Abstract
Prevalence of adynamic bone disease (ABD), characterized by low bone turnover and absence or a reduced number of osteoblasts and osteoclasts, is increasing steadily over the last years. We present a dialysis patient, with recurrent bone fractures and biopsy-proven ABD, who was treated with teriparatide. Nine months after initiation of treatment, iPTH plasma levels increased to 520 pg/mL and a second bone biopsy revealed high bone turnover, normal mineralization and normal bone volume. Two years later, iPTH was 250–350 pg/dL and bone metabolism parameters within normal range. The probable utility of teriparatide in the treatment of ABD in dialysis patients is discussed.
Keywords: adynamic bone disease, haemodialysis, teriparatide
Introduction
Adynamic bone disease (ABD) is characterized by low bone turnover and absence or a reduced number of osteoblasts and osteoclasts and its prevalence is increasing steadily over the last years [1]. This high prevalence has been attributed to many factors, such as diabetes, use of drugs down-regulating parathormone release, high calcium intake and advanced age.
Treatment of ABD imposes a serious challenge, since this condition is accompanied by a considerable increase in mortality, while large prospective controlled trials and reliable guidelines are lacking. Current treatment concepts focus on limiting daily oral calcium intake as well as intradialytic calcium, by using a dialysate calcium concentration of 1.25 mmol/L. Administration of full length or truncated 1–34 parathormone (PTH) would theoretically improve ABD in CKD patients, since in these patients the condition is attributed to PTH deficiency or an intrinsic bone resistance to PTH.
Teriparatide is the recombinant (1–34) N-terminal region of human parathyroid hormone (PTH1−34) and its administration is indicated for post-menopausal women patients with severe osteoporosis, who are at high risk of fracture. However, its probable effects in patients on dialysis with ABD remain unclear [2].
We present a patient on dialysis, with biopsy-proven ABD, who was treated with teriparatide and we discuss the results.
Case report
A 68-year-old woman on chronic maintenance dialysis, presented with recurrent fractures in thoracic vertebrae and ribs, over a period of 9 months.
She had chronic renal failure since she was 40 years old and was started on dialysis at the age of 56. After 1 year on dialysis she received a renal transplant from a deceased donor and the administered immunosuppressive therapy consisted of methylprednizolone, mycophenolate mofetil and cyclosporine A. The patient maintained sufficient glomerular filtration rate, up to 40 mL/min, for the next 7 years but thereafter, she experienced a gradual decline in GFR. A transplant biopsy showed evidence of chronic allograft nephropathy, renal function deteriorated gradually and she was started again on dialysis, 8 years after transplantation.
Bone disease-related biochemistry of the patient during the transplantation period is shown in Table 1. Osteoporosis prophylaxis over the first year on transplantation included alendronate 70 μg/week, followed by alphacalcidol 0.25 μg/day for the rest of the transplantation period. Six months before restarting dialysis, she complained of diffuse bone pain in the spine and dual-energy X-ray absorptiometry (DEXA) findings were compatible with osteoporosis.
Table 1.
Bone mineral disease-related biochemical parameters of the patient before and after teriparatide treatment
| During Transplantation | Biochemical parameters |
||||
|---|---|---|---|---|---|
| On HD Pre-treatment |
On HD Post-treatment |
On HD 1 year post-treatment |
Normal levels | ||
| Calcium-corrected (mg/dL) | 8.5–9.5 | 8.9 | 9.8–10.3 | 8.9–9.8 | 8.8–10.6 |
| Phosphorus (mg/dL) | 3.0–4.5 | 3.5–5 | 4.5 | 3–4.5 | 3.5–5.1 |
| Alk.phosphatase (iu/L) | 30–70 | 70–90 | 200 | 140 | 30–120 |
| i-PTH (pg/mL) | 30–50 | 30 | 520 | 250 | 7–53 |
| 1,25 (OH)2 VitD (pg/mL) | 22 | 20 | 12.3 | 20 | 19.6–54.3 |
| 25 (ΟΗ) VitD (ng/mL) | 35 | 15.2 | 9.1 | 45 | 9.0–37.6 |
Notes: Conversion factors for units: serum calcium mg/dL to mol/L, ×0.25; serum phosphorus mg/dL to mmol/L, ×0.323 iPTH. pg/mL to pmol/L ×1, 25(ΟΗ)D in ng/mL to nmol/L × 2.496, 1,25(OH)2D in pg/mL to pmol/L × 2.6.
Three months after she restarted dialysis, she complained of severe bone pain, resulting in restricted mobility. An X-ray examination revealed fractures in the 6th and 7th thoracic ribs and the 12th thoracic vertebra. Plasma iPTH and alkaline phosphatase levels were low whereas Ca, P, 25(OH)Vitamin D and 1,25(OH)2 Vitamin D were within the normal laboratory range (Table 1). Her HD therapy consisted of 4 h long sessions, using a low flux polysulfone dialyser with standard bicarbonate dialysate with 1.5 mmol/L calcium thrice weekly. Her phosphate-binding medication included sevelamer hydrochloride.
DEXA showed severe osteoporosis (Tscore −4.7). A bone biopsy revealed ABD, with low bone turnover, normal mineralization and low bone volume (Figure 1a). Static histomorphometric parameters were: [bone volume/tissue volume (BV/TV) 17.6% (normal 19.1 ± 4.5%), osteoblast surface/bone surface (ObS/BS) 0.11% (normal 1.14 ± 1.0%), osteoid volume/bone volume (OV/BV) 0.33% (normal 1.77 ± 0.08%), osteoclast surface/bone surface (OcS/BS) 0.0% (normal 0.18 ± 0.19%), bone formation rate/bone surface (BFR)/BS 0.07 (normal 24.1 ± 13.5 μm3/μm2/year).
Fig. 1.
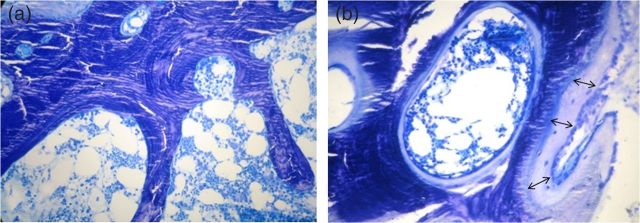
Bone biopsy toluidine blue ×20. (a) Before treatment with teriparitide, bone without osteoid, any osteoblast or osteoclast, (b) at the end of treatment, thick osteoid layer (double arrows) with many osteoblasts.
The patient received treatment with teriparatide 20 μg/day subcutaneously, oral cholecalciferol 400 IU, sevelamer and oral alphacalcidol 0.25 μg/day. Calcium concentration in the dialysis fluid was reduced from 1.5 to 1.25 mmol/L. iPTH plasma levels were measured monthly.
Six months later, iPTH plasma levels increased gradually from 30 to 520 pg/mL and remained steady, for the next 2 months. Therefore, teriparatide was withdrawn and a second bone biopsy was performed. Secondary hyperparathyroidism was revealed, with high bone turnover, normal mineralization and normal bone volume (Figure 1b). Histomorphometric parameters were: BV/TV 31.16% (normal 19.1 ± 4.5%), ObS/BS 4.4% (normal 1.14 ± 1.0%), OV/BV 12.6% (normal 1.77 ± 0.08%), OcS/BS 0.39% (normal 0.18 ± 0.19%), BFR/BS 69.16 (normal 24.1 ± 13.5 μm3/μm2/year). After cessation of teriparatide treatment, iPTH plasma levels decreased gradually over the next 2 months below 300 pg/mL, remaining quite stable between 250 and 350 pg/dL one year after treatment. Measured plasma levels of bone metabolism parameters were also within normal range (Table 1). DEXA showed an increase in bone mass (Tscore −3.8), bone pain subsided and mobility was gradually improved. The patient continued to receive low-dose cholecalciferol and alphacalcidol for the next 2 years with no significant further changes in her clinical and bone biochemistry findings.
Discussion
Our patient had many factors contributing to ABD, including advanced age (post-menopausal status), previous corticosteroid administration and a possible chronic inflammation due to chronic allograft rejection before initiation of dialysis. Bone mass was extremely low when measured with DEXA. This technique has been proved useful in healthy post-menopausal women, for estimating the mineralized bone mass and therefore the relative risk for bone fracture, but in patients on dialysis failed to identify the type of bone turnover defect [3]. As a result a bone biopsy was considered necessary in our patient and revealed low bone volume and total absence of bone turnover, consistent with low plasma levels of iPTH and alkaline phosphatase.
The patient was started on recombinant human parathyroid hormone (teriparatide) at a dose of 20 μg/day. Theoretically, parathormone administration should be ideal for up-regulating the number of osteoblasts and osteoclasts and therefore increasing bone turnover in patients with ABD, providing that there is no concurrent inflammation or cytokine up-regulation inhibiting its action. Although teriparatide is not currently approved for renal ABD, we considered that it would be beneficial to this patient, who already had recurrent bone fractures and severe bone metabolism suppression. In a pilot study in seven haemodialysis patients, use of teriparitide improved bone mineral density measured with DEXA [4].
Alternatively a dialysate calcium concentration of 1.25 mmol/L or lower could have been used, to induce hypocalcaemia and stimulate endogenous PTH release. This type of treatment alone is not associated with a controlled rise in PTH and can lead to non-reversible parathyroid gland hyperplasia [5]. Biphosphonates enhance osteoclast apoptosis and were contraindicated in this patient, since there were no osteoclasts or increased bone resorption on biopsy [6, 7].
In combination with teriparatide, we co-administered VitD to promote adequate bone calcification. We, therefore, chose to use lower calcium concentration dialysate (1.25% mmol/L) to avoid potential hypercalcaemia secondary to vitamin D administration. The patients' serum calcium was followed very closely (thrice weekly) and it was always between 8.5 and 10.0 mg/dL.
Although the recommended length of treatment with teriparatide is 1 year, we had to withdraw it after 9 months, because there was a steep rise in endogenous PTH and alkaline phosphatase plasma levels.
The reason for this unexpected rise in PTH is not clear.
One possibility is that inflammation, which usually accompanies chronic kidney allograft nephropathy, was decreased after the removal of the allograft, soon after dialysis initiation, thus promoting recovery of parathyroid function.
Using a dialysate calcium concentration of 1.25 mmol/L could also have contributed in improvement of ABD, as a transient decrease of ionized calcium levels during dialysis might stimulate PTH secretion, even though ionized calcium levels were repeatedly within normal range between dialysis sessions.
Finally, another possibility is that teriparatide may have promoted parathyroid gland recovery through stimulation of PTH receptor. Though there is really little bibliographic evidence, Lewin et al. have shown that (1–40) aminoterminal PTH peptides could possibly stimulate, through positive feedback, endogenous PTH production in rats [8]. Teriparatide could theoretically act in a similar way, as it acts on the same parathyroid gland receptor. We considered this as another hypothetical contributor to explain the post teriparatide administration rise in PTH levels.
This hypothesis is in concordance with the fact that when teriparatide was stopped, iPTH plasma levels decreased gradually over 2 months to 250 pg/mL, a value within recommended levels to maintain normal bone metabolism. The second bone biopsy proved a favourable response of bone metabolism to teriparatide administration, since bone volume was restored to normal and turnover was high.
Active (alphacalcidol) and inactive (cholecalciferol) compounds of vitamin D were administered along with teriparatide, because we considered that adequate bone mineralization would depend upon sufficient plasma levels of vitamin D, calcium and phosphate. Administration of vitamin D may have also contributed to the restoration of endogenous iPTH to lower—recommended—levels after cessation of teriparatide treatment.
Our case is unique in that, to our knowledge, it is the first case of teriparatide treatment associated with biopsy-proven bone response in a haemodialysis patient with ABD. This response included an increase of bone metabolism and bone, and though we cannot clearly dissect teriparatide's influence from other potential contributing factors, it may represent a therapeutic option for patients with ABD and severe suppression of bone metabolism.
Conflict of interest statement
The authors have no conflicts of interest. The contents of this paper have not been published previously in whole or part, except in abstract format.
References
- 1.Cannata-Andía JB, Rodriguez García M, Gómez Alonso C. Osteoporosis and adynamic bone in chronic kidney disease. J Nephrol 2013; 26: 73–80 [DOI] [PubMed] [Google Scholar]
- 2.Cejka D, Haas M. Should teriparatide ever be used for adynamic bone disease? Semin Dial 2011; 24: 431–433 [DOI] [PubMed] [Google Scholar]
- 3.Schwarz C, Sulzbacher I, Oberbauer R. Diagnosis of renal osteodystrophy. Eur J Clin Invest 2006; 36(Suppl 2): S13–S22 [DOI] [PubMed] [Google Scholar]
- 4.Cejka D, Kodras K, Bader T, et al. Treatment of hemodialysis-associated adynamic bone disease with teriparatide (PTH1-34): a pilot study. Kidney Blood Press Res 2010; 33: 221–226 [DOI] [PubMed] [Google Scholar]
- 5.Spasovski G, Gelev S, Masin-Spasovska J, et al. Improvement of bone and mineral parameters related to adynamic bone disease by diminishing dialysate calcium . Bone 2007; 41: 698–703 [DOI] [PubMed] [Google Scholar]
- 6.Haffner D, Fischer DC. Can bisphosphonates play a role in the treatment of children with chronic kidney disease? Pediatr Nephrol 2011; 26: 2111–2119 [DOI] [PubMed] [Google Scholar]
- 7.Ott S. Therapy for patients with CKD and low bone mineral density. Nat Rev Nephrol 2013; 9: 681–692 [DOI] [PubMed] [Google Scholar]
- 8.Lewin E, Garfia B, Almanden Y, et al. Autoregulation in parathyroid glands by PTH/PTHrP receptor ligands in normal and uremic rats. Kidney Int 2003; 64: 63–70 [DOI] [PubMed] [Google Scholar]


