Abstract
Emerging evidence suggests that myeloid-derived suppressor cells (MDSCs) have great potential as a novel immune intervention modality in the fields of transplantation and autoimmune diseases. Thus far, efforts to develop MDSC-based therapeutic strategies have been hampered by the lack of a reliable source of MDSCs. Here we show that functional MDSCs can be efficiently generated from mouse embryonic stem (ES) cells and bone marrow hematopoietic stem (HS) cells. In vitro-derived MDSCs encompass two homogenous subpopulations: CD115+Ly-6C+ and CD115+Ly-6C− cells. The CD115+Ly-6C+ subset is equivalent to the monocytic Gr-1+CD115+F4/80+ MDSCs found in tumor-bearing mice. In contrast, the CD115+Ly-6C− cells, a previously unreported population of MDSCs, resemble the granulocyte/macrophage progenitors developmentally. In vitro, ES- and HS-MDSCs exhibit robust suppression against T-cell proliferation induced by polyclonal stimuli or alloantigens via multiple mechanisms involving nitric oxide synthase-mediated NO production and interleukin (IL)-10. Impressively, they display even stronger suppressive activity and significantly enhance ability to induce CD4+CD25+Foxp3+ regulatory T-cell development compared with tumor-derived MDSCs. Furthermore, adoptive transfer of ES-MDSCs can effectively prevent alloreactive T-cell-mediated lethal graft-versus-host disease, leading to nearly 82% long-term survival among treated mice. The successful in vitro generation of MDSCs may represent a critical step toward potential clinical application of MDSCs.
Keywords: Myeloid-derived suppressor cells, Mouse embryonic stem cells, Mouse hematopoietic stem/progenitor cells, Differentiation, Graft-versus-host disease
Introduction
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of immature myeloid cells with immunoregulatory activity [1]. These cells, defined in mice by surface expression of CD11b (Mac-1) and Gr-1 (for granulocytic MDSCs) or CD11b, Gr-1, CD115, and F4/80 (for monocytic MDSCs), can suppress antigen-specific and nonspecific T-cell responses via diverse mechanisms [2–6]. Accumulating evidence supports that MDSCs play a pivotal role in various pathologic conditions. In animal tumor models [7, 8] and cancer patients [9–11], MDSCs, induced by tumor-derived factors, accumulate in large numbers in the blood, bone marrow, spleen, and tumor masses, mediating T-cell tolerance, thus leading to tumor escape and progression, tumor angiogenesis, and metastasis [12–16]. MDSCs, together with tumor-associated macrophages and T regulatory cells (Tregs) present in the tumor niche, are now considered the major factors responsible for the limited effectiveness or failure of cancer vaccines and other immunotherapies [17–21]. A critically important role for MDSCs in opportunistic infections, inflammation, and traumatic stress has also been described [22, 23].
The unique functional feature of MDSCs endows them with potential clinical merits. While it is widely accepted that MDSCs offer an appealing target for therapeutic intervention in cancer treatment [1, 24, 25], a growing body of evidence suggests that MDSCs per se have great therapeutic potential in the pathologic settings where deleterious or excessive immune responses need to be avoided or reduced. We (Zhou et al., manuscript submitted) and others [26] have demonstrated that upon adoptive transfer, MDSCs can efficiently prevent graft-versus-host disease (GVHD) without compromising the graft-versus-leukemia effects (GVL) following allogeneic hematopoietic stem cell transplantation (allo-HSCT) in murine models. Active induction of transplant tolerance by MDSCs has also been observed in allogeneic skin [27], kidney [28], and cardiovascular (Jordi C. Ochando, personal communication) transplant models. Recently, Haile et al. [29] assessed the role of MDSCs in inflammatory bowel disease (IBD), and found that cotransfer of MDSCs with HA-specific CD8+ T-cells into naïve VILLIN-HA mice inhibited T-cell-mediated intestinal injury and protected mice from T-cell-mediated chronic enterocolitis. These studies highlight a remarkable effect of MDSCs on the downregulation of immune response, which may provide ample therapeutic opportunities within the fields of transplantation and autoimmune diseases. However, the enthusiasm and efforts toward the use of MDSCs as a therapeutic modality have been dampened by the limited availability of these cells because of the paucity of MDSCs in healthy hosts or by the safety concerns surrounding the use of MDSCs recovered from tumor-bearing hosts. Furthermore, the lack of protocols for in vitro generation of specific MDSC subpopulations and characterization of differentiation stage of in vitro-generated MDSCs also prevents the potential clinical applications.
Embryonic stem (ES) cells have numerous attractive attributes, such as pluripotency, infinite self-renewal, and feasibility of genetic manipulation, making them an ideal source for producing large quantities of a specific cell type or tissue. Indeed, a variety of cell types, including many of hematopoietic origin, have been successfully derived from ES cells for cell replacement therapy or for other purposes. Here we described an efficient system in which functionally active MDSCs were generated from mouse ES cells. The same procedure was also applicable to the derivation of MDSCs from bone marrow hematopoietic stem (HS) cells. The ES-derived MDSCs (ESMDSCs) were comparable with those isolated from tumor-bearing mice (TD-MDSCs) in morphology, phenotypes, and development, but had stronger suppressive activity and significantly enhanced ability to induce Treg development. The in vitro differentiation of ES or HS cells into MDSCs may not only represent an important step toward the exploitation of MDSCs as a promising immunotherapeutic approach, but also prove useful in investigating the differentiation and accumulation of MDSCs.
Materials and Methods
ES Cell Maintenance
HoxB4-transduced ES cell line (HoxB4-ESs, 129SvEv background) was kindly given by Dr. George Daley (Harvard Medical School, Boston, MA). The generation of HoxB4-ES cell line has been described previously [30]. Expansion and maintenance of HoxB4-ES cells were conducted according to methods described previously [31]. J++ and V6.5 ES cell lines were generously provided by Drs. Sunita D'souza, and Valentina Fossati (Mount Sinai School of Medicine, New York, NY).
Differentiation of ES Cells into Myeloid-Derived Suppressor Cells
A three-step differentiation strategy was applied to generate myeloid suppressor cells from HoxB4-ESs as outlined in supporting information Figure S1. First, HoxB4-ESs were induced to undergo differentiation through a well-characterized process named embryonic bodies (EBs) formation. Day 6 (unless otherwise specified) EB-derived cells were seeded onto irradiated semiconfluent OP9 monolayer and cultured in Iscove's modified Dulbecco's media (IMDM) containing M1 cytokine mix consisting of 1% c-kit ligand (KL) conditioned medium (CM) [32, 33] or 5 ng/ml murine KL, 10 ng/ml murine IL-6, and 0.5% WEHI-3 (IL-3, CM) [32, 34] or 10 ng/ml murine IL-3. Two days later, the medium was removed and replaced with medium containing M2 cytokine mix consisting of 1% KL CM or 5 ng/ml murine KL, 10 ng/ml murine thrombopoietin (TPO), 40 ng/ml murine vascular endothelial growth factor (VEGF), and 50 ng/ml murine Flt-3L or M2 plus 10 ng/ml murine macrophage colony stimulating factor (M-CSF) (M2+M-CSF), and medium was changed every other day thereafter. Unless otherwise specified, nonadherent and loosely attached cells were harvested after 10-day coculture in M2+M-CSF and used for morphologic, phenotypic, developmental, and functional analysis. Murine recombinant KL, IL-6, IL-3, TPO, VEGF, fms-like tyrosine kinase receptor-3 ligand (Flt-3L), and M-CSF were purchased from PeproTech Inc. (Rocky Hill, NJ, http://www.peprotech.com).
Lineage Commitment and Colony-Forming Assay
For redifferentiation, fluorescence-activated cell sorting (FACS)-sorted CD115+Ly-6C− and CD115+Ly-6C+ cells were cultured in IMDM supplemented with 10% BenchMark™ fetal calf serum (Gemini Bio-Products, West Sacramento, CA, http://www.gembio.com), 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin, 1.5 × 10 −4 M MTG (monothioglycerol), and with the following murine growth factors: 5 ng/ml IL-6, 0.5% IL-3 CM or 10 ng/ml IL-3, 10 ng/ml IL-1β, 10 ng/ml basic fibroblast growth factor (bFGF), 10 ng/ml erythropoietin (EPO), 10 ng/ml TPO, 20 ng/ml VEGF, 25 ng/ml Flt-3L, 10 ng/ml M-CSF, and 5 ng/ml granulocyte-macrophage colony-stimulating factor (GMCSF). The resulting cells were analyzed by flow cytometry or May-Grünwald Giemsa staining. To evaluate colony-forming activity of CD115+Ly-6C− and CD115+Ly-6C+ cells in methylcellu-lose, the same culture condition for redifferentiation was used and colonies were scored at day 9 under an inverted microscope and identified as the following categories: colony-forming unit (CFU)-macrophage (M), CFU-granulocyte (G), and CFU-granulocyte, macrophage (GM). Pooled colonies from each dish were stained by May-Grünwald Giemsa for morphologic evaluation.
Flow Cytometric Analysis and Cell Sorting
All fluorochrome-labeled antimouse antibodies were purchased from eBioscience (San Diego, CA, http://www.ebioscience.com). Surface staining and intracellular staining of Foxp3 were performed as described [35]. Data were acquired on a FACS LSR-II (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com) and analyzed using Flowjo software (Tree Star, Inc., Ashland, OR, http://www.treestar.com). To purify total CD115+ cells from ES or HS cell cultures, samples were stained with anti-CD115 antibodies, and CD115 positive cells were isolated using magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany, http://www.miltenyibiotec.com). In some experiments, bulk CD115+ cells were further FACS-sorted into CD115+Ly-6C− and CD115+Ly-6C+ cells. Sorted cells with a purity of greater than 90% (for bead-sorted cells) or 98% (for FACS-sorted cells) were used.
Suppression Assay and Treg Induction
Tumor-derived MDSCs (TD-MDSCs) purified from bone marrow (BM) cells of tumor-bearing 129SvEv mice (F9 mouse embryonal carcinoma) or C57BL/6 (B6) mice (Lewis lung carcinoma) were prepared as described [35]. Briefly, BM cells were depleted of red blood cells and fractionated by Percoll (GE Healthcare, Pittsburgh, PA, https://www2.gehealthcare.com/portal/site/usen/) density gradient [2, 3]. Fraction two cells (the band between 50% and 60% Percoll) were further enriched for CD115+ cells using magnetic beads (Miltenyi Biotec). Because CD115+ cells homogeneously express Gr-1 and F4/80, CD115 positive-selected cells with a purity of greater than 90% were used as TD-MDSCs.
The suppressive activity of MDSCs was assessed either by coculturing 1 × 105 129SvEv (using B6 for HS-MDSCs comparison) splenocytes with various numbers of CD115+ cells or CD115− cells for 3 days in the presence of anti-CD3/anti-CD28 or by coculturing 2 × 105 129SvEv splenocytes (responder) with an equal number of irradiated (25 Gy) BALB/c splenocytes (stimulator) in the presence of various numbers of CD115+ or CD115− cells for 4 days. For the last 8 hours of incubation, [3H]-Thymidine was added. For Treg induction, 4 × 106/well of 129SvEv (B6 for HS-MDSCs) splenocytes and 1 × 106 CD115+ or CD115− cells were cocultured for 5 days in the presence of anti-CD3/anti-CD28 The concentration of anti-CD3 and anti-CD28 used was 0.25 μg/ml (in experiments involving ES MDSCs) or 0.5 μg/ml (in experiments involving HS-MDSCs) for each antibody. Foxp3 expression was determined by intracellular staining and NO level in the supernatant was measured by Greiss reagents (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com).
Reverse Transcription-Polymerase Chain Reaction
Magnetic bead-purified cells were left untreated (control) or stimulated with interferon-gamma (IFN-γ) (50 ng/ml) or IL-13 (40 ng/ml). Twenty-four hours later, cell pellets were lysed with TRIzol (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) for RNA isolation. A one-step reverse transcription-polymerase chain reaction (RT-PCR) kit (Qiagen, Hilden, Germany, http://www1.qiagen.com) was used for reverse transcription of RNA and amplification of cDNA (30 cycles for all analysis).
RT-PCR Primers
iNOS 5′-GAGATTGGAGTTCGAGACTTCTGTG-3′ (sense) and 5′-TGGCTAGTGCTTCAGACTTC-3′ (antisense); arginase 1 5′- CAGAGTATGACGTGAGAGACCAC-3′ (sense) and 5′-CAGCTTGTCTACTTCAGTCATGGAG-3′ (antisense); IL-10 5′-CTCTTACTGACTGGCATGAGG-3′ (sense) and 5′-CCTTGTAGACAC CTTGGTCTTGGAG-3′ (antisense); TGF-β1 5′-GTGGTATACTGAGACACCTTGG-3′ (sense) and 5′-CCTTAGTTTGGACAGGAT CTGG-3′ (antisense); and GAPDH 5′-GTGGAGATTGTTGCC ATCAACG-3′ (sense) and 5′-CAGTGGATGCAGGGATGATGTTCTG-3′ (antisense).
Allogeneic BM Transplantation
To prepare T-cell-depleted bone marrow cells (TCDBM), BM cells isolated from naïve 129SvEv mice were negatively selected using anti-Thy-1.2 antibodies, and Thy-1.2 negative cells were used as TCDBM. Donor T-cells (T) were prepared from splenocytes of naïve 129SvEv mice using a negative selection kit (R&D Systems, Minneapolis, MN, http://www.rndsystems.com). For establishment of the GVHD model, BALB/c mice (8–10 weeks old) were lethally irradiated (137Cs source, 8.5 Gy, TBI, split in two treatments with a 4-hour interval). Within 24 hours after irradiation, recipients were left untreated or reconstituted via tail vein injection with donor-derived cells as detailed in Figure 5A. ES-MDSC-treated mice were given two additional infusions of ES-MDSCs (bulk CD115+ cells, 2 × 106/mouse) on days 4 and 10, respectively. Animals were monitored daily for symptoms of GVHD and long-term survival, and were weighed every 3 4 days. For histopathologic analysis, specimens obtained on day 23 were fixed in formalin and tissue sections were stained with hematoxylin and eosin.
Figure 5.
Prevention of allo-HSCT-associated GVHD by ES-MDSCs. (A): Survival curve of recipient mice. Lethally irradiated (8 Gy, TBI) BALB/c mice were left untreated (ν, n = 6) or transplanted via tail vein injection with 129SvEv T-cell-depleted bone marrow cells (TCDBM, 5 × 106/mouse) alone (v, n = 6), or TCDBM plus purified 129SvEv splenic T-cells (T, 5 × 105/mouse) (σ, n = 10), or TCDBM plus T and ES-CD115− (2 × 106/mouse) (î, n = 4), or TCDBM plus T and ES-MDSCs (2 × 106/mouse) (λ, n = 11). Two additional treatments of ES-MDSCs (2 × 106/mouse, each) were given to ES-MDSC recipients on days 4 and 10 after the initial transplantation. Data shown are combined results from two independent experiments. (B): Changes of mean body weight in treated mice (values are mean ± SD. v, n = 6; σ, n= 3–10; λ, n= 9–11 mice). (C): Sections of livers stained with hematoxylin and eosin and harvested on day 23 from indicated groups. Representative micrographs are shown. (D): Kinetics of chimerism in the recipient mice. Spleens (SP), livers, and lymph nodes (LN) were recovered from the recipients (n = 3) on days 4, 7, and 14 after transplantation. Donor-derived T-cells (H-2Kb+) were identified by flow cytometry. Representative dot plots of peripheral T-cell chimerism from one of reproducible experiments are presented. The overall allogeneic chimerism was not apparently affected but the frequencies of donor T-cell subsets were increased in the recipients treated with MDSCs. Abbreviations: BM, bone marrow; ES, embryonic stem; LN, lymph nodes; MDSC myeloid-derived suppressor cells; SP, spleens; T, T cells; TCDBM, T-cell-depleted bone marrow.
Migration of MDSC in GVHD
TCDBM and donor T-cells were isolated from C57BL/6 mice as described. Purified Gr-1+CD115+ MDSCs were labeled with PKH26 per the manufacturer's protocols (PKH26 Red Fluorescent Cell Linker Kit, Sigma-Aldrich). Irradiated (850 rad, split into two sessions) BALB/c mice were injected, via tail vein, with 5 × 106 TCDBM cells and 0.5 × 106 donor T-cells with (n = 5) or without (n = 4) 1 × 107 PKH26 labeled MDSCs. Mice were killed on day 3 after transfer. Spleen, bone marrow, lymph nodes, liver, and lung were harvested. Livers and lungs were digested with type IV collagenase (20 U/ml, Sigma-Aldrich) in HBSS with 0.015% NaHCO3 for 2 hours at 37°C, at which time hepatocytes and alveolar cells were removed via low-speed centrifugation. Single cell suspensions were prepared from bone marrow, spleen, lymph nodes, and digested liver and lung and fractionated by Percoll density gradient [2, 3]. Fraction two cells were stained with anti-Ly-6C-FITC or isotype control followed by flow cyto-metric analysis.
Derivation of MDSCs from Marrow Hematopoietic Stem/Progenitor Cells
BM cells prepared from naïve B6 mice were depleted of lineage positive cells using antibodies against a panel of lineage antigens including CD5, CD45R CD11b, Gr-1 (Ly-6G/Ly-6C), 7-4, and Ter-119 (Miltenyi Biotec). The Lin− Cells were further fractioned into Sca1+ and Sca1− populations by FACS sorting. To derive MDSCs, we used similar culture conditions as those identified for ES cells. The highly purified Sca1+ and Sca1− Cells were separately plated at a density of 2.5 × 105/ml (24-well plate) and cultured in M1 cytokine mix for 2 to 4 days, depending on the cell number required for subsequent differentiation. The expanded stem/progenitor cells were then transferred to six-well plates (3 × 105/2 ml/well) and incubated in M2+M-CSF for various lengths of time. In experiments involving gene knockout mice, only Lin− BM cells were used for development of MDSCs from respective groups.
Results
Myeloid-Derived Suppressor Cells Can Be Generated Efficiently From ES Cells
In this study, the HoxB4 ES cell line was used for generation of MDSCs. We chose to use this cell line because HoxB4, a member of the Hox family of homeodomain transcription factors, has been shown to enhance the formation of mixed hematopoietic colonies from differentiating ES cell cultures [36] and has been implicated in self-renewal of the definitive hematopoietic stem cell (HSC) [37]. Overexpression of HoxB4 substantially facilitated the commitment to definitive HSC and skewed ES cell differentiation toward myeloid development [38]. Given that CD115+Gr-1+F4/80+ monocytic MDSCs derived from tumor-bearing mice could suppress T-cell proliferation and induced Treg development, we sought to establish a differentiation conditions conducive to the generation of this population from ES cells. To accomplish this, we applied a three-stage differentiation strategy as outlined in supporting information Figure S1, focusing on the cytokine requirements in the final step of MDSC development. After a 7-day coculture of day-6 EB-disaggregated cells with OP9 stromal cell lines, a considerable proportion of cells coexpressing CD115 and Ly-6C was detected in the cultures in the presence of M2 cocktail (KL, VEGF, Flt3L, and TPO), but absent from cultures treated with a cytokine mix consisting of KL, IL-6, IGF, IL-β or continually maintained in M1 (KL, IL-6, and IL-3) (data not shown). Kinetic analysis indicated that the CD115+Ly-6C+ population reached peak frequency on day 10 after coculture with OP9 stroma in M2 and remained at relatively high levels thereafter (Fig. 1A). Interestingly, a CD115 positive but Ly-6C negative population was also observed with different kinetics of emergences under the same culture conditions. We also compared EBs at different stages (days 5, 6, 7, and 9) and found day-6 EBs resulted in the highest percentage of CD115+ cells, especially the CD115+Ly-6C+ population (data not shown). To assess a potential role for HoxB4 in this process, we cultured ES cells by taking advantage of the tetracycline-inducible expression of hoxb4, in the presence or absence of doxycycline (DOX). As determined by FSC (forward scatter) versus SSC (side scatter) plots, cells incubated without induction of hoxb4 expression did not grow well, resulting in nearly 90% dead cells (supporting information Fig. S2, upper panel). While the development of CD115+Ly-6C− cells was not significantly affected among the residual growing cells, CD115+Ly-6C+ cells and CD115− Ly-6C+ cells were severely reduced in the absence of DOX (supporting information Fig. S2, lower panel). These results suggest that overexpression of HoxB4 can substantially enhance the proliferation and differentiation of this specific ES cell line.
Figure 1.
Directed differentiation of HoxB4 ES cells into myeloid-derived suppressor cells. (A): Kinetics of MDSC development. HoxB4 ES cells were first grown in suspension to form embryonic bodies (EB), and cells dissociated from day-6 EB were plated onto semiconfluent (irradiated) OP9 stromal cells and cultured in medium one (M1, see details in Methods). After 48 hours, M1 medium was removed and replaced with medium two (M2, see details in Methods). Floating and loosely attached cells recovered on different days after coculture in M2 were stained and analyzed by fluorescence-activated cell sorting. Data shown are representative of at least five experiments with reproducible results. (B): Opposing roles of M-CSF and GM-CSF in the generation of ES-derived CD115+ cells. Data shown are representative of three experiments with reproducible results. (C): Dose-dependent effect of M-CSF on the generation of ES-derived CD115+ cells. Error bars represent standard deviations (n = 3, triplicates). For experiments in (B) and (C), HoxB4 ES cells were differentiated using the same procedure as described in (A) except for the last step, where cells were cultured for 10 days in M2 or M2 supplanted with M-CSF or GM-CSF (B) or M2 plus various concentration of M-CSF (C). (D): Efficiency of myeloid-derived suppressor cell production: at the indicated number of dates in M2+M-CSF, floating and loosely adherent cells were retrieved and cell numbers were determined based on cell count and percentage of individual populations. Error bars represent standard deviations (n = 3, independent experiments). Abbreviations: Ctrl, control; D, days; GM-CSF, granulocyte-macrophage colony-stimulating factor; M-CSF, macrophage colony stimulating factor.
We further determined the effect of HoxB4 expression in ES cells on the generation of MDSCs and attempted to generate MDSCs from non-HoxB4-expressing ES cells (J++ and V6.5). Day-6 dissociated EB cells derived from J++ and V6.5 were subjected to the same culture condition and the resulting cells were analyzed by flow cytometry. We were able to generate Ly-6C+CD115+ monocytic MDSCs from both ES cell lines in the absence of transgenic expression of HoxB4 (supporting information Fig. S3). However, the yield of monocytic MDSC was significantly lower (0.08 × 106 and 0.05 × 106 for J++ and V6.5 cell lines, respectively, from 3 × 106 EB cells) when compared with HoxB4 ES cell line (5.7 × 106 from 3 × 106 EB cells). Therefore, ectopic expression of HoxB4 is not absolutely necessary for the generation of monocytic MDSCs from ES cells, but it significantly enhances the yield of MDSCs.
To enhance the production of ES-derived myeloid cells, we examined additional cytokines, focusing on the colony-stimulating factor family members M-CSF and GM-CSF, which are known to be involved in the development of myeloid lineages. As shown in Figure 1B, the addition of GMCSF led to a dramatic decrease in CD115+Ly-6C+ cells and a marked increase in CD115− Ly-6C+ cells (Fig. 1B, right panel), suggesting that GM-CSF might skew a progenitor population (possibly the CD115+Ly-6C− cells) toward differentiation into the CD115− Ly-6C+ cells, or alternatively into both CD115+Ly-6C+ and CD115− Ly-6C+ populations, but the former pathway (to CD115+Ly-6C+) was overwhelmingly outpaced by the latter (to CD115− Ly-6C+). In contrast, the production of both CD115+ populations was significantly augmented in the presence of M-CSF (Fig. 1B, middle panel), and the effect of M-CSF was manifested in a dose-dependent manner (Fig. 1C). Given the profound effect of M-CSF, the M2 cytokine cocktail plus 10 ng/ml of M-CSF (designated as M2+M-CSF) was used for derivation of MDSCs in subsequent experiments. Using these modified culture conditions, approximately 5.72 ± 0.68 × 106 CD115+Ly-6C+ and 3.19 ± 0.35 × 106 CD115+Ly-6C− cells could be generated from 3 × 105 EB cells (1 × 105/well, six-well plate) after a 10-day differentiation in M2+M-CSF (Fig. 1D).
ES-Derived CD115+ Cells Exhibit Strong Suppressive Capacity In Vitro
Immune suppression is the hallmark feature of MDSCs. Next we determined whether these ES-derived myeloid cells were truly functional MDSCs. We separated differentiated cells into CD115+ and CD115− cells using magnetic beads and cocultured them with splenocytes isolated from naïve 129SvEv mice. Remarkably, HoxB4 ES-derived CD115+ but not CD115− cells displayed potent suppressive activity against T-cell proliferation stimulated either by polyclonal stimulus anti-CD3 plus anti-CD28 (Fig. 2A) or by alloantigens (mixed lymphocyte reaction, [MLR]) (Fig. 2B) in a CD115 cell dose-dependent fashion. Compared with the control (splenocyte alone), addition of ES-CD115+ cells resulted in a significant inhibition of T-cell proliferation even at a high splenocytes to CD115+ cells ratio (1:0.125) (p <.01, Student's t test). Because the CD115+ fraction was composed of two populations, CD115+Ly-6C+ and CD115+Ly-6C− cells, we further purified these two subsets by FACS and conducted similar proliferation assays. Consistent with the results shown in Figure 2A and 2B, sorted CD115− Ly-6C− and CD115− Ly-6C+ populations showed minimal suppressor function. Interestingly, both CD115+Ly-6C− and CD115+Ly-6C+ populations exerted robust inhibition of T-cell proliferation, with the former showing even slightly but significantly (when a lower number of MDSC was present) higher suppressive activity (Fig. 2C, p <.05, Student's t test). We further compared the suppressive capacity of ES-derived CD115+ cells (ESCD115+) versus those isolated from tumor-bearing mice (TDCD115+). Somewhat unexpectedly, ES-CD115+ cells were even more suppressive than the TD-CD115+ cells (Fig. 2D, p <.001, Student's t test). These results clearly indicate that two functionally active MDSC populations can be generated from ES cells in vitro and designated hereafter as ES-MDSCs.
Figure 2.
Potent suppressive capacity of ES-MDSCs. (A): Inhibition of polyclonally stimulated T-cell proliferation by ES-derived CD115+ cells (CD115+ versus control, **p <.001; ***p <.0001). (B): Inhibition of alloantigen-stimulated T-cell proliferation by ES-derived CD115+ cells (CD115+ versus control, ***p <.0001). (C): Suppression of T-cell proliferation by CD115+Ly-6C+ and CD115+Ly-6C− populations (CD115+Ly-6C− versus CD115+Ly-6C+, *p <.05). (D): A comparison of suppressive activity of ES-derived CD115+ cells versus purified CD115+ cells isolated from tumor-bearing mice (TD-CD115+) (ES-CD115+ versus TD-CD115+, **p <.001). Various numbers of ES-derived cells or TD-CD115+ cells were coincubated with 129SvEv splenocytes in the presence of anti-CD3/anti-CD28 (A, C, D) or irradiated BALB/c splenocytes (B). [3H]-Thymidine was pulsed for the final 8 hours of a 3-day (A, C, D) or a 4-day (B) culture. Data shown are representatives of at least three experiments with consistent results. Abbreviations: Ctrl, Control (splenocyte alone); ES, embryonic stem; MDSC, myeloid-derived suppressor cell; TD, tumor-derived.
The Suppressor Function of ES-MDSCs Involves Multiple Pathways
Previous studies have demonstrated that MDSCs may function to suppress T-cell responses directly via diverse mechanisms, for example, expression of arginase one and inducible nitric oxide synthase (iNOS), secretion of IL-10 and TGF-β [6, 12, 39–41], or indirectly by inducing Treg development [2, 26, 42–44]. To evaluate whether these pathways play a potential role in mediating the suppression of ES-MDSCs, we first examined the ability of ES-MDSCs to induce Tregs and produce NO. Interestingly, while both ES-CD115+ (Fig. 3A, lower right panel) and TD-CD115+ (Fig. 3A, lower left panel) cells induced drastic propagation of Tregs, the percentage of Tregs was significantly higher in the presence of ES-CD115+ cells as compared with TD-CD115+ cells (21.2 ± 3.1 versus 11.8 ± 1.6, p <.001, Student's t test). In contrast, inclusion of ES-CD115− cells (Fig. 3A, upper right panel) showed no change in the frequency of Tregs when compared with splenocyte-only cultures (Fig. 3A, upper left panel) (5.2 ± 0.9 versus 5.7 ± 0.5, p >.05, Student's t test). Similarly, Both ES-CD115+ or TD-CD115+ cells produced a large amount of NO with a higher level detected in the supernatants collected from ES-CD115+ cultures (Fig. 3B, p <.05, Student's t test). We next assessed the expression of a panel of genes including iNOS, arginase 1, IL-10, and transforming growth factor (TGF)-β. As shown in Figure 3C, both ES-CD115+ and TD CD115+ cells constitutively expressed high levels of TGF-β and low levels of IL-10 that were considerably enhanced in the presence of interferon-gamma (IFN-γ). In accordance with our previous work [2], stimulation with the Th1 cytokine, IFN-γ, and the Th2 cytokine, IL-13, induced strong expression of iNOS and arginase 1, respectively, in ES-CD115+ and TD-CD115+ cells, but not ES-CD115− cells. Taken together, these data suggest that except for an enhanced ability to induce Treg and produce NO, ES-CD115+ cells are very similar to TD-CD115+ cells in the context of potential mechanisms for mediating suppressive functions.
Figure 3.
Various pathways mediating suppressor function of ES-MDSCs. (A): Treg development induced by ES-MDSCs. Data shown are representative dot plots and numbers inserted are mean ± SD from three reproducible experiments. (B): NO production by ES-MDSCs. In A and B, 1 × 106 of ES-derived CD115+ and CD115− cells or TD-CD115+ cells were cocultured with 4 × 106 129SvEv splenocytes for 5 days in the presence of anti-CD3/anti-CD28. CD4+CD25+Foxp3+ cells were analyzed by intracellular staining. NO levels in the supernatants were determined per manufacturer's protocol (n = 3 triplicate, ES-CD115+ vs. Ctrl or ES-CD115− , **p <.0001; ES-CD115+ vs. TD-CD115+, *p <.05). Data are representative of two experiments with consistent results. (C): Expression of iNOS, arginase 1, IL-10, and TGF-β by ES-MDSCs. ES-derived CD115+ and CD115− cells and TD-CD115+ cells were cultured for 24 hours in the presence or absence of IFN-γ or IL-13. We assessed mRNA expression by reverse transcription polymerase chain reaction. Abbreviations: Arg.1, arginase 1; Ctrl, control; ES, embryonic stem; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IL, interleukin; iNOS, inducible nitric oxide synthase; NO, nitric oxide; TD, tumor-derived IFN-γ, interferon-gamma; TGF-ß, transforming growth factor-beta.
ES-MDSC Sub-Populations Have Distinct Phenotype and Developmental Potentials
To better understand and define the unique properties of ESMDSCs, we further characterized them. Morphologically, in comparison with the CD115− Ly-6C+ cells (immature granulocytes as determined by their smaller size and a Gr-1highLy-6Clow phenotype; Fig. 4A, lower left panel,) and CD115− Ly-6C− cells (comprised of differentiated and undifferentiated or less-differentiated cells; Fig. 4A, lower right panel), both CD115+Ly-6C− (Fig. 4A, upper left panel) and CD115+Ly-6C+ (Fig. 4A, upper right panel) populations were highly homogeneous with a relatively larger size and a greater nuclear to cytoplasmic ratio, indicating they were not fully differentiated cells. This evaluation was supported by a characteristic phenotype of these cells expressing CD34, c-Kit, FcR-III/II, and Prominin-1 (CD133) (Fig. 4B), surface antigens normally found on myeloid progenitor cells at various stages of differentiation such as common myeloid progenitors (CMPs) and granulocyte/macrophage progenitors (GMPs). Although the two CD115-positive populations were not clearly distinguishable from each other morphologically, their differential phenotypes along with their disparate kinetics of development (Fig. 1A) suggests that they represent distinct cell populations with the CD115+Ly-6C− cells at an earlier stage of differentiation. To more precisely assess their developmental stage and lineage potentials, we redifferentiated FACS-sorted CD115+Ly-6C− and CD115+Ly-6C+ cells in vitro in the presence of a more complete cytokine combination. As shown in Figure 4C, while most of the originally seeded CD115+Ly-6C− (upper panels) and CD115+Ly-6C+ (lower panels) cells became attached and progressed gradually toward a population expressing CD115 and low levels of Ly-6C (immature macrophages as evaluated by Giemsa staining), the CD115+Ly-6C− cell cultures also gave rise to two additional minor populations, CD115+Ly-6C+ and CD115− Ly-6C+ (granulocytes), the latter lineage potential of which was entirely dependent upon GMCSF (supporting information Fig. S4). These results not only revealed a precursor-progeny relationship between the CD115+Ly-6C− and CD115+Ly-6C+ cells, but also suggest that they were the GMP- and monocyte (or promonocyte)-like cells, respectively. The distinct developmental potentials of CD115+Ly-6C− and CD115+Ly-6C+ cells were further confirmed by colony-forming assay. The CD115+Ly-6C− cells had a greater colony-forming activity in methylcellulose (Fig. 4D, upper panel) compared with CD115+Ly-6C+ cells (p <.01, Student's t test), giving rise to macrophage (M), granulocyte (G), and occasional granulocyte/macrophage (GM) mixed colonies, whereas the CD115+Ly-6C+ cells formed only M colonies (Fig. 4D, lower panel). We verified these observations by examining the morphology of colony components. Unlike the CD115+Ly-6C+ cell-derived colonies, which consisted solely of macrophages (Fig. 4E, bottom photo), colonies grown from CD115+Ly-6C− cell cultures contained both macrophages and granulocytes (Fig. 4E, top photo).
Figure 4.
Morphologic, phenotypic, and developmental characterization of ES-MDSCs. (A): Morphology of ES-derived MDSCs. Differentiated cells were sorted by fluorescence-activated cell sorting (FACS), cytospun, and stained by May-Giemsa (original magnification: 1,000). (B): Surface phenotype of various subsets differentiated from HoxB4 ESs. Cells recovered were directly stained with a panel of surface markers and analyzed by flow cytometry. (C): Distinct potentials of CD115+Ly-6C− (upper profiles) and CD115+Ly-6C+ (lower profiles) cells. FACS-sorted subsets of MDSCs were seeded into gelatinized plates and cultured in medium supplemented with the appropriate cytokine mix (see details in Methods) for various lengths of time. Differentiated cells were collected and analyzed by FACS for the expression of CD115 and Ly-6C. (D): Colony forming activity of FACS-sorted CD115+Ly-6C− and CD115+Ly-6C+ populations in methylcellulose. CD115+Ly-6C− cells, which had higher clonogenic activity than CD115+Ly-6C+ cells (upper histogram, *p <.001), gave rise to M, GM, G colonies, whereas the CD115+Ly-6C+ population generated only M colonies (lower histogram). M, macrophage colonies; GM, macrophage and granulocyte mixed colonies; G, granulocyte colonies. (E): May-Grünwald Giemsa staining of pooled colonies developed from CD115+Ly-6C− cells (upper panel, arrows and arrowheads indicated macrophages and granulocytes, respectively) and CD115+Ly-6C+ cells (lower panel). Original magnification: ×400. Cells used in (A–D) were obtained on day 10 after differentiation on OP9 in M2 MCSF. Abbreviations: D, day; G, granulocyte colony stimulating factor; GM, granulocyte macrophage colony stimulating factor; M, macrophage stimulating+factor; No., number.
ES-MDSCs Prevent GVHD Following Allogeneic Bone Marrow Transplantation
Our previous work indicated that upon adoptive transfer, MDSCs freshly isolated from tumor-bearing mice could efficiently suppress GVHD while maintaining the GVL effect in a murine model (Zhou et al., manuscript submitted). To determine whether ES-MDSCs would preserve their suppressive activity in vivo and thus be effective in preventing allo-HSCT-associated GVHD, we performed similar experiments of allo-HSCT. Specifically, lethally-irradiated hosts (BALB/c, H-2d) were left untreated or within 24 hours were adoptively transferred with 129SvEv (H-2b) TCDBM alone or TCDBM plus purified 129SvEv splenic T-cells (T) or TCDBM plus T and ES-derived CD115+ cells (ES-MDSCs). All lethally irradiated mice without treatment died within 15 days, whereas mice treated with TCDBM alone were healthy and survived the entire experimental period (Fig. 5A). As expected, mice that had received TCDBM plus donor-type T-cells developed severe signs of GVHD (loss of hair, hunched posture, diarrhea, and weight loss), and most animals were dead within 40 days after transplantation (Fig. 5A, 5B). Strikingly, when ESMDSCs were cografted along with TCDBM plus T-cells, mice were largely protected from GVHD lethality and 81.8% of the animals survived for more than 100 days (p <.0001; log-rank test). Conversely, injection of ES-CD115− cells failed to confer protection from GVHD in mice that received TCDBM plus T-cells. While the mean body weights of the ES-MDSCs recipients were slightly lower compared with TCDBM-alone-treated animals, the difference was not statistically significant (p >.05) at all time points examined (Fig. 5B). In correspondence with the survival and body weight change, histologic examination revealed dense lymphocyte infiltration in the periportal areas of the livers obtained from TCDBM plus T-cell-treated mice (Fig. 5C, right panel), in contrast with tissue samples recovered from ES-MDSC-treated mice (Fig. 5C, middle panel) or recipients of TCDBM-only transfer (Fig. 5C, left panel). Similar histologic features within respective groups were also observed in other GVHD target organs, for example, small intestine and skin (data not shown).
The ES-MDSC-treated mice exhibited peripheral mixed chimerism in the first 2 weeks after treatment and became completely chimeric thereafter (Fig. 5D). The long-term surviving mice (longer than 100 days after treatment) were fully chimeric with donor hematopoietic cells and no sign of GVHD was observed (data not shown). Taken together, these results establish that ES-MDSCs are able to prevent the development of GVHD induced by alloreactive T-cells, resulting in a great improvement in animal survival following allo-HSCT.
Since the treatment of MDSCs prevented the massive inflammation and leukocyte infiltration in the liver (Fig. 5C), we asked whether MDSCs could migrate to inflammation sites, such as the liver where GVHD occurs. To determine the homing profile of MDSCs in the recipient mice, purified PKH26-labeled MDSCs were injected, via tail vein, along with TCDBM and purified splenic T-cells from C57BL/6 mice into irradiated BALB/c mice. Three days after transfer, single-cell suspensions were prepared from spleen, bone marrow, lymph nodes, liver, and lung. The number of transferred MDSCs was assessed by Ly-6C and PKH26. As shown in Figure 6A, significantly higher numbers of PKH26-labled MDSCs were found in the spleen and liver of irradiated host mice on day 3 after transfer when compared with those without MDSC transfer (baseline) (p = .01 and p = .0016, Student's t test). Ten and 50-fold higher numbers of PKH26+MDSCs were observed in the spleen and liver, respectively, when compared with the baseline control (without transfer of PKH26-labeled MDSCs) (Fig. 6B).
Figure 6.
Migration pattern of MDSC in GVHD. Irradiated BALB/c mice were injected, via tail vein, with 5 × 106 TCDBM cells and 0.5 × 106 donor T-cells with (n = 5) or without (baseline, n = 4) 1 × 107 PKH26 labeled MDSCs. Mice were killed on day 3 after transfer. Single cell suspensions were prepared from spleen (SP), bone marrow (BM), lymph nodes (LN), liver, and lung and stained with anti-Ly-6C-FITC or isotype control followed by flow cytometric analysis. (A): Absolute numbers of transferred MDSCs in various organs. Significantly higher numbers of transferred MDSCs were found in the spleen (p = .01) and liver (p = .0016). (B): Fold changes of transferred MDSCs in various organs when compared with the baseline (without MDSC transfer). Abbreviations: BM, bone marrow; CT, control; LN, lymph nodes; SP, spleen.
Development of MDSCs from Marrow Hematopoietic Stem/Progenitor Cells
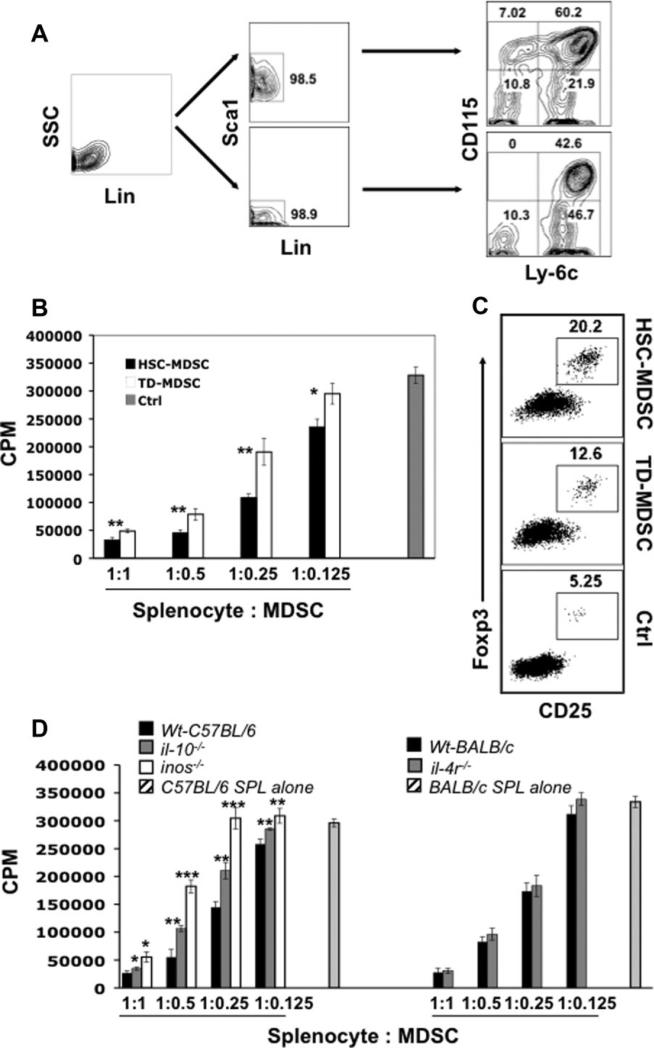
The efficient derivation of MDSCs from ES cells prompted us to examine whether the cytokine requirements identified using ES cells could be applied to the generation of MDSCs from bone marrow hematopoietic/progenitor cells. Development of such a system may not only help establish an additional source for MDSCs, but also provide a more ideal alternative platform to dissect the mechanisms underlying the differentiation and accumulation of MDSCs in the tumor-bearing host. We first depleted naïve bone marrow cells of lineage positive cells (Fig. 7A, left dot plot) followed by sorting Lin− Cells into Sca1+ and Sca1− Cells by FACS (Fig. 7A, middle panel). These highly purified hematopoietic/progenitor cells were then separately subject to differentiation as described in Methods. Surprisingly, under similar culture conditions (cytokine mix) as defined using ES cells, roughly 60% and 40% of CD115+Ly-6C+ cells were observed after an 8-day culture (3-day in M1 plus 5-day in M2+M-CSF) of the Lin− Sca1+ and Lin− Sca1− fraction cultures, respectively (Fig. 7A, right panel). The marrow Lin− Sca1+ cells appeared to be superior to the Lin− Sca1− fraction, as the former, in addition to resulting in a higher overall cell percentage, enabled sustainable production (at least 15 days in M2+M-CSF) of CD115+Ly-6C+ cells, which were dramatically decreased in the Lin− Sca1−Cell culture after 6 days, possibly because of the exhaustion of the pool of CD115+Ly-6C+ progenitors. Interestingly, only the Lin− Sca1+ but not Lin− Sca1−Cells gave rise to the CD115+Ly-6C− population, suggesting that the CD115+Ly-6C− cells develop from progenitors at an earlier stage of differentiation (compared with the precursors of CD115+Ly-6C+ cells). Using the culture conditions described here, approximately 43.6 ± 5.2 × 106 total CD115+ cells could be generated from 1 × 106 Lin−Sca1+ cells after an 8-day culture.
Figure 7.
Generation of myeloid-derived suppressor cells from marrow hematopoietic stem/progenitor cells. (A): In vitro differentiation of bone marrow hematopoietic stem/progenitor cells into MDSCs (HSMDSC). BM cells isolated from B6 mice were depleted of lineage positive cells (left panel: after depletion) and then FACS-sorted into Sca1+ and Sca1− Cells (middle panel). Purified cells were differentiated using similar culture condition used in the derivation of ES-MDSCs. (B): Comparison of suppressive activity between HS-MDSC and TD-MDSC. HS-MDSCs or TDMDSCs were coincubated with B6 splenocytes at different ratios in the presence of anti-CD3/anti-CD28 and [3H]-thymidine was pulsed for the final 8 hours of a 3-day culture. (C): Enhanced Treg induction by HS-MDSCs. MDSCs were coincubated for 5 days with B6 splenocytes at a 1:4 ratio in the presence of anti-CD3/anti-CD28. Foxp3 expression was analyzed via intra-cellular staining. (D): The role of IL-10, iNOS, and IL-4 in the suppression mediated by HS-MDSC. HS-MDSCs developed from Lin− BM cells of indicated strains of mice were coincubated with B6 (for il10−/− and inos−/− MDSCs) or BALB/c (for il-4r−/− MDSCs, BALB/c background) splenocytes at different ratios in the presence of anti-CD3/anti-CD28. [3H]-Thymi-dine was pulsed for the final 8 hours of a 3-day culture (il-10−/− MDSCs or inos−/− MDSCs versus Wt B6 MDSCs, *p <.05; **p <.01; ***p <.001). Abbreviations: BM, bone marrow; CPM, counts per minute; Ctrl, control; FACS, fluorescence-activated cell sorter; HSC, hematopoietic stem cell; Lin, lineage; MDSC myeloid-derived suppressor cells; TD-MDSC, MDSC cells generated from tumor-bearing mice; Wt, wild-type.
The suppressive activity of marrow hematopoietic stem/ progenitor cells-derived CD115+ cells (HS-MDSCs) was assessed. Compared with TD-MDSCs, HS-MDSCs, like the ES-MDSCs, not only displayed significantly stronger suppressive activity against T-cell proliferation stimulated by anti-CD3/anti-CD28 (Fig. 7B), but also were more efficient in inducing CD4+CD25+Foxp3+ Treg development (Fig. 7C). To delineate which of the aforementioned suppressive mechanisms play a major role, the functional activity of HS-MDSCs was evaluated when specific pathway(s) were interfered. Whereas arginase one inhibitor (NOHA) displayed minimal effects even at a high concentration (500 μM), the addition of L-NMMA (NG-monomethyl-L-arginine, a specific iNOS inhibitor) partially but significantly reversed the suppression of both HS- and TD-MDSCs against T-cell proliferation in a dose-dependent manner (supporting information Fig. S5). We confirmed the critical role of iNOS by examining MDSCs deficient in inos (Fig. 7D, left panel). Interestingly, il-10−/− MDSCs also showed significantly lower suppressive activity, though to a lesser extent compared with inos−/− MDSCs. In contrast, T-cell proliferation was only slightly restored in the presence of il-4r−/− MDSCs (BALB/c background) as compared with wild-type BALB/c HS-MDSC (Fig. 7D, right panel). Taken together, the results suggest that IL-10 and iNOS are involved in HS-MDSC-mediated T-cell suppression.
Discussion
We have described a protocol for directed differentiation of mouse ES cells into MDSCs, which to our knowledge represents the first attempt of its kind. With this differentiation system we demonstrated that functionally active MDSCs could be generated in vitro from mouse ES cells. Moreover, we extended the differentiation system to the bone marrow cells, enabling MDSC production at an even higher efficiency from purified marrow hematopoietic stem/progenitor cells.
Two homogenous populations of MDSCs were identified in both ES and HS cell cultures: CD115+Ly-6C+ and CD115+Ly-6C− cells. Although several reports have shown that a granulocytic subpopulation of MDSCs can be isolated from tumor-bearing hosts, the granulocytes (CD115− Ly-6C+, immature cell as evaluated based on morphology) derived from in vitro ES cultures only showed slight suppression even at a high ratio of granulocyte versus T-cells. Whether earlier (more immature) granulocytes have suppressive activity remains to be determined. Both CD115+Ly-6C+ and CD115+ Ly-6C− cells expressed CD11b (Mac-1) and Gr-1, two surface markers widely used for defining MDSCs. However, CD115 appeared to be a more specific and reliable marker for monocytic MDSCs, as firmly demonstrated here and in our previous studies [2].
A detailed examination of the morphology, phenotype, and developmental potential indicated that ES-derived CD115+Ly-6C+ cells were very similar to the ex vivo CD115+Gr-1+F4/80+ monocytic MDSCs found in tumor-bearing mice [2, 45]. The ES-CD115+Ly-6C− cells, distinctively characterized by the high expression of myeloid progenitor markers (for example, CD34, c-Kit, FcR-III/II, Prominin-1) and relatively low expression of myeloid lineage markers (for example, Mac-1 and Gr-1), closely resemble the granulocyte/macrophage progenitors (GMPs) as revealed by redifferentiation and colony-forming assays. This conclusion is further substantiated by the observation that a considerable proportion of CD115+Ly-6C− cells could be derived only from the marrow Lin− Sca1+ fraction (because of the existence of more primitive cell populations upstream of GMP, for example, HSCs, multipotential progenitors (MPPs), and/or CMPs), but not from the marrow Lin− Sca1−Cells, which themselves might be GMPs or GMP-containing population as determined by their CD115+Ly-6C+ (monocyte) and CD115− Ly-6C+ (granulocyte) cell potentials. It is generally accepted that MDSCs encompass immature dendritic cells, immature macrophages, monocyte, and myeloid cells at earlier stages [42]. Interestingly, in a recent report, CD45+ hematopoietic progenitor cells (HPS) derived from HoxB4-transduced ES cells effectively protected cardiac allografts from rejection in animal models [46]. However, the specific population within a mixture of CD45+ HPS that mediated the tolerogenic effect was not identified. It remains to be addressed in future studies whether the GMPs represent the earliest population along the myeloid lineage pathways that exhibit the suppressive function. The identification of various myeloid progenitors with a suppressor phenotype may not only extend our understanding of MDSC biology, but also provide new insight into the seminal findings, by Owen [45] and Billingham [47] over 60 years ago, of immunologic tolerance induced by hematopoietic cell infusion, which formed the basis for the bone marrow transplantation procedure.
ES-MDSCs were highly suppressive against T-cell proliferation induced by polyclonal stimuli or alloantigens in vitro. More impressively, pair-wise comparison analysis (in at least three independent experiments) indicated that ES-MDSCs showed consistently stronger suppressive activity than TDMDSCs. A similar result was also observed when comparing HS-MDSCs to TD-MDSC. The suppressive function of ESMDSCs appears to involve multiple yet similar mechanisms essential for the suppressive activities of TD-MDSCs. It is believed that arginase, an enzyme that hydrolyzes L-arginine to L-ornithine and urea, is an important molecule for the MDSC function [39]. However, the data presented here indicated that blockade of arginase one activity with NOHA failed to downregulate the suppression mediated by HS- or TD-MDSCs. One possibility for the discrepancy might be the subsets of MDSCs examined (for example, monocytic MDSC versus granulocytic MDSC) or the environmental stimuli from Th1 (INF-γ) versus Th2 (IL-4/IL-13) responses. Conversely, we demonstrated, by using specific inhibitors or MDSCs derived from knockout mice, that iNOS was involved in the suppression of HS-MDSCs, which might account in part for the observed difference in the suppressive capacity between in vitro-derived MDSCs and TD-MDSCs as the former produced significantly higher levels of NO. Consistent with our previous report that treatment with anti-IL-10 antibodies partially reversed the suppression of TD-MDSCs [2], examination of IL-10 deficient MDSCs revealed that IL-10 also played a role in the suppressive functions of HS-MDSCs. It remains to be determined whether iNOS and IL-10 are the sole mechanisms fully responsible for the function of MDSCs. In addition to their stronger suppression, both ES-MDSCs and HS-MDSCs displayed significantly enhanced ability to induce Treg development compared with TD-MDSCs. Provided that MDSCs are intermediate cells and thus doomed to undergo further differentiation leading to eventual loss of suppressive activity, the striking Treg-inducing feature of in vitro-derived MDSCs may be particularly meaningful for the full realization of their immunoregulatory functions.
One of the major goals for this study was to generate MDSCs that are capable of inducing tolerance in vivo. By using a murine major histocompatibility complex (MHC)-mismatched HSCT model, we clearly showed that adoptive transfer of bulk ES-derived CD115+cells could efficiently protect animals from development of alloreactive T-cell-mediated lethal GVHD, leading to nearly 82% long-term survival of treated mice. Our preliminary data also indicated that MDSCs derived from bone marrow stem/progenitor cells were equally effective in preventing GVHD using a similar animal model (Zhou et al., unpublished data). Although the respective effects of CD115+Ly-6C+ and CD115+Ly-6C− cells have not been examined because of the low cell amount of CD115+Ly-6C− from the culture, we reasoned that the profound protection from GVHD might be afforded compositely by these two populations, given their potent suppressive activity as revealed in functional assays in vitro and the strong inhibition of GVHD by CD115+ Gr-1+F4/80+ MDSCs (comparable with ES-CD115+Ly-6C+ cells) freshly isolated from tumor-bearing mice (Zhou et al., manuscript submitted). In these studies, MDSCs suppressed GVHD with a potency that was comparable to that of ES-MDSCs shown in this report. More importantly the beneficial GVL effect and intact immunity were maintained in the long-term surviving mice that were injected with CD115+Gr-1+F4/80+ cells (Zhou et al., manuscript submitted). The data presented here not only confirmed the exceptional immune tolerogenic capability of MDSCs, but also suggest that in vitro-derived MDSCs, by virtue of providing a safer (tumor-cell free) and renewable cell source with a greater potency, have tremendous translational potential in the clinic.
Conclusion
The present study clearly demonstrates that functional MDSCs can be efficiently derived in vitro from mouse ES cells and bone marrow hematopoietic stem/progenitor cells. Both the ES or BM systems established here could have important implications in basic research and clinical applications. While these systems may inherently serve as an ideal platform for investigating the roles of cytokines and underlying pathways leading to aberrant expansion of MDSCs in vivo, they may also be well suited for derivation of unlimited and reliable MDSCs, and particularly for development of genetically modified MDSCs or “customized” (immunologically compatible) MDSCs, which could substantially extend the applicability of MDSCs in clinical settings.
Supplementary Material
Acknowledgments
We thank Ms. Marcia Meseck for editing the manuscript. This work was supported in part by grants from the National Cancer Institute and NIDDK (National Institute of Diabetes and Digestive and Kidney Diseases) grants and Black Family Stem Cell Foundation to S.-H.C., and grant support from Susan G. Komen Breast Cancer Foundation to P.-Y.P.
Footnotes
Author contributions: Z.Z.: Designed and performed the research, analyzed data, and wrote the manuscript; D.L.F.: Participated in the development of ES-MDSC study; G.M. S.E., Y.C.: Participated in the GVHD study and data analysis; C.M.D., G.K.: Interpreted data; S.-H.C., P.-Y.P.: Designed research, interpreted data, wrote the manuscript.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors indicate no potential conflicts of interest.
See www.StemCells.com for supporting information available online.
REFERENCES
- 1.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: Recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Huang B, Pan PY, Li Q, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 3.Kusmartsev SA, Li Y, Chen SH. Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J Immunol. 2000;165:779–785. doi: 10.4049/jimmunol.165.2.779. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez PC, Hernandez CP, Quiceno D, et al. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202:931–939. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez PC, Quiceno DG, Zabaleta J, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 6.Terabe M, Matsui S, Park JM, et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: Abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melani C, Chiodoni C, Forni G, et al. Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood. 2003;102:2138–2145. doi: 10.1182/blood-2003-01-0190. [DOI] [PubMed] [Google Scholar]
- 8.Youn JI, Nagaraj S, Collazo M, et al. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: A mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 10.Diaz-Montero CM, Salem ML, Nishimura MI, et al. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young MR, Lathers DM. Myeloid progenitor cells mediate immune suppression in patients with head and neck cancers. Int J Immunopharmacol. 1999;21:241–252. doi: 10.1016/s0192-0561(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 12.Nagaraj S, Gupta K, Pisarev V, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shojaei F, Wu X, Zhong C, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–831. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- 14.Tu S, Bhagat G, Cui G, et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L, DeBusk LM, Fukuda K, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 16.Yang L, Huang J, Ren X, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Condeelis J, Pollard JW. Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 19.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 20.Shojaei F, Ferrara N. Refractoriness to antivascular endothelial growth factor treatment: Role of myeloid cells. Cancer Res. 2008;68:5501–5504. doi: 10.1158/0008-5472.CAN-08-0925. [DOI] [PubMed] [Google Scholar]
- 21.Shojaei F, Wu X, Malik AK. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+ Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 22.Bunt SK, Sinha P, Clements VK, et al. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 23.Bunt SK, Yang L, Sinha P, et al. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kujawski M, Kortylewski M, Lee H, et al. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118:3367–3377. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melani C, Sangaletti S, Barazzetta FM, et al. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res. 2007;67:11438–11446. doi: 10.1158/0008-5472.CAN-07-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacDonald KP, Rowe V, Clouston AD, et al. Cytokine expanded myeloid precursors function as regulatory antigen-presenting cells and promote tolerance through IL-10-producing regulatory T cells. J Immunol. 2005;174:1841–1850. doi: 10.4049/jimmunol.174.4.1841. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Liang S, Wu J, et al. Human inhibitory receptor immunoglobulin-like transcript 2 amplifies CD11b+Gr1+ myeloid-derived suppressor cells that promote long-term survival of allografts. Transplantation. 2008;86:1125–1134. doi: 10.1097/TP.0b013e318186fccd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dugast AS, Haudebourg T, Coulon F, et al. Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J Immunol. 2008;180:7898–7906. doi: 10.4049/jimmunol.180.12.7898. [DOI] [PubMed] [Google Scholar]
- 29.Haile LA, von Wasielewski R, Gamrekelashvili J, et al. Myeloid-derived suppressor cells in inflammatory bowel disease: A new immunoregulatory pathway. Gastroenterology. 2008;135:871–881. 881, e871–875. doi: 10.1053/j.gastro.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 30.Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymph-oid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- 31.Vogelsang GB, Lee L, Bensen-Kennedy DM. Pathogenesis and treatment of graft-versus-host disease after bone marrow transplant. Annu Rev Med. 2003;54:29–52. doi: 10.1146/annurev.med.54.101601.152339. [DOI] [PubMed] [Google Scholar]
- 32.Lacaud G, Gore L, Kennedy M, et al. Runx1 is essential for hematopoietic commitment at the hemangioblast stage of development in vitro. Blood. 2002;100:458–466. doi: 10.1182/blood-2001-12-0321. [DOI] [PubMed] [Google Scholar]
- 33.Kubo A, Chen V, Kennedy M, et al. The homeobox gene HEX regulates proliferation and differentiation of hemangioblasts and endothelial cells during ES cell differentiation. Blood. 2005;105:4590–4597. doi: 10.1182/blood-2004-10-4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JC, Hapel AJ, Ihle JN. Constitutive production of a unique lymphokine (IL 3) by the WEHI-3 cell line. J Immunol. 1982;128:2393–2398. [PubMed] [Google Scholar]
- 35.Pan PY, Wang GX, Yin B, et al. Reversion of immune tolerance in advanced malignancy: Modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;111:219–228. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helgason CD, Sauvageau G, Lawrence HJ, et al. Overexpression of HOXB4 enhances the hematopoietic potential of embryonic stem cells differentiated in vitro. Blood. 1996;87:2740–2749. [PubMed] [Google Scholar]
- 37.Sauvageau G, Thorsteinsdottir U, Eaves CJ, et al. Overexpression of HOXB4 in hematopoietic cells causes the selective expansion of more primitive populations in vitro and in vivo. Genes Dev. 1995;9:1753–1765. doi: 10.1101/gad.9.14.1753. [DOI] [PubMed] [Google Scholar]
- 38.Pilat S, Carotta S, Schiedlmeier B, et al. HOXB4 enforces equivalent fates of ES-cell-derived and adult hematopoietic cells. Proc Natl Acad Sci U S A. 2005;102:12101–12106. doi: 10.1073/pnas.0505624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 40.Rodríguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: Mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sinha P, Clements VK, Bunt SK, et al. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 42.Ghiringhelli F, Puig PE, Roux S, et al. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoechst B, Ormandy LA, Ballmaier M, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008 doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 44.Serafini P, Mgebroff S, Noonan K, et al. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439–5449. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Owen RD. Immunogenetic consequences of vascular anastomoses between bovine twins. Science. 1945;102:400–401. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- 46.Bonde S, Chan K-M, Zavazava N. ES-cell derived hematopoietic cells induce transplantation tolerance. Plos One. 2008;3:e3212. doi: 10.1371/journal.pone.0003212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.