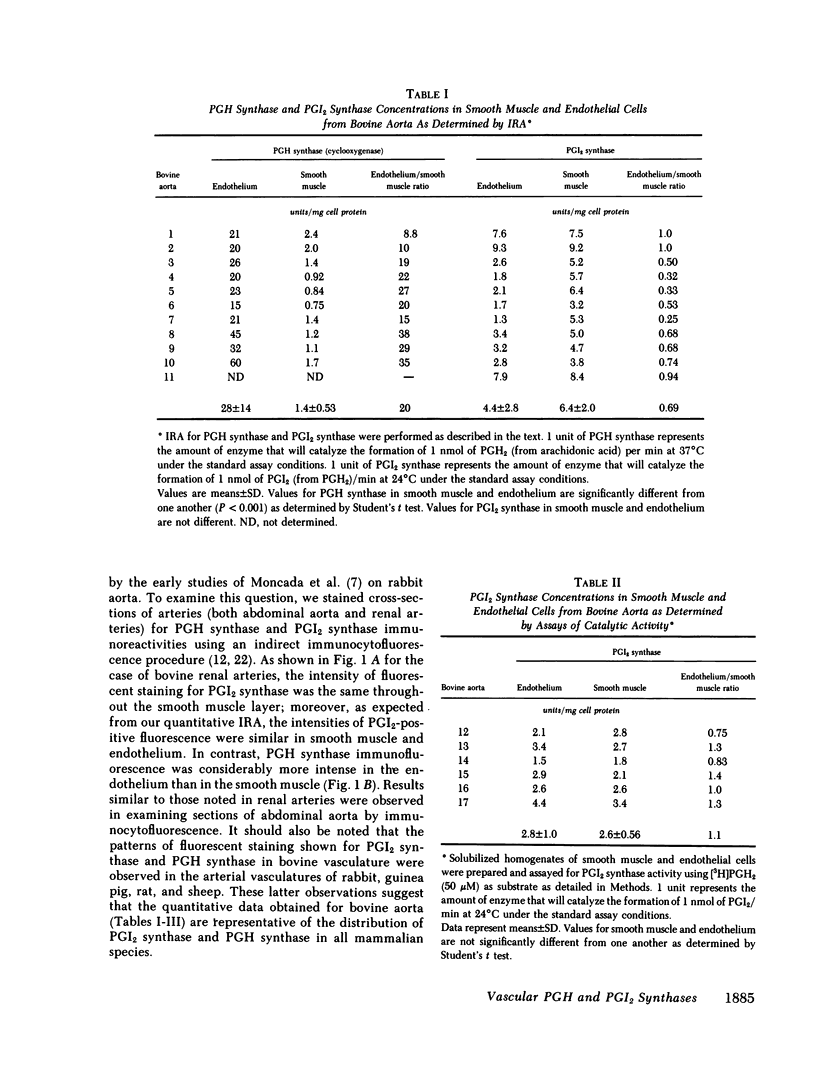
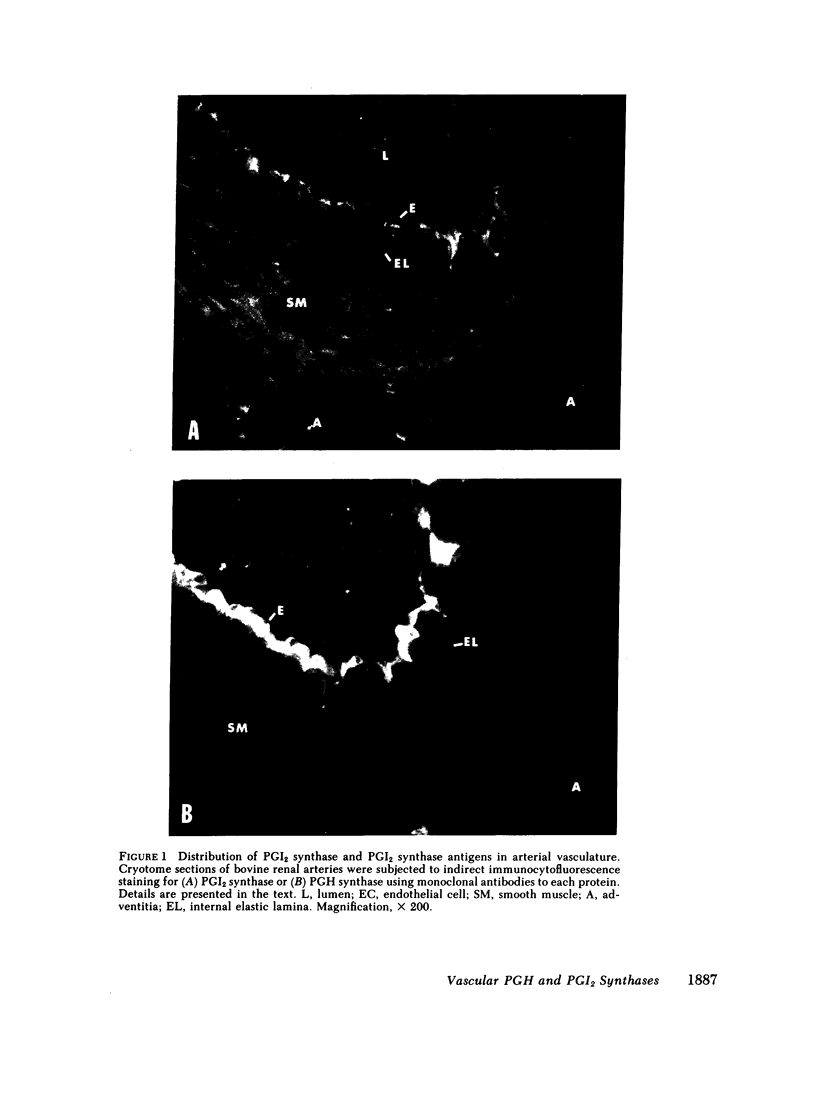
Abstract
Platelets adhere to the subendothelial layer of newly deendothelialized arteries. Attachment can be reduced with exogenous prostacyclin (PGI2). Thus, the subendothelium may be unable to produce sufficient PGI2 to prevent platelet adherence and subsequent platelet-platelet interaction. Consistent with this explanation are data from an earlier report (1977. Moncada S., A. G. Herman, E. A. Higgs, and J. R. Vane. Thromb. Res. 11:323-344) indicating that the smooth muscle layer of aorta has only 10-15% of the capacity of endothelial cells to synthesize PGI2. We have measured the concentrations of PGI2 synthase and prostaglandin endoperoxide (PGH) synthase in bovine aorta and obtained results quite different from those described in this earlier report. Tandem immunoradiometric assays for PGI2 synthase and PGH synthase antigens were used to quantitate these proteins in detergent-solubilized homogenates of endothelial cells and smooth muscle tissue prepared from 10 different bovine aorta. The concentrations of PGI2 synthase in endothelial cells and smooth muscle were found to be the same. However, the concentration of PGH synthase in endothelial cells averaged greater than 20 times that of smooth muscle. Results similar to those determined by immunoradiometric assay were also obtained when PGH synthase and PGI2 synthase catalytic activities were measured in preparations of endothelial and smooth muscle cells. Furthermore, when bovine aorta and renal arteries were subjected to immunocytofluorescence staining using monoclonal antibodies to PGI2 synthase, fluorescence staining of equivalent intensity was detected in both the endothelial cells and the smooth muscle. Moreover, the intensity of fluorescence was similar throughout cross-sections of vascular smooth muscle, indicating that there is no gradient in PGI2 synthase concentrations between the endothelium and adventitia. Our results indicate that the propensity of platelets to adhere to the subendothelium of deendothelialized arteries and form aggregates cannot be attributed simply to an inability of the denuded vasculature to produce PGI2 from PGH2, but may be a consequence of the low PGH synthase activity of smooth muscle. Consistent with this concept are the results of Eldor et al. (1981. J. Clin. Invest. 67:735-741) who reported that increases in PGH synthase activity are associated with formation of a nonthrombogenic neointima.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ager A., Gordon J. L., Moncada S., Pearson J. D., Salmon J. A., Trevethick M. A. Effects of isolation and culture on prostaglandin synthesis by porcine aortic endothelial and smooth muscle cells. J Cell Physiol. 1982 Jan;110(1):9–16. doi: 10.1002/jcp.1041100103. [DOI] [PubMed] [Google Scholar]
- Baenziger N. L., Becherer P. R., Majerus P. W. Characterization of prostacyclin synthesis in cultured human arterial smooth muscle cells, venous endothelial cells and skin fibroblasts. Cell. 1979 Apr;16(4):967–974. doi: 10.1016/0092-8674(79)90111-9. [DOI] [PubMed] [Google Scholar]
- Bourgain R. H., Andries R., Six F. Role of prostaglandin biochemical pathway in platelet-vessel wall interaction and local thrombosis. Haemostasis. 1982;11(3):133–138. doi: 10.1159/000214653. [DOI] [PubMed] [Google Scholar]
- Bunting S., Gryglewski R., Moncada S., Vane J. R. Arterial walls generate from prostaglandin endoperoxides a substance (prostaglandin X) which relaxes strips of mesenteric and coeliac ateries and inhibits platelet aggregation. Prostaglandins. 1976 Dec;12(6):897–913. doi: 10.1016/0090-6980(76)90125-8. [DOI] [PubMed] [Google Scholar]
- Cazenave J. P., Dejana E., Kinlough-Rathbone R., Packham M. A., Mustard J. F. Platelet interactions with the endothelium and the subendothelium: the role of thrombin and prostacyclin. Haemostasis. 1979;8(3-5):183–192. doi: 10.1159/000214310. [DOI] [PubMed] [Google Scholar]
- Curwen K. D., Gimbrone M. A., Jr, Handin R. I. In vitro studies of thromboresistance: the role of prostacyclin (PGI2) in platelet adhesion to cultured normal and virally transformed human vascular endothelial cells. Lab Invest. 1980 Mar;42(3):366–374. [PubMed] [Google Scholar]
- DeWitt D. L., Day J. S., Gauger J. A., Smith W. L. Monoclonal antibodies against PGH synthase: an immunoradiometric assay for quantitating the enzyme. Methods Enzymol. 1982;86:229–240. doi: 10.1016/0076-6879(82)86194-6. [DOI] [PubMed] [Google Scholar]
- DeWitt D. L., Rollins T. E., Day J. S., Gauger J. A., Smith W. L. Orientation of the active site and antigenic determinants of prostaglandin endoperoxide synthase in the endoplasmic reticulum. J Biol Chem. 1981 Oct 25;256(20):10375–10382. [PubMed] [Google Scholar]
- DeWitt D. L., Smith W. L. Monoclonal antibodies against PGI2 synthase: an immunoradiometric assay for quantitating the enzyme. Methods Enzymol. 1982;86:240–246. doi: 10.1016/0076-6879(82)86195-8. [DOI] [PubMed] [Google Scholar]
- DeWitt D. L., Smith W. L. Purification of prostacyclin synthase from bovine aorta by immunoaffinity chromatography. Evidence that the enzyme is a hemoprotein. J Biol Chem. 1983 Mar 10;258(5):3285–3293. [PubMed] [Google Scholar]
- Dejana E., Cazenave J. P., Groves H. M., Kinlough-Rathbone R. L., Richardson M., Packham M. A., Mustard J. F. The effect of aspirin inhibition of PGI2 production on platelet adherence to normal and damaged rabbit aortae. Thromb Res. 1980 Feb 1;17(3-4):453–464. doi: 10.1016/0049-3848(80)90080-8. [DOI] [PubMed] [Google Scholar]
- Eldor A., Falcone D. J., Hajjar D. P., Minick C. R., Weksler B. B. Recovery of prostacyclin production by de-endothelialized rabbit aorta. Critical role of neointimal smooth muscle cells. J Clin Invest. 1981 Mar;67(3):735–741. doi: 10.1172/JCI110090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman R. R., Fitzpatrick F. A., Miller O. V. Reciprocal regulation of human platelet cAMP levels by thromboxane A2 and prostacyclin. Adv Cyclic Nucleotide Res. 1978;9:597–609. [PubMed] [Google Scholar]
- Hemler M., Lands W. E. Purification of the cyclooxygenase that forms prostaglandins. Demonstration of two forms of iron in the holoenzyme. J Biol Chem. 1976 Sep 25;251(18):5575–5579. [PubMed] [Google Scholar]
- Higgs E. A., Moncada S., Vane J. R., Caen J. P., Michel H., Tobelem G. Effect of prostacyclin (PGI2) on platelet adhesion to rabbit arterial subendothelium. Prostaglandins. 1978 Jul;16(1):17–22. doi: 10.1016/0090-6980(78)90197-1. [DOI] [PubMed] [Google Scholar]
- Huslig R. L., Fogwell R. L., Smith W. L. The prostaglandin forming cyclooxygenase of ovine uterus: relationship to luteal function. Biol Reprod. 1979 Oct;21(3):589–600. doi: 10.1095/biolreprod21.3.589. [DOI] [PubMed] [Google Scholar]
- Ingerman-Wojenski C., Silver M. J., Smith J. B., Macarak E. Bovine endothelial cells in culture produce thromboxane as well as prostacyclin. J Clin Invest. 1981 May;67(5):1292–1296. doi: 10.1172/JCI110157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973 Nov;52(11):2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelton J. G., Hirsh J., Carter C. J., Buchanan M. R. Thrombogenic effect of high-dose aspirin in rabbits. Relationship to inhibition of vessel wall synthesis of prostaglandin I2-like activity. J Clin Invest. 1978 Oct;62(4):892–895. doi: 10.1172/JCI109203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larrue J., Rigaud M., Daret D., Demond J., Durand J., Bricaud H. Prostacyclin production by cultured smooth muscle cells from atherosclerotic rabbit aorta. Nature. 1980 Jun 12;285(5765):480–482. doi: 10.1038/285480a0. [DOI] [PubMed] [Google Scholar]
- Macarak E. J., Howard B. V., Kefalides N. A. Properties of calf endothelial cells in culture. Lab Invest. 1977 Jan;36(1):62–67. [PubMed] [Google Scholar]
- Marcus A. J., Weksler B. B., Jaffe E. A., Broekman M. J. Synthesis of prostacyclin from platelet-derived endoperoxides by cultured human endothelial cells. J Clin Invest. 1980 Nov;66(5):979–986. doi: 10.1172/JCI109967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Moncada S., Herman A. G., Higgs E. A., Vane J. R. Differential formation of prostacyclin (PGX or PGI2) by layers of the arterial wall. An explanation for the anti-thrombotic properties of vascular endothelium. Thromb Res. 1977 Sep;11(3):323–344. doi: 10.1016/0049-3848(77)90185-2. [DOI] [PubMed] [Google Scholar]
- Moncada S., Vane J. R. The role of prostacyclin in vascular tissue. Fed Proc. 1979 Jan;38(1):66–71. [PubMed] [Google Scholar]
- Murota S., Chang W. C., Koshihara Y., Morita I. Importance of cyclooxygenase induction in the biosynthesis of prostacyclin. Adv Prostaglandin Thromboxane Leukot Res. 1983;11:99–104. [PubMed] [Google Scholar]
- Salmon J. A., Smith D. R., Flower R. J., Moncada S., Vane J. R. Further studies on the enzymatic conversion of prostaglandin endoperoxide into prostacyclin by porcine aorta microsomes. Biochim Biophys Acta. 1978 Mar 14;523(1):250–262. doi: 10.1016/0005-2744(78)90028-1. [DOI] [PubMed] [Google Scholar]
- Smith W. L., DeWitt D. L., Allen M. L. Bimodal distribution of the prostaglandin I2 synthase antigen in smooth muscle cells. J Biol Chem. 1983 May 10;258(9):5922–5926. [PubMed] [Google Scholar]
- Smith W. L., Lands W. E. Oxygenation of polyunsaturated fatty acids during prostaglandin biosynthesis by sheep vesicular gland. Biochemistry. 1972 Aug 15;11(17):3276–3285. doi: 10.1021/bi00767a024. [DOI] [PubMed] [Google Scholar]
- Smith W. L., Rollins T. E. Characteristics of rabbit anti-PGH synthase antibodies and use in immunocytochemistry. Methods Enzymol. 1982;86:213–222. doi: 10.1016/0076-6879(82)86192-2. [DOI] [PubMed] [Google Scholar]
- Weiss H. J., Turitto V. T. Prostacyclin (prostaglandin I2, PGI2) inhibits platelet adhesion and thrombus formation on subendothelium. Blood. 1979 Feb;53(2):244–250. [PubMed] [Google Scholar]
- Wlodawer P., Hammarström S. Some properties of prostacyclin synthase from pig aorta. FEBS Lett. 1979 Jan 1;97(1):32–36. doi: 10.1016/0014-5793(79)80045-9. [DOI] [PubMed] [Google Scholar]