Abstract
Purpose
The effect of timing of onset of necrotizing enterocolitis (NEC) on outcomes has not been determined for the full-term infant. In this study we aimed to characterize the full-term NEC population and to evaluate onset of NEC.
Methods
We performed a two-center retrospective review of all full-term infants (≥ 37 weeks) with a diagnosis of NEC between 1990 and 2012. Patients were identified by ICD-9 and age. Early onset for NEC was ≤ 7 days and late onset after 7 days of life. Demographics, comorbidities, maternal factors, clinical factors, surgical intervention, complications, and mortality were evaluated. Wilcoxon’s test was performed on continuous variables and Fisher’s exact test on categorical data. A p-value b 0.05 was considered significant. Univariate outcomes with a p-value b 0.1 were selected for multivariable analysis.
Results
Thirty-nine patients (24 boys, 15 girls) with median EGA of 39 weeks were identified. Overall mortality was 18%. Univariate predictors of mortality included congenital heart disease and placement of an umbilical artery (UA) catheter. Multivariate analysis revealed late onset of NEC to be an independent predictor of mortality (OR 90.8, 95% CI 2.6–3121).
Conclusion
Full-term infants who develop NEC after 7 days of life, have congenital heart disease, and/or need UA catheterization have increased mortality.
Keywords: Neonate, Full-term, Term, Necrotizing enterocolitis, Mortality
The pathogenesis of necrotizing enterocolitis (NEC) in infants remains incompletely understood. Most commonly, NEC occurs in the premature infant, with less than 10% occurring in full-term neonates [1]. The common end point is dysfunction of the gut barrier with subsequent bacterial invasion, secondary injury, and progression to intestinal necrosis. In the premature infant, this is thought to be due to immature mucosal defenses, abnormalities in intestinal perfusion, and/or pathologic colonization of bacteria [2-4]. In the full-term infant, multiple clinical factors have been associated with onset of NEC, including perinatal hypoxic events, congenital heart disease, thrombotic conditions (e.g. polycythemia), endocrinopathies, perinatal sepsis, and possibly mode of delivery [1,5-7].
It is not known whether the underlying pathophysiology of NEC in the term infant is distinctly different from that in the premature infant. In addition to the frequent presence of significant co-morbidities, term infants are frequently reported to present with NEC earlier in postnatal life [1,7]. Recent evidence suggests that time of onset of NEC can result in a difference in clinical outcomes among preterm infants [8,9]. There also appears to be a similar bimodal distribution of NEC onset in term infants. To our knowledge evaluation of timing of NEC onset and its relation to outcome has not been conducted in the full-term infant. In this study we aim to evaluate differences between full-term infants with NEC who present early compared those who present late. The findings may help to better understand underlying pathophysiologic abnormalities in order to optimize care.
1. Methods
1.1. Patient Population
We performed a two-center retrospective review of all full-term infants (≥ 37 weeks) with a diagnosis of NEC between 1990 and 2012. Charts were identified by including ICD-9 codes (777.5–777.6) and were reviewed to verify diagnosis of NEC and gestational age. Term infants determined to have Bell’s stage 2 or 3 NEC were included. Term infants with possible Bell’s stage I NEC were excluded from analysis. Infants who had an estimated gestational age less than 37 weeks were also excluded from the study. The subject met inclusion criteria for NEC if he/she had evidence of Bell’s stage II or greater and had diagnosis of NEC documented in the chart with signs and symptoms that included temperature instability, apnea, bradycardia, lethargy, pneumatosis, metabolic acidosis, peritonitis, and/or pneumoperitoneum [10].
Similar to a previous publication, early-onset NEC was defined as development of NEC during the 1st week of life (≤ 7 days) and late-onset NEC as any time after day 7 of life [9]. Prior to data collection, institutional review board approval was obtained at both institutions.
1.2. Description of Variables
Multiple demographic factors including gender, gestational age, birth weight, mode of delivery (e.g. vaginal vs. cesarean section), and APGAR scores at 1 and 5 min were recorded. Additional co-morbid conditions were recorded and included congenital heart disease (cyanotic vs. acyanotic with asymptomatic lesions such as a transient PDA or PFO that are associated with transition from fetal life excluded), perinatal hypoxic event (desaturation event requiring intubation and/or cardiopulmonary resuscitation), documented hypoglycemia (defined as glucose b 40 gm/dl), presence of an endocrinopathy (e.g. hypothyroid, congenital adrenal hyperplasia), perinatal sepsis (documented by the presence of positive blood, urine, or stool cultures and presence of systemic inflammatory response syndrome (SIRS) as defined by Goldstein [11]), hypercoagulable state (defined as presence of a genetic hypercoagulable state or severe polycythemia), any congenital anomaly, and any genetic disorder. Maternal factors that were evaluated included the presence or absence of pre-eclampsia, pregnancy induced hypertension (PIH), and use of alcohol, tobacco, or elicit substances. Other assessed clinical factors included whether the child was a singleton pregnancy, had umbilical artery catheterization, age at first feed, time from first feed to full feed, use of breast milk or formula feeds, presence of milk protein allergy, Bell’s stage of NEC (II or III), age at onset of NEC, days of nasogastric decompression, medical or surgical management, any surgical complication, indication for surgical intervention, type of surgical intervention, operative intervention prior to onset of NEC, length of NICU stay, and mortality.
1.3. Statistical Analysis
All data were recorded into a standardized spreadsheet program (Microsoft Excel©, Seattle, WA). Statistical analysis was performed using the R software (www.R-project.org). Association between outcomes and covariates was analyzed using Fisher’s exact and Wilcoxon test. Multivariable analysis was done using logistic regression. A p-value b 0.05 was considered significant. Multivariable analysis was performed on variables with p b 0.1 using multiple stepwise logistic regression model, using the R software.
2. Results
Thirty-nine patients were identified (24 boys, 15 girls) with Bell’s Stage ≥ 2 at a median gestational age of 39 weeks. (Table 1) Three additional patients were determined to have Bell’s stage I NEC, and were excluded from analysis. Most children were born via spontaneous vaginal delivery (n = 27, 69%) and median APGAR scores were 7.6 at one minute and 8.2 at five minutes, respectively. More than one third (38%) of children were born to mothers with perinatal risk factors. These include illegal narcotic use (cocaine n = 3, methamphetamines n = 1), intrapartum infection (syphilis n = 2, rubella n = 1, other non-specified infection n = 3), maternal diabetes mellitus requiring insulin (n = 3), and pregnancy induced hypertension requiring treatment (n = 2). Early perinatal infection was present in the majority (64%) of these infants and included documented bacteremia (Klebsiella n = 3, Escherichia coli n = 3), suspected or unspecified bacteremia (n = 10), Rotavirus (n = 3), Clostridium difficile (n = 3), and Congenital Syphilis (n = 3). Other previously reported risk factors of necrotizing enterocolitis were also found in our cohort of patients, including perinatal hypoxic events (n = 10), congenital heart disease (cyanotic lesions (n = 5), non-cyanotic lesions (n = 4)), endocrinopathy (hypoglycemia (n = 4,) hypothyroidism (n = 2)), persistent hypertension (n = 1), milk protein allergy (n = 2), hypercoagulable state (protein S deficiency (n = 1), and polycythemia (n = 4)) (Table 1). In addition to these previously reported risk factors we identified a relatively large percentage (18%) of patients with genetic disorders (Cystic Fibrosis (n = 2), Phenylketonuria (n = 2), Trisomy 21 (n = 3), Turner syndrome (n = 1), Smith–Lemli–Opitz (n = 1)). One patient was also eventually diagnosed with Hirschsprung’s disease by suction rectal biopsy and later underwent a definitive pull-through procedure.
Table 1.
Demographics for Patient Population (N = 39).
| EGA weeks (median, IQR) | 39 (38, 40) |
| Gender (Male) | 24/39 |
| Birth Weight (kg) | 3.2 ± 0.59 |
| APGAR @ 1 min | 7.6 ± 2 |
| APGAR @ 5 min | 8.2 ± 1.8 |
| C-Section | 26/39 (67%) |
| Maternal Factor | 15/39 (38%) |
| Sepsis | 25/39 (64%) |
| Perinatal Hypoxic Event | 10/39 (26%) |
| Congenital Heart Disease | 9/39 (23%) |
| Endocrinopathy | 7/39 (18%) |
| Genetic Anomaly | 7/39 (18%) |
| IUGR | 4/39 (10%) |
| Hypercoagulable State | 2/39 (5%) |
| Age @ 1st Feed (median) | 1 day |
| Days to full feeds (median) | 5.5 days |
| Breast milk only | 13/39 (33%) |
| Milk protein allergy | 2/39 (5%) |
| Age @ NEC onset (median, IQR) | 3 (2, 8.75) |
| Days NG decompression (median) | 11 |
| Required Surgery | 26/39 (67%) |
| NICU length of stay (median, IQR) | 29.5 (20.8–60.5) days |
| Survival | 32/39 (82%) |
N (percentage).
Mean ± standard deviation.
When comparing children with early (≤ 7 days) vs. late (N 7 days) onset of disease, we found significant differences between the two groups on univariate analysis. Some characteristics of patients with early onset and late onset NEC are summarized in Table 2. The mean age of NEC onset in the early group was 2.5 ± 1.6 days and 25.1 ± 14.4 days in the late group (p b 0.001). Infants with late onset NEC were more likely to have a genetic abnormality (46% vs. 7%, p = 0.01) and to have had a previous operation prior to the development of NEC (36% vs. 0%, p = 0.004). Reasons for operation prior to NEC included repair of congenital heart disease (n = 3) and intestinal malrotation without volvulus (n = 1). There was also a trend for the late onset group to have an increase in associated congenital anomalies (55% vs. 21%, p = 0.06), non-operative or medical management (65% vs. 29%, p = 0.07), and mortality (36% vs. 11%, p = 0.08). No significant differences were found between groups with regards to age at 1st feeding, need for operative exploration, or length of stay. Multivariable analysis revealed only late onset of NEC to be an independent predictor of mortality (OR 90.8, 95% CI 2.6–3121).
Table 2.
Comparison of Early (≤ 7 days) vs. Late (> 7 days) Onset of NEC.
| Factor | Total Cohort n = 39 |
Early n = 28 |
Late n = 11 |
P |
|---|---|---|---|---|
| EGA (weeks) | 39 (38, 40) | 39 (38, 40) | 39 (37, 40) | 0.33 |
| Male gender | 24 (62%) | 18 (64%) | 6 (55%) | 0.72 |
| Birth Weight (kg) | 3.17 ± 0.59 | 3.13 ± 0.56 | 3.26 ± 0.67 | 0.56 |
| Age at NEC onset (days) | 8.7 ± 12.8 | 2.5 ± 1.6 | 26.9 ± 14.0 | <0.001 |
| C-Section Delivery | 12 (31%) | 8 (29%) | 4 (36%) | 0.69 |
| APGAR at 0 min | 8 (8, 9) | 8 (8, 9) | 7 (4, 8) | 0.10 |
| APGAR at 5 min | 9 (8, 9) | 9 (9, 9) | 8 (5, 8) | 0.01 |
| Congenital Heart Disease | 9 (23%) | 5 (17%) | 4 (40%) | 0.24 |
| Hypoxic event | 11 (28%) | 7 (25%) | 4 (36%) | 1.0 |
| Perinatal sepsis | 25 (64%) | 16 (57%) | 9 (82%) | 0.27 |
| Maternal factors | 15 (38%) | 12 (43%) | 3 (27%) | 0.48 |
| Congenital Anomalies | 12 (31%) | 6 (21%) | 6 (55%) | 0.06 |
| Genetic Anomalies | 7 (18%) | 2 (7%) | 5 (46%) | 0.01 |
| UA Catheter placed | 5 (13%) | 2 (7%) | 3 (27%) | 0.13 |
| Formula feeds only | 25 (64%) | 19 (73%) | 6 (55%) | 0.44 |
| Medical Management | 15 (38%) | 8 (29%) | 7 (64%) | 0.07 |
| Bell’s II NEC | 21 (54%) | 16 (57%) | 5 (46%) | 0.72 |
| Bell’s III NEC | 18 (46%) | 12 (43%) | 6 (55%) | 0.72 |
| OR prior to NEC | 4 (10%) | 0 (0%) | 4 (36%) | 0.004 |
| Length of NICU stay | 29.5 (21, 60) | 26.5 (20.8, 60.5) | 40 (20.5, 60) | 0.66 |
| Mortality | 7 (18%) | 3 (11%) | 4 (36%) | 0.08 |
| OR | Lower CI | Upper CI | P | |
|
| ||||
| Multivariable Analysis of Factors Associated with Late Onset NEC | ||||
| APGAR at 5 min | 0.58 | 0.31 | 1.1 | 0.09 |
| Mortality | 90.8 | 2.6 | 3121 | 0.01 |
P value for early vs. late onset of NEC.
Mean ± standard deviation.
Median (interquartile range).
N (percentage).
Risk factors for mortality from NEC in the term infant were analyzed (Table 3). Significant univariate predictors of mortality included congenital heart disease in (57% vs. 16% p = 0.04), and umbilical artery catheterization (57% vs. 3%, p = 0.002). There was a trend for increased mortality among those with significant perinatal hypoxic events (57% vs. 19%, p = 0.06), older age at onset (57% vs. 19%, p = 0.06), and those with genetic anomalies (43% vs. 13%, p = 0.09) (Table 3).
Table 3.
Univariate Predictors of Mortality in Full Term NEC.
| Factor | Alive (n = 32) |
Dead (n = 7) |
P |
|---|---|---|---|
| EGA | 39 (38, 40) | 39 (38, 49.5) | 0.66 |
| Male gender | 18 (56%) | 6 (86%) | 0.22 |
| Birth Weight (kg) | 3.1 (2.8, 3.5) | 3.3 (3.1, 3.6) | 0.32 |
| C-Section Delivery | 11 (36%) | 1 (14%) | 0.39 |
| APGAR at 0 min | 8 (8, 9) | 8 (7.3, 8.8) | 0.65 |
| APGAR at 5 min | 9 (9, 9) | 8.5 (8, 9) | 0.24 |
| Congenital Heart Disease | 5 (16%) | 4 (57%) | 0.04 |
| Hypoxic event | 6 (19%) | 4 (57%) | 0.06 |
| Perinatal sepsis | 19 (59%) | 6 (8%) | 0.39 |
| Maternal factors | 13 (41%) | 2 (29%) | 0.87 |
| Congenital Anomalies | 8 (25%) | 4 (57%) | 0.17 |
| Genetic Anomalies | 4 (13%) | 3 (43%) | 0.09 |
| Need for UA Catheter | 1 (3%) | 4 (57%) | 0.002 |
| Formula feeds only | 22 (71%) | 3 (43%) | 0.59 |
| Medical Management | 12 (37%) | 3 (43%) | 1.0 |
| Age > 7 days at time of NEC onset | 6 (19%) | 4 (57%) | 0.06 |
| Bell’s II NEC | 18 (56%) | 3 (43%) | 0.82 |
| Bell’s III NEC | 14 (44%) | 4 (57%) | 0.82 |
| OR prior to NEC | 2 (6%) | 2 (29%) | 0.28 |
| Length of NICU stay | 29.5 (21, 60.5) | 23 (14, 35.5) | 0.23 |
3. Discussion
This is the first study to evaluate and report differential outcomes between term infants who develop NEC in early (≤ 7 days) versus late (N 7 days) postnatal life. All children in this study had some unusual or significant factor that may have predisposed them to NEC. Perinatal sepsis, hypoxic events, congenital heart disease, infectious issues, and maternal factors were especially prevalent. Nearly 10% of children had documented intrauterine exposure to cocaine or methamphetamines and more than 10% were exposed to maternal infections including rubella and syphilis. Among the observed factors in this study, congenital heart disease, need for umbilical artery catheterization, and late onset of NEC were associated with increased risk for mortality Congenital heart disease has been frequently associated with increased risk for NEC. This is thought to be secondary to hypoxia and/or decreased blood flow to the mesenteric vessels. Nine of our patients had congenital heart disease with five patients in the early onset group and four patients in the late onset group. Four of the patients who died (1 early-onset NEC, 3 late-onset NEC) had a combination of congenital heart disease and other congenital anomalies. One additional patient who survived with a combination of congenital heart disease and congenital and genetic anomalies was in the late onset NEC patient. Cardiac surgery accounted for three of the surgeries performed prior to the onset of NEC in the late onset group. Poor intestinal perfusion secondary to the underlying heart disease or low flow state during cardiac surgery may have contributed to the onset of NEC. Patients who had a combination of heart disease and other anomalies appeared to be at particularly high risk for late-onset NEC and mortality. Umbilical artery (UA) catheterization was also found to be a significant risk factor for mortality in our patient population. The UA catheter itself has also been theorized to mechanically decrease mesenteric blood flow and place patients at risk for NEC. Rand et al. used duplex Doppler sonography to demonstrate decreased mesenteric blood flow in neonates with umbilical artery catheters in place [14]. The difference in mortality may also be simply because infants who are more systemically ill would be more likely to have a UA catheter placed for monitoring. These patients may include infants with congenital heart disease, hypotension, hypoxia, or other congenital anomalies that may increase their risk for development of NEC.
Previous studies have differed greatly regarding time to onset of NEC in the term and preterm infant. Studies by both Yee and Llanos demonstrated that younger preterm infants tended to present with NEC later than older preterm infants. Surgical intervention was more common in the early onset, older gestational age patients [8, 15]. In a retrospective review of 30 term infants with NEC, Lambert et al. reported the median age of NEC onset to be 12 days. They did not note a bimodal distribution [5]. They reported similar rates of congenital heart disease and that the infants in their population also had many associated illness and anomalies. Bolisetty and colleagues reported differential median onset of NEC based on presence or absence of endocrinopathy (median 20.5 days), congenital heart disease (median 4.0 days) and presence (median 8.0 days) or absence of congenital diseases (5.5 days). While their findings suggest that the underlying pathophysiology may help determine time to development of NEC, their findings did not reach significance [12]. A previous retrospective study of 10 patients with a comparable rate of surgical intervention (70%) reported that 90% of their cohort developed NEC by day of life four [13].
In our patient population of full-term infants, we have found a skewed distribution of onset of NEC with an early peak between one and seven days followed by a long tail (Fig. 1). The early onset group with age ≤ 7 days (n = 28) developed NEC at 2.5 ± 1.6 days while the late onset with group with age N 7 days (n = 11) developed NEC at 26.9 ± 14.0 days of life (p b 0.001). The median overall age of NEC onset was 8.7 ± 12.8 days. The infants in our study who developed NEC after day of life seven had a higher incidence of genetic anomalies (e.g. Down’s syndrome), associated congenital anomalies, previous surgical intervention, and a significant increased risk of mortality. NEC is thought to have a multifactorial etiology and it is possible that stressing the infant with procedures or conditions associated with intestinal hypoperfusion (e.g. surgery) in the setting of multiple anomalies may predispose infants to development NEC. Two of our late-onset NEC patients who died had hypoxic cardiac defects that would place them at higher risk for intestinal hypoperfusion.
Fig. 1.
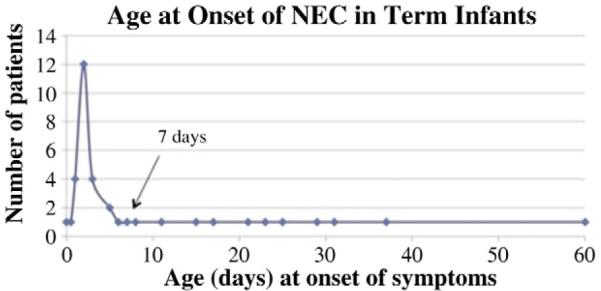
Distribution of NEC onset in the full term is skewed with an early peak between days of life one and seven followed by a long tail.
The patient population in this full-term NEC cohort was very diverse and had some diagnoses different from those found commonly found in preterm infants. Several of the patients in our study had intestinal infections not commonly associated with preterm infants. These included documented infections with C. difficile, Rotavirus, congenital syphilis, and E. coli 0157:H7 infection. This may be related to the different intestinal microbiome of the term infant as well as exposure to potential infectious sources for the infants who had been discharged to home prior to their diagnosis of NEC. In addition, at least two of the patients were thought to have NEC secondary to severe milk protein allergy. This can present in a very similar fashion with abdominal distension, pneumatosis, blood in the stool, and feeding intolerance. One patient that met the diagnostic criteria for NEC was subsequently found to have Hirschsprung’s disease as confirmed on suction rectal biopsy. The patient was treated with bowel rest, antibiotics, and irrigations and later underwent a definitive pull-through procedure. In retrospect, the patient did meet inclusion criteria for NEC, but the presenting diagnosis in this patient may have been Hirschsprung-associated enterocolitis. These patients reinforce the need to maintain a high index of suspicion when evaluating term infants with the diagnosis of NEC and to look for etiologies of NEC not commonly associated with preterm infants. Although the clinical picture of intestinal dysfunction and inflammation may be a common pathway, the etiology of NEC in the term and preterm infants may be different.
While our study reflects patients from two centers and is the largest reported series of NEC in the term infant, it has a number of limitations. It is retrospective and we are limited by the retrospective nature of the study with inherent risk of selection bias and reliance on the accuracy of the medical record. The relatively small number of patients underpowers the analysis, making it difficult to determine if other differences in demographic factors impact patient outcomes. The study also takes place over a long time period and outcomes from many patients may not reflect outcomes from current treatment algorithms. While all operative notes and radiographic images were reviewed for meeting inclusion criteria in these patients, misclassification bias is possible since some clinical features of spontaneous intestinal perforation, Hirschsprung’s associated enterocolitis, bacterial enteritis, and milk protein allergy are similar to those of NEC.
Our data demonstrate that term infants in this study who developed NEC after day of life seven were at increased risk of mortality. This group was more likely to have undergone a previous operation, have a chromosomal anomaly, and to have non-operative management of their NEC. Term infants with NEC may also have conditions not normally associated with the preterm population and may require further investigation and studies. Due to the rarity of the condition, a multicenter prospective study is planned to better understand the pathophysiology of NEC in full-term infants.
References
- [1].Ostlie DJ, Spilde TL, St Peter SD, et al. Necrotizing enterocolitis in full-term infants. J Pediatr Surg. 2003;38:1039–42. doi: 10.1016/s0022-3468(03)00187-8. [DOI] [PubMed] [Google Scholar]
- [2].Emami CN, Petrosyan M, Giuliani S, et al. Role of the host defense system and intestinal microbial flora in the pathogenesis of necrotizing enterocolitis. Surg Infect. 2009;10:407–17. doi: 10.1089/sur.2009.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Afrazi A, Sodhi CP, Richardson W, et al. New insights into the pathogenesis and treatment of necrotizing enterocolitis: toll-like receptors and beyond. Pediatr Res. 2011;69:183–8. doi: 10.1203/PDR.0b013e3182093280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Petrosyan M, Guner YS, Williams M, et al. Current concepts regarding the pathogenesis of necrotizing enterocolitis. Pediatr Surg Int. 2009;25:309–18. doi: 10.1007/s00383-009-2344-8. [DOI] [PubMed] [Google Scholar]
- [5].Lambert DK, Christensen RD, Henry E, et al. Necrotizing enterocolitis in term neonates: data from a multihospital health-care system. J Perinatol. 2007;27:437–43. doi: 10.1038/sj.jp.7211738. [DOI] [PubMed] [Google Scholar]
- [6].Ng S. Necrotizing enterocolitis in the full-term neonate. J Paediatr Child Health. 2001;37:1–4. doi: 10.1046/j.1440-1754.2001.00584.x. [DOI] [PubMed] [Google Scholar]
- [7].Maayan-Metzger A, Itzchak A, Mazkereth R, et al. Necrotizing enterocolitis in fullterm infants: case–control study and review of the literature. J Perinatol. 2004;24:494–9. doi: 10.1038/sj.jp.7211135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yee WH, Soraisham AS, Shah VS, et al. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics. 2012;129:e298–304. doi: 10.1542/peds.2011-2022. [DOI] [PubMed] [Google Scholar]
- [9].Teasdale F, Le Guennec JC, Bard H, et al. Neonatal necrotizing enterocolitis: the relation of age at the time of onset to prognosis. Can Med Assoc J. 1980;123:387–90. [PMC free article] [PubMed] [Google Scholar]
- [10].Caplan MS, Jilling T. New concepts in necrotizing enterocolitis. Curr Opin Pediatr. 2001;13:111–5. doi: 10.1097/00008480-200104000-00004. [DOI] [PubMed] [Google Scholar]
- [11].Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- [12].Bolisetty S, Lui K, Oei J, et al. A regional study of underlying congenital diseases in term neonates with necrotizing enterocolitis. Acta Paediatr. 2000;89:1226–30. doi: 10.1080/080352500750027619. [DOI] [PubMed] [Google Scholar]
- [13].Andrews DA, Sawin RS, Ledbetter DJ, et al. Necrotizing enterocolitis in term neonates. Am J Surg. 1990;159:507–9. doi: 10.1016/s0002-9610(05)81257-7. [DOI] [PubMed] [Google Scholar]


