Abstract
Background
Emerging research has identified the endothelin (ET)-1 pathway as a potential target for novel renoprotective therapies. We recently showed that selective ET-A receptor antagonism in chronic renovascular disease (RVD) improves renal function and reduces renal injury. Although ET-A and -B have opposing roles, in some clinical situations they may induce similar effects. Thus, we hypothesized that simultaneous blockade of the ET-A and -B receptors would protect the kidney during RVD.
Methods
Unilateral RVD was induced in pigs. After 6 weeks, single-kidney function was quantified in vivo using multi-detector computer tomography. Pigs were subsequently divided into untreated (RVD, n = 7) or daily-treated with the dual ET-A/B receptor antagonist macitentan (RVD + macitentan, n = 6) for 4 weeks. At 10 weeks, in vivo studies were repeated, then pigs were euthanized and ex vivo studies performed in the stenotic kidney to quantify inflammation, fibrosis, microvascular density and remodeling.
Results
Four weeks of macitentan therapy modestly improved renal blood flow (29%, P = 0.06 versus pre-treatment) and showed protective effects on the renal parenchyma by attenuating inflammation and glomerulosclerosis, reducing apoptosis and tubular casts and improving albuminuria and cortical microvessel density. No overt adverse effects were observed.
Conclusion
Possibly by inducing a pro-survival renal microenvironment, macitentan increased renal microvascular density, promoted cell survival and decreased injury, which in turn improved stenotic kidney hemodynamics in our model. Our results further support the safety of using macitentan in patients with concomitant chronic renal disease and supported the feasibility of a new strategy that may preserve the stenotic kidney in RVD.
Keywords: endothelin, imaging microcirculation, inflammation, renal hemodynamics, renovascular disease
INTRODUCTION
Chronic renovascular disease (RVD) is a major illness with a distinctly higher incidence in patients over 65 years old [1–3]. Renal artery stenosis (RAS) is the main cause of RVD. The role of RAS in RVD, hypertension and renal injury has been extensively studied following the foundational work performed by Goldblatt et al. [4], placing major efforts on understanding the mechanisms of progressive renal injury and development of treatments to recover the kidney.
Using a large clinically relevant swine model of chronic RVD that closely mimics the human condition, we showed that loss of function in the stenotic kidney, disclosed by significant reductions in renal blood flow (RBF), glomerular filtration rate (GFR) and later renal perfusion [5, 6], is progressive but partly reversible. Such progressive deterioration associates with a progressive decrease in cortical and medullary microvascular (MV) density and function and significant MV remodeling, accompanied by marked inflammation and fibrosis [7–10]. Ample experimental [11–15] and clinical evidence [16–18] showed that renal MV damage is a common feature in renal disease, supporting a role in the progressive nature of renal damage in RVD. Thus, part of our previous studies has focused on determining the feasibility of targeting the renal microcirculation as a therapeutic attempt to recover renal function [5, 6, 10].
Endothelin (ET)-1 is a potent vasoconstrictor and mitogenic peptide, which operates via two receptors, ET-A and ET-B, through a system of autocrine and paracrine signaling cascades [19, 20]. ET receptors are abundant in the kidney and widely distributed throughout the vascular, tubular and glomerular compartments, playing important roles in controlling blood pressure and renal function. Activation of the ET-A receptor promotes vasoconstriction and cell proliferation whereas activation of the ET-B receptor stimulates the generation and release of nitric oxide (NO), vasodilatation and clearance of circulating ET-1. Both receptors work in a tight balance and their deregulation may contribute to the development of pathological states. We have shown that the ET-1/ET-A pathway is up-regulated and contributes to renal pathophysiology in early atherosclerosis and chronic RVD [21–24], but such pathological changes in kidney function and damage can be largely prevented or reversed by selective ET-A receptor blockade, with distinct protective effects on the renal microvasculature [23–25].
While we [21–24] and others [26–28] have demonstrated the beneficial effects of ET-A blockade, we have also recently showed that single ET-B receptor antagonism did not confer renal protection in RVD [23]. This is of clinical interest since two (bosentan and macitentan) of the three (ambrisentan) ET receptor antagonists currently approved by the Food and Drug Administration (FDA) inhibit both ET-A and -B receptors. Macitentan is a novel dual ET-A and -B receptor antagonist that was shown to reduce morbidity and mortality in patients with pulmonary arterial hypertension [29]. Notably, although ET-A and -B receptors seem to have specific and likely opposing roles, in some pathological situations they may put forth similar detrimental effects [30] as some unforeseen synergistic effects may occur in the kidney by silencing both receptors at the same time [31]. Whether this is the case during the progression of RVD has not been investigated. Thus, this study aims to extend our previous studies [21, 23, 24] to unravel a potential therapeutic strategy for RVD. We tested the hypothesis that dual blockade of the ET-A and -B receptors using macitentan may protect the kidney in chronic RVD.
MATERIALS AND METHODS
The procedures and protocols of this study were approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center. Twenty female pre-juvenile (6 months old) domestic pigs (sus scrofa domestica) were used in this study. In 13 of them, unilateral RAS was induced by the implantation of a copper coil inside the main renal artery. We have shown that this intervention induces a gradual significant vascular stenosis within 4–6 weeks that leads to a progressive deterioration of renal function, renal damage and hypertension [7, 10]. Blood pressure was continuously measured in free moving animals over the 10 weeks of the study using telemetry (PhysioTel, Data Sciences International), every 5 min and averaged for each 24-hour period, as described [7, 10, 32].
Six weeks following the placement of the coil, all pigs were anesthetized with an intra-muscular injection of telazol (5 mg/kg) and xylazine (2 mg/kg), intubated and mechanically ventilated on room air. A mixture of ketamine (0.2 mg/kg/min) and xylazine (0.3 /mg/kg/min) was continuously administered IV to maintain anesthesia. The degree of stenosis was quantified via renal angiography as previously described [33]. A catheter was then placed in the superior vena cava and in vivo helical multi-detector computer tomography (MDCT) flow studies were performed to quantify single-kidney RBF, GFR and regional perfusion of the stenotic kidney, as previously described and validated [7, 9, 34].
Following the 6-week MDCT studies, the RVD pigs were divided into two groups: placebo (RVD, n = 7) and those treated daily with the specific dual ET-A and ET-B receptor antagonist macitentan (7.5 mg/day, RVD + macitentan, n = 6) for 4 weeks. Macitentan has been shown to be effective in a daily dose range of 1–30 mg [35]. The dose was selected after completing a pilot study in the swine model that shows renal effects without decreasing blood pressure, with the ultimate goal of avoiding confounding effects on renoprotection. The primary circulating metabolites are the active ACT-132577 and the inactive ACT-373898 [35]. The half-life of the circulating active ACT-132557 metabolite has been shown to be between 40 and 66 h following a single dose [36]. Few studies have examined the pharmacokinetics of macitentan in patients with renal disease, but Sidharta et al. demonstrated that severe renal impairment did not significantly affect exposure to circulating macitentan or its active metabolites [35]. Additional animals were used as normal controls (n = 7).
At 10 weeks, the MDCT in vivo studies were repeated and compared with the pre-treatment measurements obtained at 6 weeks. Renal vascular resistance was calculated at the 6- and 10-week time-point as previously described [5]. Plasma renin activity (PRA), serum creatinine (SCr) and plasma ET-1 were measured via enzyme-linked immunosorbent assay (ELISA, R&D Systems, Minneapolis, MN) in blood samples collected from the inferior vena cava and stenotic renal vein at 6 and 10 weeks. Urine samples were also collected during the 6- and 10-week in vivo studies for quantification of albuminuria using ELISA (Alpha Diagnostic, San Antonio, TX) according to manufacturer's instructions.
After completion of in vivo studies, pigs were allowed to recover for 48–72 h and then euthanized by an intravenous injection of sodium pentobarbital (100 mg/kg). The kidneys were harvested and immersed in heparinized saline (10 units/mL). One portion of the harvested kidneys was snap frozen in liquid nitrogen and stored at −80°C for protein expression and concentration analysis. One portion was fixed in 10% formalin for histological analysis of renal morphology and fibrosis using Masson's trichrome and hematoxylin and eosin (H&E) staining protocols. The remaining portion was perfused (Syringe Infusion Pump 22; Harvard Apparatus, Holliston, MA) with a contrast agent (Microfil MV122; Flow Tech, Inc., Carver, MA) for Micro-CT analysis, as described [10].
CT analysis
In vivo MDCT
Regions of interest (aorta, renal cortex, medulla and papilla) in the MDCT images were manually traced and time-density curves were generated. The area under each curve segment as well as the first moment of the curve was calculated using curve-fitting parameters. These values were used to calculate in vivo single-kidney RBF (mL/min), GFR (mL/min) and renal perfusion (mL/min/cc tissue), as extensively described and validated [7, 9, 34].
Ex vivo micro-CT
Microfil-perfused lobes of the stenotic kidney were scanned at 0.3° increments using a micro-CT scanner and reconstructed with a resolution of 9 µm for analysis as previously described [10]. Micro-CT images were analyzed using the Analyze Software (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN). Briefly, the cortex and medulla of the images were tomographically divided and the spatial density and distribution of the microvessels (diameter <200 µm) were calculated as previously described [8, 10].
Protein expression analysis
Western blot on renal tissue homogenates were performed following standard procedures, as previously described [33], using specific polyclonal antibodies against pro-angiogenic vascular endothelial growth factor (VEGF), phosphorylated (p)-Akt and angiopoietin (Ang)-1; anti-angiogenic endostatin and angiostatin; markers of MV permeability and remodeling such as plasminogen (Plg) and tissue transglutaminase (tTg); pro-inflammatory nuclear factor kappa (NFκ)B, tumor necrosis factor (TNF)-α, monocyte chemoattractant protein 1 (MCP-1) and interleukins 6 (IL-6), 10 (IL-10) and 12 (IL-12); pro-fibrotic transforming growth factor (TGF)-β, its specific mediator smad-4; matrix metalloproteinase 2 (MMP-2) and its inhibitor, tissue inhibitor of metalloproteinases 1 (TIMP-1); and cell survival factors such as proliferating cell nuclear antigen (p-PCNA), Caspase 3 and Caspase 8. β-actin was used as the loading control (Sigma, St Louis, MO, 1:500). Protein expression was calculated using densitometry and the average expression was determined for each group and expressed as a ratio relative to the renal expression of β-actin, as previously described [33].
Histological analysis
Mid-hilar, 5 µm kidney cross sections (1 per animal) were prepared and examined. Renal inflammation was quantified by counts of F4/80+ cells performed in selected fields following previously published protocols as a guide [37]. Briefly, 12 fields (6 random fields and 6 fields with at least one positive cell per slide, one slide per animal) were selected, cells counted and results were represented as the average count of positive cells found in all the fields. Masson's trichrome staining was quantified using a semi-automatically quantified method in 15–20 fields using a computer-aided image analysis system (NIS Element 3.0; Nikon Instruments, Melville, NY) and was reported as a mean percentage of total staining surface area over all fields per slide. In addition, renal immunoreactivity against IL-6, IL-10 and IL-12 (Abcam, Cambridge, MA, 1–50 for all) was also investigated in renal cross sections following standard procedures for immunohistochemistry. Glomerular scores were determined as previously described [10, 33] and expressed as the number of sclerotic glomeruli out of 100 counted glomeruli. Apoptosis scores were quantified as the fraction of apoptotic cells in 10 randomly selected fields per slide, per animal, as described [37].
Statistical analysis
Statistical analysis was performed using a paired Student's t-test for intragroup comparisons, while one-way ANOVA with Bonferroni correction for multiple comparisons was employed for intergroup evaluations. P values ≤0.05 are reported as significant. Results were displayed as the mean ± SEM.
RESULTS
General characteristics
The body weights were slightly lower in RVD pigs at 6 weeks (Table 1), but similar in all pigs at 10 weeks (Table 2). The degree of stenosis, hypertension and renal vascular resistance were similar in untreated RVD and RVD + macitentan groups at both time points (Tables 1 and 2). PRA was similar among the groups, whereas SCr was similarly elevated in RVD and RVD + macitentan (Tables 1 and 2). On the other hand, circulating ET-1 was higher in RVD + macitentan compared with the rest of the groups (Table 2), suggesting effective ET-B blockade [38]. Albuminuria was similarly elevated in all RVD animals at 6 weeks, remained elevated after 10 weeks in untreated RVD, but decreased to normal levels after macitentan (Tables 1 and 2).
Table 1.
Body weight, mean arterial pressure, degree of stenosis, SCr and basal parameters of single-kidney hemodynamics and function (mean ±SEM), after 6 weeks of RVD but before treatment with macitentan in normal, RVD and RVD pigs before macitentan treatment (RVD +macitentan)
| Parameter | Normal, n = 7 | RVD, n = 7 | RVD before macitentan, n = 6 |
|---|---|---|---|
| Body weight (kg) | 53.3 ± 1.7 | 45.7 ± 1.5* | 45 ± 4.2 |
| Mean arterial pressure (mmHg) | 100.8 ± 7.2 | 151.2 ± 10.7* | 147.4 ± 10.9* |
| Degree of stenosis (%) | 0.00 | 72.7 ± 6.8 | 71.2 ± 8.3 |
| SCr (μmol/L) | 74.7 ± 4.8 | 94.1 ± 7.78* | 95 ± 8.3* |
| Albuminuria (μg/mL) | 2.50 ± 1.21 | 134.68 ± 46.4* | 104.81 ± 60.5* |
| Renal vascular resistance (mmHg/mL/min) | 0.17 ± 0.02 | 0.58 ± 0.17* | 0.61 ± 0.2* |
| Renal volume (cc) | |||
| Cortex | 123.3 ± 7.1 | 62.6 ± 6.2* | 67.4 ± 3.9* |
| Medulla | 35.1 ± 2.5 | 18.4 ± 2.8* | 17.9 ± 2.9* |
| RBF (mL/min) | 589.9 ± 32.5 | 260.8 ± 59.9* | 242.9 ± 41.8* |
| Perfusion (mL/min/cc) | |||
| Cortex | 4.5 ± 0.7 | 3.2 ± 0.6 | 3.1 ± 0.5 |
| Medulla | 2.3 ± 0.4 | 2.4 ± 0.6 | 1.7 ± 0.4 |
| GFR (mL/min) | 65.9 ± 9.0 | 34.8 ± 8.1* | 42.3 ± 4.4* |
*P ≤ 0.05 versus normal.
Table 2.
Body weight, mean arterial pressure, degree of stenosis, plasmin rennin activity, circulating endothelin-1, serum creatinine and basal parameters of single-kidney hemodynamics and functions (mean ± SEM) after 10 weeks of RVD, for normal, RVD and RVD pigs after 4 weeks of macitentan treatment (RVD + macitentan)
| Parameter | Normal, n = 7 | RVD, n = 7 | RVD + macitentan, n = 6 |
|---|---|---|---|
| Body weight (kg) | 52.6 ± 2.5 | 54.6 ± 3.5 | 52.8 ± 3.8 |
| Mean arterial pressure (mmHg) | 101.4 ± 5.5 | 145.1 ± 10.4 | 147.2 ± 13.6 |
| Degree of stenosis (%) | 0.00 | 72.5 ± 8.1 | 77.9 ± 6.3 |
| PRA (ng/mL/h) | 0.19 ± 0.01 | 0.22 ± 0.04 | 0.21 ± 0.03 |
| ET-1 (pg/mL) | 0.64 ± 0.01 | 0.83 ± 0.08* | 1.1 ± 0.03*,† |
| SCr (μmol/L) | 88.5 ± 14.8 | 125.2 ± 4.2* | 121.0 ± 22.3* |
| Albuminuria (μg/mL) | 2.98 ± 1.12 | 130.2 ± 48.6 | 4.46 ± 4.24†,‡ |
| Renal vascular resistance (mmHg/mL/min) | 0.19 ± 0.02 | 0.49 ± 0.1* | 0.46 ± 0.2* |
| Renal volume (cc) | |||
| Cortex | 110.9 ± 4.78 | 64.7 ± 9.9* | 84.7 ± 4.3*,† |
| Medulla | 33.7 ± 0.8 | 19.2 ± 4.5* | 26.3 ± 3.7* |
| RBF (mL/min) | 530.5 ± 26.6 | 291.7 ± 74.7* | 313.8 ± 36.4*,^ |
| Perfusion (mL/min/cc) | |||
| Cortex | 4.3 ± 0.3 | 3.6 ± 0.7 | 3.3 ± 0.4 |
| Medulla | 1.7 ± 0.3 | 1.8 ± 0.6 | 1.6 ± 0.2 |
| GFR (mL/min) | 73.8 ± 6.4 | 44.3 ± 6.8* | 40.0 ± 6.4* |
*P < 0.05 versus normal.
†P < 0.05 versus RVD.
‡P < 0.05 versus 6 weeks.
^P = 0.06 versus 6 weeks.
Single-kidney hemodynamics and function
Following 6 weeks of RVD, pigs showed a significant reduction in MDCT-derived stenotic kidney RBF and GFR compared with normal animals (P < 0.05) at 6 and 10 weeks (Tables 1 and 2). Macitentan treatment showed a trend toward an increase in RBF compared with 6 weeks pre-treatment values (29% increase, P = 0.06; Tables 1 and 2), although GFR remained unchanged (Table 2).
Renal MV density and proliferation
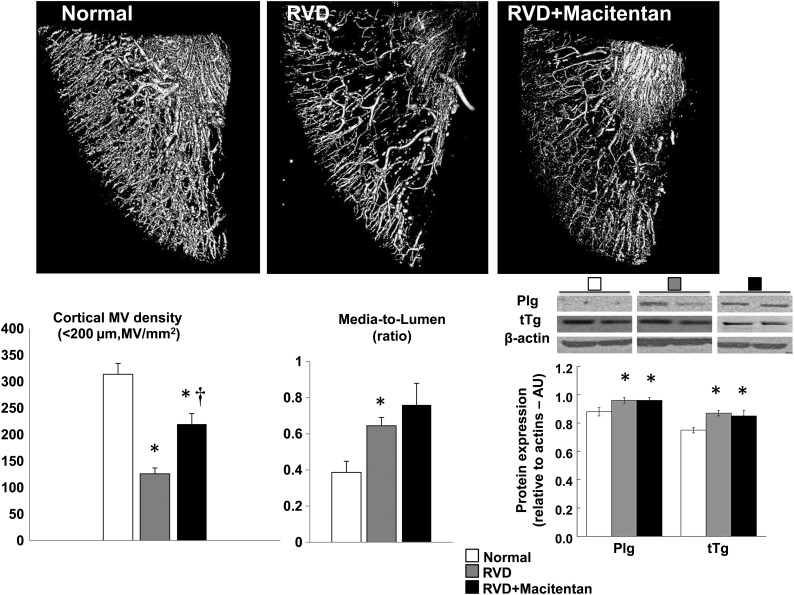
RVD induced a significant decrease in MV density of the stenotic kidney (microvessels <200 µm in diameter) compared with normal controls, accompanied by an increased media-to-lumen ratio (Figure 1). Treatment with macitentan significantly improved (albeit did not normalize) cortical MV density RVD (Figure 1), whereas medullary MV density remained attenuated (P = NS versus RVD, data not shown). However, media-to-lumen ratio, and renal expression of Plg and tTg remained unchanged after macitentan therapy, suggesting persistence of some MV damage in the stenotic kidney (Figure 1).
FIGURE 1:
Representative 3D micro-CT reconstruction (top) and quantification (bottom-left) of the cortical microvascular (MV) density (<200 µm in diameter), renal MV media-to-lumen ratio (bottom-middle) and renal expression of plasminogen (Plg) and tissue-transglutaminase (tTg) by western blot (bottom-right) from stenotic kidneys of normal, RVD and RVD + macitentan-treated animals at 10 weeks. Macitentan improved cortical MV density but not MV media-to-lumen ratio or the renal expression of Plg and tTg, indicating persistence of some MV damage in the stenotic kidney (*P < 0.05 versus normal, †P < 0.05 versus RVD).
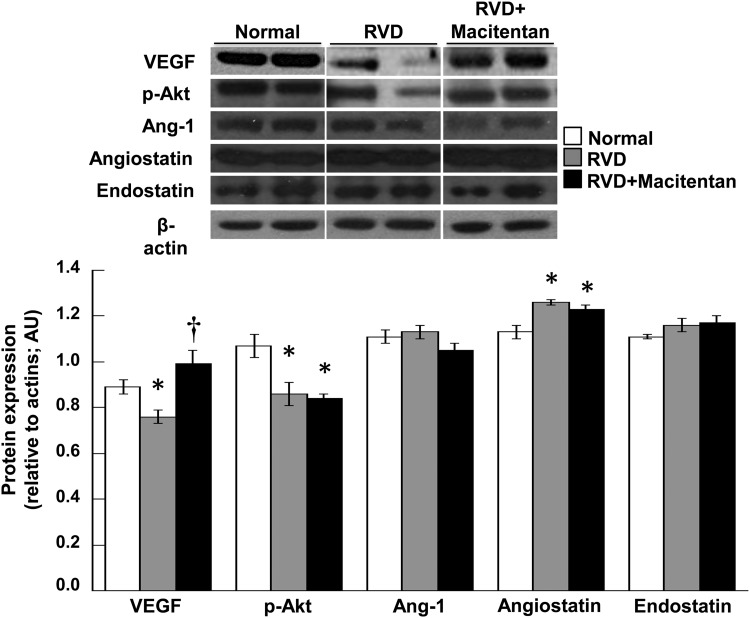
Angiogenic factors
The stenotic kidney exhibited a decreased expression in VEGF and p-Akt, an increased expression angiostatin compared with normal kidneys (Figure 2). Four weeks of macitentan significantly improved the expression of VEGF in the stenotic kidney. However, renal expression of downstream mediators of VEGF such as p-Akt and Ang-1, or anti-angiogenic angiostatin and endostatin were not significantly modified after macitentan therapy (Figure 2).
FIGURE 2:
Renal protein expression (top, n = 4–5 per group, two representative bands per animal shown) and average quantification per group (bottom) of VEGF, p-Akt, Ang-1, angiostatin and endostatin in normal, RVD and RVD + macitentan pigs. Macitentan partly improved the angiogenic cascade in the stenotic kidney after 4 weeks of treatment (*P < 0.05 versus normal, †P < 0.05 versus RVD).
Renal inflammation
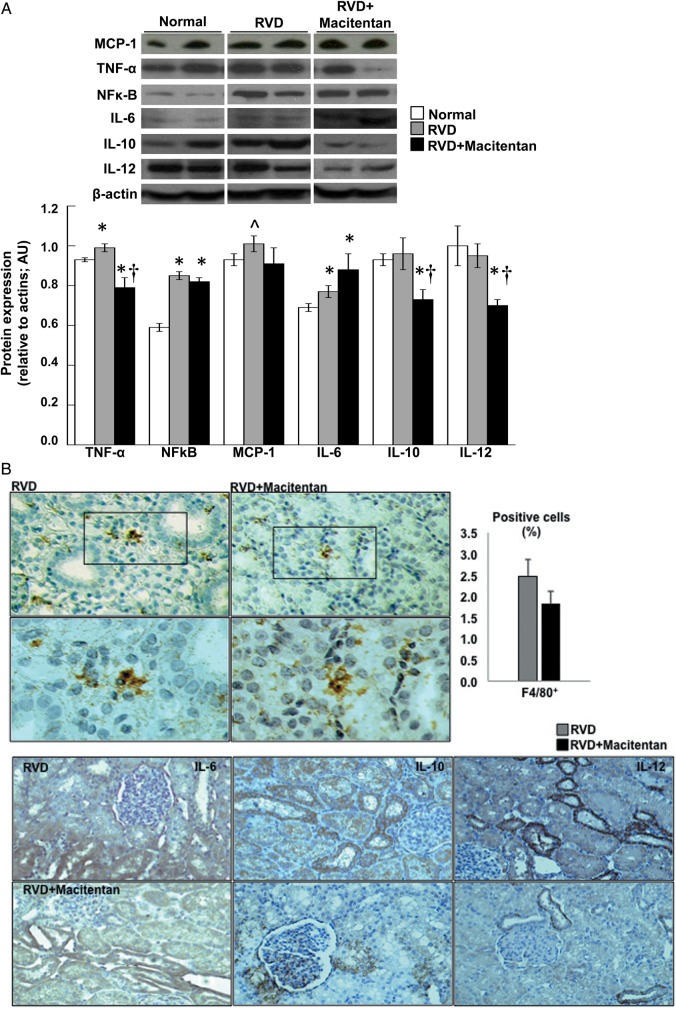
Inflammatory factors
The stenotic kidney exhibited significant increases in the expression of TNF-α, and NFκ-β indicating an augmented inflammatory response (Figure 3A). However, macitentan reduced renal expression of TNF-α but not NFκ-β. While a significant increase in the renal expression (mainly tubular) of IL-6 was observed, IL-10 and -12 were not elevated in the stenotic kidney (Figure 3A and B). Macitentan treatment did not decrease IL-6 but reduced the protein expression of IL-10 and IL-12 compared with RVD, suggesting a potential reduction in renal inflammatory activity.
FIGURE 3:
(A) Renal protein expression (n = 4–5 per group, two representative bands per animal shown) and average quantification per group (bar graphs) for tumor necrosis factor alpha (TNF-α), nuclear factor kappa (NFκ)-B, monocyte-chemoattractant protein (MCP)-1, interleukin 6 (IL-6), IL-10, and IL-12 in normal, RVD and RVD + macitentan kidneys. (B) (Top) Representative histological slides of stenotic kidneys of RVD and RVD + macitentan showing positive staining for F4/80+ cells at both ×20 and ×40 magnification (right, top and bottom rows, respectively) and average histological counts (left) of F4/80+ cells. (Bottom) Representative pictures (×20) showing immunoreactivity against IL-6, IL-10 and IL-12 from stenotic kidneys, untreated and treated with macitentan. Treatment with macitentan for 4 weeks attenuated inflammation in the stenotic kidney (*P < 0.05 versus normal, †P < 0.05 versus RVD, ^P=0.07 versus normal).
Macrophage infiltration
The expression of MCP-1 was slightly but not significantly elevated only in RVD animals (P = 0.07 versus normal, Figure 3A), accompanied by inflammatory infiltration of macrophages (F4/80+ cells), mainly evident at the tubule-interstitial compartments (Figure 3B). In line with the IL-10 and IL-12 expression data, treatment with macitentan led to a slight (albeit not significant) reduction of macrophage infiltration.
Renal morphology
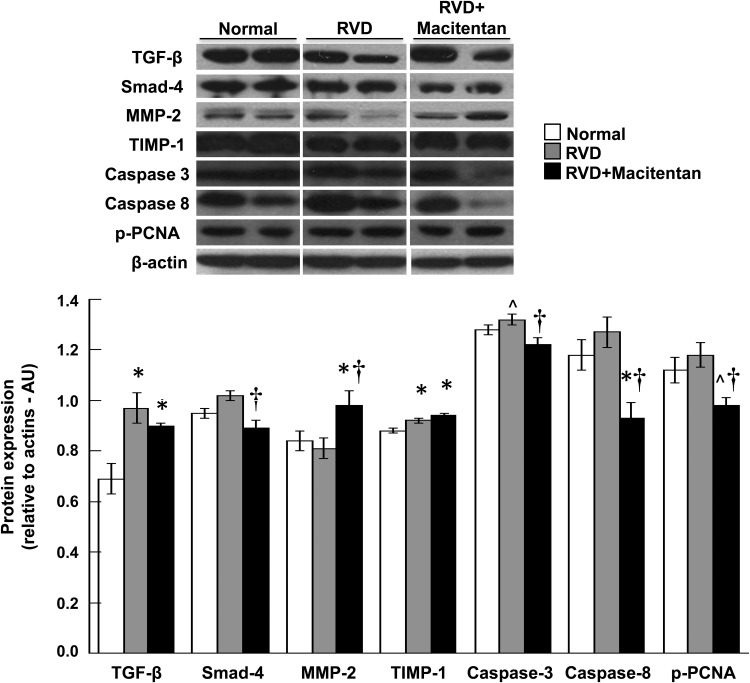
Fibrosis and cell survival
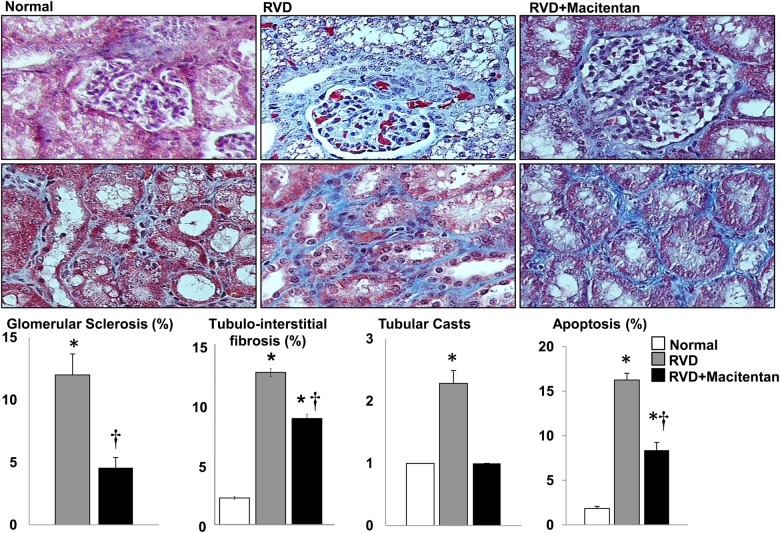
The stenotic kidney exhibited a significant increased renal expression of TGF-β (Figure 4). Macitentan treatment did not decrease TGF-β but decreased Smad-4 (Figure 4), accompanied by a greater increase in MMP-2 in the absence of a decrease in TIMP-1 (similarly increased in RVD and RVD + macitentan, Figure 4). These were accompanied by a significantly reduced expression of Caspase 3, Caspase 8 and p-PCNA, overall suggesting improved extracellular matrix turnover and attenuated injury in the stenotic kidney. Accordingly, glomerulosclerosis was attenuated by macitentan therapy (Figure 5) suggesting protection of the glomerular structure (Figure 5) that correlates with the reduction in albuminuria. Macitentan also reduced tubular casts (Figure 5) and apoptotic activity (Figures 4 and 5), further supporting the notion of protective effects on the stenotic renal parenchyma.
FIGURE 4:
Representative renal protein expression (top, n = 4–5 per group, two representative bands per animal shown) and average quantification per group (bottom) of TGF-β, smad-4, MMP-2, TIMP-1, cleaved Caspase 3 and 8 and phosphorylated proliferating cell nuclear antigen (p-PCNA) in normal, RVD and RVD + macitentan-treated kidneys at 10 weeks. Treatment with macitentan attenuated fibrotic and apoptotic activity in the stenotic kidney, indicating a reduction in kidney injury (*P < 0.05 versus normal, ^P = 0.06 versus normal, †P < 0.05 versus RVD).
FIGURE 5:
Representative pictures (trichrome) of the glomeruli (top) and tubulointerstitium (middle) and average quantification per group of renal morphometric analysis and apoptosis (bottom) of normal, RVD and RVD + macitentan. Four weeks of macitentan treatment reduced renal apoptosis and fibrosis in the stenotic kidney. (*P < 0.05 versus normal, †P < 0.05 versus RVD).
DISCUSSION
Previous studies have focused on the potential of selective or dual ET receptor antagonists to improve hypertension and renal disease [21–24, 39, 40]. Few previous studies involving antagonism of the ET-A receptor have shown promising results [23, 24, 40, 41] but, despite some encouraging evidence, transition to novel renal therapies involving chronic blockade of the ET receptors has been difficult. However, a few ET receptor antagonists have been approved for clinical use, specifically for treatment of pulmonary hypertension. Bosentan, a dual ET receptor antagonist with a ∼20 fold selection preference for ET-A, was validated in the BREATHE-1 trial in 2002 [42] and currently licensed for use. Macitentan is another dual ET-A/B blocker that was recently validated in the SERAPHIN trial involving 742 patients with pulmonary arterial hypertension. The trial tested the impact of macitentan on morbidity and mortality, recording a 45% risk reduction compared with placebo-treated patients, which helped to receive the approval by the FDA in 2013 [29].
Our previous research on the renoprotective potential of selective ET receptor antagonists in chronic RVD showed that chronic ET-A receptor blockade significantly improved RBF, GFR and redox status in early [24] and established experimental chronic RVD [23], whereas selective ET-B blockade did not offer benefits to renal health and function [23]. Since dual ET-A/B receptor blockers are currently used to treat pulmonary arterial hypertension, a disease that is frequently associated with chronic renal disease [43], elucidation of the potential impact of dual ET-A and -B blockade on renal health is of clinical relevance. Hence, this study aimed to extend our previous work by determining the effects of macitentan, a dual ET-A/B antagonist, on RVD as a model of chronic renal disease. Treatment with macitentan for 4 weeks resulted in a modest (albeit not significant) improvement in RBF without major changes on regional perfusion, hypertension, GFR or SCr compared with untreated RVD, supporting the safety of this compound for use in patients with concomitant chronic renal disease. It is relevant to emphasize that macitentan therapy did not result in overt adverse, deleterious or collateral effects in our model. We looked for signs of fluid retention, a major concern related to ET receptor antagonism [44]. We observed that macitentan-treated pigs did not show signs suggestive of edema (e.g. increase in body weight after treatment) compared with normal or untreated RVD animals, further supporting the notion that macitentan may be safe for patients with underlying renal disease.
Augmented vascularization after dual ET-A/B antagonism in hind limb ischemia was previously reported [45]. We showed the ability of ET-A blockade to restore the MV density of the stenotic kidney in established RVD while single ET-B blockade did not [23]. We observed that macitentan therapy improved rarefaction of cortical (but not medullary) microvessels <200 µm compared with untreated RVD. Although the loss of ET-B signaling in the stenotic kidney after macitentan did not fully inhibit the protective effects of ET-A blockade on the renal microcirculation [23, 24], it may have partly reduced the stimulus for MV proliferation since the renal medulla showed persistence of MV rarefaction. Macitentan increased the renal expression of VEGF, an effect likely mediated by ET-A (and not ET-B) receptors [23]. However, the lack of improvement in the expression of downstream mediators of VEGF such as p-Akt or the persistent increased expression of anti-angiogenic factors such as angiostatin [46, 47] and endostatin [48] may explain the somewhat limited effects of macitentan on renal MV proliferation that resulted in a less robust expansion of the MV architecture [10, 49]. The latter may suggest that ET-B receptors may contribute to renal angiogenesis partly by controlling anti-angiogenic factors. Furthermore, the expanded renal vasculature after 4 weeks of macitentan administration may still be immature and hence not fully functional. Therefore, the possibility of pathological MV proliferation resulting in leaky (suggested by the increased renal expression of Plg [50]) or dysfunctional vessels that may have limited their functional benefits cannot be ruled out. On the same line, blockade of ET-B signaling may have also exacerbated MV remodeling [23], suggested by the elevated expression of tTg [51] and increased media-to-lumen ratio. Thus, the somewhat limited protective effects on the renal microcirculation may have contributed to reducing the impact of macitentan treatment on the hemodynamics of the stenotic kidney.
Our previous studies have also shown that the ET-1/ET-A pathway plays a role in the progression of renal inflammation and fibrosis [22–24]. The stenotic kidney shows a pro-inflammatory state reflected by a significant increase in the expression of TNF-α and NFκ-B compared with normal controls. Macitentan reduced the expression of TNF-α but not NFκ-B in the stenotic kidney. The mechanisms underlying the lack of effect on NFκ-B are unclear. Activators of NFκ-B include chemical, microbial, mechanical and damage-associated factors [52], thus the persistent increase could be explained by the chronic nature of RVD and suggest a role of the ET-B signaling in the process since we demonstrated that the expression and activity of NFκ-B normalize after specific ET-A blockade [21, 23]. On the other hand, the improved expression of IL-10 and IL-12 implies that macitentan may have attenuated the inflammatory milieu in the stenotic kidney. Both IL-10 and IL-12 are involved in macrophage proliferation and activation, which may have contributed to the slightly (albeit not significant) reduction in the expression of MCP-1 and the attenuation in renal infiltration of F4/80+ cells. The macrophage population includes pro-inflammatory and pro-fibrotic/wound healing phenotypes [53], and it is possible that macitentan treatment may have suppressed both ‘arms’ of the macrophage response by reducing both pro-inflammatory IL-12 and pro-fibrotic IL-10 in the stenotic kidney.
Dual ET-A/B blockade may have modulated renal injury by primarily interfering with fibrosis as described by Boffa et al. who observed that bosentan, another dual antagonist, attenuated glomerular and vascular injury and improved survival without inducing significant hemodynamic effects [54]. Macitentan induced positive changes in fibrosis and cell survival markers compared with untreated animals with RVD. Despite a similar increase in the renal expression of TGF-β in RVD, macitentan reduced the downstream TGF-β signal molecule Smad-4, which may indicate transition to a less pro-fibrotic milieu. In addition, the improved renal expression of cleaved (active) MMP-2 compared with untreated RVD suggests that fibrogenic activity may have been partly counteracted by augmented extracellular matrix turnover in the stenotic kidney. These were accompanied by improvements in renal expression of Caspase 3, Caspase 8 and p-PCNA, which may suggest a decrease in apoptotic activity and a switch to a pro-survival renal milieu as TIMP-1 can also inhibit apoptosis and promote cell survival [55]. Accordingly, glomerulosclerosis and tubular casts in the stenotic kidney were attenuated, highlighting tissue-protective effects of macitentan.
Some limitations of our study should be recognized. While the swine model of RVD has been extensively validated [7, 9, 34], the pigs in this study lack the common human comorbidities of human RVD. Furthermore, the exposure to the disease and treatment is relatively brief and longer studies are needed. It is possible that tissue changes induced by macitentan might develop into a more robust functional effect after longer treatment. Despite these limitations, this study indicates that macitentan does not aggravate renal injury in a model of RVD and established renal disease and even attenuated fibrotic and inflammatory signaling in the stenotic kidney.
In summary, our study unraveled important effects of a dual ET receptor antagonist on the function and health of the stenotic kidney in RVD. While our previous research indicates that many of the benefits are probably due to antagonism of ET-A signaling, the results of this study should be placed into perspective since some parameters cannot be fully explained by ET-A blockade alone and may unravel novel actions of ET-B blockade or interactions between the two receptors. Blockade of both receptors have no significant effects on renal hemodynamics but improve other signs of renal disease in this model of RVD. When the results are examined together with our recent [23, 25] and previous [24] studies, they suggest that the beneficial effects induced by the ET-A blockade may not be as evident during macitentan administration due to a concomitant ET-B receptors antagonism. Thus, the greater beneficial effects of ET-A blockade could be partly secondary to a greater activity of ET-B receptors. However, treatment with macitentan for 4 weeks improved cortical MV density, inflammatory response and glomerulosclerosis of the stenotic kidney, without adverse events, suggesting protective effects on the stenotic renal parenchyma, possibly by inducing a pro-survival renal microenvironment, which may have contributed to the modest improvement in RBF. We believe our study concurred and also provides further supportive evidence for the renal safety and efficacy of this clinically approved ET receptor antagonist for using it in patients with concomitant chronic renal disease.
ACKNOWLEDGEMENTS
This work was supported by grant HL095638, HL5197 and P20GM104357 from the National Institute of Health, and by grant 18490005 from the American Heart Association.
CONFLICT OF INTEREST STATEMENT
Macitentan was a generous gift by Actelion Pharmaceuticals Ltd. The authors confirm that the results presented in this paper have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1.Ritchie J, Green D, Chrysochou C, et al. High-risk clinical presentations in atherosclerotic renovascular disease: prognosis and response to renal artery revascularization. Am J Kidney Dis 2014; 63: 186–197 [DOI] [PubMed] [Google Scholar]
- 2.Textor SC, Lerman LO. Reality and renovascular disease: when does renal artery stenosis warrant revascularization? Am J Kidney Dis 2014; 63: 175–177 [DOI] [PubMed] [Google Scholar]
- 3.Textor SC, Misra S, Oderich GS. Percutaneous revascularization for ischemic nephropathy: the past, present, and future. Kidney Int 2013; 83: 28–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldblatt H, Lynch J, Hanzal RF, et al. Studies on experimental hypertension: I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med 1934; 59: 347–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chade AR, Kelsen S. Reversal of renal dysfunction by targeted administration of VEGF into the stenotic kidney: a novel potential therapeutic approach. Am J Physiol Renal Physiol 2012; 302: F1342–F1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart N, Chade AR. Renoprotective effects of hepatocyte growth factor in the stenotic kidney. Am J Physiol Renal Physiol 2013; 304: F625–F633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chade AR, Rodriguez-Porcel M, Grande JP, et al. Distinct renal injury in early atherosclerosis and renovascular disease. Circulation 2002; 106: 1165–1171 [DOI] [PubMed] [Google Scholar]
- 8.Zhu XY, Chade AR, Rodriguez-Porcel M, et al. Cortical microvascular remodeling in the stenotic kidney: role of increased oxidative stress. Arterioscler Thromb Vasc Biol 2004; 24: 1854–1859 [DOI] [PubMed] [Google Scholar]
- 9.Daghini E, Primak AN, Chade AR, et al. Assessment of renal hemodynamics and function in pigs with 64-section multidetector CT: comparison with electron-beam CT. Radiology 2007; 243: 405–412 [DOI] [PubMed] [Google Scholar]
- 10.Iliescu R, Fernandez SR, Kelsen S, et al. Role of renal microcirculation in experimental renovascular disease. Nephrol Dial Transplant 2010; 25: 1079–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindenmeyer MT, Kretzler M, Boucherot A, et al. Interstitial vascular rarefaction and reduced VEGF-A expression in human diabetic nephropathy. J Am Soc Nephrol 2007; 18: 1765–1776 [DOI] [PubMed] [Google Scholar]
- 12.Basile DP, Donohoe D, Roethe K, et al. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 2001; 281: F887–F899 [DOI] [PubMed] [Google Scholar]
- 13.Hansen-Smith FM, Morris LW, Greene AS, et al. Rapid microvessel rarefaction with elevated salt intake and reduced renal mass hypertension in rats. Circ Res 1996; 79: 324–330 [DOI] [PubMed] [Google Scholar]
- 14.Gobe G, Browning J, Howard T, et al. Apoptosis occurs in endothelial cells during hypertension-induced microvascular rarefaction. J Struct Biol 1997; 118: 63–72 [DOI] [PubMed] [Google Scholar]
- 15.Maric-Bilkan C, Flynn ER, Chade AR. Microvascular disease precedes the decline in renal function in the streptozotocin-induced diabetic rat. Am J Physiol Renal Physiol 2012; 302: F308–F315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antonios TF, Singer DR, Markandu ND, et al. Rarefaction of skin capillaries in borderline essential hypertension suggests an early structural abnormality. Hypertension 1999; 34: 655–658 [DOI] [PubMed] [Google Scholar]
- 17.Antonios TF, Singer DR, Markandu ND, et al. Structural skin capillary rarefaction in essential hypertension. Hypertension 1999; 33: 998–1001 [DOI] [PubMed] [Google Scholar]
- 18.Hansen KJ, Edwards MS, Craven TE, et al. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg 2002; 36: 443–451 [DOI] [PubMed] [Google Scholar]
- 19.Yanagisawa M, Kurihara H, Kimura S, et al. A novel peptide vasoconstrictor, endothelin, is produced by vascular endothelium and modulates smooth muscle Ca2+ channels. J Hypertens Suppl 1988; 6: S188–S191 [DOI] [PubMed] [Google Scholar]
- 20.Yanagisawa M, Inoue A, Ishikawa T, et al. Primary structure, synthesis, and biological activity of rat endothelin, an endothelium-derived vasoconstrictor peptide. Proc Natl Acad Sci USA 1988; 85: 6964–6967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chade AR, Best PJ, Rodriguez-Porcel M, et al. Endothelin-1 receptor blockade prevents renal injury in experimental hypercholesterolemia. Kidney Int 2003; 64: 962–969 [DOI] [PubMed] [Google Scholar]
- 22.Chade AR, Krier JD, Textor SC, et al. Endothelin-A receptor blockade improves renal microvascular architecture and function in experimental hypercholesterolemia. J Am Soc Nephrol 2006; 17: 3394–3403 [DOI] [PubMed] [Google Scholar]
- 23.Chade AR, Stewart NJ, Peavy PR. Disparate effects of single endothelin-A and -B receptor blocker therapy on the progression of renal injury in advanced renovascular disease. Kidney Int 2014; 85: 833–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelsen S, Hall JE, Chade AR. Endothelin-A receptor blockade slows the progression of renal injury in experimental renovascular disease. Am J Physiol Renal Physiol 2011; 301: F218–FF25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chade AR, Tullos N, Stewart NJ, et al. Endothelin-A receptor antagonism after renal angioplasty enhances renal recovery in renovascular disease. J Am Soc Nephrol 2014; doi:10.1681/ASN.2014040323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benigni A, Perico N, Remuzzi G. Endothelin antagonists and renal protection. J Cardiovasc Pharmacol 2000; 35: S75–S78 [DOI] [PubMed] [Google Scholar]
- 27.Benigni A. Endothelin antagonists in renal disease. Kidney Int 2000; 57: 1778–1794 [DOI] [PubMed] [Google Scholar]
- 28.Remuzzi G, Perico N, Benigni A. New therapeutics that antagonize endothelin: promises and frustrations. Nat Rev Drug Discov 2002; 1: 986–1001 [DOI] [PubMed] [Google Scholar]
- 29.Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013; 369: 809–818 [DOI] [PubMed] [Google Scholar]
- 30.Haynes WG, Strachan FE, Webb DJ. Endothelin ETA and ETB receptors cause vasoconstriction of human resistance and capacitance vessels in vivo. Circulation 1995; 92: 357–363 [DOI] [PubMed] [Google Scholar]
- 31.Ozaki S, Ohwaki K, Ihara M, et al. Coexpression studies with endothelin receptor subtypes indicate the existence of intracellular cross-talk between ET(A) and ET(B) receptors. J Biochem 1997; 121: 440–447 [DOI] [PubMed] [Google Scholar]
- 32.Lerman LO, Schwartz RS, Grande JP, et al. Noninvasive evaluation of a novel swine model of renal artery stenosis. J Am Soc Nephrol 1999; 10: 1455–1465 [DOI] [PubMed] [Google Scholar]
- 33.Chade AR, Rodriguez-Porcel M, Grande JP, et al. Mechanisms of renal structural alterations in combined hypercholesterolemia and renal artery stenosis. Arterioscler Thromb Vasc Biol 2003; 23: 1295–1301 [DOI] [PubMed] [Google Scholar]
- 34.Krier JD, Ritman EL, Bajzer Z, et al. Noninvasive measurement of concurrent single-kidney perfusion, glomerular filtration, and tubular function. Am J Physiol Renal Physiol 2001; 281: F630–F638 [DOI] [PubMed] [Google Scholar]
- 35.Sidharta PN, Lindegger N, Ulc I, et al. Pharmacokinetics of the dual endothelin receptor antagonist macitentan in subjects with hepatic or renal impairment. J Clin Pharmacol 2013; doi:10.1002/jcph.193 [DOI] [PubMed] [Google Scholar]
- 36.Sidharta PN, van Giersbergen PL, Halabi A, et al. Macitentan: entry-into-humans study with a new endothelin receptor antagonist. Eur J Clin Pharmacol 2011; 67: 977–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelsen S, He X, Chade AR. Early superoxide scavenging accelerates renal microvascular rarefaction and damage in the stenotic kidney. Am J Physiol Renal Physiol 2012; 303: F576–F583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loffler BM, Breu V, Clozel M. Effect of different endothelin receptor antagonists and of the novel non-peptide antagonist Ro 46–2005 on endothelin levels in rat plasma. FEBS Lett 1993; 333: 108–110 [DOI] [PubMed] [Google Scholar]
- 39.Baltatu OC, Zaugg CE, Schumacher C, et al. Avosentan is protective in hypertensive nephropathy at doses not causing fluid retention. Pharmacol Res 2014; 80: 9–13 [DOI] [PubMed] [Google Scholar]
- 40.Dhaun N, Johnston NR, Goddard J, et al. Chronic selective endothelin A receptor antagonism reduces serum uric acid in hypertensive chronic kidney disease. Hypertension 2011; 58: e11–e12 [DOI] [PubMed] [Google Scholar]
- 41.Dhaun N, Melville V, Blackwell S, et al. Endothelin-A receptor antagonism modifies cardiovascular risk factors in CKD. J Am Soc Nephrol 2013; 24: 31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 2002; 346: 896–903 [DOI] [PubMed] [Google Scholar]
- 43.Pabst S, Hammerstingl C, Hundt F, et al. Pulmonary hypertension in patients with chronic kidney disease on dialysis and without dialysis: results of the PEPPER-study. PLoS One 2012; 7: e35310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mann JF, Green D, Jamerson K, et al. Avosentan for overt diabetic nephropathy. J Am Soc Nephrol 2010; 21: 527–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iglarz M, Silvestre JS, Duriez M, et al. Chronic blockade of endothelin receptors improves ischemia-induced angiogenesis in rat hindlimbs through activation of vascular endothelial growth factor-no pathway. Arterioscler Thromb Vasc Biol 2001; 21: 1598–1603 [DOI] [PubMed] [Google Scholar]
- 46.O'Reilly MS, Holmgren L, Shing Y, et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 1994; 79: 315–328 [DOI] [PubMed] [Google Scholar]
- 47.O'Reilly MS, Holmgren L, Shing Y, et al. Angiostatin: a circulating endothelial cell inhibitor that suppresses angiogenesis and tumor growth. Cold Spring Harb Symp Quant Biol 1994; 59: 471–482 [DOI] [PubMed] [Google Scholar]
- 48.O'Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 1997; 88: 277–285 [DOI] [PubMed] [Google Scholar]
- 49.Chade AR, Bentley MD, Zhu X, et al. Antioxidant intervention prevents renal neovascularization in hypercholesterolemic pigs. J Am Soc Nephrol 2004; 15: 1816–1825 [DOI] [PubMed] [Google Scholar]
- 50.Ratnoff OD. Increased vascular permeability induced by human plasmin. J Exp Med 1965; 122: 905–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bakker EN, Buus CL, Spaan JA, et al. Small artery remodeling depends on tissue-type transglutaminase. Circ Res 2005; 96: 119–126 [DOI] [PubMed] [Google Scholar]
- 52.Guijarro C, Egido J. Transcription factor-kappa B (NF-kappa B) and renal disease. Kidney Int 2001; 59: 415–424 [DOI] [PubMed] [Google Scholar]
- 53.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 2011; 11: 723–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boffa JJ, Tharaux PL, Dussaule JC, et al. Regression of renal vascular fibrosis by endothelin receptor antagonism. Hypertension 2001; 37: 490–496 [DOI] [PubMed] [Google Scholar]
- 55.Lee SJ, Yoo HJ, Bae YS, et al. TIMP-1 inhibits apoptosis in breast carcinoma cells via a pathway involving pertussis toxin-sensitive G protein and c-Src. Biochem Biophys Res Commun 2003; 312: 1196–1201 [DOI] [PubMed] [Google Scholar]