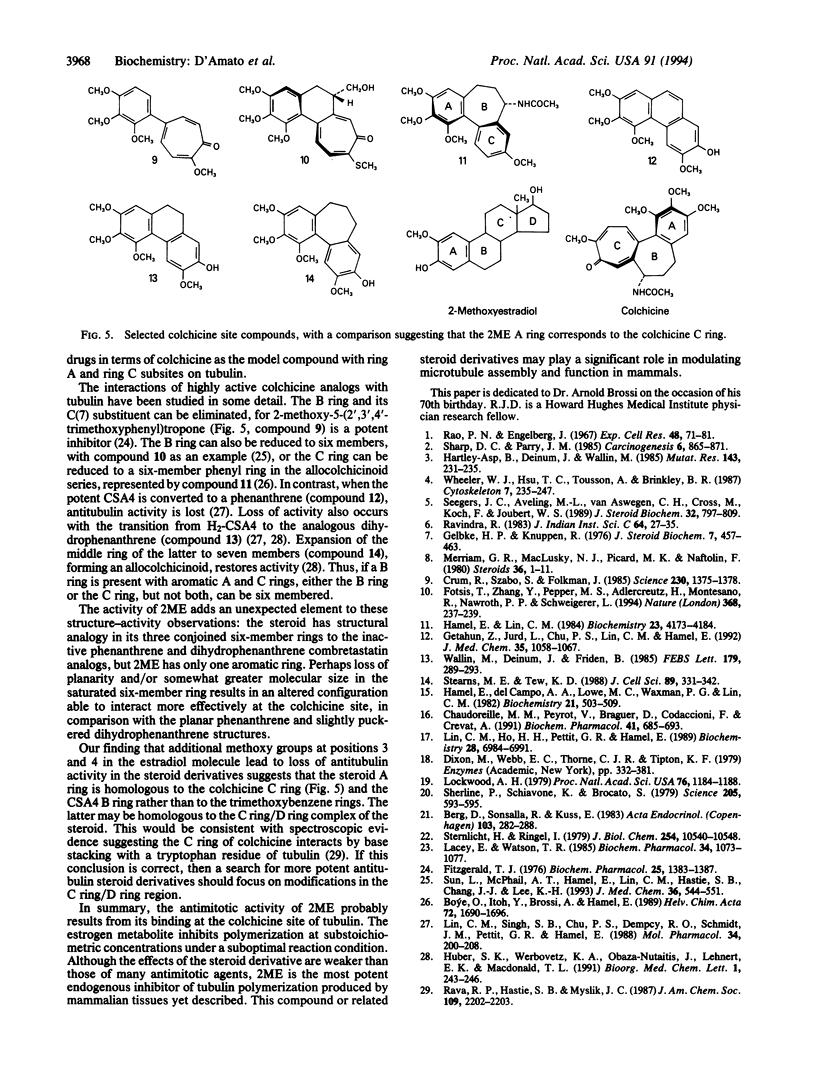
Abstract
A metabolite of estradiol, 2-methoxyestradiol (2ME), inhibits angiogenesis in the chicken embryo chorioallantoic membrane assay. Since 2ME causes mitotic perturbations, we examined its interactions with tubulin. In our standard 1.0 M glutamate system (plus 1.0 mM MgCl2 at 37 degrees C), superstoichiometric concentrations (relative to tubulin) of 2ME inhibited the nucleation and propagation phases of tubulin assembly but did not affect the reaction extent. Although polymer formed in the presence of 2ME was more cold-stable than control polymer, morphology was little changed. Under suboptimal reaction conditions (0.8 M glutamate/no MgCl2 at 26 degrees C), substoichiometric 2ME totally inhibited polymerization. No other estrogenic compound was as effective as 2ME as an inhibitor of polymerization or of the binding of colchicine to tubulin. Inhibition of colchicine binding was competitive (Ki, 22 microM). Thus, a mammalian metabolite of estradiol binds to the colchicine site of tubulin and, depending on reaction conditions, either inhibits assembly or seems to be incorporated into a polymer with altered stability properties.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg D., Sonsalla R., Kuss E. Concentrations of 2-methoxyoestrogens in human serum measured by a heterologous immunoassay with an 125I-labelled ligand. Acta Endocrinol (Copenh) 1983 Jun;103(2):282–288. doi: 10.1530/acta.0.1030282. [DOI] [PubMed] [Google Scholar]
- Chaudoreille M. M., Peyrot V., Braguer D., Codaccioni F., Crevat A. Qualitative study of the interaction mechanism of estrogenic drugs with tubulin. Biochem Pharmacol. 1991 Mar 1;41(5):685–693. doi: 10.1016/0006-2952(91)90067-f. [DOI] [PubMed] [Google Scholar]
- Crum R., Szabo S., Folkman J. A new class of steroids inhibits angiogenesis in the presence of heparin or a heparin fragment. Science. 1985 Dec 20;230(4732):1375–1378. doi: 10.1126/science.2416056. [DOI] [PubMed] [Google Scholar]
- Fitzgerald T. J. Molecular features of colchicine associated with antimitotic activity and inhibition of tubulin polymerization. Biochem Pharmacol. 1976 Jun 15;25(12):1383–1387. doi: 10.1016/0006-2952(76)90108-8. [DOI] [PubMed] [Google Scholar]
- Fotsis T., Zhang Y., Pepper M. S., Adlercreutz H., Montesano R., Nawroth P. P., Schweigerer L. The endogenous oestrogen metabolite 2-methoxyoestradiol inhibits angiogenesis and suppresses tumour growth. Nature. 1994 Mar 17;368(6468):237–239. doi: 10.1038/368237a0. [DOI] [PubMed] [Google Scholar]
- Gelbke H. P., Knuppen R. The excretion of five different 2-hydroxyoestrogen monomethyl ethers in human pregnancy urine. J Steroid Biochem. 1976 Jun-Jul;7(6-7):457–463. doi: 10.1016/0022-4731(76)90112-6. [DOI] [PubMed] [Google Scholar]
- Getahun Z., Jurd L., Chu P. S., Lin C. M., Hamel E. Synthesis of alkoxy-substituted diaryl compounds and correlation of ring separation with inhibition of tubulin polymerization: differential enhancement of inhibitory effects under suboptimal polymerization reaction conditions. J Med Chem. 1992 Mar 20;35(6):1058–1067. doi: 10.1021/jm00084a011. [DOI] [PubMed] [Google Scholar]
- Hamel E., Lin C. M. Separation of active tubulin and microtubule-associated proteins by ultracentrifugation and isolation of a component causing the formation of microtubule bundles. Biochemistry. 1984 Aug 28;23(18):4173–4184. doi: 10.1021/bi00313a026. [DOI] [PubMed] [Google Scholar]
- Hamel E., del Campo A. A., Lowe M. C., Waxman P. G., Lin C. M. Effects of organic acids on tubulin polymerization and associated guanosine 5'-triphosphate hydrolysis. Biochemistry. 1982 Feb 2;21(3):503–509. doi: 10.1021/bi00532a014. [DOI] [PubMed] [Google Scholar]
- Hartley-Asp B., Deinum J., Wallin M. Diethylstilbestrol induces metaphase arrest and inhibits microtubule assembly. Mutat Res. 1985 Aug;143(4):231–235. doi: 10.1016/0165-7992(85)90086-7. [DOI] [PubMed] [Google Scholar]
- Lacey E., Watson T. R. Structure-activity relationships of benzimidazole carbamates as inhibitors of mammalian tubulin, in vitro. Biochem Pharmacol. 1985 Apr 1;34(7):1073–1077. doi: 10.1016/0006-2952(85)90611-2. [DOI] [PubMed] [Google Scholar]
- Lin C. M., Ho H. H., Pettit G. R., Hamel E. Antimitotic natural products combretastatin A-4 and combretastatin A-2: studies on the mechanism of their inhibition of the binding of colchicine to tubulin. Biochemistry. 1989 Aug 22;28(17):6984–6991. doi: 10.1021/bi00443a031. [DOI] [PubMed] [Google Scholar]
- Lin C. M., Singh S. B., Chu P. S., Dempcy R. O., Schmidt J. M., Pettit G. R., Hamel E. Interactions of tubulin with potent natural and synthetic analogs of the antimitotic agent combretastatin: a structure-activity study. Mol Pharmacol. 1988 Aug;34(2):200–208. [PubMed] [Google Scholar]
- Lockwood A. H. Molecules in mammalian brain that interact with the colchicine site on tubulin. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1184–1188. doi: 10.1073/pnas.76.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam G. R., MacLusky N. J., Picard M. K., Naftolin F. Comparative properties of the catechol estrogens, I: methylation by catechol-O-methyltransferase and binding to cytosol estrogen receptors. Steroids. 1980 Jul;36(1):1–11. doi: 10.1016/0039-128x(80)90062-8. [DOI] [PubMed] [Google Scholar]
- Rao P. N., Engelberg J. Structural specificity of estrogens in the induction of mitotic chromatid non-disjunction in HeLa cells. Exp Cell Res. 1967 Oct;48(1):71–81. doi: 10.1016/0014-4827(67)90277-7. [DOI] [PubMed] [Google Scholar]
- Seegers J. C., Aveling M. L., Van Aswegen C. H., Cross M., Koch F., Joubert W. S. The cytotoxic effects of estradiol-17 beta, catecholestradiols and methoxyestradiols on dividing MCF-7 and HeLa cells. J Steroid Biochem. 1989 Jun;32(6):797–809. doi: 10.1016/0022-4731(89)90455-x. [DOI] [PubMed] [Google Scholar]
- Sharp D. C., Parry J. M. Diethylstilboestrol: the binding and effects of diethylstilboestrol upon the polymerisation and depolymerisation of purified microtubule protein in vitro. Carcinogenesis. 1985 Jun;6(6):865–871. doi: 10.1093/carcin/6.6.865. [DOI] [PubMed] [Google Scholar]
- Sherline P., Schiavone K., Brocato S. Endogenous inhibitor of colchicine-tubulin binding in rat brain. Science. 1979 Aug 10;205(4406):593–595. doi: 10.1126/science.451622. [DOI] [PubMed] [Google Scholar]
- Stearns M. E., Tew K. D. Estramustine binds MAP-2 to inhibit microtubule assembly in vitro. J Cell Sci. 1988 Mar;89(Pt 3):331–342. doi: 10.1242/jcs.89.3.331. [DOI] [PubMed] [Google Scholar]
- Sternlicht H., Ringel I. Colchicine inhibition of microtubule assembly via copolymer formation. J Biol Chem. 1979 Oct 25;254(20):10540–10550. [PubMed] [Google Scholar]
- Sun L., McPhail A. T., Hamel E., Lin C. M., Hastie S. B., Chang J. J., Lee K. H. Antitumor agents. 139. Synthesis and biological evaluation of thiocolchicine analogs 5,6-dihydro-6(S)-(acyloxy)- and 5,6-dihydro-6(S)-[(aroyloxy)methyl]-1,2,3-trimethoxy-9-(methylthio)-8H- cyclohepta[a]naphthalen-8-ones as novel cytotoxic and antimitotic agents. J Med Chem. 1993 Mar 5;36(5):544–551. doi: 10.1021/jm00057a004. [DOI] [PubMed] [Google Scholar]
- Wallin M., Deinum J., Fridén B. Interaction of estramustine phosphate with microtubule-associated proteins. FEBS Lett. 1985 Jan 7;179(2):289–293. doi: 10.1016/0014-5793(85)80536-6. [DOI] [PubMed] [Google Scholar]
- Wheeler W. J., Hsu T. C., Tousson A., Brinkley B. R. Mitotic inhibition and chromosome displacement induced by estradiol in Chinese hamster cells. Cell Motil Cytoskeleton. 1987;7(3):235–247. doi: 10.1002/cm.970070306. [DOI] [PubMed] [Google Scholar]