Abstract
Background
The R2CHADS2 is a new prediction rule for stroke risk in atrial fibrillation (AF) patients wherein R stands for renal risk. However, it was created from a cohort that excluded patients with advanced renal failure (defined as glomerular filtration rate of <30 mL/min). Our study extends the use of R2CHADS2 to patients with advanced renal failure and aims to compare its predictive power against the currently used CHADS and CHA2DS2VaSc.
Methods
This retrospective cohort study analyzed the 1-year risk for stroke of the 524 patients with AF at Metropolitan Hospital Center. AUC and C statistics were calculated using three groups: (i) the entire cohort including patients with advanced renal failure, (ii) a cohort excluding patients with advanced renal failure and (iii) all patients with GFR < 30 mL/min only.
Results
R2CHADS2, as a predictor for stroke risk, consistently performs better than CHADS2 and CHA2DS2VsC in groups 1 and 2. The C-statistic was highest in R2CHADS compared with CHADS or CHADSVASC in group 1 (0.718 versus 0.605 versus 0.602) and in group 2 (0.724 versus 0.584 versus 0.579). However, there was no statistically significant difference in group 3 (0.631 versus 0.629 versus 0.623).
Conclusion
Our study supports the utility of R2CHADS2 as a clinical prediction rule for stroke risk in patients with advanced renal failure.
Keywords: anticoagulation, atrial fibrillation, CKD, clinical prediction rule, stroke
Introduction
Thromboembolic stroke is a known complication of non-valvular atrial fibrillation (AF). Therefore, the need to prevent such occurrence is of paramount importance when treating patients with AF. To aid in the assessment of stroke risk, several risk stratification schemes or clinical prediction rules have been developed. The two most common risk stratification schemes validated for predicting stroke risk in non-valvular AF patients are CHADS2 (congestive heart failure, hypertension, age ≥ 75 years old, diabetes, prior TIA or stroke or thromboembolism) and the CHA2DS2VASc, which is a modification of the CHADS2 that added three additional risk factors: age 65–74, female sex and history of vascular disease [1]. Current guidelines recommend antithrombotic or anticoagulant therapy to protect AF patients from stroke based on these risk stratification algorithms [2]. Although not included in these stroke risk predictors, chronic kidney disease (CKD) has been demonstrated to be an independent risk factor for stroke in AF patients in several studies. A study by Go et al. [3] examined how CKD, evidenced either by reduced glomerular filtration rate (GFR) or proteinuria, related to stroke risk in patients with AF without anticoagulation therapy. From this study, they have shown a graded, progressive risk of stroke associated with progressively lower level of estimated GFR [3].
CKD is also an independent risk factor for cardiovascular disease (CVD) outcomes, such as hypertension, heart failure and myocardial infarction. The mechanisms by which CKD causes CVD outcomes are still not fully elucidated, but may include predisposition to early atherosclerosis [4]. In the Reasons for Geographic and Racial Differences (REGARDS) study, CKD was also shown to be associated with increased prevalence of AF in a large population-based sample of 27 000 US adults. The prevalence was highest in those with CKD stages 4 and 5, and the association persisted even after multivariable adjustment [5]. However, the incidence of stroke was significantly higher in AF patients with CKD than in those with AF alone. In a large cohort study of 132 372 patients with non-valvular AF from the Danish national registry revealed that the risk of stroke or systemic embolism was higher in those AF patients with non-end-stage CKD compared with those who did not have renal disease and even higher in those requiring dialysis [6]. CKD patients, therefore, are at an increased risk of stroke.
Despite this evidence, CKD is not a component of widely used current predictors for stroke risk in patients with non-valvular AF. The commonly used clinical prediction rules, the CHADS and CHADSVASC, do not consider decline in renal function. The main reason for this is that patients with decreased GFR were excluded in those cohorts from which these clinical prediction rules were derived, making them not optimal for CKD patients. Few studies have included renal dysfunction in their risk stratification scheme for stroke. In one study that did assess CKD as part of the risk stratification scheme, Piccini et al. validated the R2CHADS2 scoring system (where R for renal dysfunction was measured by creatinine clearance with inclusion of those <60 mL/min and >30 mL/min) in the ROCKET AF and ATRIA study cohorts. The authors concluded that reduced creatinine clearance was a strong independent predictor for stroke, second only to prior stroke and transient ischemic attack and that R2CHADS2 improved net reclassification index by 6.2% compared with CHA2DS2VASc and 8.2% compared with CHADS2 [7]. This study, however, excluded more advanced CKD patients with creatinine clearance of <30 mL/min. The current retrospective study, therefore, uses a patient population that includes more advanced CKD subgroups, including those on hemodialysis, to compare the predictive value of R2CHADS2 for stroke and other thrombosis against those of CHADS and CHA2DS2VASc and to determine the risk of developing stroke, transient ischemic attack (TIA), and other thrombosis in patients with eGFR < 30 mL/min.
Methods
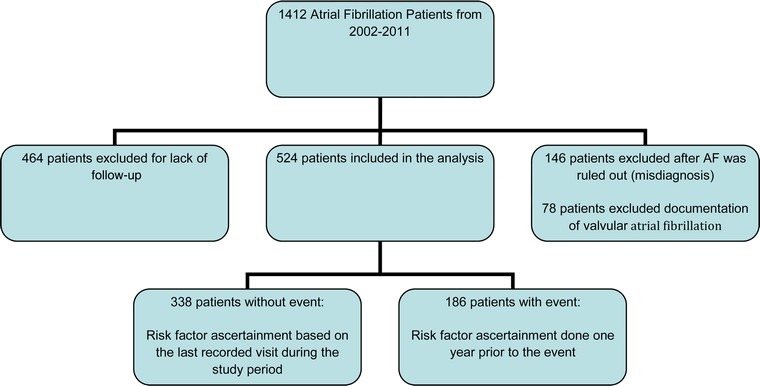
This is a 10-year retrospective analysis of all patients admitted to Metropolitan Hospital Center (MHC) in New York City with a diagnosis of non-valvular AF. The 2013 ICD-9-CM Diagnosis Code 427.31 was used to screen all hospital admissions and outpatient visits. Patients with recorded diagnoses between 1 January 2002 and 31 December 2011 were included. Inclusion criteria were any adult patient, age above 18, admitted to MHC, with a documented non-valvular AF diagnosis and at least 1-year follow-up from the time of the diagnosis. A total of 1412 adults were initially eligible for the study. One hundred and forty-six were excluded after AF was ruled out by review of their EKG and 78 after a documentation of valvular AF was discovered. In addition, 464 patients were excluded for lack of follow-up of at least 1 year from the time of AF diagnosis. The remaining 524 patients with atrial fibrillation were then analyzed and any occurrence of a primary endpoint was documented. We calculated the risk for a primary endpoint by recording the variables of interest that are present 1 year prior to the incidence of the endpoint. For patients who did not have an endpoint, we calculated their risk by recording the variables of interest present in the last recorded visit in our hospital.
Eleven variables were evaluated as potential confounders of the CKD and stroke relationship: age, sex, race/ethnicity, presence of congestive heart failure (CHF), hypertension, diabetes, prior stroke, history of vascular disease and anticoagulation treatment. All variables are categorical except for creatinine level, GFR and age. Age was evaluated both as continuous and categorical (</≥75). These variables were ascertained from the patients' medical charts.
Race/ethnicity is categorized as Hispanic, African American or others, including Caucasian. CHF is defined as a documented left ventricular ejection fraction of <40%, hypertension (HTN) as mean systolic blood pressure >160 mmHg, vascular disease as the presence of peripheral arterial disease, carotid artery disease, or coronary arterial disease. Treatment was categorized either as warfarin, aspirin, heparin, or no-treatment group.
The average creatinine over 6 months was used to measure the eGFR using the 4-variable Modification of Diet in Renal Disease (MDRD) equation, which includes age, sex, race and creatinine in the computation [8]. For inpatients with acute kidney injury, defined as an increase in the creatinine by 0.3, the creatinine level was documented prior to the incident. The eGFR levels were categorized according to the 2002 KDOQI guidelines of CKD stages. Stage 1 included eGFR > 90 mL/min, stage 2 eGFR 60–89 mL/min, stage 3 eGFR 30–59 mL/min, stage 4 eGFR 15–29 and stage 5 eGFR < 15 mL/min. Patients on hemodialysis, regardless of GFR, were allocated in a different category.
A primary endpoint event is defined as the presence of any of the following: thromboembolic stroke, transient ischemic attack (TIA), or central thrombosis such as pulmonary embolism (PE), deep venous thrombosis (DVT) or other arterial embolism. DVT was included in the primary outcome because of clinical data supporting its association with both AF and CKD [9, 10]. Hemorrhagic stroke was excluded as a primary endpoint but considered in bleeding outcomes (not shown in this paper). Stroke alone was also an outcome of interest.
Radiologic documentation of a new stroke was required prior to assigning a primary endpoint for any patient. Neurology consult was required for a TIA diagnosis. Patients with intracranial hemorrhage were not considered in the primary endpoint but recorded as a secondary endpoint.
A priori variables included in the CHADS (CHF, hypertension, age > 75, diabetes, previous stroke) and CHADSVASc (CHADS plus vascular disease, age 65–74, and female as sex category) clinical prediction rules were collected and considered for inclusion in the multivariate analyses for primary and secondary endpoints and stroke alone. Congestive heart failure is defined as left ventricle systolic ejection fraction of <40%, hypertension as the presence of multiple systolic blood pressure readings >160 mmHg, and vascular disease as PAD, CHD, or CAD. The presence of each disease was given 1 point while a prior cerebrovascular accident (CVA), defined as ischemic stroke or TIA, conferred 2 points. When CHADSVASc was used 2 points were given for age >75 and 1 point for age 65–74. The R2CHADS was calculated by the addition of renal risk factor that confers a score of 2 if the GFR < 60 mL/min.
Statistical analysis was performed using SAS version 9.3 and STATA version 12. Continuous variables were compared using the Wilcoxon–Mann–Whitney test. Categorical variables are expressed as proportions and were compared using the χ2 test or Fisher's exact test if sample size was <5. A two-sided P-value <0.05 was considered significant and significant variables were included in the multivariate relative risk regression for thromboembolic stroke and other primary composite outcomes.
The C-statistic was calculated to assess the discriminatory capacities of the prediction models for the primary composite outcome, with 95% CI generated by an approximate jackknife method. C statistics with 95% confidence interval were calculated for R2CHADS, CHADS and CHADSVASc. Sensitivity and specificity values were also computed for every score using the three clinical prediction models. The C statistics was calculated using three groups. Group 1 (n = 524) involved the entire cohort, group 2 (n = 426) excluded patients with advanced renal failure, and group 3 (n = 98) included only those patients with advanced renal failure.
This study was approved by the Institutional Review Board of New York Medical College. No author or co-author disclosed any financial interest during the course of this study.
Results
Characteristics of the patients who developed a primary endpoint
Table 1 shows the characteristics of the cohort members who developed two endpoints of interest: stroke alone; and stroke, TIA or other central thromboses, collectively termed as primary outcome. A total of 145 (27.7%) developed stroke and 186 (34%) a primary outcome. Forty-seven percent (47%) of patients with CKD developed stroke. More females (30.2%), African Americans (32%), patients older than 75 (29.6%), patients with hypertension (30.1%), diabetes (31.7%), prior stroke (63.9%), vascular disease (33.1%), and those on warfarin (30.5%) developed stroke.
Table 1.
Risk factors and characteristics of the cohort
| No stroke (n = 375) | Stroke (n = 145) | Rate % | P-value | No primary outcome (n = 338) | Primary outcome (n = 186) | Rate % | P-value | |
|---|---|---|---|---|---|---|---|---|
| CKD Stage | ||||||||
| GFR > 90 | 71 | 8 | 10.1% | ref | 68 | 11 | 13.9% | ref |
| GFR 60–90 | 186 | 26 | 12.3% | 0.61 | 176 | 36 | 17.0% | 0.53 |
| Stage 3a | 39 | 53 | 57.6% | <0.0001 | 29 | 63 | 68.5% | <0.0001 |
| Stage 3b | 22 | 21 | 48.8% | <0.0001 | 16 | 27 | 62.8% | <0.0001 |
| Stage 4 | 22 | 10 | 31.3% | 0.006 | 15 | 17 | 53.1% | <0.0001 |
| Stage 5 | 14 | 19 | 57.6% | <0.0001 | 14 | 19 | 57.6% | <0.0001 |
| Hemodialysis | 25 | 8 | 24.2% | 0.05 | 20 | 13 | 39.4% | 0.003 |
| Sex | ||||||||
| female | 164 | 71 | 30.2% | 150 | 85 | 36.2% | ||
| male | 215 | 74 | 25.6% | 0.24 | 188 | 101 | 33.8% | 0.78 |
| Ethnicity | ||||||||
| Hispanic | 196 | 66 | 25.2% | ref | 176 | 86 | 21.4% | ref |
| African American | 115 | 54 | 32.0% | 0.13 | 102 | 67 | 39.6% | 0.15 |
| Others (including Whites) | 68 | 25 | 26.9% | 0.75 | 60 | 33 | 35.5% | 0.64 |
| CHF | ||||||||
| No | 254 | 109 | 30.0% | 222 | 141 | 38.8% | ||
| Yes | 125 | 36 | 22.4% | 0.07 | 116 | 45 | 28% | 0.02 |
| Hypertension | ||||||||
| No | 93 | 22 | 19.1% | 83 | 32 | 27.8% | ||
| Yes | 286 | 123 | 30.1% | 0.02 | 255 | 154 | 37.7% | 0.052 |
| Age > 75 | ||||||||
| No | 229 | 82 | 26.4% | 206 | 105 | 33.8% | ||
| Yes | 150 | 63 | 29.6% | 0.42 | 132 | 81 | 38% | 0.32 |
| Diabetes | ||||||||
| No | 254 | 87 | 25.5% | 225 | 116 | 34% | ||
| Yes | 125 | 58 | 31.7% | 0.13 | 113 | 70 | 24.7% | 0.33 |
| Prior CVA | ||||||||
| No | 349 | 92 | 20.9% | 311 | 130 | 29.5% | ||
| Yes | 30 | 53 | 63.9% | <0.0001 | 27 | 56 | 67.5% | <0.0001 |
| Vascular | ||||||||
| No | 290 | 101 | 18.2% | 261 | 130 | 33.2% | ||
| Yes | 89 | 44 | 33.1% | 0.11 | 77 | 56 | 42% | 0.07 |
| Treatment | ||||||||
| None | 71 | 27 | 27.6% | 0.58 | 60 | 38 | 38.8% | 0.94 |
| Aspirin only | 99 | 27 | 21.4% | 0.06 | 93 | 33 | 26.2 | 0.02 |
| Warfarin | 203 | 89 | 30.5% | ref | 180 | 112 | 38.4% | ref |
| Others (including heparin) | 6 | 2 | 25.0% | 1 | 5 | 3 | 60% | 1 |
| Median | Median | P-value | Median | Median | P-value | |||
| Age (continuous) | 70 | 72 | 0.06 | 70 | 72 | 0.04 | ||
CKD and risk for a primary outcome (stroke, TIA, and other central thrombosis)
In the multivariable analysis only two variables were significantly associated with an increased risk of stroke, TIA, or other central thromboses. The risk for a primary outcome was higher in patients with a history of previous CVA and those with CKD. The presence of advanced CKD increased the risk for a primary outcome by 1.3 times (Adjusted RR 1.30, 95% CI 1.01–1.67) while a history of previous stroke increased risk for a subsequent stroke, TIA or other central thrombosis by almost three times (RR 2.9, 95% CI 2.26–3.71). Of note, CHF, hypertension, age older than 75, diabetes, vascular disease, female as sex, or treatment with warfarin were not statistically associated with a risk for a primary outcome.
ROC curves of CHADS, CHADSVASc and R2CHADS for a primary outcome
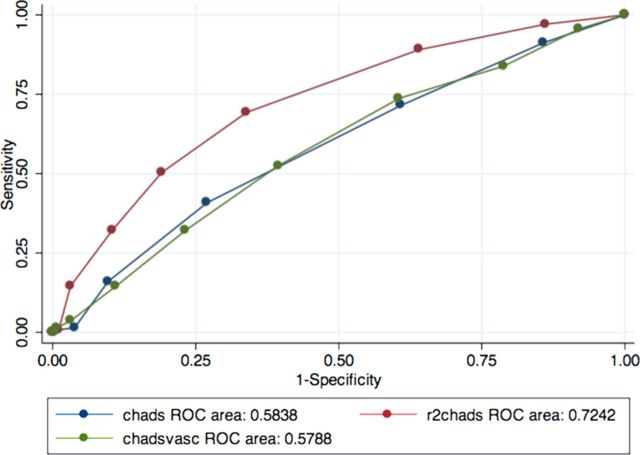
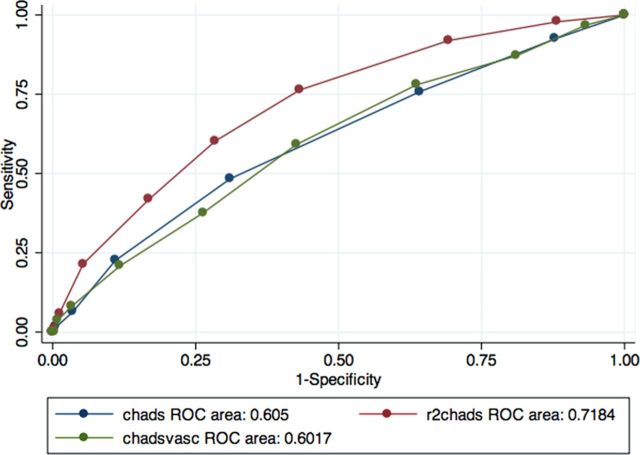
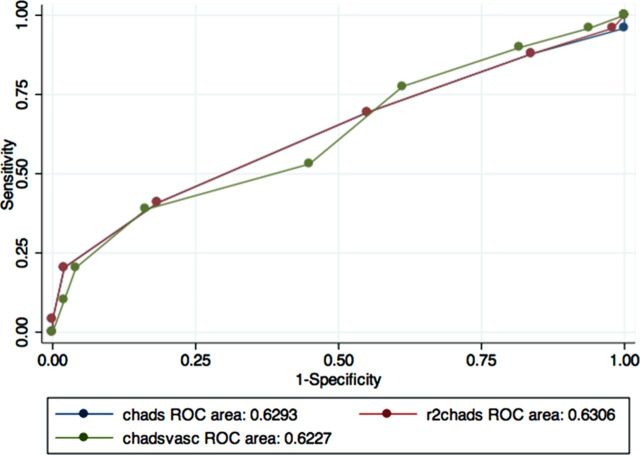
Figures 1–3 show the areas under the curve (AUC) of the R2CHADS for a primary outcome (stroke, TIA and central thrombosis) compared with CHADS and CHADSVASc. We used CKD levels as cutoffs in determining the groups for our analyses of the predictive power of the three clinical prediction rules—group 1 includes all patients, group 2 excludes patients with advanced CKD, and group 3 includes only patients with advanced CKD.
Fig. 2.
ROC curve for cohort excluding those with advanced CKD. N = 426.
Fig. 1.
ROC curve for the entire cohort. N = 524.
Fig. 3.
ROC curve for cohort with advanced CKD only. N = 98
Table 2 shows the C statistics for the three clinical prediction rules in the three groups analyzed. In group 1 the C statistics for the new clinical prediction rule R2CHADS were higher at 0.718 than for CHADS and CHADSVASc, which are only 0.605 and 0.602, respectively. In addition, when patients with advanced CKD were excluded (group 2) the C statistics for R2CHADS were still the highest, at 0.724 versus 0.584 and 0.579 for CHADS and CHADSVASc, respectively. However; in group 3, the R2CHADS has a C statistics of 0.631 versus 0.629 for CHADS and 0.623 for CHADSVASC. The C statistics for CHADSVASc, when compared with that of CHADS, were not statistically higher in the three groups, as evidenced by the overlapping confidence intervals. In the first two groups, R2CHADS outperformed CHADS and CHADSVASc, although there was no statistical difference in the performance of R2CHADS versus CHADS or CHADSVASC in group 3.
Table 2.
C statistics of clinical prediction rules for a primary outcome
| C statistics/ROC curve | Standard error | 95% confidence interval | |
|---|---|---|---|
| Group 1: All patients, n = 524 | |||
| CHADS | 0.605 | 0.0253 | 0.56–0.65 |
| CHADSVASc | 0.602 | 0.0255 | 0.55–0.65 |
| R2CHADS | 0.718a | 0.026 | 0.67–0.76 |
| Group 2: eGFR < 30 excluded, n = 426 | |||
| CHADS | 0.584 | 0.029 | 0.53–0.64 |
| CHADSVASc | 0.579 | 0.029 | 0.52–0.64 |
| R2CHADS | 0.724a | 0.026 | 0.67–0.77 |
| Group 3: Patients with eGFR < 30, n = 98 | |||
| CHADS | 0.629 | 0.025 | 0.52–0.74 |
| CHADSVASc | 0.623 | 0.025 | 0.51–0.73 |
| R2CHADS | 0.631b | 0.023 | 0.52–0.74 |
aWith statistically significant difference.
bNo statistical significant difference.
Discussion
The Rocket AF study results showed that moderate renal failure is an independent risk factor for stroke among AF patients. In addition, a new clinical prediction rule that incorporated the renal risk proved to have a better discriminatory power than the CHADS and CHADSVASc scoring systems. The ROCKET AF study, however, excluded patients with advanced renal failure, those with eGFR < 30 mL/min. In this retrospective cohort study we aimed to determine whether the R2CHADS clinical prediction rule can be applied to include patients with GFR < 30 mL/min.
Advanced CKD and primary outcome:
In this study, advanced CKD (defined by eGFR < 30 mL/min), along with a prior CVA, is one of the variables that increase the rate for stroke, TIA and other central thromboses in non-valvular AF. This is consistent with other studies that place a strong emphasis on CKD as a risk factor for thromboembolic phenomena. The exact mechanism as to how CKD increases risk for thromboembolic disease is not known but may involve a more rapid progression of atherosclerotic events, apart from other complications, such as difficult to control HTN. It is also important to note that CKD, in itself, is usually a complication of other cardiovascular risks, such as uncontrolled diabetes and hypertension, which are major factors in development of atherosclerosis and thromboembolic complications.
Our data had a stroke rate of 27.7% for the entire cohort. This rate is substantially higher when compared with previously reported rates of stroke in AF patients with or without CKD. The presence of CKD and AF confers a stroke rate that ranges from 17% in non-CKD to as high as 35% in dialysis patients [11, 12]. Our result may be explained by the fact that our cohort is exclusively inpatient, representing a sicker population and a significant fraction of our patient population includes stroke patients who are admitted to the acute inpatient rehabilitation unit. This latter fact increased the incidence of stroke in this study.
Use of R2CHADS in advanced CKD
Several studies have attempted to incorporate CKD in the clinical prediction rules for stroke in AF patients with varying results. In a study involving 978 patients, incorporating CKD in the traditional CHADS and CHADS-VASc did not result in an improved integrated discrimination index (IDI) or C-statistics [13]. Another study compared CHADS, CHADSVASc and R2CHADS in patients who underwent catheter ablation for AF (the Leipzig Heart Center AF Ablation Registry). The results showed superiority of CHADSVASc score over the other two clinical prediction models; however, the study's patient population of 2069 only included a total of 27 patients with renal failure, with an average eGFR of 100 ± 34 for controls and 88 ± 25 for cases [14]. The result of our study may have differed from those of the Leipzig study because (i) our population included more patients with advanced renal failure (18 versus 1%), (ii) the median eGFR for our cases was 50 mL/min (IQR 30–60) and 68 mL/min (IQR 49–68) for the controls, and (iii) there were no patients in our study who underwent ablation, which is known to treat the arrhythmic source in AF.
The clinical prediction rule R2CHADS, based on its published report, had a net reclassification index of 17.4%. We attempted to extend its clinical use to patients with advanced renal failure, a population excluded in the original calculation of this new risk scoring system. We calculated the area under the curve or AUC using three groups based on CKD stages. In the first two groups (entire cohort and cohort excluding advanced CKD) the AUC curve of R2CHADS was consistently greater than those of CHADs and CHADSVASc. Based on our results, R2CHADS showed good discriminatory power across all CKD levels without a decrease in C-statistics even when patients with eGFR < 30 were included (0.718 versus 0.724). Group 2 reflected the same cohort used in the ROCKET AF study and our result is consistent with what they published: R2CHADS outperformed CHADS and CHADSVASC even in patients with moderate CKD. Interestingly there was no statistically significant difference in the C statistics for the three clinical prediction rules when only the advanced CKD patients were analyzed—group 3. This lack of difference could be explained by (i) the relatively similar R2CHADS scores in these patients and (ii) that our small sample size is not adequately powered to detect a difference.
A thromboembolic stroke is the most serious complication of atrial fibrillation. The management of stroke in AF begins with proper risk stratification of the patients. Since the decision to anticoagulate depends on the risk perceived using clinical prediction rules, it is imperative that clinicians use a clinical prediction rule that has a good discriminatory power. Currently, the commonly used clinical prediction rules for stroke in AF are, at best, moderate in their discriminatory power. This problem is further magnified in CKD patients since CKD itself is an independent risk factor for stroke, with or without AF, and for bleeding, on or off anticoagulation.
Our study adds important insights into the existing literature. First it showed that CKD, as well as advanced CKD, is an independent risk factor for stroke in AF patients. Second it further supports the use of R2CHADS as a better clinical prediction rule for the risk of stroke in AF patients. Lastly our results showed that R2CHADS may be used even in patients with advanced CKD without being inferior or superior to already available clinical prediction rules CHADS and CHADSVASC. Again, the lack of statistical difference in the discriminatory power of R2CHADS when compared with CHADS and CHADSVASC in patients with advanced CKD may be explained by their homogenous risk profiles or by the small sample size. This finding must be better defined by future epidemiologic studies.
Limitations and potential biases
This study has some limitations. Owing to the retrospective nature of this study, the researchers were able to include only patients with follow-up in at least 1 year, as reflected in the electronic medical record, in the final analysis. Those who did not follow-up for any other reason (i.e. non-compliance, admission in other institution, death, etc.) within the specified time frame were excluded. This was done to address the issue of missing outcomes or drop-outs which, in a prospective study, would have been solved by intention-to-treat analysis. Because of this, however, the population size was reduced, consequently decreasing the power of the study. Increasing the time frame used to collect more samples might be done in the future to address this issue.
Despite adjusting for confounding variables, residual effects of these variables may have affected our results. Many of a priori risk factors for stroke in AF patients did not prove to be statistically significant in our results. Risk factors such as diabetes, vascular disease, age and sex were more prevalent in those with a primary endpoint but their associations were not statistically significant. A possible explanation is that our sample size is underpowered to detect a statistical significance in the risk for stroke in the presence of these risk factors. In addition, our population is homogenously inpatient and thus may limit the generalizability of our results. Patients who did not need any admission represent a healthier population and may have less risk for developing stroke. This premise should be considered before drawing conclusions from our data. Lastly, our cohort is predominantly Hispanic. The CHADS, CHADSVASC and the R2CHADS were calculated from populations that were predominantly white and racial difference is being increasingly recognized as a risk factor in stroke and other vascular diseases.
Conclusion
Advanced CKD is associated with an increased risk of stroke and other primary outcomes in non-valvular AF patients. Adding CKD in R2CHADS to predict risk for stroke in non-valvular AF results in a higher predictive and discriminatory power compared with CHADS and CHADSVASc in this retrospective study. The use of R2CHADS in predicting stroke risk in non-valvular AF will likely include more patients to be considered for primary or secondary stroke prevention, but, because of the increased risk for bleeding, the decision to anticoagulate would need careful decision-making on the part of the physician, balancing the risk-benefit obtained from such treatment. Future epidemiologic studies are needed to address this issue.
Conflict of interest statement
None declared.
References
- 1.Coppens M, Eikelboom JW, Hart RG, et al. The CHA2DS2-VASc score identifies those patients with atrial fibrillation and a CHADS2 score of 1 who are unlikely to benefit from oral anticoagulant therapy. Eur Heart J 2013; 34: 170–176 [DOI] [PubMed] [Google Scholar]
- 2.Rockson SG, Albers GW. Comparing the guidelines: anticoagulation therapy to optimize stroke prevention in patients with atrial fibrillation. J Am Coll Cardiol 2004; 43: 929–935 [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Fang MC, Udaltsova N, et al. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. Circulation 2009; 119: 1363–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 2003; 42: 1050–1065 [DOI] [PubMed] [Google Scholar]
- 5.Baber U, Howard VJ, Halperin JL, et al. Association of chronic kidney disease with atrial fibrillation among adults in the United States: REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Circ Arrhythm Electrophysiol 2011; 4: 26–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olesen JB, Lip GY, Kamper AL, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med 2012; 367: 625–635 [DOI] [PubMed] [Google Scholar]
- 7.Piccini JP, Stevens SR, Chang Y, et al. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) study cohorts. Circulation 2013; 127: 224–232 [DOI] [PubMed] [Google Scholar]
- 8.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wattanakit K, Cushman M, Stehman-breen C, et al. Chronic kidney disease increases risk for venous thromboembolism. J Am Soc Nephrol 2008; 19: 135–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang CC, Lin CL, Wang GJ, et al. Atrial fibrillation associated with increased risk of venous thromboembolism. A Population-Based Cohort Study. Thromb Haemost 2015; 113: 185–192 [DOI] [PubMed] [Google Scholar]
- 11.Marinigh R, Lane DA, Lip GY. Severe renal impairment and stroke prevention in atrial fibrillation: implications for thromboprophylaxis and bleeding risk. J Am Coll Cardiol 2011; 57: 1339–1348 [DOI] [PubMed] [Google Scholar]
- 12.Vázquez E, Sánchez-perales C, Borrego F, et al. Influence of atrial fibrillation on the morbido-mortality of patients on hemodialysis. Am Heart J 2000; 140: 886–890 [DOI] [PubMed] [Google Scholar]
- 13.Roldán V, Marín F, Manzano-fernandez S, et al. Does chronic kidney disease improve the predictive value of the CHADS2 and CHA2DS2-VASc stroke stratification risk scores for atrial fibrillation? Thromb Haemost 2013; 109: 956–960 [DOI] [PubMed] [Google Scholar]
- 14.Kornej J, Hindricks G, Kosiuk J, et al. Renal dysfunction, stroke risk scores (CHADS2, CHA2DS2-VASc, and R2CHADS2), and the risk of thromboembolic events after catheter ablation of atrial fibrillation: the Leipzig Heart Center AF Ablation Registry. Circ Arrhythm Electrophysiol 2013; 6: 868–874 [DOI] [PubMed] [Google Scholar]