Abstract
We have previously shown that accumulation of ceramide, triggered by hydrogen peroxide (H2O2), induces apoptosis of human airway epithelial (HAE) cells. Under oxidant exposure, a lung sphingomyelinase (SMase) is activated and displays continued ceramide generation and pro-apoptotic signaling, thus leading to the pathological apoptosis that causes lung injury. In a search for a specific SMase that is modulated by oxidative stress, we recently cloned nSMase2 from monkey lung tissue and HAE cells. Here, we show that this nSMase2 is up-regulated by an oxidant (H2O2) and is inhibited by an antioxidant (glutathione (GSH)). Moreover, nSMase2 subcellular localization is governed by oxidant exposure, which leads to its preferential trafficking to the plasma membrane, where it generates ceramide and induces apoptosis. On the other hand, exposure to GSH results in nSMase2 trafficking to the nucleus, where it neither generates ceramide nor induces apoptosis.
Keywords: Neutral sphingomyelinase 2, Apoptosis, Hydrogen peroxide, Glutathione, Ceramide, Lung
Reactive oxidants such as hydrogen peroxide (H2O2) cause lung injury and contribute to pulmonary diseases mainly through targeting human airway epithelial (HAE) cells. Yet, the cellular and molecular mechanisms that link these reactive oxidants to the development of lung injury and disease are not fully understood. We have shown that HAE cells exposed to reactive oxygen species (ROS) are stimulated to generate excessive ceramide, which functions as a potent inducer of apoptosis in these cells [1–6]. Although antioxidant defenses are constitutively expressed inmammalian cells [7], additional responses are mounted when the amount of environmental oxidants exceeds a threshold level, thereby becoming a threat to overall tissue integrity. Apoptosis is one such cellular adaptive response [8–11].
The pro-apoptotic effects of ceramide are mediated by a variety of mechanisms [12–15]. Ceramide is synthesized primarily through a de novo pathway involving serine palmitoyl-CoA transferase and ceramide synthase or from membrane sphingomyelin breakdown by sphingomyelinases (SMases). Sphingomyelin (N-acylsphingosin-1-phosphocholine) is a phospholipid preferentially concentrated in the plasma membrane of mammalian cells [16]. Sphingomyelin catabolism occurs via the action of sphingomyelin-specific forms of phospholipase C termed sphingomyelinases (SMases), which hydrolyze the phosphodiester bond of sphingomyelin, yielding ceramide and phosphorylcholine. Ceramide then serves as a second messenger, leading to apoptotic DNA degradation. The main forms of SMases are distinguished by their pH optima [17–20]. Human and murine acid sphingomyelinase (aSMase; pH optimum 4.5–5.0) have been cloned and determined to be the products of a conserved gene, while Mg2+-dependent or -independent neutral SMases (nSMase; pH optimum 7.4) have yet to be molecularly characterized.
Interestingly, membrane nSMase does not gain access to the signaling events activated by the lysosomal aSMase and vice versa, indicating that ceramide action may be determined by the subcellular site of its production.
Hofmann et al. [21] cloned the mammalian brain-specific Mg2+-dependent nSMase2 gene, which encodes a 71 kDa, 655-amino acid protein. The N terminus contains two putative transmembrane domains, whereas the C-terminal contains the putative catalytic domain [21]. The role of nSMase2 in sphingomyelin metabolism was demonstrated in vivo using MCF7 cells overexpressing the enzyme. In addition, it was shown that nSMase2 is activated by TNF-α and that it is involved in cell growth and regulation [22].
Very little is presently known about the regulatory mechanisms of SMases. Also, the exact cellular and molecular mechanism(s) underlying ceramide-mediated apoptosis are still unknown, and thus, the role of ceramide in the pathobiology of disease still needs to be addressed.
Excessive accumulation of reactive oxidants is toxic [23], and the intracellular level of reactive oxidants is therefore tightly regulated by several antioxidants. Depletion of glutathione (GSH), the most abundant intracellular thiolcontaining small peptide, has been suggested to precede the onset of apoptosis induced by various agents [24–30]. Moreover, it has also been suggested that depletion of GSH could be an early event in the commitment to apoptosis [24,31]. However, the mechanism by which depletion of GSH transmits apoptotic signals remains unknown.
Here, we demonstrate that nSMase2 is the specific target in airway epithelial cells that is modulated by oxidative stress to generate ceramide, and is essential for apoptosis induction in these cells when exposed to ROS. nSMase2 preferentially traffics to the plasma membrane under H2O2 exposure. This is where ceramide generation can occur, leading to elevated apoptosis.
Materials and methods
Cell culture and transfections
We are using primary, immortalized (HBE1) or transformed (A549) HAE cells. Of note is that over the years, our data regarding ceramide generation and apoptosis were similar in all these HAE cells [1–5,32]. Therefore, we are routinely utilizing either HBE1 cells or A549 cells for most of our experiments and only verify key findings in the primary cells. Culture conditions are identical for both primary and HBE1 cells, which are grown in serum-free medium supplemented with insulin (5 μg/ml), transferrin (5 μg/ml), EGF (5 ng/ml), dexamethasone (0.1 μM), cholera toxin (20 ng/ml), and bovine hypothalamus extract (15 μg/ml) [33,34]. A549 human lung carcinoma cells were maintained in F-12K (Kaighn’s modification) nutrient mixture (Invitrogen, Carlsbad, CA), supplemented with 10% fetal bovine serum (Invitrogen) and 1% penicillin/streptomycin. Transient transfections using pFLAG-nSMase2 (kindly provided by Yusuf A. Hannun, Medical University of South Carolina, Charleston) were performed using Lipofectamine Transfection Reagent (Invitrogen) according to the manufacturer’s protocol. Transfected cells were treated 48 h post-transfection with 250 μM H2O2 or 10 mM GSH. For nSMase2 silencing, HAE cells were transiently transfected using Lipofectamine 2000 Transfection Reagent (Invitrogen) according to the manufacturer’s instructions.
Real-time PCR
Real-time PCR was done as described in Khan et al. [35]. The primers for the PCR were located on both sides of the siRNA. Primers for β-actin serve as a control for normalizing of cDNA.
Immunofluorescence and confocal microscopy
A549 cells grown on coverslips were transiently transfected with pFLAG-nSMase2 using Lipofectamine with the PLUS reagent (Invitrogen) according to the manufacturer’s protocol. Forty-eight hours post-transfection, cells were exposed to 250 μMH2O2 or 10 mM GSH. Following treatment cells were stained for the raft-localized lipid GM1 with FITC-conjugated cholera toxin β-subunit (2 μg/ml, Sigma–Aldrich, St. Louis, MO), for 40 min at room temperature (RT). Then cells were washed with 1 × PBS and fixed with 4% paraformaldehyde, 4% sucrose in PBS for 10 min at RT. Cells were permeabilized for 5 min at RT with PBS containing 0.25% Triton X-100 and blocked in PBS containing 10% BSA at RT for 30 min. The coverslips were then incubated for 1 h in primary Ab (anti-FLAG M2, Sigma–Aldrich), washed three times with PBS for 3 min each, and incubated for 45 min in secondary Ab, Alexa Fluor 594 goat anti-mouse (Molecular Probes, Eugene, OR). Nuclei were stained with 1 μg/ml DAPI (Sigma–Aldrich) for 3 min. After three washes with PBS, the coverslips were mounted onto glass slides using Fluoromount-G (SouthernBiotech, Birmingham, AL). Confocal microscopy at 60× magnification was carried out using an Olympus FV1000 Fluoview confocal laser-scanning microscope. All images are representative of at least 100 cells viewed in each of three separate experiments.
Assays for nSMase activity
The enzyme activity of nSMase was determined as described [18,20]. For determining nSMase activity, an enzyme preparation (100 μg of protein) in 20 mM Tris–HCl, pH 7.4, was mixed with [14C]sphingomyelin (10 nmol/ 100,000 dpm) in 0.1% Triton X-100 in 100 mM Tris–HCl, pH 7.4, containing 10 mM MgCl2, 10mM DTT, 10 nM phosphatidylserine, and 1 mg/ml BSA. The incubation time was 1 h at 37 °C. The reaction was terminated by the addition of 1 ml of chloroform:methanol:HCl (100:100:1) followed by 0.3 ml of 1 N HCl. After phase separation, the upper phase was removed and the radioactivity was determined by liquid scintillation counting.
Apoptosis assessment
Cells were pulsed for 1 h with 250 μM H2O2. After 24 h, both non-adherent and adherent cells were collected and analyzed using BD ApoAlert kit (BD Biosciences, Palo Alto, CA) as described [3,4]. The cells that were collected were incubated in binding buffer with annexin V (FITC-conjugated) and propidium iodide (PI) for 15 min at RT to detect for early apoptosis. The cells were analyzed by flow cytometry using the FITC signal detector (FL1) and the PI detector (FL2) in FAC-Scan flow cytometer (Becton–Dickinson, Franklin Lakes, NJ). Cells negative for both annexin V and PI staining are live cells; annexin V-positive- and PI-negative-stained cells are early apoptotic cells; annexin V- and PI-positive stained cells are necrotic and/or late apoptotic cells; and PI-positive and annexin V-negative stained cells are necrotic cells.
Results
nSMase2 is present and functional in lung epithelial cells
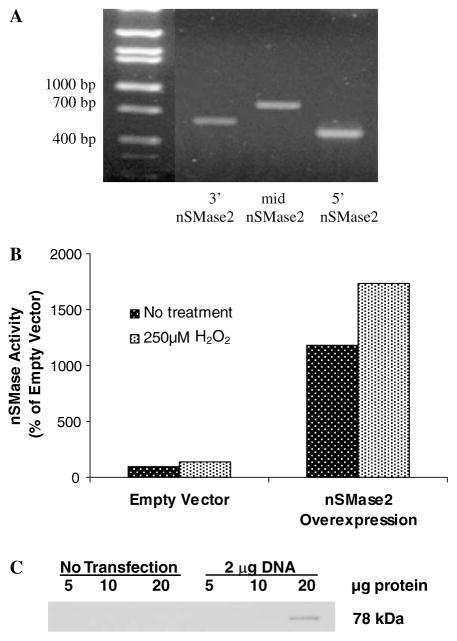
We demonstrated that nSMase2, a putative nSMase cloned on the basis of homology with bacterial SMases [21], is present and is functional in lung epithelial cells. We synthesized three sets of primers that correspond to the 5′-end, middle, and 3′-end of the open-reading frame (ORF) from the cDNA sequence of the putative human nSMase, and performed RT-PCR using RNA extracted from lung epithelial cells (HBE1 or A549) or from lung tissues (monkey or pig) and received the expected size products (Fig. 1A). Then, using the 5′ and 3′ primers, the PCR product of the expected size (approximately 1.9-kb) was cloned into pT7Blue-3, and its sequence was confirmed to be identical to the previously reported nSMase [21]. Subsequently, nSMase2 cDNA in-frame with the FLAG octapeptide epitope was transiently transfected into HAE cells to verify the functionality of this protein in lung epithelial cells by measuring its activity. As shown (Fig. 1B) cells overexpressing nSMase2 not only demonstrated 6-fold enhanced nSMase activity, but the latter was further augmented (1.5-fold) in response to oxidative stress exposure (1 h treatment of 250 μM H2O2). The transiently transfected FLAG-tagged nSMase2 was also detected by Western blotting with anti-FLAG M2 antibody (Ab), resulting in a single protein band of apparent molecular weight of 78 kDa (Fig. 1C), which was slightly higher than the previously reported size of 71–73 kDa [21,36].
Fig. 1.
nSMase2 is present and functional in lung epithelial cells. (A) The presence of nSMase2 was determined by PCR using three sets of primers to amplify the coding portion of the gene in HAE cells. Lane 1, 3′ coding region of nSMase2; lane 2, mid-region; lane 3, 5′ region. (B) HAE cells were transiently transfected with pFLAG-nSMase2. Transfected cells were treated for 1 h with 250 μMH2O2 and nSMase activity was measured. (C) The over-expression of pFLAG-nSMase2 in HAE cells, as measured by the presence of the FLAG tag in immunoblot analysis from transfected cells.
nSMase2 is the target modulated by H2O2 to induce apoptosis in HAE cells
We developed siRNA for nSMase2, which allowed us to study the role of the endogenous nSMase2 in HAE cells. These cells were transiently transfected with siRNA that specifically and efficiently knocked down nSMase2 (more than 90% silencing as determined by RT-PCR). Subsequently, the cells were treated with H2O2. The knocked down cells showed a complete elimination of the H2O2-inducible activity of nSMase compared to mock- or scrambled siRNA-transfected cells undergoing the same treatment (Fig. 2A). In addition, the increase in apoptosis typically seen in HAE cells after treatment with 250 μM H2O2 was entirely eliminated by the specific knockdown of nSMase2 (Fig. 2B).
Fig. 2.
nSMase2 is modulated by H2O2. HAE cells were transiently transfected with siRNA for nSMase2 or scrambled siRNA. (A) Forty-eight hours post-transfection the cells were treated with 250 μMH2O2 for 1 h and nSMase activity was measured compared to mock transfection. (B) Twenty-four hours post-transfection, cells were pulsed with 250 μM H2O2 for 1 h. Twenty-four hours after H2O2 exposure, cells were stained with annexin V-FITC and propidium iodide (PI), and were evaluated by FACS analysis for early apoptosis.
nSMase2 activity is modulated by GSH
Our past studies have shown that GSH treatment of HAE cells resulted in a dose-dependent decrease in nSMase activity [3]. Additionally, depletion of intracellular GSH with L-buthionine-(SR)-sulfoximine (BSO) treatment resulted in a dose-dependent increase in nSMase activity (Fig. 3A). However, after examining different enzymes in the ceramide-generating machinery, we were previously unable to identify the specific enzyme that is affected by GSH depletion. Only now we are able to demonstrate that when nSMase2 is knocked down, the modulation of nSMase activity by GSH or BSO is abolished. No changes in the enzyme activity after GSH or BSO treatment could be observed (Fig. 3B). These results further support our hypothesis that oxidative stress-induced apoptosis is coupled to ceramide generation specifically via nSMase2.
Fig. 3.
nSMase2 is modulated by glutathione (GSH). (A) HAE cells were treated by either depleting endogenous GSH (with various concentrations of BSO for 24 h) or supplementing with exogenous GSH for 1 h before nSMase activity was measured. (B) HAE cells were transiently transfected with siRNA to nSMase2. Twenty-four hours post-transfection, cells were exposed to 250 μM BSO for 24 h followed by treatment with 250 μM H2O2 for 1 h. Forty-eight hours post-transfection, another set of cells were treated with 10 mM GSH for 1 h followed by treatment with 250 μM H2O2 for 1 h. After H2O2 treatment, nSMase activity was determined.
nSMase2 subcellular localization is dictated by oxidative stress
Experiments with HAE cells overexpressing FLAG-tagged nSMase2 showed that exposure to 250 μM H2O2 resulted in the preferential translocation of nSMase2 to the plasma membrane (Fig. 4A), indicating co-localization with a lipid raft marker (cholera toxin β-subunit). On the other hand, exposure to 10 mM GSH resulted in the translocation and concentration of nSMase2 in the perinuclear area (Fig. 4B).
Fig. 4.

nSMase2 trafficking is modulated by H2O2 and by GSH. HAE cells on coverslips were transfected with pFLAG-nSMase2. (A) Twenty-four hours post-transfection, the cells were treated with 250 μM H2O2 for 1 h prior to performing anti-FLAG and anti-GM1 immunostaining. The merged image shows points where the raft-localized lipid GM1 and nSMase2 co-localize. (B) Twenty-four hours post-transfection, the cells were exposed to 10 mM GSH for 2 h. After fixation, cells were incubated with anti-FLAG Ab, followed by staining with Alexa Fluor 594-conjugated secondary antibody and nuclear staining with DAPI. Co-localization analysis was performed by confocal microscopy. (C) nSMase2 subcellular localization is modulated by oxidative stress. Under exposure to H2O2, nSMase2 traffics to the plasma membrane whereas upon exposure to GSH it translocates to the perinuclear area.
Discussion
Although there is little doubt that the ceramide-generating family of enzymes contributes to physiological cell signaling and apoptosis, much of the mechanistic and molecular understanding of these pathways is still missing.
In this study, we aimed to determine the role of nSMase2 in lung epithelial cell apoptosis. We first demonstrated that nSMase2 is present and functional in lung epithelial cells. Using loss-of-function analysis, nSMase2 activity was demonstrated to be required for the cells to undergo oxidative stress-mediated ceramide generation and apoptosis. Both basal and inducible activation of nSMase were impaired when nSMase2 was silenced, suggesting that the H2O2-induced nSMase activity is modulated by nSMase2. Moreover, it appears that H2O2-induced apoptosis in HAE cells is totally dependent on nSMase2, since H2O2 enhanced early apoptosis 2.5-fold whereas silencing of nSMase2 blocked this induction.
This study also shows that GSH is inhibiting nSMase2 activity specifically. For example, in the absence of GSH, using BSO, we see an increase in nSMase activity while by adding GSH to the cells we find a decrease in the enzyme activity. After knocking down nSMase2, we cannot find any change in the enzyme activity after depleting or adding GSH. These results support our hypothesis that nSMase2 is the specific nSMase that is modulated by GSH. It is possible that under resting and stable conditions the susceptible nSMase exists as an inactive enzyme due to the presence of ample GSH. Depletion of GSH from cells may then relieve the inhibition by GSH, resulting in activation of the nSMase accompanied by ceramide generation and apoptosis.
We further demonstrated that nSMase2 is trafficking to different cell compartments under oxidative stress or antioxidant treatments. With 250 μM H2O2 treatment we found movement of the enzyme to the plasma membrane, while treatment with 10 mM GSH resulted in its movement to the nucleus. Hofman et al. reported that nSMase2 is localized to the Golgi in rat PC-12 cells [21] while Marchesini et al. found that nSMase2 in MCF7 cells becomes progressively enriched at the plasma membrane as the cells become increasingly confluent [37]. Moreover, Karakashian et al. showed that in Hep G2 cells, nSMase2 localizes to the plasma membrane [36]. Our results suggest that the localization of nSMase2 controls its biological activity and that this trafficking of nSMase2 is modulated by oxidative stress. Trafficking of nSMase2 under oxidative stress may be a mechanism to either activate or inhibit nSMase2-mediated apoptosis. For example, plasma membrane recruitment of nSMase2 may be required for stimulating nSMase2 to generate ceramide, whereas GSH may act via a sequestering mechanism, causing nSMase2 translocation to the nuclear area where it cannot generate ceramide and participate in apoptotic signaling (Fig. 4C).
Taken together, these studies directly examined the role of the novel nSMase2 in controlling apoptosis in lung epithelial cells.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL-71871 and HL-66189 to T.G.) and Training Grant T32 HL-07013. Thanks to Dr. Elaine Khan and Will Fry for critical reading and comments.
Footnotes
Abbreviations: BSA, bovine serum albumin; BSO, L-buthionine-SRsulfoximine; DAPI, 4′,6-diamidino-2-phenylindole; GSH, glutathione; H2O2, hydrogen peroxide; HAE, human airway epithelial; nSMase, neutral sphingomyelinase; ROS, reactive oxygen species; siRNA, small interfering RNA.
References
- 1.Goldkorn T, Balaban N, Shannon M, Chea V, Matsukuma K, Gilchrist D, Wang H, Chan C. H2O2 acts on cellular membranes to generate ceramide signaling and initiate apoptosis in tracheobronchial epithelial cells. J Cell Sci. 1998;111:3209–3220. doi: 10.1242/jcs.111.21.3209. [DOI] [PubMed] [Google Scholar]
- 2.Chan C, Goldkorn T. Ceramide path in human lung cell death. Am J Respir Cell Mol Biol. 2000;22:460–468. doi: 10.1165/ajrcmb.22.4.3376. [DOI] [PubMed] [Google Scholar]
- 3.Lavrentiadou SN, Chan C, Kawcak TN, Ravid T, Tsaba A, van der Vliet A, Rasooly R, Goldkorn T. Ceramide-mediated apoptosis in lung epithelial cells is regulated by glutathione. Am J Respir Cell Mol Biol. 2001;25:676–684. doi: 10.1165/ajrcmb.25.6.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ravid T, Tsaba A, Gee P, Rasooly R, Medina EA, Goldkorn T. Ceramide accumulation precedes caspase-3 activation during apoptosis of A549 human lung adenocarcinoma cells. Am J Physiol Lung Cell Mol Physiol. 2003;284:L1082–L1092. doi: 10.1152/ajplung.00172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravid T, Goldkorn T, Medina EA. Ceramide signaling under oxidative stress. Kluwer; 2003. [Google Scholar]
- 6.Goldkorn T, Ravid T, Khan EM. Life and death decisions: ceramide generation and EGF receptor trafficking are modulated by oxidative stress. Antioxid Redox Signal. 2005;7:119–128. doi: 10.1089/ars.2005.7.119. [DOI] [PubMed] [Google Scholar]
- 7.Halliwell B, Cross CE. Oxygen-derived species: their relation to human disease and environmental stress. Environ Health Perspect. 1994;102(Suppl 10):5–12. doi: 10.1289/ehp.94102s105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 9.Whyte M, Evan G. Apoptosis. The last cut is the deepest. Nature. 1995;376:17–18. doi: 10.1038/376017a0. [DOI] [PubMed] [Google Scholar]
- 10.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 11.Wu X, Fan Z, Masui H, Rosen N, Mendelsohn J. Apoptosis induced by an anti-epidermal growth factor receptor monoclonal antibody in a human colorectal carcinoma cell line and its delay by insulin. J Clin Invest. 1995;95:1897–1905. doi: 10.1172/JCI117871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chalfant CE, Rathman K, Pinkerman RL, Wood RE, Obeid LM, Ogretmen B, Hannun YA. De novo ceramide regulates the alternative splicing of caspase 9 and Bcl-x in A549 lung adenocarcinoma cells. Dependence on protein phosphatase-1. J Biol Chem. 2002;277:12587–12595. doi: 10.1074/jbc.M112010200. [DOI] [PubMed] [Google Scholar]
- 13.Heinrich M, Wickel M, Winoto-Morbach S, Schneider-Brachert W, Weber T, Brunner J, Saftig P, Peters C, Kronke M, Schutze S. Ceramide as an activator lipid of cathepsin D. Adv Exp Med Biol. 2000;477:305–315. doi: 10.1007/0-306-46826-3_33. [DOI] [PubMed] [Google Scholar]
- 14.Gulbins E, Grassme H. Ceramide and cell death receptor clustering. Biochim Biophys Acta. 2002;1585:139–145. doi: 10.1016/s1388-1981(02)00334-7. [DOI] [PubMed] [Google Scholar]
- 15.Siskind LJ, Kolesnick RN, Colombini M. Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J Biol Chem. 2002;277:26796–26803. doi: 10.1074/jbc.M200754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merrill AH, Jr, Jones DD. An update of the enzymology and regulation of sphingomyelin metabolism. Biochim Biophys Acta. 1990;1044:1–12. doi: 10.1016/0005-2760(90)90211-f. [DOI] [PubMed] [Google Scholar]
- 17.Schutze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Kronke M. TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced “acidic” sphingomyelin breakdown. Cell. 1992;71:765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- 18.Lawler JF, Jr, Yin M, Diehl AM, Roberts E, Chatterjee S. Tumor necrosis factor-alpha stimulates the maturation of sterol regulatory element binding protein-1 in human hepatocytes through the action of neutral sphingomyelinase. J Biol Chem. 1998;273:5053–5059. doi: 10.1074/jbc.273.9.5053. [DOI] [PubMed] [Google Scholar]
- 19.Jayadev S, Liu B, Bielawska AE, Lee JY, Nazaire F, Pushkareva M, Obeid LM, Hannun YA. Role for ceramide in cell cycle arrest. J Biol Chem. 1995;270:2047–2052. doi: 10.1074/jbc.270.5.2047. [DOI] [PubMed] [Google Scholar]
- 20.Okazaki T, Bielawska A, Domae N, Bell RM, Hannun YA. Characteristics and partial purification of a novel cytosolic, magnesium-independent, neutral sphingomyelinase activated in the early signal transduction of 1 alpha,25-dihydroxyvitamin D3-induced HL-60 cell differentiation [published erratum appears in J. Biol. Chem. 1994 Jun 10;269(23):16518] J Biol Chem. 1994;269:4070–4077. [PubMed] [Google Scholar]
- 21.Hofmann K, Tomiuk S, Wolff G, Stoffel W. Cloning and characterization of the mammalian brain-specific, Mg2+-dependent neutral sphingomyelinase. Proc Natl Acad Sci USA. 2000;97:5895–5900. doi: 10.1073/pnas.97.11.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchesini N, Luberto C, Hannun YA. Biochemical properties of mammalian neutral sphingomyelinase 2 and its role in sphingolipid metabolism. J Biol Chem. 2003;278:13775–13783. doi: 10.1074/jbc.M212262200. [DOI] [PubMed] [Google Scholar]
- 23.Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994;77:817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 24.Macho A, Hirsch T, Marzo I, Marchetti P, Dallaporta B, Susin SA, Zamzami N, Kroemer G. Glutathione depletion is an early and calcium elevation is a late event of thymocyte apoptosis. J Immunol. 1997;158:4612–4619. [PubMed] [Google Scholar]
- 25.Dhanbhoora CM, Babson JR. Thiol depletion induces lethal cell injury in cultured cardiomyocytes. Arch Biochem Biophys. 1992;293:130–139. doi: 10.1016/0003-9861(92)90375-7. [DOI] [PubMed] [Google Scholar]
- 26.Beaver JP, Waring P. A decrease in intracellular glutathione concentration precedes the onset of apoptosis in murine thymocytes. Eur J Cell Biol. 1995;68:47–54. [PubMed] [Google Scholar]
- 27.Gao L, Kim KJ, Yankaskas JR, Forman HJ. Abnormal glutathione transport in cystic fibrosis airway epithelia. Am J Physiol. 1999;277:L113–L118. doi: 10.1152/ajplung.1999.277.1.L113. [DOI] [PubMed] [Google Scholar]
- 28.Ghibelli L, Coppola S, Fanelli C, Rotilio G, Civitareale P, Scovassi AI, Ciriolo MR. Glutathione depletion causes cytochrome c release even in the absence of cell commitment to apoptosis. FASEB J. 1999;13:2031–2036. doi: 10.1096/fasebj.13.14.2031. [DOI] [PubMed] [Google Scholar]
- 29.Baker A, Santos BD, Powis G. Redox control of caspase-3 activity by thioredoxin and other reduced proteins. Biochem Biophys Res Commun. 2000;268:78–81. doi: 10.1006/bbrc.1999.1908. [DOI] [PubMed] [Google Scholar]
- 30.Vahrmeijer AL, van Dierendonck JH, Schutrups J, van de Velde CJ, Mulder GJ. Effect of glutathione depletion on inhibition of cell cycle progression and induction of apoptosis by melphalan (L-phenylalanine mustard) in human colorectal cancer cells. Biochem Pharmacol. 1999;58:655–664. doi: 10.1016/s0006-2952(99)00130-6. [DOI] [PubMed] [Google Scholar]
- 31.Liu M, Pelling JC, Ju J, Chu E, Brash DE. Antioxidant action via p53-mediated apoptosis. Cancer Res. 1998;58:1723–1729. [PubMed] [Google Scholar]
- 32.Barak A, Morse LS, Goldkorn T. Ceramide: a potential mediator of apoptosis in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2001;42:247–254. [PubMed] [Google Scholar]
- 33.Robinson CB, Wu R. Culture of airway epithelial cells in serum-free medium. J Tissue Cult Methods. 1991;13:95–102. [Google Scholar]
- 34.Chen Y, Zhao YH, Wu R. Differential regulation of airway mucin gene expression and mucin secretion by extracellular nucleotide triphosphates. Am J Respir Cell Mol Biol. 2001;25:409–417. doi: 10.1165/ajrcmb.25.4.4413. [DOI] [PubMed] [Google Scholar]
- 35.Khan EM, Heidinger JM, Levy M, Lisanti MP, Ravid T, Goldkorn T. EGF receptor exposed to oxidative stress undergoes Srcand caveolin-1-dependent perinuclear trafficking. J Biol Chem. 2006 doi: 10.1074/jbc.M509332200. [DOI] [PubMed] [Google Scholar]
- 36.Karakashian AA, Giltiay NV, Smith GM, Nikolova-Karakashian MN. Expression of neutral sphingomyelinase-2 (NSMase-2) in primary rat hepatocytes modulates IL-beta-induced JNK activation. FASEB J. 2004;18:968–970. doi: 10.1096/fj.03-0875fje. [DOI] [PubMed] [Google Scholar]
- 37.Marchesini N, Osta W, Bielawski J, Luberto C, Obeid LM, Hannun YA. Role for mammalian neutral sphingomyelinase 2 in confluence-induced growth arrest of MCF7 cells. J Biol Chem. 2004;279:25101–25111. doi: 10.1074/jbc.M313662200. [DOI] [PubMed] [Google Scholar]