Abstract
Neonates and infants have a higher morbidity and mortality associated with lower respiratory tract illnesses compared with older children. To identify age-related and longitudinal differences in the cellular immune response to acute lung injury (ALI), neonatal and juvenile mice were given Escherichia coli LPS using a novel, minimally invasive aspiration technique. Neonatal and juvenile mice received between 3.75 and 7.5 mg/kg LPS by intrapharyngeal aspiration. Airway and lung cells were isolated and characterized by flow cytometry, cytokine/chemokine mRNA expression from lung homogenates was quantified, and lung morphometry and injury scores were performed. LPS-treated neonatal mice underwent adoptive transfer with adult T regulatory cells (Tregs). After LPS aspiration, lung monocytes isolated from neonatal mice had a predominant M2 phenotype, whereas lung monocytes from juvenile mice displayed a mixed M1/M2 phenotype. At 72 hours after LPS exposure, neonatal lungs were slower to resolve inflammation and expressed lower mRNA levels of CCL2, CCL5, CXCL10, and IL-10. Juvenile, but not neonatal, mice demonstrated a significant increase in airway Tregs after LPS exposure. Adoptive transfer of adult Tregs into LPS-challenged neonatal mice resulted in reduced lung inflammation and improved weight gain. These findings underscore several vulnerabilities in the neonatal immune response to LPS-induced ALI. Most striking was the deficiency in airway Tregs after LPS aspiration. Adoptive transfer of adult Tregs mitigated LPS-induced ALI in neonatal mice, highlighting the importance of age-related differences in Tregs and their response to ALI during early postnatal development.
Keywords: acute lung injury, neonate, juvenile, Escherichia coli LPS
Clinical Relevance
Findings in this study underscore vulnerabilities in the neonatal pulmonary immune response to acute inflammation, including an impaired ability to activate monocytes/macrophages and an attenuated airway/lung T regulatory cell response. Further elucidation of these immune response vulnerabilities in the neonate may help identify immunological interventions that could improve morbidity and mortality resulting from neonatal pulmonary injury.
Pediatric acute lung injury (ALI) has a mortality rate of 22%, with 11% of children dying from ALI-related pneumonias (1). Young infants, in particular, are at increased risk for poor outcomes secondary to ALI-related illnesses.
In contrast to older children, neonates and infants are more likely to develop lower respiratory tract pathology when exposed to viruses and bacteria (2). This can occur even though neonates have been shown to have near-normal Toll-like receptor (TLR) responses to bacterial and viral antigens (3–5). The adult response to ALI is characterized by an early, robust proinflammatory T helper (Th) 1 immune response (6), whereas the neonate has been reported to have a delayed inflammatory response, which can be manifested by slow clearance of airway antigens and prolonged disease symptomatology (2, 7, 8). Neonatal dendritic cells have also been shown to have a skewed Th2 response, which may contribute to the differences in immune response between neonates and adults to lower airway infections (9).
It is unclear why these developmental differences in the immune response exist. The neonatal response to ALI may be a transitional response between fetal and adult life, resulting in an “at risk” period for the development of severe lower respiratory pathology. Alternatively, an attenuated inflammatory lung response may benefit the neonate by minimizing growth inhibition during a critical period of alveologenesis (10). Choo-Wing and colleagues found that neonatal mice that overexpressed the proinflammatory cytokine, IL-6, had a higher mortality when exposed to hyperoxia compared with wild-type mice (11). In contrast, adult IL-6 transgenic mice had better survival in hyperoxia when compared with wild-type adult mice (12). Their findings may indicate that neonatal mice are less tolerant of lung inflammation, more susceptible to oxidative stress, and sensitive to growth inhibition during a critical period of lung development in early postnatal life.
Unfortunately, a delayed or attenuated immune response to a severe ALI may be detrimental to the neonate. For instance, fatal outcomes from respiratory syncytial virus have been associated with fewer alveolar macrophages and a failure to clear airway antigen (8), suggesting that the neonate is less able to mount an effective immune response when exposed to a more severe ALI. In addition, although the migration of T regulatory cells (Tregs) to areas of airway inflammation have been shown to be critical for clearance of inflammation in an adult murine model of ALI (6), the role of Tregs in the resolution of ALI in the neonate is poorly understood. Lines and colleagues (13) reported that neonatal mice infected with influenza had impaired T cell migration into the airways, whereas other murine studies have found that an impaired lymphocytic response to TLR4 activation can cause chronic or hyperresponsive inflammation in the neonate (14, 15). Thus, an ineffective lymphocytic response to ALI may delay recovery in the neonate.
The goal of this study was to characterize age-related differences in the immune response to lower airway inflammation between neonatal and juvenile mice. Employing a novel, minimally invasive method to deliver LPS to the lower airways of neonatal and juvenile mice, we identified several vulnerabilities in the neonatal immune response to high-dose LPS aspiration. Compared with juvenile mice, the lungs of neonatal mice after LPS challenge exhibited a more prolonged inflammatory response, minimal M1 activation, altered cytokine/chemokine expression, and few airway Tregs. Furthermore, adoptive transfer of adult Tregs into neonatal mice challenged with LPS resulted in reduced lung inflammation and improved weight gain. Elucidation of age-related differences in the immune response to LPS-induced ALI may provide insight into interventions that could impact clinical approaches in the management of ALI in the neonate.
Materials and Methods
Mice
Timed pregnant C57BL/6NJ mice were obtained from the National Cancer Institute (Bethesda, MD). The animals were maintained on an AIN 76A diet and water ad libitum, and housed at a temperature range of 20–23°C under 12-hour light/dark cycles. All experiments were conducted in accordance with the standards established by the United States Animal Welfare Acts, set forth in National Institutes of Health guidelines and the policy and procedures manual of the Johns Hopkins University Animal Care and Use Committee. Congenic CD45.1 mice from the Jackson Laboratory (Bar Harbor, ME) were used for adoptive transfer experiments.
Intrapharyngeal Aspiration of LPS
Pups were lightly sedated with isofluorane before delivery of Escherichia coli LPS (L2880; Sigma-Aldrich, St. Louis, MO). Forceps were used to gently pull out the tongue, and saline containing 0, 3.75, 5.0, or 7.5 mg/kg of LPS was deposited in the pharynx. Neonatal mice (2 to 5 d old) received 15 μl of fluid, and juvenile mice (12–20 d old) received 20 μl of fluid. Aspiration of fluid was directly observed. Mice were recovered under a heating lamp and returned to their litter. A preliminary study using methylene blue showed aspiration of dye in the lower airways and lung of neonatal and juvenile mice (data not shown).
Bronchoalveolar Lavage
Bronchoalveolar lavage (BAL) samples were collected with 2 × 0.1 ml of sterile PBS through a cannula inserted in the trachea. The BAL fluid was centrifuged at 3,000 rpm for 10 minutes at 4°C. A portion of the cell suspension was centrifuged onto a glass slide with a Cytospin 3 centrifuge (Thermo Scientific, Wilmington, DE). Diff-Quik (Andwin Scientific, Tyron, NC) was used to stain the cells on each slide to determine the differential. The remaining cell suspension was used to obtain total cell counts with a hemocytometer. Percentages of CD45.1 and Tregs from BAL were quantified by multicolor flow cytometry.
Adoptive Transfer of T Cells
Bulk splenic cells from adult CD45.1 congenic mice were prepared as previously described (6), and 5 × 106 were given to neonatal mice by intraperitoneal injection using a 30-gauge needle or by intrapharyngeal delivery. For the transfer of specific T cell subsets from the spleen of adult CD45.1 congenic mice, Tregs and CD8+ cells were isolated by flow cytometry as previously described (6). Cells were suspended in PBS, and 500,000 cells were administered by intraperitoneal injection.
Lung Inflation, Quantitative RT-PCR, Flow Cytometry, Isolation of Peritoneal Macrophages, and Major Histocompatibility Complex Class II Antibodies
See the online supplement.
Statistical Analysis
Differences in measured variables between treated and control groups were determined using Student’s t test (two-tailed, equal variance) or Mann-Whitney U test. Statistical significance was accepted at P less than 0.05. Error bars represent SEM.
Results
Inflammatory Response of Neonatal and Juvenile Lung to E. coli LPS
Initial experiments used 3.75 mg/kg of LPS. At 4 days after LPS aspiration, both neonatal mice and juvenile mice had a significant increase in the number of cells isolated by BAL compared with their respective age-matched saline controls (neonatal—n = 13–15; LPS, 85,067 ± 4,920 cells; saline, 13,827 ± 3,456 cells; P < 0.013; and juvenile—n = 9–10; LPS, 217,366 ± 30,942 cells; saline, 35, 833 ± 4,565 cells; P < 0.0001). The airway inflammatory response at Day 4 after 3.75 mg/kg LPS was less in neonatal mice compared with juvenile mice, as shown by the significant difference in inflammatory cell number in the BAL and lower mean percentage of airway neutrophils (neonatal LPS, 40.3% ± 8.0%; juvenile LPS, 59.4% ± 2.7%; P < 0.04). In addition, although neonatal and juvenile mice showed comparable increases in lung IL-6 and TNF-α after LPS challenge, only lung from juvenile mice had a significant increase in the neutrophil chemoattractant, CXCL1 (juvenile LPS, 2.5 ± 0.2–fold increase; neonatal LPS, 1.3 ± 0.2–fold increase above control; P < 0.001).
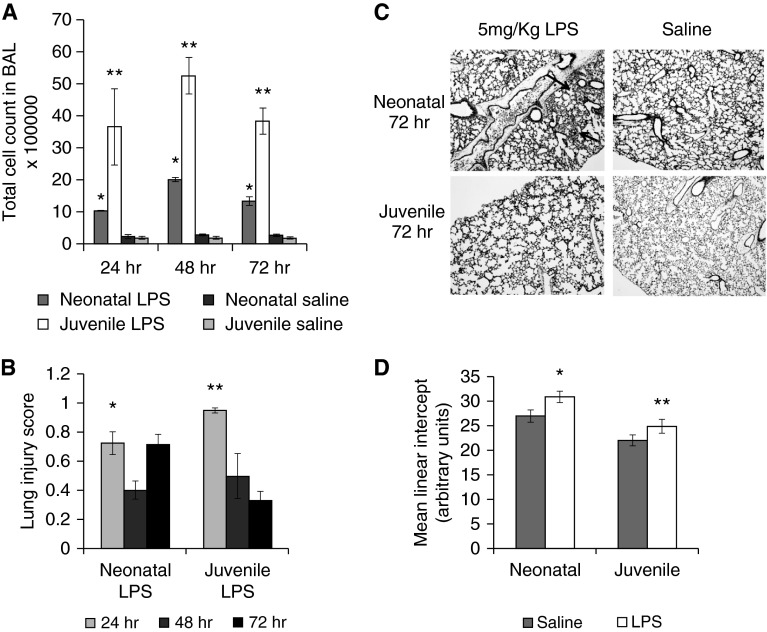
To induce a greater inflammatory response, we exposed mice to 5 mg/kg of LPS. BAL cell counts were evaluated at 24, 48, and 72 hours (Figure 1A). Both neonatal and juvenile LPS-treated mice had a significant increase in BAL cell counts compared with their respective controls at all three time points; however, the cellular response in the juvenile BAL was significantly more robust. At 6 days after LPS aspiration, neonatal mice had a higher percentage of BAL neutrophils compared with juvenile mice (neonatal mice, 51 ± 3.9%, versus juvenile mice, 22 ± 8.1%; P < 0.01; n = 5–6). Mean lung injury scores were determined at 24, 48, and 72 hours after LPS challenge. Juvenile mice had a significantly higher mean lung injury score at 24 hours after LPS aspiration compared with neonatal mice. The mean lung injury score in juvenile mice, however, declined at 48 and 72 hours after LPS, whereas neonatal mice had similar lung injury scores at 24 and 72 hours after LPS (Figures 1B and 1C). Mean linear intercepts (MLIs) were also measured at 6 days after LPS in both age groups. Both LPS-treated neonatal and juvenile mice had a modest, but significant, increase in MLI compared with age-matched saline controls (Figure 1D), indicating that both groups experienced alveolar damage.
Figure 1.
Impact of LPS aspiration on neonatal and juvenile lung. Neonatal and juvenile mice were given 5 mg/kg of LPS by intrapharyngeal aspiration. (A) Total bronchoalveolar lavage (BAL) cell counts from neonatal and juvenile mice at 24, 48, and 72 hours were significantly higher after LPS compared with age-matched saline controls (*P < 0.0001, **P < 0.04). There was also a significant difference in total BAL cell count between neonatal and juvenile mice after LPS at each time point except 24 hours (P < 0.004; n = 3–4 per group). (B) Mean lung injury scores were measured in neonatal and juvenile mice at 24, 48, and 72 hours after LPS. The mean lung injury scores of neonatal mice after LPS were significantly higher than age-matched saline controls at 24, 48, and 72 hours after aspiration (*P < 0.05). At 24 hours, juvenile mice after LPS had a higher mean lung injury score than neonatal mice after LPS (**P < 0.04). Juvenile, but not neonatal, mice had a decreased mean lung injury score at 72 hours after LPS. (C) Representative example of neonatal and juvenile lung 72 hours after intrapharyngeal Escherichia coli LPS (5 mg/kg) or saline. Black arrows point to inflammatory cells in the lungs. (D) Mean linear intercept measurements were significantly increased at 6 days after LPS in both neonatal and juvenile mice compared with age-matched controls (n = 3–7 per group). *P < 0.004, **P < 0.05. Error bars represent mean ± SEM.
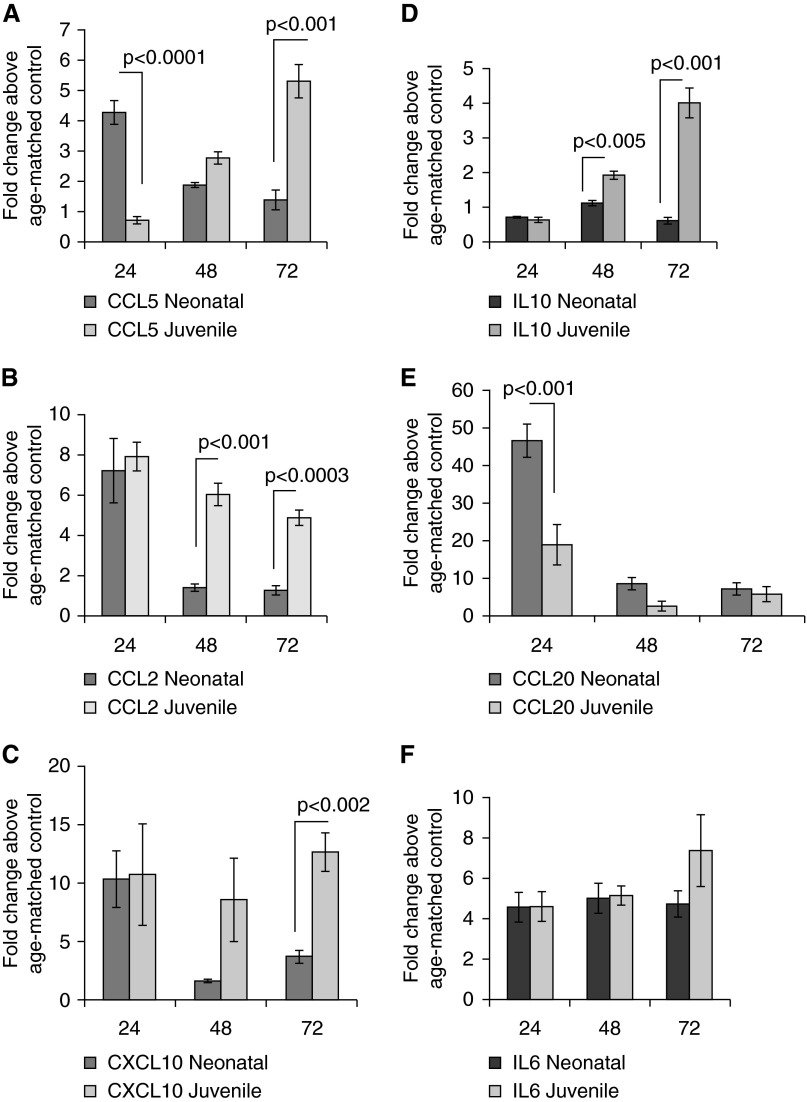
Expression of several NF-κβ–regulated genes was measured in neonatal and juvenile lung at 24, 48, and 72 hours after LPS aspiration using real-time PCR. At all three time points after LPS aspiration, IL-6 expression was significantly induced to comparable levels in neonatal and juvenile lung (Figure 2F). At 24 hours after LPS, expression of CCL5, CCL2, CXCL10, and CCL20 in neonatal lung was significantly induced above age-matched controls. However, by 72 hours after LPS, mRNA expression of CCL5, CCL2, CXCL10, and CCL20 had declined in neonatal lung (Figures 2A–2C and 2E). Juvenile mice had a different chemokine profile than that of neonatal mice after LPS. At 24 hours after LPS, juvenile mice had significantly lower expression of lung CCL5 and CCL20 compared with neonatal mice. However, expression of CCL5, CCL2, and CXCL10 in juvenile lung was significantly higher than that of neonatal lung at 72 hours after LPS. Expression of IL10 in lung of juvenile mice was also significantly higher than neonatal lung at 48 and 72 hours after LPS (Figure 2D).
Figure 2.
Neonatal and juvenile lung cytokine/chemokine expression after LPS aspiration. (A–F) Lung cytokine/chemokine levels were measured from whole-lung homogenate from neonatal and juvenile mice at 24, 48, and 72 hours after intrapharyngeal LPS (5 mg/kg). The y axis represents fold changes above age-matched saline-treated lung (n = 3–4). Error bars represent mean ± SEM.
Age-Related Phenotypic Differences in Monocytes after LPS Challenge
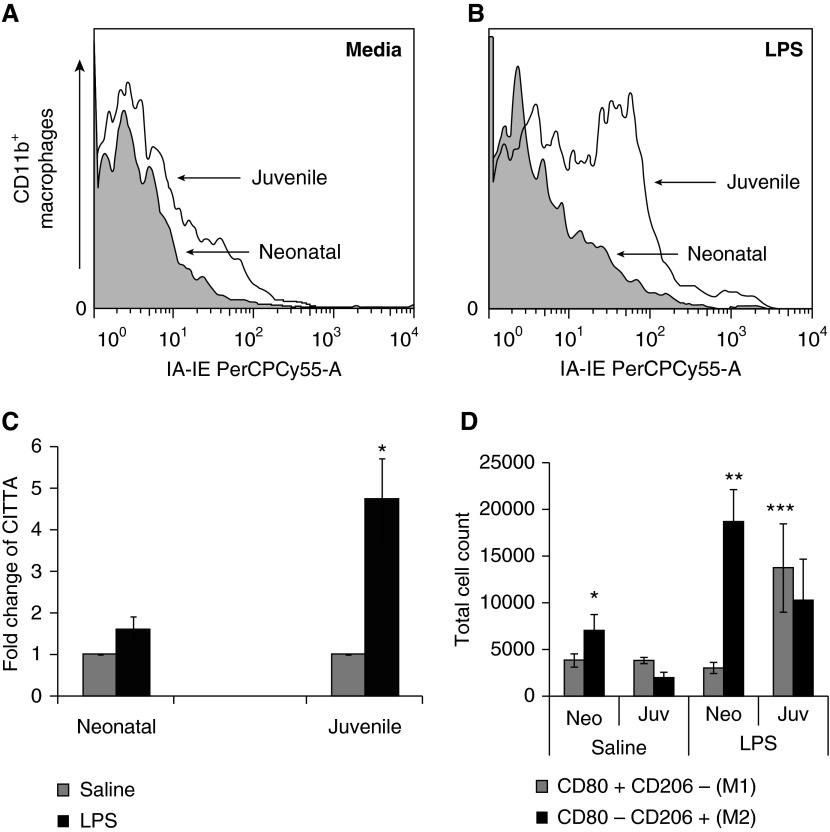
We next sought to determine if age-related phenotypic differences existed in airway monocytes after LPS challenge. CD11b+ cells isolated from the BAL of neonatal and juvenile mice 4 days after LPS had increased TLR2, CD14, and CD86 expression compared with age-matched saline controls (Table 1). In contrast, only juvenile airway CD11b+ cells had a significant increase in major histocompatibility complex (MHC) class II (IA-IE) and CD40 expression (Table 1). This difference in MHC class II expression between neonates and juveniles was not restricted to the CD11b+ cells in the lungs, as LPS induced an increase in MHC II on cultured peritoneal-derived mononuclear cells from juvenile, but not neonatal, mice (Figures 3A and 3B). Because MHC II expression has been shown to be regulated by MHC II transactivator (CITTA) (16), we measured CITTA expression from whole lung of neonatal and juvenile mice after LPS challenge. We found that juvenile, but not neonatal, lung after LPS had increased expression of CITTA, indicating that LPS-induced CITTA expression was attenuated in neonatal lung (Figure 3C). CD45+ F4/80+ monocytes were also isolated from whole-lung homogenates at Day 3 after LPS to assess M1 (CD80+CD206-) or M2 (CD80-CD206+) activation status. Monocytes isolated from neonatal mice showed a predominance of M2 activation, whereas juvenile monocytes had a mixed M1/M2 phenotype (Figure 3D). After LPS, both neonatal and juvenile lung–derived monocytes had a significant increase in the percentage of TLR2-positive cells compared with controls (juvenile LPS, 58.5 ± 2.7%, versus control, 7.8 ± 1.3, P < 0.001; and neonatal LPS, 39.2 ± 11.4%, versus control, 12.2 ± 2.3%, P < 0.03 [n = 3–4]). The percentages of TLR2-positive cells after LPS were not significantly different between the two age groups. No differences were found in the percentage of TLR4 in either age group after LPS.
Table 1.
CD14, CD86, Toll-like Receptor 2, CD40, GR-1, and IA-IE Expression of CD11b+ Monocytes Isolated from Bronchoalveolar Lavage of Neonatal and Juvenile Mice 4 Days after Intrapharyngeal Escherichia coli LPS (3.75 mg/kg) or Saline
| After Saline | After LPS | P Value | |
|---|---|---|---|
| Neonatal | |||
| CD11b+ cells | 52 ± 17.1 | 806 ± 75.7 | 0.001* |
| CD14 | 611.9 ± 284.8 | 2,488.2 ± 172.5 | 0.001* |
| CD86 | 36.2 ± 7.2 | 204.7 ± 30.8 | 0.007* |
| TLR2 | 127.7 ± 88.8 | 1,368.9 ± 55.0 | 0.001* |
| CD40 | 73.4 ± 40.9 | 110.3 ± 18.9 | 0.382 |
| GR-1 | 916.7 ± 166.0 | 690.5 ± 37.5 | 0.135 |
| IA-IE | 53.3 ± 24.0 | 32.9 ± 1.5 | 0.292 |
| Juvenile | |||
| CD11b+ cells | 2,004.7 ± 465 | 6,731.5 ± 448 | 0.001* |
| CD14 | 62.2 ± 14.0 | 958.3 ± 142.8 | 0.004* |
| CD86 | 26.9 ± 1.0 | 106.2 ± 8.9 | 0.001* |
| TLR2 | 40.1 ± 3.6 | 601.8 ± 72.0 | 0.001* |
| CD40 | 40.2 ± 13.5 | 238.2 ± 25.2 | 0.001* |
| GR-1 | 70.8 ± 10.7 | 386.2 ± 35.6 | 0.001* |
| IA-IE | 12.3 ± 1.2 | 71.2 ± 6.9 | 0.001* |
Definition of abbreviation: TLR, Toll-like receptor.
Numbers expressed as geometric means for CD14, CD86, TLR2, CD40, GR-1, and IA-IE (n = 5–6 for LPS; n = 3 for saline) (± SEM).
Significant differences between saline and LPS.
Figure 3.
Influence of LPS stimulation on major histocompatibility complex (MHC) II expression in peritoneal macrophages, MHC II transactivator (CITTA) expression in lung, and lung monocyte M1/M2 phenotype. (A) MHC class II expression (IA-IE PerCPCy55-A) from macrophages in cell media isolated from the peritoneal space of neonatal and juvenile mice treated with thioglycollate. (B) MHC class II expression in macrophages after LPS. Juvenile, but not neonatal, macrophages had a marked increase in MHC class II expression. (C) Lung CITTA levels in juvenile and neonatal mice four days after LPS (5 mg/kg) or saline aspiration. Juvenile, but not neonatal, mice had significantly increased lung expression of CITTA after LPS aspiration above age-matched saline controls (*P < 0.002, n = 5–7 in each group). (D) Neonatal saline-treated mice had significantly fewer lung M2 monocytes compared with neonatal LPS-treated mice (*P < 0.02), neonatal LPS-treated mice had significantly more lung M2 monocytes than M1 monocytes (**P < 0.001) and juvenile LPS-treated mice had significantly more lung M1 monocytes than neonatal LPS-treated mice (***P < 0.04). Error bars represent mean ± SEM. Juv, juvenile; Neo, neonatal.
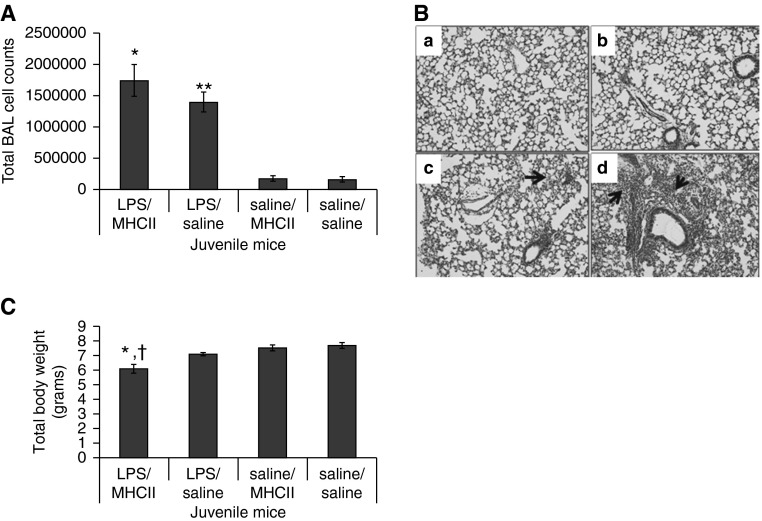
Significant induction of MHC II molecules occurred in juvenile, but not neonatal, airway CD11b+ cells after LPS aspiration. To examine the association between MHC II induction and LPS-induced lung injury, juvenile mice were given MHC II antibodies (MHC II) starting 2 days before LPS aspiration. At 4 days after LPS aspiration, the LPS/MHC II– and LPS/saline–treated mice had higher BAL cell counts compared with controls (Figure 4A), and more lung inflammation (Figure 4B). Although the LPS/MHC II mice had significantly lower weights compared with LPS/saline, saline/saline, and saline/MHC II mice (Figure 4C), the LPS/MHC II and LPS/saline mean lung injury scores were similar, with both having significantly higher injury scores than saline/saline and saline/MHC II mice (P < 0.01).
Figure 4.
Juvenile mice treated with MHC class II antibodies. (A) Total BAL cell counts were higher in LPS/MHC II and LPS/saline mice compared with saline/MHC II and saline/saline mice (*P < 0.001, **P < 0.001). (B) Representative example of lung in saline/saline (a), LPS/saline (b), saline/MHC II (c), and LPS/MHC II (d) mice. Arrows point to inflammatory cells in the lungs. (C) LPS/MHC II mice had significantly lower weights than LPS/saline mice (†P < 0.01), saline/MHC II mice, and saline/saline mice (*P < 0.001) at 4 days after LPS or saline aspiration (n = 7–9). Error bars represent mean ± SEM.
Tregs and ALI in Neonatal and Juvenile Mice
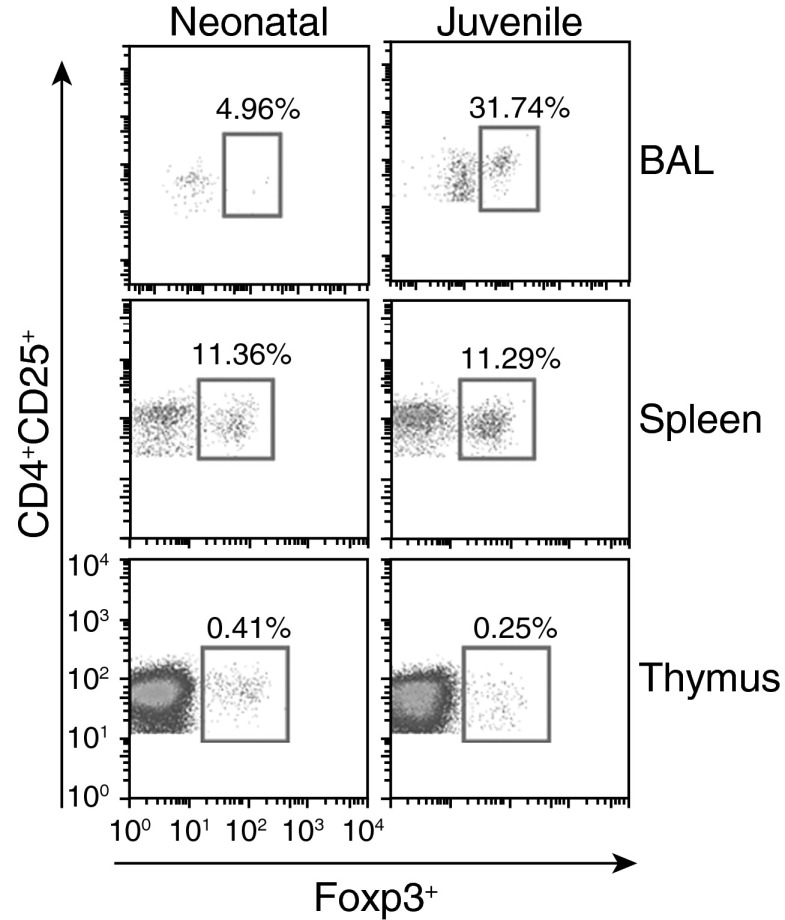
CCL2, CCL5, and CXCL10 have previously been reported to function as T cell chemoattractants (17–19). Given that neonatal lung exhibited a decline in CCL2, CCL5, and CXCL10 expression at 72 hours after LPS challenge, we examined the role of airway Tregs in neonatal ALI. After LPS challenge, juvenile mice were found to have a significant increase in airway Tregs (juvenile LPS, 65.4 ± 5.3 cells, versus control, 4.3 ± 0.3 cells, P < 0.001), whereas neonatal mice did not (neonatal LPS, 1.4 ± 0.5 cells, versus control, 3 ± 1.7 cells, P < 0.31). With higher-dose LPS (5 mg/kg) challenge, we found a low percentage of airway Tregs in the BAL of the neonate compared with the juvenile, but similar percentages of Tregs in spleen and thymus of both neonatal and juvenile mice (Figure 5). From Tregs isolated from neonatal and juvenile lung, we measured, by flow cytometry, the percentage of CD103, an integrin expressed in a subset of Tregs with enhanced migration and recruitment (20). Interestingly, after LPS challenge, we found a higher percentage of CD103 in juvenile Tregs compared with neonatal Tregs (juvenile CD103, 39.9 ± 0.1.6%, versus neonatal CD103, 7.2 ± 0.5%, P < 0.0001), suggesting that migration of Tregs into the airways after LPS is influenced by age-related phenotypic and functional differences.
Figure 5.
Higher percentage of T regulatory cells (Tregs; CD4+CD25+Foxp3+) found in juvenile mice after LPS aspiration compared with neonatal mice. Juvenile mice had a higher percentage of Tregs in the BAL compartment compared with neonatal mice at 6 days after LPS aspiration (5 mg/kg). In the spleen and thymus, the percentages of Tregs between the two age groups were similar.
Migration of Adult CD45.1+ Splenocytes into Airways of Neonatal Mice after LPS Challenge
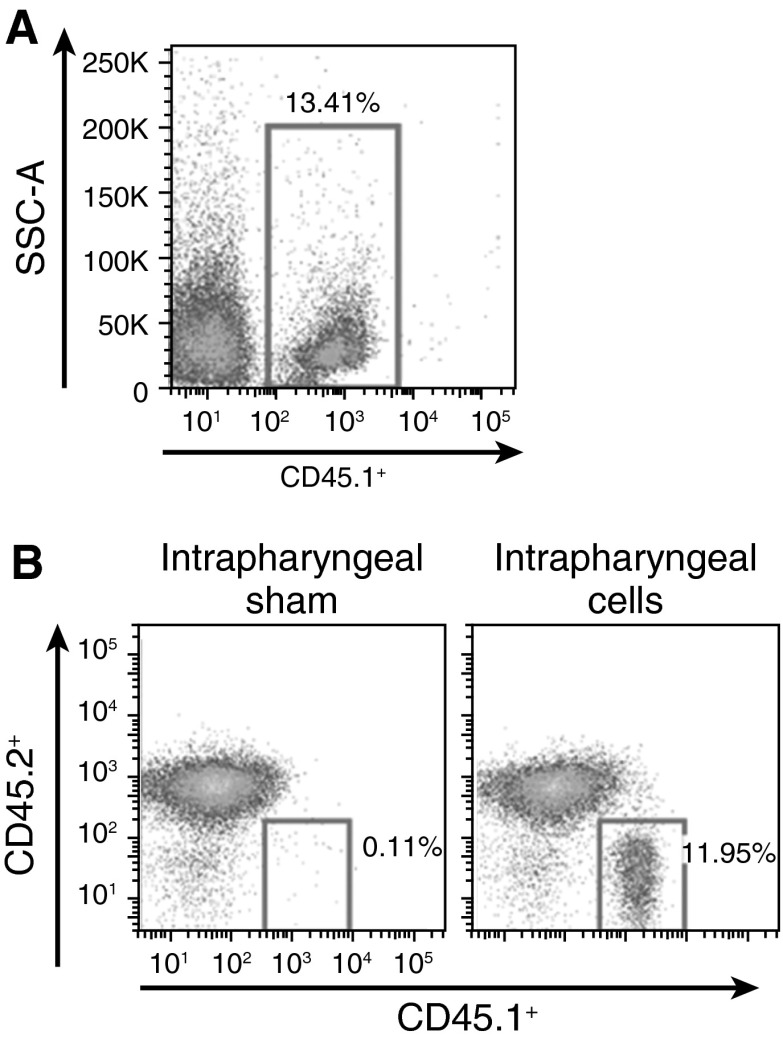
In adult mice, Tregs were found to be instrumental in the resolution of ALI (6). In the neonate, few airway Tregs were found after LPS challenge, indicating impaired airway migration of Tregs in response to neonatal ALI. To determine if adult Tregs could migrate into neonatal airways after LPS aspiration, we isolated splenic cells from adult CD45.1 congenic mice. Adult CD45.1 splenocytes were given by adoptive transfer into LPS-challenged neonatal CD45.2 mice by intraperitoneal delivery. The percentage of adult splenic CD45.1+ donor cells in the BAL of saline-treated neonatal mice was 3.7 (±1.0)% (n = 3), whereas LPS-treated neonatal mice had between 13.4 and 50%, respectively (Figure 6A). We also gave LPS-challenged neonatal mice adult CD45.1 splenocytes by intrapharyngeal delivery to determine the utility of this mode of delivery. At 6 days after LPS, CD45.1 cells were isolated from the BAL of neonatal mice, indicating that adult splenocytes given by intrapharyngeal delivery can migrate and persist in the airways of neonatal mice after LPS (Figure 6B, right panel).
Figure 6.
Adult CD45.1+ splenocytes in the BAL of neonatal mice. (A) CD45.1 splenocytes in the BAL of a CD45.2 neonatal mouse 6 days after LPS aspiration and adoptive transfer of adult CD45.1 splenocytes by intraperitoneal delivery. SSC-A, side scatter. (B) CD45.1 splenocytes in the BAL of LPS-treated CD45.2 mice given either CD45.1 splenocytes by intrapharyngeal adoptive transfer (right panel) or saline (sham; left panel).
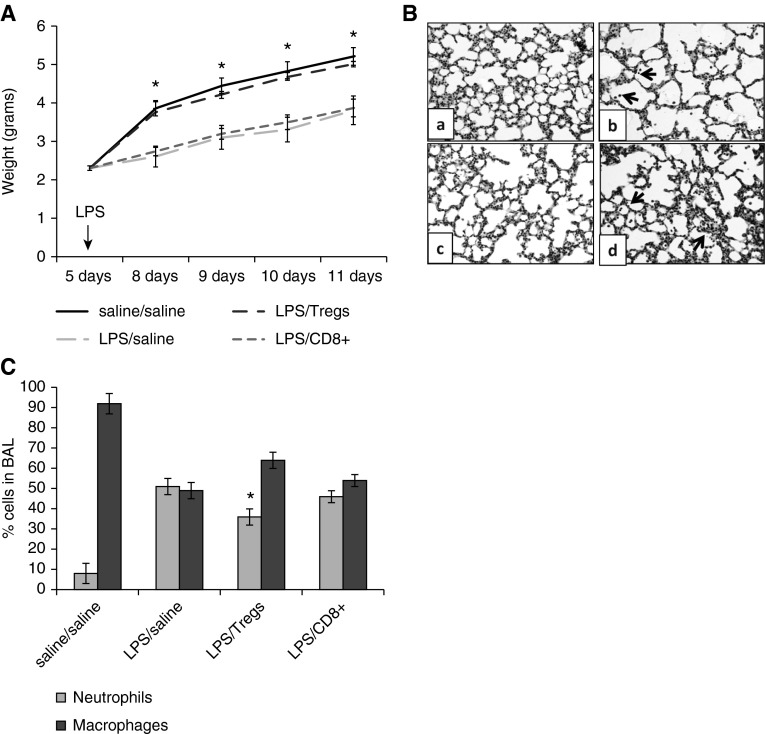
After demonstrating that adult splenocytes could migrate into the airways of neonatal mice after LPS challenge, Tregs and CD8+ cells were isolated from adult spleens and used for adoptive transfer experiments in neonatal mice. Neonatal mice challenged with LPS and given adult Tregs by intraperitoneal adoptive transfer (LPS/Tregs) gained weight significantly faster than neonatal mice challenged with LPS and given CD8+ cells by adoptive transfer (LPS/CD8+) or saline alone (LPS/saline) (Figure 7A). A modest, but significant, increase in mean MLI was found in the LPS/saline mice compared with LPS/Treg and saline/saline mice (LPS/saline, 30.7 ± 0.7, versus LPS/Tregs, 28.6 ± 0.2 and versus saline/saline, 27.0 ± 1.3; P < 0.04 and P < 0.05, respectively), indicating greater alveolar damage (Figure 7B). At Day 6 after LPS, the mean percentage of neutrophils in the BAL of LPS/Treg neonatal mice was significantly less than the LPS/saline mice (Figure 7C), suggesting that adoptive transfer of Tregs could modestly attenuate LPS-induced lung inflammation in the neonate.
Figure 7.
Adoptive transfer of adult splenic Tregs, but not CD8+ cells, improved weight gain in neonatal mice after LPS aspiration. (A) Neonatal mice with adoptive transfer of adult Tregs after LPS aspiration had significantly better weight gain than neonatal mice that received LPS/saline or LPS/CD8+ cells by adoptive transfer (*P < 0.01). (B) Representative example of lung from saline/saline (a), LPS/saline (b), LPS/Tregs (c), and LPS/CD8+ (d) mice. Arrows point to increased numbers of inflammatory cells in the lungs of LPS/saline and LPS/CD8+ mice. (C) The percentage of neutrophils in the BAL of LPS/Treg neonatal mice was significantly less than that in LPS/saline mice (*P < 0.02) and trended toward significance with the LPS/CD8+ mice (P < 0.08; n = 4–7). Error bars represent mean ± SEM.
Discussion
Neonates and infants with ALI have a higher morbidity and mortality compared with older children (21). In this study, we used a novel, minimally invasive aspiration technique to model LPS-induced ALI in neonatal and juvenile mice. High-dose LPS delivery in neonatal mice caused an inflammatory lung response characterized by a predominance of M2 lung monocytes, with no increase in airway Tregs. Conversely, juvenile mice displayed a mixed monocyte M1/M2 phenotype, a significant increase in airway Tregs, and more rapid resolution of lung inflammation. We were able to demonstrate that adult splenocytes given by intraperitoneal or intrapharyneal adoptive transfer can migrate and persist in the airways of LPS-challenged neonatal mice. Our finding, that intrapharygeal adoptive transfer is a feasible way to deliver adult splenocytes, opens up the possibility of using this mode of delivery for therapeutic interventions. In addition, we found that adoptive transfer of adult Tregs partially rescued the LPS-induced ALI phenotype in neonatal mice. Taken together, our findings indicate that neonatal mice fail to mount an airway Treg response to ALI, contributing to an inadequate injury repair response, and that adoptive transfer of adult Tregs can mitigate lung inflammation. These findings suggest that interventions that enable Tregs to enter neonatal airways may improve respiratory outcomes.
During in utero development, a Th2 bias exists to minimize inflammation and decrease fetal loss (22). This skewing can persist into neonatal life, with neonatal monocytes reported to express cytokines that support a Th2- and Th17-type immune response (23). In the neonate, an attenuated inflammatory lung response to low-dose LPS may be beneficial by minimizing growth inhibition during a critical period of postnatal lung development. Nevertheless, neonatal rodent models have reported both hypo- and hyperimmune responses to LPS. Whereas Martin and colleagues (7) reported an early attenuated immune response to LPS-induced lung injury in neonatal rats, Zhao and colleagues (14) found that neonatal mice developed a hyperresponsive inflammatory response to LPS-induced sepsis associated with a high mortality that was modified by adoptive transfer of adult T cells. These immune response differences are likely influenced by LPS dose, route of delivery, and an immature immune system. Consistent with a bias toward a Th2 response, we found that CCL20 was markedly up-regulated at 24 hours after LPS aspiration in neonatal lung. CCL20 has been shown to be induced in fetal lung explants by E. coli LPS, induced by IL-17 in bronchial epithelial cells, and increased in the BAL of patients with allergic asthma (24–26).
Differences in the expression of lung chemokines to LPS challenge may influence immune responses to ALI. In our study, we found that juvenile, but not neonatal, mice had increased expression of lung CCL5, CCL2, and CXCL10 at 72 hours after LPS. These chemokines have been reported to be monocyte and T cell chemoattractants (27). As such, their expression may influence T cell migration. We found that juvenile mice after LPS challenge exhibited a significant increase in the percentage of airway Tregs after LPS challenge, whereas no increase in airway Tregs was found in neonatal mice. Taken together, these findings suggest that a difference in lung chemokine response to ALI in neonatal mice may influence airway recruitment and migration of neonatal Tregs. In addition, we found phenotypic differences between juvenile and neonatal lung Tregs after LPS challenge. Neonatal lung Tregs had a significantly lower percentage of CD103, an integrin expressed in a subset of Tregs with enhanced migration and recruitment, when compared with juvenile Tregs (20). Furthermore, we found that adult Tregs could migrate into the airways of neonatal mice after LPS and partially attenuate lung inflammation when given by adoptive transfer, underscoring the importance of airway Tregs in resolving lung inflammation.
Little is known regarding the role of Tregs in resolving lung inflammation in the neonate. Influenza-infected neonatal mice have been shown to have few airway T cells, suggesting a defect in T cell migration; however, the mechanism for this was not determined (13). Another study reported a paucity of liver Tregs in neonatal mice with rotavirus-induced bilary atresia, and that adoptive transfer of CD4+ cells before infection improved weight gain and survival (28). Similarly, we found that adoptive transfer of Tregs significantly improved weight gain and reduced lung inflammation in neonatal mice after LPS aspiration. Although the mechanism by which adult Tregs attenuated LPS-induced ALI in our neonatal murine model will require further investigation, our studies indicate that neonatal Tregs have an impaired ability to migrate into airways after ALI. It is not yet clear if neonatal Tregs are functionally similar to adult Tregs and would be able to migrate into airways and attenuate lung injury if exposed to appropriate adult immune signaling from the injured lung, or if amplifying systemic numbers of Tregs would allow adequate migration into the inflamed airways of the neonate. If neonatal Tregs are intrinsically different from adult Tregs, then further maturation through external stimuli may be required before they could effectively migrate into inflamed airways and modify lung injury. Furthermore, it is not known if increasing the number of peripheral Tregs in a neonate would prolong viral shedding or promote bacterial replication during an infection. Nevertheless, examining the effect of expanding neonatal Treg numbers, inducing neonatal Treg maturation, or promoting migration of neonatal Tregs by altering cytokine/chemokine lung profiles may provide insights into alternative therapies to improve outcomes in neonatal ALI.
Other innate immune responses in the neonatal lung may influence injury responses to ALI. In contrast to juvenile mice, airway CD11b+ monocytes of neonatal mice had minimal induction of MHC II expression when challenged with LPS. In addition, neonatal mice had minimal induction of lung CITTA with LPS, indicating a maturational defect. Lee and colleagues also found decreased MHC II expression in neonatal alveolar macrophages in response to IFN-γ stimulation (29). Another study reported lower IL-6 and TNF-α production from neonatal monocytes given low-dose LPS compared with adult monocytes (30).
Our study was novel in that we were able to deliver LPS to the lungs of neonatal mice in a minimally invasive way, allowing us to assess the longitudinal impact of LPS and avoid the high mortality and stress of a surgical procedure (7). In a previous study by Martin and colleagues (7), neonatal rats were treated with LPS through a surgical incision in the trachea. At 6 hours after LPS, they found that neonatal rats had fewer total neutrophils and fewer total number of BAL cells compared with adult rats, consistent with an early blunted inflammatory response in the neonates. Unlike our study, however, later time points were not examined because of the high mortality in the neonatal rats after tracheal surgery. Using our intrapharyngeal technique, we were able to characterize lung inflammation and resolution of LPS-induced lung injury in the neonate and juvenile mouse over a longer time course. We were also able to demonstrate that neonatal mice with LPS-induced ALI tolerated adoptive transfer of adult splenic cells by intraperitoneal or intrapharyngeal delivery, that adult splenocytes could migrate into inflamed airways of neonatal mice, and that adult splenocytes were detected in the BAL of neonatal mice 6 days after adoptive transfer. As a consequence of using the adoptive transfer techniques in neonatal mice, we were able to demonstrate that LPS-challenged neonatal mice that received adult Tregs had better weight gain and attenuated lung inflammation.
In summary, our findings underscore the presence of several vulnerabilities in the neonatal immune response to LPS-induced ALI. Adoptive transfer of adult Tregs mitigated LPS-induced ALI in neonatal mice, highlighting the importance of age-related differences in Tregs and their response to ALI during early postnatal development. We believe that understanding the age-related differences in Treg cell responses to ALI is an essential step toward identifying translational interventions that could improve morbidity and mortality in the vulnerable neonatal population.
Acknowledgments
Acknowledgments
The authors express gratitude to Anne Jedlicka and the Johns Hopkins School of Public Health Genomic Analysis and Sequencing Core for technical assistance in the expression analyses, Thomas Lauer for his technical assistance with the animal work, and Raeffello Cimbro and Joe Crest for their assistance in the Johns Hopkins Bayview Flow Cytometry Core.
Footnotes
This work was supported by the Flight Attendant Medical Research Institute (S.A.M.-M.), the American Academy of Pediatrics (S.A.M.-M. and J.M.C.), and by National Institutes of Health grants RHL114800A (S.A.M.-M.) and R00HL103793 (F.D.).
Author Contributions: concept and design—S.A.M.-M., F.D., M.J.S., and A.S.; analysis and interpretation—S.L., K.G., E.N., A.L., and J.M.C.; drafting the manuscript for important intellectual content—S.A.M.-M., A.S., and F.D.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0100OC on July 28, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Flori HR, Glidden DV, Rutherford GW, Matthay MA. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171:995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 2.Levy O. Innate immunity of the human newborn: distinct cytokine responses to LPS and other Toll-like receptor agonists. J Endotoxin Res. 2005;11:113–116. doi: 10.1179/096805105X37376. [DOI] [PubMed] [Google Scholar]
- 3.Strunk T, Currie A, Richmond P, Simmer K, Burgner D. Innate immunity in human newborn infants: prematurity means more than immaturity. J Matern Fetal Neonatal Med. 2011;24:25–31. doi: 10.3109/14767058.2010.482605. [DOI] [PubMed] [Google Scholar]
- 4.Strunk T, Power Coombs MR, Currie AJ, Richmond P, Golenbock DT, Stoler-Barak L, Gallington LC, Otto M, Burgner D, Levy O. TLR2 mediates recognition of live Staphylococcus epidermidis and clearance of bacteremia. PLoS One. 2010;5:e10111. doi: 10.1371/journal.pone.0010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang JP, Yang Y, Levy O, Chen C. Human neonatal peripheral blood leukocytes demonstrate pathogen-specific coordinate expression of TLR2, TLR4/MD2, and MyD88 during bacterial infection in vivo. Pediatr Res. 2010;68:479–483. doi: 10.1203/PDR.0b013e3181f90810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119:2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin TR, Ruzinski JT, Wilson CB, Skerrett SJ. Effects of endotoxin in the lungs of neonatal rats: age-dependent impairment of the inflammatory response. J Infect Dis. 1995;171:134–144. doi: 10.1093/infdis/171.1.134. [DOI] [PubMed] [Google Scholar]
- 8.Reed JL, Brewah YA, Delaney T, Welliver T, Burwell T, Benjamin E, Kuta E, Kozhich A, McKinney L, Suzich J, et al. Macrophage impairment underlies airway occlusion in primary respiratory syncytial virus bronchiolitis. J Infect Dis. 2008;198:1783–1793. doi: 10.1086/593173. [DOI] [PubMed] [Google Scholar]
- 9.Langrish CL, Buddle JC, Thrasher AJ, Goldblatt D. Neonatal dendritic cells are intrinsically biased against Th-1 immune responses. Clin Exp Immunol. 2002;128:118–123. doi: 10.1046/j.1365-2249.2002.01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilgendorff A, Reiss I, Ehrhardt H, Eickelberg O, Alvira CM. Chronic lung disease in the preterm infant: lessons learned from animal models. Am J Respir Cell Mol Biol. 2014;50:233–245. doi: 10.1165/rcmb.2013-0014TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choo-Wing R, Nedrelow JH, Homer RJ, Elias JA, Bhandari V. Developmental differences in the responses of IL-6 and IL-13 transgenic mice exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2007;293:L142–L150. doi: 10.1152/ajplung.00434.2006. [DOI] [PubMed] [Google Scholar]
- 12.Ward NS, Waxman AB, Homer RJ, Mantell LL, Einarsson O, Du Y, Elias JA. Interleukin-6–induced protection in hyperoxic acute lung injury. Am J Respir Cell Mol Biol. 2000;22:535–542. doi: 10.1165/ajrcmb.22.5.3808. [DOI] [PubMed] [Google Scholar]
- 13.Lines JL, Hoskins S, Hollifield M, Cauley LS, Garvy BA. The migration of T cells in response to influenza virus is altered in neonatal mice. J Immunol. 2010;185:2980–2988. doi: 10.4049/jimmunol.0903075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao J, Kim KD, Yang X, Auh S, Fu YX, Tang H. Hyper innate responses in neonates lead to increased morbidity and mortality after infection. Proc Natl Acad Sci USA. 2008;105:7528–7533. doi: 10.1073/pnas.0800152105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai J, Liu B, Ngoi SM, Sun S, Vella AT, Li Z. TLR4 hyperresponsiveness via cell surface expression of heat shock protein gp96 potentiates suppressive function of regulatory T cells. J Immunol. 2007;178:3219–3225. doi: 10.4049/jimmunol.178.5.3219. [DOI] [PubMed] [Google Scholar]
- 16.Reith W, LeibundGut-Landmann S, Waldburger JM. Regulation of MHC class II gene expression by the class II transactivator. Nat Rev Immunol. 2005;5:793–806. doi: 10.1038/nri1708. [DOI] [PubMed] [Google Scholar]
- 17.Gu L, Rutledge B, Fiorillo J, Ernst C, Grewal I, Flavell R, Gladue R, Rollins B. In vivo properties of monocyte chemoattractant protein-1. J Leukoc Biol. 1997;62:577–580. doi: 10.1002/jlb.62.5.577. [DOI] [PubMed] [Google Scholar]
- 18.Lane TE, Hardison JL, Walsh KB. Functional diversity of chemokines and chemokine receptors in response to viral infection of the central nervous system. Curr Top Microbiol Immunol. 2006;303:1–27. doi: 10.1007/978-3-540-33397-5_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kudo-Saito C, Shirako H, Ohike M, Tsukamoto N, Kawakami Y. CCL2 is critical for immunosuppression to promote cancer metastasis. Clin Exp Metastasis. 2013;30:393–405. doi: 10.1007/s10585-012-9545-6. [DOI] [PubMed] [Google Scholar]
- 20.Liston A, Gray DH. Homeostatic control of regulatory T cell diversity. Nat Rev Immunol. 2014;14:154–165. doi: 10.1038/nri3605. [DOI] [PubMed] [Google Scholar]
- 21.Osrin D, Vergnano S, Costello A. Serious bacterial infections in newborn infants in developing countries. Curr Opin Infect Dis. 2004;17:217–224. doi: 10.1097/00001432-200406000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Diesner SC, Förster-Waldl E, Olivera A, Pollak A, Jensen-Jarolim E, Untersmayr E. Perspectives on immunomodulation early in life. Pediatr Allergy Immunol. 2012;23:210–223. doi: 10.1111/j.1399-3038.2011.01259.x. [DOI] [PubMed] [Google Scholar]
- 23.Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, Lavoie PM, Furlong J, Fortuno ES, III, Hajjar AM, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183:7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nozato K, Fujita J, Kawaguchi M, Ohara G, Morishima Y, Ishii Y, Huang SK, Kokubu F, Satoh H, Hizawa N. IL-17F induces CCL20 in bronchial epithelial cells. J Allergy (Cairo) 2011;2011:587204. doi: 10.1155/2011/587204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pichavant M, Charbonnier AS, Taront S, Brichet A, Wallaert B, Pestel J, Tonnel AB, Gosset P. Asthmatic bronchial epithelium activated by the proteolytic allergen Der p 1 increases selective dendritic cell recruitment. J Allergy Clin Immunol. 2005;115:771–778. doi: 10.1016/j.jaci.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 26.Starner TD, Barker CK, Jia HP, Kang Y, McCray PB., Jr CCL20 is an inducible product of human airway epithelia with innate immune properties. Am J Respir Cell Mol Biol. 2003;29:627–633. doi: 10.1165/rcmb.2002-0272OC. [DOI] [PubMed] [Google Scholar]
- 27.Gilbert FB, Cunha P, Jensen K, Glass EJ, Foucras G, Robert-Granié C, Rupp R, Rainard P. Differential response of bovine mammary epithelial cells to Staphylococcus aureus or Escherichia coli agonists of the innate immune system. Vet Res. 2013;44:40. doi: 10.1186/1297-9716-44-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miethke AG, Saxena V, Shivakumar P, Sabla GE, Simmons J, Chougnet CA. Post-natal paucity of regulatory T cells and control of NK cell activation in experimental biliary atresia. J Hepatol. 2010;52:718–726. doi: 10.1016/j.jhep.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee PT, Holt PG, McWilliam AS. Failure of MHC class II expression in neonatal alveolar macrophages: potential role of class II transactivator. Eur J Immunol. 2001;31:2347–2356. doi: 10.1002/1521-4141(200108)31:8<2347::aid-immu2347>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 30.Bessler H, Komlos L, Punsky I, Ntambi JA, Bergman M, Straussberg R, Sirota L. CD14 receptor expression and lipopolysaccharide-induced cytokine production in preterm and term neonates. Biol Neonate. 2001;80:186–192. doi: 10.1159/000047141. [DOI] [PubMed] [Google Scholar]