Abstract
Background
An estimated 18 million adults in the United States meet the clinical criteria for diagnosis of alcohol abuse or alcoholism, a disorder ranked as the third leading cause of preventable death. In addition to brain pathology, heavy alcohol consumption is co-morbid with damage to major organs including heart, lungs, liver, pancreas and kidneys. Much of what is known about risk for and consequences of heavy consumption derive from rodent or retrospective human studies. The neurobiological effects of chronic intake in rodent studies may not easily translate to humans due to key differences in brain structure and organization between species, including a lack of higher-order cognitive functions, and differences in underlying prefrontal cortical neural structures that characterize the primate brain. Further, rodents do not voluntarily consume large quantities of EtOH and they metabolize it more rapidly than primates.
Methods
The basis of the Monkey Alcohol Tissue Research Resource (MATRR) is that nonhuman primates (NHPs), specifically monkeys, show a range of drinking excessive amounts of alcohol (>3.0 g/kg or a 12 drink equivalent/day) over long periods of time (12–30 months) with concomitant pathological changes in endocrine, hepatic and central nervous system (CNS) processes. The patterns and range of alcohol intake that monkeys voluntarily consume parallel what is observed in humans with alcohol use disorders and the longitudinal experimental design spans stages of drinking from the ethanol-naïve state to early exposure through chronic abuse. Age- and sex-matched control animals self-administer an isocaloric solution under identical operant procedures.
Results
The MATRR is a unique post-mortem tissue bank that provides CNS and peripheral tissues, and associated bioinformatics from monkeys that self-administer ethanol using a standardized experimental paradigm to the broader alcohol research community.
Conclusions
This resource provides a translational platform from which we can better understand the disease processes associated with alcoholism.
Keywords: monkey, alcohol, self-administration, tissue repository, bioinformatics
Introduction
Excessive alcohol consumption is co-morbid with disorders affecting the brain and behavior and other organ systems. Much of what is known about the risk for and consequences of heavy alcohol consumption derive from rodent studies or retrospective pre- and post-mortem human accounts. Rodent studies do not readily recapitulate the human condition due to species differences. Additionally, rodents do not typically consume excessive amounts of alcohol voluntarily or drink to a state of physical dependence. Postmortem findings or studies in human alcoholics are typically conducted in clinical samples composed largely of older individuals, and, therefore, may not generalize to the overall alcoholic population, many of whom are younger, asymptomatic and do not seek treatment. Moreover, many of these studies are confounded by numerous comorbidities such as a well-documented interaction between the aging brain and alcohol (reviewed in Pfefferbaum et al., 2002). Human studies are also complicated by heterogeneous genetic and environmental factors that contribute to the development of alcohol-related problems (e.g. family background, socioeconomic status, education, stress), combined with such factors as polydrug abuse, comorbid psychiatric illness and other pathologic conditions that make it difficult to confidently identify alcohol-specific neurobiological changes. This has the ultimate effect of obstructing the development of strategies for prevention, diagnosis and treatment. An appropriate animal model that provides a translational neurobiological and behavioral platform that also parallels the patterns and levels of alcohol intake seen clinically would facilitate research into alcohol-related problems (Sheedy et al., 2008).
Nonhuman primates offer unique advantages to address questions not readily addressed in rodent or postmortem tissue studies. Monkeys are phylogenetically similar to humans, sharing many anatomic, neurophysiological and complex behavioral characteristics. A major feature distinguishing NHPs and humans from other animals is their brain structure and function, specifically expansion of the cerebral cortex, which enables complex cognitive processing and behavioral flexibility. Importantly, monkeys will voluntarily consume ethanol in levels and patterns of intake that closely parallel those observed in human alcoholics (Cuzon-Carlson et al., 2011). The experimental design has face validity with known individual differences in alcohol consumption as well as a distribution of drinking phenotypes that represent the extreme ends of the alcoholic drinking spectrum (Grant et al., 2008a, Mello and Mendelson, 1970). Specifically, “spree” drinking patterns from this monkey model closely parallel patterns and levels of ethanol intake observed in alcoholics (Monti et al., 2004; Grant et al., 2008a, Mello and Mendelson, 1970). Further, alcohol drinking typologies (i.e., “gulping” vs. “sipping”) seen during ethanol induction are highly predictive of subsequent daily intake in the monkeys (Grant et al., 2008a) and parallel patterns that have been documented in alcoholics (Nathan et al., 1971). Monkeys show a range of excessive consumption, with some exceeding >3.0 g/kg (12 drink equivalent/day) over 12–30 months. This leads to pathological changes in endocrine, hepatic and central nervous system (CNS) processes like those seen in humans. A distinct advantage of an NHP model of ethanol abuse is the longitudinal design that spans “stages of drinking” beginning with ethanol-naïve animals, and continuing through periods of early exposure to chronic drinking. This cannot be accomplished in human studies for both practical and ethical reasons. The CNS and peripheral organs from these animals therefore comprise unique translational resources for mechanistic, genetic, proteomic and metabolomic studies of ethanol-induced pathologies without the variables confounding studies conducted in human subjects.
Currently there are brain banks that provide CNS tissues for research on neurological conditions including Alzheimer’s, schizophrenia, stroke, and head injuries. The New South Wales tissue research center (TRC) is one of the only human brain banks in existence primarily focused on alcohol-related disorders. In its relatively short existence the New South Wales TRC has proven pivotal for research into alcohol-related brain damage. As described by Sheedy and colleagues (2008), this brain bank has established interactions with a large number of alcohol researchers and sponsored the dissemination of research findings through multiple symposia and scientific presentations. As successful as human brain banks have been, they are limited by multiple variables inherent in any human-based clinical study, some of which are difficult to accurately document in humans. These variables can be closely managed in animal models. Our experimental design allows complete control over access to alcohol, nutritional status, housing conditions, medical care, other drug use (for veterinary care) which are critical advantages of appropriate animal models.
To take advantage of the tissues generated in our drinking studies, we recently (2010) formally established the MATRR. The MATRR is a multi-institutional collaboration that was initially developed in response to numerous tissue requests for intramural research projects designed to determine the functional consequences of long-term ethanol exposure using in vitro electrophysiology and voltammetry methods. The tissues are derived from a standard protocol of ethanol self-administration (Vivian et al., 2001; Grant et al., 2008a, 2008b) in 3 species of monkeys; cynomolgus (Macaca fascicularis), rhesus (Macaca mulatta) macaques and African green vervets (Chlorocebus aethiops). Animals self-administer ethanol (EtOH group) or an isocaloric maltose-dextrose solution (control group) under identical operant procedures. A state-of-the-art necropsy protocol is standardized across geographic sites for the collection and banking of tissues from these animals (Davenport et al., 2013).
The MATRR is an interactive tissue bank that provides a platform for integration of precise alcohol drinking data with multiple assessments of ex vivo and in vitro tissue analyses with the underlying genetics of the monkeys. The design is intended to optimize use of these valuable tissues by the greater alcohol research community. The number of NHPs used in biomedical research is limited by time, expense and ethical considerations, making it unfeasible to propose separate groups of monkeys for each specific hypothesis (e.g., gene expression in a single region of the brain; fat deposition in the liver). Our primary focus is to make tissue available to laboratories examining mechanisms underlying alcohol use disorders but lacking the infrastructure or expertise to conduct longitudinal alcohol studies in NHPs. As such, the MATRR makes tissue and associated bioinformatics tools readily available to the alcohol research community. Specifically, this resource provides highly characterized tissues and complementary data sets for hypothesis testing relating the risk for and consequences of alcohol consumption, and serves to bridge the gap between rodent and human studies. The ultimate goal of this tissue resource is to facilitate correlation of the exquisite behavioral data set generated by this paradigm with cutting edge molecular, cellular and physiological indices. The MATRR offers bioinformatics via a web-based interactive platform. This platform permits hypothesis testing across multiple tissues to probe the genetic bases of cellular, physiological and behavioral outcomes, as well as for correlating different aspects of the phenotypic data. The bioinformatics resource is available at www.matrr.com and is accessible to the greater alcohol research community. This paper is intended to parallel a recent report by Sheedy et al., (2008) which reviewed the TRC. One of our goals is to demonstrate how the MATRR complements the TRC and to highlight additional advantages of an appropriate animal model for alcohol use disorders. We review our current protocols and procedures and provide examples of outcomes of various methodologies.
Material and Methods
Animals
Male and female monkeys (4–13 yr old depending on study) were subjects in identical self-administration paradigms (Vivian et al., 2001; Grant et al., 2008a), funded as separate projects by the National Institute of Alcohol Abuse and Alcoholism (NIAAA) to examine the behavioral and neurobiological impact of chronic ethanol abuse. All animals were healthy and treatment-naïve prior to entering the studies. No medications were administered to block or prevent reinstatement of EtOH. Only medications related to clinical care were administered. All procedures were approved by the Institutional Animal Care and Use Committees of Oregon Health and Science University (OHSU) and Wake Forest School of Medicine (WFSM).
Apparatus
After acclimation to the laboratory, all monkeys are trained on operant panels that allow access to all fluid and food via responses controlled by a computerized system (Vivian et al., 2001; Grant et al., 2008a). A custom data acquisition program creates a data record each time a pellet is delivered, drinking occurs (fluid displacement measured), or a specific condition takes place (achieving the required induction volume or the end of an experimental state). Drinking records are assigned as a drink (continuous fluid displacement with < 5 sec between records) and a bout (< 5 min between records) and numbered consecutively to evaluate the temporal pattern of drinking behavior. During data analysis, consumption amounts and timings are processed to achieve totals for each drink and bout as well as full session totals which allows for determination of precise daily intakes and patterns of intake. This ability to document precise levels and patterns of intake offers a unique advantage over self-reports of alcohol intake in humans that are retrospective in nature and can be error prone.
Induction of Ethanol Self-Administration
Once trained on the operant panel, animals undergo a step-wise induction paradigm during which they are first induced to drink water followed by a series of escalating doses of ethanol (4% w/v in water). Age- and sex-matched controls self-administer an isocaloric maltose-dextrin solution rather than ethanol. During the induction phase, 1 g banana-flavored pellets (Granville Milling Co., Creedmoor, NC) are delivered every 5 min until the monkeys drink a predetermined volume of water or 4% (w/v) ethanol. Initially, the animals are induced to drink water for 30 days (the volume corresponding to an ethanol dose of 1.5 g/kg (4% w/v) ethanol). After 30 days, ethanol induction begins with ethanol as the only fluid available. The animals are induced to drink 0.5 g/kg/day, 1.0 g/kg/day and finally 1.5 g/kg/day for 30 days at each dose. Once the required volume of fluid is consumed, scheduled pellet delivery is discontinued and only water is available for 2 hrs. After the 2 hr time-out, any remaining food is available under a fixed ratio of one response per pellet.
Chronic Ethanol Self-Administration
Following induction, the open access 22 hour daily self-administration stage begins and ethanol and water are available ad libitum and food is available in at least 3 meals (Vivian et al., 2001; Grant et al., 2008a). The monkeys are trained to comply with awake venipuncture and blood samples (3 ml) are collected 1–3 times per week and banked for hormonal, metabolic and proteomic analyses. In addition, 20ul blood samples for determination of blood ethanol concentrations are collected every fifth day just prior to lights off in the room and approximately 7 hr following the onset of the session. At the conclusion of the self-administration studies (12 months minimum and up to 30 months depending on the study), the animals are taken to necropsy for collection of all CNS and peripheral tissues.
Two sites supply monkeys to the MATRR using an identical ethanol self-administration design. OHSU (Oregon National Primate Research Center) supplies the majority of cynomolgus and rhesus macaques. WFSM supplies rhesus macaques and African green (vervet) monkeys. IACUC approval for all studies is obtained prior to each experimental procedure. Table 1 outlines the number, species and sex of animals that comprise the MATRR resource. The number of tissues available per species is based on whole, non-subdivided tisses since specific regions are available at multiple levels. Though available, blood and serum are not included in this table. The ratio of age- and sex-matched controls to drinkers is 40–100% depending on the cohort.
Table 1.
MATRR Resource Species Availability. Monkey species categorized by sex, treatment group for animals that are currently available in the tissue bank, currently in progress or for assay development purposes.
| Species/Gender | Control (n) | Ethanol (n) |
|---|---|---|
| Available | ||
| Macaca fascicularis (Cynomolgus) | ||
| male | 26 | 32 |
| female | 9 | 15 |
| Macaca mulatta (Rhesus) | ||
| male | 31 | 49 |
| female | 6 | 11 |
| Chlorocebus aethiops (African Green Vervet) | ||
| male | 8 | 12 |
| Available Total | 80 | 119 |
| In Progress | ||
| Macaca mulatta (Rhesus) | ||
| male | 6 | |
| female | 12 | |
| Available for Assay Development | ||
| Macaca fascicularis (Cynomolgus) | ||
| male | 9 | |
| female | 1 | |
| Macaca mulatta (Rhesus) | ||
| male | 1 | |
| Chlorocebus aethiops (African Green Vervet) | ||
| male | 1 |
Collection of Central Nervous System and Peripheral Tissues
We utilize a state-of-the-art necropsy protocol, standardized across sites, to collect CNS and peripheral tissues (Davenport et al., 2013). The protocol details how the entire brain is blocked or sectioned, including discrete brain regions that are micro dissected at necropsy. In addition to gross examination of the brain at necropsy for obvious abnormalities, pre-mortem structural MRIs guide how the brain is blocked (Daunais et al, 2010). Every brain region is available unless requested for specific assays. Brain regions that are not available for animals in a particular cohort are indicated as ‘unavailable’ on the website. The vast majority of tissues are flash-frozen unless otherwise requested prior to necropsy.
Blood, CSF and urine are collected immediately prior to necropsy. Immediately following the perfusion and removal of the brain, all peripheral tissues are harvested (Table 2). The typical post-mortem interval (PMI) for brain extraction is less than 5 min. PMI ranges 1–30 min for peripheral tissues depending on the tissue. New tissues are added to the list from future subjects as requested. As studies using the self-administration procedure are funded and completed, additional animals are added to the resource.
Table 2.
Peripheral tissues collected prior to or after necropsy. As additional tissues are requested, they are added to this list.
| Serum | Fat (Retroperitoneal) | Lymph node (Inguinal) |
| Adrenal (lt, rt) | Fat (Subcutaneous) | Lymph node (Mesenteric) |
| Aorta (descending) | Gall bladder | Mammary Gland (lt, rt) |
| Aortic arch | Gonads (testes, ovaries) | Muscle (Biceps) |
| Bile | Hair (Without skin) | Muscle (Gastrocnemius) |
| Blood | Hair (With skin) | Muscle (Quadriceps) |
| Bone (Be specific) | Heart (Lt Atrium) | Muscle (Be specific) |
| Bone (Femur) | Heart (Lt ventricle) | Nails (finger, toe) |
| Bone (Shoulder) | Heart (Rt Atrium) | Nerve (Femoral) |
| Bone (Tibia) | Heart (Rt ventricle) | Nerve (Inguinal) |
| Bone Marrow (From Femur) | Illium | Nerve (Sciatic) |
| Cerebrospinal fluid | Jejunum | Pancreas |
| Cheek | Kidney (lt, rt) | Plasma |
| Colon | Large intestine | Prostate |
| Duodenum | Liver | Rectum |
| Esophagus | Lung (Accessory Lobe) | Skin |
| Eyes | Lung (Lt lower lobe) | Small intestine |
| Fat (Abdominal) | Lung (Lt middle lobe) | Spleen |
| Fat (Brown) | Lung (Lt upper lobe) | Stomach |
| Fat (Mesenteric) | Lung (Rt lower lobe) | Thymus |
| Fat (Pericardial) | Lung (Rt middle lobe) | Thyroid |
| Fat (Perigonadal) | Lung (Rt upper lobe) | Tongue |
| Fat (Perirenal) | Lymph node (Axial) | Urine |
Fresh frozen tissues are stored under −80°C conditions until shipped on dry ice. If requested prior to necropsy, tissues are post-fixed following the harvest and handled as instructed by the requesting investigator. Material Transfer Agreements (MTAs) are required before shipping tissues. Package tracking number and delivery time are provided to ensure presence of receiving personnel. Once tissue is shipped, we require notification of tissue receipt including date and status of the tissue. Research updates are required within 90 days of tissue receipt and again every 180 days until study completion. Research updates are uploaded on the MATRR website and automated emails are sent to alert when updates are due. Approval of additional or future tissue requests is contingent upon submission of updates.
Necropsy reports
Complete medical and experimental histories on each animal are documented and archived including necropsy date and time, species, animal number, sex, age at necropsy, ACUC protocol number and clinical history. Additional information includes amount and time of ketamine sedation and pentobarbital administration, start time for the craniotomy and PMI for harvest of each tissue type.
Medical and experimental history
This animal model offers the ability to control and longitudinally track every aspect of each animal’s experimental history from the naïve state through chronic exposure including diet and housing. For ethical and logistical reasons, this cannot be done in humans. Longitudinal measures include blood ethanol concentrations and clinical chemistry measures during the study (Table 3). Medical history, including all medications for clinical purposes, is recorded throughout the duration of the study. Procedures to which animals are subjected are recorded and tracked, including sedation for imaging procedures.
Table 3.
Clinical Chemistry values (unit of measure and range) measured every 4–6 months during the self-administration protocol
|
Gamma- Glutamyltransferase IU/L 0–55 |
Glucose mg/dl 45–90 |
Sodium mmol/L 140–155 |
RBC 106/mm3 5.0–6.5 |
WBC 103/mm3 6.0–15.0 |
Protein g/dl 6.0–8.0 |
RDW % 10.0–20.0 |
|
Alkaline phosphate IU/L 30–120 |
Triglyceride mg/dl 30–200 |
Potassium mmol/L 3.4–4.2 |
HGB g/dl 11.0–14.0 |
NEU 103/mm3 2.0–8.0 |
Albumin g/dl 3.0–4.1 |
PLT 103/mm3 250–650 |
|
SGOT (AST) IU/L 5.0–40 |
Cholesterol mg/dl <200 |
Chloride mmol/L 100–115 |
HCT % 35–45 |
LYM 103/mm3 1.0–5.0 |
Globulin g/dl 2.8–3.8 |
MPV µm3 6.0–10.0 |
|
SGPT (ALT) IU/L 0–37 |
LDH IU/L 90–240 |
Calcium mg/dl 8.8–10.3 |
MCV µm3 65–80 |
MON 103/mm3 0.1–1.0 |
A/G ratio 0.6–2.8 |
|
|
Urea Nitrogen (BUN) mg/dl 9.0–24 |
Total Bilirubin Mg/dl 0.2–1.2 |
Phosphorus mg/dl 3.0–5.5 |
MCH pg 20–34 |
EOS 103/mm3 0.0–0.4 |
||
|
Creatinine mg/dl 0.3–1.0 |
Direct Bilirubin mg/dl 0.0–0.2 |
Iron µg/dl 45–160 |
MCHC g/dl 28–36 |
BAS 103/mm3 0.0–0.2 |
||
|
BUN/Creatinine ratio 8.0–24 |
RNA quality assurance
Tissue is assayed regularly to ensure RNA and protein integrity using a 2100 Bioanalyzer (Agilent Technologies) and NanoDrop 1000 (Nanodrop, Wilmington, DE) to assess size, purity and distribution of proteins and RNA. Samples are tested from blocks of tissue from a control and ethanol animal of each cohort. Electropherograms and concentration data are documented and made available to approved investigators. Total RNA is isolated as described (Hemby et al., 2005; O’Conner et al., 2006). Following RNA isolation, sample integrity is assessed by capillary gel electrophoresis with the Agilent Bioanalyzer 2100 and RNA 6000 NanoLabChips (Agilent, Palo Alto, CA). Total RNA concentration is determined by UV spectrophotometric analysis (A260/A280 ratio; Nanodrop 1000, Wilmington, DE).
Additional quality control measures taken by the recipient are included in the annual progress report. Records of RNA quality measured at the tissue bank are archived and subsequent results from the recipient investigator are incorporated into the MATRR informatics in order to track tissue integrity. Current policy of the MATRR is to isolate RNA and DNA which are then distributed as requested in order to better control for the quality of samples rather than depend on different isolation methods across laboratories.
Protein concentration can be assayed by isolating total protein homogenate and quantifying using the bicinchoninic acid (BCA) protein assay. Samples are diluted in Laemmli sample buffer to achieve the equivalent final protein concentrations, electrophoresed on SDS-PAGE gels and transferred to PVDF membranes. Membranes are stained with Ponceau stain to assess size distribution. Membranes are blocked using Odyssey blocking buffer. Protein visualization is accomplished with AlexFluor680 and IRDye800 labeled secondary antibodies using a Licor Odyssey infrared scanner. Signals can be quantified with Odyssey software. Equal protein loading is confirmed by probing the blots with a monoclonal β-tubulin antibody.
Bioinformatics
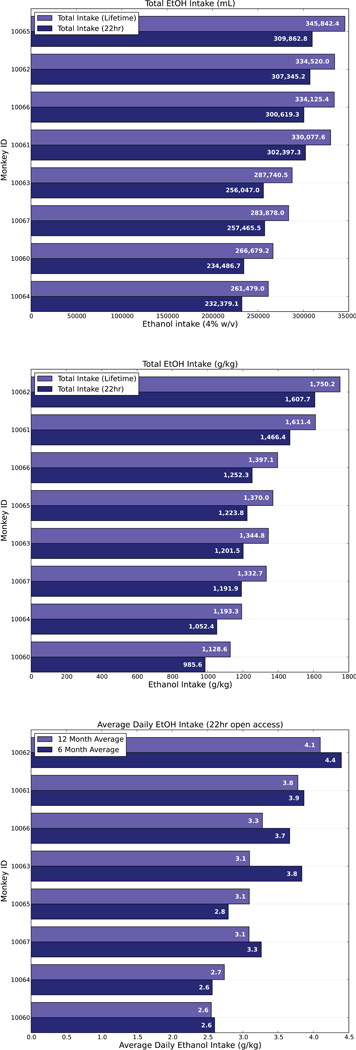
The MATRR website is supported by a web-accessible portal available to all researchers pending administrative approval and verification. Demographic and experimental data are compiled on all animals. Currently, the MATRR maintains information derived from 17 publically available cohorts including animal ID, birthdate and sex. Weight, necropsy date and drinking category for each animal is archived under ‘Cohort Details’. Individual cohort plots provide total lifetime intake in mls, g/kg and average g/kg intake over the 22 hr free access phase (Figure 1). Length of ethanol or control solution exposure, including start and end dates are archived under ‘Cohort Timeline’. Publications arising from tissue use or drinking data are linked to the relevant cohort. CNS and peripheral tissues are also available for assay development. Tissue requests are approved by a Steering Committee. If a request collision (multiple requests for the same tissue) cannot be resolved by the Steering Committee, a Scientific Advisory Board ensures that the distribution of tissues occurs with a minimum of bias. The PIs of the MATRR are currently conducting our own hypothesis testing on tissues using Next Generation Sequencing and will release the results once the data are published. In order to partially offset costs of dissecting and handling of tissues, users are charged a use/handling fee when requesting experimental tissue. There is no charge if tissue is requested for assay development. Users are required to cover shipping costs.
Figure 1.
Plots demonstrating total lifetime ethanol intake measured in mls, g/kg and average g/kg intake over the 22 hr free access phase. Plots are available on an individual cohort basis.
While all verified users can request tissues, access to raw and derived data sets are more tightly controlled. Typical users have access to compiled animal metadata for the purposes of allowing researchers to make better-informed decisions on which cohorts or tissues to select for a study, but users can request more granular raw data based on collaborative research needs or proposed research study, as determined by the Steering Committee. A unique feature of the resource is its bidirectional nature. Once investigators complete a study and publish their findings, they agree to submit appropriate data back into the MATRR in order to reintegrate data derived from the analysis of distributed tissues and to build a more complete profile on each cohort.
The environment relies on an open source and extensible architecture, including a Postgres database backend and the Django web framework and ORM. The database is versioned daily and archived every 90 days, and all MATRR source code resides within a Git repository, an open source distributed version control system. From a data perspective, the resource attempts to collect and harmonize data across a broad range of experimental domains and data types, currently including behavioral drinking data, necropsy data, hormonal and protein data, tissue inventory, relevant publications, and links to external analysis tool sets for functional genomics, such as Gene Weaver. The resource is extensible to collect genetic data, metabolomics and electrophysiological data. Each data point references particular animals and is presented and maintained with the primary objective to provide pertinent information to individuals requesting tissues.
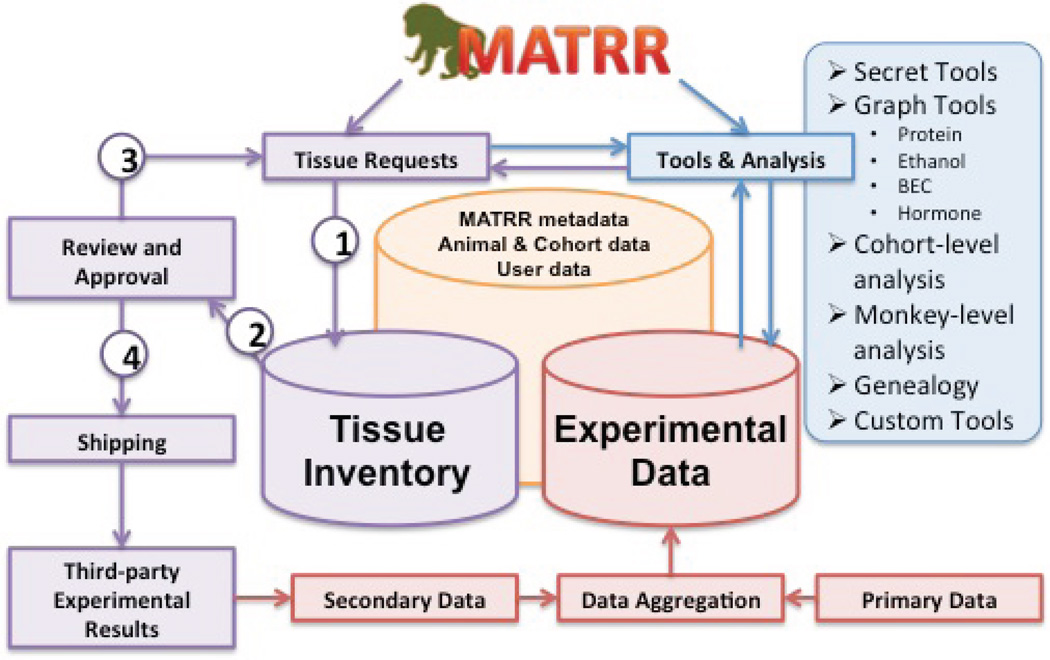
Operationally, the environment is designed to dynamically support three general categories of functionality (Figure 2). First, MATRR offers fully functional user account support and management, including qualified researcher information, MTA tracking, tissue requests, reporting, and related information. In addition, MATRR offers a detailed methodology for administrators to track inventory from collection through shipment, monitor and evaluate tissue requests, and monitor diagnostics related to cohort progress. Secondly, MATRR is designed to track third party experimental outcomes as a result of distributed tissues and, once a study is completed and published, encourage the PI to provide a summary of the results, including relevant data, for inclusion back into the core MATRR data set. Finally, MATRR resources support a broad range of data analysis and data mining functionality. While the central mandate encompassed by providing tissue request and sample management represents a compelling need for this community resource, the added value of coupling data analysis within the request platform provides a means to directly leverage experimental results for hypothesis driven inquiry.
Figure 2. Schematic outline of the Monkey Alcohol and Tissue Research Resource.
Operationally, the MATRR web interface segregates responsibility into three distinct features. The primary features involve data dedicated to inventory tracking, user and administrative management of tissue requests and shipping (purple). Pipelines allow users to (1) request tissues that are (2) reviewed and approved by the scientific committee. Processed requests can be (3) denied or returned for modifications or (4) accepted and shipped to participating investigators. Other parts of the site (blue) contain a variety of analysis tools while the third section (red) is intended to aggregate data from primary behavioral protocols and data returned from tissue recipients.
In order to aid global analysis while maintaining functionality, tools are divided into two layers. One layer faces the public interface and allows users to identify cohorts or animals based on broad characteristics of drinking behaviors, such as ethanol intakes, BEC data, and, in some cases, protein and hormonal information. This gives researchers the ability to rapidly identify possible animals, tissues or cohorts of interest. In other cases, such as mining multivariate associative data or analyzing non-published primary data, tools are made available only to a subset of users by request. In many cases, both public and private facing pages offer static representations of the data due to query and processing time. Even with certain results pre-computed, users can gain enormous insight into how animals behave collectively and independently. An additional advanced search interface attempts to filter a range of possible results of interest by a variety of pre-computed metrics.
Results
To date we have banked all CNS and peripheral tissues from approximately 200 cases with known experimental and medical histories (Table 2). Tissues have also been banked for assay development from animals with documented histories but in unrelated studies. Prior to formal funding by NIAAA in 2010, the predominant means of supplying tissues to collaborators was accomplished through direct correspondence and preset collaborations. Initial interactions involved 6 investigators at Wake Forest School of Medicine. Through the subsequent years this resource has grown dramatically to include interactions with over 90 investigators across more than 25 institutions in the US and across the globe. A decade ago when we first began harvesting tissue, the majority of CNS tissue requests utilized fresh tissue for in vitro electrophysiology and voltammetry studies. These functional studies comprised approximately 75% of the tissue requests, the remainder of which utilized molecular approaches. Currently, 75–85% of current tissue requests utilize frozen tissues for molecular assays while 10–15% utilize fresh tissue for recording studies. These functional studies highlight a distinct advantage of our animal model – living tissue can be utilized for recording studies. This is not feasible in tissue acquired from human brain banks. A fraction of tissue requests requires tissue that has been post-fixed in paraformaldehyde or formalin for histological studies.
The resources generated from MATRR have been in high demand. Being limited to banking and disseminating tissue minimized the larger potential for this resource to inform on disease processes associated with excessive ethanol self-administration through integrative analysis across all tissue recipients’ data. In short, each monkey carries a unique gene set that can be informative of the risk for developing excessive drinking as well as the tissue response that results in disease states. Now these tissues and associated bioinformatics are widely available to the larger alcohol research community through the interactive MATRR website. Since its inception, the MATRR has contributed resources that have been cited in 70 peer-reviewed publications, including reports of the functional (Ariwodola et al., 2003; Budygin et al., 2003; Floyd et al., 2004; Alexander et al., 2006; Carden et al., 2006; Anderson et al., 2007; Cuzon-Carlson et al., 2012; Welsh et al., 2011), genomic (Hemby et al., 2006; Acosta et al., 2009; Burnett et al., 2012), proteomic (Freeman et al., 2006, 2010, 2011), biochemical (Ivester et al., 2003; 2007; Lebold et al., 2010) and cardiac (Cheng et al., 2010) consequences of long-term, excessive ethanol consumption. The pathophysiological effects of chronic EtOH on the hypothalamic-pituitary-adrenal axis have also been well-characterized (Morrow et al., 2006; Porcu et al., 2006a, b, 2010; Ferguson et al., 2012; Helms et al., 2012a, b, c, 2013). More importantly perhaps, since the initial tissue harvest, our interactions have resulted in the funding of over 70 intramural and extramural grants, spanning a wide array of mechanisms including R01, U01, R03, R21 and foundation grants at the individual investigator level and multiple P-type awards including P01, P20 and P50 mechanisms. Additional grants are currently pending review.
Discussion
Appropriate animal models are critical for establishing a better understanding of mechanisms underlying human pathologies, especially substance abuse disorders. Animal models of drug self-administration are valid predictors of human drug abuse (Griffiths et al., 1980; Johanson and Fischman, 1989; Johanson and Schuster, 1981). The ethanol self-administration paradigm that is the basis of the MATRR results in consumption that parallel patterns and levels of intake observed in alcoholics (Vivian et al., 2001; Monti et al., 2004; Grant et al., 2008a).
Reliance on self-report can be problematic and error-prone in assessing lifetime intake, especially following decades of abuse in human alcoholics. Our experimental design allows exquisite control over all experimental variables that mediate ethanol intake and allows us to track and document precise amounts of intake and drinking behavior in ways that are not possible in human studies. We control factors such as nutritional status, housing conditions, medical care and exposure to drugs used for veterinary care. In addition, recent technological advances have made it possible to fully genotype animals, thus encouraging intricate analyses and correlations to be conducted related to phenotype. Genotyping information is available for many of the animals upon request by emailing the MATRR administration.
A multidisciplinary team comprised of anatomists, pathologists, biostatisticians, bioinformatics specialists, molecular biologists and researchers with expertise in behavioral pharmacology are involved in obtaining tissues and data for the MATRR. Consistency among personnel and standard operating procedures are necessary for optimizing the end results and maintaining quality control. Standardized EtOH self-administration and tissue collection procedures are followed across sites. Annual updates and training sessions are implemented in order to protect against drift in standard procedures. The consistency of identical self-administration and tissue harvest procedures contrasts with the heterogeneous nature of tissues that comprise human brain banks, and reduces variables that can confound human studies. Another critical advantage of our animal model is that all studies include age-matched control animals. To address the shortage of control tissue, some human tissue banks utilize a strategy of encouraging pre-mortem tissue donations through such donor programs as the “Using our Brains” initiative that was started by the New South Wales Tissue Resource Center at the University of Sydney, Australia (Sheedy et al., 2008).
Our primary goal is to build and distribute the resources of this state-of-the-art tissue bank, along with associated bioinformatics to the broader alcohol research community. It is clear from the number of publications and funded grants that the MATTR has provided a significant return on the initial investment. The opportunity to conduct hypotheses–driven investigations using this NHP model of chronic EtOH abuse helps bridge the gap between rodent models of alcohol abuse and human alcoholism and furthers our understanding of alcohol use disorders. The ability to track neuro- and patho-physiological consequences of alcohol abuse beginning with naïve animals that progress to the abuse state provides a level of analyses not possible with human studies. This is in addition to the obvious advantage of conducting various assays in viable CNS tissue. The experimental design ultimately allows various behavioral and pharmacological interventions to be applied at various stages of drinking. The self-administration model elicits consistent results across three NHP species regardless of age or sex of the animals. The longitudinal nature and the uniformity of the experimental design combined with broad data collection and aggregation allows for future expansion into co-morbid drug studies with depressants and stimulants, highlighting one translational potential of this resource.
Acknowledgements
Supported by AA019431 (KAG, EJB, JBD) and AA013510 (KAG).
REFERENCES
- Acosta G, Hasenkamp W, Daunais JB, Friedman DP, Grant KA, Hemby SE. Ethanol self-administration modulation of NMSA receptor subunit and related synaptic protein mRNA expression in prefrontal cortical fields. Brain Res. 2010;1318:144–154. doi: 10.1016/j.brainres.2009.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GM, Carden WB, Mu J, Kurukulasuriya NC, McCool BA, Norskog BK, Friedman DP, Daunais JB, Grant KA, Godwin DW. The native T-type calcium current in relay neurons of the primate thalamus. Neuroscience. 2006;141(1):453–461. doi: 10.1016/j.neuroscience.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Anderson NJ, Daunais JB, Friedman DP, Grant KA, McCool BA. Long-term ethanol self-administration by the nonhuman primate, Macaca fascicularis, decreases benzodiazepine sensitivity of amygdala GABAA receptors. Alcohol Clin Exp. Res. 2007;31:1061–1070. doi: 10.1111/j.1530-0277.2007.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariwodola OJ, Crowder TL, Grant KA, Daunais JB, Friedman DP, Weiner JL. Ethanol modulation of excitatory and inhibitory synaptic transmission in rat and monkey dentate granule neurons. Alcohol Clin Exp. Res. 2003;27:1632–1639. doi: 10.1097/01.ALC.0000089956.43262.17. [DOI] [PubMed] [Google Scholar]
- Budygin EA, John CE, Mateo Y, Daunais JB, Friedman DP, Grant KA, Jones SR. Chronic ethanol exposure alters presynaptic dopamine function in the striatum of monkeys: a preliminary study. Synapse. 2003;50:266–268. doi: 10.1002/syn.10269. [DOI] [PubMed] [Google Scholar]
- Burnett EJ, Davenport AT, Grant KA, Friedman DP. The effects of chronic ethanol self–administration on hippocampal serotonin transporter density in monkeys. Frontiers Psychiatry. 2012 doi: 10.3389/fpsyt.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carden WB, Alexander GM, Friedman DP, Daunais JB, Grant KA, Mu J, Godwin DW. Chronic ethanol drinking reduces native T-type calcium current in the thalamus of nonhuman primates. Brain Res. 2006;1089(1):92–100. doi: 10.1016/j.brainres.2006.02.135. [DOI] [PubMed] [Google Scholar]
- Cheng HJ, Grant KA, Han QH, Daunais JB, Friedman DP, Masutani S, Little WC, Cheng CP. Up-regulation and functional effect of cardiac β3-adrenoreceptors in alcoholic monkeys. Alcohol Clin Exp. Res. 2010;34:1171–1181. doi: 10.1111/j.1530-0277.2010.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzon-Carlson VC, Seabold GK, Helms CM, Garg N, Odagiri M, Rau AR, Daunais J, Alvarez VA, Lovinger DM, Grant KA. Synaptic and Morphological Neuroadaptations in the Putamen Associated with Long-Term, Relapsing Alcohol Drinking in Primates. Neuropsychopharmacol. 2011;36(12):2513–28. doi: 10.1038/npp.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport AT, Grant KA, Szeliga KT, Friedman DP, Daunais JB. Standardized Method for the Harvest of Nonhuman Primate Tissue Optimized for Multiple Modes of Analyses. Cell Tissue Banking. 2013 doi: 10.1007/s10561-013-9380-2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daunais JB, Kraft RA, Davenport AT, Burnett EJ, Maxey VM, Szeliga KT, Flory GS, Hemby SE, Kroenke CD, Grant KA, Friedman DP. MRI-guided dissection of the nonhuman primate brain: a case study. Methods. 2010;50:199–204. doi: 10.1016/j.ymeth.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd DW, Friedman DP, Daunais JB, Pierre PJ, Grant KA, McCool BA. Long-term Ethanol Self-administration by Cynomolgus Macaques Alters the Pharmacology and Expression of GABA(A) Receptors Expressed in Basolateral Amygdala. J Pharmacol Exp Ther. 2004;311:1071–1079. doi: 10.1124/jpet.104.072025. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Gooch RS, Lull ME, Worst TJ, Walker SJ, Xu AS, Green H, Pierre PJ, Grant KA, Vrana KE. Apo-AII is an elevated biomarker of chronic non-human primate ethanol self-administration. Alcohol Alcohol. 2006;41(3):300–5. doi: 10.1093/alcalc/agl021. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Salzberg AC, Gonzales SW, Grant KA, Vrana KE. Classification of alcohol abuse by plasma protein biomarkers. Biol Psych. 2010;68(3):219–222. doi: 10.1016/j.biopsych.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman WM, Vanguilder HD, Guidone E, Krystal JH, Grant KA, Vrana KE. Plasma proteomic alterations in non-human primates and humans after chronic alcohol self-administration. Int J Neuropsychopharmacol. 2011;14(7):899–911. doi: 10.1017/S1461145711000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson B, Hunter JE, Luty J, Street SL, Woodall A, Grant KA. Genetic load is associated with hypothalamic-pituitary-adrenal axis dysregulation in macaques. Genes Brain Behav. 2012 doi: 10.1111/j.1601-183X.2012.00856.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Leng XY, Green HL, Rogers LSM, Szeliga K, Gonzales SW. Drinking typography established by scheduled-induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp. Res. 2008a;32(10):1–31. doi: 10.1111/j.1530-0277.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Stafford J, Thiede A, Kiley C, Odagiri M, Ferguson B. Who’s at risk? Population characterization of alcohol self-administration in nonhuman primates helps identify pathways to dependence. Alcohol Research and Health. 2008b;31(4) [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Liebson I, Kaliszak JE. Drug preference in humans: double-blind choice comparison of pentobarbital, diazepam and placebo. J Pharmacol Exp Ther. 1980;215(3):649–61. [PubMed] [Google Scholar]
- Helms CM, Messaoudi I, Jeng S, Freeman WM, Vrana KE, Grant KA. A longitudinal analysis of circulating stress-related proteins and chronic ethanol self-administration in cynomolgus macaques. Alcohol Clin Exp. Res. 2012a;36(6):995–1003. doi: 10.1111/j.1530-0277.2011.01685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, McClintick MN, Grant KA. Social rank, chronic ethanol self-administration and diurnal pituitary-adrenal activity in cynomolgus monkeys. Psychopharmacol. 2012b;224(1):133–143. doi: 10.1007/s00213-012-2707-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Rossi D, Grant KA. Neurosteroid effects on sensitivity to ethanol. Front Endocrinol (Lausanne) 2012c;3:10. doi: 10.3389/fendo.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Gonzales SW, Green HL, Szeliga KT, Rogers LS, Grant KA. Diurnal pituitary-adrenal activity during schedule-induced polydipsia of water and ethanol in cynomolgus monkeys (Macaca fascicularis) Psychopharmacology. 2013 doi: 10.1007/s00213-013-3052-6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Tang W, Horman B. Regulation of glutamate receptor subunits following cocaine self- administration. Brain Research. 2005;1064:75–82. doi: 10.1016/j.brainres.2005.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, O’Conner JA, Acosta G, Floyd D, Anderson N, McCool BA, Friedman D, Grant KA. Ethanol-induced regulation of GABA-A subunit mRNAs in prefrontal fields of cynomolgus monkeys. Alcohol Clin Exp. Res. 2006;30(12):1978–1985. doi: 10.1111/j.1530-0277.2006.00254.x. [DOI] [PubMed] [Google Scholar]
- Ivester P, Shively CA, Register TC, Grant KA, Cunningham C. The effects of moderate ethanol consumption on the liver of the monkey, Macaca fascicularis. Alcohol Clin Exp. Res. 2003;27(11):1831–1837. doi: 10.1097/01.ALC.0000095633.26284.FA. [DOI] [PubMed] [Google Scholar]
- Ivester P, Young T, Vivian J, Lees C, Daunais J, Friedman D, Rippe RA, Parsons C, Grant KA, Cunningham CC. Ethanol self-administration and alterations in the livers of the cynomolgus monkey. Alcohol Clin Exp Res. 2007;31:144–155. doi: 10.1111/j.1530-0277.2006.00276.x. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Schuster CR. An analysis of drug-seeking behavior in animals. Neurosci Biobehav Rev Autumn. 1981;5(3):315–323. doi: 10.1016/0149-7634(81)90026-9. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Fischman MW. The pharmacology of cocaine related to its abuse. Pharmacol Rev. 1989;41:3–52. [PubMed] [Google Scholar]
- Lebold KM, Grant KA, Freeman WM, Wiren KM, Miller GW, Kiley C, Leonard SW, Traber MG. Individual Differences in Hyperlipidemia and Vitamin E Status in Response to Chronic Alcohol Self-Administration in Cynomolgus Monkeys. Alcohol Clin Exp Res. 2011;35(3):474–483. doi: 10.1111/j.1530-0277.2010.01364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Experimentally induced intoxication in alcoholics: A comparison between programmed and spontaneous drinking. J Pharmacol Exper Therap. 1970;173:101–116. [PubMed] [Google Scholar]
- Monti PM, Tidey J, Czachowski CL, Grant KA, Rohsenow DJ, Sayette M, Maners N, Pierre P. Building bridges: the transdisciplinary study of craving from the animal laboratory to the lamppost. Alcohol Clin Exp. Res. 2004;28(2):279–287. doi: 10.1097/01.alc.0000113422.04849.fa. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Porcu P, Boyd KN, Grant KA. Hypothalamic-pituitary-adrenal axis modulation of GABAergic neuroactive steroids influences ethanol sensitivity and drinking behavior. Dialogues Clin Neurosci. 2006;8:463–477. doi: 10.31887/DCNS.2006.8.4/amorrow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan PE, O’Brien JS, Norton D. In: Comparative studies of the interpersonal and affective behavior of alcoholics and nonalcoholics during prolonged experimental drinking, in Recent Advances in Studies of Alcoholism. Mello NK, Mendelson JH, editors. Washington, DC: U.S. Gov. Printing Office (Pub. No 71–9045; 1971. pp. 619–646. [Google Scholar]
- O’Connor J, Hasenkamp W, Horman B, Muly EC, Hemby SE. Region specific regulation of NR1 subunit in rhesus monkeys following chronic antipsychotic drug administration. Biological Psychiatry. 2006;60:659–662. doi: 10.1016/j.biopsych.2006.03.044. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Sullivan EV. Alcoholism and AIDS: magnetic resonance imaging approaches for detecting interactive neuropathology. Alcohol Clin Exp. Res. 2002;26:1031–1046. doi: 10.1097/01.ALC.0000021146.01778.55. [DOI] [PubMed] [Google Scholar]
- Porcu P, Grant KA, Green HL, Rogers LS, Morrow AL. Hypothalamic-pituitary-adrenal axis and ethanol modulation of deoxycorticosterone levels in cynomolgus monkeys. Psychopharmacol. 2006a;186(3):293–301. doi: 10.1007/s00213-005-0132-2. [DOI] [PubMed] [Google Scholar]
- Porcu P, Rogers LS, Morrow AL, Grant KA. Plasma pregnenolone levels in cynomolgus monkeys following pharmacological challenges of the hypothalamic-pituitary-adrenal axis. Pharmacol Biochem Behav. 2006b;84(4):618–27. doi: 10.1016/j.pbb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Porcu P, O’Buckley TK, Alward SE, Song SC, Grant KA, de Wit H, Morrow AL. Differential effects of ethanol on serum GABAergic 3α,5α/3α,5β neuroactive steroids in mice, rats, cynomolgus monkeys and humans. Alcohol Clin Exp. Res. 2010;34:432–442. doi: 10.1111/j.1530-0277.2009.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheedy D, Garrick T, Dedova I, Hunt C, Miller R, Sundqvist N, Harper C. An Australian brain bank: a critical investment with a high return! Cell Tissue Banking. 2008;9:205–216. doi: 10.1007/s10561-008-9076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majersky LJ, Thomas BW, Shively CA, MA Nader, Grant KA. Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): Long-term characterization of sex and Individual differences. Alcohol Clin Exp. Res. 2001;25:1087–1097. [PubMed] [Google Scholar]
- Welsh J, Han VV, Rossi D, Mohr C, Odigari M, Daunais J, Grant K. Bidirectional plasticity in the primate inferior olive induced by chronic ethanol intoxication and sustained abstinence. PNAS. 2011;108:10314–10319. doi: 10.1073/pnas.1017079108. [DOI] [PMC free article] [PubMed] [Google Scholar]