Abstract
Leveraging advances in consumer electronics and wireless telecommunications, low-cost, portable optical imaging devices have the potential to improve screening and detection of disease at the point of care in primary health care settings in both low- and high-resource countries. Similarly, real-time optical imaging technologies can improve diagnosis and treatment at the point of procedure by circumventing the need for biopsy and analysis by expert pathologists, who are scarce in developing countries. Although many optical imaging technologies have been translated from bench to bedside, industry support is needed to commercialize and broadly disseminate these from the patient level to the population level to transform the standard of care. This review provides an overview of promising optical imaging technologies, the infrastructure needed to integrate them into widespread clinical use, and the challenges that must be addressed to harness the potential of these technologies to improve health care systems around the world.
MOTIVATION FOR IMAGING AT THE FRONT LINE
Technological advances in imaging and computation have improved the quality of health care. Biomedical imaging, perhaps, represents the most recognized interface between engineering and medicine, improving clinicians’ abilities to screen for and diagnose disease, and monitor the result of treatments. Biomedical imaging modalities are used across many size scales, ranging from whole-body x-ray computed tomography to the cellular and molecular scales by the microscopic investigation of tissue sections in pathology, or immunohistochemical staining of tissue to reveal the presence and distribution of specific molecules. In general, these imaging modalities are used by specialists in radiology and pathology; however, there exists a more general need for all physicians and health care providers the world over to visualize tissue, cells, and molecules to aid in diagnosis at the point of care (PoC) or to guide surgical and medical procedures at the point of procedure (PoP).
The ability to routinely provide image-based data in all resource settings to aid in screening, diagnosis, and treatment monitoring could transform global health care and the systems that provide this care. Optical imaging technologies offer many advantages over radiological instruments, namely, being low-cost and portable yet offering real-time, high-resolution imaging capabilities of tissues, cells, and molecules (1, 2). Currently, there exist numerous techniques and instruments that rely on sensing biophysical, biochemical, or biological components or properties from biological samples, with many being ideally suited for PoC screening and diagnostics. Optical techniques, however, can provide an additional means for imaging these properties or biomarkers, providing multiplexed, spatially resolved information across cell or tissue samples or specimens, as well as providing in vivo screening and diagnostic capabilities. When integrated with mobile cell phone platforms and networks, these image data can be shared remotely with experts for medical decision-making.
The development and dissemination of new PoC/PoP imaging technologies can be beneficial across all countries and health care settings of differing resources and infrastructures. The initial patient encounter, whether it be in a developed or developing country, remains largely the same: an experience between a patient and a health care provider that involves a physical exam; questioning; keen observational, listening, and problem-solving skills; and relatively simplistic instruments, such as the stethoscope or reflex hammer. Somewhat more sophisticated instruments would include the otoscope, ophthalmoscope, and sphygmomanometer. Because the PoC is critical for identifying disease early, an effective strategy would be to apply imaging technology at this front line, where diseases could be detected and appropriate treatment could be initiated more rapidly.
In high-resource settings, primary care is usually provided by physicians or nurse practitioners with ready access to reference laboratories. Delays in receiving diagnostic test results from these laboratories can lead to additional costs and/or delays in initiating treatment. Some patients may be lost to follow-up if they do not return to receive their test results. In low-resource settings, especially in rural areas, there is a shortage of trained medical personnel, and patients may have to travel long distances to receive primary health care. Frontline health care is often provided by community health workers working in settings with intermittent power, limited access to clean water, and essentially no access to central laboratory services (3). It is conceivable that real-time PoC microscopic imaging can be done by community health workers during the initial patient encounter, with compact, low-cost imaging systems that can be linked across cell phone networks and connected to expert providers located miles, countries, or continents away.
In addition to imaging at the PoC, imaging at the PoP offers the opportunity to shift the microscopic assessment of tissues, cells, and molecules from the pathology laboratory to the operating suite, for example, in the case of breast cancer surgery, where surgeons could use real-time feedback to alter their interventional care and reduce re-operation owing to positive margins (4, 5). Even as surgical procedures become more advanced with robotic guidance, there remains a need for imaging to guide and complete the procedures (6). In low-resource settings, pathology services are often unavailable owing to a lack of trained pathologists, histotechnologists, and laboratory equipment. The inability to obtain accurate diagnostic and staging information and to assess tumor margins compromises the ability to deliver effective care (7).
Despite differences in infrastructure and resources, the gaps in care for screening, diagnosis, and image-guided interventions or therapy are similar in both low- and high-resource settings (Fig. 1, outer ring). Inadequate screening in primary care settings leads to inappropriate referral, where opportunities for early detection and treatment can be missed, or patients can be unnecessarily referred to a higher level of care, increasing associated costs of care. Inadequate diagnosis can further delay treatment. At the PoP, inadequate treatment worsens outcomes and leads to the need for additional treatment visits. At the population level, poor knowledge of health conditions makes it difficult to plan for the needs of the health care system and to assess whether resources are appropriately deployed. To improve this process of screening, diagnosis, and referrals, there is an opportunity to leverage advances in telecommunications technology and consumer electronics to develop imaging technologies for the PoC/PoP that promote equitable access to health-improving technologies, regardless of the country or resource setting (Fig. 1, inner ring).
Fig. 1. Gaps in PoC and PoP that may be addressed by imaging.
The outer ring illustrates current gaps at the frontline primary care, referral care, and health care system levels. The inner ring illustrates how optical imaging technologies designed to be used at the PoC/PoP can fill these gaps.
Although large medical imaging systems that can only be used in specialty care settings are high in cost, the need at the PoC/PoP is to minimize the cost of the imaging device itself and, more importantly, the per-test cost of the imaging procedure. Cost minimization is particularly important in low-resource settings, where designers must also consider supply chain limitations and the frequent lack of infrastructure (power, water, disinfection protocols, extreme environmental conditions). With networked results, epidemiologic monitoring can be improved and health care systems strengthened. Broad dissemination of such systems to ultimately improve care at the PoC/PoP requires commercialization through industry, philanthropic, or government support. If these goals are met, there is the opportunity to demonstrate a new generation of PoC/PoP imaging platforms that could improve the quality and efficiency of care and reduce health care costs.
This review motivates the need for PoC/PoP imaging technologies, provides representative examples of current technological innovations and applications, outlines steps by which these changes can be financially supported and implemented, and concludes with future challenges and directions. Just as technological advances like genome sequencing and electronic health records have revolutionized the practice and quality of health care, we are at another juncture of opportunity to leverage the convergence of imaging systems with consumer electronics, wireless communication networks, and advanced computation and automation to improve health globally, in all settings.
POINT-OF-CARE PLATFORMS
PoC imaging is an alternative approach to laboratory-based analyses that provide diagnostic information in an outpatient setting, thereby reducing the time and infrastructure necessary for clinical decision-making (8). In resource-limited settings, PoC testing offers important clinical benefits, including reduced clinical infrastructure and fewer patients lost to follow-up. Nevertheless, it is essential that the benefits outweigh the costs of PoC testing, especially in settings where there are limited economic and personnel resources. Recognizing this need, the World Health Organization (WHO) called for PoC tests to meet specific ASSURED criteria: Affordable, Sensitive, Specific, User-friendly, Rapid and robust, Equipment-free, and Deliverable to end-users (9). Additional criteria for an ideal PoC test in resource-limited settings are that it allows quick decision-making, can be used at the clinical PoC by health care workers, is of low average cost per test, provides result during the clinic visit, has acceptable test efficacy, and is cost-effective (8). Here, we list some platforms that have shown great promise for fulfilling the above criteria.
Microscopy
To meet these needs, several imaging-based PoC diagnostic tools have been developed and translated into PoC applications. Leveraging advances in light-emitting diodes (LEDs) and inexpensive high-resolution image sensors, several approaches have focused on reducing the cost and expertise necessary for microscopy so that it can be performed at the PoC. For example, fluorescence microscopy has been shown to improve diagnostic sensitivity for the diagnosis of Mycobacterium tuberculosis compared to bright-field microscopy (10, 11), but laboratory-grade fluorescence microscopes are too costly for many low-resource settings. In response, commercially available LED-based fluorescence microscopes have been developed for low-resource settings, including a stand-alone fluorescence microscope from the Foundation for Innovative New Diagnostics (FIND; http://www.finddiagnostics.org), and adaptors have been designed to add fluorescence capability to traditional bright-field microscopes (10). Field tests with clinical samples in Uganda have shown that these LED-based fluorescence systems improve sensitivity and reduce examination time compared with bright-field microscopes (10). Furthermore, owing to the high performance and low cost of these systems—ranging from US$700 to US$1750—the WHO now recommends that LED fluorescence microscopy should replace conventional fluorescence microscopy. Miller and colleagues (11) developed an even lower-cost (US$240), stand-alone, battery-powered fluorescence field microscope that uses battery-operated LED flashlights. In a small pilot study of diagnostic accuracy for M. tuberculosis in clinical samples, concordant results were obtained with the field fluorescence microscope and a laboratory-grade fluorescence microscope in >98% of cases examined (11).
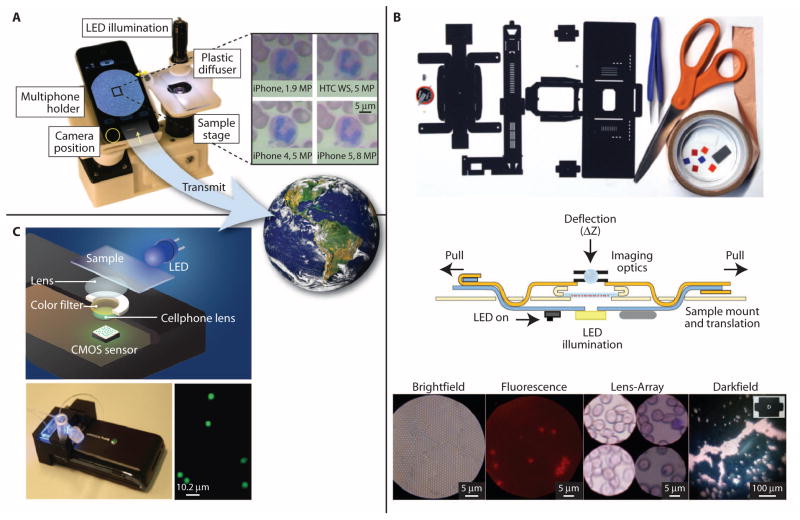
A modular, portable fluorescence microscope has been designed and evaluated in the laboratory for measuring white blood cell count and a three-part differential from a small drop of capillary blood applied to a disposable cartridge containing fluorescent dye (12). Mobile phone–based clinical microscopy has also shown promise for PoC testing in global health applications (13). A mobile phone–mounted microscope was used in the laboratory to image Plasmodium falciparum–infected and sickled red blood cells from patient samples in bright-field mode, and M. tuberculosis–infected sputum cells from patient samples in fluorescence mode using the camera in the phone and a liquid crystal display (LCD) screen to capture and display the images (13) (Fig. 2A). This approach opens the possibility of processing and storing images locally or transmitting them to a central location for interpretation and storage. However, quantitative image analysis can be complicated by proprietary and rapidly evolving image processing algorithms present across a diverse array of commercially available phones, such as iPhones (Apple) and HTCs (Windows, Android). For example, automatic focus can alter effective magnification, resulting in changes in apparent feature size, and built-in sharpening algorithms can produce halos around high-contrast structures. Strategies, therefore, have to be developed to control for these factors across a broad range of smartphones (14).
Fig. 2. PoC microscopes and flow cytometers.
(A) A cell phone–based microscope. Four different cell phones were used to obtain images of a Wright-stained blood smear (14), illustrating the resulting differences in image resolution, color, and brightness. For global health care, these images can be viewed locally and transmitted remotely for storage or further analysis. [IMAGE: Visible Earth, NASA.] (B) Foldscope components, tools, and instructions used in its assembly. The cross-section view shows sample mount and translation mechanism, LED illumination, and imaging optics. The sample is inserted from the side. Images were acquired from 1-μm polystyrene beads in bright field, 2-μm fluorescent beads in fluorescence, a Giemsa-stained blood smear in lens array, and 6-μm polystyrene beads in dark field modes (15). (C) Schematic diagram and photo of a cell phone–based flow cytometer. The spatial resolution of the system is about 2 μm, and images acquired with the system show that it can resolve 2- and 4-μm-diameter fluorescent beads (18). Images reproduced from (14, 15, 18) with permission.
An ultra-low-cost, origami-based approach has recently been reported for developing bright-field, dark-field, and fluorescence microscopes that can withstand harsh field conditions (15). This “Foldscope” (http://www.foldscope.com) is assembled from a flat piece of paper, which is folded to hold an illumination system containing LEDs; imaging optics composed of microlenses; and a sample mounting system to hold, translate, and focus on a slide containing the specimen of interest. Images are viewable by eye or can be projected on a screen (Fig. 2B). Early bench tests show that the Foldscope can be used to visualize parasites such as Giardia lamblia and Trypanosoma cruzi in cultured samples (15). In a related approach, a lens-free cell phone microscope that uses in-line digital holography has been used to image biological samples with large field of view (for example, 20 to 30 mm2), without a trade-off in spatial resolution (16). Promising results have been obtained in the laboratory for blood smears, dilute samples of fixed waterborne parasites, and cytologic specimens from patients (17).
Flow-based systems
As an alternative to microscopy, several groups have proposed the use of cell phone–based imaging to develop on-chip flow cytometry systems (17–20). Ozcan and colleagues developed an imaging cytometry platform in which fluorescently labeled cells are flowed through a disposable microfluidic channel positioned above a cell phone camera (Fig. 2C). Excitation light from an LED is directed through the microfluidic channel to excite fluorescence, and spatial resolution is about 2 μm. Movies are acquired as objects flow through the microchannel and are processed to quantify the number and density of labeled particles. Pilot laboratory testing showed that whole blood labeled with a fluorescent dye could be used to estimate white blood cell density in reasonable agreement with a gold standard hematology analyzer: for 12 patient samples with white blood cell concentrations ranging from 4000 to 8000 cells/μl, the 95% limits of agreement were 1026 cells/μl (lower) and 347 cells/μl (upper) (18).
Flow-based systems provide an alternative approach to obtain the needed spatial resolution in a lens-free system. Sub-pixel resolution has been obtained by using an optofluidic system to flow specimens across an image sensor (21). Image reconstruction algorithms can yield submicron spatial resolution, and automated scanning of large sample volumes is possible. Bench testing showed that color images of red blood cells could be acquired to aid in recognizing cells infected with P. falciparum (22). In an alternate approach, a moving light source was used to illuminate the sample and create multiple shadow images of the sample over a light sensor. These low-resolution images were processed to yield a high-resolution image; proof-of-concept images of three types of cultured waterborne parasites were obtained using this approach (23). Currently, full-image reconstruction requires a few minutes of processing time using a personal computer with an Intel i3 processor. Alternative processing strategies must be developed to support field use in low-resource settings.
Depth-resolved imaging
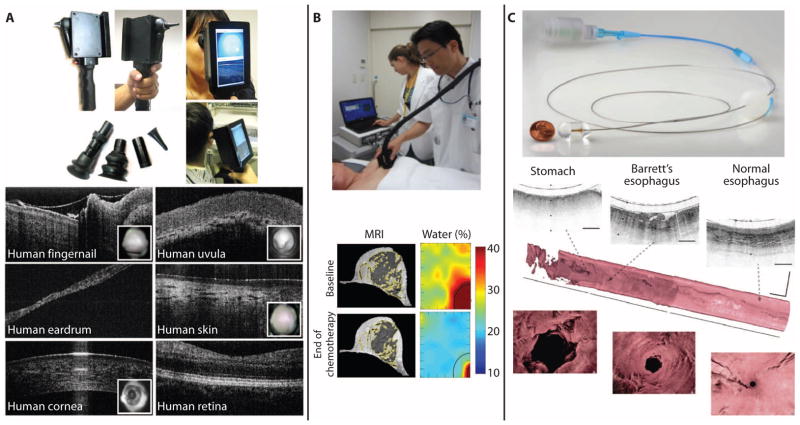
Technological advances are also being made to add two-dimensional (2D) and 3D depth-resolved optical imaging capabilities to the PoC in a platform resembling the handheld otoscope and ophthalmoscope—two instruments that are ubiquitous in primary care medicine. For example, optical coherence tomography (OCT) (for depth-resolved imaging) has been integrated with video-based surface imaging in a handheld scanner with interchangeable tips to provide real-time microscopic assessment of tissue sites commonly examined in primary care, including the ears, eyes, skin, oral mucosa, and teeth (Fig. 3A) (24, 25). Several laboratory and translational clinical human studies in adults and children have demonstrated noninvasive identification of middle-ear biofilms and their relationship to the severity of otitis media (ear infections) (26)—one of the most common diseases in children that can often lead to hearing loss, speech and language delays, and lifelong disability, if not treated appropriately. Technologies such as these could not only provide high-resolution imaging for screening and diagnosis at the PoC but also be used to monitor treatment efficacy for guiding procedures and interventions.
Fig. 3. PoC and PoP imaging systems.
(A) Handheld OCT scanner for primary care imaging. The handheld unit with a built-in screen shows both the surface video image and the depth-resolved OCT images in real time. Interchangeable tips are used for imaging various tissue sites. Reproduced from (24, 25) with permission. (B) A handheld diffuse optical spectroscopy scanner generates images based on water, deoxyhemoglobin, and fat content in the human breast for early identification of treatment response during chemotherapy. When compared to breast MRI, the low-cost and portable optical imaging system data strongly correlate with breast density and provide additional functional information. Reproduced from (37) with permission. (C) A tethered imaging capsule coupled to a portable OCT system enables rapid high-resolution 3D imaging of the human esophagus for screening and surveillance of premalignant changes in Barrett’s esophagus patients. The tethered imaging capsule is swallowed, then pulled back during image acquisition to collect the 3D image data. Reproduced from (41) with permission.
POINT-OF-PROCEDURE PLATFORMS
The care of a patient often involves medical and surgical procedures performed by primary care providers and specialists. Universally, visualization is essential for a successful surgery, whether it be by naked eye or aided by imaging systems such as video-guided endoscopy or colonoscopy, ultrasound- or magnetic resonance–guided needle biopsy, or robotic-assisted procedures that rely on video imaging for internal manipulation of tissue. Most of these examples rely on either large imaging systems for whole-body imaging or video-based imaging to visualize surface features of tissues (in other words, not at the cellular or molecular scale). Histopathological assessment of tissue, as the gold standard, can provide cellular or molecular information. However, pathologists work on excised tissue removed at the PoP and assess the tissue within hours, with the full report days later.
Within medicine and surgery, there remain opportunities for PoP optical imaging to essentially bring the microscopic cellular and molecular imaging capabilities to health care providers in real time. This could be especially useful in situations where it is not safe or practical to remove tissue for histopathological assessment. Here, noninvasive or minimally invasive methods to evaluate the cellular- or molecular-level changes in tissue in situ are needed. Moreover, many low-resource settings lack the infrastructure and expertise needed for diagnostic pathology, so real-time diagnosis with robust, affordable systems would greatly improve care.
Intraoperative imaging
PoP imaging applications in surgery, particularly in the surgical treatment of breast cancer, have been an active area of research in recent years, largely because of the high 38% reoperation rate after breast conserving surgery (27). This high rate occurs because the surgical margins on the resected tissue/tumor specimen are often found to harbor tumor cells, implying that tumor cells remain within the surgical tumor cavity of the patient. Detecting these microscopically currently takes place in the pathology laboratory, often days after the surgical procedure has ended. OCT has been developed as a label-free PoP imaging technology to provide real-time intraoperative microscopic assessment of breast tumor margins and lymph nodes (28, 29). OCT relies on the inherent differences in optical scattering between tissue types, and can be performed using compact chip-based optical sources instead of large and costly lasers, making future translation of OCT into PoC applications in low-resource settings feasible. Because the biomechanical properties of tissues also change in disease processes, an imaging needle capable of both OCT and elastography measurements has been developed for probing breast tissue to localize the tumor boundary (30).
Several fluorescence-based techniques have also been reported to identify tumor tissue and cells along surgical margins, using endogenous autofluorescence (31) and the addition of exogenous fluorescent contrast agents injected intravenously (32) or at the tumor site, or even sprayed onto the surface of the tumor cavity during the open surgery (33). These exogenous agents may target to specific molecular receptors overexpressed on the tumor cells. The stronger fluorescence emission from exogenous agents permits wide-field imaging across the surgical field, which is challenging for point-scanning methods. These techniques can also identify spectroscopic molecular differences between tissue types, but weak autofluorescence can often require seconds of acquisition time per point. Real-time confocal microscopy, as a label-free modality, has been used to guide laser ablation of basal cell carcinoma and assess resected skin tumor and tissue specimens during Mohs surgeries, which replaces the time and expense of frequent histological processing of resected specimens (34).
Outside the surgical suite
Many medical procedures take place outside the surgical suite, and PoP imaging technologies have advanced these to provide real-time feedback for screening and diagnostic purposes, ahead of the results from the histopathological examination of tissue specimens acquired during biopsy. Fluorescence and spectroscopic methods have been widely used for disease diagnosis and treatment monitoring, often with image-based representations of these signals to spatially resolve changes in tissue. Portable handheld devices have enabled access to sampling and imaging the water, deoxyhemoglobin, and fat content of breast tissue in humans (Fig. 3B), with clear differences between normal and tumor tissue, as well as differences that predict early on the treatment response after chemotherapy (35, 36). A study involving nine breast cancer patients undergoing chemotherapy showed strong correlations between the functional optical image data and magnetic resonance imaging (MRI) breast density, suggesting that this low-cost and portable optical imaging system could be used at the bedside to assess response to therapy (37).
Coherent optical methods can be used to collect scattering signatures from sub-resolution subcellular structures and, in turn, enable assessment of the local tissue microenvironment and the “field effect” in tissue (38). For colon and esophageal cancers, especially, the field effect could inform diagnosis and treatment options without the need to physically biopsy, retrieve, and process tissue specimens (39, 40). In other procedures in the gastrointestinal tract, a tethered capsule for OCT scanning of the esophagus could help screen patients with Barrett’s esophagus for progression to high-grade dysplasia and adenocarcinoma, with the goal of reducing the need to take random tissue biopsies (Fig. 3C) (41). Compact, battery-powered, low-cost imaging systems, some based on consumer-grade cameras, allow for PoP visualization of cells and tissues acquired from patients to diagnose disease earlier, such as precancer (42–44). These devices have been used for cervical precancer detection in low-resource settings in Botswana (45) and China (43). New peptide-targeted fluorescent dyes have been developed to target esophageal high-grade dysplasia and adenocarcinoma; in vivo studies in 25 patients with Barrett’s esophagus show increased fluorescence in neoplastic regions and suggest that molecular PoP imaging may improve targeted biopsy and early detection (46).
SCREENING VERSUS DIAGNOSTICS
Although the above platforms can be divided into applications for PoC and PoP imaging, it is also possible to consider the roles of these technologies along the spectrum of patient care, namely, screening for disease, diagnosing a disease, and monitoring the treatment or intervention of a disease. Imaging technologies for screening applications are targeted toward use in the general population, which consists primarily of healthy individuals that do not have any obvious signs or symptoms of a disease, who are being seen for preventative health care and wellness visits. Here, the goal is to rapidly and inexpensively identify abnormal findings from normal variants, with less emphasis on identifying precisely what disease may be present. Screening PoC and PoP imaging technologies are most needed at this front line of our health care system because early detection of disease generally results in early treatment and more effective outcomes. In designing early detection programs, it is important to balance the potential benefits of early detection against the potential harms associated with screening. For example, a recent review of breast cancer screening outcomes in Europe noted that for every 1000 women screened biennially between the ages of 50 and 69 years, an estimated 7 to 9 breast cancer deaths are avoided; however, 200 women have at least one false-positive screening result, and 30 of those women undergo an invasive procedure, such as a needle biopsy that yields a negative result (47).
Diagnostic PoC or PoP technologies are used for patients that have a disease, with the need to determine the specific type to select or guide treatment. Although the gold standard for diagnosis is histopathology, PoC/PoP imaging technologies can shift the point of diagnosis into real time, when this information can be used during care and during procedure interventions. Finally, PoC/PoP imaging can be used for treatment monitoring to determine if the therapy is or will be effective, with the potential to save time and costs, and open further options for alternative treatments. For example, new functional and metabolic imaging techniques have shown promise as early predictors of tumor response to therapy, potentially enabling treatment regimens tailored for maximum response and minimal toxicity (48, 49). Diffusion-weighted MRI, dynamic contrast-enhanced MRI, and 18-fluorodeoxyglucose positron emission tomography all have shown promise as early response indicators, but are still at the investigational stage (48). Imaging tumor hypoxia and adaptation to hypoxia also appears predictive of response to therapy (49) and can be measured with optical instrumentation that is suitable for use at the PoP (50).
These example platforms also represent different stages along the path of development, commercialization, and integration. Early-stage technologies are identified as those with engineered and functional systems placed in clinical settings for initial first-to-human or small clinical feasibility studies. Representative early-stage PoC technologies include OCT as a screening and diagnostic technique for primary care applications (24, 25) and the innovative low-cost Foldscope platform for assembling a working microscope (15) (Figs. 2 and 3). Among the early-stage PoP technologies are different optical imaging modalities and methods, including fluorescence (32), spectroscopy (31), and OCT (28, 29) for tumor margin assessment. Mid-stage technologies are identified as those currently involved in larger multi-institutional trials in either low- and high-resource countries. A representative example of a mid-stage PoC technology is the implementation of fluorescence microscopy as a replacement for bright-field microscopy (10). Representative mid-stage PoP technologies include the handheld scanner for spectroscopic imaging of breast tumors and tissue for assessing treatment response (37, 51), as well as the array of optical techniques to evaluate the field effect of carcinogenesis during gastrointestinal imaging procedures (38). Late-stage technologies are those that have been widely evaluated in clinical trials and have been adopted by health care providers and integrated into health care systems. At the PoP, the use of visual examination has been successfully used to detect and enable immediate treatment of cervical precancer in low-resource settings (52), and optical technologies to improve specificity of visual inspection are in early-stage development (43). Currently, despite a large and increasing number of new PoC imaging technologies, none can be considered late-stage. However, as described later, the successful implementation of the rapid HIV test is a non-imaging example for a late-stage PoC technology (53) and one that perhaps establishes a pathway for the imaging-based technologies to follow.
CHANGING THE HEALTH CARE INFRASTRUCTURE
What might the opportunities be if we could redesign our frontline PoC and PoP health care system enterprise from scratch? This opportunity exists in low-resource countries, and lessons learned from such a transformation could be brought back to improve the standard of care—and potentially reduce costs—in high-resource countries. Currently, in high-resource settings, new technologies are almost always incorporated as an addition to the standard of care; so, even though they may improve care, it is often at a higher cost (54). Low-resource settings face the necessity of defining a new standard of care, one where new technologies can be developed to provide the high-quality care for great value (55). Similar to the rapid expansion of cell phone networks in developing countries, which leapfrogged land lines, new technology—or health care models based on systems engineering principles—may leapfrog traditional medical exams, tools, and visits (56).
In both settings, it is important to ensure that screening and diagnostic technologies are implemented at the PoC in ways that maximize their potential benefit (57). Decision analysis provides a tool to examine the health and economic costs of trade-offs in sensitivity and specificity (58). The Bill & Melinda Gates Foundation convened the Global Health Diagnostics Forum to estimate the potential impact of new PoC diagnostic tools for a broad range of infectious diseases (57). Researchers developed a model to estimate the potential impact of new diagnostics for bacterial pneumonia, HIV/AIDS, diarrheal diseases, malaria, tuberculosis, and sexually transmitted infections, and determined the test sensitivity, specificity, and infrastructure requirements necessary to achieve these benefits (57, 58). Results showed that substantial benefits are possible from new diagnostics, and that to achieve maximum benefit, tests should not require clean water, electricity, or trained staff. Improvements in access to new diagnostics were forecast to have greater health impact than improvements in test accuracy (59).
The use of decision analysis to predict health impacts can guide the development and optimization of new imaging tools for use at the PoC and PoP, and can explicitly include health outcomes and costs associated with trade-offs between sensitivity and specificity. For example, a new test for bacterial pneumonia with 95% sensitivity and 85% specificity in a setting with minimal laboratory infrastructure could save an estimated 405,000 lives if accompanied by greater access to treatment (58, 60). The greatest number of lives can be saved if a new test requires only minimal infrastructure. In Africa, a test with 90% sensitivity could save 117,000 lives, but a more sensitive test is required in Asia and Latin America because treatment based on clinical symptoms would instead be directed by a less sensitive test (60). In regions where overuse of antibiotics is common, a test that has 85% specificity could save 253,000 lives by reducing overtreatment. A 1% increase in sensitivity could save an additional 14,000 lives, whereas a 1% increase in specificity could save an additional 8000 lives (60).
Cancer has a rising incidence in low-income countries. Although a noncommunicable disease, there is a distinct challenge in improving cancer diagnosis and care because it is recognized at a late stage owing to the stigmas associated with disease as well as the lack of tools and education for routine screening and diagnostics (61–63). In 2012, 60% of those diagnosed with cancer lived in Africa, Asia, and Central and South America (61). By 2030, the cancer burden in sub-Saharan Africa is expected to increase by more than 85% (62). In most low-income countries, those diagnosed with cancer have advanced disease (62). Cancer care requires an infrastructure that does not exist in many low-income countries. For example, only 24 of 53 African countries report availability of radiation therapy, and in Ethiopia, there are only two radiation machines for a population of more than 60 million people (62).
Early diagnosis is essential to reduce cancer morbidity and mortality in these regions. The first essential step is confirmed histologic diagnosis, but pathology facilities are inadequate in most African settings (7, 62). It has been estimated that countries in sub-Saharan Africa have about 1/10th of the pathology coverage that exists in high-resource settings. Indeed, with the exception of Botswana and South Africa, all countries in the region have less than 1 pathologist per 500,000 people (7). Inadequate access to pathology services can lead to a cycle of ineffective health care practice and gaps in the ability of clinicians to treat patients when treatment would be most effective and least costly, painful, and debilitating (7).
As an example, breast cancer is the most common cancer in women in the world today (excluding nonmelanoma skin cancers), and incidence rates are rising by as much as 5% annually in low-resource settings. The Breast Global Health Initiative, a public-private partnership that aims to improve breast health care for women around the world, has held several consensus meetings to identify breast cancer control issues and implementation strategies for low- and middle-income countries (64). The greatest challenges identified for low-income countries are poor community awareness that breast cancer is treatable, inadequate diagnostic pathology services, and fragmented treatment options, typically involving only surgery or broad chemotherapy (64). In high-resource settings, diagnostic pathology relies on core needle biopsy, but in low-resource settings, needle biopsy is often not available because of costs and lack of pathologists. There is debate about whether fine-needle aspiration cytology could be used as a more affordable alternative, but this also requires access to trained cytologists (64). Confocal fluorescence microscopy of proflavine-stained core needle breast biopsies shows promise to enable real-time assessment of the presence of neoplasia with high accuracy because it can produce images of architectural features in breast tissue comparable with conventional histology, with minimal processing (65).
Finally, twinning partnerships—where health care institutions in high-resource countries offer expertise and mentorship to partner institutions in low-resource countries—have been formed to help improve cancer care (61). Such partnerships could offer a model to connect technology developers in low- and high-resource countries to support the development of appropriate technologies to help provide and improve cancer care in both low- and high-resource settings. One benefit of this strategy is that local researchers are often highly effective conduits to facilitate interactions with local policy-makers (66).
COMMERCIALIZATION PATHS
PoC and PoP imaging technologies, like most technologies in biomedical engineering, are driven forward by goals for translating ideas from “bench to bedside,” where laboratory-based techniques or systems are first used in limited clinical studies to demonstrate preliminary feasibility. Federal agencies such as the National Institutes of Health (NIH) have been instrumental in supporting this translational research, and in particular, the National Institute for Biomedical Imaging and Bioengineering (NIBIB) and the National Cancer Institute (NCI) Cancer Imaging Program have supported many imaging technology– and tool-driven research projects. Although there has been an emphasis on translational research outcomes, there remains a critical need for furthering the clinical integration or adoption of new technologies into our health care systems, both in developed and developing countries.
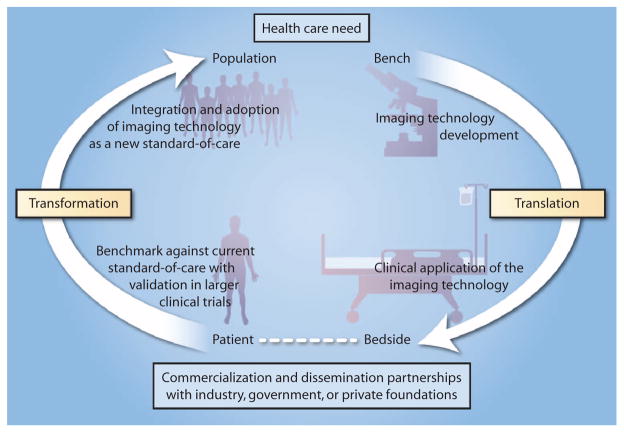
Fundamentally, there is a need for these new technologies not only to be demonstrated in clinical studies but also to change clinical practice or the standard of care for disease screening, diagnostics, and treatment monitoring. As a scientific community, we should consider opportunities for transformational research, or research outcomes that become more widely disseminated and adopted, and transform the standard of care (Fig. 4). Driven first by an identified health care need, imaging technology development is demonstrated and refined at the laboratory bench, then translated toward the patient bedside, or the clinical application that addresses the health care need. We need to consider research projects and programs, however, that do not stop at this stage of patient feasibility, but expand from patient studies to population studies through expanded clinical trials and comparison and validation against current methods or practices. To expand from translational research to transformational research is to develop partnerships with industry, governments, or private foundations to commercialize the technologies developed in academic research settings and facilitate their broader dissemination and use. Successful dissemination, implementation, adoption, and integration of imaging technologies at this final stage would likely result in a new standard of care (Fig. 4).
Fig. 4. Translational research and transformational changes to health care.
Driven by health care needs, PoC and PoP imaging technologies are increasingly being translated from the bench to the patient bedside via technology development and initial clinical application studies. However, these initial translational efforts frequently stall without advancing toward commercialization and wider dissemination for larger populations of patients unless supported and funded by industry, government, and/or private foundations. With such support, transformational changes can occur by first validating the imaging technology against the current standard of care, followed by successfully overcoming the challenges associated with the integration and adoption of the imaging technology as the new standard of care.
Rapid HIV tests are an example of the kind of transformational change that is possible with improved PoC testing (53). The majority of people living with HIV are in sub-Saharan Africa, where health care is accessed largely through centers that lack the capacity to implement early laboratory diagnostic methods based on enzyme-linked immunosorbent assay or Western blot. In the 1990s, lateral flow–based HIV tests were developed; these accurate, inexpensive, easy-to-use tests enabled health center personnel to offer rapid HIV testing. Patients could be immediately triaged for treatment without loss to follow-up (53), and pregnant women could be tested and treated to help prevent transmission from mother to child (67). Today, HIV testing in low-resource settings is often provided by specially trained expert patients or community health workers (53). It is difficult to understate the impact of improved PoC testing on the HIV pandemic; indeed, in the last decade, expanded global access to antiretroviral treatment has reduced the annual global number of deaths due to HIV from 3.2 million in 2001 to 2.5 million in 2011, as well as the annual number of new HIV infections from 1.9 million to 1.7 million during the same period (53).
Although PoC/PoP imaging technologies have yet to achieve such transformational impact, several opportunities show similar promise. For example, proof-of-concept optical and imaging technologies have recently been demonstrated to measure several different components of a complete blood count (CBC), including hemoglobin concentration (68, 69), white blood cell count (12, 18), and differential (12, 70). The CBC is an extremely valuable clinical measurement used to identify anemia, to differentiate between bacterial and viral causes of infection, and to help direct the appropriate use of antibiotics (71). In high-resource settings, a CBC is usually measured by flow cytometry to count cells of different sizes. Alternatively, image analysis techniques are used to count stained cells, using a thin blood smear. Some tools exist to measure components of the CBC at the PoC; however, the per-test costs exceed $1—a price that is not affordable in many low-resource settings (68). PoC technologies in development have the potential to reduce both the hardware cost and the per-test cost by one to two orders of magnitude; if realized, this could transform the practice of primary care in low-resource settings, helping to more effectively diagnose and treat some of the most common causes of child and maternal morbidity and potentially reduce overuse of antibiotics that contribute to the development of drug resistance.
Similarly, new PoC and PoP technologies to improve early detection of cancer could have a transformative effect. More than one-third of Americans will be diagnosed with cancer at some time in their lives, and costs of cancer in the United States exceeded $219 billion in 2008 (72). In the next 20 years, the number of global deaths due to cancer will double (73). Detecting and treating cancer before metastasis markedly improves odds of survival; when detected late, treatment is less effective, has greater morbidity, and is more expensive. Early detection and treatment may be the best, most cost-effective means to improve survival and quality of life. Although improvements in early detection have helped reduce cancer mortality, the full potential of early detection has yet to be realized. Furthermore, cancer patients who are uninsured are more likely to be diagnosed later and have lower survival than patients with insurance (72). At a time when the global incidence of cancer is rapidly increasing and 13% of Americans lack health insurance, there is an urgent need for effective and affordable tools to facilitate early detection and management of cancer. Translating promising PoC and PoP technologies into routine clinical practice will require multicenter, prospective validation trials; commercial approval of cost-effective platforms; and reimbursement strategies that support their adoption.
With the potential to rapidly generate images using these PoC and PoP technologies, and given the shortage or inaccessibility of expert radiologists or pathologists trained to interpret these images for medical decision-making, another transformative effect will be the implementation of automated image interpretation algorithms that could locally identify and flag suspicious features in images or identify image-based indicators of disease. Technologies already in use include the automated, image-based interpretation of Pap smear slides to prescreen slides and identify those for closer examination by a cytopathologist. A study involving 70,522 Pap smears, divided between automated screening using the Thin Prep Imaging System and manual screening, showed similar rates of detection for high-grade lesions (74). Computer-aided detection algorithms are also in use for assisting radiologists in identifying suspicious masses on x-ray mammograms. These were developed because of the high false-positive recall rates that reduce the efficacy of screening mammography, lead to additional invasive biopsy procedures, and increase overall health care costs. By using image feature analysis schemes with artificial neural network classifiers tested on databases of digital mammography images, it is possible to reduce false-positive recall rates (75). Such automated analyses can be supported and run on computer-based platforms or increasingly powerful smartphone computing hardware. The ability to transmit image data across communication networks can facilitate either automated interpretation at remote sites linked to extensive databases of images with known diagnoses or direct interpretation of the transmitted image data by experts around the world (Fig. 2A).
There are several paths toward commercialization and dissemination. Innovation and entrepreneurship have been an established pathway for technology transfer, and in 2012, nearly 100 companies were marketing or developing tools for PoC testing (8). New start-up companies, often founded by faculty, researchers, and students from academic research laboratories, have leveraged investments from venture capital firms, angel investors, larger companies, and U.S. government–funded Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) programs. To help bridge the gap between early-stage technology and commercialization for photonics-based technologies, organizations such as Open Photonics Inc. have introduced new grant programs to show proof of concept, develop prototypes, and move toward commercialization (http://www.open-photonics.com). Given the rapidly developing interest in mobile health platforms and portable PoC/PoP systems, there are for-profit motivations that are driving technological development and commercialization in the space of PoC/PoP imaging technologies in developed countries, which have the potential to shift these commercialized technology to developing countries and low-resource settings.
The use and integration of large-volume, low-cost, consumer-driven electronics and technologies, including smartphones and their communication networks, have fueled technological innovation to use these technologies for new PoC and PoP imaging applications (76, 77). However, much of this same technology is driven by a large consumer user base demanding ever-increasing capabilities and performance, which results in rapid outdating of technologies that may only be a year or two old. This poses problems for the developing world, where technology is adopted more slowly, and more importantly, for researchers trying to adopt these technologies for imaging platforms. Also, given the competitive marketplace in this technology space, there exist many different platforms that may restrict cross-platform compatibility. Therefore, new ideas for establishing a long-term implementation and use model are critically needed if we are to fully leverage the advantages in consumer electronics. Partnerships with major manufacturers of these consumer electronics (Fig. 4), such as cell phone manufacturers, could ensure long-term support for specific product lines that would only facilitate their adoption and long-term use in these PoC/PoP technologies, particularly for those distributed to low-resource global health care settings.
A second route toward commercialization and dissemination is driven by philanthropic support from private foundations (Fig. 4), such as the Bill & Melinda Gates Foundation, the Rockefeller Foundation, the Clinton Foundation, the Lemelson Foundation, and the Doris Duke Charitable Foundation, typically for devices targeting developing countries. Many of these contributions have been directed toward addressing challenges in infectious diseases and women’s and children’s health, with some emphasis on chronic diseases such as obesity, diabetes, heart disease, and cancer. Nevertheless, these foundations do support advances in PoC/PoP imaging technologies, which are inherently adaptable for targeting chronic diseases.
A third route, either separately or in conjunction with the other routes, is via the support of local, regional, and national governments (Fig. 4). This support is essential not only for adoption and integration of PoC/PoP imaging technologies but also for providing the needed infrastructure to use and maintain the new technologies. Needed support is not only financial but also through personnel, facilities, policies, and agencies. For example, the NIBIB has partnered with the Department of Science and Technology in India to leverage resources of the two countries to fund research leading to the development of new low-cost medical technologies, such as those for measuring blood pressure that automatically provide frequent feedback to both patients and health care workers. Collectively, the paths for commercialization and dissemination require a coordination of these different routes, a consensus for improving health and the health care systems, and resources to support these efforts. Development assistance for health, which includes support from public and private funders to improve health in low-resource settings, increased rapidly from $5.8 billion in 1990 to $28.8 billion in 2010 (both in 2011 USD). However, with the global economic crisis in 2010 to 2012, this assistance stagnated. Additional investments of more than $23 billion/year are needed to scale up health technologies and systems to close the gap in the rates of infectious disease–based child and maternal mortality between the best-performing middle-income countries and other low- and middle-income countries by 2035 (78).
CHALLENGES AND POTENTIAL OUTCOMES
In low- and high-resource settings alike, there is an important need to change the way we think about imaging technologies. Rather than relegating imaging to the pathology laboratory or the radiology department, imaging technologies can improve frontline health care, resulting in more rapid and accurate diagnoses and more immediate and appropriate therapies. However, there are challenges to this lofty goal. To be effective at the PoC/PoP, such technologies need to be simple. Incorporating the consumer electronics industry into novel PoC/PoP device development affords the opportunity to leverage millions of dollars of investment to include imaging, processing, and networking capabilities in a single device. However, the rapid evolution of such devices and the use of proprietary image processing algorithms (14) may make it difficult to secure Food and Drug Administration approval for technologies incorporating mobile phones or other consumer-grade technologies. One way to address this challenge is to standardize such platforms. Manufacturers may be motivated to do this because it ensures deeper penetration into remote markets for longer periods.
The technical limitations of optical imaging techniques can be difficult to define because these are often specific to the application and to the value of the information received. In general, an optical imaging platform would be considered successful if the sensitivity and specificity of its measurement added clinical value. However, for many of the early- and mid-stage PoC and PoP technologies reported here, the clinical value for the use of the technology has yet to be determined from larger clinical trials and tracking outcomes. In Frost’s book, Access: How Do Good Health Technologies Get to Poor People in Poor Countries?, three limitations were identified that must be overcome for success (79). The technology must be (i) affordable, so that there are low barriers for purchase or use; (ii) available, so that it can be readily made, approved, and implemented; and (iii) adoptable, so that there is a need and willingness to change current practices or habits. These limitations are likely to be more challenging to overcome than the specific technical challenges that may exist.
Major limitations in infrastructure remain in developing countries. Even technologies that can be properly used by minimally trained personnel and that do not require external power or water cannot improve health in the absence of a functional supply chain for delivery, good training programs, clinical guidelines, and routine maintenance programs to support their effective use (80). There are well-documented challenges associated with sustained implementation of new technologies in low-resource settings (79, 80). Developing a new, PoC/PoP technology–based standard of care requires a base of evidence to understand what models of deployment are most cost-effective and what infrastructure is necessary to support these models. Researchers, funders, and policy-makers must invest additional effort to ensure that evidence-based recommendations are actually implemented at scale in communities with the greatest need (66).
The inherent costs associated with new intellectual property for devices targeted toward use in low-resource communities are often viewed by commercial partners as a challenge to innovation. However, in a recent study exploring research and development investments by country revealed that many of the sectors with growth are emerging economies that will likely exert a major pull for growth in the medical technology industry (81). Given the economic differences between low- and high-resource countries, it is expected that much of the intellectual property interest, protection, and market forces will be driven by high-resource countries, and be profitable in these countries. However, we envision that these technological developments, effectively implemented, would carry over into low-resource countries and communities, and in a cyclical and reciprocal manner, the experiences in low-resource settings would serve to refine the technological solutions for low-cost, portable, user-friendly, and diagnostically useful devices that will improve the quality of health care in high-resource countries. In the long term, sustaining access to a new technology requires that the technology be affordable, that it is available at the point of use, and that payers, providers, and patients are willing to adopt the technology (79). Access is facilitated when technology developers and policy-makers cooperate throughout the early stages of test development and assessment, regulatory approval, and introduction to the health system to ensure that all three conditions are met (79).
Technological breakthroughs are needed to overcome many of the challenges and limitations that we have identified. Advances in imaging resolution, acquisition rates, automated detection and diagnosis algorithms, and hardware configurations will always be welcomed, but none of these would constitute breakthroughs needed for success. In fact, complex, highly engineered systems often exceed basic requirements to visualize cells and tissues at microscopic levels for medical decision-making. Given the large number of technologies that exist, why is it so challenging to put these into clinical practice and change the standard of care? The breakthroughs must come in the practical implementation of our technologies, focusing on what has been called “implementation science” (80). By more effectively understanding the science behind the implementation of these technologies and by reengineering the processes by which these technologies are implemented, we will be able to put optical imaging technologies into global practice at the PoC and PoP to improve early detection and effective treatment of disease in ways that improve both access to care and health outcomes.
Acknowledgments
We thank all of our colleagues working toward these shared goals, and acknowledge all of those individuals we could not cite because of space limitations. We also appreciate the public, private, and industrial support provided for developing PoC and PoP technologies, and for sharing the vision for improving health care globally.
Funding: The NIH, NIBIB R01 EB013723 and R01 EB012479 (to S.A.B.), and NCI R01CA140257 and R01CA103830 (to R.R.-K.).
Footnotes
Competing interests: S.A.B. is cofounder of Diagnostic Photonics and PhotoniCare, which are developing optical imaging technologies for surgical guidance and primary care diagnostics.
REFERENCES AND NOTES
- 1.Taruttis A, Ntziachristos V. Translational optical imaging. AJR Am J Roentgenol. 2012;199:263–271. doi: 10.2214/AJR.11.8431. [DOI] [PubMed] [Google Scholar]
- 2.Moriyama EH, Zheng G, Wilson BC. Optical molecular imaging: From single cell to patient. Clin Pharmacol Ther. 2008;84:267–271. doi: 10.1038/clpt.2008.58. [DOI] [PubMed] [Google Scholar]
- 3.Justman JE, Koblavi-Deme S, Tanuri A, Goldberg A, Gonzalez LF, Gwynn CR. Developing laboratory systems and infrastructure for HIV scale-up: A tool for health systems strengthening in resource-limited settings. J Acquir Immune Defic Syndr. 2009;52(Suppl 1):S30–S33. doi: 10.1097/QAI.0b013e3181bbc9f5. [DOI] [PubMed] [Google Scholar]
- 4.Anscher MS, Jones P, Prosnitz LR, Blackstock W, Hebert M, Reddick R, Tucker A, Dodge R, Leight G, Jr, Iglehart JD, Rosenman J. Local failure and margin status in early-stage breast carcinoma treated with conservation surgery and radiation therapy. Ann Surg. 1993;218:22–28. doi: 10.1097/00000658-199307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pleijhuis RG, Graafland M, de Vries J, Bart J, de Jong JS, van Dam GM. Obtaining adequate surgical margins in breast-conserving therapy for patients with early-stage breast cancer: Current modalities and future directions. Ann Surg Oncol. 2009;16:2717–2730. doi: 10.1245/s10434-009-0609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sridhar AN, Hughes-Hallett A, Mayer EK, Pratt PJ, Edwards PJ, Yang GZ, Darzi AW, Vale JA. Image-guided robotic interventions for prostate cancer. Nat Rev Urol. 2013;10:452–462. doi: 10.1038/nrurol.2013.129. [DOI] [PubMed] [Google Scholar]
- 7.Adesina A, Chumba D, Nelson AM, Orem J, Roberts DJ, Wabinga H, Wilson M, Rebbeck TR. Improvement of pathology in sub-Saharan Africa. Lancet Oncol. 2013;14:e152–e157. doi: 10.1016/S1470-2045(12)70598-3. [DOI] [PubMed] [Google Scholar]
- 8.Drain PK, Hyle EP, Noubary F, Freedberg KA, Wilson D, Bishai WR, Rodriguez W, Bassett IV. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect Dis. 2014;14:239–249. doi: 10.1016/S1473-3099(13)70250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peeling RW, Holmes KK, Mabey D, Ronald A. Rapid tests for sexually transmitted infections (STIs): The way forward. Sex Transm Infect. 2006;82(Suppl 5):v1–v6. doi: 10.1136/sti.2006.024265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albert H, Manabe Y, Lukyamuzi G, Ademun P, Mukkada S, Nyesiga B, Joloba M, Paramasivan CN, Perkins MD. Performance of three LED-based fluorescence microscopy systems for detection of tuberculosis in Uganda. PLOS One. 2010;5:e15206. doi: 10.1371/journal.pone.0015206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller AR, Davis GL, Oden ZM, Razavi MR, Fateh A, Ghazanfari M, Abdolrahimi F, Poorazar S, Sakhaie F, Olsen RJ, Bahrmand AR, Pierce MC, Graviss EA, Richards-Kortum R. Portable, battery-operated, low-cost, bright field and fluorescence microscope. PLOS One. 2010;5:e11890. doi: 10.1371/journal.pone.0011890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majors CE, Pawlowski ME, Tkaczyk T, Richards-Kortum RR. IEEE Point-of-Care Technologies. IEEE; New York, NY: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breslauer DN, Maamari RN, Switz NA, Lam WA, Fletcher DA. Mobile phone based clinical microscopy for global health applications. PLOS One. 2009;4:e6320. doi: 10.1371/journal.pone.0006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skandarajah A, Reber CD, Switz NA, Fletcher DA. Quantitative imaging with a mobile phone microscope. PLOS One. 2014;9:e96906. doi: 10.1371/journal.pone.0096906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cybulski JS, Clements J, Prakash M. Foldscope: Origami-based paper microscope. PLOS One. 2014;9:e98781. doi: 10.1371/journal.pone.0098781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenbaum A, Luo W, Su TW, Göröcs Z, Xue L, Isikman SO, Coskun AF, Mudanyali O, Ozcan A. Imaging without lenses: Achievements and remaining challenges of wide-field on-chip microscopy. Nat Methods. 2012;9:889–895. doi: 10.1038/nmeth.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coskun AF, Ozcan A. Computational imaging, sensing and diagnostics for global health applications. Curr Opin Biotechnol. 2014;25:8–16. doi: 10.1016/j.copbio.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu H, Mavandadi S, Coskun AF, Yaglidere O, Ozcan A. Optofluidic fluorescent imaging cytometry on a cell phone. Anal Chem. 2011;83:6641–6647. doi: 10.1021/ac201587a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee WG, Kim YG, Chung BG, Demirci U, Khademhosseini A. Nano/microfluidics for diagnosis of infectious diseases in developing countries. Adv Drug Deliv Rev. 2010;62:449–457. doi: 10.1016/j.addr.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watkins NN, Hassan U, Damhorst G, Ni H, Vaid A, Rodriguez W, Bashir R. Microfluidic CD4+ and CD8+ T lymphocyte counters for point-of-care HIV diagnostics using whole blood. Sci Transl Med. 2013;5:214ra170. doi: 10.1126/scitranslmed.3006870. [DOI] [PubMed] [Google Scholar]
- 21.Zheng G, Lee SA, Yang S, Yang C. Sub-pixel resolving optofluidic microscope for on-chip cell imaging. Lab Chip. 2010;10:3125–3129. doi: 10.1039/c0lc00213e. [DOI] [PubMed] [Google Scholar]
- 22.Lee SA, Leitao R, Zheng G, Yang S, Rodriguez A, Yang C. Color capable sub-pixel resolving optofluidic microscope and its application to blood cell imaging for malaria diagnosis. PLOS One. 2011;6:e26127. doi: 10.1371/journal.pone.0026127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SA, Erath J, Zheng G, Ou X, Willems P, Eichinger D, Rodriguez A, Yang C. Imaging and identification of waterborne parasites using a chip-scale microscope. PLOS One. 2014;9:e89712. doi: 10.1371/journal.pone.0089712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung W, Kim J, Jeon M, Chaney EJ, Stewart CN, Boppart SA. Handheld optical coherence tomography scanner for primary care diagnostics. IEEE Trans Biomed Eng. 2011;58:741–744. doi: 10.1109/TBME.2010.2096816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shelton RL, Jung W, Sayegh SI, McCormick DT, Kim J, Boppart SA. Optical coherence tomography for advanced screening in the primary care office. J Biophotonics. 2014;7:525–533. doi: 10.1002/jbio.201200243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen CT, Jung W, Kim J, Chaney EJ, Novak M, Stewart CN, Boppart SA. Non-invasive in vivo optical detection of biofilm in the human middle ear. Proc Natl Acad Sci USA. 2012;109:9529–9534. doi: 10.1073/pnas.1201592109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrow M, Jagsi R, Alderman AK, Griggs JJ, Hawley ST, Hamilton AS, Graff JJ, Katz SJ. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. JAMA. 2009;302:1551–1556. doi: 10.1001/jama.2009.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen FT, Zysk AM, Chaney EJ, Kotynek JG, Oliphant UJ, Bellafiore FJ, Rowland KM, Johnson PA, Boppart SA. Intraoperative evaluation of breast tumor margins with optical coherence tomography. Cancer Res. 2009;69:8790–8796. doi: 10.1158/0008-5472.CAN-08-4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen FT, Zysk AM, Chaney EJ, Adie SG, Kotynek JG, Oliphant UJ, Bellafiore FJ, Rowland KM, Johnson PA, Boppart SA. Optical coherence tomography: The intra-operative assessment of lymph nodes in breast cancer. IEEE Eng Med Biol Mag. 2010;29:63–70. doi: 10.1109/MEMB.2009.935722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennedy KM, McLaughlin RA, Kennedy BF, Tien A, Latham B, Saunders CM, Sampson DD. Needle optical coherence elastography for the measurement of microscale mechanical contrast deep within human breast tissues. J Biomed Opt. 2013;18:121510. doi: 10.1117/1.JBO.18.12.121510. [DOI] [PubMed] [Google Scholar]
- 31.Brown JQ, Bydlon TM, Kennedy SA, Caldwell ML, Gallagher JE, Junker M, Wilke LG, Barry WT, Geradts J, Ramanujam N. Optical spectral surveillance of breast tissue landscapes for detection of residual disease in breast tumor margins. PLOS One. 2013;8:e69906. doi: 10.1371/journal.pone.0069906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tummers QR, Verbeek FP, Schaafsma BE, Boonstra MC, van der Vorst JR, Liefers GJ, van de Velde CJ, Frangioni JV, Vahrmeijer AL. Real-time intraoperative detection of breast cancer using near-infrared fluorescence imaging and methylene blue. Eur J Surg Oncol. 2014;40:850–858. doi: 10.1016/j.ejso.2014.02.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urano Y, Sakabe M, Kosaka N, Ogawa M, Mitsunaga M, Asanuma D, Kamiya M, Young MR, Nagano T, Choyke PL, Kobayashi H. Rapid cancer detection by topically spraying a γ-glutamyltranspeptidase–activated fluorescent probe. Sci Transl Med. 2011;3:110ra119. doi: 10.1126/scitranslmed.3002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen CS, Sierra H, Cordova M, Rajadhyaksha M. Confocal microscopy–guided laser ablation for superficial and early nodular basal cell carcinoma: A promising surgical alternative for superficial skin cancers. JAMA Dermatol. 2014 doi: 10.1001/jamadermatol.2013.10225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leproux A, Durkin A, Compton M, Cerussi AE, Gratton E, Tromberg BJ. Assessing tumor contrast in radiographically dense breast tissue using Diffuse Optical Spectroscopic Imaging (DOSI) Breast Cancer Res. 2013;15:R89. doi: 10.1186/bcr3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tromberg BJ, Cerussi A, Shah N, Compton M, Durkin A, Hsiang D, Butler J, Mehta R. Imaging in breast cancer: Diffuse optics in breast cancer: Detecting tumors in pre-menopausal women and monitoring neoadjuvant chemotherapy. Breast Cancer Res. 2005;7:279–285. doi: 10.1186/bcr1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Sullivan TD, Leproux A, Chen JH, Bahri S, Matlock A, Roblyer D, McLaren CE, Chen WP, Cerussi AE, Su MY, Tromberg BJ. Optical imaging correlates with magnetic resonance imaging breast density and reveals composition changes during neoadjuvant chemotherapy. Breast Cancer Res. 2013;15:R14. doi: 10.1186/bcr3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roy HK, Turzhitsky V, Kim Y, Goldberg MJ, Watson P, Rogers JD, Gomes AJ, Kromine A, Brand RE, Jameel M, Bogovejic A, Pradhan P, Backman V. Association between rectal optical signatures and colonic neoplasia: Potential applications for screening. Cancer Res. 2009;69:4476–4483. doi: 10.1158/0008-5472.CAN-08-4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yi J, Radosevich AJ, Stypula-Cyrus Y, Mutyal NN, Azarin SM, Horcher E, Goldberg MJ, Bianchi LK, Bajaj S, Roy HK, Backman V. Spatially resolved optical and ultrastructural properties of colorectal and pancreatic field carcinogenesis observed by inverse spectroscopic optical coherence tomography. J Biomed Opt. 2014;19:36013. doi: 10.1117/1.JBO.19.3.036013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Konda VJ, Cherkezyan L, Subramanian H, Wroblewski K, Damania D, Becker V, Gonzalez MH, Koons A, Goldberg M, Ferguson MK, Waxman I, Roy HK, Backman V. Nanoscale markers of esophageal field carcinogenesis: Potential implications for esophageal cancer screening. Endoscopy. 2013;45:983–988. doi: 10.1055/s-0033-1344617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gora MJ, Sauk JS, Carruth RW, Gallagher KA, Suter MJ, Nishioka NS, Kava LE, Rosenberg M, Bouma BE, Tearney GJ. Tethered capsule endomicroscopy enables less invasive imaging of gastrointestinal tract microstructure. Nat Med. 2013;19:238–240. doi: 10.1038/nm.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pierce M, Yu D, Richards-Kortum R. High-resolution fiber-optic microendoscopy for in situ cellular imaging. J Vis Exp. 2011;2306 doi: 10.3791/2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pierce MC, Guan Y, Quinn MK, Zhang X, Zhang WH, Qiao YL, Castle P, Richards-Kortum R. A pilot study of low-cost, high-resolution microendoscopy as a tool for identifying women with cervical precancer. Cancer Prev Res. 2012;5:1273–1279. doi: 10.1158/1940-6207.CAPR-12-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shin D, Pierce MC, Gillenwater AM, Williams MD, Richards-Kortum RR. A fiber-optic fluorescence microscope using a consumer-grade digital camera for in vivo cellular imaging. PLOS One. 2010;5:e11218. doi: 10.1371/journal.pone.0011218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quinn MK, Bubi TC, Pierce MC, Kayembe MK, Ramogola-Masire D, Richards-Kortum R. High-resolution microendoscopy for the detection of cervical neoplasia in low-resource settings. PLOS One. 2012;7:e44924. doi: 10.1371/journal.pone.0044924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sturm MB, Joshi BP, Lu S, Piraka C, Khondee S, Elmunzer BJ, Kwon RS, Beer DG, Appelman HD, Turgeon DK, Wang TD. Targeted imaging of esophageal neoplasia with a fluorescently labeled peptide: First-in-human results. Sci Transl Med. 2013;5:184ra161. doi: 10.1126/scitranslmed.3004733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paci E, Broeders M, Hofvind S, Puliti D, Duffy SW. EUROSCREEN Working Group, European breast cancer service screening outcomes: A first balance sheet of the benefits and harms. Cancer Epidemiol Biomarkers Prev. 2014;23:1159–1163. doi: 10.1158/1055-9965.EPI-13-0320. [DOI] [PubMed] [Google Scholar]
- 48.Harry VN, Semple SI, Parkin DE, Gilbert FJ. Use of new imaging techniques to predict tumour response to therapy. Lancet Oncol. 2010;11:92–102. doi: 10.1016/S1470-2045(09)70190-1. [DOI] [PubMed] [Google Scholar]
- 49.Kyle SD, Law WP, Miles KA. Predicting tumour response. Cancer Imaging. 2013;13:381–390. doi: 10.1102/1470-7330.2013.9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ueda S, Roblyer D, Cerussi A, Durkin A, Leproux A, Santoro Y, Xu S, O’Sullivan TD, Hsiang D, Mehta R, Butler J, Tromberg BJ. Baseline tumor oxygen saturation correlates with a pathologic complete response in breast cancer patients undergoing neoadjuvant chemotherapy. Cancer Res. 2012;72:4318–4328. doi: 10.1158/0008-5472.CAN-12-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erickson SJ, Martinez SL, DeCerce J, Romero A, Caldera L, Godavarty A. Three-dimensional fluorescence tomography of human breast tissues in vivo using a hand-held optical imager. Phys Med Biol. 2013;58:1563–1579. doi: 10.1088/0031-9155/58/5/1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sankaranarayanan R, Esmy PO, Rajkumar R, Muwonge R, Swaminathan R, Shanthakumari S, Fayette JM, Cherian J. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: A cluster-randomised trial. Lancet. 2007;370:398–406. doi: 10.1016/S0140-6736(07)61195-7. [DOI] [PubMed] [Google Scholar]
- 53.Reid SD, Fidler SJ, Cooke GS. Tracking the progress of HIV: The impact of point-of-care tests on antiretroviral therapy. Clin Epidemiol. 2013;5:387–396. doi: 10.2147/CLEP.S37069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Business Report: A Cure For Health-Care Costs. MIT Technology Review. 2013 Sep; www.technologyreview.com.
- 55.Richards-Kortum R, Oden M. Engineering. Devices for low-resource health care. Science. 2013;342:1055–1057. doi: 10.1126/science.1243473. [DOI] [PubMed] [Google Scholar]
- 56.Sinha SR, Barry M. Health technologies and innovation in the global health arena. N Engl J Med. 2011;365:779–782. doi: 10.1056/NEJMp1108040. [DOI] [PubMed] [Google Scholar]
- 57.Hay Burgess DC, Wasserman J, Dahl CA. Global health diagnostics. Nature. 2006;444(Suppl 1):1–2. doi: 10.1038/nature05440. [DOI] [PubMed] [Google Scholar]
- 58.Girosi F, Olmsted SS, Keeler E, Hay Burgess DC, Lim YW, Aledort JE, Rafael ME, Ricci KA, Boer R, Hilborne L, Derose KP, Shea MV, Beighley CM, Dahl CA, Wasserman J. Developing and interpreting models to improve diagnostics in developing countries. Nature. 2006;444(Suppl 1):3–8. doi: 10.1038/nature05441. [DOI] [PubMed] [Google Scholar]
- 59.Urdea M, Penny LA, Olmsted SS, Giovanni MY, Kaspar P, Shepherd A, Wilson P, Dahl CA, Buchsbaum S, Moeller G, Hay Burgess DC. Requirements for high impact diagnostics in the developing world. Nature. 2006;444(Suppl 1):73–79. doi: 10.1038/nature05448. [DOI] [PubMed] [Google Scholar]
- 60.Lim YW, Steinhoff M, Girosi F, Holtzman D, Campbell H, Boer R, Black R, Mulholland K. Reducing the global burden of acute lower respiratory infections in children: The contribution of new diagnostics. Nature. 2006;444(Suppl 1):9–18. doi: 10.1038/nature05442. [DOI] [PubMed] [Google Scholar]
- 61.Bender E. Developing world: Global warning. Nature. 2014;509:S64–S65. doi: 10.1038/509S64a. [DOI] [PubMed] [Google Scholar]
- 62.Morhason-Bello IO, Odedina F, Rebbeck TR, Harford J, Dangou JM, Denny L, Adewole IF. Challenges and opportunities in cancer control in Africa: A perspective from the African Organisation for Research and Training in Cancer. Lancet Oncol. 2013;14:e142–e151. doi: 10.1016/S1470-2045(12)70482-5. [DOI] [PubMed] [Google Scholar]
- 63.Varmus H, Trimble EL. Integrating cancer control into global health. Sci Transl Med. 2011;3:101cm128. doi: 10.1126/scitranslmed.3002321. [DOI] [PubMed] [Google Scholar]
- 64.Anderson BO, Cazap E, El Saghir NS, Yip CH, Khaled HM, Otero IV, Adebamowo CA, Badwe RA, Harford JB. Optimisation of breast cancer management in low-resource and middle-resource countries: Executive summary of the Breast Health Global Initiative consensus, 2010. Lancet Oncol. 2011;12:387–398. doi: 10.1016/S1470-2045(11)70031-6. [DOI] [PubMed] [Google Scholar]
- 65.Dobbs JL, Ding H, Benveniste AP, Kuerer HM, Krishnamurthy S, Yang W, Richards-Kortum R. Feasibility of confocal fluorescence microscopy for real-time evaluation of neoplasia in fresh human breast tissue. J Biomed Opt. 2013;18:106016. doi: 10.1117/1.JBO.18.10.106016. [DOI] [PubMed] [Google Scholar]
- 66.Nabyonga J, Orem J. From knowledge to policy: Lessons from Africa. Sci Transl Med. 2014;6:240ed213. doi: 10.1126/scitranslmed.3008852. [DOI] [PubMed] [Google Scholar]
- 67.Pai NP, Tulsky JP, Cohan D, Colford JM, Jr, Reingold AL. Rapid point-of-care HIV testing in pregnant women: A systematic review and meta-analysis. Trop Med Int Health. 2007;12:162–173. doi: 10.1111/j.1365-3156.2006.01812.x. [DOI] [PubMed] [Google Scholar]
- 68.Bond M, Elguea C, Yan JS, Pawlowski M, Williams J, Wahed A, Oden M, Tkaczyk TS, Richards-Kortum R. Chromatography paper as a low-cost medium for accurate spectro-photometric assessment of blood hemoglobin concentration. Lab Chip. 2013;13:2381–2388. doi: 10.1039/c3lc40908b. [DOI] [PubMed] [Google Scholar]
- 69.Yang X, Piety NZ, Vignes SM, Benton MS, Kanter J, Shevkoplyas SS. Simple paper-based test for measuring blood hemoglobin concentration in resource-limited settings. Clin Chem. 2013;59:1506–1513. doi: 10.1373/clinchem.2013.204701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith ZJ, Gao T, Chu K, Lane SM, Matthews DL, Dwyre DM, Hood J, Tatsukawa K, Heifetz L, Wachsmann-Hogiu S. Single-step preparation and image-based counting of minute volumes of human blood. Lab Chip. 2014;14:3029–3036. doi: 10.1039/c4lc00567h. [DOI] [PubMed] [Google Scholar]
- 71.George-Gay B, Parker K. Understanding the complete blood count with differential. J Perianesth Nurs. 2003;18:96–114. doi: 10.1053/jpan.2003.50013. quiz 115–117. [DOI] [PubMed] [Google Scholar]
- 72.American Cancer Society. Cancer Facts & Figures. American Cancer Society; Atlanta: 2008. [Google Scholar]
- 73.Boyle P, Levin B. World Cancer Report. IARC; Lyon, France: 2008. [Google Scholar]
- 74.Koltz BR, Russell DK, Lu N, Bonfiglio TA, Varghese S. Effect of Thin Prep® imaging system on laboratory rate and relative sensitivity of atypical squamous cells, high-grade squamous intraepithelial lesion not excluded and high-grade squamous intraepithelial lesion interpretations. Cytojournal. 2013;10:6. doi: 10.4103/1742-6413.109720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tan M, Pu J, Zheng B. Reduction of false-positive recalls using a computerized mammographic image feature analysis scheme. Phys Med Biol. 2014;59:4357–4373. doi: 10.1088/0031-9155/59/15/4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Glynn LG, Hayes PS, Casey M, Glynn F, Alvarez-Iglesias A, Newell J, Ólaighin G, Heaney D, Murphy AW. SMART MOVE—A smartphone-based intervention to promote physical activity in primary care: Study protocol for a randomized controlled trial. Trials. 2013;14:157. doi: 10.1186/1745-6215-14-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boulos MN, Wheeler S, Tavares C, Jones R. How smartphones are changing the face of mobile and participatory healthcare: An overview, with example from eCAALYX. Biomed Eng Online. 2011;10:24. doi: 10.1186/1475-925X-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jamison DT, Summers LH, Alleyne G, Arrow KJ, Berkley S, Binagwaho A, Bustreo F, Evans D, Feachem RG, Frenk J, Ghosh G, Goldie SJ, Guo Y, Gupta S, Horton R, Kruk ME, Mahmoud A, Mohohlo LK, Ncube M, Pablos-Mendez A, Reddy KS, Saxenian H, Soucat A, Ulltveit-Moe KH, Yamey G. Global health 2035: A world converging within a generation. Lancet. 2013;382:1898–1955. doi: 10.1016/S0140-6736(13)62105-4. [DOI] [PubMed] [Google Scholar]
- 79.Frost LJ, Reich MR. Access: How Do Good Health Technologies Get to Poor People in Poor Countries? Harvard Center for Population and Development Studies; Cambridge, MA: 2008. [Google Scholar]
- 80.Madon T, Hofman KJ, Kupfer L, Glass RI. Public health. Implementation science. Science. 2007;318:1728–1729. doi: 10.1126/science.1150009. [DOI] [PubMed] [Google Scholar]
- 81.Chakma J, Sun GH, Steinberg JD, Sammut SM, Jagsi R. Asia’s ascent—Global trends in biomedical R&D expenditures. N Engl J Med. 2014;370:3–6. doi: 10.1056/NEJMp1311068. [DOI] [PubMed] [Google Scholar]