Abstract
BACKGROUND
In vitro data suggest that erythrocytes undergo storage time-dependent degradation, eventuating in hemolysis. We hypothesize that transfusion of old blood, as compared with newer blood, results in a smaller increment in hematocrit.
METHODS
We performed an analysis of packed red blood cell transfusions administered in the surgical intensive care unit. Age of blood was analyzed as continuous, dichotomized at 14 days (old vs new), and grouped by weeks old.
RESULTS
A total of 136 U of packed red blood cells were given to 52 patients; 110 (80.9%) were 14 days old or more. A linear, inverse correlation was observed between the age of blood and the increment in hematocrit (r2 = −.18, P = .04). The increment in hematocrit was greater after transfusion of new as compared with old blood (5.6% vs 3.5%, respectively; P = .005). A linear relationship also was observed between the age of transfused blood in weeks and the increment in hematocrit (P = .02).
CONCLUSIONS
There is an inverse relationship between the age of blood and the increment in hematocrit. The age of blood should be considered before transfusion of surgical patients with intensive care unit anemia.
Keywords: Transfusion, Age of blood, Critical illness, Outcomes
Nearly all critically ill surgical patients are anemic within 72 hours of intensive care unit (ICU) admission.1 Although most anemic ICU patients receive a packed red blood cell (pRBC) transfusion, such transfusions are associated independently with worse outcomes and have been shown to afford questionable benefit in terms of oxygen consumption.2 Many of the adverse effects of pRBC transfusions are believed to be the result of a storage lesion, which is characterized by time-dependent accumulation of inflammatory mediators as well as depletion of essential nutrients such as adenosine triphosphate and 2,3-diphosphoglycerate. Storage of pRBCs also impairs both erythrocyte deformability and oxygen offloading, as well as results in time-dependent hemolysis.3
Based on these experimental findings, we sought to investigate the relationship between the age of transfused blood and the resultant change in recipient hematocrit level among critically ill surgical patients. Because the primary goal of pRBC transfusion is to increase oxygen delivery via an increase in the hematocrit level, this relationship is of clinical relevance. However, despite numerous investigations concerning the adverse effects of the storage lesion, the specific relationship between age of blood and change in hematocrit level has not been investigated among critically ill patients. The hypothesis of this study was that transfusion of older blood, as compared with newer blood, results in a lesser increment in the hematocrit level. Furthermore, to assess the clinical relevance of the increment in hematocrit, we hypothesized that a lesser increment in post-transfusion hematocrit level would be associated with the need for a subsequent pRBC transfusion.
Methods
This study was a cross-sectional analysis of all leukocyte-reduced, pRBC transfusions administered to critically ill patients from January through June 2009 in a 20-bed academic surgical ICU. Transfusions abstracted were limited to those administered for ICU anemia. Transfusions given for acute hemorrhage were identified by the terms “bleeding” or “hemorrhage” used in the medical record and excluded. The hemoglobin transfusion trigger for ICU anemia in our surgical ICU is 7.0 g/dL, although deviation from this standard may occur as a result of specific patient circumstances and at the discretion of the attending intensivist (eg, acute coronary syndrome). All transfusions for ICU anemia are given as single units.
The primary predictor variable was the age of the transfused blood identified using blood bank records. The outcome variable was the change in hematocrit level after transfusion (percentage points, continuous). The change in hematocrit level was calculated as the difference between the first post-transfusion hematocrit value (Hctpost) and the last pretransfusion hematocrit value (Hctpre). The time from Hctpre determination to the blood transfusion (Tpre, hours), as well as the time from the blood transfusion to Hctpost determination (Tpost, hours) also were abstracted. For standardization purposes, only instances in which a single unit of pRBCs was transfused between Hctpre and Hctpost were included. Transfusion of additional blood products (eg, fresh-frozen plasma, platelets, cryoprecipitate) along with pRBCs also was recorded.
The relationship between the age of stored blood and the change in hematocrit level after transfusion was explored in several ways. First, the age of stored blood was analyzed as a continuous variable (days). Next, the age of blood was dichotomized as either old (≥14 days) or new (<14 days). The 14-day cut-off value was selected based on several experimental studies indicating that the deleterious effects of blood transfusion become evident after this point.4,5 Finally, the age of blood was categorized by weeks old (≤7 days, 8–14 days, 15–21 days, 22–28 days, 29–35 days, and ≥35 days). Additional covariates abstracted included recipient patient age (years), sex, diagnosis (trauma vs other), time from admission to transfusion (days), ICU length of stay (days), and mortality. Finally, we abstracted the occurrence of any subsequent transfusions.
All statistical analyses were performed using SAS version 9.2 (SAS, Inc, Cary, NC). The correlation between the age of transfused blood (days) and the resultant change in recipient hematocrit level when both variables were analyzed continuously was calculated using the r2 statistic. Differences in categoric variables were compared using the chi-squared test. When expected cell counts were fewer than 10, the Fisher exact test was used. When more than 2 groups were compared, analysis of variance was used. Differences in continuous variables were compared using the Student t test. When analyzing the age of transfused blood by weeks old, the chi-squared trend statistic was used to assess for a linear relationship. The α error level was set as .05, with a P value of less than .05 considered statistically significant. This study was approved by the Colorado Multiple Institutional Review Board (COMIRB protocol #10-0028).
Results
A total of 215 U of pRBCs were administered during the study period. Of these 215, 186 (86.5%) U were given for an indication of ICU anemia. Of these 186, 136 (73.1%) U were given to 52 patients as single-unit transfusions and served as the final sample size.
The distribution of the age of transfused blood is shown in Fig. 1; most units transfused were 14 days old or more (n = 110 units; 80.9%). The mean Hctpre was 21.5% (range, 14.8%–30.3%). Recipient patient demographics as a function of receiving either old or new blood are shown in Table 1. Recipient patient age (P = .78), sex (P = .43), admission diagnosis (P = .92), and time from admission to first transfusion (P = .86) did not vary by age of blood transfused.
Figure 1.
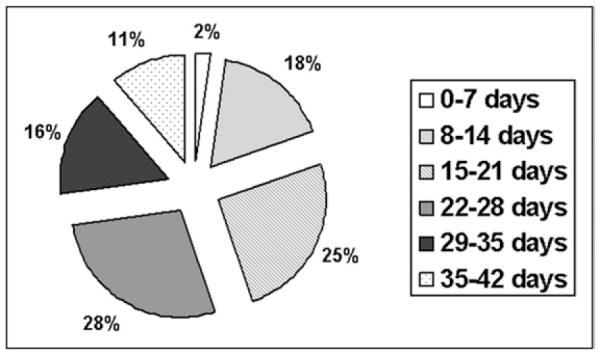
Distribution of the age of red blood cell units transfused. The majority (80.0%) of transfused packed red blood cell units were more than 14 days old.
Table 1.
Recipient demographics as a function of the age of transfused blood
| Variable | Old blood (n = 110 units) |
New blood (n = 26 units) |
P |
|---|---|---|---|
| Age, y | 48.8 | 47.1 | .64 |
| Male | 72.7% | 80.8% | .43 |
| Admission diagnosis of trauma |
64.5% | 65.4% | .92 |
| Time from ICU admission to transfusion, d |
8.3 | 8.7 | .86 |
Data related to the transfusion itself as a function of the age of blood are shown in Table 2. Neither Tpre (4.4 hours for old blood vs 4.9 hours for new blood; P = .64) nor Tpost (4.0 hours for old blood vs 4.5 hours for new blood; P = .31) varied by age of blood. Furthermore, no difference in either the pretransfusion or post-transfusion hematocrit level was observed between the old and new blood groups. In 14 instances (10.3%) additional blood products were administered with the pRBC transfusion (n = 7 fresh-frozen plasma, n = 6 platelets, n = 1 cryoprecipitate). The likelihood of additional products being administered along with the pRBC transfusion did not vary by the age of blood (12 of 110 [10.9%] for old blood vs 2 of 26 [7.8%] for new blood; P = .46).
Table 2.
Transfusion data as a function of the age of transfused blood
| Variable | Old blood (n = 110 units) |
New blood (n = 26 units) |
P |
|---|---|---|---|
| Age of transfused blood, d |
26.0 | 10.2 | <.001 |
| Tpre, h | 4.4 | 4.9 | .64 |
| Tpost, h | 4.0 | 4.5 | .42 |
| Pretransfusion hematocrit level, % |
21.8 | 20.4 | .31 |
| Post-transfusion hematocrit level, % |
25.6 | 25.9 | .91 |
| Change in Hct, % | 3.5 | 5.6 | .005 |
| Change in hemoglobin level, g/dL |
1.3 | 2.0 | .03 |
The mean change in hematocrit leve after transfusion was 4.1% (range, 3.2%–12.2%). The change in hematocrit level after the index transfusion was associated significantly with the likelihood of a subsequent transfusion. Specifically, the change in hematocrit level for the index transfusion was 3.3% in cases of a subsequent transfusion as compared with 4.6% in cases of no subsequent transfusion (P = .04).
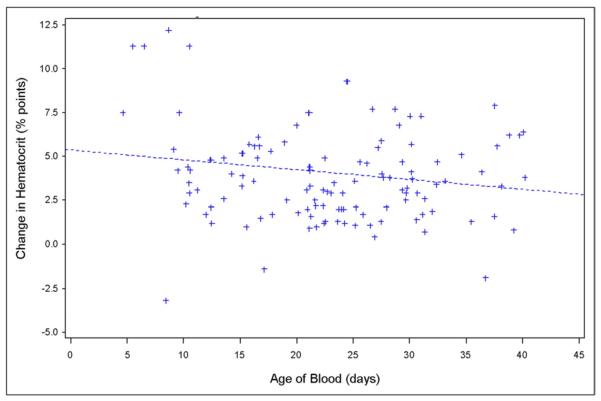
When the age of transfused blood was analyzed as a continuous variable, a significant, inverse correlation with the change in hematocrit level was observed (r2 = −.18; P = .04) (Fig. 2). Furthermore, the change in hematocrit level was significantly greater after transfusion of new blood (<14 days old) as compared with old blood (≥14 days old) (5.6% points vs 3.5% points, respectively; P = .005). Finally, when grouped by weeks old, a linear relationship was observed between the age of transfused blood and the change in recipient hematocrit level (Fig. 3; P = .02). Specifically, blood that was fewer than 8 days old increased the recipient hematocrit level a mean of 10.0 percentage points, blood that was more than 35 days old increased the hematocrit level a mean of only 2.6 percentage points.
Figure 2.
Scatter plot of the relationship between the age of blood (days, continuous) and the change in recipient hematocrit level (percentage points, continuous). A significant, inverse relationship was observed (r2 = −.18; P = .04).
Figure 3.
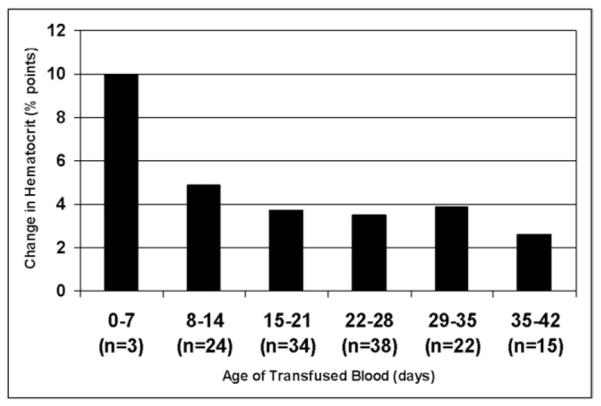
Relationship between the age of blood (grouped by weeks old) and the change in recipient hematocrit level (percentage points, continuous).
Comments
In this cross-sectional analysis of anemic, critically ill surgical patients, we observed an inverse relationship between the age of transfused blood and change in hematocrit level after transfusion. This relationship was significant when analyzing the age of the transfused blood as a continuous variable, a binary variable dichotomized at 14 days, and as an ordinal categoric variable grouped by weeks old. In this last case, the newest blood increased the hematocrit level approximately 4 times as much as the oldest blood. Finally, the change in hematocrit level was associated significantly with the occurrence of a subsequent transfusion.
Although pRBC transfusions can be live-saving in the face of hemorrhagic shock, the deleterious effects of such transfusions have become increasingly apparent over the past 3 decades. During this time, much attention has focused on the immunomodulatory effects of blood transfusion leading to inflammation, infection, and organ dysfunction.6–10 These risks are believed to be potentiated by increased length of storage, which leads to both neutrophil priming11 and the accumulation of inflammatory cytokines.12 In addition to these risks, the efficacy of pRBC transfusions also is reduced as a function of storage time, secondary to impaired erythrocyte survival and function. As storage length increases, pRBCs are depleted of essential nutrients, resulting in decreased deformability, impaired oxygen offloading, and hemolysis.13
Studies addressing the efficacy of pRBC transfusions among the critically ill are few and have focused primarily on the outcomes of oxygen delivery and consumption. Not surprisingly, although some studies suggest that pRBC transfusion increases oxygen delivery, nearly all studies have failed to document a corresponding increase in oxygen consumption.14 The lack of an increase in oxygen consumption is particularly apparent after transfusion of older blood.15 Because pRBC transfusion increases oxygen delivery specifically by an increase in the hematocrit level, we chose to specify this parameter as our primary outcome variable. Furthermore, the change in hematocrit level is an inexpensive marker of transfusion efficacy that is measured routinely. Our observation that there is a significant inverse relationship between the age of stored blood and the resultant change in recipient hematocrit level is consistent with experimental evidence reporting decreased pRBC longevity with storage, as well as decreased oxygen consumption.
Out study was limited by a retrospective design. We were unable to capture additional variables that may have influenced the change in recipient hematocrit level irrespective of the age of transfused blood. However, patient demographics, Hctpre, and Tpre were all similar between groups. Furthermore, we were unable to abstract either the hematocrit level of the pRBC unit before transfusion or the resultant change in both oxygen delivery and consumption after transfusion. Laboratory markers of hemolysis (eg, lactate dehydrogenase, hemoglobinuria) were not measured routinely. A prospective analysis of the relationship between pRBC storage time, change in hematocrit level, and laboratory markers of hemolysis is currently underway at our institution. Finally, this preliminary study comprised a relatively small sample size, and further large-scale validation studies are needed. However, because the groups were well matched with respect to the variables analyzed, we were able to perform a univariate comparison, increasing statistical power and minimizing the occurrence of type I error inherent to multiple comparisons.
Our study had several clinical implications. First, we have identified the change in hematocrit level after pRBC transfusion as a clinically relevant marker of the efficacy of transfusion that is associated with both the likelihood of an additional transfusion and the time to that transfusion. Second, we have shown a significant inverse relationship between the age of transfused blood and the resultant change in hematocrit level after transfusion. Because of both the increased incidence of adverse events observed with transfusion of older blood, as well as the decrement in hematocrit level increase observed in the current study, caution should be exercised before transfusion of older blood to critically ill patients. Specifically, we believe that the age of pRBC units should become a standard variable used in determining the risks and benefits of the proposed transfusion, and ultimately influencing the decision to transfuse. Avoidance of transfusion of older blood to hemodynamically normal, critically ill patients with a marginal hematocrit level (21%–24%) may be regarded as an additional means by which a restrictive transfusion policy is implemented. The mean Hctpre observed in our sample (21.5%) suggests an improving but persistent discrepancy with current evidence regarding hematocrit transfusion thresholds.16 Determination of the age of available pRBCs units may become increasingly important as additional studies document the adverse effects and limited efficacy of transfusion of older blood.17
In addition to avoidance of unnecessary transfusion of older blood, continued research into decreasing the risk: benefit ratio of such transfusions is necessary. One promising strategy that is the focus of current investigations involves prestorage washing of pRBCs (leukoreduction) to remove not only leukocytes but leukocyte and platelet fragments that are believed to improve pRBC morphology and decrease hemolysis during storage.18,19
In conclusion, most of the blood transfused to our critically ill surgical patients with ICU anemia was more than 14 days old. We observed a significant, inverse relationship between the age of blood and the change in hematocrit level after transfusion, such that older blood is less effective in achieving an increase in hematocrit level. The change in hematocrit level after transfusion, in turn, was predictive of the need for a subsequent transfusion. The age of banked blood should be considered before transfusion of surgical patients with ICU anemia.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Vincent JL, Baron JF, Reinhart K, et al. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499–507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 2.Napolitano LM, Corwin HL. Efficacy of red blood cell transfusion in the critically ill. Crit Care Clin. 2004;20:255–68. doi: 10.1016/j.ccc.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Tinmouth A, Chin-Yee I. The clinical consequences of the red cell storage lesion. Transfus Med Rev. 2001;15:91–107. doi: 10.1053/tmrv.2001.22613. [DOI] [PubMed] [Google Scholar]
- 4.Kirkpatrick UJ, Adams RA, Lardi A, et al. Rheological properties and function of blood cells in stored bank blood and salvaged blood. Br J Haematol. 1998;101:364–8. doi: 10.1046/j.1365-2141.1998.00689.x. [DOI] [PubMed] [Google Scholar]
- 5.Chin-Yee I, Arya N, d’Almeida MS. The red cell storage lesion and its implication for transfusion. Transfus Sci. 1997;18:447–58. doi: 10.1016/S0955-3886(97)00043-X. [DOI] [PubMed] [Google Scholar]
- 6.Napolitano LM. Current status of blood component therapy in surgical critical care. Curr Opin Crit Care. 2004;10:311–7. doi: 10.1097/01.ccx.0000140948.98019.8a. [DOI] [PubMed] [Google Scholar]
- 7.Silliman CC, Moore EE, Johnson JL, et al. Transfusion of the injured patient: proceed with caution. Shock. 2004;21:291–9. doi: 10.1097/00024382-200404000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Blajchman MA, Dzik S, Vamvakas EC, et al. Clinical and molecular basis of transfusion-induced immunomodulation: summary of the proceedings of a state-of-the-art conference. Transfus Med Rev. 2001;15:108–35. doi: 10.1053/tmrv.2001.22614. [DOI] [PubMed] [Google Scholar]
- 9.Croce MA, Tolley EA, Claridge JA, et al. Transfusions result in pulmonary morbidity and death after a moderate degree of injury. J Trauma. 2005;59:19–23. doi: 10.1097/01.ta.0000171459.21450.dc. discussion, 23-4. [DOI] [PubMed] [Google Scholar]
- 10.Barnett CC, Jr, Beck AW, Holloway SE, et al. Intravenous delivery of the plasma fraction of stored packed erythrocytes promotes pancreatic cancer growth in immunocompetent mice. Cancer. 2010;116:3862–74. doi: 10.1002/cncr.25140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chin-Yee I, Keeney M, Krueger L, et al. Supernatant from stored red cells activates neutrophils. Transfus Med. 1998;8:49–56. doi: 10.1046/j.1365-3148.1998.00125.x. [DOI] [PubMed] [Google Scholar]
- 12.Shanwell A, Kristiansson M, Remberger M, et al. Generation of cytokines in red cell concentrates during storage is prevented by prestorage white cell reduction. Transfusion. 1997;37:678–84. doi: 10.1046/j.1537-2995.1997.37797369441.x. [DOI] [PubMed] [Google Scholar]
- 13.Tinmouth A, Fergusson D, Yee IC, et al. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46:2014–27. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 14.Napolitano LM, Kurek S, Luchette FA, et al. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Crit Care Med. 2009;37:3124–57. doi: 10.1097/CCM.0b013e3181b39f1b. [DOI] [PubMed] [Google Scholar]
- 15.Kiraly LN, Underwood S, Differding JA, et al. Transfusion of aged packed red blood cells results in decreased tissue oxygenation in critically injured trauma patients. J Trauma. 2009;67:29–32. doi: 10.1097/TA.0b013e3181af6a8c. [DOI] [PubMed] [Google Scholar]
- 16.Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 17.Hassan M, Pham TN, Cuschieri J, et al. The association between the transfusion of older blood and outcomes after trauma. Shock. 2011;35:3–8. doi: 10.1097/SHK.0b013e3181e76274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blumberg N, Heal JM, Murphy P, et al. Association between transfusion of whole blood and recurrence of cancer. Br Med J (Clin Res Ed) 1986;293:530–3. doi: 10.1136/bmj.293.6546.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bordin JO, Chiba AK, Carvalho KI, et al. The effect of unmodified or prestorage white cell-reduced allogeneic red cell transfusions on the immune responsiveness in orthopedic surgery patients. Transfusion. 1999;39:718–23. doi: 10.1046/j.1537-2995.1999.39070718.x. [DOI] [PubMed] [Google Scholar]