Abstract
The concept that the intestine and chronic kidney disease influence each other, emerged only recently. The problem is multifaceted and bidirectional. On one hand, the composition of the intestinal microbiota impacts uraemic retention solute production, resulting in the generation of essentially protein-bound uraemic toxins with strong biological impact such as vascular damage and progression of kidney failure. On the other hand, the uraemic status affects the composition of intestinal microbiota, the generation of uraemic retention solutes and their precursors and causes disturbances in the protective epithelial barrier of the intestine and the translocation of intestinal microbiota into the body. All these elements together contribute to the disruption of the metabolic equilibrium and homeostasis typical to uraemia. Several measures with putative impact on intestinal status have recently been tested for their influence on the generation or concentration of uraemic toxins. These include dietary measures, prebiotics, probiotics, synbiotics and intestinal sorbents. Unfortunately, the quality and the evidence base of many of these studies are debatable, especially in uraemia, and often results within one study or among studies are contradictory. Nevertheless, intestinal uraemic metabolite generation remains an interesting target to obtain in the future as an alternative or additive to dialysis to decrease uraemic toxin generation. In the present review, we aim to summarize (i) the role of the intestine in uraemia by producing uraemic toxins and by generating pathophysiologically relevant changes, (ii) the role of uraemia in modifying intestinal physiology and (iii) the therapeutic options that could help to modify these effects and the studies that have assessed the impact of these therapies.
Keywords: uraemic toxins, CKD, haemodialysis, creatinine, systematic review
Introduction: the intestine in health and disease
The previous view that the gut is a largely inert organ has been challenged for more than a decade, as intestinal microbiota provide essential functions which the human body by itself is unable to supply [1], such as the harvesting of essential food products, energy saving, metabolism of xenobiotics, protection against invasion by alien microbes, renewal of the intestinal epithelium, generation of vitamins and balancing of immune function [1, 2].
The definition of the ‘normal’ microbiome with its striking inter-individual differences, sometimes due to day-by-day elements such as diet or exercise [3, 4], raised the obvious question about what happens during disease [5, 6]. Already in 2003, intestinal microbiome changes were described for multi-organ failure, colon cancer and inflammatory bowel disease [2]. In the last decade, this list was completed by a long series of highly prevalent chronic diseases. The relation between liver disease and gut microbiome could be expected by their juxtaposition [7]. However, also a link was demonstrated, as cause or consequence, with diabetes mellitus, obesity, insulin resistance, the metabolic syndrome, ageing, micro-inflammation and cardiovascular disease [6, 8, 9].
The awareness that also chronic kidney disease (CKD) is related to the intestinal status came relatively late [10] and stemmed in part from the insight that several toxins active in the uraemic syndrome originate from the intestine [11, 12].
Many of the above-mentioned chronic conditions linked to changes in the gut microbiome are also associated with renal failure or are considered as a cause of it. The question has been raised whether the gut microbiome might be one of the common denominators linking all these elements together [13, 14]. It could be possible that some of the solutes generated in the gut and currently called uraemic toxins exert their toxicity even with normally functioning kidneys [15] and thus are not only uraemic toxins but toxins in general. This hypothesis, however, remains to be confirmed.
Thus, there are good reasons to take a close look at the interaction between the gut and kidneys, especially since understanding the mechanisms might create opportunities to develop novel targeted therapeutic interventions [13, 16]. In this narrative review, we will consider the current state of the art on this topic, including the bidirectional interaction between gut and uraemic toxicity, a proof of concept of the role of the intestine in uraemic toxin generation, the evidence of the toxicity of the generated molecules and a comprehensive review of the available therapeutic options, considering uraemic toxicity/toxins as key elements.
The bidirectional interference between the gut and uraemia
The gut is responsible for the generation of uraemic retention compounds with pathophysiologic impact, whereas uraemia as such causes structural and functional intestinal changes which indirectly cause morbidity and mortality [17] by inducing inflammation via diverse pathways [10]. Thus, the crosstalk between uraemia and the intestine is based on two different elements, but the consequences are the same, based on a double effect: induction of micro-inflammation going along with an incompetence of the pre-activated immune system to cope with stimuli [10, 18], resulting in an enhanced risk of infection which at the end in its turn again is pro-inflammatory. Inflammation remains one of the major causes of complications and death in CKD but also in non-CKD conditions.
The gut as a source of uraemic toxins
Uraemic toxins are generated in several ways (Figure 1): (i) some of them (e.g. peptides) are produced in the body without the contribution of the gut; (ii) others (e.g. the advanced glycation end products—AGEs) enter the body via unmodified absorption of nutritional elements by the intestine; (iii) finally, essentially protein-bound solutes such as the phenols, indoles or hippurates but also trimethylamine oxide (TMAO) are generated metabolically from precursors originating from microbial fermentation [12, 19]. A number of retention solutes (e.g. AGEs, uric acid) originate partially in the intestine and partially in the body.
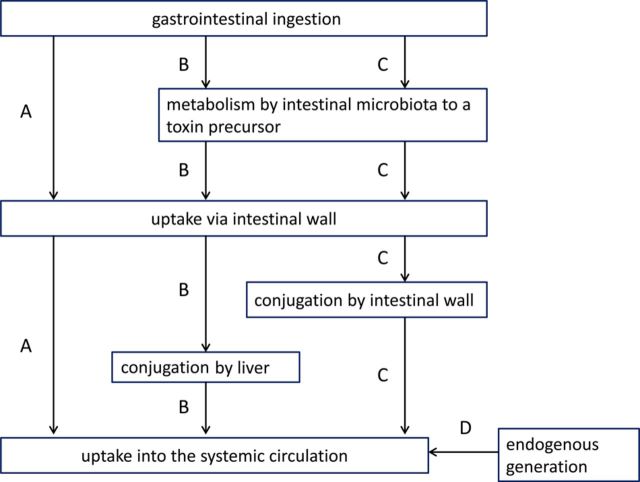
Fig. 1.
Main metabolic pathways involved in uraemic toxin generation. (A) ingestion via the gastrointestinal tract and direct unmodified uptake via the intestinal wall into the systemic circulation (e.g. advanced glycation end products); (B) gastrointestinal uptake of a precursor (e.g. an amino acid), that is transformed by the intestinal microbiota into another precursor (e.g. indole); after its uptake via the intestinal wall and portal vein, this precursor is further conjugated by the liver (e.g. indoxyl sulphate) before its transfer into the systemic circulation; (C) gastrointestinal uptake of a precursor (e.g. an amino acid), that is transformed by the intestinal microbiota into another precursor (e.g. indole); the precursor is conjugated during its uptake via the intestinal wall (e.g. indoxyl sulphate) and then passes into the systemic circulation via the liver without further modification; (D) endogenous generation without contribution of the gastro-intestinal tract (e.g. β2-microglobulin). Modified from Schepers et al. [12].
The active role of the intestine is especially prominent in the generation of the protein-bound uraemic toxins (e.g. indoxyl sulphate and p-cresyl sulphate) (Figure 1, pathways B and C). At the origin are amino acids (in casu tryptophan and tyrosine) resulting from the digestion of proteins that are transformed by the intestinal microbiota into precursors of uraemic toxins like indole and p-cresol). Those are then conjugated during absorption in the intestinal wall or further downstream in the liver before their transfer into the systemic circulation [12]. The most abundant conjugates are the sulphates although many other structural variants are possible (Table 1). In addition, functional groups, such as methyl or hydroxyl, may be added in different positions. Some compounds remain unmodified (e.g. kynurenine).
Table 1.
Precursors and conjugates composing intestinally generated uraemic metabolitesa
| Precursors |
| Indole |
| Phenol |
| Cresol |
| Hippurate |
| Methylamine |
| Conjugates |
| Sulphate |
| Lactate |
| Acetate |
| Glucuronide |
| Propionate |
| Acetylglycine |
| Propionylglycine |
| Oxide |
aThe list is not exhaustive.
Additional contributing factors
In uraemia, a number of further intestinal modifications involved in the so-called assimilation process cause an increase in the generation of uraemic toxins compared with the normal condition. First, amino acids, if less well absorbed along the gastrointestinal tract, present more substrate to the intestine for microbial metabolism [12]. Second, the balance of intestinal microbiota is shifted in favour of proteolytic microorganisms producing more toxin precursor by fermentation (see below) [20–22]. Finally, abnormal intestinal motility prolonging colon transit [23, 24] deprives the colon from carbohydrates inducing upstream expansion of proteolytic species increasing generation of bacterial toxins and increasing time for their absorption [23]. In an elegant study, Bammens et al. assessed the urinary excretion of p-cresol, the precursor of cresyl conjugates and a surrogate of their generation: approximately twice as much p-cresol was excreted per 24 h in the urine of advanced CKD patients than that of controls with normal kidney function, and urinary p-cresol excretion was negatively correlated with GFR [25].
It is an upside-down world: changes of the intestine due to uraemia
The intestine does not only play a role in the generation of uraemic toxins, but also the other way around uraemia (and by extension uraemic toxins) may cause functional and structural modifications in the intestine with patho-physiologic consequences.
First, bacterial species that are prone to generate uraemic toxins by fermentation are privileged in uraemia (Table 2) and outnumber protective species such as lactobacilli [20, 21]. Up till now, only three studies compared the composition of the intestinal microbiota of uraemic versus control patients by bacterial genomic analysis: all found a disbalance in the proportional distribution of intestinal bacteria with a predominance of species with a pathologic impact and/or a shortage of those having no deleterious effect [21, 26, 27], identical to what had been demonstrated before in uraemic rats [26]. Earlier studies using non-genomic techniques had also defined that a number of bacterial species prone to proteolytic fermentation, among which the clostridia species, were more abundant [20].
Table 2.
Fermenting intestinal bacterial speciesa
| Clostridia |
| Clostridium bifermentans |
| Clostridium sporogenes |
| Clostridium clostridiforme |
| Clostridium leptum |
| Peptostreptococcus asaccholyticus |
| Peptostreptococcus indolicus |
| Bacteroidetes |
| Bacteroides thetalotaomicron |
| Bacteroides putredinis |
| Other |
| Fusobacterium nucleatum |
| Actinomyces israelii |
| Megalosfaera elsdinii |
| Propionibacterium acnes |
aBased on Smith and MacFarlane [20].
Second, colon bacteria in uraemia tend to move to sections of the body where they normally are not present unless in very low quantities (translocation). This includes migration of microbes into the jejunum [23], into the mesenteric lymph nodes and further downstream into the blood and the rest of the body [22, 28–31]. Bossola et al. found bacterial-derived DNA fragments in the blood of haemodialysis patients, but since bacteria were also detected in dialysate, an extra-intestinal source, i.e. contaminated dialysate, could not be excluded [28]. However, Wang et al. reported similar findings in non-dialysed CKD patients, pointing to the fact that also sources other than dialysate can be responsible for these DNA fragments in uraemic patients, and in this case, the intestine is a primary candidate culprit [22]. More convincingly even, all bacterial DNA found in the blood was the same as that from genera present in the gut [22]. In uraemic rats, bacterial DNA of enteric species was demonstrated in the mesenteric lymph nodes, blood and even in liver and spleen [30]. Both in humans and in animals, this bacterial overgrowth and translocation was associated to micro-inflammation [22, 30].
Third, compelling arguments favour a loss of the intestinal protective barrier in uraemia. After the first results in this direction published in 1991 [32], Vaziri and co-workers demonstrated in vitro and ex vivo that the uraemic milieu disrupted intestinal epithelial protection [21, 33] by depleting protein constituents of the tight junctions (Zona Occludens-1 (ZO-1), Claudin-1 and Occludin). The ensuing leakage could cause inflammation by propagating endotoxin transfer into the bloodstream. Vaziri et al. could also demonstrate that urea created the same effect [34], with a further enhancement by adding urease, suggesting that urease producing bacteria generating ammonia amplify this effect [34]. The same authors also demonstrated that the administration of the sorbent AST-120 (KremezinR) protected the intestinal barrier in uraemic rats [35]. As AST-120 is essentially known for decreasing the concentration of protein-bound uraemic solutes rather than that of urea, it can reasonably be assumed that also other uraemic solutes than urea [36], or their precursors, disrupt the intestinal barrier.
Thus, in summary, uraemia causes a series of intestinal changes (bacterial translocation, leakiness of the intestinal barrier and the generation of protein-bound uraemic toxins) that all lead to inflammation, a major cause of morbidity and mortality, both in CKD and the non-CKD population.
Proof of concept
The theory that intestinal microbiota contribute to uraemic toxin generation by metabolizing breakdown products derived from dietary protein intake was confirmed in a number of proof-of-concept studies.
In 1965, Einheber and Carter demonstrated that germfree rats made anephric survived longer and lost less weight than their counterparts with intestinal microorganisms [37]. In 1982, Yokoyama et al. demonstrated that sterilizing the intestine by a combination of antibiotics in weanling pigs with normal kidneys decreased the faecal and urinary excretion of phenolic and aromatic bacterial metabolites and increased their body weight [38]. A recent study demonstrated that antibiotic prophlaxis for episodes of neutropenia in patients having received an allogeneic stem cell transplantation resulted in a shift of intestinal microbiota and in a dramatic decrease of urinary indoxyl sulphate concentration [39].
Recently, Wikoff et al. compared by untargeted metabolomic mass spectrometry the plasma from germ-free mice with normal kidney function with that of animals with normal faecal microbiota and could indicate a number of discriminators, among which are several compounds known as protein-bound uraemic toxins such as indoxyl sulphate, hippuric acid and phenylacetic acid [40], which are also a substrate of renal tubular organic anion transporters (OATs) explaining their retention in kidney failure if the tubules are damaged [41].
Aronov et al. applied metabolomic analysis by mass spectrometry to compare concentration of plasma solutes of six haemodialysis patients without a colon, to that of nine patients with a colon [42], with as aim the identification of metabolites discriminating between these two groups. Among the 30 discriminators were several protein-bound uraemic retention products. In most cases, a ratio of plasma concentrations of <0.05 for those without versus with colon was found; for p-cresyl sulphate, the ratio was even 0.01 [42], indicating that concentration was ∼100 times higher in patients with a colon.
Patel et al. found in healthy vegetarians a strikingly lower urinary excretion of indoxyl sulphate and p-cresyl sulphate, as equivalents of their intestinal generation, than that in subjects with a more conventional food intake [43]. A similar study of >20 years before had shown lower serum concentrations and faecal losses of phenol and p-cresol in veganists [44]. Increasing dietary fibre intake by administration of resistant starch for 6 weeks in a randomized controlled trial (RCT) in haemodialysis patients decreased plasma-free indoxyl sulphate whereas there was a trend for a decrease of free p-cresyl sulphate [45].
Eloot et al. assessed the relationship between the pre-dialysis concentration of several essentially protein-bound uraemic toxins and a number of potential influencing factors such as Kt/Vurea as an index of dialysis adequacy, normalized protein catabolic rate (nPCR) as an index of dietary protein intake, residual renal function, age, diabetes, gender, body weight and dialysis vintage [46]. A correlation was found, essentially for residual renal function and nPCR, and not for the other factors, including Kt/Vurea, again suggesting a role for digestion of dietary proteins in uraemic toxin generation and concentration, even overruling the impact of dialysis adequacy.
Toxicity of the compounds of intestinal origin
The toxicity of the protein-bound solutes, which compose a large fraction of the intestinally generated compounds, remains a matter of debate, as in many experimental studies too high concentrations resulted in an overestimation of potential toxicity [47]. In analogy with drugs, such toxicity should reasonably be exerted by the free, unbound fraction. A recent systematic review, however, unravelled 27 studies where correct free concentrations of two prototypic protein-bound solutes, p-cresyl sulphate and indoxyl sulphate, had been applied [48]. Interference was shown with several key metabolic processes involved in the uraemic syndrome, such as reactive oxygen species generation, endothelial dysfunction, epithelial-to-mesenchymal transition, leukocyte–endothelial interaction, deterioration of cardiac cell functional capacity, renal tubular cell senescence, expression of tissue factor and sensitivity to insulin. These data refer to the core of the morbidity and mortality of uraemia, i.e. cardiovascular disease and progression of renal failure. Together with observational studies showing a highly significant association between concentration of protein-bound toxins with hard endpoints such as cardio-vascular events, progression of renal failure and mortality [49–54], these data offer strong arguments in favour of a key role of indoxyl sulphate and p-cresyl sulphate in the uraemic syndrome.
Without following the same rigid approach as applied in the systematic review mentioned [48], we found at least five more recent publications conforming the inclusion criteria of the latter study, covering inhibition of drug metabolism [55]; increased crosstalk between leukocytes and endothelium, glycocalyx degradation and vascular leakage [56]; apoptosis of osteoblasts [57]; induction of tubular endothelial growth factor receptor leading to tissue remodelling [58] and inhibition of breakdown of angiotensin II [59].
Similar effects were also described for other protein-bound toxins [60] such as hippuric acid [61–63], indole acetic acid [61, 62, 64–67], phenyl acetic acid [61, 67, 68]; p-cresylglucuronide [56, 67, 69] and kynurenic acid [61, 67] (Table 3).
Table 3.
Protein-bound uraemic toxins other than indoxyl sulphate and p-cresyl sulphate with toxic impacta
| Affected biological function | Toxic solute |
|---|---|
| Protein binding of drugs [63] | Hippuric acid |
| Expression of drug transporters [62] | Hippuric acid |
| Indole acetic acid | |
| Renal tubular efflux pumps [61] | Hippuric acid |
| Indole acetic acid | |
| Phenyl acetic acid | |
| Kynurenic acid | |
| Tissue factor expression [64, 66] | Indole acetic acid |
| Glucuronidation [67] | Indole acetic acid |
| Phenyl acetic acid | |
| Kynurenic acid | |
| Mitochondrial metabolism [67] | Indole acetic acid |
| Phenyl acetic acid | |
| P-cresyl glucuronide | |
| Nitric oxide synthase [68] | Phenyl acetic acid |
| Leukocyte-free radical production [69] | P-cresyl glucuronideb |
| Endothelial albumin leakage [56] | P-cresyl glucuronideb |
| Endothelial Cox-2 mRNA expression [65] | Indole acetic acid |
aList is not exhaustive.
bIn combination with p-cresyl sulphate.
The link of AGEs with inflammation has been emphasized before [70]. Nutritional AGEs contribute to this effect since AGE-rich food administered to diabetics increased endothelial free radical production and decreased arterial responsiveness to vasodilatory stimuli [71].
Several arguments thus plead in favour of a broad array of biological effects exerted by uraemic solutes of intestinal origin, and it seems rational to explore novel strategies to decrease their concentration, especially since removal even with the most efficient dialysis strategies is deceiving.
Therapeutic options
Several approaches have the potential to decrease the capacity of intestinal microbiota to generate uraemic solutes and their precursors (Table 4) [12, 72]. Following hypothesis-generating experimental studies, several clinical studies have recently resulted in a few reviews expressing an affirmative opinion about such therapies [13, 14, 16]. However, those clinical studies are to be checked critically with regard to their evidence base, with an increasing order of importance given to (i) uncontrolled concentration studies in the normal population, (ii) uncontrolled concentration studies in uraemics, (iii) uncontrolled hard outcome trials, (iv) RCTs with toxin concentration as outcomes and (v) hard outcome RCTs (Table 4).
Table 4.
Summary of studies on therapeutic options affecting uraemic toxin concentration/generation via intestinal pathways in humans
| Uncontrolled |
RCT |
||||||
|---|---|---|---|---|---|---|---|
| Concentration |
Outcomes | Concentration |
Outcomes | ||||
| Intervention | Target molecule | Healthy | Uraemia | Uraemia | Healthy | Uraemia | Uraemia |
| Diet | |||||||
| Vegetable/vegetarian [43, 44] | PBUT | + | |||||
| Phenols | + | ||||||
| Phosphate | + | ||||||
| AGE-free diet [19] | AGE | + | + | ||||
| Oxalate-controlling diet [73] | Oxalate | + | |||||
| Prebiotics | |||||||
| Resistant starch [45, 74] | Phenols | + | |||||
| Ammonia | + | ||||||
| IS | + | ||||||
| pCS | − | ||||||
| Inulin [75] | p-Cresola | + | |||||
| Phenol | − | ||||||
| Lactulose [76] | p-Cresol | + | |||||
| OF-IN [76–79] | p-Cresol | + | + | ||||
| pCS | + | ||||||
| IS | − | ||||||
| AXOS [80] | p-Cresol | +/−b | |||||
| Phenol | − | ||||||
| Arabinogalactan [81] | Creatinine | − | |||||
| Urea | + | ||||||
| Isphagula husk [81] | Creatinine | − | |||||
| Urea | + | ||||||
| Gum Arabic supplement [82] | Creatinine | − | |||||
| Urea | + | ||||||
| Fermentable carbohydrate [83] | Urea | + | |||||
| probiotics | |||||||
| L. casei Shirota [77, 78, 84] | Urea | +/−c | |||||
| Creatinine | − | ||||||
| p-Cresol | + | ||||||
| L. acidophilus, B. longum, S. thermophilus, enteric coated [85] | Urea | + | |||||
| Creatinine | − | ||||||
| Uric acid | − | ||||||
| B. breve [77, 78] | p-Cresol | + | |||||
| Lebenin® [86] | IS | + | |||||
| Phenol | − | ||||||
| p-Cresol | − | ||||||
| Bifidobacteria, gastro-resistant capsule [87–89] | IS | +d | −/+e | ||||
| Hcy | + | ||||||
| Renadyl® [90] | IS | − | −f | ||||
| IG | − | ||||||
| pCS | − | ||||||
| pCG | − | ||||||
| Pentosidine | − | ||||||
| Β2-M | − | ||||||
| Oxalobacter formigenes [91] | Oxalate | + | |||||
| Lactic acid bacteria [73, 92–95] | Oxalate | +/±/− | − | ||||
| Synbiotics | |||||||
| L. casei, B. breve, galacto-oligosachharides [96] | p-Cresol | + | |||||
| phenol | − | ||||||
| IS | − | ||||||
| L. casei Shirota, OF-IN [78] | p-Cresol | + | |||||
| Probinul-neutro® [97] | p-Cresol | + | |||||
| Prebiotic–probiotic mixture [98] | +g | ||||||
| AKSB [73] | oxalate | − | |||||
| Sorbents | |||||||
| Sevelamer [99, 100] | Oxalate | − | |||||
| PBUT | − | ||||||
| AST-120 [101–106] | PBUT | + | + | +/−h | |||
| Chitosan [107] | IS | + | +i | ||||
| Phosphate | + | ||||||
| Others | |||||||
| Acarbose [108] | p-Cresol | + | |||||
The plus symbols indicate that the study has a positive impact; thus in general a decrease in concentration or generation or a protective effect against morbidity; the negative symbols indicate that there was no impact; plus or minus symbols indicate contradictory results; blank spaces indicate that no valid data were found to include in the table.
PBUT, protein-bound uraemic toxins; AGEs, advanced glycation end products; pCS, p-cresyl sulphate; pCG, p-cresyl glucuronide; IS, indoxyl sulphate; IG, indoxyl glucuronide; β2-M, β2-microglobulin; Hcy, homocysteine; OF-IN, oligofructose-enriched inulin; AXOS, arabinoxylan-oligosaccharides; Lebenin®, antibiotic-resistant Bifidobacterium infantis, Lactobacillus acidophilus, Enterococcus fecalis; Renadyl®, Streptococcus thermophilus, L. acidophilus, B. longum; Probinul-neutroR, mixture of several Lactobacilli and Bifidobacteria, S. thermophilus, inulin and tapioca-resistant starch; AKSB, fructo-oligosaccharide, E. faecium, S. cerivisiae.
ap-cresol in all studies surrogate for p-cresyl sulphate + p-cresyl glucuronide.
bPositive after 2 weeks but negative after 3 weeks.
cPositive at high dose, negative at low dose, no statistical comparison between high and low dose.
dOnly if included in gastro-resistant capsule—not in powder formulation.
eOverall negative; only an inhibitory effect on CKD progression in the subgroup with the worst kidney function.
fInflammation and quality of life.
gDecline of eGFR.
hSmall Japanese randomized trials showed a benefit on progression of CKD, which could, however, not be confirmed in a large European–American RCT; one RCT showed an improved response to ESAs.
iOxidative stress parameters.
Diet
With protein as the source of amino acids, decreasing protein intake should diminish generation of uraemic retention solutes. A shift from a vegetable to meat diet in healthy subjects indeed impacts the intestinal balance of microbiota [4] and increases urinary excretion of phenols and ammonia [109]. Veganistic and vegetarian diets in non-uraemic populations decrease the concentration and generation of protein-bound uraemic solutes or their precursors [43, 44] but remain a matter of concern in the malnourished uraemic population. Although low-protein diet has a nephroprotective effect, it should be taken into account that a large section of CKD patients, not even on dialysis, suffer from protein malnourishment. On the other hand, what is currently a standard diet in Western countries often contains excessive quantities of protein.
Selective diets excluding the sources of specific toxins are an alternative. When AGE-rich food was administered to both non-uraemic and uraemic diabetics, AGE concentration and cross-linking in plasma increased compared with AGE-poor diet [19]. In the run-in phase of an RCT on oxalate decreasing intervention in hyperoxaluria, a diet controlling oxalate intake decreased urinary oxalate compared with a free-choice diet [73].
Prebiotics
Prebiotics are non-digestible compounds improving the composition and/or function of the intestinal microbiota [110].
An animal study by Koppe et al. showed that CKD as well as p-cresyl sulphate caused insulin resistance, disturbances of adipose tissue metabolism and reallocation of fat throughout the body [111]. When arabinoxylan-oligosaccharides (AXOS) were administered to CKD animals, p-cresyl sulphate concentration decreased while insulin sensitivity was restored [111]. In 5/6 nephrectomized rats, galacto-oligosaccharides decreased caecal indoles and serum indoxyl sulphate and improved renal injury, stress markers and apoptosis [112].
In healthy humans, resistant starch decreased faecal phenol and ammonia [74]. When assessing solute generation out of radiolabelled tyrosine, urinary excretion of p-cresol was decreased by inulin [75]. A similar effect on p-cresol was seen also for oligofructose-enriched inulin (OF-IN) and lactulose [76], a finding confirmed for OF-IN in two RCTs [77, 78]. In another RCT assessing AXOS, urinary p-cresol was decreased after 2 but not 3 weeks, and no change was shown for phenol [80].
In uraemia, a few early studies only focused on the concentration of the water-soluble compounds creatinine and/or urea [81–83,113]. In 1984, Rampton et al. studied the effect of arabinogalactan and of Isphagula husk on blood urea and creatinine [81]. The study contained no control group, and no effect was seen on creatinine. The therapeutic decrease in urea claimed by the authors is doubtful in view of the far from stable baseline [81]. Bliss et al. randomized non-dialysed CKD patients on low-protein diet to gum Arabic supplement or placebo and found a decrease in urea concentration but not of creatinine, while also nitrogen in stools increased [82]. In 2006, Younes et al. confirmed their earlier findings in animals [113] in men, indicating a decrease of urea after administration of fermentable carbohydrate supplements. The study, however, contained no controls [83].
More recently, in a single-centre, non-randomized, open-label study with an escalating dose of prebiotic over 4 weeks, followed by a 4-week washout, OF-IN [79] has been shown in maintenance haemodialysis patients to impact plasma concentration of p-cresyl sulphate, but not of indoxyl sulphate [79]. In an RCT in haemodialysis patients, the administration of resistant starch decreased plasma-free indoxyl sulphate [45]. The study was, however, characterized by a substantial number of dropouts, both in the control and the verum group (29%) [45].
Probiotics
Probiotics are living organisms, administered as food components or supplements, which provide specific benefits by themselves, essentially by creating a more favourable balance in the composition of intestinal microbiota [114].
Urea
Urease-expressing bacteria may decrease blood urea. Most studies in this context are experimental, either in vivo in rats after 5/6 nephrectomy or acetaminophen-induced kidney failure or in vitro [115–117]. The probiotics not only decreased urea but also other markers of kidney failure like creatinine or oxidative stress, and they prolonged life span [116, 117]. In a rat study assessing five different probiotic combinations, only two (one containing Bacillus pasteuri and one Lactobacillus sporogenes) decreased BUN and serum creatinine [118]. A matter of concern is that degradation of urea by urease probably generates ammonia, which is now considered a uraemic toxin by itself [119].
In an uncontrolled study in CKD 3-4, high-concentration Lactobacillus casei Shirota (16 × 109 CFU/mL) but not low-dose (8 × 109) decreased blood urea only while creatinine remained unmodified [84]. The decrease of urea was only by 11%.
In an RCT assessing in CKD patients, the effect of an enteric-coated probiotic combination of Lactobacillus acidophilus, Bifidobacterium longum and Streptococcus thermophilus on the evolution of BUN, serum creatinine and uric acid, only a borderline significant decrease in BUN could be observed, which remained absent in close to 40% of subjects [85].
Protein-bound uraemic toxins
Essentially lactic acid bacteria including Lactobacilli and Bifidobacteria have been studied.
In two placebo-controlled RCTs in healthy subjects, Lactobacillus casei Shirota and Bifidobacterium breve both reduced urinary p-cresol in the urine after 4 weeks [77, 78].
In haemodialysis patients, Lebenin®, a mixture of antibiotic-resistant lactic acid bacteria, resulted in a decrease in plasma indoxyl sulphate but not in phenol and p-cresol [86]. In a study by Takayama et al., Bifidobacterium in a gastro-resistant capsule but not in powder formulation decreased indoxyl sulphate in haemodialysis patients [87]. The same encapsulated variant also decreased homocysteinemia and increased serum folate [88]. None of these studies, however, checked hard endpoints or were even placebo controlled. The study by Takayama et al. contained a non-randomly selected group, treated with powdered Bifidobacterium without showing any effect [87]. This might be considered as a sort of control, but, if true, these data cast doubts on other studies with unencapsuled lactic acid bacteria that were successful.
In an uncontrolled Japanese hard-endpoint study evaluating an enteric capsule preparation of Bifidobacterium longum, no effect was seen on progression of renal failure [89]. An RCT in end-stage CKD patients studying Renadyl® showed no differences in protein-bound uraemic toxin concentration nor in surrogate endpoints such as inflammatory and quality-of-life parameters [90].
Oxalate
A specific area of interest is the research on oxalate degrading bacteria, mostly in the context of oxalate urolithiasis and of hyperoxaluria, either because of genetic reasons or of medical predisposition (bowel disease). Of note, apart from being a reason for CKD in extreme forms of hyperoxaluria or because of kidney stones, oxalate is also a uraemic retention compound by itself.
A number of uncontrolled clinical trials in subjects without manifest renal failure with Oxalobacter formigenes or with lactic acid bacteria showed a decrease in oxalate plasma concentration and/or generation [91,92], but another uncontrolled trial with a mixture of lactic acid bacteria gave contradictory results, with a decrease for the lower but not for the higher dose [93]. Another uncontrolled study showed no effect al all [94], which was also the case for two RCTs [73, 95].
Synbiotics
Synbiotics contain a mixture of prebiotics and probiotics.
In an uncontrolled study in HD patients, a preparation combining Lactobacillus casei, Bifidobacterium breve and galacto-oligosaccharides decreased p-cresol but not indoxyl sulphate or phenol [96]. Patients with high p-cresol tended to have defaecation difficulties, which were normalized together with the decrease in p-cresol after 2 weeks of symbiotic treatment.
In a crossover RCT in healthy subjects, co-administration of Lactobacillus casei Shirota and OF-IN resulted in a reduction of urinary p-cresol excretion [78]. An RCT applying Probinul-neutro® in CKD 3-4 showed a decrease of plasma p-cresol versus baseline and placebo [97]. In placebo controls, however, plasma p-cresol spontaneously rose by >50% during a short follow-up of only 1 month [97], suggesting unidentified confounders forcing the results to a difference favouring synbiotics. A randomized open-label trial allotting CKD 3-4 patients to either protein restrictive diet alone or a combination of protein restriction, prebiotics and probiotics, showed a slower decline of eGFR in patients on the combined regimen [98]. A placebo-controlled RCT with crossover after 4-week washout has recently been developed to assess the effect on indoxyl sulphate and p-cresyl sulphate next to cardiovascular risk parameters and indices of renal damage, quality of life and gastro-intestinal status [120].
Finally, in an RCT assessing next to diet and two prebiotic formulations, also the symbiotic AKSB (fructo-oligosaccharide, Enterococcus faecium and Saccharomyces cerivisiae) for their effect on oxalate levels could show no effect [73].
Absorption
The possibility that orally administered sorbents could remove uraemic solutes or their precursor by binding them in the intestine has long since been recognized, but not extensively explored. In fact, all phosphate binders are a classical example of formulations capturing in the intestine an actor associated with negative outcomes of uraemia.
One of the novel phosphate binders, sevelamer, absorbs also other compounds such as cholesterol [121] and antibiotics [122]. It has been hypothesized to absorb oxalate, but this could not be confirmed in an open-label non-randomized clinical trial [99]. Studies exploring the capacity of sevelamer to absorb protein-bound uraemic solutes or their precursors turned out negative, both in animals [123] and in an RCT in haemodialysis patients [100].
Probably the most successful results in the context of intestinal absorption have been obtained with the sorbent AST-120, which in a randomized trial dose-dependently decreased plasma indoxyl sulphate [101], while also other protein-bound compounds are likely to be removed by AST-120 [124]. Protein-bound compounds have been associated in both experimental [48] and clinical studies [53] to progression of CKD. Animal studies and retrospective clinical studies have suggested a nephroprotective effect for AST-120 [125, 126], which was confirmed in a number of small Japanese RCTs [102–104]. A subsequent large RCT in the USA and Europe could, however, not confirm those findings [105]. In a recent animal study, AST-120 protected against cardiorenal fibrosis subsequent to myocardial infarction [127]. In an RCT, AST-120 potentiated the activity of erythropiesis-stimulating agents (ESAs) in stage 5 CKD patients [106].
In an uncontrolled longitudinal follow-up study in haemodialysis patients, a decrease of indoxyl sulphate, phosphate and oxidative stress parameters versus baseline was seen with the sorbent chitosan [107].
Other interventions
Oral administration of sulfotransferase inhibitors to rats with acute renal failure suppressed indoxyl sulphate accumulation and had a positive effect on kidney function [128]. This study, however, raises the question of what happens to the mother compound p-cresol if it is not sulphated. Either it may exert its own toxicity [129], or it may be metabolized into the other main conjugate p-cresylglucuronide, which also has biological effects on its own [69]. Also meclofenamate strongly inhibited hepatic generation of indoxyl sulphate and had a nephroprotective effect [130]. In human volunteers, acarbose, an α-glucosidase inhibitor increasing colonic availability of carbohydrates, decreased urinary p-cresol excretion [108].
Summary of interventional studies
A dietary approach by restricting protein intake helps reducing the generation of uraemic toxins. Of note, protein restriction has been considered for a long time as nephroprotective [131], while at the same time reducing the generation of protein-bound uraemic toxins which are likely to play a role in progression of kidney failure. Nevertheless, in view of the possible link to malnutrition, such regimes should be tested in controlled studies, before they really can be considered of clinical value in uraemia. Keto-analogues may neutralize the negative impact of protein intake, but their effect on uraemic toxins has not yet been studied.
Prebiotics, probiotics and synbiotics could play a role in reducing the generation of uraemic toxins, but the results of clinical studies have been deceiving, with sometimes contradictory results [45, 73, 75, 79, 80, 84, 86, 95, 96, 118], e.g. if several uraemic toxins of similar origin were studied or if the same solute was assessed in several different studies. The two hard outcome studies that we retrieved assessing progression of kidney failure were negative at primary analysis [89, 90]. Of note, our literature search was not systematic, but a systematic review and meta-analysis on the topic a few years ago came to the same conclusions and classified the quality of the retrieved papers from moderate to very low [72, 89].
In view of the concern that probiotics might lose their effect if they do not survive the acidic milieu of the stomach, encapsulated formulations have been considered to offer new perspectives [16]. A few studies showed a decrease in uraemic toxin concentration but were non-randomized [85, 87, 88]. The only hard outcome study on encapsulated probiotics was not randomized either and, as mentioned earlier, the results were negative [89].
The studies in uraemia are scarce, and mostly not randomized, or if randomized, results are often inconsistent. The number of RCTs in healthy subjects is larger, but here only urinary excretion and no plasma levels were checked.
Consistent data have been reported on AST-120 as far as reduction of protein-bound solutes is concerned. A few small Japanese RCTs of variable quality suggested that this sorbent could refrain progression of CKD [102–104]. A large RCT could, however, not confirm these promising results [105].
In brief, what is needed in this area is well conceived, sufficiently powered, randomized placebo-controlled trials, performed in comparable groups (e.g. in regard to kidney function) and under standardized conditions of food intake, adherence and other influencing factors. If promising data are obtained with regard to toxin concentration, these should be followed by hard outcome studies.
Conclusions
A host of data that have been acquired lately pointing to a strong bidirectional link between renal failure and the intestine, whereby in the intestine, uraemic toxins are generated which cause inflammation and are deleterious to body functions, while uraemia affects intestinal functions and protective barriers, which in their turn lead to inflammation (Figure 2). Several recent studies deliver proof of concept of the crosstalk between the intestine and the uraemic condition. In spite of the availability of a number of therapeutic options to interfere with this process, results of clinical trials remain unconvincing, partly due to the design of the studies and partly to the lack of a hard outcome evidence base.
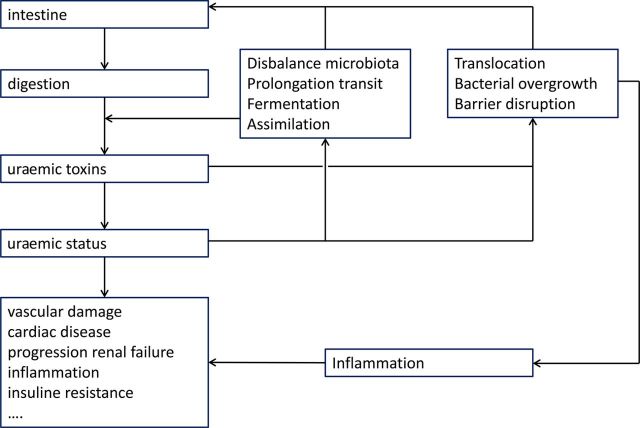
Fig. 2.
The dual interference between the intestine and uraemia. The intestine is responsible for generating and absorbing uraemic toxins with a deleterious impact on body functions. Uraemia in its turn modifies intestinal functions with a further increase in uraemic toxin generation and pro-inflammatory modifications which again are deleterious to body functions.
Future therapeutic clinical studies in this context should focus on a more rigid controlled setting, with hard outcomes and a decrease of target uraemic solutes as primary endpoints, including a control of compliance and if possible of dietary elements with potential influence. However, first we need more insight in the basic mechanisms and bacteriological underpinning of the gut–kidney crosstalk. Maybe single interventions are not efficient enough, and combination therapies should be considered.
Conflict of interest statement. None declared.
References
- 1.Turnbaugh PJ, Ley RE, Hamady M, et al. The human microbiome project. Nature 2007; 449: 804–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet 2003; 361: 512–519 [DOI] [PubMed] [Google Scholar]
- 3.Choi JJ, Eum SY, Rampersaud E, et al. Exercise attenuates PCB-induced changes in the mouse gut microbiome. Environ Health Perspect 2013; 121: 725–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014; 505: 559–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lam V, Su J, Koprowski S, et al. Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J 2012; 26: 1727–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006; 444: 1027–1031 [DOI] [PubMed] [Google Scholar]
- 7.Cesaro C, Tiso A, Del Prete A, et al. Gut microbiota and probiotics in chronic liver diseases. Dig Liver Dis 2011; 43: 431–438 [DOI] [PubMed] [Google Scholar]
- 8.Hopkins MJ, Sharp R, Macfarlane GT. Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut 2001; 48: 198–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature 2009; 457: 480–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anders HJ, Andersen K, Stecher B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int 2013; 83: 1010–1016 [DOI] [PubMed] [Google Scholar]
- 11.Evenepoel P, Meijers BK, Bammens BR, et al. Uremic toxins originating from colonic microbial metabolism. Kidney Int Suppl 2009; 76(Suppl 114): S12–S19 [DOI] [PubMed] [Google Scholar]
- 12.Schepers E, Glorieux G, Vanholder R. The gut: the forgotten organ in uremia? Blood Purif 2010; 29: 130–136 [DOI] [PubMed] [Google Scholar]
- 13.Ramezani A, Raj DS. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol 2014; 25: 657–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vitetta L, Linnane AW, Gobe GC. From the gastrointestinal tract (GIT) to the kidneys: live bacterial cultures (probiotics) mediating reductions of uremic toxin levels via free radical signaling. Toxins (Basel) 2013; 5: 2042–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massy ZA, Barreto DV, Barreto FC, et al. Uraemic toxins for consideration by the cardiologist-Beyond traditional and non-traditional cardiovascular risk factors. Atherosclerosis 2010; 211: 381–383 [DOI] [PubMed] [Google Scholar]
- 16.Di Cerbo A, Pezzuto F, Palmieri L, et al. Clinical and experimental use of probiotic formulations for management of end-stage renal disease: an update. Int Urol Nephrol 2013; 45: 1569–1576 [DOI] [PubMed] [Google Scholar]
- 17.Poesen R, Meijers B, Evenepoel P. The colon: an overlooked site for therapeutics in dialysis patients. Semin Dial 2013; 26: 323–332 [DOI] [PubMed] [Google Scholar]
- 18.Glorieux G, Vanholder R, Lameire N. Advanced glycation and the immune system: stimulation, inhibition or both? Eur J Clin Invest 2001; 31: 1015–1018 [DOI] [PubMed] [Google Scholar]
- 19.Koschinsky T, He CJ, Mitsuhashi T, et al. Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci USA 1997; 94: 6474–6479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith EA, MacFarlane GT. Enumeration of amino acid fermenting bacteria in the human large intestine: effects of pH and starch on peptide metabolism and dissimilation of amino acids. FEMS Microbiol Ecol 1998; 25: 355–368 [Google Scholar]
- 21.Vaziri ND, Wong J, Pahl M, et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int 2013; 83: 308–315 [DOI] [PubMed] [Google Scholar]
- 22.Wang F, Jiang H, Shi K, et al. Gut bacterial translocation is associated with microinflammation in end-stage renal disease patients. Nephrology (Carlton) 2012; 17: 733–738 [DOI] [PubMed] [Google Scholar]
- 23.Strid H, Simren M, Stotzer PO, et al. Patients with chronic renal failure have abnormal small intestinal motility and a high prevalence of small intestinal bacterial overgrowth. Digestion 2003; 67: 129–137 [DOI] [PubMed] [Google Scholar]
- 24.Wu MJ, Chang CS, Cheng CH, et al. Colonic transit time in long-term dialysis patients. Am J Kidney Dis 2004; 44: 322–327 [DOI] [PubMed] [Google Scholar]
- 25.Bammens B, Verbeke K, Vanrenterghem Y, et al. Evidence for impaired assimilation of protein in chronic renal failure. Kidney Int 2003; 64: 2196–2203 [DOI] [PubMed] [Google Scholar]
- 26.Wang IK, Lai HC, Yu CJ, et al. Real-time PCR analysis of the intestinal microbiotas in peritoneal dialysis patients. Appl Environ Microbiol 2012; 78: 1107–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong J, Piceno YM, DeSantis TZ, et al. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol 2014; 39: 230–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bossola M, Sanguinetti M, Scribano D, et al. Circulating bacterial-derived DNA fragments and markers of inflammation in chronic hemodialysis patients. Clin J Am Soc Nephrol 2009; 4: 379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Almeida Duarte JB, de Aguilar-Nascimento JE, Nascimento M, et al. Bacterial translocation in experimental uremia. Urol Res 2004; 32: 266–270 [DOI] [PubMed] [Google Scholar]
- 30.Wang F, Zhang P, Jiang H, et al. Gut bacterial translocation contributes to microinflammation in experimental uremia. Dig Dis Sci 2012; 57: 2856–2862 [DOI] [PubMed] [Google Scholar]
- 31.Wei M, Wang Z, Liu H, et al. Probiotic Bifidobacterium animalis subsp. lactis Bi-07 alleviates bacterial translocation and ameliorates microinflammation in experimental uraemia. Nephrology (Carlton) 2014; 19: 500–506 [DOI] [PubMed] [Google Scholar]
- 32.Magnusson M, Magnusson KE, Sundqvist T, et al. Impaired intestinal barrier function measured by differently sized polyethylene glycols in patients with chronic renal failure. Gut 1991; 32: 754–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaziri ND, Yuan J, Rahimi A, et al. Disintegration of colonic epithelial tight junction in uremia: a likely cause of CKD-associated inflammation. Nephrol Dial Transplant 2012; 27: 2686–2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaziri ND, Yuan J, Norris K. Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney disease. Am J Nephrol 2013; 37: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaziri ND, Yuan J, Khazaeli M, et al. Oral activated charcoal adsorbent (AST-120) ameliorates chronic kidney disease-induced intestinal epithelial barrier disruption. Am J Nephrol 2013; 37: 518–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niwa T, Emoto Y, Maeda K, et al. Oral sorbent suppresses accumulation of albumin-bound indoxyl sulphate in serum of haemodialysis patients. Nephrol Dial Transplant 1991; 6: 105–109 [DOI] [PubMed] [Google Scholar]
- 37.Einheber A, Carter D. The role of the microbial flora in uremia. I. Survival times of germfree, limited-flora, and conventionalized rats after bilateral nephrectomy and fasting. J Exp Med 1966; 123: 239–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokoyama MT, Tabori C, Miller ER, et al. The effects of antibiotics in the weanling pig diet on growth and the excretion of volatile phenolic and aromatic bacterial metabolites. Am J Clin Nutr 1982; 35: 1417–1424 [DOI] [PubMed] [Google Scholar]
- 39.Holler E, Butzhammer P, Schmid K, et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant 2014; 20: 640–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wikoff WR, Anfora AT, Liu J, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA 2009; 106: 3698–3703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wikoff WR, Nagle MA, Kouznetsova VL, et al. Untargeted metabolomics identifies enterobiome metabolites and putative uremic toxins as substrates of organic anion transporter 1 (Oat1). J Proteome Res 2011; 10: 2842–2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aronov PA, Luo FJ, Plummer NS, et al. Colonic contribution to uremic solutes. J Am Soc Nephrol 2011; 22: 1769–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel KP, Luo FJ, Plummer NS, et al. The production of p-cresol sulfate and indoxyl sulfate in vegetarians versus omnivores. Clin J Am Soc Nephrol 2012; 7: 982–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ling WH, Hanninen O. Shifting from a conventional diet to an uncooked vegan diet reversibly alters fecal hydrolytic activities in humans. J Nutr 1992; 122: 924–930 [DOI] [PubMed] [Google Scholar]
- 45.Sirich TL, Plummer NS, Gardner CD, et al. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin J Am Soc Nephrol 2014; 9: 1603–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eloot S, Van Biesen W, Glorieux G, et al. Does the adequacy parameter Kt/V(urea) reflect uremic toxin concentrations in hemodialysis patients? PLoS One 2013; 8: e76838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sirich T, Meyer TW. Indoxyl sulfate: long suspected but not yet proven guilty. Clin J Am Soc Nephrol 2011; 6: 3–4 [DOI] [PubMed] [Google Scholar]
- 48.Vanholder R, Schepers E, Pletinck A, et al. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J Am Soc Nephrol 2014; 25: 1897–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barreto FC, Barreto DV, Liabeuf S, et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 2009; 4: 1551–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiu CA, Lu LF, Yu TH, et al. Increased levels of total P-Cresylsulphate and indoxyl sulphate are associated with coronary artery disease in patients with diabetic nephropathy. Rev Diabet Stud 2010; 7: 275–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liabeuf S, Barreto DV, Barreto FC, et al. Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant 2010; 25: 1183–1191 [DOI] [PubMed] [Google Scholar]
- 52.Wang CP, Lu LF, Yu TH, et al. Serum levels of total p-cresylsulphate are associated with angiographic coronary atherosclerosis severity in stable angina patients with early stage of renal failure. Atherosclerosis 2010; 211: 579–583 [DOI] [PubMed] [Google Scholar]
- 53.Wu IW, Hsu KH, Lee CC, et al. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol Dial Transplant 2011; 26: 938–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu IW, Hsu KH, Hsu HJ, et al. Serum free p-cresyl sulfate levels predict cardiovascular and all-cause mortality in elderly hemodialysis patients--a Prospective Cohort Study. Nephrol Dial Transplant 2012; 27: 1169–1175 [DOI] [PubMed] [Google Scholar]
- 55.Barnes KJ, Rowland A, Polasek TM, et al. Inhibition of human drug-metabolising cytochrome P450 and UDP-glucuronosyltransferase enzyme activities in vitro by uremic toxins. Eur J Clin Pharmacol 2014; 70: 1097–1106 [DOI] [PubMed] [Google Scholar]
- 56.Pletinck A, Glorieux G, Schepers E, et al. Protein-bound uremic toxins stimulate crosstalk between leukocytes and vessel wall. J Am Soc Nephrol 2013; 24: 1981–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim YH, Kwak KA, Gil HW, et al. Indoxyl sulfate promotes apoptosis in cultured osteoblast cells. BMC Pharmacol Toxicol 2013; 14: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun CY, Young GH, Hsieh YT, et al. Protein-bound uremic toxins induce tissue remodeling by targeting the EGF receptor. J Am Soc Nephrol 2014; doi:10.1681/ASN2014010021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ng HY, Yisireyili M, Saito S, et al. Indoxyl sulfate downregulates expression of Mas receptor via OAT3/AhR/Stat3 pathway in proximal tubular cells. PLoS One 2014; 9: e91517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jourde-Chiche N, Dou L, Cerini C, et al. Protein-bound toxins--update 2009. Semin Dial 2009; 22: 334–339 [DOI] [PubMed] [Google Scholar]
- 61.Mutsaers HA, van den Heuvel LP, Ringens LH, et al. Uremic toxins inhibit transport by breast cancer resistance protein and multidrug resistance protein 4 at clinically relevant concentrations. PLoS One 2011; 6: e18438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsujimoto M, Hatozaki D, Shima D, et al. Influence of serum in hemodialysis patients on the expression of intestinal and hepatic transporters for the excretion of pravastatin. Ther Apher Dial 2012; 16: 580–587 [DOI] [PubMed] [Google Scholar]
- 63.Vanholder R, Van Landschoot N, De Smet R, et al. Drug protein binding in chronic renal failure: evaluation of nine drugs. Kidney Int 1988; 33: 996–1004 [DOI] [PubMed] [Google Scholar]
- 64.Chitalia VC, Shivanna S, Martorell J, et al. Uremic serum and solutes increase post-vascular interventional thrombotic risk through altered stability of smooth muscle cell tissue factor. Circulation 2013; 127: 365–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dou L, Sallee M, Cerini C, et al. The cardiovascular effect of the uremic solute indole-3 acetic acid. J Am Soc Nephrol 2014; doi 10.1681/ASN 2013121283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gondouin B, Cerini C, Dou L, et al. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int 2013; 84: 733–744 [DOI] [PubMed] [Google Scholar]
- 67.Mutsaers HA, Wilmer MJ, Reijnders D, et al. Uremic toxins inhibit renal metabolic capacity through interference with glucuronidation and mitochondrial respiration. Biochim Biophys Acta 2013; 1832: 142–150 [DOI] [PubMed] [Google Scholar]
- 68.Jankowski J, van der Giet M, Jankowski V, et al. Increased plasma phenylacetic acid in patients with end-stage renal failure inhibits iNOS expression. J Clin Invest 2003; 112: 256–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meert N, Schepers E, Glorieux G, et al. Novel method for simultaneous determination of p-cresylsulphate and p-cresylglucuronide: clinical data and pathophysiological implications. Nephrol Dial Transplant 2012; 27: 2388–2396 [DOI] [PubMed] [Google Scholar]
- 70.Glorieux G, Helling R, Henle T, et al. In vitro evidence for immune activating effect of specific AGE structures retained in uremia. Kidney Int 2004; 66: 1873–1880 [DOI] [PubMed] [Google Scholar]
- 71.Uribarri J, Stirban A, Sander D, et al. Single oral challenge by advanced glycation end products acutely impairs endothelial function in diabetic and nondiabetic subjects. Diabetes Care 2007; 30: 2579–2582 [DOI] [PubMed] [Google Scholar]
- 72.Rossi M, Klein K, Johnson DW, et al. Pre-, pro-, and synbiotics: do they have a role in reducing uremic toxins? A systematic review and meta-analysis. Int J Nephrol 2012; 2012: 673631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lieske JC, Tremaine WJ, De Simone C, et al. Diet, but not oral probiotics, effectively reduces urinary oxalate excretion and calcium oxalate supersaturation. Kidney Int 2010; 78: 1178–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Birkett A, Muir J, Phillips J, et al. Resistant starch lowers fecal concentrations of ammonia and phenols in humans. Am J Clin Nutr 1996; 63: 766–772 [DOI] [PubMed] [Google Scholar]
- 75.Geboes KP, De Hertogh G, De Preter V, et al. The influence of inulin on the absorption of nitrogen and the production of metabolites of protein fermentation in the colon. Br J Nutr 2006; 96: 1078–1086 [DOI] [PubMed] [Google Scholar]
- 76.De Preter V, Vanhoutte T, Huys G, et al. Baseline microbiota activity and initial bifidobacteria counts influence responses to prebiotic dosing in healthy subjects. Aliment Pharmacol Ther 2008; 27: 504–513 [DOI] [PubMed] [Google Scholar]
- 77.De Preter V, Geboes K, Verbrugghe K, et al. The in vivo use of the stable isotope-labelled biomarkers lactose-[15N]ureide and [2H4]tyrosine to assess the effects of pro- and prebiotics on the intestinal flora of healthy human volunteers. Br J Nutr 2004; 92: 439–446 [DOI] [PubMed] [Google Scholar]
- 78.De Preter V, Vanhoutte T, Huys G, et al. Effects of Lactobacillus casei Shirota, Bifidobacterium breve, and oligofructose-enriched inulin on colonic nitrogen-protein metabolism in healthy humans. Am J Physiol Gastrointest Liver Physiol 2007; 292: G358–G368 [DOI] [PubMed] [Google Scholar]
- 79.Meijers BK, De Preter V, Verbeke K, et al. p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol Dial Transplant 2010; 25: 219–224 [DOI] [PubMed] [Google Scholar]
- 80.Cloetens L, Broekaert WF, Delaedt Y, et al. Tolerance of arabinoxylan-oligosaccharides and their prebiotic activity in healthy subjects: a randomised, placebo-controlled cross-over study. Br J Nutr 2010; 103: 703–713 [DOI] [PubMed] [Google Scholar]
- 81.Rampton DS, Cohen SL, Crammond VD, et al. Treatment of chronic renal failure with dietary fiber. Clin Nephrol 1984; 21: 159–163 [PubMed] [Google Scholar]
- 82.Bliss DZ, Stein TP, Schleifer CR, et al. Supplementation with gum Arabic fiber increases fecal nitrogen excretion and lowers serum urea nitrogen concentration in chronic renal failure patients consuming a low-protein diet. Am J Clin Nutr 1996; 63: 392–398 [DOI] [PubMed] [Google Scholar]
- 83.Younes H, Egret N, Hadj-Abdelkader M, et al. Fermentable carbohydrate supplementation alters nitrogen excretion in chronic renal failure. J Ren Nutr 2006; 16: 67–74 [DOI] [PubMed] [Google Scholar]
- 84.Miranda Alatriste PV, Urbina AR, Gomez Espinosa CO, et al. Effect of probiotics on human blood urea levels in patients with chronic renal failure. Nutr Hosp 2014; 29: 582–590 [DOI] [PubMed] [Google Scholar]
- 85.Ranganathan N, Ranganathan P, Friedman EA, et al. Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Adv Ther 2010; 27: 634–647 [DOI] [PubMed] [Google Scholar]
- 86.Hida M, Aiba Y, Sawamura S, et al. Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron 1996; 74: 349–355 [DOI] [PubMed] [Google Scholar]
- 87.Takayama F, Taki K, Niwa T. Bifidobacterium in gastro-resistant seamless capsule reduces serum levels of indoxyl sulfate in patients on hemodialysis. Am J Kidney Dis 2003; 41: S142–S145 [DOI] [PubMed] [Google Scholar]
- 88.Taki K, Takayama F, Niwa T. Beneficial effects of Bifidobacteria in a gastroresistant seamless capsule on hyperhomocysteinemia in hemodialysis patients. J Ren Nutr 2005; 15: 77–80 [DOI] [PubMed] [Google Scholar]
- 89.Ando Y, Miyata Y, Tanba K, et al. Effect of oral intake of an enteric capsule preparation containing Bifidobacterium longum on the progression of chronic renal failure. Nihon Jinzo Gakkai Shi 2003; 45: 759–764 [PubMed] [Google Scholar]
- 90.Natarajan R, Pechenyak B, Vyas U, et al. Randomized controlled trial of strain-specific probiotic formulation (renadyl) in dialysis patients. Biomed Res Int 2014; 2014: 568571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hoppe B, Dittlich K, Fehrenbach H, et al. Reduction of plasma oxalate levels by oral application of Oxalobacter formigenes in 2 patients with infantile oxalosis. Am J Kidney Dis 2011; 58: 453–455 [DOI] [PubMed] [Google Scholar]
- 92.Campieri C, Campieri M, Bertuzzi V, et al. Reduction of oxaluria after an oral course of lactic acid bacteria at high concentration. Kidney Int 2001; 60: 1097–1105 [DOI] [PubMed] [Google Scholar]
- 93.Lieske JC, Goldfarb DS, De Simone C, et al. Use of a probiotic to decrease enteric hyperoxaluria. Kidney Int 2005; 68: 1244–1249 [DOI] [PubMed] [Google Scholar]
- 94.Ferraz RR, Marques NC, Froeder L, et al. Effects of Lactobacillus casei and Bifidobacterium breve on urinary oxalate excretion in nephrolithiasis patients. Urol Res 2009; 37: 95–100 [DOI] [PubMed] [Google Scholar]
- 95.Goldfarb DS, Modersitzki F, Asplin JR. A randomized, controlled trial of lactic acid bacteria for idiopathic hyperoxaluria. Clin J Am Soc Nephrol 2007; 2: 745–749 [DOI] [PubMed] [Google Scholar]
- 96.Nakabayashi I, Nakamura M, Kawakami K, et al. Effects of synbiotic treatment on serum level of p-cresol in haemodialysis patients: a Preliminary Study. Nephrol Dial Transplant 2011; 26: 1094–1098 [DOI] [PubMed] [Google Scholar]
- 97.Guida B, Germano R, Trio R, et al. Effect of short-term synbiotic treatment on plasma p-cresol levels in patients with chronic renal failure: a randomized clinical trial. Nutr Metab Cardiovasc Dis 2014; 24: 1043–1049 [DOI] [PubMed] [Google Scholar]
- 98.Pavan M. Influence of prebiotic and probiotic supplementation on the progression of chronic kidney disease. Minerva Urol Nefrol 2014; in press [PubMed] [Google Scholar]
- 99.Lieske JC, Regnier C, Dillon JJ. Use of sevelamer hydrochloride as an oxalate binder. J Urol 2008; 179: 1407–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brandenburg VM, Schlieper G, Heussen N, et al. Serological cardiovascular and mortality risk predictors in dialysis patients receiving sevelamer: a prospective study. Nephrol Dial Transplant 2010; 25: 2672–2679 [DOI] [PubMed] [Google Scholar]
- 101.Schulman G, Agarwal R, Acharya M, et al. A multicenter, randomized, double-blind, placebo-controlled, dose-ranging study of AST-120 (Kremezin) in patients with moderate to severe CKD. Am J Kidney Dis 2006; 47: 565–577 [DOI] [PubMed] [Google Scholar]
- 102.Akizawa T, Asano Y, Morita S, et al. Effect of a carbonaceous oral adsorbent on the progression of CKD: a multicenter, randomized, controlled trial. Am J Kidney Dis 2009; 54: 459–467 [DOI] [PubMed] [Google Scholar]
- 103.Konishi K, Nakano S, Tsuda S, et al. AST-120 (Kremezin) initiated in early stage chronic kidney disease stunts the progression of renal dysfunction in type 2 diabetic subjects. Diabetes Res Clin Pract 2008; 81: 310–315 [DOI] [PubMed] [Google Scholar]
- 104.Shoji T, Wada A, Inoue K, et al. Prospective randomized study evaluating the efficacy of the spherical adsorptive carbon AST-120 in chronic kidney disease patients with moderate decrease in renal function. Nephron Clin Pract 2007; 105: c99–107 [DOI] [PubMed] [Google Scholar]
- 105.Schulman G, Berl T, Beck GJ, et al. Randomized placebo-controlled EPPIC trials of AST-120 in CKD. J Am Soc Nephrol 2014; doi:10.1681/ASN2014010042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu IW, Hsu KH, Sun CY, et al. Oral adsorbent AST-120 potentiates the effect of erythropoietin-stimulating agents on Stage 5 chronic kidney disease patients: a Randomized Crossover Study. Nephrol Dial Transplant 2014; 29: 1719–1727 [DOI] [PubMed] [Google Scholar]
- 107.Anraku M, Tanaka M, Hiraga A, et al. Effects of chitosan on oxidative stress and related factors in hemodialysis patients. Carbohydr Polym 2014; 112: 152–157 [DOI] [PubMed] [Google Scholar]
- 108.Evenepoel P, Bammens B, Verbeke K, et al. Acarbose treatment lowers generation and serum concentrations of the protein-bound solute p-cresol: a Pilot Study. Kidney Int 2006; 70: 192–198 [DOI] [PubMed] [Google Scholar]
- 109.Cummings JH, Hill MJ, Bone ES, et al. The effect of meat protein and dietary fiber on colonic function and metabolism. II. Bacterial metabolites in feces and urine. Am J Clin Nutr 1979; 32: 2094–2101 [DOI] [PubMed] [Google Scholar]
- 110.Gibson G, Scott K, Rastall R. Dietary Prebitoics: current status and new defintion. IFRS Functional FoodsBulletin 2010; 7: 1–19 [Google Scholar]
- 111.Koppe L, Pillon NJ, Vella RE, et al. p-Cresyl sulfate promotes insulin resistance associated with CKD. J Am Soc Nephrol 2013; 24: 88–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Furuse SU, Ohse T, Jo-Watanabe A, et al. Galacto-oligosaccharides attenuate renal injury with microbiota modification. Physiol Rep 2014; 2 doi:10.14814/phy2.12029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Younes H, Alphonse JC, Behr SR, et al. Role of fermentable carbohydrate supplements with a low-protein diet in the course of chronic renal failure: experimental bases. Am J Kidney Dis 1999; 33: 633–646 [DOI] [PubMed] [Google Scholar]
- 114.Sanders ME. Probiotics: definition, sources, selection, and uses. Clin Infect Dis 2008; 46(Suppl 2): S58–S61 [DOI] [PubMed] [Google Scholar]
- 115.Mandal A, Mandal S, Roy S, et al. Assessment of efficacy of a potential probiotic strain and its antiuremic and antoxidative activities. e-SPEN J 2013; 8: e155–e163 [Google Scholar]
- 116.Mandal A, Das K, Roy S, et al. In vivo assessment of bacteriotherapy on acetaminophen-induced uremic rats. J Nephrol 2013; 26: 228–236 [DOI] [PubMed] [Google Scholar]
- 117.Ranganathan N, Patel BG, Ranganathan P, et al. In vitro and in vivo assessment of intraintestinal bacteriotherapy in chronic kidney disease. ASAIO J 2006; 52: 70–79 [DOI] [PubMed] [Google Scholar]
- 118.Ranganathan N, Patel B, Ranganathan P, et al. Probiotic amelioration of azotemia in 5/6th nephrectomized Sprague-Dawley rats. Scientific World J 2005; 5: 652–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jankowski J, Westhof T, Vaziri ND, et al. Gases as uremic toxins: is there something in the air? Semin Nephrol 2014; 34: 135–150 [DOI] [PubMed] [Google Scholar]
- 120.Rossi M, Johnson DW, Morrison M, et al. SYNbiotics easing renal failure by improving Gut microbiologY (SYNERGY): a protocol of placebo-controlled randomised cross-over trial. BMC Nephrol 2014; 15: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Barna MM, Kapoian T, O'Mara NB. Sevelamer carbonate. Ann Pharmacother 2010; 44: 127–134 [DOI] [PubMed] [Google Scholar]
- 122.Kays MB, Overholser BR, Mueller BA, et al. Effects of sevelamer hydrochloride and calcium acetate on the oral bioavailability of ciprofloxacin. Am J Kidney Dis 2003; 42: 1253–1259 [DOI] [PubMed] [Google Scholar]
- 123.Phan O, Ivanovski O, Nguyen-Khoa T, et al. Sevelamer prevents uremia-enhanced atherosclerosis progression in apolipoprotein E-deficient mice. Circulation 2005; 112: 2875–2882 [DOI] [PubMed] [Google Scholar]
- 124.Kikuchi K, Itoh Y, Tateoka R, et al. Metabolomic search for uremic toxins as indicators of the effect of an oral sorbent AST-120 by liquid chromatography/tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2010; 878: 2997–3002 [DOI] [PubMed] [Google Scholar]
- 125.Schulman G, Vanholder R, Niwa T. AST-120 for the management of progression of chronic kidney disease. Int J Nephrol Renovasc Dis 2014; 7: 49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ueda H, Shibahara N, Takagi S, et al. AST-120, an oral adsorbent, delays the initiation of dialysis in patients with chronic kidney diseases. Ther Apher Dial 2007; 11: 189–195 [DOI] [PubMed] [Google Scholar]
- 127.Lekawanvijit S, Kumfu S, Wang BH, et al. The uremic toxin adsorbent AST-120 abrogates cardiorenal injury following myocardial infarction. PLoS One 2013; 8: e83687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Saito H, Yoshimura M, Saigo C, et al. Hepatic sulfotransferase as a nephropreventing target by suppression of the uremic toxin indoxyl sulfate accumulation in ischemic acute kidney injury. Toxicol Sci 2014; 141: 206–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vanholder R, De Smet R, Waterloos MA, et al. Mechanisms of uremic inhibition of phagocyte reactive species production: characterization of the role of p-cresol. Kidney Int 1995; 47: 510–517 [DOI] [PubMed] [Google Scholar]
- 130.Saigo C, Nomura Y, Yamamoto Y, et al. Meclofenamate elicits a nephropreventing effect in a rat model of ischemic acute kidney injury by suppressing indoxyl sulfate production and restoring renal organic anion transporters. Drug Des Devel Ther 2014; 8: 1073–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Levey AS, Adler S, Caggiula AW, et al. Effects of dietary protein restriction on the progression of advanced renal disease in the Modification of Diet in Renal Disease Study. Am J Kidney Dis 1996; 27: 652–663 [DOI] [PubMed] [Google Scholar]