalveolar lavage fluid (BALF) supernatant were measured with commercially available multiplex immunoassays according to the manufacturer’s instructions (Millipore, St. Charles, MO).
Measurement of t-TUCB Concentration
Blood (10 μl) was diluted with 0.1% aqueous EDTA (50 μl) and mixed vigorously. Samples were then extracted using 200 μl of ethyl acetate twice and dried by Speedvac (Thermo Scientific, Waltham, MA). The residue was reconstituted to 50 μl of internal standard solution and measured by LC/MS/MS.
Regulatory Lipid Mediator Profiling
Profiles of regulatory mediators were measured using the LC/MS/MS method as described previously (30). Aliquots of plasma (250 μl), BAL supernatant (2 ml), or lung tissue (∼ 100 mg) were used for the measurements, respectively. Further details are provided in the online supplement.
Quantitative Real-Time Reverse Transcription PCR
Total RNA was isolated from lung tissue using Trizol and a Quick-RNA Mini prep isolation kit (Zymo Research, Irvine, CA). cDNA synthesis and the RT-PCR processes are described in the online supplement.
Statistical Analysis
Data are presented as means ± SEM. Data were analyzed using unpaired values compared by two-tailed Student's t test or one-way or two-way ANOVA with Tukey's post test where appropriate, using the Prism 5.0 software package (GraphPad, Inc., San Diego, CA), with statistical significance defined as P ≤ 0.05.
Results
sEHI Was Successfully Delivered and Well Engaged
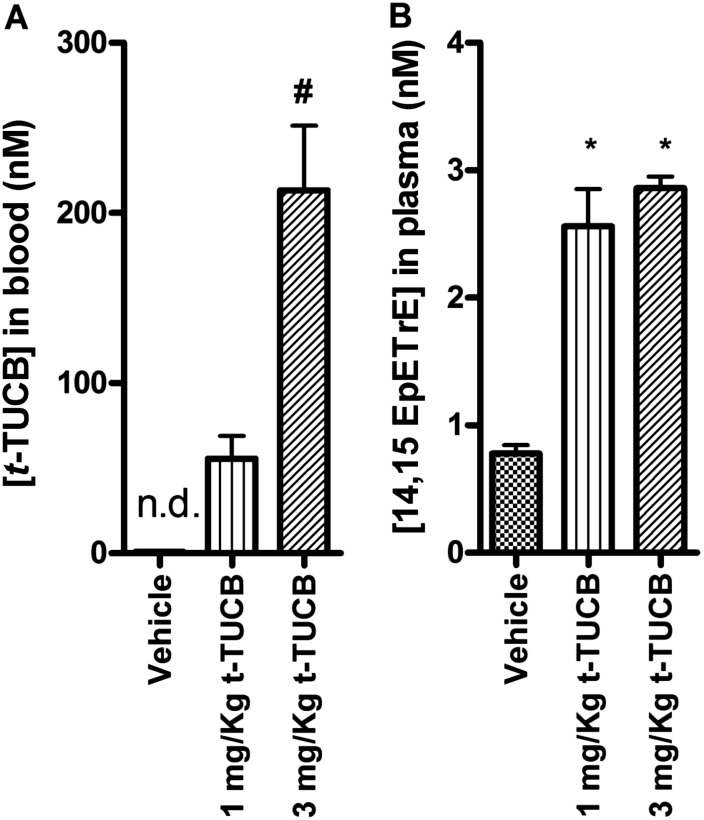
After 14 days of subcutaneous injection of t-TUCB, concentrations of t-TUCB in blood (Figure 2A) reached 55.6 ± 13.2 nM (1 mg/kg) and 213 ± 38 nM (3 mg/kg), which are 42.8 and 164 times higher, respectively, than the IC50 of t-TUCB in mouse on the murine recombinant sEH (1.3 nM) using trans-diphenylpropene oxide as substrate (17). As a result of enzyme inhibition, the concentrations of 14,15-EpETrE, an endogenous substrate of sEH, increased by 3- to 3.6-fold from 0.78 nM to 2.56 or 2.86 nM with the administration of 1 or 3 mg/kg t-TUCB compared with the vehicle control (Figure 2B). Increased concentrations of 14,15-EpETrE after exposure to t-TUCB confirmed the efficacy of t-TUCB as an sEHI in this study.
Figure 2.
The concentration of t-TUCB in blood (A) and 14,15-EpETrE concentration in plasma (B) suggest that the inhibitor was delivered successfully in vivo and was well engaged. Blood was drawn 2 to 6 hours after administration of the last dose. *Significant difference from vehicle group. #Significant difference between the 1 mg/kg and 3 mg/kg t-TUCB groups. All three groups were exposed to OVA (n = 5 in all groups, except n = 4 in the Air+Vehicle group).
sEHI Administration Increased Antiinflammatory Mediator Concentrations and Decreased Proinflammatory Mediator Concentrations
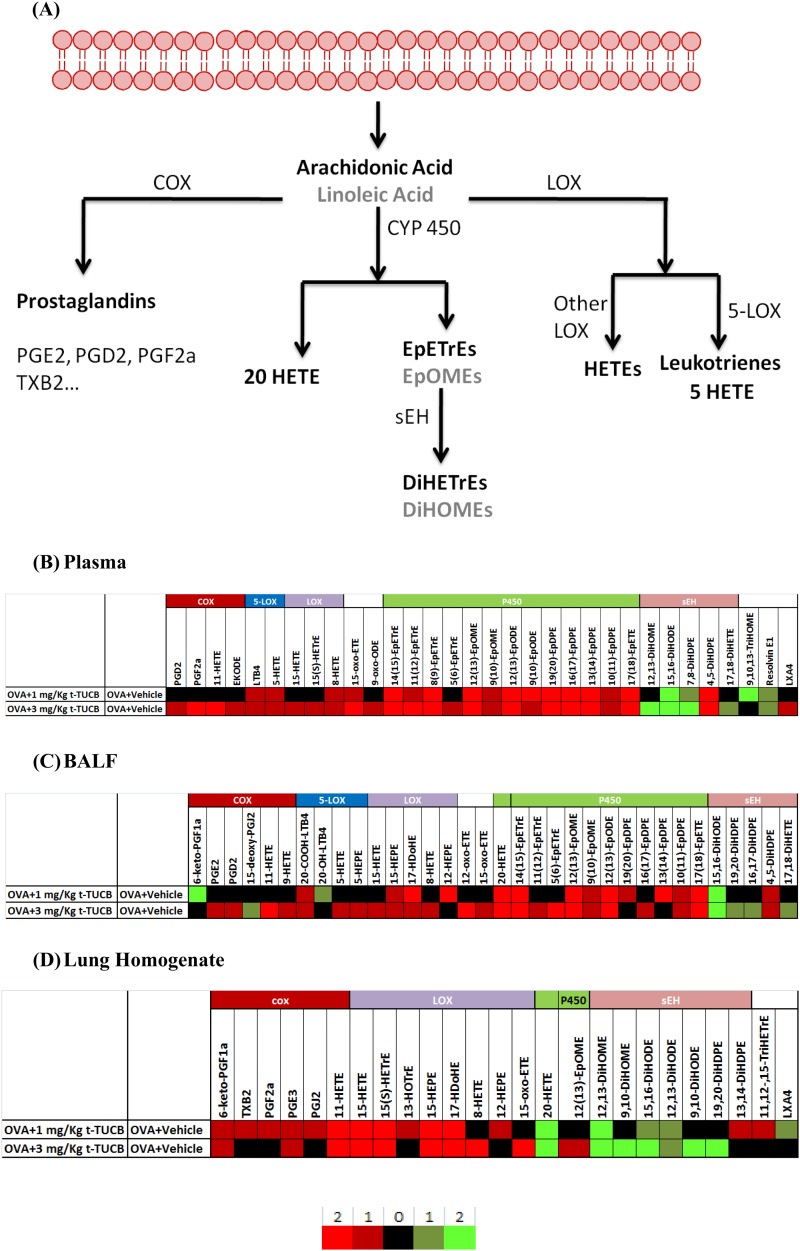
We analyzed the regulatory lipid mediators from BALF, plasma, and lung tissue homogenate using LC/MS/MS. Figure 3 shows the results presented as heatmaps, including a simplified arachidonic acid cascade listing the major lipid mediators (Figure 3A) and the significantly changed regulatory lipid mediators after t-TUCB administration (Figures 3B–3D). In general, for the P450 and sEH pathways, sEHI administration increased epoxides in plasma and BALF and decreased diols in BALF and lung homogenates. In plasma, sEHI administration increased some COX and LOX metabolites (11-HETE, 9-HETE, 5-HETE, and 5-HEPE). The low dose of sEHI significantly reduced the proinflammatory mediators 6-keto-PGF1α (the metabolite and surrogate of prostacyclin-PGI2) and LTB4. In BALF, sEHI administration increased LOX metabolites, including 11-HETE, 9-HETE, 5-HETE, 15-HEPE, 17HDoHE, and 8-HETE. In lung homogenates, sEHI administration increased the COX metabolites 6-keto-PGF1α, TXB2, PGF2α, PGE3, PGJ2, and 11-HETE and increased the LOX metabolites, including 15-HETE, 15(s) HETrE, 15-HEPE, 17-hydroxy docosahexaenoic acid, and 8-HETE.
Figure 3.
Heatmaps generated from regulatory lipid mediators show that t-TUCB administration increased antiinflammatory lipid mediators and decreased proinflammatory lipid mediators in vivo. (A) A simplified depiction of the arachidonic acid cascade. (B–D) Heatmaps based on regulatory lipid mediators in plasma (B), bronchoalveolar lavage fluid (BALF) (C), and lung homogenates (D). The color corresponds to the fold change as shown in the legend. Bright red means an increase of more than 2 times significantly (P < 0.05); dark red means an increase of less than 2 times significantly; black means no significant change; dark green means a decrease of less than 2 times significantly; and bright green means a decrease of more than 2 times significantly. For plasma and BALF, n = 5 in all groups, except n = 4 in the Air+Vehicle group; for lung homogenates results, n = 4 in all groups, except n = 3 in the Air+Vehicle group.
sEHI Administration Reduced Th2 Cytokines and Chemokines
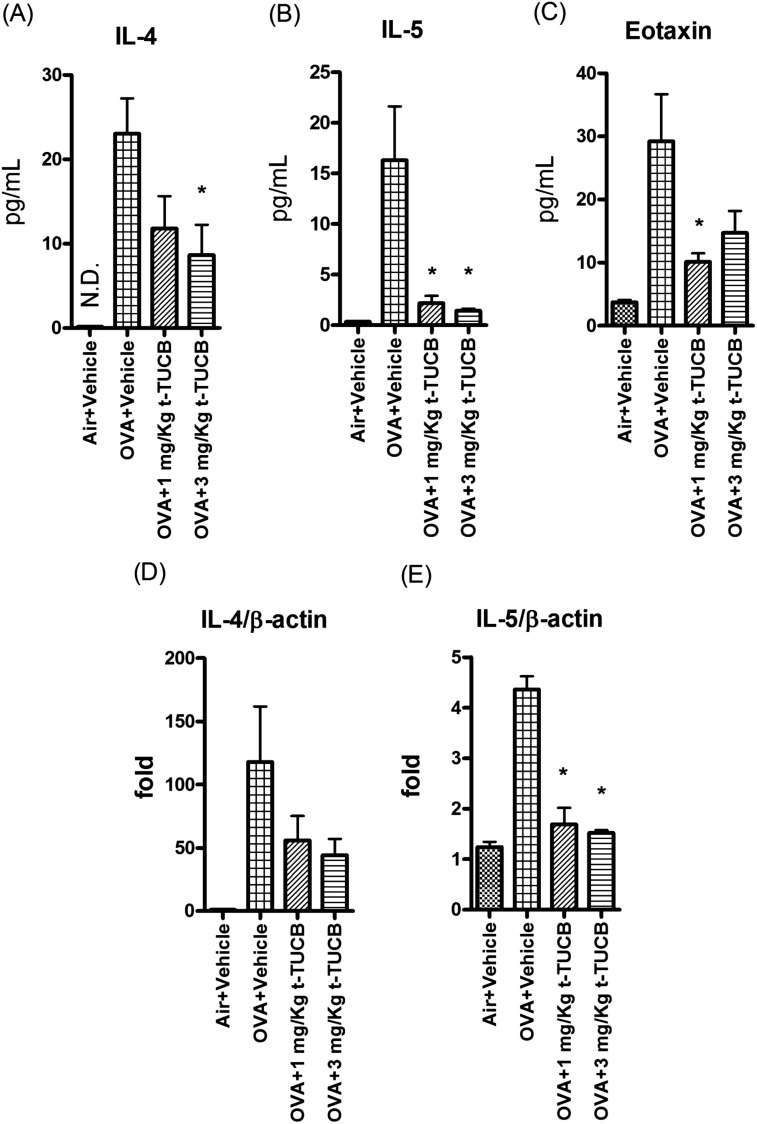
Several inflammatory cytokines were induced after OVA exposure (Figure 4 and Figure E1). After sEHI administration, IL-4 and IL-5 in lung lavage fluid decreased to almost the baseline level of the control animals (Figures 4A and 4B). By contrast, there were no clear trends for Th1 and innate immune cytokines assayed (Figure E1). Because of the methodological issues involved in IL-13 detection, we could not make firm conclusions regarding the involvement of IL-13 in this model system. The chemokine eotaxin was induced after OVA exposure, and its levels were blunted by inhibition of sEH (Figure 4C). These data suggest that inhibition of sEH could reduce the Th2-specific cytokines and chemokines, which are important in eosinophil trafficking, recruitment, and maturation in airways. Lung expression of IL-4 and IL-5 also showed that RNA levels of these Th2 cytokines were down-regulated by sEHI administration (Figures 4D and 4E).
Figure 4.
t-TUCB administration dramatically decreased Th2 cytokines IL-4 and IL-5 (A and B) and chemokine (Eotaxin) (C) production in BALF and down-regulated the gene expression of these Th2 cytokines (D and E) in lung homogenates. *Significant difference from the OVA+vehicle group (n = 5 in all groups, except n = 4 in nonimmunized+Vehicle group).
sEHI Administration Reduced Inflammatory Cell Infiltration in Lung Tissues and Lavage Fluid
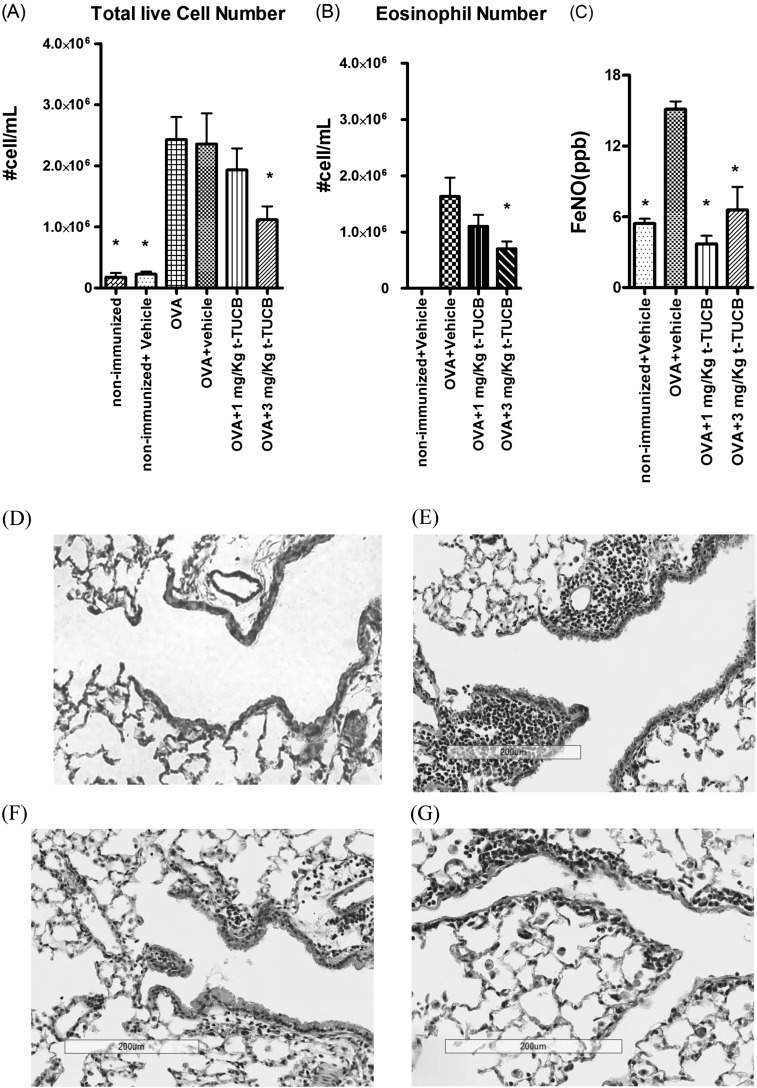
Sensitization and exposure of mice to OVA induced significant inflammatory cell infiltration into the airway (Figure 5A). The total cell count in BALF reached approximately 2.6 × 106 cells/ml. t-TUCB administration at 1 mg/kg dose decreased the number of inflammatory cells infiltrated into the BALF. Furthermore, 3 mg/kg t-TUCB significantly reduced the total cell number in BALF to approximately 46.2%. ANOVA showed that there was significant reduction of total live cell numbers after sEHI administration (P = 0.04).
Figure 5.
(A) t-TUCB administration reduced the infiltrated inflammatory cells (cells/ml) in BALF. (B) Differential cell counts by Hema-3 stain shows that soluble epoxide hydrolase inhibitor (sEHI) reduced the number of eosinophil (cells/ml) in BALF. (C) t-TUCB administration reduced the inflammatory marker FeNO. (D–G) Hematoxylin and eosin stain results of lung tissues shows that sEHI reduces the infiltration of inflammatory cells into lung tissues. Shown are results from the air control group (D), the vehicle control group after OVA exposure (E), the 1 mg/kg t-TUCB–treated group (F), and the 3 mg/kg t-TUCB–treated group (G). *Significant difference from the OVA+vehicle group.
Figure 5B shows the differential cell counts determined by the Hema-3 stain set. After OVA exposure, the eosinophil is the dominant inflammatory cell type in BALF, comprising up to 75% of the total inflammatory cell infiltrate. After sEHI administration, the percentage of eosinophils was reduced to 65%. t-TUCB at a dose of 3 mg/kg significantly reduced eosinophil infiltration into lung lavage from 1.63 × 106 to 7.05 × 105. ANOVA shows that there is significant reduction of total live cell number after sEHI administration (P = 0.049), indicating that sEHI not only reduced the total inflammatory cell infiltration into the airway but also altered the ratio of inflammatory cells present in the BALF (eosinophil/macrophage ratio from 4.39 to 2.30). This result also corresponds to the reduction of Th2 cytokines in the lavage and lung tissue. Exhaled nitric oxide (NO) is a biomarker of airway eosinophil inflammation and was increased from 5.43 to 15.1 ppb after OVA exposure (Figure 5C). Treatment with 1 and 3 mg/kg of t-TUCB decreased the induction of this inflammatory biomarker to 3.68 and 6.57 ppb, respectively. ANOVA with Bonferroni post test shows that there is significant reduction of FeNO after sEHI administration (P = 0.0006). In lung tissues, there was marked inflammatory cell influx in the peribronchiolar space after OVA exposure, as shown in the hematoxylin and eosin stain result (Figures 5D and 5E), which is consistent with the lung lavage data (Figures 5A and 5B). After inhibition of sEH, inflammatory cells in the lung tissue were reduced in a dose-dependent manner (Figures 5F and 5G). These figures show that sEHI administration reduced the lung inflammatory cell infiltration, which is consistent with the inflammatory cell results in the BALF cell counts.
Our results reveal severe eosinophil-dominant inflammation in the airway and alveolar of the mice after OVA exposure. sEHI administration reduced this inflammation, as shown in total live cell number, inflammatory cell differentiation, hematoxylin and eosin–stained lung tissue, and FeNO.
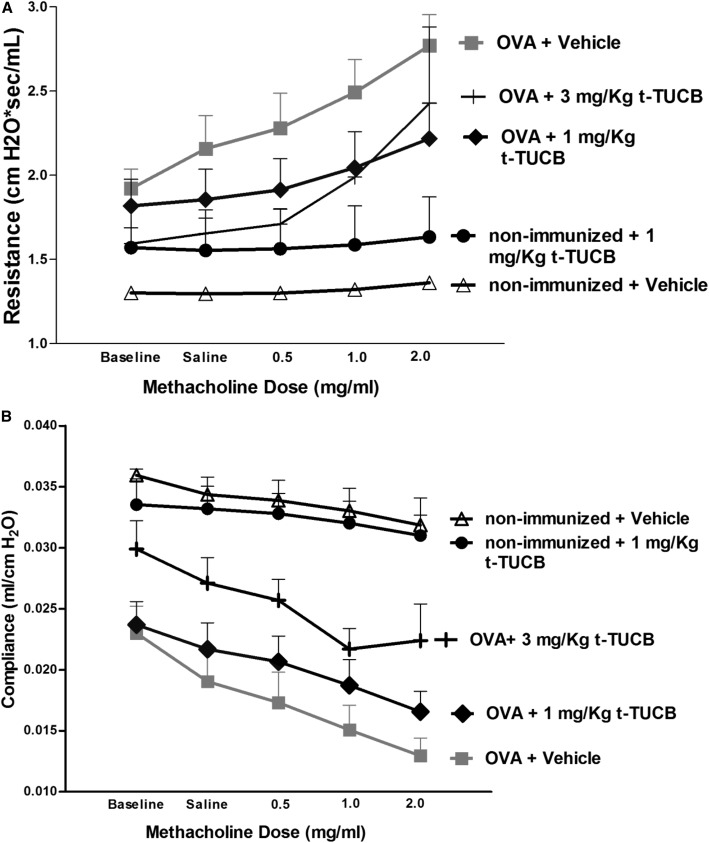
sEHI Reduced Methacholine-Induced Changes in Resistance and Dynamic Compliance of the Respiratory System
Exposure to OVA reduced the baseline compliance from 0.036 ml/cm H2O (filter air group) to 0.023 ml/cm H2O (OVA+vehicle). Administration of 3 mg/kg sEHI rescued the baseline compliance to 0.03 ml/cm H2O (Figure 6B). Although 1 mg/kg sEHI did not rescue the baseline compliance, both sEHI treatment groups helped to prevent the change in compliance with methacholine challenge (P < 0.0001 for overall effect by treatment group and P = 0.0005 for the doses; assessed by two-way ANOVA).
Figure 6.
Airway hyperreacitivity (A) and Dynamic lung compliance (B) results show that t-TUCB administration rescued OVA-induced asthmatic airway hyperresponsiveness (n = 4 for OVA+Vehicle group and the OVA+3 mg/kg t-TUCB group; n = 3 for the nonummunized+Vehicle group and the OVA+1 mg/kg t-TUCB group).
OVA exposure increased baseline resistance and the slope of resistance change in response to methacholine challenge(Figure 6A). sEHI administration rescued the baseline resistance in a dose-dependent manner (P = 0.0035 for overall effect by treatment group and P = 0.0018 for the doses of methacholine, as assessed by two-way ANOVA). At 1 mg/kg, sEHI reduced the slope of resistance change along with methacholine, whereas sEHI at a dose of 3 mg/kg shows a more complex pattern: a shallow initial slope with low methacholine dose, then a rapid increase in slope with the higher dose of methacholine.
Discussion
We demonstrated that administration of an sEHI markedly attenuates the allergic airway inflammation and AHR caused by exposing mice to OVA. Specifically, the sEHI reduced the total inflammatory cell number by 50%. Compared with two clinically available compounds (montelukast and dexamethasone) tested in the similar animal model, sEHI had a greater effect on reduction of the inflammatory cells infiltration than montelukast and dexamethasone did (40 and 28%) (31). In addition, sEHI reduced IL-5 levels to 12.5% of those mice treated with OVA and vehicle. Taken together, these findings indicate that sEHI is a promising potential candidate drug to treat allergic asthma.
We and others have previously shown the antiinflammatory effects of sEHIs in different disease models (9, 21, 22, 32). The mechanism is believed to be through stabilizing of the antiinflammatory EETs, which regulate NF-κB translocation (33), or through reducing production of proinflammatory diols, including DHOMEs (34) and dihydroxy eicosatrienoic acids (DHETs) (35). Here, our data show that the concentration of sEHI in the plasma significantly altered the circulating EET and DHET levels present in plasma, BALF, and lung homogenates (Figures 3C and 3D). Node (33) reported that EETs (EpETrEs) can reduce endothelial cell VCAM-1 expression in response to TNF-α, IL-1α, and LPS. At the same time, sEHI administration significantly reduced proinflammatory DHETs in BALF and lung homogenate (Figures 3C and 3D). The DHETs were reported as essential for monocyte chemotaxis to MCP-1 (35). In particular, sEHI reduced the DHOMEs, metabolites of leukotoxin, and iso-leukotoxin. It was found that these DHOMEs are more toxic than EpOMEs and are associated with multiple organ failure and adult respiratory distress syndrome (34). Taken together, these findings indicate that the effect t-TUCB on the concentrations of EETs and DHETs may contribute to the antiinflammatory effects of sEHI in this murine asthmatic model.
In this study, we also observed that additional lipid mediators were affected upon t-TUCB administration. The proinflammatory lipid mediator LTB4 was decreased in the plasma after low-dose sEHI administration. It was reported that LTB4 participates in the allergic sensitization process in animal models (36). Therefore, the effect of inhibition of sEH might also benefit from a reduction of proinflammatory LTB4. Another lipid mediator, 17HDoHE, which is a precursor to resolvins and possesses biological activity that inhibits TNF-α–induced IL-1β expression (37), was increased in lavage and lung homogenates after sEHI administration. Resolvins have been reported to promote the resolution of the allergic airways response (38).
Our findings add to those reported elsewhere by describing the effects of sEH inhibition on allergic airway inflammation. Previous studies (10, 21) have demonstrated that sEHI can reduce the inflammatory cytokines IL-1β, IL-6, and INF-γ. In the present study, we found that sEHI reduced Th2 cytokines and chemokines, which are known to play major roles in the asthmatic immune response (39). Specifically, the pronounced effect of sEHI on IL-5 and eotaxin-1, a key cytokine and a chemokine responsible for the release of eosinophil from the bone marrow and homing of eosinophil to the lung, is rather intriguing. Indeed, mepolizumab, a monoclonal antibody against IL-5 used for the treatment of severe asthma, is garnering significant attention in the clinical realm (40). The inhibition of sEH may be an alternative strategy for decreasing IL-5 levels in concert with other key mediators of lung inflammation.
There are reports indicating that some of these regulatory lipid mediators, such as EETs, have direct function on the bronchi (6, 41). We observed that sEHI administration increased EETs in the BALF (Figure 5C), which may directly rescue airway hyperreactivity. However, inflammation may also play a role in regulating lung compliance and resistance. The antiinflammatory effects of sEHI might have contributed to the improvement of lung function after sEHI administration in this acute model of asthma. In addition, lipid mediators such as HETEs are reported to have effects on the airways (42). Alterations in the levels of various lipid mediators may explain why treatment with 3 mg/kg of sEHI did not show improved rescue (in comparison to treatment with 1 mg/kg) of the resistance induced by 2.0 mg/ml of methacholine (Figure 6B). Direct pulmonary administration of a sEHI would provide additional evidence on how sEHI regulates lung compliance and resistance. We have not developed an effective system for administering sEHI directly to the lung.
In this study, neither COX-2 nor 5-LOX in lung homogenates was significantly suppressed by sEHI administration in OVA-exposed mice (Figure E2). These findings suggest, at least in lung homogenates, that the major effects of sEHI are unlikely due to the NF-κB pathway.
sEHI administration increased the antiinflammatory mediators systemically and in the airways, as indicated by the lipid mediator levels from the lavage, while simultaneously decreasing proinflammatory mediators in the lung tissues and airways. These changes in lipid mediators influenced the reduction and down-regulation of Th2 cytokines and chemokine expression in the airways. The reduction of these Th2 cytokines and chemokines further decreased the recruitment of inflammatory cells infiltration into the lungs and airways. The reduction in overall lung inflammation and the increase of EETs in airway contributes to the alleviation of AHR.
The data are consistent with the sEH (EPHX2) being primarily responsible for the conversion of fatty acid epoxides to the corresponding diols in the lung and with t-TUCB inhibiting this catalytic activity. The activity of mEH (EPHX1) on this substrate is low, as are pulmonary levels of the mEH. We have found no evidence for catalytic activity of EH3 (EPHX3) or EH4 in the lung. Although there is no evidence of inhibition of EPHX1, -3, or -4 or an unknown enzyme by t-TUCB, we cannot exclude the possibility of off-target effects.
Among the limitations of this study is that we used only prophylactic and not therapeutic treatment. Long-term pulmonary inflammation, including asthma, leads to chronic changes in the lung. However, our short-term model did not assess the effects of sEHI on fibrotic biomarkers because this would require a longer-term exposure to inflammation, particularly one that is associated with chronic diseases involving the use of multiple drugs with different mechanisms of action. Future studies need to address the possible beneficial and detrimental effects of long-term sEHI use.
Footnotes
This work was supported by American Asthma Association grant AAF 09-0269, National Institute of Environmental Health Sciences grant R01 ES002710, and National Institute of Environmental Health Sciences Superfund Research Program grant P42 ES004699. Analytical work was partially supported by the National Institutes of Health and by National Institute of Diabetes and Digestive and Kidney Diseases grant U24 DK097154. National Institutes of Health grant HL105573 provided the NO work equipment. This work was also supported by a Fellowship from Cystic Fibrosis Research, Inc. (J.Y.), by #UL1TR000002 and CTSC NIH KL2 (K12) Award TR000134 (A.A.Z.), and by T32HL007013 (J.B.).
Author Contributions: Conception and design: B.D.H., J.Y., and N.J.K. Analysis and interpretation: J.Y., J.B., L.F., J.-Y.L., G.Z., A.A.Z., C.F.A.V., K.W., H.D., Y.L., and S.H.H. Drafting the manuscript: J.Y., B.D.H., and N.J.K.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0440OC on June 12, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Masoli M, Fabian D, Holt S, Beasley R Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 3.Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008;8:218–230. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- 4.Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kersting U, Kersting D, Spring KR. Ketoconazole activates Cl- conductance and blocks Cl- and fluid absorption by cultured cystic fibrosis (CFPAC-1) cells. Proc Natl Acad Sci USA. 1993;90:4047–4051. doi: 10.1073/pnas.90.9.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pascual JM, McKenzie A, Yankaskas JR, Falck JR, Zeldin DC. Epoxygenase metabolites of arachidonic acid affect electrophysiologic properties of rat tracheal epithelial cells1. J Pharmacol Exp Ther. 1998;286:772–779. [PubMed] [Google Scholar]
- 7.Salvail D, Dumoulin M, Rousseau E. Direct modulation of tracheal Cl- channel activity by 5,6- and 11,12-EET. Am J Physiol. 1998;275:L432–L441. doi: 10.1152/ajplung.1998.275.3.L432. [DOI] [PubMed] [Google Scholar]
- 8.Zeldin DC, Plitman JD, Kobayashi J, Miller RF, Snapper JR, Falck JR, Szarek JL, Philpot RM, Capdevila JH. The rabbit pulmonary cytochrome P450 arachidonic acid metabolic pathway: characterization and significance. J Clin Invest. 1995;95:2150–2160. doi: 10.1172/JCI117904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith KR, Pinkerton KE, Watanabe T, Pedersen TL, Ma SJ, Hammock BD. Attenuation of tobacco smoke-induced lung inflammation by treatment with a soluble epoxide hydrolase inhibitor. Proc Natl Acad Sci USA. 2005;102:2186–2191. doi: 10.1073/pnas.0409591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Yang J, Guo L, Uyeminami D, Dong H, Hammock BD, Pinkerton KE. Use of a soluble epoxide hydrolase inhibitor in smoke-induced chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2012;46:614–622. doi: 10.1165/rcmb.2011-0359OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvarez DF, Gjerde EA, Townsley MI. Role of EETs in regulation of endothelial permeability in rat lung. Am J Physiol Lung Cell Mol Physiol. 2004;286:L445–L451. doi: 10.1152/ajplung.00150.2003. [DOI] [PubMed] [Google Scholar]
- 12.Fleming I. Cytochrome P450-dependent eicosanoid production and crosstalk. Curr Opin Lipidol. 2011;22:403–409. doi: 10.1097/MOL.0b013e32834a9790. [DOI] [PubMed] [Google Scholar]
- 13.Loot AE, Fleming I. Cytochrome P450-derived epoxyeicosatrienoic acids and pulmonary hypertension: central role of transient receptor potential C6 channels. J Cardiovasc Pharmacol. 2011;57:140–147. doi: 10.1097/FJC.0b013e3181ed088d. [DOI] [PubMed] [Google Scholar]
- 14.Shen HC, Hammock BD. Discovery of inhibitors of soluble epoxide hydrolase: a target with multiple potential therapeutic indications. J Med Chem. 2012;55:1789–1808. doi: 10.1021/jm201468j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rose TE, Morisseau C, Liu JY, Inceoglu B, Jones PD, Sanborn JR, Hammock BD. 1-Aryl-3-(1-acylpiperidin-4-yl)urea inhibitors of human and murine soluble epoxide hydrolase: structure-activity relationships, pharmacokinetics, and reduction of inflammatory pain. J Med Chem. 2010;53:7067–7075. doi: 10.1021/jm100691c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim IH, Tsai HJ, Nishi K, Kasagami T, Morisseau C, Hammock BD. 1,3-disubstituted ureas functionalized with ether groups are potent inhibitors of the soluble epoxide hydrolase with improved pharmacokinetic properties. J Med Chem. 2007;50:5217–5226. doi: 10.1021/jm070705c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang SH, Tsai HJ, Liu JY, Morisseau C, Hammock BD. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J Med Chem. 2007;50:3825–3840. doi: 10.1021/jm070270t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu D, Li N, He Y, Timofeyev V, Lu L, Tsai HJ, Kim IH, Tuteja D, Mateo RK, Singapuri A, et al. Prevention and reversal of cardiac hypertrophy by soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci USA. 2006;103:18733–18738. doi: 10.1073/pnas.0609158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ai D, Fu Y, Guo D, Tanaka H, Wang N, Tang C, Hammock BD, Shyy JY, Zhu Y. Angiotensin II up-regulates soluble epoxide hydrolase in vascular endothelium in vitro and in vivo. Proc Natl Acad Sci USA. 2007;104:9018–9023. doi: 10.1073/pnas.0703229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ai D, Pang W, Li N, Xu M, Jones PD, Yang J, Zhang Y, Chiamvimonvat N, Shyy JY, Hammock BD, et al. Soluble epoxide hydrolase plays an essential role in angiotensin II-induced cardiac hypertrophy. Proc Natl Acad Sci USA. 2009;106:564–569. doi: 10.1073/pnas.0811022106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu JY, Yang J, Inceoglu B, Qiu H, Ulu A, Hwang SH, Chiamvimonvat N, Hammock BD. Inhibition of soluble epoxide hydrolase enhances the anti-inflammatory effects of aspirin and 5-lipoxygenase activation protein inhibitor in a murine model. Biochem Pharmacol. 2010;79:880–887. doi: 10.1016/j.bcp.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci USA. 2005;102:9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inceoglu B, Jinks SL, Ulu A, Hegedus CM, Georgi K, Schmelzer KR, Wagner K, Jones PD, Morisseau C, Hammock BD. Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proc Natl Acad Sci USA. 2008;105:18901–18906. doi: 10.1073/pnas.0809765105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inceoglu B, Wagner K, Schebb NH, Morisseau C, Jinks SL, Ulu A, Hegedus C, Rose T, Brosnan R, Hammock BD. Analgesia mediated by soluble epoxide hydrolase inhibitors is dependent on cAMP. Proc Natl Acad Sci USA. 2011;108:5093–5097. doi: 10.1073/pnas.1101073108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inceoglu B, Wagner KM, Yang J, Bettaieb A, Schebb NH, Hwang SH, Morisseau C, Haj FG, Hammock BD. Acute augmentation of epoxygenated fatty acid levels rapidly reduces pain-related behavior in a rat model of type I diabetes. Proc Natl Acad Sci USA. 2012;109:11390–11395. doi: 10.1073/pnas.1208708109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keserü B, Barbosa-Sicard E, Schermuly RT, Tanaka H, Hammock BD, Weissmann N, Fisslthaler B, Fleming I. Hypoxia-induced pulmonary hypertension: comparison of soluble epoxide hydrolase deletion vs. inhibition. Cardiovasc Res. 2010;85:232–240. doi: 10.1093/cvr/cvp281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Revermann M, Barbosa-Sicard E, Dony E, Schermuly RT, Morisseau C, Geisslinger G, Fleming I, Hammock BD, Brandes RP. Inhibition of the soluble epoxide hydrolase attenuates monocrotaline-induced pulmonary hypertension in rats. J Hypertens. 2009;27:322–331. doi: 10.1097/hjh.0b013e32831aedfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bratt JM, Franzi LM, Linderholm AL, Last MS, Kenyon NJ, Last JA. Arginase enzymes in isolated airways from normal and nitric oxide synthase 2-knockout mice exposed to ovalbumin. Toxicol Appl Pharmacol. 2009;234:273–280. doi: 10.1016/j.taap.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bratt JM, Williams K, Rabowsky MF, Last MS, Franzi LM, Last JA, Kenyon NJ.Nitric oxide synthase enzymes in the airways of mice exposed to ovalbumin: Nos2 expression is nos3 dependent. Mediators Inflam2010;2010:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Chem. 2009;81:8085–8093. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamabayashi C, Koya T, Kagamu H, Kawakami H, Kimura Y, Furukawa T, Sakagami T, Hasegawa T, Sakai Y, Matsumoto K, et al. A novel prostacyclin agonist protects against airway hyperresponsiveness and remodeling in mice. Am J Respir Cell Mol Biol. 2012;47:170–177. doi: 10.1165/rcmb.2011-0350OC. [DOI] [PubMed] [Google Scholar]
- 32.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moghaddam MF, Grant DF, Cheek JM, Greene JF, Williamson KC, Hammock BD. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat Med. 1997;3:562–566. doi: 10.1038/nm0597-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kundu S, Roome T, Bhattacharjee A, Carnevale KA. Yakubenko VP, Zhang R, Hwang SH, Hammock BD, Cathcart MK. Metabolic projects of soluble epoxide hydrolase are essential for monocyte chemotaxis to MCP-1 in vitro and in vivo. J Lipid Res. 2013;54:436–447. doi: 10.1194/jlr.M031914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hallstrand TS, Henderson WR., Jr An update on the role of leukotrienes in asthma. Curr Opin Allergy Clin Immunol. 2010;10:60–66. doi: 10.1097/ACI.0b013e32833489c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rogerio AP, Haworth O, Croze R, Oh SF, Uddin M, Carlo T, Pfeffer MA, Priluck R, Serhan CN, Levy BD. Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. J Immunol. 2012;189:1983–1991. doi: 10.4049/jimmunol.1101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Locksley RM. Asthma and allergic inflammation. Cell. 2010;140:777–783. doi: 10.1016/j.cell.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O’Byrne PM. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 41.Jacobs ER, Zeldin DC. The lung HETEs (and EETs) up. Am J Physiol Heart Circ Physiol. 2001;280:H1–H10. doi: 10.1152/ajpheart.2001.280.1.H1. [DOI] [PubMed] [Google Scholar]
- 42.Copas JL, Borgeat P, Gardiner PJ. The actions of 5-, 12-, and 15-HETE on tracheobronchial smooth muscle. Prostaglandins Leukot Med. 1982;8:105–114. doi: 10.1016/s0262-1746(82)80002-4. [DOI] [PubMed] [Google Scholar]