Abstract
Our previous studies showed that cigarette smokers who are exposed to wood smoke (WS) are at an increased risk for chronic bronchitis and reduced lung function. The present study was undertaken to determine the mechanisms for WS-induced adverse effects. We studied the effect of WS exposure using four cohorts of mice. C57Bl/6 mice were exposed for 4 or 12 weeks to filtered air, to 10 mg/m3 WS for 2 h/d, to 250 mg/m3 cigarette smoke (CS) for 6 h/d, or to CS followed by WS (CW). Inflammation was absent in the filtered air and WS groups, but enhanced by twofold in the bronchoalveolar lavage of the CW compared with CS group as measured by neutrophil numbers and levels of the neutrophil chemoattractant, keratinocyte-derived chemokine. The levels of the anti-inflammatory lipoxin, lipoxin A4, were reduced by threefold along with cyclo-oxygenase (COX)-2 and microsomal prostaglandin E synthase (mPGES)-1 in airway epithelial cells and PGE2 levels in the bronchoalveolar lavage of CW compared with CS mice. We replicated, in primary human airway epithelial cells, the changes observed in mice. Immunoprecipitations showed that WS blocked the interaction of aryl hydrocarbon receptor (AHR) with AHR nuclear transporter to reduce expression of COX-2 and mPGES-1 by increasing expression of AHR repressor (AHRR). Collectively, these studies show that exposure to low concentrations of WS enhanced CS-induced inflammation by inducing AHRR expression to suppress AHR, COX-2, and mPGES-1 expression, and levels of PGE2 and lipoxin A4. Therefore, AHRR is a potential therapeutic target for WS-associated exacerbations of CS-induced inflammation.
Keywords: arachidonic acid pathway, air pollution, neutrophilic inflammation, exacerbation, lipoxin
Clinical Relevance
Recently, the negative effects of low-level wood smoke (WS) exposure on health outcomes of cigarette smokers were reported. However, the mechanisms of this adverse effect of WS are not understood. This study identified a protein called aryl hydrocarbon receptor repressor (AHRR) that is induced by WS exposure. AHRR suppresses the anti-inflammatory action of the arachidonic acid pathway. WS-induced expression of AHRR suppresses expression of cyclo-oxygenase-2 and microsomal prostaglandin E synthase-1 and thereby enhances inflammation. Therefore, AHRR may be a good target for reversing the adverse effects of WS exposure in cigarette smokers.
Clinical studies have demonstrated that exposure to wood smoke (WS) causes chronic obstructive pulmonary disease (COPD), acute respiratory tract infection, and lung cancer (1). Although many studies have focused on high WS concentrations as those found primarily in developing countries (2), recent studies in Europe (3), Canada (4), and in the United States (5) have documented that exposure to even low levels of WS affects pulmonary function in susceptible individuals. In a population of cigarette smokers in the United States, we found that heavy smokers are at increased risk for chronic bronchitis and reduced lung function when exposed to WS (5), similar to those with pre-existing cardiopulmonary conditions (6). However, the mechanisms for the enhancement of CS-induced inflammatory responses by WS remain unknown.
The arachidonic acid (AA) metabolites of the cyclo-oxygenase (COX) and lipoxygenase pathway are among the mediators that play an important role in the initial inflammatory responses to CS (7). CS induces COX-2 expression that leads to prostaglandin (PG) synthesis in epithelial cells, lymphocytes, and fibroblasts (8). Although COX-2 is important in the initial inflammatory response, and its pharmacological inhibition, such as by celecoxib, reduces the development of an emphysema-like phenotype in vivo (9), the enzyme and its derivatives are more recently shown to play a major role in the resolution of both acute and chronic inflammation (10, 11). Selective pharmacologic inhibition of COX-2 delays resolution of peritoneal inflammation (12, 13), acute lung injury (14), perpetuates chronic inflammation in infectious and autoimmune arthritis (15, 16), exacerbates inflammation of the colon (17), and enhances chronic inflammation in a carrageenan-induced paw injury (18). COX-2–derived PGE2 can induce 15-lipoxygenase (15-LO) expression to promote lipoxin A4 (LXA4) biosynthesis, which mediates the resolution of inflammatory responses (13). Because both COX-2 and PGE2 are transcriptionally regulated by aryl hydrocarbon receptor (AHR), this receptor can play an important role in activating the resolution of inflammation (19).
CS-induced COPD is initiated by inflammation caused by the airway epithelium producing a number of cytokines and chemokines, and recruitment of macrophages and neutrophils to the airways (20). Cigarette smoking combined with viral infections or exposure to increased levels of airway pollutants can cause COPD exacerbations. However, although the mechanism of virus-induced augmentation of CS-induced inflammation has been investigated (21), the mechanisms for WS-induced enhancement of CS-induced inflammation have not been explored.
Therefore, the purpose of the present study was to elucidate the cellular and molecular mechanisms by which WS enhances CS-induced airway inflammation. We show that WS exposure suppresses CS-induced COX-2 as well as anti-inflammatory lipid mediators, such as LXA4. WS blocked AHR’s suppression of inflammation by inducing expression of an AHR repressor (AHRR) that competes for the interaction between AHR and a nuclear transporter.
Materials and Methods
Animals
Male-specific, pathogen-free, wild-type C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and exposed 5 d/wk to filtered air (FA), cigarette smoke (CS) 6 h/d at 250 mg/m3 total particulate matter (PM), WS for 2 h/d at 10 mg/m3 total PM, or CS for 6 hours followed by WS for 2 hours (CW) for 4 and 12 weeks. All studies were preapproved by the Institutional Animal Care and Use Committee and the Environmental Safety and Health department.
Cell Culture
Two immortalized human airway epithelial cells (HAECs), N1 and N3, were provided by S. Randell (University of North Carolina Chapel Hill, Chapel Hill, NC) (22). Primary HAECs from several donors and N1 and N3 cells were maintained in bronchial epithelial growth medium BEBM (Lonza, Walkersville, MD) supplemented with growth factors (BEGM Singlequots; Lonza). Cells were cultured to 60–70% confluency before treatment with various concentrations of CS and/or WS extracts for different periods of time.
Cytokine/Chemokine Detection in Bronchoalveolar Lavage Fluid
Lungs of mice were lavaged three times with 0.5 ml of PBS via the tracheal tube, and bronchoalveolar lavage fluid (BALF) was stored at −80°C until use. A standard multiplex assay kit (Lincoplex panel; Linco Research, Inc., St. Charles, MO) was used with the Luminex Flowmetrix system (Luminex, Austin, TX) to determine the levels of cytokines/chemokines IL-1β, IL-6, TNF-α, keratinocyte-derived chemokine (KC), vascular endothelial growth factor, and IFN-γ. PGE2 and LXA4 in the BALF were quantitated by ELISA according to the manufacturer’s instructions (Neogen, Lansing, MI).
Quantitative RT-PCR
Total RNA from cultured cells and mouse lung tissues was extracted and analyzed as described previously (23).
Retroviral Silencing Using Short Hairpin RNA
Retroviral silencing vector encoding for AHRR short hairpin RNA (shRNA) and control (Ctrl) vector were used. AHRR-specific shRNA plasmids and control plasmids (OriGene Technologies, Rockville, MD) were packaged into retroviral particles using Phoenix cells as specified by the manufacturer’s instructions. The harvested virus was then used to infect HAECs and to generate stable HAECs expressing the AHRR or Ctrl shRNAs by puromycin selection of transfected cells according to the manufacturer’s instructions. The knockdown efficiency of the shRNA in HAECs was confirmed by both quantitative PCR and Western blot analyses.
Immunoprecipitation and Western Blot Analysis
Total protein lysates or the cytosolic and nuclear extracts were prepared, and protein was analyzed by Western blotting as described previously (23). For immunoprecipitation using the crosslink immunoprecipitation kit (Thermo Fisher Scientific, Waltham, MA), cells were rinsed twice with cold PBS, scraped into cold PBS plus protease inhibitors, and analyzed per the manufacturer’s instructions.
Immunofluorescence
Immunofluorescent staining and image analysis of HAECs and lung tissues from mice were performed as described previously (24).
Statistical Analysis
Grouped results from at least three different experiments were expressed as means (± SEM). Differences among groups were examined by ANOVA and t tests using Prism statistical analysis software (GraphPad Software Inc., La Jolla, CA) and by application of Tukey’s test for multiple comparisons. Differences were considered significant at a P value less than 0.05.
Results
WS Exacerbates CS-Induced Inflammation in Mice
Although the WS concentrations used did not affect body weight gain (see Figure E1A in the online supplement) or the number of inflammatory cells in the BALF at 4 weeks (Figures 1A–1D) or 12 weeks (Figures E1B and E1C) of exposure, these WS concentrations, when combined with CS, synergistically increased neutrophil counts compared with CS mice both at 4 weeks (Figure 1C) and 12 weeks (Figure 1D) of exposure. Histological examination of lung tissue sections showed that the number of macrophages in CS, WS, and CW mice was significantly increased compared with FA controls, with the CW group displaying more macrophages per alveolus than the CS group at 12 weeks of exposure (Figure E1D). In addition, macrophage activation was apparent, as displayed by increased size and multinucleation in mice exposed to CS or CW, with CW having a significantly higher number of nuclei per cell compared with the CS group (Figures 1E and E1E).
Figure 1.
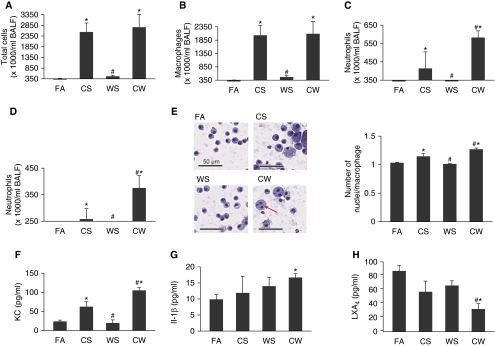
Wood smoke (WS) exacerbates cigarette smoke (CS)-induced inflammation in mice. Total (A) and differential cell counts of macrophages (B), neutrophils at 4 weeks (C), and 12 weeks (D) of exposure, in mice exposed to filtered air (FA), CS, WS, or CS followed by WS (CW). (E) Changes in macrophage phenotypes in the cytospins of bronchoalveolar lavage (BAL) cell pellets from mice exposed to FA, CS, WS, or CW at 4 weeks. Increased size and multinucleation of macrophages in CS and CW mice (left) and higher number of nuclei per macrophage in CW than CS mice are observed. Multinucleated cells are marked with a red arrow. Scale bars, 50 μm. BAL fluid (BALF) levels of the proinflammatory mediators, keratinocyte-derived chemokine (KC) after 12 weeks (F), IL-1β after 12 weeks (G), and the anti-inflammatory lipid mediator, lipoxin A4 (LXA4) after 4 weeks (H) of exposure of mice to FA, CS, WS, or CW and as measured by ELISA (n = 9 mice for each treatment). Data are presented as mean ± SEM. *P < 0.05 compared with FA; #P < 0.05 compared with CS.
Consistent with the higher neutrophilic inflammation, CW mice had higher levels of the neutrophil chemoattractant chemokine, KC, than CS animals, and had the highest level of IL-1β at 12 weeks of exposure (Figures 1F and 1G). The vascular endothelial growth factor concentrations were also elevated in the BALF from CS and CW mice (Figure E1F), although no differences were observed between the two groups; LXA4 levels were significantly lower in CW mice compared with all other groups at 4 weeks (Figure 1H) and lower, but not statistically so, at 12 weeks of exposure (Figure E1G), suggesting that WS exacerbates CS-induced inflammation in mice, in part, by affecting the AA pathway.
WS Modifies CS-Induced Expression of AA Pathway Genes
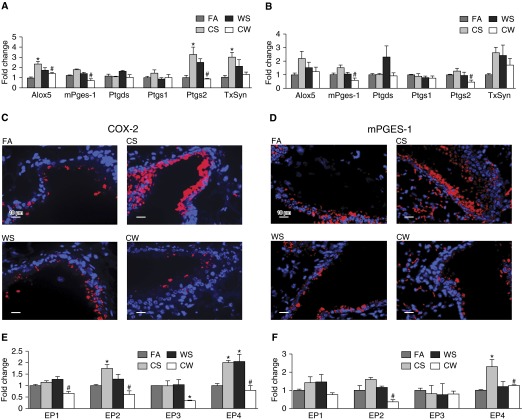
Because LXA4 levels were reduced in the CW group, we initially investigated the levels of several COX and lipoxygenase family of genes involved in AA metabolism, and found that mRNA levels for COX-2 at 4 weeks, and protein levels of COX-2 and microsomal PGE synthase (mPGES)-1 at 4 and 12 weeks, were consistently elevated in the lungs of CS mice compared with the FA group, but were reduced in CW mice compared with CS mice at both 4 and 12 weeks of exposure (Figures 2A–2D). Immunostaining revealed that expression of these proteins was predominantly restricted to the bronchial epithelium, suggesting that the bronchial epithelium may play a major role in WS-induced enhancement of inflammation.
Figure 2.
WS modifies CS-induced expression of genes within the arachidonic acid (AA) pathway. mRNA levels of arachidonate 5-lipoxygenase (ALOX-5), prostaglandin (PG)–endoperoxide synthase 1 (PTGS-1; or cyclo-oxygenase [COX]-1), PTGS-2 (or COX-2), thromboxane synthase (TXsyn), PGD synthase (PTGDS), and microsomal PGE synthase (mPGES)-1 in whole lung tissues of mice exposed to FA, CS, WS, or CW for 4 (A) and 12 (B) weeks, and as determined by quantitative real-time PCR (qRT-PCR; n = 9 for each treatment). Immunofluorescence analysis of COX-2 (C) and mPGES-1 (D) proteins in lung tissue sections showing increased expression of both proteins predominantly in the bronchial epithelium of CS airways, and significantly reduced immunostaining in CW airways compared with CS airways. Scale bars, 10 μm. mRNAs for the four E-prostanoid (EP) receptors 1–4 in whole lung tissues of FA, CS, WS, and CW mice at 4 (E) and 12 weeks (F) of exposure, as determined by qRT-PCR. Data are presented as means ± SEM. *P < 0.05 compared with FA; #P < 0.05 compared with CS.
Consistent with previous reports that COX-2–derived PGH2 is metabolized by mPGES-1 to produce PGE2 (25), CW mice had lower PGE2 levels in the BALF (3.8 ± 0.5 ng/mg protein) than CS animals (5.6 ± 0.6 ng/mg protein) (P = 0.02). Expression of E-prostanoid (EP) 2 receptor at 4 weeks and EP4 receptor at 4 and 12 weeks of exposures were significantly up-regulated in CS compared with FA mice, but was significantly reduced in CW compared with CS mice at both 4 and 12 weeks of exposures, whereas expression of EP1 and EP3 did not show this pattern (Figures 2E and 2F). These findings suggested that WS increases CS-induced airway inflammation in a synergistic fashion by suppressing COX-2/PGE2 and the anti-inflammatory lipid mediator, LXA4.
We next developed a cell culture model that mimics the in vivo conditions to facilitate investigation of the mechanisms involved. Primary HAECs from at least three donors were differentiated on air–liquid interface Transwell cultures and treated with extracts prepared from CS and/or WS. CS extract at 4 μg/ml increased COX-2 mRNA and protein levels in a bimodal fashion at 3 and 24 hours, whereas mPGES-1 levels did not increase until 24 hours after treatment (Figures E2A and E2B). We selected 4 μg/ml CS concentration and a treatment time of 40 hours, because these conditions consistently increased both COX-2 and mPGES-1 levels in two immortalized HAECs (N1 and N3 cells) and in primary HAECs from seven different individuals compared with nontreated controls (Figures E2C and E2D).
We determined that exposure to 10 ng/ml WS extract for 3 hours lead to minor, nonsignificant increases in COX-2 and mPGES-1 mRNA levels (Figure E2E). Therefore, for the combination treatments, WS at 10 ng/ml and CS at 4 μg/ml were used to treat HAECs in the following manner to mimic exposure conditions in mice: (1) growth medium for 40 hours followed by 3 hours fresh growth medium nontreated (NT); (2) CS for 40 hours followed by 3 hours fresh growth medium (CS); (3) CS for 40 hours followed by 3 hours WS extract (CW). We added a treatment of CS for 40 hours followed by 3 hours CS (10 ng/ml) (CC) to test whether the effect of WS is distinct from that of CS at low concentrations. COX-2 and mPGES-1 mRNA and protein levels were increased in the CS and CC compared with the NT group, whereas CW treatment significantly reduced them to levels observed in the NT group (Figures 3A and 3B). Differences in PGE2 levels in the supernatants of CS and NT cells were not significant, and, given the very short half-life of PGE2, it is possible that CS-induced PGE2 might be degraded during the 3 hours after CS-containing medium was replaced with normal growth medium. However, supernatants from CC cells had higher levels of PGE2 compared with NT cells (6.67 ± 1.49 versus 3.19 ± 1.38 ng/mg protein; P = 0.03), whereas no differences were observed in PGE2 levels in supernatants of CW and NT cells.
Figure 3.
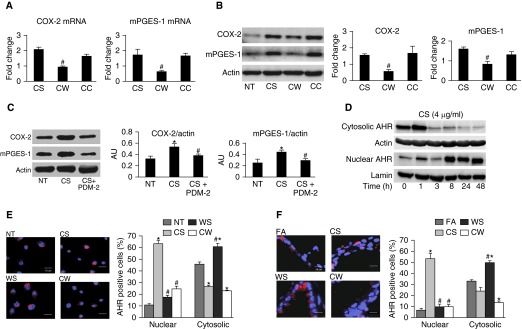
WS down-regulates CS-induced COX-2 and mPGES-1 levels in an aryl hydrocarbon receptor (AHR)–dependent manner. mRNA (A) and protein (B) levels in N3 cells treated with: (1) normal growth medium for 40 hours followed by a 3-hour treatment with fresh growth medium (nontreated (NT)); (2) CS (4 μg/ml) for 40 hours followed by a 3-hour treatment with normal growth medium (CS); (3) CS (4 μg/ml) for 40 hours followed by a 3-hour treatment with WS (10 ng/ml) (CW); or (4) CS (4 μg/ml) for 40 hours followed by a 3-hour treatment with CS (10 ng/ml) (CC) as an additional control. Means ± SEM (n ≥ 3 repeats for each treatment). #P < 0.05 compared with CS. (C) Inhibition of AHR with POU domain protein 2 (PDM-2) treatment reduced CS-induced COX-2 and mPGES-1 protein levels. Human airway epithelial cells (HAECs) were untreated (NT), treated with CS (4 μg/ml) for 40 hours, or pretreated with the selective AHR antagonist (PDM-2), followed by CS (4 μg/ml) for 40 hours (CS + PDM-2). AU, arbitrary units. (D) CS induces translocation of AHR from the cytoplasm to the nucleus in a time-dependent manner. Primary HAECs were treated with CS (4 μg/ml) for indicated times and AHR levels in the cytosolic and nuclear fractions were measured by Western blot (WB) analysis. Actin and lamin were used as loading controls. (E) WS down-regulates CS-induced AHR nuclear translocation in HAECs. Primary HAECs were treated as described previously here with NT, CS, CW, or WS (10 ng/ml) for 3 hours and immunofluorescence staining performed for AHR protein (red) and nuclei (blue). The percentage of cells showing positive staining for cytosolic and nuclear AHR is shown. Scale bars, 10 μm. (F) WS down-regulates CS-induced AHR nuclear translocation in airway cells of mice. Immunostaining and quantification for AHR protein expression was performed using lung tissues from mice exposed to FA, CS, CW, and WS, as described previously here for HAECs. Scale bars, 10 μm. Data presented are means ± SEM (n ≥ 3 repeats experiment for each treatment. *P < 0.05 compared with NT; #P < 0.05 compared with CS.
AHR Mediates Down-Regulation of CS-Induced COX-2 and mPGES-1 by WS
AHR signal transduction pathway is involved in CS-induced expression of COX-2 and PGE2, and AHR activity is primarily driven by nuclear localization (19). Consistent with these reports, we found that, in HAECs, CS-induced COX-2 and mPGES-1 mRNA levels were significantly down-regulated by treating cells with 1 μM POU domain protein 2 (PDM-2) or 5 μM C223191, two potent and selective AHR antagonists, for 1 hour before the start of CS (Figures E3A and E3B). CS-induced protein levels of both COX-2 and mPGES were also reduced by prior treatment with PDM-2 (Figure 3C). CS-induced CYP1A1 mRNA levels, measured as additional readouts of AHR activity (Figures E3C, E3F, and E3G), also showed reduction with prior treatment with PDM-2 or CH223191 (Figure E3C).
Furthermore, CS induced a time-dependent depletion of the cytosolic AHR while increasing nuclear AHR levels (Figure 3D). In HAECs from three individuals, total AHR protein levels were increased by CS compared with NT controls, but significantly down-regulated in cells treated with CW (Figure E3C). This observation was confirmed by immunofluorescence staining of NT-, CS-, WS-, and CW-treated HAECs (Figure 3E). A higher proportion of CS cells (∼63%) compared with NT cells (∼11%) were positive for nuclear AHR, whereas the opposite applied for cytosolic AHR. In contrast, cytosolic AHR was much higher (∼61%) in the WS cells and NT cells. Nuclear localization of AHR was markedly reduced from approximately 63% in CS-treated cells to 25% in CW cells. Similarly, AHR protein expression was significantly up-regulated in bronchial epithelia of mice exposed to CS or WS compared with FA control mice (Figures 3F and E3E), and a higher percentage of airway cells from CS (∼53%) compared with WS (∼10%) mice were positive for nuclear AHR. In contrast, more airway cells were positive for cytosolic AHR in WS (∼50%) compared with CS (∼24%) or FA (∼33%) mice, suggesting that CS and WS cause nuclear and cytosolic localization of AHR, respectively. In addition, AHR protein expression was remarkably down-regulated in CW mice—only roughly 10% and roughly 14% of cells immunostained for nuclear and cytosolic AHR, respectively—suggesting that WS affected either nuclear translocation or turnover of AHR.
WS Increases AHRR Expression to Suppress CS-Induced Transcriptional Activity of AHR
We next determined if the CW treatment affected nuclear translocation or degradation of AHR. AHR turnover is mediated by the ubiquitin-26S proteasome–mediated proteolysis, which can occur either in the cytoplasm or nuclear compartments (26, 27). In the presence of the proteasome inhibitor, MG132 (10 μM), CW cells had a higher proportion of cells expressing nuclear AHR protein compared with CW cells without MG132 (Figure E4A), whereas exposure to MG132 alone had no effect on nuclear AHR levels in NT cells (data not shown). These findings suggest that low levels of nuclear AHR in CW cells were due to a higher turnover of AHR, and not to an inhibitory effect of WS on the nuclear translocation machinery.
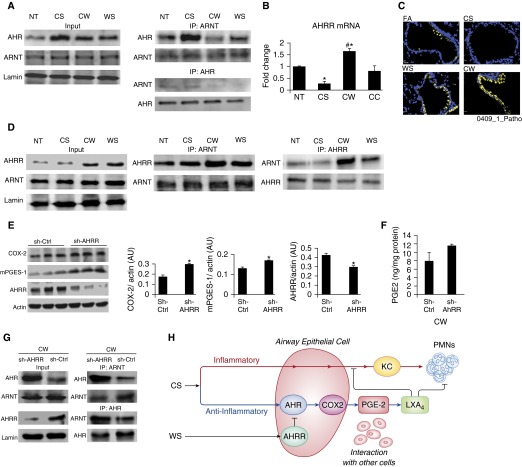
The transcriptional activity of AHR is dependent on the availability of its protein partner, AHR nuclear transporter (ARNT) (28), but no significant changes were observed in ARNT levels over 0–48 hours with CS or WS (Figure E4C). However, immunoprecipitation using both AHR and ARNT antibodies showed increased interaction of AHR and ARNT in CS cells, whereas this interaction was significantly reduced in CW cells compared with levels observed in WS or NT cells (Figure 4A). Because ARNT levels remained unchanged after CS or WS treatment, we postulated that CW suppressed the formation of a functional AHR–ARNT heterodimer by sequestering ARNT away from AHR and thereby affecting the nuclear residence time of AHR. Because the AHRR can decrease the concentration of ARNT available for AHR dimerization (29, 30), we measured steady-state levels of AHRR mRNA in CS or WS cells over 0–48 hours (Figure E4B). CS (4 μg/ml) induced AHRR levels by twofold at 3 hours, but these levels declined over 6 hours, and were reduced to levels lower than the basal levels at 48 hours. However, WS at a much lower concentration (10 ng/ml) induced a threefold increase in AHRR levels at 3 hours, and these levels further increased to fivefold at 6 hours before declining over 48 hours to levels lower than at baseline. AHRR protein expression was consistent with the patterns observed for the AHRR mRNA (Figure E4D). AHRR expression was up-regulated in CW, but was reduced by CS compared with NT cells; no differences were observed between NT and CC cells (Figure 4B). Immunostaining of lung tissues from mice exposed to FA, CS, CW, and WS with anti-AHRR antibody showed findings essentially identical to those observed in HAECs (Figure 4C).
Figure 4.
WS increases AHR repressor (AHRR) expression to suppress CS-induced AHR activity and suppress COX-2 and mPGES-1 expression, and AHRR deficiency mitigates the WS effect. (A) WS affects CS-induced AHR–AHR nuclear transporter (ARNT) interaction. HAECs were treated as described in Figure 3. Nuclear extracts were prepared and immunoprecipitation (IP)-immunoblot (IB) analyses were performed using antibodies to both AHR and ARNT. (B) CW induces higher AHRR mRNA expression than CS. The levels of AHRR mRNA were measured and normalized by cyclin-dependent kinase inhibitor 1 β (CDKN1β) levels (n ≥ 3). *P < 0.05 compared with NT; #P < 0.05 compared with CS. (C) WS increases AHRR protein expression in mice. Lung tissue sections from FA, CS, CW, and WS mice were stained with AHRR antibodies. Scale bars, 10 μm. (D) WS enhances AHRR–ARNT interaction. Nuclear extracts were prepared from HAECs treated as described in (A) with FA, CS, CW, and WS, and IP–IB analyses were performed using antibodies to both AHRR and ARNT. (E) Short hairpin–AHRR and sh control (sh-Ctrl) cells were treated with CW and COX-2, and mPGES-1 protein levels were measured by Western blotting. HAECs with reduced AHRR protein levels were generated by infecting parental cells with a retroviral vector expressing shRNA against AHRR (sh-AHRR). Control cells were infected with vector only (sh-Ctrl). (F) Supernatants from CW-treated cells showed a near-significant (P = 0.059) increase in PGE2 levels in sh-AHRR compared with sh-Ctrl cells. (G) Nuclear extracts were prepared from sh-AHRR and sh-Ctrl cells treated with CW, and IP followed by IB analyses were performed using antibodies to both ARNT and AHR. Nuclear extract was analyzed for AHR, ARNT, and AHRR by Western blotting (input). sh-AHRR cells show a greater AHR–ARNT interaction than sh-Ctrl cells. Data represent means ± SEM (n = 3). *P < 0.05 compared with sh-Ctrl. (H) Proposed model for WS-induced enhancement of CS-induced inflammation. CS induces KC expression and neutrophilic inflammation, but also induces AHR to increase COX-2 expression that activates other cell types to produce LXA4 to limit neutrophilic inflammation and KC production by airway epithelial cells. WS blocks this anti-inflammatory pathway of CS by up-regulating AHRR and suppressing AHR activity. PMNs, polymorphonuclear cells.
AHRR Deficiency in HAECs Mitigates WS Effect
Immunoprecipitation using anti-AHRR antibodies from nuclear fractions from NT, CS, CW, and WS cells showed an enhanced association between AHRR and ARNT in CW and WS compared with NT and CS cells (Figure 4D). Similar findings were observed for immunoprecipitations using anti-ARNT antibodies (Figure 4D). Taken together, these findings suggest that CW-induced up-regulation of AHRR mediates the observed suppression of CS-induced AHR signaling by WS. The role of AHRR was further confirmed by generating HAECs with reduced AHRR protein levels using retroviral vectors expressing shRNA against AHRR (sh-AHRR); control cells were infected with vector only (sh-Ctrl) (Figure 4E). Consistent with the notion that AHRR suppressed the transcriptional activity of AHR, CW treatment increased both COX-2 and mPGES-1 levels in sh-AHRR compared with sh-Ctrl cells (Figure 4E). In addition, after CW treatment, a near-significant increase in the level of PGE2 was observed in the supernatant of sh-AHRR compared with sh-Ctrl cells (Figure 4F). Furthermore, immunoprecipitation of nuclear extracts from CW cells using both anti-AHR and anti-ARNT antibodies revealed a greater AHR–ARNT interaction in sh-AHRR cells compared with sh-Ctrl cells (Figure 4G). Thus, efficient silencing of AHRR in HAECs mitigated WS effect on CS-induced AHR activity and COX-2 and mPGES-1 expression.
Discussion
CS causes inflammation in the lung by inducing production of the neutrophil chemoattractant, KC, by airway epithelial cells. Prolonged exposure to CS also activates AHR to initiate the anti-inflammatory pathway involving COX-2 and mPGES-1 to increase LXA4 production through interaction with other cell types in the lung. Biosynthesis of lipoxins can occur through at least three pathways: first, epithelial cell– or monocyte-derived 15-LO converts AA into 15(S)-hydroperoxyeicosatetraenoic acid that is converted by neutrophilic 5-LO to lipoxins; second, 5-LO found in cells of myeloid origin can produce leukotriene A4; and third, aspirin-triggered synthesis of 15-epi lipoxins, which involves acetylation of COX-2 and action of LOs present in endothelial/epithelial cells and leukocytes (reviewed in Ref. 31). In an autocrine fashion, LXA4 blocks the production of KC (32, 33). The present studies demonstrate that exposure to even low concentrations of WS enhances CS-induced inflammation by inducing AHRR expression to suppress AHR, dampen the anti-inflammatory COX-2 and mPGES-1 pathway, and decrease LXA4 levels to enhance CS-induced inflammation (Figure 4G). In addition to its protective action through LXA4 biosynthesis, COX-2–derived PGE2 has direct anti-inflammatory and antifibrotic roles in the resolution of inflammation, including control of 5-LO–generated leukotriene B4 production by polymorphonuclear neutrophils and inhibition of fibroblast proliferation, collagen synthesis, chemotaxis, fibroblast-to-myofibroblast differentiation, and connective tissue growth factor expression (13, 25; reviewed in Ref. 34). Therefore, loss of PGE2 in CW mice may also have contributed directly to the enhanced inflammation in these animals.
The WS concentrations used in the present study were too low to show any inflammatory changes. Exposure of healthy rodents (35–37) and humans (38) to similarly low WS concentrations shows either no or only mild inflammatory responses. However, these low concentrations of WS enhanced CS-induced inflammation, as reflected by a higher number of BAL neutrophils, increased size and number of multinucleated alveolar macrophages, and a lower concentration of LXA4 in the BALF. The change in numbers and phenotype of alveolar macrophages in CW mice may be the result of enhanced proliferation, activation, or phagocytosis, as was reported for long-term intratracheal exposure (> 4–5 wk) to silica or asbestos in mice (39). In addition, in chronic inflammatory environments, macrophages can undergo fusion with other macrophages to form multinucleated giant cells, which is a feature of the mannose receptor–mediated phagocytic process with participation of the endoplasmic reticulum (40).
CS and WS are very similar in chemical composition (41), and both cause activation of AHR. However, WS at 400-fold–lower concentrations induces more sustained AHRR expression compared with CS. These observations suggest that WS components may activate pathways other than AHR signaling to induce AHRR expression. The 5′ upstream region of the AHRR gene contains regulatory DNA sequences, such as GC boxes and NF-κB–binding site, that regulate constitutive and AHR ligand–induced AHRR transcription in addition to the AHR response elements (42). WS may activate NF-κB and induce AHRR expression more potently than CS, or may modify the overall structure of activated AHR to affect coactivator recruitment and thereby differentially modulate the biological response of the receptor (29). Previous reports, showing that hazardous free radicals in WS are chemically active 40 times longer than those from CS (43), and that WS PM generates more DNA damage than traffic-generated PM per unit mass in human cell lines (44), support the idea that components in WS have effects more potent than those in CS.
CS induced COX-2 and mPGES-1 expression in the airway epithelial cells of mice and in HAECs in vitro. Besides acting as a physical barrier from inhaled particles and pollutants, the airway epithelium is increasingly recognized as an active participant in the innate defense mechanisms of the lung. The epithelial layer also initiates other mechanisms to reduce oxidant injury and prevent extensive inflammation through the production of antioxidants, anti-inflammatory cytokines, mucus, defensins, matrix proteins, and lipid molecules (45, 46). COX-2–derived PGE2 exerts its effect through one of the four cognate receptors (EP1–4) (47). We found increased expression of the EP2 and EP4 receptors in the lungs of CS mice, whereas, in CW mice, EP2 and EP4 expression were significantly reduced along with LXA4 levels in the BALF. Therefore, it is possible that EP2 and EP4 are involved in limiting the inflammatory process. This is also supported by previous studies showing that PGE2 has both anti-inflammatory and bronchodilator effects in animal models and in subjects with asthma (48, 49) mediated through activation of the EP2 and/or the EP4 receptor subtypes, which inhibits neutrophil and eosinophil chemotaxis (47). Up-regulation of COX-2 and COX-2–derived mediators have been associated with antiinflammatory effects in various in vitro and in vivo models of acute and chronic inflammation. For example, COX-2–derived mediators promoted in vivo resolution of carrageenan-induced pleurisy (12), acid-triggered lung injury (10, 14), bleomycin-induced lung injury (34), acid-induced gastric injury (50), allergen-induced eosinophilic inflammation (47), and infectious and autoimmune arthritis (15, 16).
Our findings demonstrate that, although CS drives nuclear localization of AHR, WS promotes cytosolic localization of AHR, both in HAECs in vitro and in mouse airway epithelia. The immunoprecipitation studies support the idea that WS drives the cytosolic localization by inducing AHRR expression to competitively remove AHR from the CS-induced AHR/ARNT complex. Whether the disruption facilitates AHR transport to the cytosol is not clear, because the percentage of cells with cytosolic AHR, although increased by WS only, was not increased in the CW group. The percentage of cells with nuclear AHR in the CW group was reduced compared with the CS group. These findings suggest that AHR may be degraded either in the nucleus or in the cytosol once it is removed from the ARNT complex by AHRR. This WS-induced inactivation of nuclear AHR is of importance for the observed enhanced inflammation, because several observations directly link CS-induced AHR activation and COX-2/mPGES-1 expression. First, immunofluorescence of HAECs and airway epithelia of mice showed a correlation with COX-2/mPGES-1 expression in NT-, CS-, and CW-treated conditions. Second, the AHR-specific inhibitors, PDM-2 or C223191, suppressed CS-induced COX-2/mPGES-1 mRNA or protein expression. Third, the effect of CW on AHRR-induced down-regulation of AHR activity was confirmed by suppressing AHRR using shRNA that reversed AHR activity and increased COX-2 and mPGES-1 protein expression. Consistent with our studies, AHR−/− compared with AHR+/+ mice produce lower levels of LXA4 (51), AHR antagonists reduce COX-2 production (52), and inhibition of COX-2 results in a reduction of 15-S-hydroxyeicosatetraenoic acid (15-(S)-HETE) by approximately 50%, thereby decreasing LXA4 (53).
Our results suggest that reduction of LXA4 levels in CW mice was associated with enhanced neutrophilic inflammation. Consistent with this finding, the anti-inflammatory and proresolution activities of LXA4 include inhibition of neutrophil chemotaxis and activation (25). Because lipoxins, in turn, can reduce KC release from human airway cells (10) and in mice (33), reduced LXA4 further enhances neutrophilic inflammation. Defects in LXA4 production and signaling have been associated with persistence of various inflammatory diseases. For example, reduced levels of lipoxins were found in airways of patients suffering from asthma, cystic fibrosis, and chronic bronchitis (54–56). Furthermore, COX-2 inhibition blocked LXA4 production, resulted in exacerbation of acute lung injury with longer recovery times (14), and perpetuated inflammation in arthritis (16), and repletion with PGE2 analogs restored LXA4 production and promoted resolution (14, 16).
Collectively, the current studies demonstrate that exposure to low concentrations of WS enhances CS-induced inflammation by inducing AHRR expression to suppress the anti-inflammatory AHR, COX-2, and mPGES-1 expression, and levels of PGE2 and LXA4. Therefore, AHRR is a potential therapeutic target for WS-associated exacerbations of CS-induced inflammation.
Acknowledgments
Acknowledgments
The authors thank Fred Kleinschnitz for assistance with animal exposures and Thomas Gagliano for assistance in preparing the figures.
Footnotes
This work was supported by National Institutes of Health grants HL68111, ES015482, HL68669, GM095467, and HL007633, and by the Brigham and Women’s Hospital–Lovelace Respiratory Research Institute Consortium.
Author Contributions: design and conception of study—Y.T. and E.G.A.; conducting the experiments—S.B., E.G.A., H.C., Y.M., K.R.S., and J.K.C.; analysis and interpretation—E.G.A., H.C., S.B., Y.M., K.R.S., J.K.C., B.D.L., and Y.T.; drafting the manuscript for intellectual content—E.G.A., H.C., S.B., Y.M., J.K.C., B.D.L., and Y.T.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0142OC on August 19, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Diette GB, Accinelli RA, Balmes JR, Buist AS, Checkley W, Garbe P, Hansel NN, Kapil V, Gordon S, Lagat DK, et al. Obstructive lung disease and exposure to burning biomass fuel in the indoor environment. Glob Heart. 2012;7:265–270. doi: 10.1016/j.gheart.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larson TV, Koenig JQ. Wood smoke: emissions and noncancer respiratory effects. Annu Rev Public Health. 1994;15:133–156. doi: 10.1146/annurev.pu.15.050194.001025. [DOI] [PubMed] [Google Scholar]
- 3.Orozco-Levi M, Garcia-Aymerich J, Villar J, Ramírez-Sarmiento A, Antó JM, Gea J. Wood smoke exposure and risk of chronic obstructive pulmonary disease. Eur Respir J. 2006;27:542–546. doi: 10.1183/09031936.06.00052705. [DOI] [PubMed] [Google Scholar]
- 4.Gan WQ, FitzGerald JM, Carlsten C, Sadatsafavi M, Brauer M. Associations of ambient air pollution with chronic obstructive pulmonary disease hospitalization and mortality. Am J Respir Crit Care Med. 2013;187:721–727. doi: 10.1164/rccm.201211-2004OC. [DOI] [PubMed] [Google Scholar]
- 5.Sood A, Petersen H, Blanchette CM, Meek P, Picchi MA, Belinsky SA, Tesfaigzi Y. Wood smoke exposure and gene promoter methylation are associated with increased risk for COPD in smokers. Am J Respir Crit Care Med. 2010;182:1098–1104. doi: 10.1164/rccm.201002-0222OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pérez-Padilla R, Regalado J, Vedal S, Paré P, Chapela R, Sansores R, Selman M. Exposure to biomass smoke and chronic airway disease in Mexican women: a case–control study. Am J Respir Crit Care Med. 1996;154:701–706. doi: 10.1164/ajrccm.154.3.8810608. [DOI] [PubMed] [Google Scholar]
- 7.Profita M, Sala A, Bonanno A, Riccobono L, Ferraro M, La Grutta S, Albano GD, Montalbano AM, Gjomarkaj M. Chronic obstructive pulmonary disease and neutrophil infiltration: role of cigarette smoke and cyclooxygenase products. Am J Physiol Lung Cell Mol Physiol. 2010;298:L261–L269. doi: 10.1152/ajplung.90593.2008. [DOI] [PubMed] [Google Scholar]
- 8.Baglole CJ, Maggirwar SB, Gasiewicz TA, Thatcher TH, Phipps RP, Sime PJ. The aryl hydrocarbon receptor attenuates tobacco smoke–induced cyclooxygenase-2 and prostaglandin production in lung fibroblasts through regulation of the NF-kappaB family member RelB. J Biol Chem. 2008;283:28944–28957. doi: 10.1074/jbc.M800685200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roh GS, Yi CO, Cho YJ, Jeon BT, Nizamudtinova IT, Kim HJ, Kim JH, Oh YM, Huh JW, Lee JH, et al. Anti-inflammatory effects of celecoxib in rat lungs with smoke-induced emphysema. Am J Physiol Lung Cell Mol Physiol. 2010;299:L184–L191. doi: 10.1152/ajplung.00303.2009. [DOI] [PubMed] [Google Scholar]
- 10.Bonnans C, Fukunaga K, Levy MA, Levy BD. Lipoxin A(4) regulates bronchial epithelial cell responses to acid injury. Am J Pathol. 2006;168:1064–1072. doi: 10.2353/ajpath.2006.051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 12.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 13.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 14.Fukunaga K, Kohli P, Bonnans C, Fredenburgh LE, Levy BD. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J Immunol. 2005;174:5033–5039. doi: 10.4049/jimmunol.174.8.5033. [DOI] [PubMed] [Google Scholar]
- 15.Blaho VA, Mitchell WJ, Brown CR. Arthritis develops but fails to resolve during inhibition of cyclooxygenase 2 in a murine model of Lyme disease. Arthritis Rheum. 2008;58:1485–1495. doi: 10.1002/art.23371. [DOI] [PubMed] [Google Scholar]
- 16.Chan MM, Moore AR. Resolution of inflammation in murine autoimmune arthritis is disrupted by cyclooxygenase-2 inhibition and restored by prostaglandin E2–mediated lipoxin A4 production. J Immunol. 2010;184:6418–6426. doi: 10.4049/jimmunol.0903816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reuter BK, Asfaha S, Buret A, Sharkey KA, Wallace JL. Exacerbation of inflammation-associated colonic injury in rat through inhibition of cyclooxygenase-2. J Clin Invest. 1996;98:2076–2085. doi: 10.1172/JCI119013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallace JL. Selective COX-2 inhibitors: is the water becoming muddy? Trends Pharmacol Sci. 1999;20:4–6. doi: 10.1016/s0165-6147(98)01283-8. [DOI] [PubMed] [Google Scholar]
- 19.Martey CA, Pollock SJ, Turner CK, O’Reilly KM, Baglole CJ, Phipps RP, Sime PJ. Cigarette smoke induces cyclooxygenase-2 and microsomal prostaglandin E2 synthase in human lung fibroblasts: implications for lung inflammation and cancer. Am J Physiol Lung Cell Mol Physiol. 2004;287:L981–L991. doi: 10.1152/ajplung.00239.2003. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida T, Tuder RM. Pathobiology of cigarette smoke–induced chronic obstructive pulmonary disease. Physiol Rev. 2007;87:1047–1082. doi: 10.1152/physrev.00048.2006. [DOI] [PubMed] [Google Scholar]
- 21.Kang MJ, Lee CG, Lee JY, Dela Cruz CS, Chen ZJ, Enelow R, Elias JA. Cigarette smoke selectively enhances viral PAMP- and virus-induced pulmonary innate immune and remodeling responses in mice. J Clin Invest. 2008;118:2771–2784. doi: 10.1172/JCI32709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med. 2005;107:183–206. doi: 10.1385/1-59259-861-7:183. [DOI] [PubMed] [Google Scholar]
- 23.Mebratu YA, Dickey BF, Evans C, Tesfaigzi Y. The BH3-only protein Bik/Blk/Nbk inhibits nuclear translocation of activated ERK1/2 to mediate IFNgamma-induced cell death. J Cell Biol. 2008;183:429–439. doi: 10.1083/jcb.200801186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Contreras AU, Mebratu Y, Delgado M, Montano G, Hu CA, Ryter SW, Choi AM, Lin Y, Xiang J, Chand H, et al. Deacetylation of p53 induces autophagy by suppressing Bmf expression. J Cell Biol. 2013;201:427–437. doi: 10.1083/jcb.201205064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 26.Lees MJ, Peet DJ, Whitelaw ML. Defining the role for XAP2 in stabilization of the dioxin receptor. J Biol Chem. 2003;278:35878–35888. doi: 10.1074/jbc.M302430200. [DOI] [PubMed] [Google Scholar]
- 27.Song J, Clagett-Dame M, Peterson RE, Hahn ME, Westler WM, Sicinski RR, DeLuca HF. A ligand for the aryl hydrocarbon receptor isolated from lung. Proc Natl Acad Sci USA. 2002;99:14694–14699. doi: 10.1073/pnas.232562899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollenz RS, Barbour ER. Analysis of the complex relationship between nuclear export and aryl hydrocarbon receptor–mediated gene regulation. Mol Cell Biol. 2000;20:6095–6104. doi: 10.1128/mcb.20.16.6095-6104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denison MS, Soshilov AA, He G, DeGroot DE, Zhao B. Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol Sci. 2011;124:1–22. doi: 10.1093/toxsci/kfr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hahn ME, Allan LL, Sherr DH. Regulation of constitutive and inducible AHR signaling: complex interactions involving the AHR repressor. Biochem Pharmacol. 2009;77:485–497. doi: 10.1016/j.bcp.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMahon B, Godson C. Lipoxins: endogenous regulators of inflammation. Am J Physiol Renal Physiol. 2004;286:F189–F201. doi: 10.1152/ajprenal.00224.2003. [DOI] [PubMed] [Google Scholar]
- 32.He R, Sang H, Ye RD. Serum amyloid A induces IL-8 secretion through a G protein–coupled receptor, FPRL1/LXA4R. Blood. 2003;101:1572–1581. doi: 10.1182/blood-2002-05-1431. [DOI] [PubMed] [Google Scholar]
- 33.Bozinovski S, Uddin M, Vlahos R, Thompson M, McQualter JL, Merritt AS, Wark PA, Hutchinson A, Irving LB, Levy BD, et al. Serum amyloid A opposes lipoxin A₄ to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proc Natl Acad Sci USA. 2012;109:935–940. doi: 10.1073/pnas.1109382109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodges RJ, Jenkins RG, Wheeler-Jones CP, Copeman DM, Bottoms SE, Bellingan GJ, Nanthakumar CB, Laurent GJ, Hart SL, Foster ML, et al. Severity of lung injury in cyclooxygenase-2–deficient mice is dependent on reduced prostaglandin E(2) production. Am J Pathol. 2004;165:1663–1676. doi: 10.1016/S0002-9440(10)63423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Danielsen PH, Loft S, Jacobsen NR, Jensen KA, Autrup H, Ravanat JL, Wallin H, Møller P. Oxidative stress, inflammation, and DNA damage in rats after intratracheal instillation or oral exposure to ambient air and wood smoke particulate matter. Toxicol Sci. 2010;118:574–585. doi: 10.1093/toxsci/kfq290. [DOI] [PubMed] [Google Scholar]
- 36.Tesfaigzi Y, Singh SP, Foster JE, Kubatko J, Barr EB, Fine PM, McDonald JD, Hahn FF, Mauderly JL. Health effects of subchronic exposure to low levels of wood smoke in rats. Toxicol Sci. 2002;65:115–125. doi: 10.1093/toxsci/65.1.115. [DOI] [PubMed] [Google Scholar]
- 37.Tesfaigzi Y, McDonald JD, Reed MD, Singh SP, De Sanctis GT, Eynott PR, Hahn FF, Campen MJ, Mauderly JL. Low-level subchronic exposure to wood smoke exacerbates inflammatory responses in allergic rats. Toxicol Sci. 2005;88:505–513. doi: 10.1093/toxsci/kfi317. [DOI] [PubMed] [Google Scholar]
- 38.Riddervold IS, Bønløkke JH, Olin AC, Grønborg TK, Schlünssen V, Skogstrand K, Hougaard D, Massling A, Sigsgaard T. Effects of wood smoke particles from wood-burning stoves on the respiratory health of atopic humans. Part Fibre Toxicol. 2012;9:12. doi: 10.1186/1743-8977-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warheit DB, Sayes CM, Frame SR, Reed KL. Pulmonary exposures to Sepiolite nanoclay particulates in rats: resolution following multinucleate giant cell formation. Toxicol Lett. 2010;192:286–293. doi: 10.1016/j.toxlet.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 40.McNally AK, Anderson JM. Macrophage fusion and multinucleated giant cells of inflammation. Adv Exp Med Biol. 2011;713:97–111. doi: 10.1007/978-94-007-0763-4_7. [DOI] [PubMed] [Google Scholar]
- 41.Tesfaigzi Y, Fischer MJ, Daheshia M, Green FH, De Sanctis GT, Wilder JA. Bax is crucial for IFN-γ–induced resolution of allergen-induced mucus cell metaplasia. J Immunol. 2002;169:5919–5925. doi: 10.4049/jimmunol.169.10.5919. [DOI] [PubMed] [Google Scholar]
- 42.Baba T, Mimura J, Gradin K, Kuroiwa A, Watanabe T, Matsuda Y, Inazawa J, Sogawa K, Fujii-Kuriyama Y. Structure and expression of the Ah receptor repressor gene. J Biol Chem. 2001;276:33101–33110. doi: 10.1074/jbc.M011497200. [DOI] [PubMed] [Google Scholar]
- 43.Lachocki TM, Church DF, Pryor WA. Persistent free radicals in woodsmoke: an ESR spin trapping study. Free Radic Biol Med. 1989;7:17–21. doi: 10.1016/0891-5849(89)90095-6. [DOI] [PubMed] [Google Scholar]
- 44.Danielsen PH, Loft S, Kocbach A, Schwarze PE, Møller P. Oxidative damage to DNA and repair induced by Norwegian wood smoke particles in human A549 and THP-1 cell lines. Mutat Res. 2009;674:116–122. doi: 10.1016/j.mrgentox.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 45.Kuhn C, III, Homer RJ, Zhu Z, Ward N, Flavell RA, Geba GP, Elias JA. Airway hyperresponsiveness and airway obstruction in transgenic mice: morphologic correlates in mice overexpressing interleukin (IL)-11 and IL-6 in the lung. Am J Respir Cell Mol Biol. 2000;22:289–295. doi: 10.1165/ajrcmb.22.3.3690. [DOI] [PubMed] [Google Scholar]
- 46.Mercer BA, Lemaitre V, Powell CA, D'Armiento J. The epithelial cell in lung health and emphysema pathogenesis. Curr Respir Med Rev. 2006;2:101–142. doi: 10.2174/157339806776843085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sturm EM, Schratl P, Schuligoi R, Konya V, Sturm GJ, Lippe IT, Peskar BA, Heinemann A. Prostaglandin E2 inhibits eosinophil trafficking through E-prostanoid 2 receptors. J Immunol. 2008;181:7273–7283. doi: 10.4049/jimmunol.181.10.7273. [DOI] [PubMed] [Google Scholar]
- 48.Gauvreau GM, Watson RM, O’Byrne PM. Protective effects of inhaled PGE2 on allergen-induced airway responses and airway inflammation. Am J Respir Crit Care Med. 1999;159:31–36. doi: 10.1164/ajrccm.159.1.9804030. [DOI] [PubMed] [Google Scholar]
- 49.Pavord ID, Wong CS, Williams J, Tattersfield AE. Effect of inhaled prostaglandin E2 on allergen-induced asthma. Am Rev Respir Dis. 1993;148:87–90. doi: 10.1164/ajrccm/148.1.87. [DOI] [PubMed] [Google Scholar]
- 50.Davies NM, Sharkey KA, Asfaha S, Macnaughton WK, Wallace JL. Aspirin causes rapid up-regulation of cyclo-oxygenase-2 expression in the stomach of rats. Aliment Pharmacol Ther. 1997;11:1101–1108. doi: 10.1046/j.1365-2036.1997.00247.x. [DOI] [PubMed] [Google Scholar]
- 51.Elizondo G, Rodríguez-Sosa M, Estrada-Muñiz E, Gonzalez FJ, Vega L. Deletion of the aryl hydrocarbon receptor enhances the inflammatory response to Leishmania major infection. Int J Biol Sci. 2011;7:1220–1229. doi: 10.7150/ijbs.7.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Degner SC, Papoutsis AJ, Selmin O, Romagnolo DF. Targeting of aryl hydrocarbon receptor–mediated activation of cyclooxygenase-2 expression by the indole-3-carbinol metabolite 3,3′-diindolylmethane in breast cancer cells. J Nutr. 2009;139:26–32. doi: 10.3945/jn.108.099259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clària J, Lee MH, Serhan CN. Aspirin-triggered lipoxins (15-epi-LX) are generated by the human lung adenocarcinoma cell line (A549)–neutrophil interactions and are potent inhibitors of cell proliferation. Mol Med. 1996;2:583–596. [PMC free article] [PubMed] [Google Scholar]
- 54.Levy BD, Bonnans C, Silverman ES, Palmer LJ, Marigowda G, Israel E Severe Asthma Research Program, National Heart, Lung, and Blood Institute. Diminished lipoxin biosynthesis in severe asthma. Am J Respir Crit Care Med. 2005;172:824–830. doi: 10.1164/rccm.200410-1413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karp CL, Flick LM, Park KW, Softic S, Greer TM, Keledjian R, Yang R, Uddin J, Guggino WB, Atabani SF, et al. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat Immunol. 2004;5:388–392. doi: 10.1038/ni1056. [DOI] [PubMed] [Google Scholar]
- 56.Starosta V, Ratjen F, Rietschel E, Paul K, Griese M. Anti-inflammatory cytokines in cystic fibrosis lung disease. Eur Respir J. 2006;28:581–587. doi: 10.1183/09031936.06.00071405. [DOI] [PubMed] [Google Scholar]