Abstract
Asthma is a disease of acute and chronic inflammation in which cytokines play a critical role in orchestrating the allergic inflammatory response. IL-13 and transforming growth factor (TGF)-β promote fibrotic airway remodeling, a major contributor to disease severity. Improved understanding is needed, because current therapies are inadequate for suppressing development of airway fibrosis. IL-13 is known to stimulate respiratory epithelial cells to produce TGF-β, but the mechanism through which this occurs is unknown. Here, we tested the hypothesis that reactive oxygen species (ROS) are a critical signaling intermediary between IL-13 or allergen stimulation and TGF-β–dependent airway remodeling. We used cultured human bronchial epithelial cells and an in vivo mouse model of allergic asthma to map a pathway where allergens enhanced mitochondrial ROS, which is an essential upstream signal for TGF-β activation and enhanced collagen production and deposition in airway fibroblasts. We show that mitochondria in airway epithelium are an essential source of ROS that activate TGF-β expression and activity. TGF-β from airway epithelium stimulates collagen expression in fibroblasts, contributing to an early fibrotic response to allergen exposure in cultured human airway cells and in ovalbumin-challenged mice. Treatment with the mitochondrial-targeted antioxidant, (2-(2,2,6,6-Tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl)triphenylphosphonium chloride (mitoTEMPO), significantly attenuated mitochondrial ROS, TGF-β, and collagen deposition in OVA-challenged mice and in cultured human epithelial cells. Our findings suggest that mitochondria are a critical source of ROS for promoting TGF-β activity that contributes to airway remodeling in allergic asthma. Mitochondrial-targeted antioxidants may be a novel approach for future asthma therapies.
Keywords: airway remodeling, asthma, reactive oxygen species, mitochondria
Clinical Relevance
Although IL-13 is known to promote airway remodeling via production of transforming growth factor (TGF)-β, the mechanism by which IL-13 stimulates TGF-β remains unknown. The current study shows that mitochondria-derived reactive oxygen species (ROS) are a critical signaling intermediary between IL-13 or allergen stimulation and TGF-β–dependent airway remodeling. We found that a mitochondrial-targeted antioxidant therapy was effective in preventing early airway remodeling in vivo and reducing TGF-β activity in cultured cells, suggesting that targeting mitochondrial ROS in airway epithelium is a novel therapeutic approach for asthma.
Asthma is a disease of acute and chronic inflammation and increased oxidative stress in which cytokines play a critical role in orchestrating the allergic inflammatory response. Chronic inflammation leads to subepithelial fibrosis and airway remodeling (1, 2). IL-13 and transforming growth factor (TGF)-β are central signals that promote airway fibrosis (3, 4) and, when expressed in bronchial epithelium, IL-13 leads to the development of several characteristic features of airway remodeling, including subepithelial fibrosis through TGF-β–dependent pathways (5). Although IL-13 promotes TGF-β production in respiratory epithelium (2), the mechanism through which this occurs remains unknown.
IL-13 is known to increase reactive oxygen species (ROS) (6–8), and the airways of asthma patients and allergen-challenged mice contain high levels of ROS that appear to contribute to asthma (9–13). ROS are known to be important in TGF-β signaling through a number of mechanisms. Superoxide radicals increase TGF-β transcription in alveolar epithelial cells (14) and promote TGF-β activity and collagen deposition from human lung fibroblasts (15). Mitochondrial ROS have recently been shown to be required for downstream TGF-β16 signaling, and mitochondrial dysfunction is a feature of asthmatic airway cells (17–19). Given that IL-13 and allergens both induce ROS, which have been shown to increase transcription, activation, and downstream TGF-β signaling, we hypothesized that mitochondrial ROS are a critical signaling intermediary between IL-13 stimulation and TGF-β–dependent airway remodeling.
Although mitochondrial dysfunction and oxidative stress are characteristic factors in patients with allergic asthma and fibrotic lung diseases (20, 21), nontargeted antioxidants are ineffective in patients with asthma (22). To test if a targeted approach to antioxidant therapy might be successful, we performed proof-of-concept studies to determine if infusion with (2-(2,2,6,6-Tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl)triphenylphosphonium chloride (mitoTEMPO), a mitochondrial-targeted antioxidant (23), could prevent or reduce key asthma characteristics in a murine model of atopic asthma. MitoTEMPO compartmentalizes to the mitochondria by virtue of its positively charged lipophilic cation, triphenylphosphonium (TPP) (24).
Here, we provide new evidence that IL-13 and ovalbumin (OVA) increase mitochondrial ROS, and that reduced mitochondrial ROS by mitoTEMPO infusion results in improvement of markers of airway remodeling in OVA-challenged mice. We used osmotic minipumps to infuse mitoTEMPO into mice treated with OVA or saline. We show that mitochondrial-targeted antioxidant therapy inhibits IL-13–induced mitochondrial ROS and TGF-β in cultured human airway epithelium, and significantly attenuates mitochondrial ROS, TGF-β, and collagen deposition in OVA-challenged mice in vivo. We used a spatially targeted, conditional TGF-β knockout mouse model to show that epithelial TGF-β is required for subepithelial collagen deposition in response to OVA. Our findings suggest that mitochondria are a critical source of ROS for promoting TGF-β signaling and pathological airway responses to OVA, and that targeted antioxidant therapy may be a novel approach for future asthma therapies.
Materials and Methods
Animals
All animal care and housing requirements of the National Institutes of Health Committee on Care and Use of Laboratory Animals were followed. All protocols were reviewed and approved by the University of Iowa Animal Care and Use Committee. B6D2 background wild-type mice were obtained from Jackson Laboratories (Bar Harbor, ME). TGF-β-floxed (fl/fl) CC10 estrogen conditional Cre recombinase (Cre-ER) mice were generated by obtaining TGF-βfl/fl (Jackson Laboratories) and crossing with CC10 Cre-ER mice (25).
A 20-mg/ml tamoxifen stock solution was dissolved in corn oil. TGF-βfl/fl CC10 Cre-ER was injected with 0.25 mg/g body weight tamoxifen every day for 5 days. Control mice were injected with corn oil alone. Mice were used for OVA challenge 7 days after the last injection.
MitoTEMPO Treatment
MitoTEMPO (Enzo Life Sciences, Farmingdale, NY) was delivered in vivo using a micro-osmotic minipump (Alzet, Cupertino, CA). Control animals were implanted with saline-filled minipumps. The pumps were implanted 3 days before OVA challenge and delivered drug at a dose of 0.7 mg/kg/d, as previously described (23).
OVA Sensitization and Challenge
Male and female B6D2 mice (6–8 wk old; Jackson Laboratories) were sensitized by intraperitoneal injection of 10 μg OVA (Sigma, St. Louis, MO) mixed with 1 mg of alum, (or saline alone, for control mice) at Days 0 and 7. Mice were subsequently challenged with inhaled OVA (1% solution in 0.9% saline, 40-min challenge), or saline on Days 15, 17, and 19, as previously described (26). Animals were killed on Day 20.
Bronchoalveolar Lavage
The trachea was cannulated and PBS washings were collected (bronchoalveolar lavage [BAL]), for total and differential counts of lavage cells, ELISA measurements, and conditioned media experiments.
Epithelial Brushings
Epithelial-specific brushing was used to evaluate gene expression in airway epithelial cells, as previously described (27). Brushes were made from 60-grit sandpaper–polished polyethylene PE-10 tube (BD Biosciences, San Jose, CA) with an inserted stainless wire (0.095 mm diameter). The brush was inserted through an incision into the midsection of the trachea, and harvested epithelium was collected in 350 μl RNeasy lysis buffer (Buffer RLT; Qiagen, Valencia, CA) for RNA preparation.
Lung Histology
Lungs were fixed with 4% paraformaldehyde and then processed by paraffin embedding. Tissue sections (5 μm) were cut and stained using Masson’s trichrome. Images were taken on an Olympus BX-61 light microscope (Olympus, Center Valley, PA) at 20×.
Morphometric Measurement of Fibrosis
Masson’s trichrome–stained slides were quantified by observers blinded to treatment status to estimate subepithelial collagen content. Subepithelial thickness was measured in four sections of each of airway. Airway collagen area was measured using ImageJ and normalized to airway lumen area.
Hydroxyproline Assay
Lung tissue was homogenized, dried to a stable weight, and then acidified with 6 N HCl, and hydrolyzed by heating at 120°C for 24 hours. Hydroxyproline measurements were determined, as described previously, and normalized to dry lung weight (28, 29).
Cytokine Determinations
TGF-β and IL-13 in BAL fluid and conditioned media of cell culture experiments were measured by cytokine-specific ELISA Duo Set kits (R&D Systems, Minneapolis, MN). BAL cytokines were normalized to total protein levels.
Quantitative Real-Time PCR
Total RNA was isolated using the Qiagen RNeasy column based kits. Complementary DNA was prepared using the Superscript III reverse transcription system (Invitrogen, Carlsbad, CA), with random nanomer primers. Expression of mRNA was quantified using the iQ Lightcycler (Bio-Rad, Hercules, CA) and SYBR green dye system, normalized to acidic ribosomal phosphoprotein mRNA. Primer sequences are provided in the supplemental Materials and Methods section.
Epithelial Cell Culture
Human bronchial epithelial cells (30) were grown in keratinocyte serum-free media (Gibco, Grand Island, NY). Cells were grown until 70% confluence and then treated with TPP, 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPOL), or mitoTEMPO at 10 μM or vehicle (DMSO). After 24 hours of pretreatment, cells were challenged with IL-13 (25 ng/ml) for 48 hours. Cells were then used to image mitochondria or perform conditioned media experiments. Conditioned media were removed from IL-13–challenged epithelial cells and added to confluent human lung fibroblast-1 (ATCC, Manassas, VA) for 48 hours. Conditioned media were also made by adding BAL fluid from saline or OVA-challenged mice (100 μg total protein) to keratinocyte serum-free media and adding to human lung fibroblasts. An Amicon Ultra-4 3K Centrifugal filter device (Millipore, Darmstadt, Germany) was used to remove residual mitoTEMPO from our media for selected studies.
Plasmids and Luciferase Assays
The 5′-flanking sequence of the human TGF-β1 promoter in the Plightswitch luciferase reporter plasmid (S72025; Switch Gear Genomics, Carlsbad, CA) was used to assess TGF-β1 promoter activity. The promoter-driven pGL4.13 firefly vector (Promega, Madison, WI) was used to control for transfection efficiency. Cells were cotransfected with plasmid using ExtremeGene9 Transfection Reagent (Roche, Basel, Switzerland) according to the manufacturer’s directions. At 4 hours after transfection, medium was replaced with fresh serum–containing medium, and cells were allowed to recover for 24 hours. Renilla and firefly luciferase activity was determined in cell lysates using the Dual Luciferase reporter assay kit (Promega) and normalized to control (firefly).
ROS Detection
ROS were measured in live cells using dihydroethidium derivative (mitoSOX) red (5 μM, D1168; Invitrogen). The mitochondrial localization of staining was confirmed by colocalizing mitoTracker green (50 nM; Invitrogen). Cells were imaged using a LSM 510 confocal microscope (Carl Zeiss, Oberkochen, Germany), and analyzed with ImageJ (National Institutes of Health, Bethesda, MD). All images were taken at the same time and using the same imaging settings. Data are presented as mean fluorescent intensity per square micron.
ROS levels from mouse lung sections were assessed using dihydroethidium (DHE) staining (5 μM; Invitrogen). Lungs were snap frozen, and 10-μm sections were stained with DHE as described previously (31). Sections were imaged using the LSM 510 confocal microscope, and analyzed with ImageJ. All images were taken at the same time and using the same imaging settings. Values were obtained from the epithelial layer of three airways per section, measuring under 500 μm in length at 200× magnification. Data are presented as mean fluorescent intensity per square micron.
Mitochondria Isolation
Isolation of mitochondria was performed on ice. Freshly collected human bronchial epithelial cells suspended in mannitol, sucrose, EGTA (MSE) buffer (5 mM 3-(N-morpholino)propanesulfonic acid, 70 mM sucrose, 2 mM ethyleneglycol-bis-(β-aminoethyl ether)-N,N′-tetraacetic acid, 220 mM mannitol, pH 7.2, with KOH), then homogenized. Nuclei and unbroken cells were pelleted by centrifugation twice at 600 × g for 10 minutes. The crude mitochondrial and cytosolic fraction was obtained from the supernatant by centrifugation at 8,500 × g for 20 minutes. The pellet was further purified by washing twice in MSE buffer, and was then resuspended in 200 μl MSE with protease inhibitors.
Lucigenin Assay
The rate of superoxide anion formation was determined by lucigenin (bis-N-methylacridinium nitrate; Sigma) as described previously (32). Briefly, 100 μl of substrate nicotinamide adenine dinucleotide phosphate reduced (100 μM) was added to 20 μg of mitochondrial protein, lucigenin (5 μM). Chemiluminescence was monitored every 15 seconds for 10 minutes, and the rate of change was expressed as relative light units per second.
Immunoblots
Media were removed from human lung fibroblast-1 cells and total protein amount was measured using detergent compatible assay (Bio-Rad). Soluble proteins (20 μg) were separated by SDS-PAGE in nondenaturing conditions and blotted against collagen I (Ab765; Millipore).
Statistical Analysis
Data are shown as means (± SE) unless otherwise stated. Groups were compared using ANOVA and two-tailed nonparametric Mann-Whitney U tests. The GraphPad Prism statistical software program (GraphPad Inc., La Jolla, CA) was used for the analyses. A P value less than 0.05 was regarded as statistically significant. In figures, asterisks represent significance versus saline or vehicle control.
Results
mitoTEMPO Attenuates IL-13– and OVA-Induced Mitochondrial ROS in Respiratory Epithelium
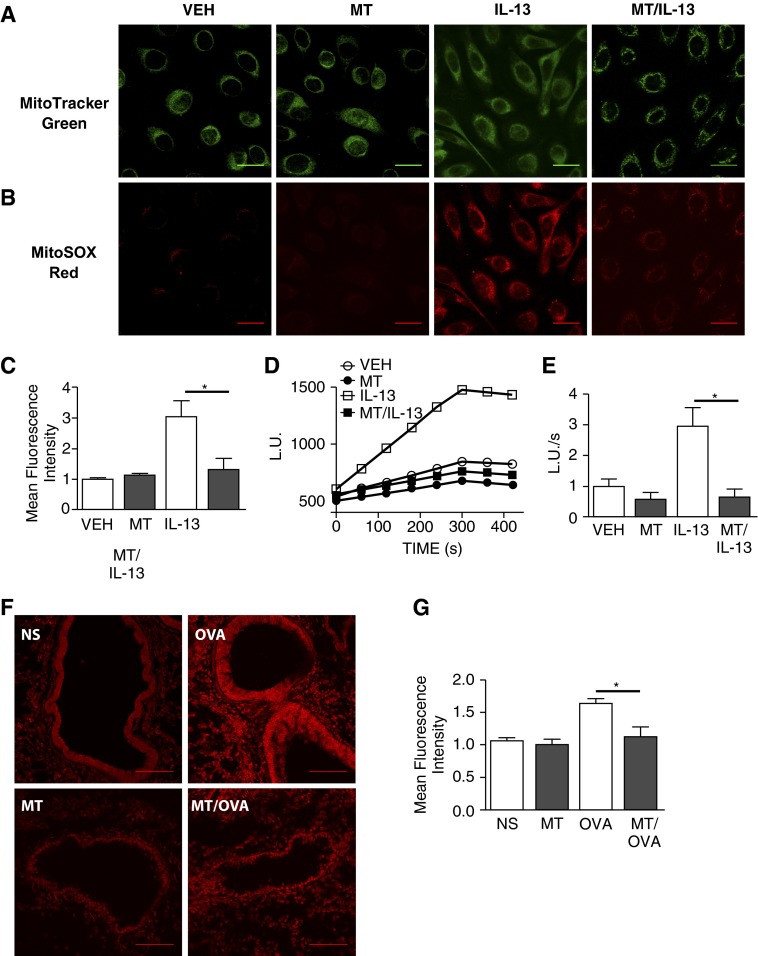
IL-13–mediated inflammation and mitochondrial dysfunction are believed to play important roles in the pathogenesis of allergic asthma (3, 18). Inflammation and tissue remodeling with pathologic fibrosis are common consequences of IL-13–mediated T helper type 2 responses in the lung and other organs (5). Based on these associations, we first tested a possible connection between mitochondrial ROS production and IL-13 treatment by measuring mitochondrial ROS in cultured human airway epithelial cells after IL-13 challenge. We assessed ROS levels using mitoSOX staining (23), a fluorescent mitochondrial ROS reporter, and found a significant increase in ROS after IL-13 treatment (Figures 1A–1C). We found that mitoTEMPO significantly reduced the IL-13–induced increase in ROS, consistent with the concept that IL-13 was increasing ROS by actions on mitochondria. TPP and TEMPOL did not have a significant effect on mitochondrial ROS as measured by mitoSOX (see Figure E2 in the online supplement). To further quantify oxidative stress, we challenged epithelial cells with IL-13 and measured the rate of O2·− production by lucigenin chemiluminescence. We found significantly higher O2·− production in mitochondria from cells treated with vehicle and challenged with IL-13 compared with cells treated with mitoTEMPO and challenged with IL-13 (Figures 1D and 1E). Based on these findings in cultured cells, we next asked if mitochondrial ROS-targeted antioxidant therapy could attenuate ROS in vivo. We used DHE to measure ROS in vehicle- or mitoTEMPO-infused mice challenged with OVA (Figure 1F). MitoTEMPO treatment significantly reduced the amount of superoxide in OVA-challenged compared with saline-treated OVA-challenged animals (Figure 1G). TPP infusion alone, however, did not reduce the amount of ROS measured from OVA-challenged mice (Figure E3). These findings suggest that mitochondrial ROS is increased by IL-13 in airway epithelia, and that IL-13– and OVA-evoked increases in mitochondrial ROS can be prevented or substantially reduced by mitoTEMPO treatment.
Figure 1.
(2-(2,2,6,6-Tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl)triphenylphosphonium chloride (mitoTEMPO) attenuates IL-13–induced mitochondrial reactive oxygen species (ROS) in respiratory epithelium. (A) Representative images of human bronchial epithelial cells treated with DMSO or mitoTEMPO (MT) and challenged with IL-13, stained with mitoTRACKER to show mitochondrial localization (B) Costaining with mitoSOX to show mitochondrial ROS; scale bars, 50 μm. (C) quantification mitoSOX staining (n = 6 in each group, *P < 0.001). (D) Representative tracing of O2·− detection using lucigenin in isolated mitochondria (E) O2·− production rate (n = 6 in each group, *P < 0.05). (F) Representative images of dihydroethidium (DHE)-stained airways in normal saline (NS), mitoTEMPO (MT), ovalbumin (OVA), and MT/OVA mice; scale bars, 25 μm. (G) Quantification of DHE staining (n = 6 in each group, *P < 0.05).
Attenuation of Allergen-Induced Subepithelial Collagen Deposition by mitoTEMPO
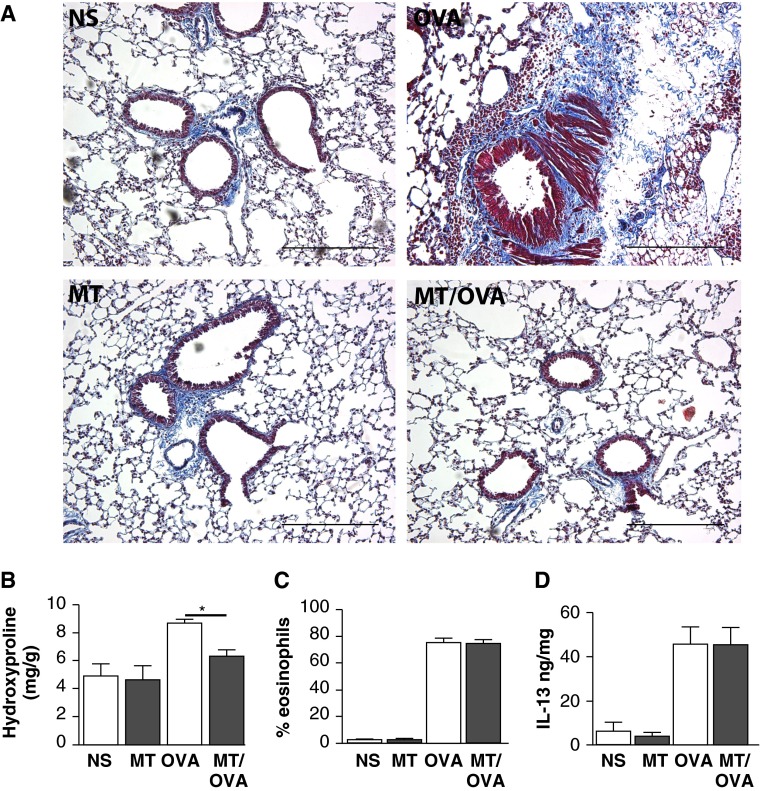
Lungs obtained from saline- or TPP-infused OVA-challenged mice showed significantly increased subepithelial collagen deposition compared with lungs obtained from mice treated with mitoTEMPO and OVA challenged (Figures 2A, E4, and E5). The histological changes were confirmed biochemically by hydroxyproline quantification. The hydroxyproline content in the lungs was significantly less in the mice treated with mitoTEMPO and challenged with OVA compared with the vehicle treated OVA challenged mice (Figure 2B). These data show that systemic mitoTEMPO treatment is beneficial in reducing subepithelial remodeling responses to allergic inflammation.
Figure 2.
Attenuation of allergen-induced subepithelial collagen deposition by mitoTEMPO. (A) Representative images of Masson’s trichrome stain of mice infused with mitoTEMPO (MT) or normal saline (NS) and challenged with OVA or saline (NS) (100×); scale bars, 100 μm. (B) Whole-lung hydroxyproline in mice treated with mitoTEMPO (n = 6 per group, *P < 0.05). (C) Bronchoalveolar lavage (BAL) eosinophil percentage (n = 10 per group). (D) BAL IL-13 (ng cytokine/mg total lavage protein; n = 10 per group).
We also measured common effectors of T helper type 2 mediated inflammation that are often correlated with allergic asthma (3). Interestingly, we did not find any difference in BAL IL-13 levels or eosinophils in vehicle treated and OVA challenged animals compared with mitoTEMPO treated OVA challenged animals (Figures 2C and 2D). These data suggest that the effects of mitochondrial ROS on fibrosis occur downstream to IL-13 signaling and inflammation.
IL-13–Challenged Respiratory Epithelial Cell Culture Media Supernatant Induced Collagen Production in Human Lung Fibroblasts
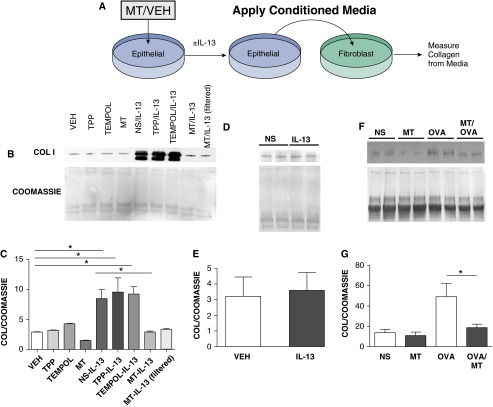
To test our hypothesis that mitochondrial ROS has downstream effects on epithelial pro-fibrotic signaling, we asked if epithelial cells contributed to adverse airway remodeling by promoting collagen production in lung fibroblasts. We challenged epithelial cells with IL-13 in the presence of either vehicle, TPP, TEMPOL, or mitoTEMPO. The conditioned media from the epithelial cells was placed on cultured human lung fibroblasts for 48 h prior to measuring collagen content in the media (Figure 3A). We found that lung fibroblasts cultured in conditioned media from vehicle, TPP, or TEMPOL treated, IL-13 challenged epithelial cells produced significantly higher amounts of collagen compared with those in conditioned media from mitoTEMPO treated, IL-13 challenged epithelial cells (Figures 3B and 3C). We saw the same reduction in collagen production when mitoTEMPO was filtered from the conditioned media, suggesting the reduced profibrotic potential of media from mitoTEMPO treated, IL-13 challenged epithelial cells was the result of a secreted factor rather than from mitoTEMPO. Importantly, fibroblasts directly treated with media containing IL-13 did not have increased collagen production (Figures 3D and 3E) further suggesting that epithelial cells were contributing an essential factor for fibroblast activation that was downstream of IL-13. Taken together we interpreted these data to suggest that IL-13 acts on epithelial cells to produce a mitochondrial ROS dependent mitogen, which in turn activates fibroblasts to produce collagen. Our studies also suggested that mitochondrial targeted anti-oxidant treatment is sufficient to inhibit this paracrine signaling mechanism.
Figure 3.
Media from IL-13–challenged respiratory epithelial cells induces collagen production in human lung fibroblasts. (A) Schematic of work flow in the conditioned media experiment. (B) Collagen I detected by immunoblotting media from cultured human lung fibroblasts. (C) Quantification of collagen I immunoblots from (B) (three immunoblots, n = 6; *P < 0.05). (D) Collagen I immunoblot from media-harvested human lung fibroblasts challenged with IL-13 directly. (E) Quantification of collagen I immunoblot from (D) (n = 4). (F) Collagen I Western blot from human lung fibroblasts challenged with lavage from saline, MT controls, OVA-challenged, or OVA/MT mice. (G) Quantification of collagen I blots from (F) (2 immunoblots, n = 4; *P < 0.05).
To determine if a transferable factor contributing to fibrosis was also present in OVA mice, we challenged human lung fibroblasts with lavage fluid from our vehicle treated and OVA challenged or mitoTEMPO treated and OVA challenged mice and measured collagen content in media by immunoblot analysis (Figures 3F and 3G). We found that fibroblasts exposed to lavage fluid from mitoTEMPO treated animals produced significantly lower amounts of collagen. These observations suggest that the paracrine signaling observed in our conditioned media experiments between epithelium and fibroblasts occurs in vivo.
Next we treated epithelial cells with IL-13 to determine if mitoTEMPO may have interfered with upstream IL-13 receptor signaling. We found that IL-13 mediated STAT6 phosphorylation was independent of mitoTEMPO treatment indicating that mitoTEMPO was not globally preventing IL-13 agonist actions on epithelial cells (Figure E1). These in vitro and in vivo data suggested that IL-13 and OVA induce a humoral signal promoting fibroblasts to elaborate collagen by a pathway involving mitochondrial ROS.
mitoTEMPO Reduces TGF-β Expression and Activity
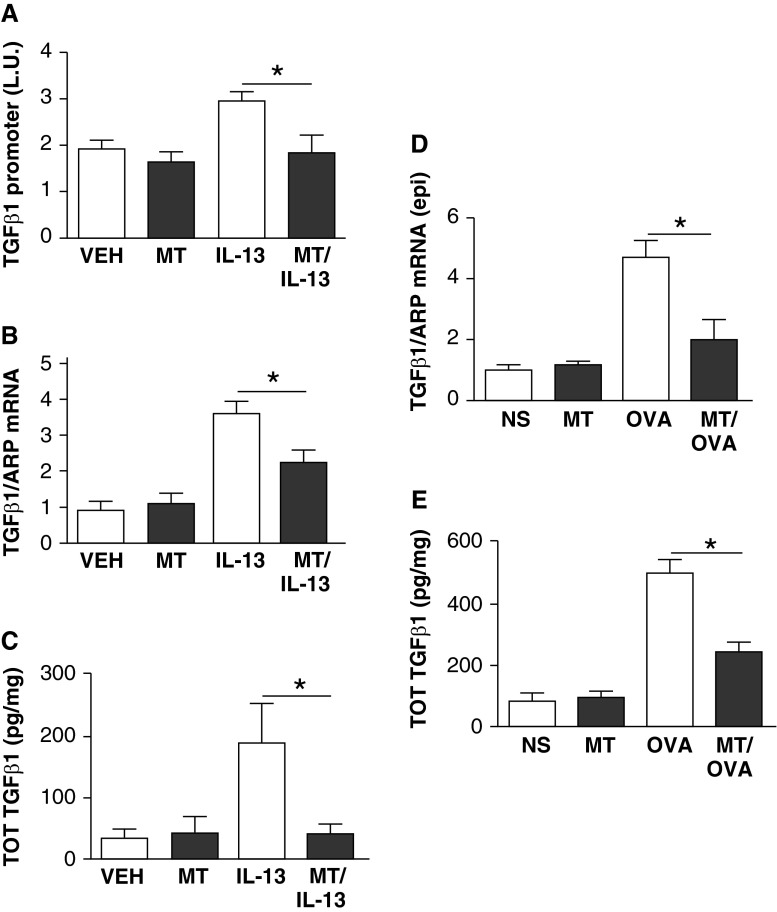
IL-13 expression in epithelial cells induces tissue fibrosis by activating TGF-β5, and mitochondrial ROS are required for TGF-β signaling (16). Based on these considerations and our findings, we hypothesized that scavenging mitochondrial-derived ROS would decrease allergen- or IL-13– induced TGF-β production from epithelial cells. To test this concept, we treated TGF-β luciferase reporter transfected airway epithelial cells with mitoTEMPO or vehicle for 24 hours, and then challenged them with IL-13 for 48 hours. We found that IL-13 mediated a significant increase in TGF-β promoter activity, and that pretreatment with mitoTEMPO significantly reduced IL-13–mediated TGF-β promoter activity (Figure 4A). Correspondingly, pretreatment with mitoTEMPO resulted in significant reductions in TGF-β promoter activity compared with vehicle-treated cells (Figure 4B). The reduced promoter and transcriptional activity seen in mitoTEMPO-treated cells aligned with the lower TGF-β concentrations in the media of mitoTEMP-treated cells (Figure 4C). These data suggest that antioxidant treatment can reduce IL-13–mediated TGF-β gene expression.
Figure 4.
mitoTEMPO reduces transforming growth factor (TGF)-β activity and expression. (A) TGF-β1 promoter activity from epithelial cells challenged with IL-13/mitoTEMPO is significantly (*P < 0.05) reduced compared with IL-13/vehicle (n = 6). (B) TGF-β1 mRNA from epithelial cells challenged with IL-13/mitoTEMPO is significantly (*P < 0.05) reduced compared with IL-13/vehicle (n = 6). (C) TGF-β from media of epithelial cells challenged with IL-13/mitoTEMPO is significantly (*P < 0.05) reduced compared with media from IL-13/vehicle (n = 6). (D) TGF-β mRNA in epithelial brushings from OVA/mitoTEMPO–challenged mice is significantly (*P < 0.05) reduced compared with OVA/saline mice (n = 8). (E) Total TGF-β in BAL from OVA/mitoTEMPO–challenged mice is significantly (*P < 0.05) reduced compared with OVA/saline mice (n = 8 in each group). ARP, acidic ribosomal phosphoprotein; epi, epithelial; L.U., luminescence units; TOT, total.
To determine the effect of allergen challenge on in vivo TGF-β activity, we measured TGF-β transcriptional activity in epithelial cells from OVA-challenged mice. TGF-β mRNA from isolated lung epithelial cells revealed that OVA allergen challenge significantly increased TGF-β gene expression in airway epithelium (Figure 4D). MitoTEMPO treatment had a trend toward reduced latent TGF-β in the BAL of OVA-challenged mice, and had significant reductions in active and total TGF-β (Figures 4E and E6). We also measured phospho-SMAD 2/3 as an indicator of downstream TGF-β signaling, and found significant reductions in phospho-SMAD 2/3 in animals treated with mitoTEMPO (Figure E7). Taken together, these findings suggest that mitochondrial ROS are important mediators of allergen-induced TGF-β signaling and collagen deposition in airway epithelium after OVA or IL-13 challenge.
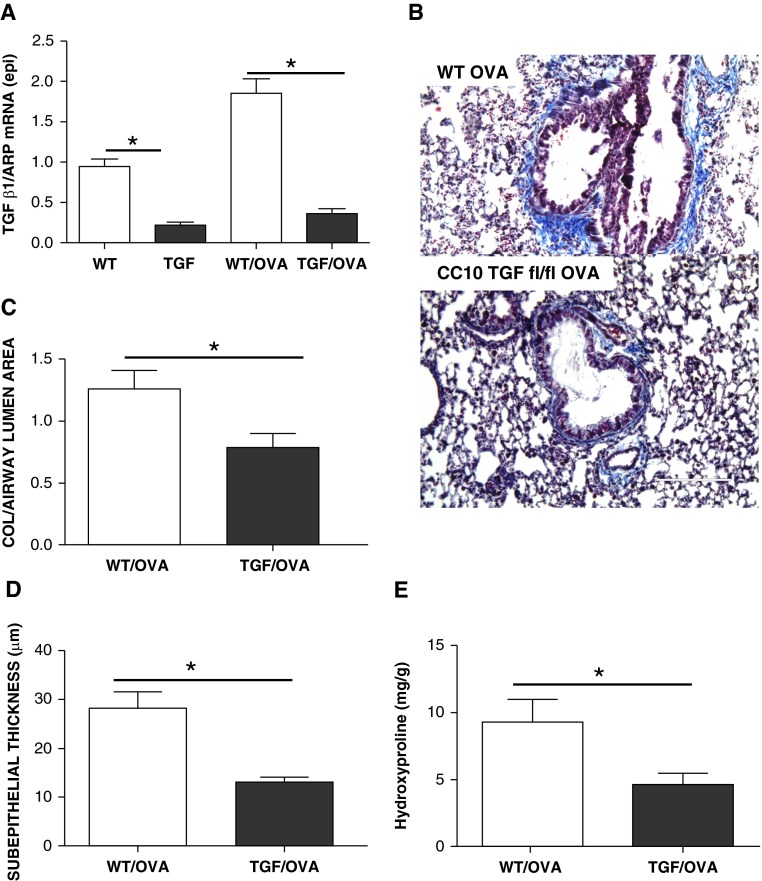
Epithelial TGF-β Is Required for Subepithelial Collagen Deposition
To determine the effect of epithelial TGF-β on subepithelial collagen deposition in vivo, we used a tamoxifen-inducible, epithelial-specific TGF-β knockout mouse. We measured epithelial TGF-β before and after OVA challenge in these mice and found significant reductions in epithelial TGF-β expression (Figure 5A). Lungs obtained from epithelial TGF-β knockout mice challenged with OVA had significantly reduced subepithelial collagen deposition compared with lungs obtained from control, OVA-challenged mice (Figures 5B–5D). The histological changes were confirmed biochemically by hydroxyproline quantification. The hydroxyproline content in the lungs was significantly less in the TGF-β knockout, OVA-challenged mice compared with the control OVA-challenged mice (Figure 5E). These data support our hypothesis that allergen challenge results in increased epithelial cell TGF-β activity that promotes subepithelial collagen deposition.
Figure 5.
TGF-β is required for subepithelial collagen deposition. (A) TGF-β1 mRNA from epithelial cells of epithelial TGF-β1 knockout animals is significantly (*P < 0.05; n = 4) reduced compared with controls. (B) Representative images of Masson’s trichrome stain of littermate control (wild-type [WT]) compared to CC10 TGF-β1 knockout mice challenged with OVA. Original magnification, 200×; scale bars, 200 μm. (C) Subepithelial collagen area (normalized to airway lumen area) is significantly reduced in TGF-β1 knockout OVA-challenged animals compared with control OVA-challenged animals (*P < 0.05; n = 4). (D) Subepithelial thickness is significantly reduced in TGF-β1 knockout OVA-challenged animals compared with control OVA-challenged animals (*P < 0.05; n = 4). (E) Whole-lung hydroxyproline is significantly (*P < 0.05) reduced in TGF-β1 knockout OVA-challenged animals compared with control OVA-challenged animals (n = 4). fl/fl, floxed/floxed.
Discussion
This study supports five novel conclusions with respect to airway remodeling in allergic airways disease. First, IL-13 induces mitochondrial ROS. Second, mitochondrial ROS generation is required for TGF-β gene transcription downstream of IL-13 receptor binding. Third, IL-13–challenged human airway epithelium releases a factor capable of promoting collagen synthesis in fibroblasts, consistent with a paracrine influence of epithelium on mesenchymal cells. Fourth, mitoTEMPO antioxidant therapy is sufficient to attenuate mitochondrial ROS, TGF-β increases to IL-13 and OVA challenge, and the fibrotic airway remodeling in OVA-challenged mice. Fifth, epithelial TGF-β is required for subepithelial collagen deposition.
We used lucigenin, DHE, and mitoSOX to detect ROS. Although the validity of lucigenin as a chemiluminescence probe has been questioned, due to the redox cycling of lucigenin, this is less important in cellular systems that produce significant amounts of O2·− (33). DHE reacts with O2·− to form ethidium and a very specific product, 2-hydroxyethidium. Although DHE fluorescence is limited in detecting the exact source of ROS, it does seem to reflect the redox status of the cell (34).
Other groups have reported that IL-13 induces TGF-β expression; however the cellular mechanisms remain unclear (3, 35, 36). Consistent with these reports, we detected an increase in TGF-β from respiratory epithelium exposed to IL-13. Using oxidant-sensitive probes, we saw that IL-13 increased mitochondrial ROS generation. Although ROS are involved in multiple aspects of TGF-β signaling, including the conversion of latent TGF-β to active TGF-β (21), we found that IL-13 stimulated ROS increased TGF-β expression and activated the TGF-β promoter, leading to enhanced TGF-β transcription. Although the ROS responsive signals that activate TGF-β need further study, the calcium and calmodulin–dependent protein kinase II (CaMKII) has been shown to contribute to a proasthmatic phenotype when oxidized (9), and so could be a mediator between ROS and TGF-β signaling in airways diseases. CaMKII has been described to activate AP-1, a known transcriptional activator of TGF-β (36). Oxidation of CaMKII is known to promote a fibrotic phenotype in cardiac tissue (37), but further studies will be required to determine if oxidation of CaMKII leads to a profibrotic phenotype in asthmatic airways.
Asthma is a complex disease with marked heterogeneity in its clinical course and response to treatment. Current asthma therapies, including corticosteroids, alleviate inflammation and improve pulmonary airflow in mild to moderate asthma; however, their efficacy in reversing structural remodeling in the airways of patients with asthma is disappointing (38). IL-13 induces a corticosteroid-insensitive stimulation of TGF-β release from bronchial epithelial cells (2), suggesting that novel therapies directed toward limiting airway remodeling by corticosteroid-independent mechanisms will be necessary. IL-13 antagonist therapy has been shown to be beneficial in patients with high serum periostin (39), but does not improve lung function in patients not taking inhaled corticosteroids (40). Understanding the complex and varied pathophysiology of the disease is vital for the future of research and clinical practice. Recent data suggest that altered TGF-β signaling can predispose to allergic phenotypes in humans, and underscores a prominent role for TGF-β in directing immune responses to antigens present in the environment (41). This paradigm may be relevant to nonsyndromic presentations of allergic disease, and highlights the potential therapeutic benefit of strategies that inhibit TGF-β signaling.
Our study shows that ROS is an upstream signal in allergic airways disease that modifies downstream airway remodeling phenotypes. Our study does not identify these ROS targets that mediate downstream effects on TGF-β expression, which is a goal for future investigation. Unfortunately global, nontargeted antioxidants have not shown a strong therapeutic benefit in patients with asthma, and might be harmful in some situations (20, 42), but, to our knowledge, mitochondrially targeted antioxidants have not been tested clinically in asthma. Our findings suggest that mitochondrial-derived ROS might be important targets for the treatment of subepithelial fibrosis in asthma.
Acknowledgments
Acknowledgments
The authors are grateful to Gary Buettner and Brett Wagner, Department of Radiation Biology, University of Iowa, for constructive criticism and helpful discussions. They are also grateful to David Meyerholz, University of Iowa Comparative Pathology Laboratory, for his assistance with interpretation of histology, and to Chantal Allamargot, Central Microscopy Core, for her assistance with staininggrateful, as well as Shawn Roach, Medical Imaging, University of Iowa, for his assistance with figure layout.
Footnotes
This work was supported by a grant from the Sandler Program for Asthma Research and the American Asthma Foundation, the University of Iowa Research Foundation, Spear Family Pulmonary Research Fund, by National Institutes of Health (NIH) Fellowship Training Grant T32 HL007121, NIH grant 2R01ES015981-06 (A.B.C.), and by Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development Merit Review Grant 1BX001135-01 (A.B.C.).
Author Contributions: M.E.A., I.M.G., and A.B.C. described the initial hypothesis; M.E.A., A.B.C. J.N.K., J.Z., I.M.G., and O.A.J. designed the experiments and M.E.A., I.M.G., A.B.C., and O.A.J., analyzed the data and wrote the manuscript. Most data in this paper were generated by O.A.J., with the exception of, immunofluorescence imaging of human cells and murine tissue for reactive oxygen species staining (O.A.J. and M.E.D.), hydroxyproline measurements (O.A.J., S.M., C.J.W., A.G.R.), bronchoalveolar lavage differential cell counts (O.A.J., M.E.D., and C.J.W.), immunoblots (P.N.S., A.M.P., C.J.W., and O.A.J.), luciferase assays (P.N.S. and A.J.R.), human epithelial cell work (P.N.S., O.A.J., M.E.D., and C.J.W.), and mRNA analysis (O.A.J. and M.E.D.); the transforming growth factor-β promoter construct was designed by A.J.R.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0519OC on July 2, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Holgate ST, Davies DE, Puddicombe S, Richter A, Lackie P, Lordan J, Howarth P. Mechanisms of airway epithelial damage: epithelial–mesenchymal interactions in the pathogenesis of asthma. Eur Respir J Suppl. 2003;44:24s–29s. doi: 10.1183/09031936.03.00000803. [DOI] [PubMed] [Google Scholar]
- 2.Richter A, Puddicombe SM, Lordan JL, Bucchieri F, Wilson SJ, Djukanovic R, Dent G, Holgate ST, Davies DE. The contribution of interleukin (IL)-4 and IL-13 to the epithelial–mesenchymal trophic unit in asthma. Am J Respir Cell Mol Biol. 2001;25:385–391. doi: 10.1165/ajrcmb.25.3.4437. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheppard D. Transforming growth factor beta: a central modulator of pulmonary and airway inflammation and fibrosis. Proc Am Thorac Soc. 2006;3:413–417. doi: 10.1513/pats.200601-008AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1) J Exp Med. 2001;194:809–821. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandal D, Fu P, Levine AD. REDOX regulation of IL-13 signaling in intestinal epithelial cells: usage of alternate pathways mediates distinct gene expression patterns. Cell Signal. 2010;22:1485–1494. doi: 10.1016/j.cellsig.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Park KW, Baik HH, Jin BK. IL-13–induced oxidative stress via microglial NADPH oxidase contributes to death of hippocampal neurons in vivo. J Immunol. 2009;183:4666–4674. doi: 10.4049/jimmunol.0803392. [DOI] [PubMed] [Google Scholar]
- 8.Yadav UC, Aguilera-Aguirre L, Ramana KV, Boldogh I, Srivastava SK. Aldose reductase inhibition prevents metaplasia of airway epithelial cells. PLoS ONE. 2010;5:e14440. doi: 10.1371/journal.pone.0014440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders PN, Koval OM, Jaffer OA, Prasad AM, Businga TR, Scott JA, Hayden PJ, Luczak ED, Dickey DD, Allamargot C, et al. CaMKII is essential for the proasthmatic effects of oxidation. Sci Transl Med. 2013;5:195ra97. doi: 10.1126/scitranslmed.3006135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarjour NN, Calhoun WJ. Enhanced production of oxygen radicals in asthma. J Lab Clin Med. 1994;123:131–136. [PubMed] [Google Scholar]
- 11.Casalino-Matsuda SM, Monzon ME, Forteza RM. Epidermal growth factor receptor activation by epidermal growth factor mediates oxidant-induced goblet cell metaplasia in human airway epithelium. Am J Respir Cell Mol Biol. 2006;34:581–591. doi: 10.1165/rcmb.2005-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdala-Valencia H, Earwood J, Bansal S, Jansen M, Babcock G, Garvy B, Wills-Karp M, Cook-Mills JM. Nonhematopoietic NADPH oxidase regulation of lung eosinophilia and airway hyperresponsiveness in experimentally induced asthma. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1111–L1125. doi: 10.1152/ajplung.00208.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hulsmann AR, Raatgeep HR, den Hollander JC, Stijnen T, Saxena PR, Kerrebijn KF, de Jongste JC. Oxidative epithelial damage produces hyperresponsiveness of human peripheral airways. Am J Respir Crit Care Med. 1994;149:519–525. doi: 10.1164/ajrccm.149.2.8306055. [DOI] [PubMed] [Google Scholar]
- 14.Bellocq A, Azoulay E, Marullo S, Flahault A, Fouqueray B, Philippe C, Cadranel J, Baud L. Reactive oxygen and nitrogen intermediates increase transforming growth factor-beta1 release from human epithelial alveolar cells through two different mechanisms. Am J Respir Cell Mol Biol. 1999;21:128–136. doi: 10.1165/ajrcmb.21.1.3379. [DOI] [PubMed] [Google Scholar]
- 15.Qi S, den Hartog GJ, Bast A. Superoxide radicals increase transforming growth factor-beta1 and collagen release from human lung fibroblasts via cellular influx through chloride channels. Toxicol Appl Pharmacol. 2009;237:111–118. doi: 10.1016/j.taap.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 16.Jain M, Rivera S, Monclus EA, Synenki L, Zirk A, Eisenbart J, Feghali-Bostwick C, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial reactive oxygen species regulate transforming growth factor-beta signaling. J Biol Chem. 2013;288:770–777. doi: 10.1074/jbc.M112.431973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konradova V, Copova C, Sukova B, Houstek J. Ultrastructure of the bronchial epithelium in three children with asthma. Pediatr Pulmonol. 1985;1:182–187. doi: 10.1002/ppul.1950010403. [DOI] [PubMed] [Google Scholar]
- 18.Aguilera-Aguirre L, Bacsi A, Saavedra-Molina A, Kurosky A, Sur S, Boldogh I. Mitochondrial dysfunction increases allergic airway inflammation. J Immunol. 2009;183:5379–5387. doi: 10.4049/jimmunol.0900228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mabalirajan U, Dinda AK, Kumar S, Roshan R, Gupta P, Sharma SK, Ghosh B. Mitochondrial structural changes and dysfunction are associated with experimental allergic asthma. J Immunol. 2008;181:3540–3548. doi: 10.4049/jimmunol.181.5.3540. [DOI] [PubMed] [Google Scholar]
- 20.Reddy PH. Mitochondrial dysfunction and oxidative stress in asthma: implications for mitochondria-targeted antioxidant therapeutics. Pharmaceuticals (Basel) 2011;4:429–456. doi: 10.3390/ph4030429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu RM, Gaston Pravia KA. Oxidative stress and glutathione in TGF-beta–mediated fibrogenesis. Free Radic Biol Med. 2010;48:1–15. doi: 10.1016/j.freeradbiomed.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearson PJ, Lewis SA, Britton J, Fogarty A. Vitamin E supplements in asthma: a parallel group randomised placebo controlled trial. Thorax. 2004;59:652–656. doi: 10.1136/thx.2004.022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 25.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kline JN, Kitagaki K, Businga TR, Jain VV. Treatment of established asthma in a murine model using CpG oligodeoxynucleotides. Am J Physiol Lung Cell Mol Physiol. 2002;283:L170–L179. doi: 10.1152/ajplung.00402.2001. [DOI] [PubMed] [Google Scholar]
- 27.Sugimoto K, Kudo M, Sundaram A, Ren X, Huang K, Bernstein X, Wang Y, Raymond WW, Erle DJ, Abrink M, et al. The alphavbeta6 integrin modulates airway hyperresponsiveness in mice by regulating intraepithelial mast cells. J Clin Invest. 2012;122:748–758. doi: 10.1172/JCI58815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 29.Murthy S, Adamcakova-Dodd A, Perry SS, Tephly LA, Keller RM, Metwali N, Meyerholz DK, Wang Y, Glogauer M, Thorne PS, et al. Modulation of reactive oxygen species by Rac1 or catalase prevents asbestos-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2009;297:L846–L855. doi: 10.1152/ajplung.90590.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramirez RD, Sheridan S, Girard L, Sato M, Kim Y, Pollack J, Peyton M, Zou Y, Kurie JM, Dimaio JM, et al. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res. 2004;64:9027–9034. doi: 10.1158/0008-5472.CAN-04-3703. [DOI] [PubMed] [Google Scholar]
- 31.Grobe AC, Wells SM, Benavidez E, Oishi P, Azakie A, Fineman JR, Black SM. Increased oxidative stress in lambs with increased pulmonary blood flow and pulmonary hypertension: role of NADPH oxidase and endothelial NO synthase. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1069–L77. doi: 10.1152/ajplung.00408.2005. [DOI] [PubMed] [Google Scholar]
- 32.He C, Murthy S, McCormick ML, Spitz DR, Ryan AJ, Carter AB. Mitochondrial Cu,Zn-superoxide dismutase mediates pulmonary fibrosis by augmenting H2O2 generation. J Biol Chem. 2011;286:15597–15607. doi: 10.1074/jbc.M110.187377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myhre O, Andersen JM, Aarnes H, Fonnum F. Evaluation of the probes 2′,7′-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem Pharmacol. 2003;65:1575–1582. doi: 10.1016/s0006-2952(03)00083-2. [DOI] [PubMed] [Google Scholar]
- 34.Dikalov S, Griendling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension. 2007;49:717–727. doi: 10.1161/01.HYP.0000258594.87211.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holgate ST, Davies DE, Lackie PM, Wilson SJ, Puddicombe SM, Lordan JL. Epithelial–mesenchymal interactions in the pathogenesis of asthma. J Allergy Clin Immunol. 2000;105:193–204. doi: 10.1016/s0091-6749(00)90066-6. [DOI] [PubMed] [Google Scholar]
- 36.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 37.Swaminathan PD, Purohit A, Soni S, Voigt N, Singh M, Glukhov A, Gao Z, He J, Luczak E, Joiner M, et al. Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J Clin Invest. 2011;121:3277–3288. doi: 10.1172/JCI57833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Homer RJ, Elias JA. Airway remodeling in asthma: therapeutic implications of mechanisms. Physiology (Bethesda) 2005;20:28–35. doi: 10.1152/physiol.00035.2004. [DOI] [PubMed] [Google Scholar]
- 39.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, Harris JM, Scheerens H, Wu LC, Su Z, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 40.Noonan M, Korenblat P, Mosesova S, Scheerens H, Arron JR, Zheng Y, Putnam WS, Parsey MV, Bohen SP, Matthews JG.Dose-ranging study of lebrikizumab in asthmatic patients not receiving inhaled steroids J Allergy Clin Immunol 2013132567–574.e12 [DOI] [PubMed] [Google Scholar]
- 41.Frischmeyer-Guerrerio PA, Guerrerio AL, Oswald G, Chichester K, Myers L, Halushka MK, Oliva-Hemker M, Wood RA, Dietz HC. TGF β receptor mutations impose a strong predisposition for human allergic disease. Sci Transl Med. 2013;5:195ra94. doi: 10.1126/scitranslmed.3006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO. Antioxidants accelerate lung cancer progression in mice. Sci Transl Med. 2014;6:221ra15. doi: 10.1126/scitranslmed.3007653. [DOI] [PubMed] [Google Scholar]