Abstract
Although there have been many advancements in the treatment of patients with chronic kidney disease (CKD) over the last 50 years, in terms of reducing cardiovascular risk, mortality remains unacceptably high, particularly for those patients who progress to stage 5 CKD and initiate dialysis (CKD5d). As mortality risk increases exponentially with progressive CKD stage, the question arises as to whether preservation of residual renal function once dialysis has been initiated can reduce mortality risk. Observational studies to date have reported an association between even small amounts of residual renal function and improved patient survival and quality of life. Dialysis therapies predominantly provide clearance for small water-soluble solutes, volume and acid-base control, but cannot reproduce the metabolic functions of the kidney. As such, protein-bound solutes, advanced glycosylation end-products, middle molecules and other azotaemic toxins accumulate over time in the anuric CKD5d patient. Apart from avoiding potential nephrotoxic insults, observational and interventional trials have suggested that a number of interventions and treatments may potentially reduce the progression of earlier stages of CKD, including targeted blood pressure control, reducing proteinuria and dietary intervention using combinations of protein restriction with keto acid supplementation. However, many interventions which have been proven to be effective in the general population have not been equally effective in the CKD5d patient, and so the question arises as to whether these treatment options are equally applicable to CKD5d patients. As strategies to help preserve residual renal function in CKD5d patients are not well established, we have reviewed the evidence for preserving or losing residual renal function in peritoneal dialysis patients, as urine collections are routinely collected, whereas few centres regularly collect urine from haemodialysis patients, and haemodialysis dialysis patients are at risk of sudden intravascular volume shifts associated with dialysis treatments. On the other hand, peritoneal dialysis patients are exposed to a variety of hypertonic dialysates and episodes of peritonitis. Whereas blood pressure control, using an angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB), and low-protein diets along with keto acid supplementation have been shown to reduce the rate of progression in patients with earlier stages of CKD, the strategies to preserve residual renal function (RRF) in dialysis patients are not well established. For peritoneal dialysis patients, there are additional technical factors that might aggravate the rate of loss of residual renal function including peritoneal dialysis prescriptions and modality, bio-incompatible dialysis fluid and over ultrafiltration of fluid causing dehydration. In this review, we aim to evaluate the evidence of interventions and treatments, which may sustain residual renal function in peritoneal dialysis patients.
Keywords: biocompatible dialysate ACEI, peritoneal dialysis, residual renal function
Importance of RRF in peritoneal dialysis
Peritoneal dialysis technique survival varies throughout the world, depending upon access to transplantation and haemodialysis, and centre practices [1]. Besides loss of patients to transplantation, peritonitis remains the commonest cause for transfer to haemodialysis in many countries [2], and due to the relatively high turnover of peritoneal dialysis patients, it was only in the 1990s that reports of the importance of maintaining residual renal function (RRF) started to appear [3]. Maiorca et al. reported a 50% reduction in mortality in peritoneal dialysis (PD) patients with RRF [4]. These encouraging results were supported by later larger prospective observational studies, with Diaz-Buxo et al., reporting that residual renal creatinine clearance (CrCl) was strongly associated with PD patient survival, whereas peritoneal clearance did not affect outcome. Moreover, they observed in their cohort of some 2686 PD patients, a dose response association between RRF and PD patient survival, with each CrCl 5 L/week/1.73 m2 increase in renal creatinine clearance associated with a 10% decrease in mortality, whereas there was no association between peritoneal CrCl and mortality [5].
Similarly, Rocco et al. reported that for each 10 L/week/1.73 m2 increase in renal CrCl there was a 40% reduced risk for death and also that for each increase in weekly renal Kt/Vurea of 0.1 there was a 12% reduction in the risk for death from a multicentre prospective cohort study of 1446 prevalent PD patients [6]. Once again there was no effect of peritoneal solute clearances on survival. These findings were not limited to North America or Europe [7, 8], with observational reports from Hong Kong [9] and Turkey also confirming that for every 1 mL/min increase in residual GFR mortality risk reduced by 35–47% [10, 11]. These studies emphasized the fact that the residual renal clearance may have greater beneficial effects than comparable peritoneal small solute clearance and as such these clearances are not simply equivalent. This led to the reanalysis of the CANUSA study [12], the landmark multicentre prospective cohort of 680 incident PD patients in Canada and USA, which reported that for each increment of residual renal GFR of 5 L/week/1.73 m2 there was a 12% reduction in the risk for death and that for each 250 mL increase in urine volume there was a 36% decreased risk for death. Once again neither peritoneal small solute clearances nor peritoneal ultrafiltration volume were associated with patient survival. Subsequent secondary analysis of ADEMEX study additionally confirmed an advantage for RRF on mortality [13]. This cornerstone multicentre prospective randomized controlled trial of 965 Mexican PD patients, reported that for each increase in RRF of CrCl 10 L/week/1.73 m2 was associated with an 11% decrease in mortality, and an increase in renal Kt/Vurea of 0.1 a 6% decrease in mortality. More recently, additional studies from the Netherlands, Sweden, Australia and New Zealand have all confirmed the importance of RRF on mortality in PD patients (Table 1) [14–16]. In addition these studies all reported additional benefits for patients with preserved RRF, ranging from improved quality of life to reduced inflammatory markers [14, 15].
Table 1.
Summary of studies reported beneficial of RRF on mortality
| Reference (year) | Study design | Number, characteristics and modality of subjects | Measurement of RRF | RR or OR of mortality per increase of RRF (CI or P-value) |
|---|---|---|---|---|
| Maiorca et al. (1995) [4] | 3-year prospective single centre | Prevalent 68 CAPD and 34 HD | GFR 10 L/week/1.73 m2 | 0.4 (P < 0.001) |
| Diaz-Buxo et al. (1999) [5] | 1-year prospective single centre | Prevalent 2686 CAPD or CCPD | Renal CrCl 10 L/week/1.73 m2 | 0.89 (P = 0.003) |
| Rocco et al. (2000) [6] | 7-month prospective multicentre | Prevalent 1446 CAPD or CCPD | Renal CrCl 10 L/week/1.73 m2 | 0.6 (0.4–0.8) |
| Szeto et al. (2000) [10] | 3-year prospective single centre | Prevalent 270 CAPD | GFR 1 mL/min/1.73 m2 | 0.65 (0.45–0.94) |
| Ates et al. (2001) [11] | 3-year prospective single centre | Incident 125 CAPD | GFR 1 mL/min/1.73 m2 | 0.53 (0.31–0.92) |
| Bargman et al. (2001) [12] | 2-year prospective multicentre | Prevalent 680 CAPD | GFR 5 L/week/1.73 m2 Urine volume > 250 mL/day |
0.88 (0.83–0.94) 0.64 (0.51–0.8) |
| Paniagua et al. (2002) [13] | 2-year multicentre randomized controlled | Incident 965 CAPD | Renal CrCl 10 L/week/1.73 m2 Renal Kt/V 0.1 unit |
0.89 (P = 0.01) 0.94 (P = 0.01) |
| Termorshuizen et al. (2003) [14] | 3-year prospective multicentre | Incident 413 CAPD | GFR 1 mL/min/1.73 m2 | 0.88 (0.79–0.99) |
| Chung et al. (2003) [15] | 2-year retrospective | Incident 117 CAPD | GFR 1 mL/min/1.73 m2 | 0.79 (0.62–0.99) |
| Szeto et al. (2004) [3] | 5-year prospective single centre | Prevalent 270 CAPD | GFR 1 mL/min/1.73 m2 | 0.8 (0.73–0.88) |
| Rumpsfeld et al. (2009) [16] | 3-year retrospective | Incident 2434 CAPD or APD | GFR 10 L/week/1.73 m2 | 0.93 (P = 0.01) |
Although the evidence from these large observational and interventional trials is strongly weighted to an association between preservation of residual renal function and improved patient survival, they do not prove a causal effect. One potential confounder to all these studies is one of lead-time bias, in that patients with greater residual renal function may have initiated dialysis at a relatively earlier time than those with lower residual renal function. Similarly, some patients with CKD may have been started on peritoneal dialysis after an episode of acute kidney injury, followed by some recovery of RRF.
Measurement of residual renal function
Simply estimating RRF by measuring urine volume is inaccurate in patients with CKD [17]. Although the clearance of inulin, isotopes and radiocontrast agents (51chromium ethylenediaminetetra-acetic acid (EDTA) and iothalamate) are more accurate for determining residual renal function in patients with CKD than urine collections [18], these add costs and are impractical for routine clinical practice. As such, most centres use 24-h urine collections, and as urinary urea falls in CKD and underestimates inulin clearance, and conversely the relative ratio of tubular secreted to glomerular filtered creatinine increases urinary creatinine [19, 20], current guidelines advocate calculating the mean of creatinine and urea clearance, and then normalizing clearance to a body surface area of 1.73 m2 [21]. However dialysis patients may suffer from sarcopenia, and as such changes in body composition [22, 23].
However, both urea and creatinine are influenced by dietary protein intake, particularly meat, and creatine production depends upon both hepatic synthetic function and muscle mass and physical activity, and changes in intestinal bacteria flora alter urea and creatine gastrointestinal losses [24]. As such, these factors add potential confounders when reviewing serial measurements of RRF from CKD5d patients over time.
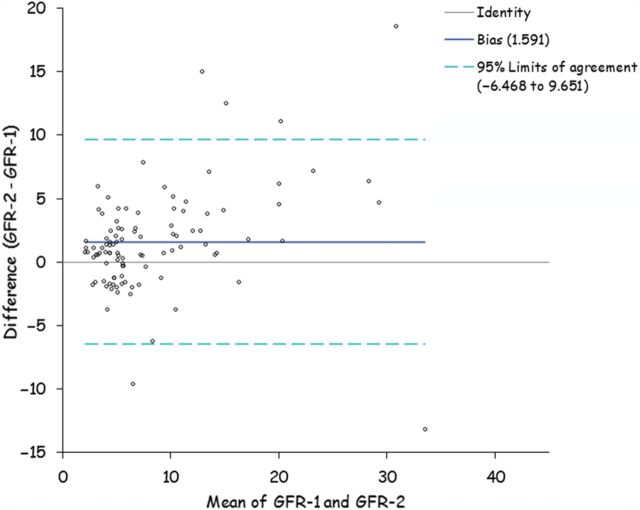
The commonest method for estimating creatinine remains the colourimetric Jaffe-based reaction. In kidney disease, chromogens accumulate which can interfere with this assay [25], and as such creatinine estimations vary between laboratories. Enzymatic methods of creatinine measurement, which are less affected, are more reliable. In addition to these technical aspects which affect measurement of RRF, urine volumes and urinary urea, creatinine and protein vary in 24-h urine collections in CKD patients not only consequent upon hydration status but also on patient compliance with completeness of the collection [25]. Although 24-h urine collections remain the standard method to determine RRF in clinical practice, there is not only inter-patient and interlaboratory variation but also intrapatient variability (Figure. 1).
Fig. 1.
Bland Altman plot showing variation in glomerular filtration rate calculated from sequential 24 h urine collections in 100 peritoneal dialysis patients.
The effect of the original cause of kidney disease on loss of residual renal function in the PD patient
The original cause of kidney disease can certainly have an impact on CKD progression, for example, most CKD5d patients with antiglomerular basement membrane disease initiate dialysis virtually anuric whereas children with nephronophthisis may be polyuric. Haynes et al. reported an annual rate of decline in residual renal function of 3.8 ± 2.5, 2.5 ± 4.8 and 1.9 ± 3.6 mL/min/1.73 m2 for patients with cystic kidney disease, diabetic kidney disease and glomerulonephritis, respectively [26], and Liao et al. also noted that PD patients with diabetic nephropathy had a more rapid progressive loss of RRF [27]. However, these observations were not supported by USRDS data [28], although this study may have been confounded by including haemodialysis patients and so introducing other factors such as repeated intradialytic hypotensive episodes [29]. More recently, another study from Hong Kong reported that patients with proteinuric renal diseases were more likely to have a faster loss of RRF [30], as were those with peripheral and cardiovascular disease [31] and patients initiating PD with less RFF [30]. Loss of RRF in returning kidney transplant patients may vary with centre practices, in terms of immunosuppressive policy and reducing or stopping these medications when starting PD.
As underlying primary renal disease, and baseline GFR at PD initiation appear to have a major effect on determining loss of RRF, these factors should be considered when designing prospective interventional studies designed to preserve RRF.
Strategies for preserving RRF in patients with progressive CKD
Dietary intervention
Increased protein intake increases both the glomerular filtration rate and increases renal tubular acid excretion in the normal kidney. As hyperfiltration and increased renal tubular work load to maintain acid-base homeostasis have both been proposed as mechanisms for continued renal injury, protein restriction may potentially reduce the rate of loss of RRF. The potential benefits of dietary protein restriction (0.58 g/kg/day versus a normal dietary protein intake of 1.3 g/kg/day) to slow the progression of chronic kidney disease (CKD) were reported in the Modification of Diet in Renal Disease (MDRD) study [32], which demonstrated that a low protein diet had a modest effect when compared with blood pressure control in patients with CKD stages 3–4 (eGFR 25–55 mL/min/1.73 m2). Follow-up suggested that there may have been a continuing effect, predominantly for those with diabetic kidney disease [33]. In contrast, very low protein diets with keto acid supplements (protein intake 0.28 g/kg/day and ketoacids 0.28 g/kg/day) did not reduce progression in patients with CKD stage 4–5 (eGFR 13–24 mL/min/1.73 m2) and were associated with increased mortality when compared with those on a low-protein diet [34]. There are limited data in PD patients, although a small single centre trial reported that RRF was better maintained in incident PD patients with a urine output ≥800 mL/day or an eGFR ≥2 mL/min/1.73 m2, over 12 months prescribed a low-protein diet with supplemental ketoacids (protein intake 0.6–0.8 g/kg/day with keto acids 0.12 g/kg/day) versus a low- 0.6–0.8 g/kg/day and a high-protein diet group 1.0–1.2 g/kg/day [35]. Reducing dietary protein intake reduces serum creatinine, but how this affects measurement of residual renal function in patients with CKD5d is unknown, as it may increase the ratio of creatinine secreted by the tubule compared to that filtered thus giving a ‘higher’ creatinine-based estimate of RRF. Similarly it is unknown whether reducing dietary protein intake affects gastrointestinal creatinine loss. In addition, none of these studies assessed dietary sodium or phosphate intake, which are linked to dietary protein ingestion, and lower protein diets supplemented with keto acids would have been expected to contain lower sodium and phosphate content. Thus, although an observational study reporting faster loss of RRF in PD patients with higher dietary protein intake [36] may have been due to greater protein intake, it may have been confounded by higher dietary sodium and phosphate intake.
Effects of blood pressure control and RRF
The protective effects of blood pressure control to slow the progression of CKD are the basis of medical management. However, the role of blood pressure control in preserving RRF in PD patients remains inconclusive. Moist et al. reported no association between blood pressure control and the rate of loss of RRF in their retrospective review of 1032 incident PD patients from the USRDS database [28]. However, this apparent difference with CKD may be confounded by clinicians treating hypertension on one hand, and on the other patients with low blood pressure secondary to cardiac dysfunction, or those patients with hypertensive kidney disease with blood pressures below their autoregulatory range are more prone to episodes of acute kidney injury with more rapid loss of residual renal function [37].
Effects of renin-angiotensin-aldosterone system blockage
Although angiotensin converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) have been convincingly demonstrated to reduce the rate of progression and proteinuria in CKD patients [38], it is unclear whether they have a benefit in the PD patient [39]. Although ACEIs and ARBs improve survival in patients with chronic heart failure they reduce renal function [40], and similarly in PD patients, any potential benefit may be abrogated by an increased risk of hypotension and acute kidney injury [41]. The results of observational studies have been mixed, with a large retrospective study from USRDS on incident and prevalent PD reporting ACEIs had a protective effect on RRF [28], whereas a study of 160 incident PD patients from Australia, and 451 from the Netherlands showed no benefit [36, 42], although more diabetics were treated with ACEIs in the latter study. A recent observational study reported a small protective effect for ACEIs, but when corrected for other factors showed no statistical advantage for ACEIs [30]. Two small randomized trials have reported better preservation of RRF with ACEIs/ARBs. First, Li et al. studied 60 PD patients and reported the rate of decline in RRF over 12 months with ramipril was 2.07 mL/min per 1.73 m2 versus 3.0 mL/min per 1.73 m2 for the control group, although there was no difference in RRF between the groups at 3, 6 and 9 months, and the only difference was at the end of the study when a number of patients had dropped out and some had stopped taking ramipril due to side effects. Interestingly, the hazard ratio for anuria was higher in the ramipril-treated group at 3, 6, 9 months, which may be explained by the haemodynamic side effects of ACEIs [43]. Suzuki et al. reported on 34 patients randomized to valsartan or other antihypertensives and the ARB group had lower loss of RRF over 2 years from 3.2 ± 0.3 to 4.3 ± 0.7 mL/min/1.73 m2 compared with 5.9 ± 0.5 to 2.8 ± 0.4 mL/min/1.73 m2 in the control group [44].
Unexpectedly, RRF improved after ARB administration and was higher at 6 months than prior to starting ARBs, suggesting that some patients had regained RRF after an acute decline which had initiated starting PD treatment. Neither study showed any effect of ACEI/ARB on proteinuria. A small prospective trial showed no difference between ARBs and ACEIs on RRF [45].
More recently, a systematic review from the Cochrane library reported that ACEIs or ARBs may provide some protection in preserving RRF in PD patients, but did not reduce proteinuria. However, as the number of studies and quality of studies, in terms of potential confounders was markedly limited, no recommendation that ACEIs/ARBS should be the antihypertensive agents of choice for PD patients could be made [46]. On the other hand ACEIs/ARBs did not increase serum potassium, although the combination of ACEIs and ARBs may potentiate hyperkalaemia and oliguria in PD patients [47].
The effect of loop diuretics on RRF
In clinical practice, diuretics are commonly prescribed to PD to aid volume control, but hypovolaemia may lead to acute kidney injury and loss of RRF. Observational studies have either reported no effect on RRF [28, 36], or a loss of RRF [26, 30]. On the other hand, Van Olden et al. observed that in the short term, high dose furosemide increased both free water and sodium excretion but without affecting urea or creatinine clearance [48]. In a randomized trial, Medcalf et al. compared the effect of 250 mg/day of furosemide over 12 months in 61 incident PD patients [49] and showed that although treatment with furosemide improved fluid balance and increased urine volume and sodium excretion there was no benefit on preserving RRF. As such there is no convincing data that loop diuretics, such as furosemide maintain RRF, whereas they increase urine output and sodium excretion and as such may benefit volume overloaded patients.
Effects of peritoneal dialysis modality and RRF
Around the world, the proportion of patients treated by continuous ambulatory peritoneal dialysis (CAPD) and automated peritoneal dialysis overnight cyclers (APD) varies markedly. There has been a debate as to whether APD therapy leads to earlier loss of RRF. APD patients are generally exposed to higher glucose dialysates compared with CAPD, and glucose exposure has been reported to be associated with faster loss of RRF [36]. In addition, blood pressure tends to fall when peritoneal dialysate is drained out and then increases during infill, and it has been hypothesized that these changes in blood pressure and cardiac filling could predispose to renal hypoperfusion and earlier loss of RRF [50]. Hiroshige and Hufnagel were the first to report more rapid loss of RRF with APD in small single-centre series [51, 52]. However, others reported no difference between the two modalities in small trials [53, 54] and large observational databases and registries [26, 28, 39, 53, 55] (Table 2). More recently, registry data from the NECOSAD study reported a higher risk for loss of RRF with APD, particularly in the first year of treatment, with an adjusted hazard ratio 2.66 (confidence interval 1.60–2.44) [65]. Apart from the NECOSADS study [65], nearly all of the other studies are potentially confounded by patient selection bias and underlying original kidney disease, as generally the older more comorbid patients were treated by CAPD [54]. APD therapy using high glucose dialysates may potentially increase the risk of hypovolaemic episodes and renal ischaemia, and as such lead to an earlier loss of RRF.
Table 2.
Summary of studies reported effect of dialysis modality on RRF
| Reference (year) | Study design | Subject characteristics | Favour CAPD | Details |
|---|---|---|---|---|
| Hiroshige et al. (1996) [51] | 6-month prospective | Prevalent 8 NIPD, 5 CCPD, 5 CAPD | Yes | Rate of change of RRF in −0.29 (NIPD) versus −0.34 (CCPD) versus +0.01 (CAPD) mL/min/month |
| Rodriguez et al. (1998) [56] | 3-year prospective | Prevalent 25 CAPD, 20 APD | No | |
| Hufnagel et al. (1999) [52] | 18-month prospective | Incident 6 NIPD, 12 CCPD, 18 CAPD | Yes | Rate of change of RRF in −0.26 (APD) versus −0.13 (CAPD) mL/min/month |
| Bro et al. (1999) [57] | 6-month randomized controlled trial | Prevalent 13 CAPD, 12 APD | No | |
| Moist et al. (2000) [28] | 3-year retrospective | Incident 722 CAPD, 310 APD | No | |
| De Fijter et al. (2000) [53] | 2-year randomized controlled trial | Incident 13 CCPD, 11 CAPD | No | |
| Gallar et al. (2000) [58] | 1-year prospective | Incident 11 CAPD, 9 APD | No | |
| Singhal et al. (2000) [59] | 4-year prospective | Incident 211 CAPD, 31 APD | No | |
| Holley et al. (2001) [60] | 9-year retrospective | Incident 11 CAPD, 9 APD | No | |
| Jansen et al. (2002) [39] | 1-year prospective | Incident 243 PD subjects | No | |
| Hidaka et al. (2003) [61] | 6-year prospective | Incident 27 CAPD, 7 APD | Yes | Approximate time to decrease 50% of RRF in CAPD is 15 months versus APD 4 months, P < 0.001 |
| Johnson et al. (2003) [36] | 6-year prospective | Incident 134 CAPD, 12 APD | No | |
| Rodriguez-Carmona (2004) [62] | 1-year prospective | Incident 53 CAPD, 51 APD | Yes | Hazard ratio of APD versus CAPD = −1.2 (−2.25 to −0.15, P = 0.02) |
| Rabindranath (2007) [63] | Systematic review of 3 RCT | 49 PD subjects | No | |
| Liao (2009) [27] | 10-year retrospective | Incident 188 CAPD, 82 APD | No | |
| Su et al. (2010) [64] | 9-year retrospective | Prevalent 140 CAPD, 32 APD | No | |
| Cnossen et al. (2010) [55] | 7-year retrospective | Incident 179 CAPD, 441 APD | No | |
| Balasubramanian et al. (2011) [54] | 5-year retrospective | Incident 178 CAPD, 13 APD | No | |
| Michels et al. (2011) [65] | 3-year retrospective | Incident 505 CAPD, 78 APD | Yes | Higher risk of loss of RRF in APD compared to CAPD in first year of treatment (adjusted hazard ratio 2.66, CI 1.66–4.44) |
Effects of biocompatible peritoneal fluid and RRF
Conventional peritoneal dialysis fluids are hypertonic and acidic, containing lactate as a base equivalent and glucose degradation products (GDPs). The newer neutral pH, lower GDP PD fluids, may better preserve RRF as they may cause less intraperitoneal inflammation, and so reduce peritoneal ultrafiltration and fluid losses [55]. This concept is supported by a short-term European study which reported greater urine volume and both urinary urea and creatinine clearance with the neutral pH low GDP glucose containing dialysates [66]. These beneficial effects of neutral pH dialysates were confirmed by a number of clinical trials, which reported better preservation of RRF with the less bioincompatible dialysates [66, 67]. However, these beneficial effects on RRF were not substantiated by a greater number of later studies and trials [68–76] (Table 3). The most recent randomized prospective trial, which recruited patients who were well matched for original kidney disease and comorbidity, reported no beneficial effect for low GDP neutral pH dialysates on preserving RRF [73]. A recent Cochrane report commented that the number of good quality trials with appropriate patient numbers was somewhat limited. Their meta-analysis reported that overall, although the use of neutral pH low GDP glucose-containing dialysates were associated with increased urine volumes compared to standard dialysates [83], there was no overall statistically significant benefit in terms of maintaining RRF or benefit at 12 or 24 months of treatment, although there was potential benefit after 2 years. As such, with the relatively high turnover of PD patients, the majority of PD patients would not benefit from the prescription of these less bio-incompatible dialysates in terms of preserving RRF.
Table 3.
Summary of studies reported effect of biocompatible peritoneal solution on RRF
| Reference (year) | Study design | Subject characteristics | Favour balance solution | Details |
|---|---|---|---|---|
| Feriani et al. (1998) [76] | 6-month randomized controlled trial | Prevalent 33 lactate base, 36 bicarbonate base | No | |
| Coles et al. (1998) [77] | 2-month randomized controlled trial | Prevalent 3 arms, 19 lactate base, 20 lactate/bicarbonate, 20 bicarbonate | No | |
| Tranaeus et al. (2000) [78] | 1-year randomized controlled trial | Prevalence 106 CAPD, 70 (bicarbonate/lactate), 36 (lactate) | No | |
| Rippe et al. (2001) [69] | 2-year randomized controlled trial | Prevalent 40 conventional, 40 neutral pH dialysate | No | |
| Williams et al. (2004) [66] | 6-month randomized crossover | Prevalent 86 CAPD subjects | Yes | Renal CrCl and urea clearance increase when using balance solution and decrease when using standard solution |
| Montenegro et al. (2006) [79] | 1-year randomized controlled trial | Incident 36 CAPD, 18 (lactate base), 18 (bicarbonate base) | Yes | GFR decline in lactate base group, but preserved in bicarbonate group |
| Szeto et al. (2007) [70] | 1-year randomized controlled trial | Incident 25 conventional, 25 neutral | No | |
| Fan et al. (2008) [71] | 1-year randomized controlled trial | Incident 61 CAPD or APD for conventional fluid, 57 CAPD or APD for neutral fluid | No | |
| Choi et al. (2008) [74] | 1-year randomized controlled trial | Prevalent, 104 CAPD, 51(neutral), 53 (conventional) | No | |
| Weiss et al. (2009) [80] | 6-month prospective crossover | Prevalent 53 CAPD | Yes | Improvement of GFR when using bicarbonate base solution |
| Pajek et al. (2009) [74] | 6-month prospective crossover | Prevalent 26 CAPD | No | |
| Haag-Weber et al. (2010) [81] | 18-month randomized controlled | Prevalent 69 CAPD, 43 (neutral), 26 (conventional) | Yes | Monthly RRF change faster in conventional group, −4.3% versus −1.5% (P = 0.04) |
| Bajo et al. (2011) [72] | 2-year prospective | Incident 20 standard, 13 balance fluid | No | |
| Johnson et al. (2012) [73] | 2-year randomized controlled trial | Incident 93 conventional, 92 balance fluid | No | |
| Kim et al. (2012) [82] | 2-year randomized controlled trial | Incident 91 CAPD, 48 (balance), 43 (conventional) | Yes | Residual renal function significantly higher in balance solution at the end of study |
| Cho et al. (2013) [76] | 1-year randomized controlled trial | Incident CAPD, 32 (balance), 28 (conventional) | No |
Effects of icodextrin and RRF
Icodextrin 7.5% is a dialysis solution containing an iso-osmolar glucose polymer with greater ultrafiltration capacity than 22.7 g/L glucose dialysates [77] and is typically used for the long day dwell period with APD or the nighttime dwell for CAPD patients. As icodextrin decreases extracellular water (ECW) [84], there have been concerns that it may lead to dehydration and loss of RRF [85]. One randomized study measuring body composition and ECW reported that icodextrin reduced ECW, but also reduced urine output and GFR [85]. Only one small single-centre study reported that icodextrin usage helped preserve RRF [86], whereas five other studies showed no effect [87–91] (Table 4). As such, a recent Cochrane meta-analysis concluded that whereas icodextrin increased ultrafiltration compared with a standard 22.7 g/L glucose exchange, there was no effect on RRF [83]. However, as icodextrin can lead to a reduction in ECW, patients could potentially be at increased risk of dehydration and acute kidney injury, as dehydration is linked to loss of RRF [36].
Table 4.
Summary of studies reported effect of icodextrin peritoneal solution on RRF
| Reference (year) | Study design | Subject characteristics | Favour icodextrin solution | Details |
|---|---|---|---|---|
| Posthuma et al. (1997) [87] | 2-year randomized controlled trial | Prevalent, CCPD, 11 (icodextrin), 10 (lowest glucose) | No | |
| Plum et al. (2002) [88] | 3-month randomized controlled trial | Prevalent, APD, 20 (icodextrin), 19 (2.27% glucose) | No | |
| Konings et al. (2003) [85] | 4-month randomized controlled trial | Prevalent, CAPD and CCPD, 22 (icodextrin), 18 (glucose) | No | GFR significantly decrease in icodextrin treated group, but maintain in control group |
| Adachi et al. (2006) [86] | 2-year retrospective | Prevalence case matched control APD, 10 (icodextrin), 12 (glucose) | Yes | GFR significantly decrease in control group, but maintain in icodextrin treated group |
| Takatori et al. (2011) [90] | 2-year randomized controlled trial | Incident, CAPD and APD, 21 (icodextrin), 20 (glucose) | No |
Effects of volume status and RRF
Intravascular volume depletion has been widely accepted as a cause of loss of RRF in PD patients [36]. However many studies using bioimpedance techniques have reported that PD patients generally have an increased ECW volume [92]. Although faster transporters may be expected to potentially be at greater risk of hypervolaemia due to the more rapid fall in the osmotic glucose gradient, cross-sectional studies have not reported any differences in ECW volume with transporter status in healthy APD outpatients and CAPD outpatients using 7.5% icodextrin [93].
Many clinicians err on the side of volume expansion for PD patients in the belief that this will help maintain RRF. Studies using the less bioincompatible PD dialysates reported lower peritoneal ultrafiltration, and so the question arose as to whether any benefit in maintaining urine volume or RRF was secondary to prevention of dehydration, rather than any effect of the dialysate per se. On the other hand, sustained hypervolaemia will result in hypertension, left ventricular hypertrophy [94] and may lead to an increased risk for cardiovascular mortality. In a cross-sectional study of 550 prevalent stable PD patients which defined hypervolaemic status, as ratio of ECW to TBW measured by multifrequency electrical bioimpedance assessments (MFBIA), urine output was lower in hypervolaemic patients [95]. Although MFBIA measures ECW, other causes of ECW expansion include inflammation and hypovolaemia [96]. However, the association between natriuretic peptides and ECW in PD patients suggests that many are volume expanded [97]. McCafferty et al. examined the association of annual measurement of MFBIA and loss of RRF in 237 prevalent PD patients [98] and reported that there were no differences in the change in RRF with respect to absolute or relative changes in ECW/TBW ratio. Importantly, this study showed that maintaining a hypervolaemic state did not preserve RRF, and this needs to be confirmed by a prospective blinded study.
Effects of nephrotoxic insults and RRF
Nephrotoxic agents such as non-steroidal anti-inflammatory drugs, aminoglycoside antibiotics and radio-contrast iodine are recognized to increase the risk of acute kidney injury in patients with CKD [41]. PD patients are at risk of peritonitis [1], and peritonitis may lead to hypotension and relative hypovolaemia with an increased risk of loss of RFF [30, 36]. In addition, treatment with aminoglycosides might potentially lead to a more rapid loss of RFF, and this was observed by Shemin et al. [99]. Nonetheless, several subsequent studies reported that aminoglycoside treatment did not adversely affect RRF compared with other antibiotic regimes [100–102]. Lui et al. randomly assigned 102 CAPD patients to once daily intraperitoneal cefazolin and netilmicin versus cefazolin and ceftazidime for 14 days. Although RRF was significantly reduced at Day 14 in the aminoglycoside group, RRF recovered to baseline by 6 weeks [102]. Registry data from Australia did not show any effect of aminoglycosides on the rate of decline of RRF in some 1400 PD patients who had suffered one of more episodes of PD peritonitis [103]. Thus, although once daily short courses of aminoglycoside may cause a transient reduction in RRF, they do not appear to have a discernable adverse long-term effect, when dosages are adjusted to maintain therapeutic and toxic levels avoided. Radiocontrast-induced acute kidney injury is more common in patients with underlying CKD. However, reports from retrospective observational studies have not noted a major effect of contrast exposure on loss of RRF [59,103]. As PD patients generally have an expanded ECW, this may help protect against radiocontrast injury in patients with appropriate periprocedural hydration.
Dittrich et al. prospectively investigated the effect of radiocontrast media on RRF in a small number of PD patients and noted that the combination of a reduced volume of non-ionic hypo-osmolar contrast media and oral hydration did not adversely affect RRF after 30 days [104]. Likewise, Moranne et al. reported no difference in RRF measured 2 weeks after radiocontrast exposure when PD patients were hydrated with 1 L of 0.9% saline preprocedure compared with PD controls [105]. Although radiocontrast exposure may cause an acute deterioration in RRF with appropriate hydration and minimizing radiocontrast dosage does not appear to have a permanent deleterious effect on RRF.
Conclusion
Preservation of RRF is an important determinant of both PD technique and patient survival. However, loss of RRF is dependent upon the primary renal disease and patient comorbidities and is affected by lead-time bias in terms of when patients initiate PD and also whether starting PD an episode of acute kidney injury, which is then followed by partial recovery of RRF. As there are significant errors in measuring RRF in PD patients, the question arises as to whether the effects of treatments designed to preserve RRF can be truly assessed above the background variation in RRF and patient risk factors. As such, there is little if any current convincing evidence to confirm the effect of strict blood pressure control or support the use of ACEI/ARBs compared with other antihypertensive medicines or less bioincompatible peritoneal dialysates in preserving RRF. Avoidance of dehydration and episodes of acute kidney injury associated with peritonitis appear important in preserving RRF, but on the other hand deliberately keeping PD patients overhydrated, as assessed by bioimpedance [106] does not appear to preserve RRF. Further carefully designed large scale prospective studies are warranted to prove the benefits of drug and other interventions to preserve RRF.
Conflict of interest statement
None declared.
Acknowledgements
This work was supported by Royal Free Hospital.
References
- 1.Chung SH, Heimbürger O, Lindholm B, et al. Peritoneal dialysis patient survival: a comparison between a Swedish and a Korean centre. Nephrol Dial Transplant 2005; 20: 1207–1213 [DOI] [PubMed] [Google Scholar]
- 2.Davenport A. Peritonitis remains the major clinical complication of peritoneal dialysis: the London, UK, peritonitis audit 2002–2003. Perit Dial Int 2009; 29: 297–302 [PubMed] [Google Scholar]
- 3.Szeto CC, Wong TY, Chow KM, et al. Independent effects of renal and peritoneal clearances on the mortality of peritoneal dialysis patients. Perit Dial Int 2004; 24: 58–64 [PubMed] [Google Scholar]
- 4.Maiorca R, Brunori G, Zubani R, et al. Predictive value of dialysis adequacy and nutritional indices for mortality and morbidity in CAPD and HD patients. A longitudinal study. Nephrol Dial Transplant 1995; 10: 2295–2305 [DOI] [PubMed] [Google Scholar]
- 5.Diaz-Buxo JA, Lowrie EG, Lew NL, et al. Associates of mortality among peritoneal dialysis patients with special reference to peritoneal transport rates and solute clearance. Am J Kidney Dis 1999; 33: 523–534 [DOI] [PubMed] [Google Scholar]
- 6.Rocco M, Soucie JM, Pastan S, et al. Peritoneal dialysis adequacy and risk of death. Kidney Int 2000; 58: 446–457 [DOI] [PubMed] [Google Scholar]
- 7.Shemin D, Bostom AG, Lambert C, et al. Residual renal function in a large cohort of peritoneal dialysis patients: change over time, impact on mortality and nutrition. Perit Dial Int 2000; 20: 439–444 [PubMed] [Google Scholar]
- 8.Rocco MV, Frankenfield DL, Prowant B, et al. ; Centers for Medicare & Medicaid Services Peritoneal Dialysis Core Indicators Study Group. Risk factors for early mortality in U.S. peritoneal dialysis patients: impact of residual renal function. Perit Dial Int 2002; 22: 371–379 [PubMed] [Google Scholar]
- 9.Szeto CC, Wong TY, Chow KM, et al. Independent effects of renal and peritoneal clearances on the mortality of peritoneal dialysis patients. Perit Dial Int 2004; 24: 58–64 [PubMed] [Google Scholar]
- 10.Szeto CC, Wong TY, Leung CB, et al. Importance of dialysis adequacy in mortality and morbidity of Chinese CAPD patients. Kidney Int 2000; 58: 400–407 [DOI] [PubMed] [Google Scholar]
- 11.Ateş K, Nergizoğlu G, Keven K, et al. Effect of fluid and sodium removal on mortality in peritoneal dialysis patients. Kidney Int 2001; 60: 767–776 [DOI] [PubMed] [Google Scholar]
- 12.Bargman JM, Thorpe KE, Churchill DN, et al. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol 2001; 12: 2158–2162 [DOI] [PubMed] [Google Scholar]
- 13.Paniagua R, Amato D, Vonesh E, et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol 2002; 13: 1307–1320 [DOI] [PubMed] [Google Scholar]
- 14.Termorshuizen F, Korevaar JC, Dekker FW, et al. The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. Am J Kidney Dis 2003; 41: 1293–1302 [DOI] [PubMed] [Google Scholar]
- 15.Chung SH, Heimburger O, Stenvinkel P, et al. Association between residual renal function, inflammation and patient survival in new peritoneal dialysis patients. Nephrol Dial Transplant 2003; 18: 590–597 [DOI] [PubMed] [Google Scholar]
- 16.Rumpsfeld M, McDonald SP, Johnson DW. Peritoneal small solute clearance is nonlinearly related to patient survival in the Australian and New Zealand peritoneal dialysis patient populations. Perit Dial Int 2009; 29: 637–646 [PubMed] [Google Scholar]
- 17.Suda T, Hiroshige K, Ohta T, et al. The contribution of residual renal function to overall nutritional status in chronic haemodialysis patients. Nephrol Dial Transplant 2000; 15: 396–401 [DOI] [PubMed] [Google Scholar]
- 18.Davenport A, Cholongitas E, Xirouchakis E, et al. Pitfalls in assessing renal function in patients with cirrhosis—potential inequity for access to treatment of hepatorenal failure and liver transplantation. Nephrol Dial Transplant 2011; 26: 2735–2742 [DOI] [PubMed] [Google Scholar]
- 19.van Olden RW, Krediet RT, Struijk DG, et al. Measurement of residual renal function in patients treated with continuous ambulatory peritoneal dialysis. J Am Soc Nephrol 1996; 7: 745–750 [DOI] [PubMed] [Google Scholar]
- 20.Caravaca F, Arrobas M, Pizarro JL, et al. Uraemic symptoms, nutritional status and renal function in pre-dialysis end-stage renal failure patients. Nephrol Dial Transplant 2001; 16: 776–782 [DOI] [PubMed] [Google Scholar]
- 21.Peritoneal Dialysis Adequacy Work Group: NKF-K/DOQI Clinical Practice Guidelines for peritoneal dialysis adequacy: update 2006 Am J Kidney Dis 2006; 48(Suppl 1): S98–S129 [DOI] [PubMed] [Google Scholar]
- 22.Fürstenberg A, Davenport A. Assessment of body composition in peritoneal dialysis patients using bioelectrical impedance and dual-energy x-ray absorptiometry. Am J Nephrol 2011; 33: 150–156 [DOI] [PubMed] [Google Scholar]
- 23.Davenport A. AKI in a patient with cirrhosis and ascites. Clin J Am Soc Nephrol 2012; 7: 2041–2048 [DOI] [PubMed] [Google Scholar]
- 24.Hoste EA, Cruz DN, Davenport A, et al. The epidemiology of cardiac surgery-associated acute kidney injury. Int J Artif Organs 2008; 31: 158–165 [DOI] [PubMed] [Google Scholar]
- 25.Agarwal R. Reproducibility of renal function measurements in adult men with diabetic nephropathy: research and clinical implications. Am J Nephrol 2007; 27: 92–100 [DOI] [PubMed] [Google Scholar]
- 26.Haynes R, Staplin N, Emberson J, et al. Evaluating the contribution of the cause of kidney disease to prognosis in CKD: results from the Study of Heart and Renal Protection (SHARP). Am J Kidney Dis 2014; 64: 40–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao CT, Chen YM, Shiao CC, et al. Rate of decline of residual renal function is associated with all-cause mortality and technique failure in patients on long-term peritoneal dialysis. Nephrol Dial Transplant 2009; 24: 2909–2914 [DOI] [PubMed] [Google Scholar]
- 28.Moist LM, Port FK, Orzol SM, et al. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol 2000; 11: 556–564 [DOI] [PubMed] [Google Scholar]
- 29.Davenport A. Intradialytic complications during hemodialysis. Hemodial Int 2006; 10: 162–167 [DOI] [PubMed] [Google Scholar]
- 30.Szeto CC, Kwan BC, Chow KM, et al. Predictors of residual renal function decline in patients undergoing continuous ambulatory peritoneal dialysis. Perit Dial Int 2014; PMID: 24497594 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palomo-Piñón S, Mora-Villalpando CJ, Del Carmen Prado-Uribe M, et al. Inflammation and myocardial damage markers influence loss of residual renal function in peritoneal dialysis patients. Arch Med Res 2014. pii: S0188-4409(14)00156-8 [DOI] [PubMed] [Google Scholar]
- 32.Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med 1994; 330: 877–884 [DOI] [PubMed] [Google Scholar]
- 33.Levey AS, Greene T, Sarnak MJ, et al. Effect of dietary protein restriction on the progression of kidney disease: long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am J Kidney Dis 2006; 48: 879–888 [DOI] [PubMed] [Google Scholar]
- 34.Menon V, Kopple JD, Wang X, et al. Effect of a very low-protein diet on outcomes: long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am J Kidney Dis 2009; 53: 208–217 [DOI] [PubMed] [Google Scholar]
- 35.Jiang N, Qian J, Sun W, et al. Better preservation of residual renal function in peritoneal dialysis patients treated with a low-protein diet supplemented with keto acids: a prospective, randomized trial. Nephrol Dial Transplant 2009; 24: 2551–2558 [DOI] [PubMed] [Google Scholar]
- 36.Johnson DW, Mudge DW, Sturtevant JM, et al. Predictors of decline of residual renal function in new peritoneal dialysis patients. Perit Dial Int 2003; 23: 276–283 [PubMed] [Google Scholar]
- 37.House AA, Anand I, Bellomo R, et al. ; Acute Dialysis Quality Initiative Consensus Group. Definition and classification of Cardio-Renal Syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant 2010; 25: 1416–1420 [DOI] [PubMed] [Google Scholar]
- 38.Marcantoni C, Jafar TH, Oldrizzi L, et al. The role of systemic hypertension in the progression of nondiabetic renal disease. Kidney Int Suppl 2000; 75: S44–S48 [PubMed] [Google Scholar]
- 39.Jansen MA, Hart AA, Korevaar JC, et al. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int 2002; 62: 1046–1053 [DOI] [PubMed] [Google Scholar]
- 40.Davenport A, Anker SD, Mebazaa A, et al. ; Acute Dialysis Quality Initiative (ADQI) consensus group. ADQI 7: the clinical management of the Cardio-Renal syndromes: work group statements from the 7th ADQI consensus conference. Nephrol Dial Transplant 2010; 25: 2077–2089 [DOI] [PubMed] [Google Scholar]
- 41.Siew E, Davenport A. The growth of acute kidney injury. A rising tide of just attention to injury? Kidney Int 2014; doi:10.1038/ki.2014.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolesnyk I, Noordzij M, Dekker FW, et al. Treatment with angiotensin II inhibitors and residual renal function in peritoneal dialysis patients. Perit Dial Int 2011; 31: 53–59 [DOI] [PubMed] [Google Scholar]
- 43.Li PK, Chow KM, Wong TY, et al. Effects of an angiotensin-converting enzyme inhibitor on residual renal function in patients receiving peritoneal dialysis. A randomized, controlled study. Ann Intern Med 2003; 139: 105–112 [DOI] [PubMed] [Google Scholar]
- 44.Suzuki H, Kanno Y, Sugahara S, et al. Effects of an angiotensin II receptor blocker, valsartan, on residual renal function in patients on CAPD. Am J Kidney Dis 2004; 43: 1056–1064 [DOI] [PubMed] [Google Scholar]
- 45.Reyes-Marin FA, Calzada C, Ballesteros A, et al. Comparative study of enalapril vs. losartan on residual renal function preservation in automated peritoneal dialysis. A randomized controlled study. Res Invest Clin 2012; 64: 315–321 [PubMed] [Google Scholar]
- 46.Zhang L, Zeng X, Fu P, et al. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for preserving residual kidney function in peritoneal dialysis patients. Cochrane Database Syst Rev 2014; 6: CD009120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phakdeekitcharoen B, Leelasa-nguan P. Effects of an ACE inhibitor or angiotensin receptor blocker on potassium in CAPD patients. Am J Kidney Dis 2004; 44: 738–746 [PubMed] [Google Scholar]
- 48.van Olden RW, Guchelaar HJ, Struijk DG, et al. Acute effects of high-dose furosemide on residual renal function in CAPD patients. Perit Dial Int 2003; 23: 339–347 [PubMed] [Google Scholar]
- 49.Medcalf JF, Harris KP, Walls J. Role of diuretics in the preservation of residual renal function in patients on continuous ambulatory peritoneal dialysis. Kidney Int 2001; 59: 1128–1133 [DOI] [PubMed] [Google Scholar]
- 50.Selby NM, Fonseca S, Hulme L, et al. Automated peritoneal dialysis has significant effects on systemic hemodynamics. Perit Dial Int 2006; 26: 328–335 [PubMed] [Google Scholar]
- 51.Hiroshige K, Yuu K, Soujima M, et al. Rapid decline of residual renal function in patients on automated peritoneal dialysis. Perit Dial Int 1996; 16: 307–315 [PubMed] [Google Scholar]
- 52.Hufnagel G, Michel C, Queffeulou G, et al. The influence of automated peritoneal dialysis on the decrease in residual renal function. Nephrol Dial Transplant 1999; 14: 1224–1228 [DOI] [PubMed] [Google Scholar]
- 53.de Fijter CW, ter Wee PM, Donker AJ. The influence of automated peritoneal dialysis on the decrease in residual renal function. Nephrol Dial Transplant 2000; 15: 1094–1096 [DOI] [PubMed] [Google Scholar]
- 54.Balasubramanian G, McKitty K, Fan SL. Comparing automated peritoneal dialysis with continuous ambulatory peritoneal dialysis: survival and quality of life differences? Nephrol Dial Transplant 2011; 26: 1702–1708 [DOI] [PubMed] [Google Scholar]
- 55.Cnossen TT, Usvyat L, Kotanko P, et al. Comparison of outcomes on continuous ambulatory peritoneal dialysis versus automated peritoneal dialysis: results from a USA database. Perit Dial Int 2011; 31: 679–684 [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez AM, Diaz NV, Cubillo LP, et al. Automated peritoneal dialysis: a Spanish multicentre study. Nephrol Dial Transplant 1998; 13: 2335–2340 [DOI] [PubMed] [Google Scholar]
- 57.Bro S, Bjorner JB, Tofte-Jensen P, et al. A prospective, randomized multicenter study comparing APD and CAPD treatment. Perit Dial Int 1999; 19: 526–533 [PubMed] [Google Scholar]
- 58.Gallar P, Ortega O, Carreno A, et al. Rate of decline in residual renal function is equal in CAPD and automated peritoneal dialysis patients. Perit Dial Int 2000; 20: 803–805 [PubMed] [Google Scholar]
- 59.Singhal MK, Bhaskaran S, Vidgen E, et al. Rate of decline of residual renal function in patients on continuous peritoneal dialysis and factors affecting it. Perit Dial Int 2000; 20: 429–438 [PubMed] [Google Scholar]
- 60.Holley JL, Aslam N, Bernardini J, et al. The influence of demographic factors and modality on loss of residual renal function in incident peritoneal dialysis patients. Perit Dial Int 2001; 21: 302–305 [PubMed] [Google Scholar]
- 61.Hidaka H, Nakao T. Preservation of residual renal function and factors affecting its decline in patients on peritoneal dialysis. Nephrology 2003; 8: 184–191 [DOI] [PubMed] [Google Scholar]
- 62.Rodriguez-Carmona A, Perez-Fontan M, Garca-Naveiro R, et al. Compared time profiles of ultrafiltration, sodium removal, and renal function in incident CAPD and automated peritoneal dialysis patients. Am J Kidney Dis 2004; 44: 132–145 [DOI] [PubMed] [Google Scholar]
- 63.Rabindranath KS, Adams J, Ali TZ, et al. Automated vs continuous ambulatory peritoneal dialysis: a systematic review of randomized controlled trials. Nephrol Dial Transplant 2007; 22: 2991–2998 [DOI] [PubMed] [Google Scholar]
- 64.Su YJ, Lee CT, Chuang FR, et al. Comparison of clinical characteristics between automated peritoneal dialysis and continuous ambulatory peritoneal dialysis: a 2-year single-center observational study. Ren Fail 2010; 32: 817–824 [DOI] [PubMed] [Google Scholar]
- 65.Michels WM, Verduijn M, Grootendorst DC, et al. Decline in residual renal function in automated compared with continuous ambulatory peritoneal dialysis. Clin J Am Soc Nephrol 2011; 6: 537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Williams JD, Topley N, Craig KJ, et al. The Euro-Balance Trial: the effect of a new biocompatible peritoneal dialysis fluid (balance) on the peritoneal membrane. Kidney Int 2004; 66: 408–418 [DOI] [PubMed] [Google Scholar]
- 67.Feriani M, Kirchgessner J, La Greca G, et al. Randomized long-term evaluation of bicarbonate-buffered CAPD solution. Kidney Int 1998; 54: 1731–1738 [DOI] [PubMed] [Google Scholar]
- 68.Coles GA, O'Donoghue DJ, Pritchard N, et al. A controlled trial of two bicarbonate-containing dialysis fluids for CAPD—final report. Nephrol Dial Transplant 1998; 13: 3165–3171 [DOI] [PubMed] [Google Scholar]
- 69.Rippe B, Simonsen O, Heimburger O, et al. Long-term clinical effects of a peritoneal dialysis fluid with less glucose degradation products. Kidney Int 2001; 59: 348–357 [DOI] [PubMed] [Google Scholar]
- 70.Szeto CC, Chow KM, Lam CW, et al. Clinical biocompatibility of a neutral peritoneal dialysis solution with minimal glucose-degradation products—a 1-year randomized control trial. Nephrol Dial Transplant 2007; 22: 552–559 [DOI] [PubMed] [Google Scholar]
- 71.Fan SL, Pile T, Punzalan S, et al. Randomized controlled study of biocompatible peritoneal dialysis solutions: effect on residual renal function. Kidney Int 2008; 73: 200–206 [DOI] [PubMed] [Google Scholar]
- 72.Bajo MA, Perez-Lozano ML, Albar-Vizcaino P, et al. Low-GDP peritoneal dialysis fluid (‘balance’) has less impact in vitro and ex vivo on epithelial-to-mesenchymal transition (EMT) of mesothelial cells than a standard fluid. Nephrol Dial Transplant 2011; 26: 282–291 [DOI] [PubMed] [Google Scholar]
- 73.Johnson DW, Brown FG, Clarke M, et al. Effects of biocompatible versus standard fluid on peritoneal dialysis outcomes. J Am Soc Nephrol 2012; 23: 1097–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choi HY, Kim DK, Lee TH, et al. The clinical usefulness of peritoneal dialysis fluids with neutral pH and low glucose degradation product concentration: an open randomized prospective trial. Perit Dial Int 2008; 28: 174–182 [PubMed] [Google Scholar]
- 75.Cho KH, Do JY, Park JW, et al. The effect of low-GDP solution on ultrafiltration and solute transport in continuous ambulatory peritoneal dialysis patients. Perit Dial Int 2013; 33: 382–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pajek J, Kveder R, Bren A, et al. Short-term effects of bicarbonate/lactate-buffered and conventional lactate-buffered dialysis solutions on peritoneal ultrafiltration: a comparative crossover study. Nephrol Dial Transplant 2009; 24: 1617–1625 [DOI] [PubMed] [Google Scholar]
- 77.Davies SJ, Woodrow G, Donovan K, et al. Icodextrin improves the fluid status of peritoneal dialysis patients: results of a double-blind randomized controlled trial. J Am Soc Nephrol 2003; 14: 2338–2344 [DOI] [PubMed] [Google Scholar]
- 78.Tranaeus A. A long-term study of a bicarbonate/lactate-based peritoneal dialysis solution—clinical benefits. The Bicarbonate/Lactate Study Group. Perit Dial Int 2000; 20: 516–523 [PubMed] [Google Scholar]
- 79.Montenegro J, Saracho RM, Martinez IM, et al. Long-term clinical experience with pure bicarbonate peritoneal dialysis solutions. Perit Dial Int 2006; 26: 89–94 [PubMed] [Google Scholar]
- 80.Weiss L, Stegmayr B, Malmsten G, et al. Biocompatibility and tolerability of a purely bicarbonate-buffered peritoneal dialysis solution. Perit Dial Int 2009; 29: 647–655 [PubMed] [Google Scholar]
- 81.Haag-Weber M, Kramer R, Haake R, et al. Low-GDP fluid (Gambrosol trio) attenuates decline of residual renal function in PD patients: a prospective randomized study. Nephrol Dial Transplant 2010; 25: 2288–2296 [DOI] [PubMed] [Google Scholar]
- 82.Kim S, Oh KH, Oh J, et al. Biocompatible peritoneal dialysis solution preserves residual renal function. Am J Nephrol 2012; 36: 305–316 [DOI] [PubMed] [Google Scholar]
- 83.Cho Y, Johnson DW, Craig JC, et al. Biocompatible dialysis fluids for peritoneal dialysis. Cochrane Database Syst Rev 2014; 3: CD007554. [DOI] [PubMed] [Google Scholar]
- 84.Davies SJ, Garcia Lopez E, Woodrow G, et al. Longitudinal relationships between fluid status, inflammation, urine volume and plasma metabolites of icodextrin in patients randomized to glucose or icodextrin for the long exchange. Nephrol Dial Transplant 2008; 23: 2982–2988 [DOI] [PubMed] [Google Scholar]
- 85.Konings CJ, Kooman JP, Schonck M, et al. Effect of icodextrin on volume status, blood pressure and echocardiographic parameters: a randomized study. Kidney Int 2003; 63: 1556–1563 [DOI] [PubMed] [Google Scholar]
- 86.Adachi Y, Nakagawa Y, Nishio A. Icodextrin preserves residual renal function in patients treated with automated peritoneal dialysis. Perit Dial Int 2006; 26: 405–407 [PubMed] [Google Scholar]
- 87.Posthuma N, ter Wee PM, Verbrugh HA, et al. Icodextrin instead of glucose during the daytime dwell in CCPD increases ultrafiltration and 24-h dialysate creatinine clearance. Nephrol Dial Transplant 1997; 12: 550–553 [DOI] [PubMed] [Google Scholar]
- 88.Plum J, Gentile S, Verger C, et al. Efficacy and safety of a 7.5% icodextrin peritoneal dialysis solution in patients treated with automated peritoneal dialysis. Am J Kidney Dis 2002; 39: 862–871 [DOI] [PubMed] [Google Scholar]
- 89.Wolfson M, Piraino B, Hamburger RJ, et al. A randomized controlled trial to evaluate the efficacy and safety of icodextrin in peritoneal dialysis. Am J Kidney Dis 2002; 40: 1055–1065 [DOI] [PubMed] [Google Scholar]
- 90.Takatori Y, Akagi S, Sugiyama H, et al. Icodextrin increases technique survival rate in peritoneal dialysis patients with diabetic nephropathy by improving body fluid management: a randomized controlled trial. Clin J Am Soc Nephrol 2011; 6: 1337–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Posthuma N, ter Wee PM, Donker AJ, et al. Assessment of the effectiveness, safety, and biocompatibility of icodextrin in automated peritoneal dialysis. The Dextrin in APD in Amsterdam (DIANA) Group. Perit Dial Int 2000; 20(Suppl 2): S106–S113 [PubMed] [Google Scholar]
- 92.Papakrivopoulou E, Booth J, Pinney J, et al. Comparison of volume status in asymptomatic haemodialysis and peritoneal dialysis outpatients. Nephron Extra 2012; 2: 48–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Davenport A, Willicombe M. Comparison of fluid status in patients treated by different modalities of peritoneal dialysis using multi-frequency bioimpedance. Int J Artif Organs 2009; 32: 779–786 [DOI] [PubMed] [Google Scholar]
- 94.Inal S, Erten Y, Okyay GU, et al. Association between bioimpedance analysis parameters and left ventricular hypertrophy in peritoneal dialysis patients. Int Urol Nephrol 2014; 46: 1851–1856 [DOI] [PubMed] [Google Scholar]
- 95.Davenport A, Sayed RH, Fan S. Is extracellular volume expansion of peritoneal dialysis patients associated with greater urine output? Blood Purif 2011; 32: 226–231 [DOI] [PubMed] [Google Scholar]
- 96.Fan S, Sayed RH, Davenport A. Extracellular volume expansion in peritoneal dialysis patients. Int J Artif Organs 2012; 35: 338–345 [DOI] [PubMed] [Google Scholar]
- 97.Papakrivopoulou E, Lillywhite S, Davenport A. Is N-terminal probrain-type natriuretic peptide a clinically useful biomarker of volume overload in peritoneal dialysis patients? Nephrol Dial Transplant 2012; 27: 396–401 [DOI] [PubMed] [Google Scholar]
- 98.McCafferty K, Fan S, Davenport A. Extracellular volume expansion, measured by multifrequency bioimpedance, does not help preserve residual renal function in peritoneal dialysis patients. Kidney Int 2014; 85: 151–157 [DOI] [PubMed] [Google Scholar]
- 99.Shemin D, Maaz D, St Pierre D, et al. Effect of aminoglycoside use on residual renal function in peritoneal dialysis patients. Am J Kidney Dis 1999; 34: 14–20 [DOI] [PubMed] [Google Scholar]
- 100.Baker RJ, Senior H, Clemenger M, et al. Empirical aminoglycosides for peritonitis do not affect residual renal function. Am J Kidney Dis 2003; 41: 670–675 [DOI] [PubMed] [Google Scholar]
- 101.Badve SV, Hawley CM, McDonald SP, et al. Use of aminoglycosides for peritoneal dialysis-associated peritonitis does not affect residual renal function. Nephrol Dial Transplant 2012; 27: 381–387 [DOI] [PubMed] [Google Scholar]
- 102.Lui SL, Cheng SW, Ng F, et al. Cefazolin plus netilmicin versus cefazolin plus ceftazidime for treating CAPD peritonitis: effect on residual renal function. Kidney Int 2005; 68: 2375–2380 [DOI] [PubMed] [Google Scholar]
- 103.Weisbord SD, Bernardini J, Mor MK, et al. The effect of coronary angiography on residual renal function in patients on peritoneal dialysis. Clin Cardiol 2006; 29: 494–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dittrich E, Puttinger H, Schillinger M, et al. Effect of radio contrast media on residual renal function in peritoneal dialysis patients—a prospective study. Nephrol Dial Transplant 2006; 21: 1334–1339 [DOI] [PubMed] [Google Scholar]
- 105.Moranne O, Willoteaux S, Pagniez D, et al. Effect of iodinated contrast agents on residual renal function in PD patients. Nephrol Dial Transplant 2006; 21: 1040–1045 [DOI] [PubMed] [Google Scholar]
- 106.Davies SJ, Davenport A. The role of bioimpedance and biomarkers in helping to aid clinical decision-making of volume assessments in dialysis patients. Kidney Int 2014. Jun 11. doi: 10.1038/ki.2014.207 [DOI] [PubMed] [Google Scholar]