Abstract
CD43, a surface glycoprotein, regulates Mycobacterium tuberculosis macrophage binding, replication, and proinflammatory cytokine induction in a murine model. We hypothesized that single-nucleotide polymorphisms (SNPs) in the CD43 gene region are associated with human tuberculosis (TB) susceptibility. We performed a case-population study in discovery (352 TB cases and 382 control subjects) and validation cohorts (339 TB cases and 376 control subjects). We examined whether 11 haplotype-tagging SNPs in the CD43 gene region were associated with tuberculous meningitis (TBM) and pulmonary TB (PTB) in Vietnam. Three SNPs from the CD43 gene region were associated with TB susceptibility with a genotypic model. The association fit a recessive genetic model and was greater for TBM than for PTB (for TBM: rs4788172, odds ratio [OR], 1.64; 95% confidence interval [CI], 1.04–2.59, rs17842268 [OR, 2.20; 95% CI, 1.29–3.76, and rs12596308 [OR, 2.38; 95% CI, 1.47–3.89]). Among TBM cases, rs17842268 was associated with decreased survival (hazard ratio, 2.7; 95% CI, 1.1–6.5; P = 0.011). In addition, rs12596308 and rs17842268 were associated with focal neurologic deficit at TBM presentation. Our data suggest that CD43 polymorphisms are associated with TB susceptibility, disease manifestations, and worse outcomes. To our knowledge, this is the first report that links CD43 genetic variants with susceptibility and outcome from a disease.
Keywords: tuberculosis, CD43, mortality, single-nucleotide polymorphism, tuberculous meningitis
Clinical Relevance
This is the first study on CD43 and tuberculosis in humans. We found that a common variation in CD43 that is associated with susceptibility to tuberculosis is also associated with increased mortality. This has potential for translational impact due to the high frequency of death and disability caused by tuberculous meningitis.
In 2011, tuberculosis (TB) caused over 1.4 million deaths, and there were 8.7 million new cases of TB disease (1). Tuberculous meningitis (TBM) is the most severe form of the disease, with mortality around 25% in HIV-uninfected and 60% in HIV-infected adults (2). The genetic determinants of susceptibility to the most severe forms of TB, such as TBM, and their pathogenesis are not well understood.
Studies of the host genetic susceptibility to TB encompass twin (3, 4), linkage, candidate gene association, and genome-wide association studies (5–12). We and others have identified associations between common polymorphisms in innate immunity genes and susceptibility to TB and clinical phenotypes (6, 8, 11, 13). Most of this work has focused on macrophage receptors that initiate phagocytosis and mediate specific host immune responses. Numerous macrophage receptors that recognize MTB have been described, including Toll-like receptors (TLR1, -2, -4, -6, -8, and -9) (14), nucleotide binding oligomerization domain protein 2 (NOD2) (15), RIG-I–Like receptors (STING), C-type lectin receptors (DC-SIGN and CLEC4E/Mincle) (16, 17), and CD43 (18–20). The details of how human genetic variation of receptors modulates mycobacterium TB (MTB) uptake and subsequent signaling events are largely unknown.
The gene for CD43 has two exons (21) encoding one 381-amino acid polypeptide (22) that functions as a cell surface sialoglycoprotein, abundantly expressed on monocytes and T cells. Although CD43 is associated in the development of several types of cancer (23–25), it is also involved in the immune response to several pathogens, such as Trypanosoma cruzi, HIV, and MTB (26–28). CD43 may bind specifically to Mycobacteria but not to other types of bacteria (18, 19, 29). Moreover, Hickey and colleagues described the MTB molecular chaperone Cpn60.2 as a mycobacterial surface ligand that interacts with CD43 to stabilize the interaction between the mycobacteria and the macrophage (30). CD43 has also been shown to regulate intracellular MTB growth and TNF secretion (19), reducing TNF secretion in response to infection and shifting macrophage’s cell death away from apoptosis to necrosis (20). Together, these findings indicate that CD43 binds Mycobacterial species and regulates macrophage signaling and mechanisms of cell death. However, it is not known if CD43, or its genetic variants, regulate susceptibility to human TB.
Using a case-population design, we investigated whether CD43 genetic polymorphisms are associated with susceptibility to TB and influence clinical presentation and outcome.
Materials and Methods
Study Population
TB cases were recruited from 1997 until 2008 from study sites in Ho Chi Minh City, Vietnam, as previously described (31–33). In brief, study subjects were recruited from Pham Ngoc Thach Hospital for Tuberculosis and the Hospital for Tropical Diseases. Umbilical cord blood samples were used as control samples. Cases were divided in discovery and validation cohorts. In the discovery cohort, “Definite TBM” cases and PTB cases recruited in 2003 and 2004 were included. The validation cohort included “Definite TBM” and “Probable TBM” cases as well as inpatient and outpatient cases with PTB recruited between 2006 and 2008 (see Table E1 in the online supplement). Approval for human study protocols was obtained from the human subjects review boards at the Hospital for Tropical Diseases and Pham Ngoc Thach hospitals, Health Services of Ho Chi Minh City, Hung Vuong Hospital, Oxford Tropical Research Ethics Committee, and the University of Washington.
Single-Nucleotide Polymorphism Selection and Assessment of Linkage Disequilibrium
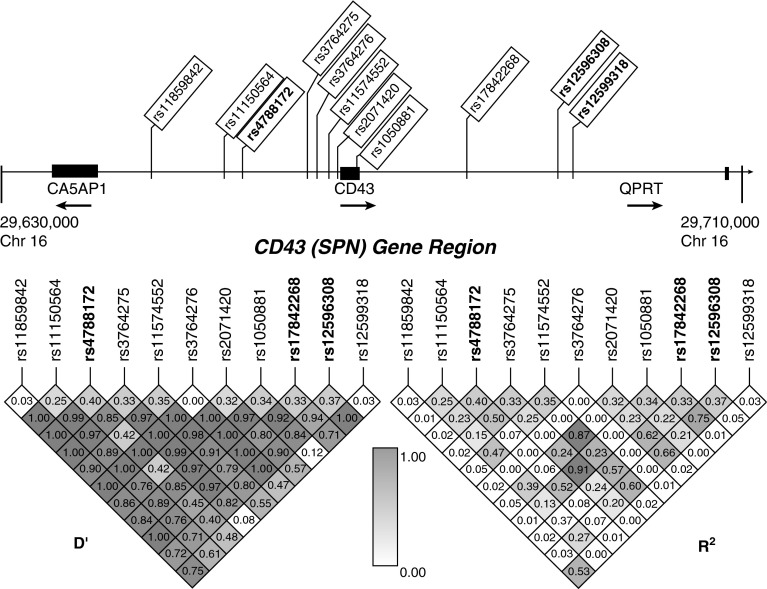
We selected haplotype-tagging single-nucleotide polymorphisms (SNPs) from the Han Chinese population using International HapMap Project data from the Genome Variation Server. We searched a region 13,000 kb upstream and downstream of the CD43 gene on the 16p11.2 chromosome for tagged SNPs using an R2 cutoff of 0.8 for linkage disequilibrium. We selected 11 SNPs in the CD43 region, which is located between the carbonic anhydrase VA pseudogene 1 (CA5AP1) and quinolinate phosphoribosyltransferase (QPRT) genes (Figure 1). Three SNPs were intronic (rs2071420, rs12596308, and rs12599318), one was in a synonymous coding region (rs1050881), and the rest were intergenic (rs376642725, rs376642727, rs11574552, rs11859842, rs11150564, rs4788172, and rs17842268).
Figure 1.
Linkage disequilibrium plot of CD43 gene region. Genomic positions of 11 single-nucleotide polymorphisms (SNPs) in the 5′ untranslated region and intronic regions of the CD43 gene. 2 exons are shown as black rectangles. D′ and R2 values were calculated from control subjects in the discovery cohort. Values are shown numerically and by shading based upon the legend in the middle. The minor allele frequency is shown below each corresponding SNP. SNPs in bold type had significant associations in this study. CA5AP1, carbonic anhydrase VA pseudogene 1; QPRT, quinolinate phosphoribosyltransferase.
Genomic Techniques
Genomic DNA was purified from peripheral blood samples using the QIAamp DNA blood kit (Qiagen). In the discovery cohort, subjects were genotyped using Sequenom, a chip-based, matrix-assisted laser desorption/ionization time-of-flight mass array technique as previously described (34). For genotyping in the validation cohort, we used a TaqMan Assay (Life Technologies). Cluster plots were visually inspected to ensure accurate genotyping calls. The call rate for each SNP exceeded 94% in the discovery cohort. All candidate SNPs were in Hardy-Weinberg equilibrium (P > 0.05) among control subjects according to a χ2 goodness-of-fit test and were further evaluated for association with TB.
Statistical Methods
To determine whether CD43 polymorphism frequencies in our discovery cohort were associated with TB in a genotypic model, we used Stata 11 software and the package “genass.” SNP rs17842268 was also genotyped in the validation cohort.
SNPs were investigated under additional genetic models (dominant, recessive, and heterozygous advantage) for association with the clinical subtypes of TB. We used logistic regression models to estimate associations of SNPs and characteristics of disease presentation: cranial nerve palsy, hemiplegia, focal neurologic deficit, TBM British Medical Research Council Grade, and clinical outcomes (death and neurologic disability). We used Cox regression models for survival analysis.
Results
CD43 Region SNPs Are Associated With Susceptibility to TB
To determine whether polymorphisms in the CD43 gene region were associated with susceptibility to TB, we examined a discovery cohort of 352 HIV-uninfected adult subjects with TB (182 PTB, 170 TBM) and 382 population control subjects. We also examined a validation cohort of 339 patients with TB (212 PTB, 127 TBM) and 376 control subjects. The clinical characteristics of these two cohorts have been described previously (31).
In our primary analysis, we examined whether 11 haplotype-tagging SNPs in the CD43 region were associated with all forms of TB in our discovery cohort. Using a genotypic model, we identified that 3 of the 11 SNPs were significantly associated with susceptibility to TB (rs12596308 [P = 0.003], rs17842268 [P = 0.004], and rs4788172 [P = 0.004]) (Table 1). An additional two SNPs had a trend toward statistically significant association (rs11574552 and rs1050881). All three SNPs remained significant after a Bonferroni correction for multiple comparisons (conservative adjusted threshold of P < 0.05/11 = 0.0045). The linkage disequilibrium patterns showed moderate to high linkage disequilibrium between rs17842268 and rs12596308 (R2 = 0.75) but not rs4788172 (R2 = 0.08 and 0.07, respectively) in the control population (Figure 1). We replicated these results in the validation cohort for SNP rs17842268 (genotypic model χ2 = 7.84; P = 0.02) (Table E2). We next considered whether these associations were confounded by population admixture. All subjects were unrelated, and more than 95% were of the Vietnamese Kinh ethnicity. We previously genotyped a panel of 24 control SNPs to look for evidence of admixture and found no significant differences in genotype frequencies between cases and control subjects (31). A principal component analysis by Khor and colleagues confirmed the genetic homogeneity of this population (35).
Table 1.
CD43 Polymorphism Frequencies in Control and Tuberculosis Groups
| SNP,* Group | Subjects with Genotype (n [%]) |
Genotypic Comparison |
HWE P Value | |||
|---|---|---|---|---|---|---|
| 00 | 01 | 11 | χ2 | P Value | ||
| rs11859842 T/C | ||||||
| Control | 364 (0.953) | 18 (0.047) | 0 (0) | 0.637 | ||
| All TB† | 347 (0.934) | 21 (0.061) | 2 (0.006) | 2.883 | 0.237 | |
| rs11150564 G/A | ||||||
| Control | 218 (0.578) | 137 (0.363) | 22 (0.058) | 0.938 | ||
| All TB | 196 (0.578) | 116 (0.342) | 27 (0.080) | 1.410 | 0.494 | |
| rs4788172 G/A | ||||||
| Control | 126 (0.335) | 187 (0.497) | 63 (0.168) | 0.650 | ||
| All TB | 122 (0.378) | 123 (0.381) | 78 (0.241) | 10.92 | 0.004 | |
| rs3764275 C/A | ||||||
| Control | 163 (0.429) | 176 (0.463) | 41 (0.108) | 0.523 | ||
| All TB | 161 (0.491) | 126 (0.384) | 41 (0.125) | 4.496 | 0.106 | |
| rs11574552 T/C | ||||||
| Control | 171 (0.451) | 165 (0.476) | 43 (0.113) | 0.738 | ||
| All TB | 143 (0.427) | 133 (0.397) | 59 (0.176) | 5.753 | 0.056 | |
| rs3764276 T/C | ||||||
| Control | 381 (0.997) | 1 (0.003) | 0 (0) | 0.980 | ||
| All TB | 348 (0.997) | 1 (0.003) | 0 (0) | 0.004 | 0.949 | |
| rs2071420 A/G | ||||||
| Control | 179 (0.469) | 165 (0.432) | 38 (0.099) | 0.998 | ||
| All TB | 154 (0.467) | 132 (0.400) | 44 (0.133) | 2.197 | 0.333 | |
| rs1050881 C/T | ||||||
| Control | 161 (0.429) | 172 (0.459) | 42 (0.112) | 0.698 | ||
| All TB | 146 (0.465) | 119 (0.379) | 49 (0.156) | 5.567 | 0.062 | |
| rs17842268 T/C | ||||||
| Control | 165 (0.440) | 176 (0.469) | 34 (0.091) | 0.181 | ||
| All TB | 161 (0.407) | 127 (0.371) | 54 (0.158) | 11.02 | 0.004 | |
| rs12596308 T/C | ||||||
| Control | 156 (0.413) | 180 (0.476) | 42 (0.111) | 0.354 | ||
| All TB | 137 (0.408) | 132 (0.393) | 67 (0.199) | 11.92 | 0.003 | |
| rs12599318 G/A | ||||||
| Control | 361 (0.953) | 18 (0.047) | 0 (0) | 0.636 | ||
| All TB | 315 (0.938) | 19 (0.057) | 2 (0.057) | 2.581 | 0.275 | |
Definition of abbreviations: HWE, Hardy Weinberg equilibrium P value in control subjects; SNP, single-nucteotide polymorphism; TB, tuberculosis.
SNPs are listed by reference SNP ID in genomic order on chromosome 16. 0, common allele; 1, minor allele.
All TB includes pulmonary tuberculosis and tuberculous meningitis cases from the discovery cohort.
Analysis of rs4788172, rs17842268, and rs12596308
We next performed several secondary analyses. The association of the tree significant SNPs (rs4788172, rs17842268, and rs12596308) with susceptibility to all cases of TB was consistent with a recessive model (genotype AA: odds ratio [OR], 1.58; 95% confidence interval [CI], 1.07–2.34; P = 0.015; genotype CC: OR, 1.88; 95% CI, 1.16–3.07; P = 0.006; and genotype TT: OR, 1.99; 95% CI, 1.29–3.10; P = 0.001, respectively) rather than a dominant, heterozygous advantage, or additive model (P = NS) (Table 2 and data not shown). When examining the two clinical phenotypes of TB, we found that the association was stronger with TBM than with PTB (P = 0.032, P = 0.003, and P < 0.001 versus P = 0.061, P = 0.088, and P = 0.049, respectively) (Table 2). These data indicate that three CD43 SNPs are associated with increased susceptibility to TB with a recessive model of inheritance and a stronger association with TBM.
Table 2.
CD43 Polymorphisms, Genetic Models, and Tuberculosis Phenotypes
| SNP* | Group | Subjects with Genotype (n [%]) |
Genotypic Model |
Recessive Model |
Dominant Model |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 00 | 01 | 11 | χ2 | P value | OR | 95% CI | P value | OR | 95% CI | P value | ||
| rs4788172 | Control | 126 (0.335) | 187 (0.497) | 63 (0.168) | ||||||||
| G/A | All TB | 122 (0.378) | 123 (0.381) | 78 (0.241) | 10.92 | 0.004 | 1.58 | 1.09–2.29 | 0.015 | 1.38 | 0.88–1.64 | 0.241 |
| PTB | 58 (0.341) | 72 (0.424) | 40 (0.235) | 4.21 | 0.122 | 1.53 | 0.98–2.39 | 0.061 | 0.02 | 0.70–1.51 | 0.889 | |
| TBM | 64 (0.418) | 51 (0.333) | 38 (0.248) | 12.32 | 0.002 | 1.64 | 1.04–2.59 | 0.032 | 2.80 | 0.97–2.10 | 0.094 | |
| rs17842268 | Control | 165 (0.440) | 176 (0.469) | 34 (0.091) | ||||||||
| T/C | All TB | 161 (0.471) | 127 (0.371) | 54 (0.158) | 11.02 | 0.004 | 1.88 | 1.34–2.63 | 0.006 | 0.68 | 0.94–1.36 | 0.409 |
| PTB | 85 (0.470) | 71 (0.392) | 25 (0.138) | 4.46 | 0.107 | 1.61 | 0.93–2.79 | 0.088 | 0.43 | 0.79–1.61 | 0.511 | |
| TBM | 76 (0.472) | 56 (0.348) | 29 (0.180) | 11.77 | 0.003 | 2.20 | 1.29–3.76 | 0.003 | 0.37 | 0.79–1.65 | 0.542 | |
| rs12596308 | Control | 156 (0.413) | 180 (0.476) | 42 (0.111) | ||||||||
| T/C | All TB | 137 (0.408) | 132 (0.393) | 67 (0.199) | 11.92 | 0.003 | 1.99 | 1.48–2.69 | 0.001 | 0.01 | 1.60–2.43 | 0.893 |
| PTB | 69 (0.394) | 76 (0.434) | 30 (0.171) | 3.90 | 0.143 | 1.65 | 1.00–2.75 | 0.049 | 0.17 | 0.64–1.34 | 0.682 | |
| TBM | 68 (0.422) | 56 (0.348) | 37 (0.230) | 15.13 | 0.001 | 2.39 | 1.47–3.89 | <0.001 | 1.04 | 0.72–1.51 | 0.835 | |
Definition of abbreviations: CI, confidence interval; OR, odds ratio; PTB, pulmonary tuberculosis; SNP, single-nucteotide polymorphism; TB, tuberculosis; TBM, tuberculous meningitis.
SNPs are listed by reference SNP ID. For OR calculations, each group was compared with the control group. 0, common allele; 1, minor frequency allele.
Haplotype Analysis of rs4788172, rs17842268, and rs12596308
We next constructed haplotypes of the SNPs that were significantly associated with TB (rs4788172, rs17842268, and rs12596308) to analyze whether there were additive associations using a different combination of the alleles (Table E3). We found one haplotype (001) that was associated with all types of TB combined (OR, 2.06; P = 0.04). In addition, we found that haplotypes 001 and 010 were associated with PTB (001: OR, 2.34; P = 0.03 and 010: OR, 4.31; P = 0.02). However, overall the haplotype analysis did not reveal that combinations of polymorphisms had a different pattern or magnitude of association with susceptibility to TB.
TBM Disease Severity in Individuals with rs17842268
Due to the stronger association with TBM, we next examined whether CD43 SNPs were associated with features of TBM disease severity, including meningitis grade, Glasgow coma scale, and focal neurologic deficit (Table 3). There was no association of SNP rs4788172 and the presence of TBM disease manifestations. Genotypes rs17842268 and rs12596308, which are in high linkage disequilibrium, were significantly associated with focal deficit (OR, 4.02; P = 0.004 and OR, 2.34; P = 0.05, respectively) (Table 3). This relationship was maintained after adjusting for age and gender. None of the SNPs was associated with TBM grade or Glasgow coma scale in the adjusted analysis. In addition, the SNPs were not associated with the median pretreatment leukocyte counts or protein in cerebrospinal fluid (data not shown).
Table 3.
CD43 Single-Nucleotide Polymorphisms and Tuberculous Meningitis Disease Presentation
| SNP | Unadjusted Model |
Adjusted for Age and Gender |
||||
|---|---|---|---|---|---|---|
| OR* | 95% CI | P Value | OR | 95% CI | P Value | |
| rs4788172 G/A, n = 147 | ||||||
| TBM grade | 1.49 | 1.04–2.12 | 0.03 | 1.19 | 0.81–1.77 | 0.37 |
| Glasgow coma scale | 1.74 | 1.17–2.60 | 0.01 | 1.53 | 0.99–2.37 | 0.06 |
| Focal deficit | 0.91 | 0.47–1.75 | 0.77 | 0.91 | 0.47–1.77 | 0.78 |
| rs17842268 T/C, n = 155 | ||||||
| TBM grade | 1.72 | 1.05–2.81 | 0.03 | 1.33 | 0.76–2.33 | 0.32 |
| Glasgow coma scale | 1.49 | 0.92–2.43 | 0.11 | 1.01 | 0.59–1.74 | 0.96 |
| Focal deficit | 4.02 | 1.58–10.2 | 0.004 | 4.46 | 1.71–11.6 | 0.002 |
| rs12596308 T/C, n = 100 | ||||||
| TBM grade | 1.65 | 1.02–2.68 | 0.04 | 1.39 | 0.77–2.33 | 0.31 |
| Glasgow coma scale | 1.46 | 0.91–2.36 | 0.12 | 0.99 | 0.58–1.71 | 0.98 |
| Focal deficit | 2.34 | 0.99–5.50 | 0.05 | 2.59 | 1.06–6.33 | 0.04 |
Definition of abbreviations: CI, confidence interval; OR, odds ratio; SNP, single-nucleotide polymorphism; TBM, tuberculous meningitis.
Odds ratio estimated following recessive model.
Together, these data suggest that there are likely two independent CD43 genetic loci associated with susceptibility to TB disease but different associations with TBM disease severity and survival.
CD43 rs17842268 Is Associated with Decreased Survival
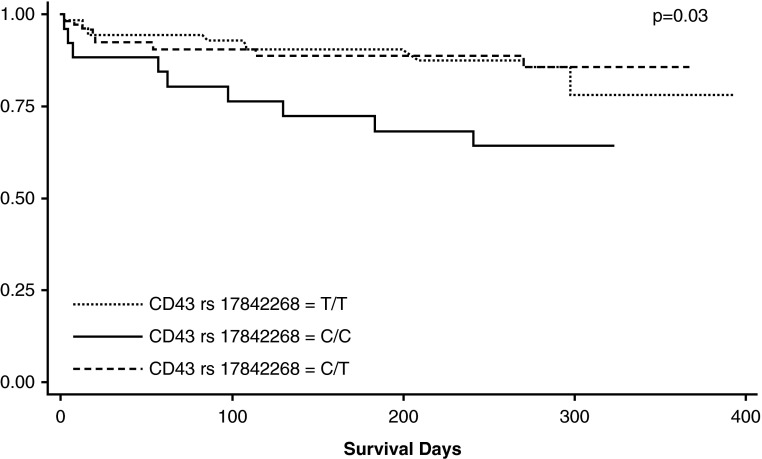
We examined whether any of the SNPs were associated with survival. We found that among TBM cases, rs17842268 genotype CC was significantly associated with decreased survival compared with genotype TT and CT, fitting a recessive model (hazard ratio, 2.82; 95% CI, 1.3–6.3; P = 0.01) (Figure 2). In contrast, SNPs rs4788172 and rs12596308 were not associated with decreased survival. These data suggest that SNP rs17842268 is associated with more severe presentation and survival in patients with TBM.
Figure 2.
CD43 SNP and survival estimates in patients with tuberculosis meningitis. A Kaplan-Meier curve shows the association between rs17842268 genotype CC and decreased mortality among 156 tuberculous meningitis cases. The log-rank P value is 0.028 for the genotypic model and 0.0079 for the recessive model.
Discussion
The primary finding of our study is that CD43 polymorphisms rs4788172, rs12596308, and rs17842268 are associated with susceptibility to TB, and to TBM in particular, in Vietnam. In addition, rs12596308 and rs17842268 were associated with TBM disease severity at presentation, and rs17842268 was associated with death. To our knowledge, this is the first report demonstrating an association of common CD43 SNPs with susceptibility to, and outcome from, a human infectious disease.
In addition to overall susceptibility, one CD43 SNP was associated with disease severity and mortality. To our knowledge, only one gene (LTA4H) has a polymorphism that has been associated with TBM mortality (11). Among the limited number of studies evaluating host genetic susceptibility to TBM, our group has previously found several innate immune gene variants (TLR2, TIRAP/Mal, and LTA4H) that are associated with susceptibility to TBM (6, 8, 11). TLR2 and TIRAP regulate MTB-induced activation of NF-κB and secretion of proinflammatory cytokines, including TNF. CD43 may similarly affect TB disease manifestations by regulating proinflammatory cascades. Leukotriene (LT)A4H encodes LTA4 hydrolase, an enzyme that regulates synthesis of LTB4, a potent chemoattractant and proinflammatory eicosanoid that induces TNF production. CD43 and LTA4H regulate TNF levels, a common pathway that could lead to increased mortality from TB due to adverse effects from inflammatory sequelae. Although there is some overlap of the functional effects of CD43, TLR2, TIRAP, and LTA4H on proinflammatory pathways, the details and regulatory mechanisms of how the expression and function of each of these molecules interact are unknown.
The mechanism by which CD43 SNPs regulates human susceptibility to TB is unknown. However, CD43 has a significant role controlling the growth of MTB demonstrated not only within macrophages but also in CD43-deficient mice (18, 19). This process appears to occur via two different mechanisms: binding and uptake of mycobacteria and regulation of TNF production. TNF restricts mycobacterial growth through activation of macrophages, regulation of granuloma formation, Th1 cytokine expression (36), and the initiation of apoptosis. MTB-stimulated Cd43−/− macrophages had decreased TNF production and showed lower levels of Caspase-3 mediated apoptosis in comparison to wild-type cells. By adding recombinant TNF to Cd43−/− macrophages, apoptosis levels increased, and growth of MTB was controlled to levels seen in CD43-expressing cells (20). In addition to decreased production of TNF, Cd43−/− macrophages also had decreased production of proinflammatory cytokines, such as IL-12 (which is involved in TH1 T cell differentiation and restricting mycobacterial growth in vivo in mice) (37) and IL-6 (which is involved in the acute response to MTB) (38). These findings provide evidence that CD43 regulates the immune response to MTB not only through direct binding of the mycobacteria but also by regulating of the production of proinflammatory and T cell–polarizing cytokines.
In addition to innate immune mechanisms, previous studies suggest that CD43 is involved primarily in three main functions: (1) cellular adhesion due to structural characteristics of the extracellular domain, which has O-glycosylated mucin-like aminoacids and sialic acid residues (39, 40); (2) T cell activation and migration via cleavage of the CD43 extracellular domain, which interacts with endogenous lectin coreceptors (41) and proteins of the cytoskeleton (42) (CD43 uses E-selectin as a ligand on endothelial cells to regulate migration to sites of inflammation [41, 43]); and (3) CD43 regulates T cell trafficking to lymph nodes via phosphorylation of a specific serine residue in the CD43 intracellular domain (42, 44, 45). Moreover, CD43 forms clusters via thiol group oxidation on lymphocytes. These clusters are recognized by macrophages as early apoptotic cells (46). In a mouse model of infection with lymphocytic choriomeningitis virus, CD43 regulated generation of virus-specific CD8 T cells as well as trafficking of T cells to the central nervous system (45). It is possible that CD43 genetic variants modulate susceptibility to TB in humans by regulating generation and trafficking of MTB-specific T cell responses in the brain.
Our study has several limitations. First, the association findings may not be due to genetic variants within the CD43 gene. The haplotype-tagging SNPs span a region that includes two flanking genes: (1) QPRT, which encodes a key enzyme in catabolism of quinolate, a potent neurotoxin linked to neurodegenerative disorders (47), and (2) CA5AP1, which encodes a liver enzyme that catalyzes rapid conversion of carbon dioxide and water to bicarbonate and protons (48). Although we cannot exclude these as candidate genes, there is substantial evidence that CD43 plays a crucial role in the host immune response to TB, whereas the functions of CA5AP1 and QPRT are primarily related to metabolic pathways. Therefore, it is likely that these three SNPs of interest are in linkage disequilibrium with functional SNPs localized in the CD43 gene. Second, we chose cord-blood subjects as controls to be able to compare background population genotype frequencies with the adult cases. These cord blood subjects may develop TB later in life, which may represent a misclassification of controls, leading to an underestimation of the genetic risk of SNPs. We expect that the misclassification rate would be low given that only 10% of individuals progress from latent to active disease and that an even lower number develop meningitis (6, 31). Lastly, population substructure acts as a confounder in candidate gene association studies. Our study population, the Vietnamese Kinh, is a relatively homogenous population in Southeast Asia in which we previously found no evidence of population stratification using control genomic SNPs (34).
In conclusion, we found that three common genetic variants localized within the CD43 gene region are associated with susceptibility to developing TB. Furthermore, among patients with TBM, individuals with genotype CC for rs17842268 had decreased survival. These results support a role for human CD43 in the immunopathogenesis of TB. Further studies are needed to dissect the mechanism by which CD43 increases susceptibility to TB disease.
Acknowledgments
Acknowledgments
The authors thank the clinical staff from the Hospital of Tropical Diseases and Pham Ngoc Thach Hospital who initially diagnosed and studied the patients with TBM and PTB; Dr. Nguyen Thi Hieu from Hung Vuong Obstetric Hospital Vietnam, Dr. Tran Tinh Hien from the Hospital for Tropical Diseases Vietnam, and all the Vietnamese doctors and patients who participated in this study; Drs. Alan Aderem and Marta Janer from the Institute for Systems Biology for advice and Sarah Li for technical assistance; and Glenna Peterson, Rick Wells, Randi Simmons, and Dr. Chetan Seshadri from the University of Washington for assistance.
Footnotes
This study was supported by a grant from the Firland Foundation (M.C.), by National Institute of Allergy and Infectious Diseases/National Institutes of Health grant K24AI089794 (T.R.H.), by the Burroughs Wellcome Foundation (T.R.H.), and by the Wellcome Trust of Great Britain (J.J.F.).
Author Contributions: Initial conception and design of clinical cohort and sample collection: S.D., J.F., M. Caws, N.D.B., N.N.L., T.T.H.C., N.T.T., and G.E.T. Conception and design of presented experiments: M. Campo, T.R.H., S.D., J.F., G.E.T., and A.K.R. Analysis and interpretation: M. Campo, D.J.H., and T.R.H. Drafting of the manuscript: M. Campo and T.R.H. Edits and approval of final manuscript: M. Campo, D.J.H., A.K.R., S.D., J.F., M. Caws, N.D.B., N.N.L., T.T.H.C., N.T.T., G.E.T., and T.R.H.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0114OC on July 31, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thwaites GE, Simmons CP, Than Ha Quyen N, Thi Hong Chau T, Phuong Mai P, Thi Dung N, Hoan Phu N, White NP, Tinh Hien T, Farrar JJ. Pathophysiology and prognosis in Vietnamese adults with tuberculous meningitis. J Infect Dis. 2003;188:1105–1115. doi: 10.1086/378642. [DOI] [PubMed] [Google Scholar]
- 3.Comstock GW. Tuberculosis in twins: a re-analysis of the Prophit survey. Am Rev Respir Dis. 1978;117:621–624. doi: 10.1164/arrd.1978.117.4.621. [DOI] [PubMed] [Google Scholar]
- 4.van der Eijk EA, van de Vosse E, Vandenbroucke JP, van Dissel JT. Heredity versus environment in tuberculosis in twins: the 1950s UK Prophit Survey Simonds and Comstock revisited. Am J Respir Crit Care Med. 2007;176:1281–1288. doi: 10.1164/rccm.200703-435OC. [DOI] [PubMed] [Google Scholar]
- 5.Berrington WR, Hawn TR. Mycobacterium tuberculosis, macrophages, and the innate immune response: does common variation matter? Immunol Rev. 2007;219:167–186. doi: 10.1111/j.1600-065X.2007.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawn TR, Dunstan SJ, Thwaites GE, Simmons CP, Thuong NT, Lan NT, Quy HT, Chau TT, Hieu NT, Rodrigues S, et al. A polymorphism in toll-interleukin 1 receptor domain containing adaptor protein is associated with susceptibility to meningeal tuberculosis. J Infect Dis. 2006;194:1127–1134. doi: 10.1086/507907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Png E, Alisjahbana B, Sahiratmadja E, Marzuki S, Nelwan R, Balabanova Y, Nikolayevskyy V, Drobniewski F, Nejentsev S, Adnan I, et al. A genome wide association study of pulmonary tuberculosis susceptibility in Indonesians. BMC Med Genet. 2012;13:5. doi: 10.1186/1471-2350-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thuong NT, Hawn TR, Thwaites GE, Chau TT, Lan NT, Quy HT, Hieu NT, Aderem A, Hien TT, Farrar JJ, et al. A polymorphism in human tlr2 is associated with increased susceptibility to tuberculous meningitis. Genes Immun. 2007;8:422–428. doi: 10.1038/sj.gene.6364405. [DOI] [PubMed] [Google Scholar]
- 9.Thye T, Owusu-Dabo E, Vannberg FO, van Crevel R, Curtis J, Sahiratmadja E, Balabanova Y, Ehmen C, Muntau B, Ruge G, et al. Common variants at 11p13 are associated with susceptibility to tuberculosis. Nat Genet. 2012;44:257–259. doi: 10.1038/ng.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thye T, Vannberg FO, Wong SH, Owusu-Dabo E, Osei I, Gyapong J, Sirugo G, Sisay-Joof F, Enimil A, Chinbuah MA, et al. Genome-wide association analyses identifies a susceptibility locus for tuberculosis on chromosome 18q11.2. Nat Genet. 2010;42:739–741. doi: 10.1038/ng.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tobin DM, Vary JC, Jr, Ray JP, Walsh GS, Dunstan SJ, Bang ND, Hagge DA, Khadge S, King MC, Hawn TR, et al. The LTA4H locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell. 2010;140:717–730. doi: 10.1016/j.cell.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vannberg FO, Chapman SJ, Hill AV. Human genetic susceptibility to intracellular pathogens. Immunol Rev. 2011;240:105–116. doi: 10.1111/j.1600-065X.2010.00996.x. [DOI] [PubMed] [Google Scholar]
- 13.Azad AK, Sadee W, Schlesinger LS. Innate immune gene polymorphisms in tuberculosis. Infect Immun. 2012;80:3343–3359. doi: 10.1128/IAI.00443-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawn TR, Misch EA, Dunstan SJ, Thwaites GE, Lan NT, Quy HT, Chau TT, Rodrigues S, Nachman A, Janer M, et al. A common human tlr1 polymorphism regulates the innate immune response to lipopeptides. Eur J Immunol. 2007;37:2280–2289. doi: 10.1002/eji.200737034. [DOI] [PubMed] [Google Scholar]
- 15.Juarez E, Carranza C, Hernandez-Sanchez F, Leon-Contreras JC, Hernandez-Pando R, Escobedo D, Torres M, Sada E. Nod2 enhances the innate response of alveolar macrophages to Mycobacterium tuberculosis in humans. Eur J Immunol. 2012;42:880–889. doi: 10.1002/eji.201142105. [DOI] [PubMed] [Google Scholar]
- 16.Ernst JD, Hanekom W, Hawn T, Kampmann B, Rengarajan J. Meeting report: the international conference on human immunity to tuberculosis. Tuberculosis (Edinb) 2012;92:440–444. doi: 10.1016/j.tube.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Ottenhoff TH. The knowns and unknowns of the immunopathogenesis of tuberculosis. Int J Tuberc Lung Dis. 2012;16:1424–1432. doi: 10.5588/ijtld.12.0479. [DOI] [PubMed] [Google Scholar]
- 18.Fratazzi C, Manjunath N, Arbeit RD, Carini C, Gerken TA, Ardman B, Remold-O'Donnell E, Remold HG. A macrophage invasion mechanism for mycobacteria implicating the extracellular domain of cd43. J Exp Med. 2000;192:183–192. doi: 10.1084/jem.192.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Randhawa AK. Ziltener HJ, Merzaban JS, Stokes RW. Cd43 is required for optimal growth inhibition of Mycobacterium tuberculosis in macrophages and in mice. J Immunol. 2005;175:1805–1812. doi: 10.4049/jimmunol.175.3.1805. [DOI] [PubMed] [Google Scholar]
- 20.Randhawa AK, Ziltener HJ, Stokes RW. Cd43 controls the intracellular growth of Mycobacterium tuberculosis through the induction of tnf-alpha-mediated apoptosis. Cell Microbiol. 2008;10:2105–2117. doi: 10.1111/j.1462-5822.2008.01194.x. [DOI] [PubMed] [Google Scholar]
- 21.Shelley CS, Remold-O'Donnell E, Rosen FS, Whitehead AS. Structure of the human sialophorin (cd43) gene: identification of features atypical of genes encoding integral membrane proteins. Biochem J. 1990;270:569–576. doi: 10.1042/bj2700569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Laurentiis A, Gaspari M, Palmieri C, Falcone C, Iaccino E, Fiume G, Massa O, Masullo M, Tuccillo FM, Roveda L, et al. Mass spectrometry-based identification of the tumor antigen un1 as the transmembrane cd43 sialoglycoprotein. Mol Cell Proteomics. 2011;10:M111007898. doi: 10.1074/mcp.M111.007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu Q, Cash SE, Andersen JJ, Kennedy CR, Oldenburg DG, Zander VB, Foley GR, Simon Shelley C. Cd43 in the nucleus and cytoplasm of lung cancer is a potential therapeutic target. Int J Cancer. 2013;132:1761–1770. doi: 10.1002/ijc.27873. [DOI] [PubMed] [Google Scholar]
- 24.Kadaja L, Laos S, Maimets T. Overexpression of leukocyte marker cd43 causes activation of the tumor suppressor proteins p53 and arf. Oncogene. 2004;23:2523–2530. doi: 10.1038/sj.onc.1207359. [DOI] [PubMed] [Google Scholar]
- 25.Park WS, Kim HJ, Lee GK, Son HS, Bae Y. Anti-adhesive functions of cd43 expressed on colon carcinoma cells through the modulation of integrins. Exp Mol Pathol. 2012;92:82–89. doi: 10.1016/j.yexmp.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Giordanengo V, Limouse M, Desroys du Roure L, Cottalorda J, Doglio A, Passeron A, Fuzibet JG, Lefebvre JC. Autoantibodies directed against cd43 molecules with an altered glycosylation status on human immunodeficiency virus type 1 (HIV-1)-infected cem cells are found in all HIV-1+ individuals. Blood. 1995;86:2302–2311. [PubMed] [Google Scholar]
- 27.Lefebvre JC, Giordanengo V, Limouse M, Doglio A, Cucchiarini M, Monpoux F, Mariani R, Peyron JF. Altered glycosylation of leukosialin, cd43, in HIV-1-infected cells of the cem line. J Exp Med. 1994;180:1609–1617. doi: 10.1084/jem.180.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Todeschini AR, Nunes MP, Pires RS, Lopes MF, Previato JO, Mendonca-Previato L, DosReis GA. Costimulation of host t lymphocytes by a trypanosomal trans-sialidase: involvement of cd43 signaling. J Immunol. 2002;168:5192–5198. doi: 10.4049/jimmunol.168.10.5192. [DOI] [PubMed] [Google Scholar]
- 29.Urano-Tashiro Y, Yajima A, Takashima E, Takahashi Y, Konishi K. Binding of the streptococcus gordonii dl1 surface protein hsa to the host cell membrane glycoproteins cd11b, cd43, and cd50. Infect Immun. 2008;76:4686–4691. doi: 10.1128/IAI.00238-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hickey TB, Ziltener HJ, Speert DP, Stokes RW. Mycobacterium tuberculosis employs cpn60.2 as an adhesin that binds cd43 on the macrophage surface. Cell Microbiol. 2010;12:1634–1647. doi: 10.1111/j.1462-5822.2010.01496.x. [DOI] [PubMed] [Google Scholar]
- 31.Horne DJ, Randhawa AK, Chau TT, Bang ND, Yen NT, Farrar JJ, Dunstan SJ, Hawn TR. Common polymorphisms in the pkp3-sigirr-tmem16j gene region are associated with susceptibility to tuberculosis. J Infect Dis. 2012;205:586–594. doi: 10.1093/infdis/jir785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah JA, Vary JC, Chau TT, Bang ND, Yen NT, Farrar JJ, Dunstan SJ, Hawn TR. Human tollip regulates tlr2 and tlr4 signaling and its polymorphisms are associated with susceptibility to tuberculosis. J Immunol. 2012;189:1737–1746. doi: 10.4049/jimmunol.1103541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thwaites GE, Nguyen DB, Nguyen HD, Hoang TQ, Do TT, Nguyen TC, Nguyen QH, Nguyen TT, Nguyen NH, Nguyen TN, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351:1741–1751. doi: 10.1056/NEJMoa040573. [DOI] [PubMed] [Google Scholar]
- 34.Storm N, Darnhofer-Patel B, van den Boom D, Rodi CP. Maldi-tof mass spectrometry-based snp genotyping. Methods Mol Biol. 2003;212:241–262. doi: 10.1385/1-59259-327-5:241. [DOI] [PubMed] [Google Scholar]
- 35.Khor CC, Chau TN, Pang J, Davila S, Long HT, Ong RT, Dunstan SJ, Wills B, Farrar J, Van Tram T, et al. Genome-wide association study identifies susceptibility loci for dengue shock syndrome at micb and plce1. Nat Genet. 2011;43:1139–1141. doi: 10.1038/ng.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flynn JL, Chan J. Tuberculosis: latency and reactivation. Infect Immun. 2001;69:4195–4201. doi: 10.1128/IAI.69.7.4195-4201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper AM, Roberts AD, Rhoades ER, Callahan JE, Getzy DM, Orme IM. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology. 1995;84:423–432. [PMC free article] [PubMed] [Google Scholar]
- 38.Saunders BM, Frank AA, Orme IM, Cooper AM. Interleukin-6 induces early gamma interferon production in the infected lung but is not required for generation of specific immunity to Mycobacterium tuberculosis infection. Infect Immun. 2000;68:3322–3326. doi: 10.1128/iai.68.6.3322-3326.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ostberg JR, Barth RK, Frelinger JG. The Roman god Janus: a paradigm for the function of cd43. Immunol Today. 1998;19:546–550. doi: 10.1016/s0167-5699(98)01343-7. [DOI] [PubMed] [Google Scholar]
- 40.Ardman B, Sikorski MA, Staunton DE. Cd43 interferes with t-lymphocyte adhesion. Proc Natl Acad Sci USA. 1992;89:5001–5005. doi: 10.1073/pnas.89.11.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsumoto M, Shigeta A, Furukawa Y, Tanaka T, Miyasaka M, Hirata T. Cd43 collaborates with p-selectin glycoprotein ligand-1 to mediate e-selectin-dependent t cell migration into inflamed skin. J Immunol. 2007;178:2499–2506. doi: 10.4049/jimmunol.178.4.2499. [DOI] [PubMed] [Google Scholar]
- 42.Cannon JL, Mody PD, Blaine KM, Chen EJ, Nelson AD, Sayles LJ, Moore TV, Clay BS, Dulin NO, Shilling RA, et al. Cd43 interaction with ezrin-radixin-moesin (erm) proteins regulates t-cell trafficking and cd43 phosphorylation. Mol Biol Cell. 2011;22:954–963. doi: 10.1091/mbc.E10-07-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsumoto M, Atarashi K, Umemoto E, Furukawa Y, Shigeta A, Miyasaka M, Hirata T. Cd43 functions as a ligand for e-selectin on activated T cells. J Immunol. 2005;175:8042–8050. doi: 10.4049/jimmunol.175.12.8042. [DOI] [PubMed] [Google Scholar]
- 44.Mody PD, Cannon JL, Bandukwala HS, Blaine KM, Schilling AB, Swier K, Sperling AI. Signaling through cd43 regulates cd4 t-cell trafficking. Blood. 2007;110:2974–2982. doi: 10.1182/blood-2007-01-065276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Onami TM, Harrington LE, Williams MA, Galvan M, Larsen CP, Pearson TC, Manjunath N, Baum LG, Pearce BD, Ahmed R. Dynamic regulation of t cell immunity by cd43. J Immunol. 2002;168:6022–6031. doi: 10.4049/jimmunol.168.12.6022. [DOI] [PubMed] [Google Scholar]
- 46.Eda S, Yamanaka M, Beppu M. Carbohydrate-mediated phagocytic recognition of early apoptotic cells undergoing transient capping of cd43 glycoprotein. J Biol Chem. 2004;279:5967–5974. doi: 10.1074/jbc.M310805200. [DOI] [PubMed] [Google Scholar]
- 47.Foster AC, Whetsell WO, Jr, Bird ED, Schwarcz R. Quinolinic acid phosphoribosyltransferase in human and rat brain: activity in huntington's disease and in quinolinate-lesioned rat striatum. Brain Res. 1985;336:207–214. doi: 10.1016/0006-8993(85)90647-x. [DOI] [PubMed] [Google Scholar]
- 48.Nagao Y, Batanian JR, Clemente MF, Sly WS. Genomic organization of the human gene (ca5) and pseudogene for mitochondrial carbonic anhydrase v and their localization to chromosomes 16q and 16p. Genomics. 1995;28:477–484. doi: 10.1006/geno.1995.1177. [DOI] [PubMed] [Google Scholar]