Abstract
We present a series of short, multidomain peptides as biocompatible solubilizing agents of single-walled carbon nanotubes (SWCNTs). These peptides are organized into an ABA block motif, where the A block is composed of charged amino acids, such as glutamic acid, and the B block is composed of alternating hydrophilic and hydrophobic residues. The hydrophobic amino acid residues interact with SWCNT sidewalls, while the hydrophilic residues interact primarily with water in an aqueous solution. When many peptides assemble along the length of the nanotube, it becomes effectively encapsulated within a peptide nanofiber. This noncovalent interaction between the peptide and nanotube solubilizes SWCNTs while keeping the electronic structure of the nanotube intact, thereby preserving the optical and electrical properties that make SWCNTs promising for use in biological applications. To assess the toxicity of these peptide coatings, they were added to cultures of NIH 3T3 mouse fibroblasts and the effect on cell viability was measured. Toxicity was found to be far lower than for ionic surfactants typically used for SWCNT suspension, and similar to Pluronics. The near-IR fluorescence intensity of SWCNTs in peptide suspensions was comparable to that in Pluronics. Five surfactants were tested for their effect on the proliferation of NIH 3T3 cells with and without SWCNTs. Although some differences were observed among surfactants, in no case did the presence of SWCNTs make a statistically significant difference. Based on their ability to solubilize SWCNTs, the fluorescence of the suspended tubes, their minimal impact on cell viability, and their potential for easy chemical modification, multidomain peptides have been found to have excellent potential as a biocompatible surfactant for suspension of SWCNTs.
Keywords: peptide, multidomain, surfactant, carbon nanotube, fluorescence, toxicity
Introduction
Single-walled carbon nanotubes (SWCNTs) have been recognized for their unique optical and electronic properties 1. As fluorophores, they possess good photostability, sharp spectra, adequate brightness, and an absence of fluorescence intermittency 2. They also emit in the near-infrared (NIR), a region in which most biological tissues are transparent, giving them potential for use as sensors inside living cells 3. SWCNTs irradiated in vitro or in vivo at optical or radiofrequency wavelengths can also selectively heat their immediate cellular environment, providing a method for directed cell death 4. These properties make carbon nanotubes promising for use in biological applications ranging from biosensors to pharmaceuticals 5-6. However, in such applications, single-walled carbon nanotubes must be functionalized to allow solubility in aqueous media 7. This functionalization may be covalent or noncovalent. Although covalent derivatization of SWCNTs has produced stable aqueous SWCNT solutions 8, chemical modification of the SWCNT sidewall locally perturbs the sp2 structure of the tube, compromising the electronic and optical properties that make SWCNTs unique. Therefore, for many applications noncovalent functionalization of SWCNTs is preferable. This generally involves the use of surfactant coatings or polymeric wrappings 9. Solubilization of single-walled carbon nanotubes in surfactants that are compatible with biological environments has also been a challenging problem. Aqueous dispersions of SWCNTs have been prepared with many surfactants, including various detergents and polymers 9. However, traditional anionic surfactants, such as sodium dodecylsulfate (SDS) and sodium dodecylbenzenesulfonate (SDBS), are toxic to mammalian cells due to surfactant-assisted dissolution of the lipid bilayer of the cell membrane 10-11. Surfactants that are traditionally considered to be biocompatible, such as the Pluronic series 12-13, do not dissolve the cell membrane and are therefore relatively nontoxic. Pluronic surfactants consist of a non-charged triblock polymer that noncovalently wraps SWCNTs 9. However, the Pluronics do not easily permit selective chemical modifications that would allow the surfactant to perform other duties in vivo, such as cell-specific targeting. Therefore, in order to effectively and safely introduce disaggregated pristine SWCNTs into biological systems, new biocompatible surfactants are needed.
Because of their ease of synthesis, biocompatibility, degradability and straightforward modification and functionalization chemistry, peptides have shown promise as SWCNT surfactants 14-15. Peptides are generally less cytotoxic than detergents such as SDBS and are potentially able to be degraded by cellular enzymes. Specifically, we have designed a class of multidomain peptides that are engineered to be amphiphilic and to suspend SWCNTs. These multidomain peptides have three distinct regions: a hydrophilic face, a hydrophobic face, and charged residues on the peptide termini 16. The hydrophilic face is generally composed of amino acids such as glutamine, and the hydrophobic face is derived from leucine residues. In addition, charged domains at the termini of the peptide allow greater solubility and mitigate aggregation. When self-assembled, such multidomain peptides form β-sheets in which there is a uniform hydrophilic face on one side of the peptide and a uniform hydrophobic face on the other. The hydrophobic face is thought to associate with the single-walled nanotube by van der Waals interactions. Multiple peptides coat a single nanotube in a self-assembled structure in which the peptide backbone lies perpendicular to the axis of the nanotube and hydrogen bonds form between adjacent peptide strands (Figure 1). This extensive β-sheet network constitutes a peptide nanofiber with the SWCNT in the center.
Figure 1.
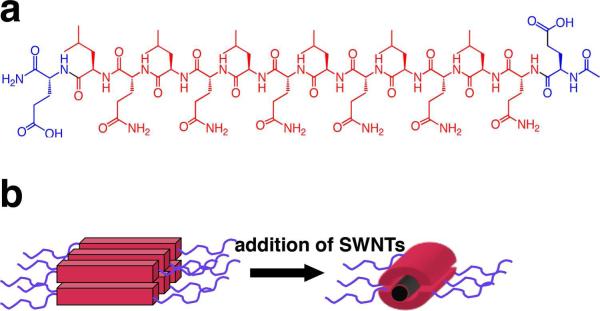
Structure of multidomain peptide (a) E(QL)6E and(b) associated nanofibers.
Besides the need for an appropriate surfactant, another obstacle hindering the use of single-walled carbon nanotubes in biological systems is potential cytotoxicity of the nanotubes. SWCNT toxicity may depend on many factors, including the method of nanotube production, impurity content, nanotube length, aggregation state, and the nanotube surface coating. In this study we examine the cytotoxicity of carbon nanotubes in vitro and the contribution of surfactant to the apparent cytotoxicity.
Our previous work has shown that multidomain peptides belonging to the series Km(QL)nKm show a promising ability to suspend nanotubes17. Peptides in this series suspend SWCNTs in aqueous solutions as individuals with brighter fluorescence emission per nanotube than Pluronic surfactants. Here we examine the growth and viability of NIH 3T3 mouse fibroblasts when cultured in the presence of two multidomain peptides, E(QL)6E and E(QL)6EGRGDS. The latter peptide incorporates the known cell-adhesion sequence RGD in order to allow the possibility of better adhesion to cell surfaces. These two peptide surfactants were tested for cytotoxicity in vitro, both alone and with carbon nanotubes in suspension. Cytotoxicities of these two surfactants were compared to SDBS, Pluronic F68, and Pluronic F127, with and without nanotubes. We chose two different surfactants in the Pluronic series because they have different molecular weights and differing abilities to suspend single-walled nanotubes.
Experimental Section
Cell Culture
NIH 3T3 mouse fibroblasts were obtained from the American Type Culture Collection and were cultured in Corning 75cm2 cell culture flasks. Dulbecco's Modified Eagle Medium (Gibco) was supplemented with 10% FBS and 1% penicillin-streptomycin v/v. Cells were cultured in an incubator at 37°C at 5% CO2 and the medium was changed approximately every 2 days. Propagation of this cell line required cells to be passaged every 6-7 days. For the toxicity experiments, 5 × 104 cells were placed in wells in a 24-well plate and allowed to incubate overnight. A 15 mM stock solution of peptide or peptide-SWCNT suspension was added to reach the desired concentrations of 150, 300, and 1000 μM. The cells were then incubated for an additional 48 h. At 48 h, cells were detached from the wells with trypsin and stained with Trypan Blue. Viable cells exclude Trypan blue from the cell, while the dye leaks into dead or injured cells. Cells were then counted using a hemocytometer (Hausser Scientific) and Nikon Eclipse E400 optical microscope.
Peptide Synthesis and Purification
Peptides were synthesized on a 0.15 mmol scale with an Advanced Chemtech Apex 396 peptide synthesizer using standard Fmoc chemistry. Cleavage and side chain deprotection were performed using a 27:1:1:1 mixture of TFA, anisole, water, and triisopropylsilane by volume. Peptides were purified by first dissolving the peptide in deionized water at pH 7. Once the peptide was completely dissolved, the pH was adjusted to 1-2 and the suspension was centrifuged at 3750 rpm for 10 min, yielding the desired peptide as a pellet. The supernatant was discarded and the pellet was then redissolved in deionized water adjusted to pH 10 with NaOH. This solution was again centrifuged for 10 min and the supernatant containing the peptide was decanted and lyophilized. The peptides were subsequently characterized by MALDI-TOF mass spectrometry.
Peptide-Single-Walled-Carbon-Nanotube Suspension Preparation
Peptides were dissolved in ultrapure water at pH 5 at a concentration of 10 mg/mL. Single-walled carbon nanotubes were obtained from the Rice University HiPco reactor and were used without further purification. SWCNTs samples of ~1 mg were weighed out and added to a 1.5 mL Eppendorf tube. To this vial was added 1 mL peptide solution. Vials were sonicated for 11 s using a Microson XL 2000 tip sonicator at a power level of 5 W. The tip of the sonicator probe was placed at approximately one-half the depth of the solution. Samples were then centrifuged for 5 min in a Sorvall Biofuge pico centrifuge at 13,000 rpm. The supernatant was decanted and centrifuged for an additional 10 min. Decanting and centrifugation were repeated once more for 10 min. Pellets were discarded. For spectral measurements and cell culture studies, the pH of the resulting peptide-nanotube solutions was adjusted to 7. This yielded gray or black suspensions of SWCNTs that were stable for weeks or months without visible aggregation. The final concentration of SWCNTs is difficult to measure, since UV absorbance values cannot tell us the number of individual SWCNTs in solution, since the exact lengths of SWCNTs in these samples are unknown. The amount of SWCNT material in each solution is, in all cases, less than 1 mg/mL.
Atomic Force Microscopy (AFM)
SWCNT-peptide solutions were diluted 20-fold with ultrapure water and were dropped onto freshly cleaved mica while spinning on a Headway Research, Inc. photo-resist spinner. Samples were rinsed with deionized water for 1-2 s and then spun for an additional 10 min. AFM images were collected in air at ambient temperature on a Digital Instruments Nanoscope IIIa atomic force microscope in tapping mode. Height profiles were found using Nanoscope software.
Vis-near-IR Absorbance and near-IR Fluorescence
Bulk absorbance and fluorescence measurements were collected on an NS1 NanoSpectralyzer (Applied NanoFluorescence, LLC). Approximately 1 mL of peptide-SWCNT suspension was transferred into a plastic cuvette for measurements. Fluorescence spectra were collected at excitation wavelengths of 659 and 784 nm and were normalized against NIR absorbance at 1034 nm.
Near-IR imaging of 3T3 cells exposed to SWCNT suspensions
Near-IR fluorescence imaging of 3T3 cells was performed using a custom-built apparatus based on an inverted Nikon TE-2000U microscope with a Nikon PlanApo VC 60× /1.4 NA oil-immersion objective 18. A combination of a dichroic beamsplitter and a dielectric 946 nm long-pass filter was used to select emission wavelengths greater than 950 nm. A liquid nitrogen cooled InGaAs camera (Roper Scientific OMA-V 2D) sensitive between 900 and 1600 nm was installed on one microscope output port. Another output port was coupled via fiberoptic cable to the input slit of a J-Y C140 spectrograph equipped with a 512-element InGaAs array (OMA-V, Roper Scientific). In this way near-IR emission spectra could be acquired from a spatial region of ~1.5 × 1.5 m at the sample. We excited samples with circularly polarized beams from diode lasers emitting at 658 and 785 nm. The circular polarization ellipticity was ≥ 0.95.
1 and 2-way ANOVA
Analysis of variance (ANOVA) was performed using Prism software. 2-way ANOVA was performed to determine whether the interaction of SWCNTs with the surfactant affected the solution toxicity, while 1-way ANOVA and Tukey tests were used to compare each condition with every other.
Results and Discussion
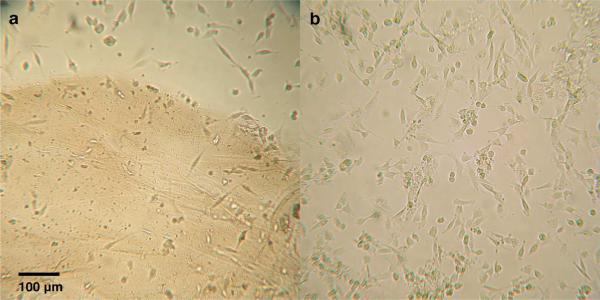
When multidomain peptides from the series Kn(QL)mKn were added to cell culture medium to assess their compatibility with biological systems, it was found that peptides bearing the positively charged residue lysine in the charged domain had a tendency to aggregate in the culture medium. Aggregation occured regardless of whether single-walled carbon nanotubes were present. It is possible that negatively charged phosphate ions in the cell culture medium interacted with positively charged lysine residues, effectively screening the charge on these terminal residues. As the charge on the terminal residues was reduced, the individual peptides no longer repelled one another and self-assembled into aggregates. When a suspension of SWCNTs in aqueous K2(QL)5K2 was added to a culture of NIH 3T3 cells, the aggregates formed were large enough to be easily visible with a standard light microscope (Figure 2). To avoid this problem, we prepared analogous peptides in which the positively charged lysine was replaced by the negatively charged glutamic acid. When such peptides displaying glutamic acid as the terminal residues were added to cell culture medium, no visible aggregates were detectable via light microscopy. For cytotoxicity studies, we therefore chose to use two such peptides: E(QL)6E and E(QL)6EGRGDS. The stability of these peptide-SWCNT suspensions both alone and in cell culture medium can be found in the Supporting Information.
Figure 2.
Light microscope images of NIH 3T3 cells incubated with SWCNT suspensions in (a) K2(QL)5K2 and (b) E(QL)6E. The brown area in the lower portion of (a) is an aggregation of peptide-coated SWCNTs.
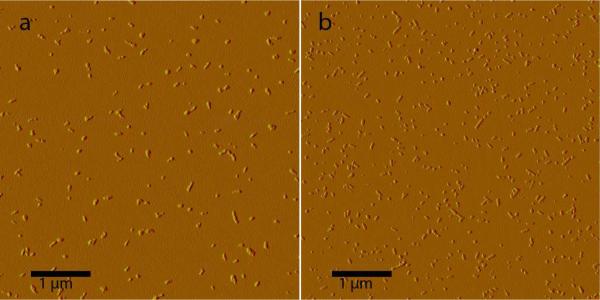
To ensure that the SWCNT suspensions being added to cell cultures contained individual SWCNTs and not aggregates, AFM was performed (Figure 3). This also allowed visualization of the peptide nanofibers to confirm that the predicted self-assembly of the peptides was taking place. The absence of large bundles of either peptide or SWCNTs in the AFM images indicated that the SWCNTs were well-dispersed by the peptide. Furthermore, height profiles measured from AFM images were consistent with the dimensions of individual SWCNTs coated by peptides (see Supporting Information). It was noticed that peptide-suspended SWCNTs visible by AFM appear to be shorter than would be predicted by the length distribution of HiPCO-produced SWCNTs. AFM of SWCNTs suspended in SDBS (but prepared under identical suspension conditions to those of peptide-SWCNT samples) show some longer SWCNTs, though these nanotubes appear somewhat aggregated (see Supporting Information). This suggests that these multidomain peptides are selectively suspending shorter SWCNTs. We also measured near-IR fluorescence spectra of each freshly prepared SWCNT suspension, using laser excitation at 659 and 784 nm (Figure 4). These measurements provide additional information about the aggregation state of the suspended nanotubes, since SWCNT bundling quenches fluorescence. Fluorescence spectra normalized to sample absorbance values reveal that of the biocompatible surfactants, E(QL)6E allows better fluorescence efficiency than either of the Pluronic surfactants, while E(QL)6EGRGDS is intermediate to the two Pluronics.
Figure 3.
AFM images of SWCNTs suspended in (a) E(QL)6E and(b) E(QL)6EGRGDS.
Figure 4.
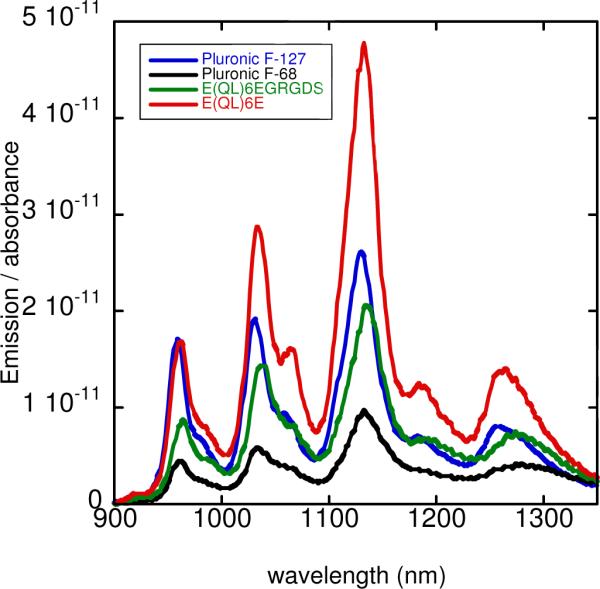
Fluorescence emission spectra of SWCNT suspensions in different surfactants, with 659 nm excitation. Curves have been normalized according to the sample absorbance to show relative fluorescence efficiencies.
In our cytotoxicity studies, NIH 3T3 fibroblasts were seeded in each well of a 24 cell well plate and allowed to incubate overnight. The following day, the peptide or peptide-SWCNT solutions were added directly to the cell wells. Cells were detached from the cell culture plate with trypsin at 48 h, stained with Trypan blue, and then counted using a hemocytometer. For every concentration of surfactant or surfactant-SWCNT suspension tested, there was a negative control, which consisted of NIH 3T3 cells with no solutions added. Cell viabilities were normalized against these negative controls for each condition. We analyzed the data by 2-way ANOVA, and the more subtle differences between specific surfactants were analyzed by 1-way ANOVA (Figure 5). The 1-way ANOVA results indicated that at the lowest concentration (150 μM) SDBS did not show statistically significant toxicity. At 300 μM and 1 mM concentrations, however, cell viability for SDBS-incubated cultures dropped to zero, a toxicity that was statistically significant compared with every other surfactant. This confirmed that SDBS is highly cytotoxic and unsuitable for use in mammalian cell culture. Although the multidomain peptides E(QL)6E and E(QL)6EGRGDS did appear to show some dose-dependent cytotoxicity, this cytotoxicity was not significantly different from the Pluronic series at 150 and 300 μM concentrations. That is, T-tests comparing cell viability between different surfactants at 150 and 300 μM yielded P values above 0.05 for every possible comparison of two surfactants, except for the comparison of SDBS-incubated cultures with all other cultures at 300 μM (as SDBS shows substantial toxicity at this concentration). As Pluronic and peptide toxicities are not significantly different from one another at or below 300 μM, the peptides explored here provide viable options for delivering nanotubes in vitro at low concentrations. At 1 mM surfactant concentration, however, the toxicity of the multidomain peptides becomes more apparent, with both peptides showing toxicities significantly higher than the Pluronics (P < 0.05).
Figure 5.
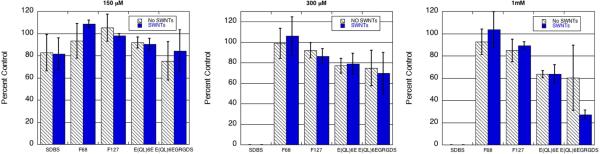
Cell viability of NIH 3T3 cells incubated for 48 hours with 150, 300 μM, and 1 mM surfactants. Half of the groups were incubated with single-walled nanotubes (shown in blue) and half of the groups were incubated in the absence of nanotubes (shown in black and white).
Analysis by 2-way ANOVA makes it possible to distinguish which set of variable factors (surfactants or the presence of SWCNTs) is responsible for deviations from the control, or whether the observed toxicity is caused by a combination of these two sets of factors. This analysis revealed that single-walled carbon nanotubes showed no significant cytotoxicity, regardless of the identity or concentration of the surfactant. We found that the 2-way ANOVA yielded a P-value above 0.05 for all comparisons collectively of SWCNT and non-SWCNT incubated cells (P = 0.5342, 0.9501, and 0.4345 for 150 μM, 300 μM, and 1 mM, respectively). The 2-way ANOVA analyses also suggest that the combination of peptide with SWCNT is not responsible for any toxicity observed at surfactant concentrations of 150 or 300 μM (P > 0.05). A P-value of 0.0438 was obtained for the interaction of peptide surfactants with SWCNTs at the 1 mM concentration. However, SWCNTs themselves showed no toxicity at this concentration, casting doubt on the practical significance of this result. Thus, we conclude that there is no evidence for synergistic toxicity effects involving single-walled carbon nanotubes and peptide surfactants, at least at 150 μM and 300 μM concentrations.
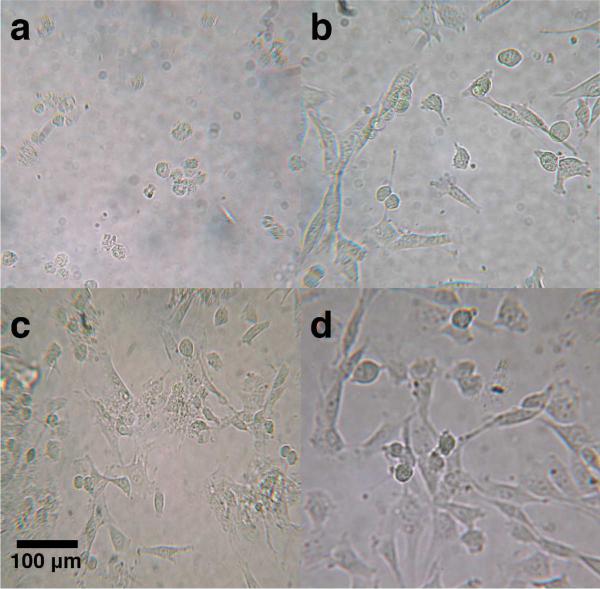
Examination of cell morphology by light microscopy revealed that cells incubated in solutions of SDBS, with or without nanotubes, showed a rounded morphology indicative of a lack of cellular anchoring to the cell well plate (Figure 6). A rounded morphology precedes cell death, and indeed, cultures incubated at SDBS concentrations of 300 μM or higher experienced total cell death. By contrast, cell morphology in the presence of Pluronic F68, F127, E(QL)6E, and E(QL)6EGRGDS appeared normal. Figure 6 shows healthy cells in each of these conditions. These cells are spread out and anchored to the cell culture wells, morphology that is typical for fibroblasts.
Figure 6.
Cell morphology of NIH 3T3 cells after 24 hours of incubation with (a) SDBS, (b) Pluronic F68, (c) E(QL)6E, and (d) E(QL)6EGRGDS.
After 48 hours, cells were imaged with a near-IR fluorescence microscope. Imaging was performed through the cell culture plates so as not to disturb cell morphology. This allowed us to assess the interaction of SWCNTs with the cells as well as the amount of soluble peptide-wrapped SWCNTs remaining in solution. It was found that at 48 h, significant amounts of nanotubes still remained in solution. Interactions between SWCNTs and 3T3 cells were also observed (Figure 7).
Figure 7.
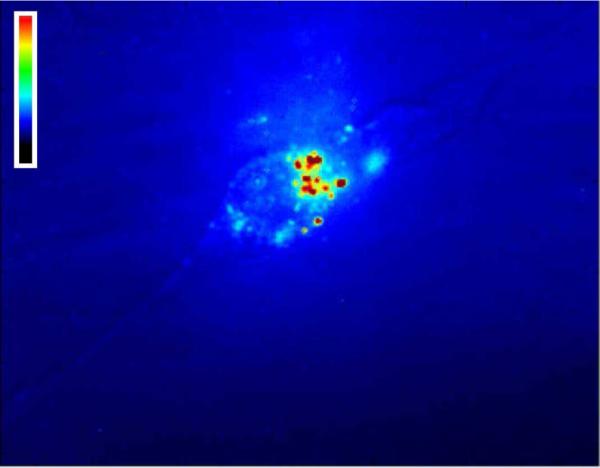
NIH 3T3 cell in the presence of single-walled carbon nanotubes suspended in E(QL)6EGRGDS. The image shows near-IR fluorescence from the nanotubes, false-colored according to intensity. Bright spots indicate carbon nanotube clusters on the cell surface.
Conclusions
In order for single-walled carbon nanotubes to be used in many biological applications, suitable biocompatible surfactant coatings that noncovalently functionalize the SWCNTs are needed. Noncovalent surfactants wrap and solubilize carbon nanotubes while preserving their unusual electronic structure and optical properties. Standard ionic surfactants are highly cytotoxic, whereas traditional “biocompatible” surfactants such as Pluronic do not readily allow chemical modification. The alternatives presented here are customizable multidomain peptides that noncovalently modify SWCNTs by wrapping the nanotubes inside β-sheet nanofibers. The cytotoxicity of carbon nanotubes remains controversial. Some reports show a lack of toxicity 19-20, while others find the opposite effect 21-22. In comparative studies, we find statistically significant differences between the viabilities of cultured cells incubated with SWCNTs suspended by different surfactants. However, analysis indicates that the cytotoxicity arises from the surfactants rather from the SWCNTs or from synergistic effects between nanotubes and surfactants. We therefore suggest that it is not necessarily the SWCNTs that may be cytotoxic, but rather the agents in which they are suspended and their aggregation state. Our 2-way ANOVA analysis shows that, within the concentration range used here, disaggregated SWCNTs do not influence the viability of the 3T3 cells in culture. Thus, single-walled carbon nanotubes seem to be acceptably nontoxic for in vitro biological applications as long as the surfactants used to suspend them are effective and nontoxic. We find that the new multidomain peptides are approximately as nontoxic as the Pluronic surfactants, and that they allow comparable SWCNT fluorescence efficiency while offering options for simple chemical customization. Our qualitative findings are summarized in Table 1. Because peptides allow for a wide range of chemical functionalities, one can design surfactants that are both nontoxic and provide biological specificity, such as enhanced integrin binding through the RGD sequence on the C-terminus of E(QL)6EGRGDS. We have shown that such cell-adhesion sequences may be incorporated without compromising the ability of the peptide to self-assemble. This suggests that other cell-adhesion sequences and targeting moieties can also be incorporated into the multidomain peptides, enabling important new biological applications of carbon nanotubes. Furthermore, peptides may undergo hydrolysis in vivo through the action of various peptidases naturally found in the body, providing a starting point for metabolism of peptide-based surfactants. This biodegradation is essential if a potentially toxic buildup of surfactant is to be avoided. These qualities extend the general range of applications of peptide surfactants far beyond that of traditional Pluronic surfactants.
Table 1.
Summary of properties of different single-walled carbon nanotube surfactants. Peptides bearing glutamic acid residues on the termini score well in all categories.
Supplementary Material
Acknowledgements
The authors thank the Rice University NanoCarbon Center for supplying SWCNTs and Dr. Richard Gomer for assistance with Prism software. This work was supported by the National Institutes of Health under NIH Grant No.5T32 GM008362, by the Welch Foundation (grants C-0807 and C-1557), and by Enscyse Biosciences.
Footnotes
Supporting Information Available. Peptide characterization, AFM images, near-IR fluorescence data, and statistical analyses are available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.Bachilo SM, Strano MS, Kittrell C, Hauge RH, Smalley RE, Weisman RB. Science. 2002;298:2361–2366. doi: 10.1126/science.1078727. [DOI] [PubMed] [Google Scholar]
- 2.Hartschuh A, Pedrosa HN, Novotny L, Krauss TD. Science. 2003;301:1354–1356. doi: 10.1126/science.1087118. [DOI] [PubMed] [Google Scholar]; Cognet L, Tsyboulski D, Rocha J-DR, Doyle CD, Tour JM, Weisman RB. Science. 2007;316:1465–1468. doi: 10.1126/science.1141316. [DOI] [PubMed] [Google Scholar]
- 3.Welsher K, Liu Z, Daranciang D, Dai H. Nano Lett. 2008;8:586–590. doi: 10.1021/nl072949q. [DOI] [PubMed] [Google Scholar]
- 4.Yehia HN, Draper RK, Mikoryak C, Walker EK, Bajaj P, Musselman IH, Daigrepont MC, Dieckmann GR, Pantano PJ. Nanobiotech. 2007;5:8–24. doi: 10.1186/1477-3155-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen RJ, Bangsaruntip S, Drouvalakis KA, Shi Kam NW, Shim M, Li Y, Kim W, Utz PJ, Dai H. Proc. Natl. Acad. Sci. USA. 2003;100:4984–4989. doi: 10.1073/pnas.0837064100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zanello LP, Zhao B, Hu H, Haddon RC. Nano Lett. 2006;6:562–567. doi: 10.1021/nl051861e. [DOI] [PubMed] [Google Scholar]
- 7.Lin Y, Taylor S, Li H, Fernando KAS, Qu L, Wang W, Gu L, Zhou B, Sun Y-PJ. Mater. Chem. 2004;14:527–541. [Google Scholar]
- 8.Bahr JL, Tour JM. J. Mater. Chem. 2002;12:1952–1958. [Google Scholar]
- 9.Moore VC, Strano MS, Haroz EH, Hauge RH, Smalley RE. Nano Lett. 2003;3:1379–1382. [Google Scholar]
- 10.Grant RL, Acosta D. Fundam. Appl. Toxicol. 1996;33:71–82. doi: 10.1006/faat.1996.0144. [DOI] [PubMed] [Google Scholar]
- 11.Dong L, Joseph KL, Witkowski CM, Craig MM. Nanotechnology. 2008;19:255702. doi: 10.1088/0957-4484/19/25/255702. [DOI] [PubMed] [Google Scholar]
- 12.Hellung-Larsen P, Assaad F, Pankratova S, Saietz BL, Skovgaard LT. J. Biotechnol. 2000;76:185–195. doi: 10.1016/s0168-1656(99)00188-1. [DOI] [PubMed] [Google Scholar]
- 13.Ma N, Chalmers JJ, Aunins JG, Zhou W, Xie L. Biotech. Prog. 2004;20:1183–1191. doi: 10.1021/bp0342405. [DOI] [PubMed] [Google Scholar]
- 14.Witus LS, Rocha JDR, Yuwono VM, Paramonov SE, Weisman RB, Hartgerink JD. J. Mater. Chem. 2007;17:1909–1915. [Google Scholar]
- 15.Ortiz-Acevedo A, Xie H, Zorbas V, Sampson WM, Dalton AB, Baughman RH, Draper RK, Musselman IH, Dieckmann GR. J. Am. Chem. Soc. 2005;127:9512–9517. doi: 10.1021/ja050507f. [DOI] [PubMed] [Google Scholar]
- 16.Dong H, Paramonov SE, Aulisa L, Bakota EL, Hartgerink JD. J. Am. Chem. Soc. 2007;129:12468–12472. doi: 10.1021/ja072536r. [DOI] [PubMed] [Google Scholar]; Dong H, Paramonov SE, Aulisa L, Bakota EL, Hartgerink JD. J. Am. Chem. Soc. 2007;129:12468–12472. doi: 10.1021/ja072536r. [DOI] [PubMed] [Google Scholar]
- 17.Tsyboulski D, Bakota EL, Witus LS, Rocha JDR, Hartgerink JD, Weisman RB. J. Am. Chem. Soc. 2008;150:17134–17140. doi: 10.1021/ja807224x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsyboulski DA, Bachilo SM, Weisman RB. Nano Lett. 2005;5:975–979. doi: 10.1021/nl050366f. [DOI] [PubMed] [Google Scholar]
- 19.Correa-Duarte MA, Wagner N, Rojas-Chapana J, Morsczeck C, Thie M, Giersig M. Nano Lett. 2004;4:2233–2236. [Google Scholar]
- 20.Cherukuri P, Bachilo SM, Litovsky SH, Weisman RB. J. Am. Chem. Soc. 2004;126:15638–15639. doi: 10.1021/ja0466311. [DOI] [PubMed] [Google Scholar]
- 21.Tian F, Cui D, Schwarz H, Estrada GG, Kobayashi H. Toxicol. in Vitro. 2006;20:1202–1212. doi: 10.1016/j.tiv.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Magrez A, Kasas S, Salicio V, Pasquier N, Seo JW, Celio M, Catsicas S, Schwaller B, Forro L. Nano Lett. 2006;6:1121–1125. doi: 10.1021/nl060162e. [DOI] [PubMed] [Google Scholar]
- 23.Nimmagadda A, Thurston K, Nollert MU, McFetridge PS. J. Biomed. Mat. Res. A. 2005;76:614–625. doi: 10.1002/jbm.a.30577. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.