Abstract
BACKGROUND
Fibrinolysis is a physiologic process maintaining patency of the microvasculature. Maladaptive overactivation of this essential function (hyperfibrinolysis) is proposed as a pathologic mechanism of trauma-induced coagulopathy. Conversely, the shutdown of fibrinolysis has also been observed as a pathologic phenomenon. We hypothesize that there is a level of fibrinolysis between these two extremes that have a survival benefit for the severely injured patients.
METHODS
Thrombelastography and clinical data were prospectively collected on trauma patients admitted to our Level I trauma center from 2010 to 2013. Patients with an Injury Severity Score (ISS) of 15 or greater were evaluated. The percentage of fibrinolysis at 30 minutes by thrombelastography was used to stratify three groups as follows: hyperfibrinolysis (Q3%), physiologic (0.081-2.9%), and shutdown (0-0.08%). The threshold for hyperfibrinolysis was based on existing literature. The remaining groups were established on a cutoff of 0.8%, determined by the highest point of specificity and sensitivity for mortality on a receiver operating characteristic curve.
RESULTS
One hundred eighty patients were included in the study. The median age was 42 years (interquartile range [IQR], 28Y55 years), 70% were male, and 21% had penetrating injuries. The median ISS was 29 (IQR, 22-36), and the median base deficit was 9 mEq/L (IQR, 6-13 mEq/L). Distribution of fibrinolysis was as follows: shutdown, 64% (115 of 180); physiologic, 18% (32 of 180); and hyperfibrinolysis, 18% (33 of 180). Mortality rates were lower for the physiologic group (3%) compared with the hyperfibrinolysis (44%) and shutdown (17%) groups (p = 0.001).
CONCLUSION
We have identified a U-shaped distribution of death related to the fibrinolysis system in response to major trauma, with a nadir in mortality, with level of fibrinolysis after 30 minutes between 0.81% and 2.9%. Exogenous inhibition of the fibrinolysis system in severely injured patients requires careful selection, as it may have an adverse affect on survival.
LEVEL OF EVIDENCE
Prognostic study, level III.
Keywords: Fibrinolysis, fibrinolysis shutdown, hyperfibrinolysis, antifibrinolytic, severe trauma
Fibrinolysis is the physiologic counterbalance of coagulation, functioning to maintain vasculature patency.1 With historical accounts of bleeding dysfunction related to trauma2 dating back to 1794 and a hundred-year gap before the identification of fibrinolysis,3 it is apparent that this system is complex. A complete understanding of the regulation of the fibrinolytic system remains elusive. Pathologic hyperfibrinolysis, excessive fibrinolytic activity, was appreciated by Starzl et al.4 in 1963 using thrombelastography (TEG) during the early operative phase of liver transplantation. In the original series of transplantation in humans, these authors recommended empiric antifibrinolytics to reduce bleeding. However, 6 years later, when evaluating the coagulopathy of liver transplantation, Starzl’s group5 retracted their statement advocating empiric antifibrinolytics when they appreciated increased mortality from venous thromboembolism (VTE).
As a result of the recent widespread use of TEG in trauma, hyperfibrinolysis has been identified in severely injured patients, and this has been proposed as integral in trauma-induced coagulopathy (TIC).6,7 Acutely injured patients with severe hyperfibrinolysis (915% fibrinolysis after 30 minutes [L-30]) are reported to have mortality rates exceeding 70%.8-10 These findings prompted the Clinical Randomization of an Antifibrinolytic in Significant Hemorrhage (CRASH-2) trial11 to determine the potential benefit of empiric antifibrinolytic therapy in injured patients. While there was a statistical improvement in survival with antifibrinolytics, less than half of the patients received a blood transfusion, and there was no overall reduction in blood product transfusions between treatment and placebo groups.11 Perhaps the most concerning finding was increased mortality if the antifibrinolytic was given 3 hours after injury.12 Despite the unclear mechanistic link of antifibrinolytic therapy (presumed inhibition of plasminogen activation) to reduced mortality despite no impact on transfusion requirements, centers in the United Kingdom have advocated empiric use for all trauma patients requiring resuscitation.13 Activated protein CYmediated TIC has been associated with fibrinolysis.7 However, recent component analyses of patients with TIC have identified a phenotypic distinction between those with global factor deficiency versus those with hyperfibrinolysis,14,15 questioning the mechanistic relationship.
On the other end of the spectrum is inhibition of fibrinolysis. During the 1960s, studies of coagulation following elective surgery identified the inhibition of fibrinolysis and termed this clinical phenomenon shutdown.2,3 Fibrinolysis shutdown has been associated with orthopedic surgery, resulting in increasing risk for deep venous thrombosis16 and increased sepsis-provoked multiple organ failure.17 We have observed in swine and rodent models that hemorrhagic shock and tissue injury have opposite effects on systemic fibrinolysis; that is, ischemia provokes hyperfibrinolysis, while tissue disruption inhibits fibrinolysis. Interestingly, the middle ground between the extremes of fibrinolysis (i.e., the potential benefit of physiologic fibrinolysis) has received little attention. Collectively, these findings indicate what appears to be a spectrum of fibrinolysis. We hypothesize that tissue injury and hemorrhagic shock produce distinct and opposing phenotypic effects on fibrinolysis, that untimely inhibition or hyperactivation may result in increased mortality, and that, between the two, there is a survival benefit.
PATIENTS AND METHODS
Study Population
TEG and clinical data were prospectively collected on acutely injured patients admitted to our Level I trauma center (Denver Health Medical Center) from 2010 to 2013 under Colorado Multiple Institutional Review Board protocol number 10-0477. Patients were excluded from the analysis if they had an Injury Severity Score (ISS) of less than 15, if the first TEG was obtained more than 12 hours after injury, or if they had taken preinjury anticoagulant medication. Patient demographics, emergency department vital signs, and initial laboratory values were obtained from this prospective registry. Blood product administration was prospectively recorded in the same registry. Cause of death was determined based on the hospital mortality and morbidity conference records as well as death certificate data.
Thrombelastography
Blood was collected from patients in 2.7-mL buffered sodium citrate (3.2%) sample tubes (Vacutainer, Becton-Dickinson, Franklin Lakes, NJ). The median time for blood sampling was 96 minutes after injury (interquartile range, 46-260 minutes). Samples were run within 2 hours of collection. Citrated Kaolin TEG assays were recalcified and ran according to the manufacturer’s instructions on a TEG 5000 Thrombelastograph Hemostasis Analyzer (Haemonetics, Niles, IL). Fibrinolysis was evaluated based on the percentage of clot lysis at 30 minutes after the clot achieved maximum strength (Ly30).
Data Analysis
Ly30 was stratified into three groups as follows: hyperfibrinolysis (Q3%), physiologic (0.81-2.9%), and shutdown (0-0.8%). The 3% cutoff was based on previously published studies that demonstrated increased mortality and blood product consumption higher than this value.9,18 Ly30 lower than this level has not received as much attention, and we were unable to find literature to support a threshold for fibrinolysis shutdown. Thus, to identify a potential threshold for fibrinolysis shutdown, a receiver operating characteristic curve for mortality was generated using the remaining patients with an Ly30 of less than 3% . The point with the highest specificity and sensitivity for mortality on the receiver operating characteristic curve was 0.8%, based on the Youden index.19 This Ly30 value was used as our upper boundary for fibrinolysis shutdown. The remaining patients with an Ly30 greater than this range and less than 3% were categorized as physiologic. Massive transfusion (MT) was defined as administration of red blood cells (RBCs) greater than 10 U within 6 hours of injury.
Data analysis was performed using the SPSS Statistics 22 software (IBM Corp., Somers, NY). Significance was set at an > of 0.05 with a two-tailed distribution. The Kruskal-Wallis test was used for continuous variables; when the experiment-wise p value was significant, pairwise comparisons were performed; the pairwise comparison p value was adjusted using the Bonferroni method. The W2 test was used for categorical variables. The Fisher’s exact test was used for categorical variables when the frequency of 5 or less was represented in a stratum comparison (cause-specific mortality between pathologic fibrinolysis groups). Kaplan-Meier survival curves were compared using the log-rank and Wilcoxon tests.
RESULTS
The study cohort of 180 patients represented a severely injured population from an urban trauma center. The median age was 43 years (interquartile range [IQR], 28-55), 70% were men, and most injuries were due to motor vehicle crashes (Table 1). The median ISS was 29 (IQR, 22-36), with a median initial base deficit of 9 (IQR, 6-13). Overall mortality was 20%, with 67% occurring within 24 hours.
TABLE 1.
Stratified Fibrinolysis Phenotypes
| Hyperfibrinolysis |
Physiologic |
Shutdown |
All Patients |
p* | |
|---|---|---|---|---|---|
| Ly30 > 2.9%, n = 34 (18%) | Ly30, 0.81–2.9%; n = 38 (19%) | Ly30 < 0.8%, n = 121 (63%) | N = 193 | ||
| Age, y | 40.5 (31–49) | 37 (28–7.5) | 46 (29–57) | 42 (28–55) | 0.16 |
| Male | 82% | 71% | 64% | 70% | 0.22 |
| ISS | 30 (25–36) | 28 (22.5–35) | 29 (20–38) | 29 (22–36) | 0.64 |
| Penetrating | 24% | 21% | 19% | 21% | 0.9 |
| GSW | 21% | 8% | 9% | 10% | 0.13 |
| MVC | 18% | 40% | 37% | 30% | 0.08 |
| AutoPed | 24% | 13% | 16% | 15% | 0.46 |
| pH | 7.23 (7.09–7.32) | 7.24 (7.19–7.30) | 7.26 (7.17–7.33) | 7.25 (7.16–7.33) | 0.37 |
| BD, mEq/L | 10 (8–16) | 11 (6.5–14) | 9 (6–12) | 9 (6–13) | 0.27 |
| Lactate, mmol/L | 6.4 (4–10.3) | 4.4 (2.6–5.1) | 4.1 (2.7–6.5) | 4.4 (2.7–7.0) | 0.07 |
| INR | 1.4 (1.1–1.6) | 1.3 (1.2–1.4) | 1.2 (1.1–1.4) | 1.3 (1.1–1.5) | 0.44 |
| Temp, °C | 36.5 (35.7–36.9) | 36.6 (35.9–37) | 36.6 (36.1–37.1) | 36.6 (36.1–37.1) | 0.83 |
| SBP, mm Hg | 92 (16–102) | 99 (86–129) | 110 (88–138) | 104 (82–130) | 0.005 |
| GCS score | 4 (3–15) | 14 (6–15) | 14 (3–15) | 13 (3–15) | 0.14 |
| RBC | 7 (0–24) | 2 (0–6) | 2 (0–10) | 2 (0–10) | 0.15 |
| FFP | 0 (0–5) | 0 (0–4) | 0 (0–6) | 0 (0–6) | 0.14 |
| CRYO | 0 (0–2) | 0 (0–0) | 0 (0–1) | 0 (0–1) | 0.65 |
| PLTS | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.57 |
| MT | 44% | 5% | 26% | 28% | 0.025 |
X2 test for categorical variables and Kruskal-Wallis test for continuous variables.
Continuous variables are represented as median (interquartile range).
AutoPed, auto-pedestrian crash; BD, base deficit; CRYO, cryoprecipitate units for 24 hours; FFP, fresh frozen plasma units for 24 hours; GCS, Glasgow Coma Scale; GSW, gunshot wound; INR, international normalized ratio; MT, 10 or greater RBC units within 24 hours; MVC, motor vehicle crash; pH, arterial pH; PLTS, platelet units for 24 hours; RBC, packed RBC units for 24 hours; Temp, temperature.
The spectrum of fibrinolysis was divided into three groups as previously defined. Fibrinolysis shutdown was the most prevalent, representing 64% (n = 115), while physiologic fibrinolysis (18% [n = 32]) and hyperfibrinolysis (18% [n = 33]) were similarly distributed. The fibrinolysis groups had similar age, ISS, and base deficit (Table 1). Transfusion between phenotypes had a similar distribution. However, when evaluating patients who received at least 1 U of RBCs, the hyperfibrinolytic phenotype had increased RBC (p = 0.001) and plasma transfusions (p = 0.002) compared with the other phenotypes, which predictably correlated to an increased rate of MTs (p = 0.002) (Fig. 1).
Figure 1.
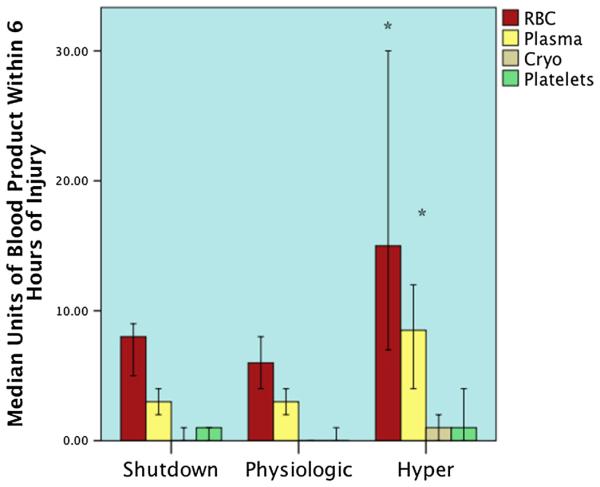
Blood product transfusions between phenotypes. The y axis represents the number of specific blood products transfused within 6 hours of injury. The figure includes only patients who received a transfusion. Overall RBC and plasma transfusion units were higher in the hyperfibrinolysis phenotype and remained statistically significant after pairwise adjustment between both the physiologic and shutdown groups. *p < 0.05 after pairwise adjustment. Cryo, croprecipitate.
Mortality between groups had a U-shaped distribution, with the lowest rate in the physiologic group, and the highest rates of mortality were seen at the extremes of shutdown and hyperfibrinolysis (p < 0.001) (Fig. 2). Pairwise adjustment retained statistical significance for a decreased mortality rate in the physiologic phenotype versus shutdown (p = 0.042). The cause of mortality specific to the shutdown and hyperfibrinolysis groups had different patterns (Fig. 3). Exsanguination represented 66% of deaths in the hyperfibrinolysis group, which was significantly higher than the shutdown phenotype (15%, p = 0.004). The converse was appreciated in which the shutdown group experienced a higher percentage of mortality attributable to multiple organ failure (40% vs. 7%, p = 0.048). Death from traumatic brain injury was higher in the shutdown group, 45% versus 26%, but did not reach statistical significance (p = 0.31). Survival time also differed greatly between groups (Fig. 4). The hyperfibrinolysis group had a significant early drop in survival compared with the shutdown cohort, which had delayed mortality (p = 0.001).
Figure 2.
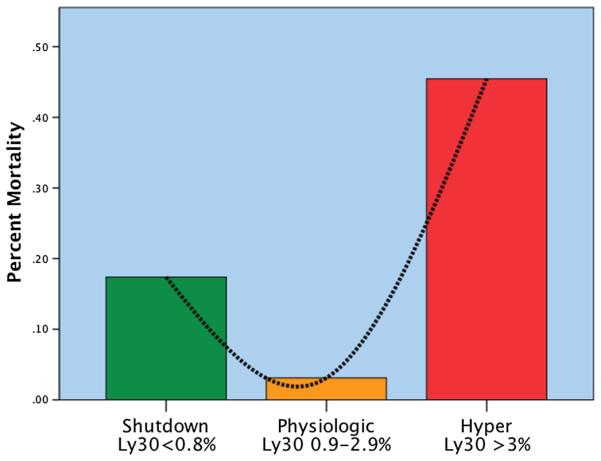
U-shaped distribution of mortality related to fibrinolysis phenotype. The y axis represents the percentage of mortality per phenotype. There is a U-shaped distribution of mortality, with a nadir in mortality identified in the physiologic group (Ly30 between 0.9% and 2.8%). Percentage of Ly30 higher and lower than this range had statistical increases in mortality. Hyper, hyperfibrinolysis; Ly30, percentage of fibrinolysis 30 minutes after reaching maximum amplitude measured by thombelastography; Physiologic, physiologic fibrinolysis; Shutdown, fibrinolysis shutdown.
Figure 3.
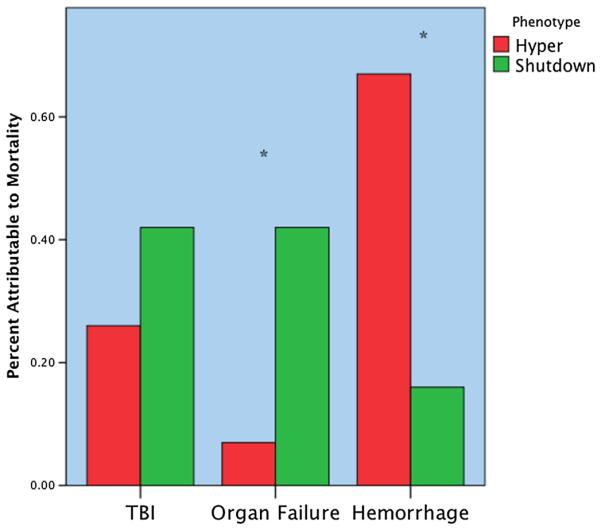
Distribution of mortality according to fibrinolytic phenotype. The y axis represents the percentage of total mortality per phenotype. The hyperfibrinolytic phenotype had a high frequency of mortality associated with hemorrhage. The shutdown phenotype has a high frequency of organ failureYrelated death. TBI did not reach statistical difference between phenotypes but was more common in the shutdown cohort. *p < 0.05. Hyper, hyperfibrinolysis; Shutdown, fibrinolysis shutdown; TBI, traumatic brain injury.
Figure 4.
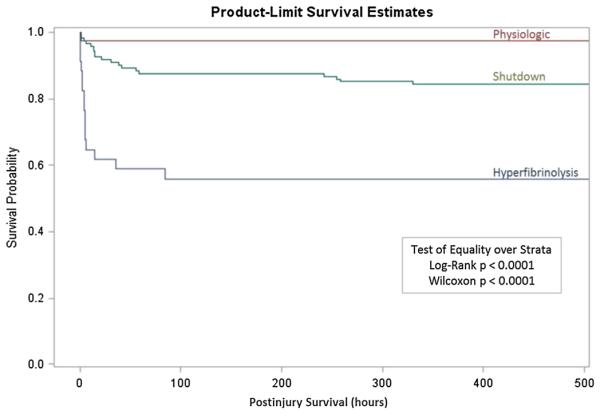
Survival curve of different phenotypes of fibrinolysis. Curve demonstrates the time from injury to survival patterns between the fibrinolysis phenotypes. The y axis represents the percentage of survival, and the x axis represents hours from injury. Survival, hours after injury.
DISCUSSION
We have identified three distinct phenotypes of fibrinolysis in response to trauma. Despite having similar demographics and injury patterns (Table 1), mortality differs between groups (Fig. 2). Modest levels of fibrinolysis seem to be protective, compared with overactive fibrinolysis, as there is a nadir in morality between the 0.8% and 2.9% range. Hyperfibrinolytic patients die of exsanguination and early after injury, whereas shutdown patients have a delayed mortality more frequently from organ failure (Fig. 4). These findings support our experimental observation that tissue injury and hemorrhagic shock have opposing impact on systemic fibrinolysis.
The degree of fibrinolysis and trauma does not have a normal distribution. In our study population of severely injured patients, only 18% of the population had hyperfibrinolysis. Our results are consistent with previous reports in the literature that hyperfibrinolysis is relatively infrequent.9,10,18 Interestingly, the more common response to injury is fibrinolysis shutdown, which accounted for more than 60% of our patients. While the concept of fibrinolysis shutdown during the acute injury phase in trauma has not received much attention, previous literature supports this concept. Raza et al.20 identified a group of patients with elevated plasmin/antiplasmin complexes and minimal detectable fibrinolysis activity using an analogous TEG device. Elevated plasmin/antiplasmin complexes and lack of hyperfibrinolysis were identified in 57% of their patient population and were associated with increased mortality. In their article, perhaps paradoxically titled, “The incidence and magnitude of fibrinolytic activation in trauma patients,” we believe that this group has elegantly shown the shutdown of fibrinolysis and confirmed a high prevalence.
Hyperfibrinolysis and shutdown have diverse pathologic sequelae. Hyperfibrinolysis is associated with early death from exsanguination, and shutdown is associated with delayed death ue to organ failure (Fig. 4). It is interesting that both groups ave similar distribution of demographics, injury patterns, and mergency department vital signs except systolic blood presure (SBP) (Table 1). Kutcher et al.21 also struggled to identify nique differences in patients who present with hyperfirinolysis. Lower SBP differed between fibrinolysis phenoypes. These findings are consistent with the recent finding that rehospital cardiopulmonary resuscitation is associated with high rate of hyperfibrinolysis.22 Circulatory arrest clearly romotes fibrinolysis but is not uniform, as the previously entioned study identified 36% of patients in circulatory arrest ith hyperfibrinolysis. In our study, 14 patients presented in irculatory arrest, and 64% had hyperfibrinolysis. Our group’s npublished work with swine indicates that hemorrhagic shock s contributory to activation of fibrinolysis, although whether this is attributable to ischemia and/or no flow remains unclear. Interestingly, tissue injury in the swine produces fibrinolysis shutdown.
In our study, we failed to identify a specific injury pattern or patient demographics that predisposed patients to fibrinolysis shutdown. This is likely due to our lack of ability to quantify and specify tissue injury. Plasminogen activator inhibitor 1 (PAI-1) is produced rapidly after tissue injury during surgery,23 and certain ratios of plasminogen inhibitors to activators to inhibitor may shut down the system. Bone fractures may be a dominant factor. Endothelial cells cultured in the plasma of postoperative hip replacement patients produced high levels of PAI-1.24 Subsequently, prospective evaluation has identified elevated PAI-1 as a risk of postoperative deep venous thrombosis in orthopedic surgery.16 The complexity of regulation of fibrinolysis is also compounded by at least a dozen receptors known to interact with plasminogen,25 and there are likely many other unknown factors we currently overlook.
The shutdown of fibrinolysis is associated with organ failure. Nearly 90% of patients in our study who died of organ dysfunction had fibrinolysis shutdown. Acute lung injury provides an example of the failure of fibrinolysis resulting in organ dysfunction. Since the early 1980s, it has been known that fibrin deposition in the pulmonary vasculature is pathologic.26 Idell et al.27 in 1991 identified tissue factor as the perpetrator for excessive fibrin deposition that remained present for weeks because of decreased fibrinolysis activity. Ostrowski et al.28 recently used TEG to demonstrate fibrinolysis shutdown in healthy volunteers by administrations of endotoxin, suggesting the cross talk between inflammation and fibrinolysis. These observations are not new, as it was appreciated in the 1960s that “stress” impacts the fibrinolytic system and that there is a complex interaction with inflammation.29 Disseminated intravascular coagulopathy (DIC) may be an extreme example of fibrinolysis shutdown. Trauma patients who progressed through acute lung injury to multiple organ failures and ultimately death from DIC have been found to have progressively elevated levels of PAI-1,30 indicative of fibrinolysis shutdown.
It seems intuitive that preventing clot degradation during the acute injury phase would have a survival benefit. Survival trends from our data support this concept, as shutdown of fibrinolysis is less lethal than hyperfibrinolysis (Fig. 3). However, between the two extremes, there is a survival advantage. This may represent a physiologic protection following injury (Fig. 2). The Blood Conservation Using Antiibrinolytics in a Randomized Trial (BART) is an example of patients at high risk for bleeding who had increased mortality from medically inhibited fibrinolysis that may otherwise have been protective. There was an increased rate of coronary graft failure, renal failure, and death with empiric antifibrinolytics.31 Even Groth et al.5 retracted their position about the use of empiric antifibrinolytics during liver transplantation after 6 years of critically evaluating their experience and observing increased VTE. In fact, fibrinolysis shutdown may be the missing link in the pathogenesis of postinjury VTE in the surgical intensive care unit. Of note, Starzl’s group advocated point-of-care testing for selective use of antifibrinolytics in 1969.
As with every retrospective clinical study, there are limitations. We were unable to identify previous reports in the literature that defined a physiologic protective level of fibrinolysis in response to trauma assayed with TEG. As a result, our current range of Ly30 from 0.81 to 2.9 is derived from a single-center study, and a multicenter trial would provide external validity. Because of the inherent variability of the TEG device, this range may differ between centers. We have recently developed a novel TEG assay that quantifies the degree of fibrinolysis shutdown. With this assay, we can more definitively differentiate shutdown from the physiologic phenotype (Supplemental Digital Content 1: tPA TEG assay, http://links.lww.com/TA/A457). Data from plasma processed within 1 hour of injury collected since the completion of this study demonstrate that PAI-1 levels in the shutdown phenotype are 20-fold higher than in healthy controls and suggest a mechanism for impairment of the fibrinolytic system. Because of the retrospective nature of this study, time from injury to TEG was different between groups. However, most of the TEGs were obtained within 3 hours of injury in all groups. More importantly, pre-TEG fluid and blood product transfusions were not different between groups (Table 1). With our ongoing prospective study of admission hospital blood values, we will reassess a threshold for defining cutoff points for the three phenotypes of fibrinolysis, augmented by our tPA TEG assay.
Severely injured patients were included in the study and eliminated more than half of all patients who had prospectively been evaluated during the past 3 years. We selected a patient population with severe injury, as our animal data suggested that tissue injury provokes the shutdown of fibrinolysis. The clinical importance of identifying the fibrinolysis shutdown is in patients at risk for organ failure and VTE. The ISS cutoff yielded a population of patients in which 98% of patients were either dead or required ICU care. Of patients who survived their initial resuscitation, 75% required ventilator support. We believe that this was an ideal population to identify the shutdown phenotype and to determine its relationship with organ failure.
It is possible that less injured patients have a different physiologic requirement of fibrinolysis, as they are not responding to as much stress and may not require a higher level of fibrinolytic activity to maintain the microvasculature patency. A previous report from Japan identified increased fibrinolysis activity to have a survival advantage during DIC.32 This group measured proteins involved in the fibrinolysis pathway and did not use TEG. In our study, the focus was to identify clinical information to discuss fibrinolytic phenotypes. Our finding that the hyperfibrinolysis group predominantly bled to death was not surprising. We cannot definitively state that, had these patients survived, they would not have developed organ failure. However, it is intriguing that the physiologic levels of fibrinolysis lacked death from organ failure, and this poses the question of whether the shutdown population would benefit from fibrinolytic therapy, as described by Hardaway et al.33 more than a decade ago.
EDITORIAL CRITIQUE.
Drs. Moore and colleagues have performed a prospective observational study evaluating the association between early fibrinolysis as measured by LY30 on thrombelastogram (TEG) and clinical outcomes in a cohort of 186 severely injured patients. They have defined 3 ranges of fibrinolysis (shutdown, physiologic fibrinolysis and hyperfibrinolysis) that correlate with clinical outcomes. Mortality was increased in patients with shutdown and hyperfibrinolysis and patients with shutdown were more likely to die from multiple organ failure whereas patients with hyperfibrinolysis were more likely to die from exsanguination.
The study is an important contribution to the literature that appeals to the reader’s common sense and introduces the concept of fibrinolysis shutdown with its potential negative effects. It also raises several concerns that need to be addressed in further study before the results can be widely accepted. The sample size is small with only 32 patients in the physiologic range and 33 patients with hyperfibrinolysis. The range of LY30 that spans across the distribution from fibrinolysis shutdown to hyperfibrinolysis is only 3% with fibrinolysis shutdown being defined as an LY30 from 0Y0.8%. It is not clear that TEG technology can supply this degree of accuracy and reproducibility. Performance of the assays in duplicate with different machines would determine if the results are reproducible.
The median time to performance of the test was 96 minutes after injury with the interquartile range extending to 260 minutes. This indicates significant variability in timing suggesting that patients were in different phases of resuscitation. The authors report that there was no difference in the volume of fluid and blood products given to the 3 groups however they only report the volume of blood products given in the results. The study would be more powerful if the TEGs were performed upon admission of the patients prior to significant resuscitation.
In summary, the authors have potentially identified the very important phenomenon of fibrinolysis shutdown and associated it with the development of multiple organ failure. This has significant implications for the use of tranexamic acid in trauma patients. This preliminary study is highly suggestive however additional work is needed to confirm these findings.
Martin A. Schreiber, MD
Oregon Health & Science University
Portland, Oregon
ACKNOWLEDGMENT
The authors thank Sarah Ammons, James Chandler, and Arsen Ghasabyan for their invaluable assistance in data procurement.
This study was presented at the 44th Annual Meeting of the Western Trauma Association, March 3, 2014, in Steamboat Springs, Colorado, and at the Annual Meeting of the American College of Surgeons’ Committee on Trauma (ACS COT), March 20, 2014, in Philadelphia, Pennsylvania. The first author (H.B.M.) received the Best Clinical Research Manuscript award in ACS COT’s 2014 Resident Trauma Papers Competition.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
DISCLOSURE
This study was supported in part by National Institutes of Health grants T32-GM008315 (National Institute of General Medical Sciences), P50-GM0492221 (National Institute of General Medical Sciences), and UM 1HL120877 (National Heart, Lung, and Blood Institute) and in part by Colorado Clinical and Translational Science Award Grant UL1 TR001082 (National Center for Advancing Translational Science). Additional research support was provided by Haemonetics.
REFERENCES
- 1.Fearnley GR. Fibrinolysis. Ann R Coll Surg Engl. 1967;41(1):51–54. [PMC free article] [PubMed] [Google Scholar]
- 2.Chakrabarti R, Hocking ED, Fearnley GR. Reaction pattern to three stressesVelectroplexy, surgery, and myocardial infarctionVof fibrinolysis and plasma fibrinogen. J Clin Pathol. 1969;22(6):659–662. doi: 10.1136/jcp.22.6.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffiths NJ. Factors affecting the fibrinolytic response to surgery. Ann R Coll Surg Engl. 1979;61(1):12–16. [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Marchioro TL, Vonkaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659–676. [PMC free article] [PubMed] [Google Scholar]
- 5.Groth CG, Pechet L, Starzl TE. Coagulation during and after orthotopic transplantation of the human liver. Arch Surg. 1969;98(1):31–34. doi: 10.1001/archsurg.1969.01340070049006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kashuk JL, Moore EE, Sawyer M, Wohlauer M, Pezold M, Barnett C, Biffl WL, Burlew CC, Johnson JL, Sauaia A. Primary fibrinolysis is integral in the pathogenesis of the acute coagulopathy of trauma. Ann Surg. 2010;252(3):434–442. doi: 10.1097/SLA.0b013e3181f09191. discussion 443-444. [DOI] [PubMed] [Google Scholar]
- 7.Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care. 2007;13(6):680–685. doi: 10.1097/MCC.0b013e3282f1e78f. [DOI] [PubMed] [Google Scholar]
- 8.Ives C, Inaba K, Branco BC, Okoye O, Schochl H, Talving P, Lam L, Shulman I, Nelson J, Demetriades D. Hyperfibrinolysis elicited via thromboelastography predicts mortality in trauma. J Am Coll Surg. 2012;215(4):496–502. doi: 10.1016/j.jamcollsurg.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Cotton BA, Harvin JA, Kostousouv V, Minei KM, Radwan ZA, Schöchl H, Wade CE, Holcomb JB, Matijevic N. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. J Trauma Acute Care Surg. 2012;73(2):365–370. doi: 10.1097/TA.0b013e31825c1234. discussion 370. [DOI] [PubMed] [Google Scholar]
- 10.Schöchl H, Frietsch T, Pavelka M, Jámbor C. Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thrombelastometry. J Trauma. 2009;67(1):125–131. doi: 10.1097/TA.0b013e31818b2483. [DOI] [PubMed] [Google Scholar]
- 11.CRASH-2 Trial Collaborators. Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, El-Sayed H, Gogichaishvili T, Gupta S, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 12.Napolitano LM, Cohen MJ, Cotton BA, Schreiber MA, Moore EE. Tranexamic acid in trauma: how should we use it? J Trauma Acute Care Surg. 2013;74(6):1575–1586. doi: 10.1097/TA.0b013e318292cc54. [DOI] [PubMed] [Google Scholar]
- 13.Bozzette A, Aeron-Thomas A. Reducing trauma deaths in the UK. Lancet. 2013;382(9888):208. doi: 10.1016/S0140-6736(13)61597-4. [DOI] [PubMed] [Google Scholar]
- 14.Chin TL, Moore EE, Moore HB, Gonzalez E, Chapman MP, Stringham JR, Ramos CR, Banerjee A, Sauaia A. A principal component analysis of postinjury viscoelastic assays: clotting factor depletion versus fibrinolysis. Surgery. 2014 doi: 10.1016/j.surg.2014.04.030. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kutcher ME, Ferguson AR, Cohen MJ. A principal component analysis of coagulation after trauma. J Trauma Acute Care Surg. 2013;74(5):1223–1229. doi: 10.1097/TA.0b013e31828b7fa1. discussion 1229-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yukizawa Y, Inaba Y, Watanabe S, Yajima S, Kobayashi N, Ishida T, Iwamoto N, Choe H, Saito T. Association between venous thromboembolism and plasma levels of both soluble fibrin and plasminogen-activator inhibitor 1 in 170 patients undergoing total hip arthroplasty. Acta Orthop. 2012;83(1):14–21. doi: 10.3109/17453674.2011.652886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayakawa M, Sawamura A, Gando S, Jesmin S, Naito S, Ieko M. A low TAFI activity and insufficient activation of fibrinolysis by both plasmin and neutrophil elastase promote organ dysfunction in disseminated intravascular coagulation associated with sepsis. Thromb Res. 2012;130(6):906–913. doi: 10.1016/j.thromres.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Chapman MP, Moore EE, Ramos CR, Ghasabyan A, Harr JN, Chin TL, Stringham JR, Sauaia A, Silliman CC, Banerjee A. Fibrinolysis greater than 3% is the critical value for initiation of antifibrinolytic therapy. J Trauma Acute Care Surg. 2013;75(6):961–967. doi: 10.1097/TA.0b013e3182aa9c9f. discussion 967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schisterman EF, Perkins NJ, Liu A, Bondell H. Optimal cut-point and its corresponding Youden Index to discriminate individuals using pooled blood samples. Epidemiology. 2005;16(1):73–81. doi: 10.1097/01.ede.0000147512.81966.ba. [DOI] [PubMed] [Google Scholar]
- 20.Raza I, Davenport R, Rourke C, Platton S, Manson J, Spoors C, Khan S, De’Ath HD, Allard S, Hart DP, et al. The incidence and magnitude of fibrinolytic activation in trauma patients. J Thromb Haemost. 2013;11(2):307–314. doi: 10.1111/jth.12078. [DOI] [PubMed] [Google Scholar]
- 21.Kutcher ME, Cripps MW, McCreery RC, Crane IM, Greenberg MD, Cachola LM, Redick BJ, Nelson MF, Cohen MJ. Criteria for empiric treatment of hyperfibrinolysis after trauma. J Trauma Acute Care Surg. 2012;73(1):87–93. doi: 10.1097/TA.0b013e3182598c70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schöchl H, Cadamuro J, Seidl S, Franz A, Solomon C, Schlimp CJ, Ziegler B. Hyperfibrinolysis is common in out-of-hospital cardiac arrest: results from a prospective observational thromboelastometry study. Resuscitation. 2013;84(4):454–459. doi: 10.1016/j.resuscitation.2012.08.318. [DOI] [PubMed] [Google Scholar]
- 23.Kluft C, Verheijen JH, Jie AF, Rijken DC, Preston FE, Sue-Ling HM, Jespersen J, Aasen AO. The postoperative fibrinolytic shutdown: a rapidly reverting acute phase pattern for the fast-acting inhibitor of tissue-type plasminogen activator after trauma. Scand J Clin Lab Invest. 1985;45(7):605–610. doi: 10.3109/00365518509155267. [DOI] [PubMed] [Google Scholar]
- 24.Kassis J, Hirsh J, Podor TJ. Evidence that postoperative fibrinolytic shutdown is mediated by plasma factors that stimulate endothelial cell type I plasminogen activator inhibitor biosynthesis. Blood. 1992;80(7):1758–1764. [PubMed] [Google Scholar]
- 25.Plow EF, Doeuvre L, Das R. So many plasminogen receptors: why? J Biomed Biotechnol. 2012;2012:141806. doi: 10.1155/2012/141806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bachofen M, Weibel ER. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med. 1982;3(1):35–56. [PubMed] [Google Scholar]
- 27.Idell S, Koenig KB, Fair DS, Martin TR, McLarty J, Maunder RJ. Serial abnormalities of fibrin turnover in evolving adult respiratory distress syndrome. Am J Physiol. 1991;2614:L2400–L2408. doi: 10.1152/ajplung.1991.261.4.L240. Pt 1. [DOI] [PubMed] [Google Scholar]
- 28.Ostrowski SR, Berg RM, Windeløv NA, Meyer MA, Plovsing RR, Møller K, Johansson PI. Discrepant fibrinolytic response in plasma and whole blood during experimental endotoxemia in healthy volunteers. PLoS One. 2013;8(3):e59368. doi: 10.1371/journal.pone.0059368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogston D, Ogston CM, Ratnoff OD, Forbes CD. Studies on a complex mechanism for the activation of plasminogen by kaolin and by chloroform: the participation of Hageman factor and additional cofactors. J Clin Invest. 1969;48(10):1786–1801. doi: 10.1172/JCI106145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gando S, Nakanishi Y, Tedo I. Cytokines and plasminogen activator inhibitor-1 in posttrauma disseminated intravascular coagulation: relationship to multiple organ dysfunction syndrome. Crit Care Med. 1995;23(11):1835–1842. doi: 10.1097/00003246-199511000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Schneeweiss S, Seeger JD, Landon J, Walker AM. Aprotinin during coronary-artery bypass grafting and risk of death. N Engl J Med. 2008;358(8):771–783. doi: 10.1056/NEJMoa0707571. [DOI] [PubMed] [Google Scholar]
- 32.Asakura H, Ontachi Y, Mizutani T, Kato M, Saito M, Kumabashiri I, Morishita E, Yamazaki M, Aoshima K, Nakao S. An enhanced fibrinolysis prevents the development of multiple organ failure in disseminated intravascular coagulation in spite of much activation of blood coagulation. Crit Care Med. 2001;29(6):1164–1168. doi: 10.1097/00003246-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 33.Hardaway RM, Harke H, Tyroch AH, Williams CH, Vazquez Y, Krause GF. Treatment of severe acute respiratory distress syndrome: a final report on a phase I study. Am Surg. 2001;67(4):377–382. [PubMed] [Google Scholar]


