Abstract
Calcitonin is a 32 amino acid hormone secreted by the C-cells of the thyroid gland. Calcitonin has been preserved during the transition from ocean-based life to land dwellers and is phylogenetically older than parathyroid hormone. Calcitonin secretion is stimulated by increases in the serum calcium concentration and calcitonin protects against the development of hypercalcemia. Calcitonin is also stimulated by gastrointestinal hormones such as gastrin. This has led to the unproven hypothesis that postprandial calcitonin stimulation could play a role in the deposition of calcium and phosphate in bone after feeding. However, no bone or other abnormalities have been described in states of calcitonin deficiency or excess except for diarrhea in a few patients with medullary thyroid carcinoma. Calcitonin is known to stimulate renal 1,25 (OH)2 vitamin D (1,25D) production at a site in the proximal tubule different from parathyroid hormone and hypophosphatemia. During pregnancy and lactation, both calcitonin and 1,25D are increased. The increases in calcitonin and 1,25D may be important in the transfer of maternal calcium to the fetus/infant and in the prevention and recovery of maternal bone loss. Calcitonin has an immediate effect on decreasing osteoclast activity and has been used for treatment of hypercalcemia. Recent studies in the calcitonin gene knockout mouse have shown increases in bone mass and bone formation. This last result together with the presence of calcitonin receptors on the osteocyte suggests that calcitonin could possibly affect osteocyte products which affect bone formation. In summary, a precise role for calcitonin remains elusive more than 50 years after its discovery.
Keywords: bone, calcitonin, calcium, parathyroid hormone, vitamin D
Introduction
The discovery of calcitonin (CT), a hormone that is released in hypercalcemia and lowers the serum calcium, was first made by Copp et al. as a result of perfusing isolated thyroid-parathyroid gland preparations in the anesthetized dog [1, 2]. Initially, the source of CT was mistakenly thought to be the parathyroid gland, but a thyroid origin was subsequently established [3, 4]. Pearse showed that the origin of CT was the C-cells of the thyroid gland [5]. Potts et al. determined the amino acid sequence of human and salmon CT, which led to the synthesis and commercial use of the more potent salmon CT [6, 7]. The hormone was first named thyrocalcitonin, but subsequently has been called CT. Since its discovery more than 50 years ago, little progress has been made in understanding its pathophysiologic role in humans, in part because a deficiency or excess of CT does not result in abnormalities except for diarrhea in a few patients with medullary thyroid carcinoma. In this review, we will discuss the role of CT in maintaining the serum calcium and regulating 1,25 (OH)2 vitamin D (1,25D) production in pregnancy and lactation and also consider potential bone effects and links to the gastrointestinal hormone gastrin. When appropriate a comparison between CT secretion and function and that of parathyroid hormone (PTH), the major hormone protecting against hypocalcemia, will be highlighted.
CT is a 32 amino acid hormone secreted by the C-cells of the thyroid gland. In species in which the structure of CT has been determined, common features include a 1–7 amino terminal disulfide bridge with cysteine at positions 1 and 7 and proline at the carboxy-terminal [6]. Divergence is seen in the interior 10–27 amino acids. Non-mammalian CTs such as salmon have the greatest potency. Salmon CT, which differs from human CT in 16 amino acids, has been used for treatment of hypercalcemia. CT is primarily metabolized by the kidney [8–10]. The gene for CT is on the short arm of chromosome 11 and encodes CT and calcitonin gene-related peptide (CGRP). CT is present in large amounts only in the C-cells of the thyroid while CGRP, a potent vasodilator, is present not only in the thyroid, but also in central and peripheral nervous tissue. CT is present in ocean fish which live in a high calcium environment with the need to expel calcium. CT is thus older than parathyroid hormone (PTH) which was first recognized in early land-dwelling animals when conservation rather than expulsion of calcium became important.
Besides modifying increases in serum calcium and increasing 1,25D production, several potential roles for CT have been suggested and important observations about CT have been made. In azotemic and non-azotemic animal models, CT has been shown to decrease the magnitude of hypercalcemia during calcium loading [1–3, 11]. Studies in the 1980s showed that CT increased renal production of 1,25D [12, 13]. In contrast to other stimuli of 1,25D production such as PTH and hypophosphatemia in which 1,25D production occurs in the convoluted proximal tubule, stimulation of 1,25D by CT occurs in the straight proximal tubule [12]. Similar to the reciprocal relationships between both PTH and 1,25D and FGF23 and 1,25D, CT stimulates 1,25D secretion while 1,25D suppresses CT secretion [14, 15]. But during pregnancy and lactation, both 1,25D and CT levels are increased [16–18]. The effectiveness of CT in the treatment of hypercalcemia is attributed to reducing osteoclast activity [19]. But there is also a subsequent escape from the calcium-lowering effect of CT [20]. Besides decreasing osteoclast activity, CT has been suggested to facilitate deposition of calcium and phosphorus in bone especially in the post-prandial state [21]. Whether CT is stimulated by the ingestion of food through gastrin stimulation and consequently affects bone has been a subject of interest [21, 22]. However, no differences in bone mass has been shown when CT is absent in congenital hypothyroidism provided thyroid hormone is replaced [23, 24]. Gender and age differences in CT have been shown with women having lower values than men and with decreasing CT values with age in some, but not all studies [9, 25]. CT screening has been shown to be a useful tool for the diagnosis of medullary thyroid carcinoma [26]. However, despite several attractive and plausible hypotheses, CT has not been shown to have an important physiologic role in humans more than 50 years after its discovery.
Calcitonin and acute hypercalcemia
Studies during the past 50 years have shown that CT modifies the development of hypercalcemia. In 1960, a calcium infusion in thyroparathyroidectomized dogs was shown to result in a greater magnitude of hypercalcemia and a longer time to return to baseline values than in normal dogs [27]. Subsequently, Hirsch et al. showed that the calcemic response to parathyroid extract was greater in thyroparathyroidectomized rats than in parathyroidectomized or sham-operated rats demonstrating the thyroid origin of CT [3]. In rats with hypercalcemia from NH4Cl-induced metabolic acidosis, CT administration decreased the serum calcium concentration [28]. As shown in Figure 1, the absence of CT in both azotemic and non-azotemic rats enhanced the calcemic response to PTH during a 24 and 48 h infusion of PTH [11]. In the CT receptor knockout mouse, 1,25D-induced hypercalcemia was greater than in the control mouse [29]. In a mouse study consisting of PTH knockout, PTH and calcium-sensing receptor (CaSR) double knockouts, and wildtype mice, it was shown that both CaSR-mediated CT secretion and enhanced renal calcium excretion were important for preventing the development of hypercalcemia while inhibition of PTH secretion was not required for a robust defense against hypercalcemia [30]. In summary, strong evidence exists that CT is an important modifier of the hypercalcemic effect of acute calcium loading.
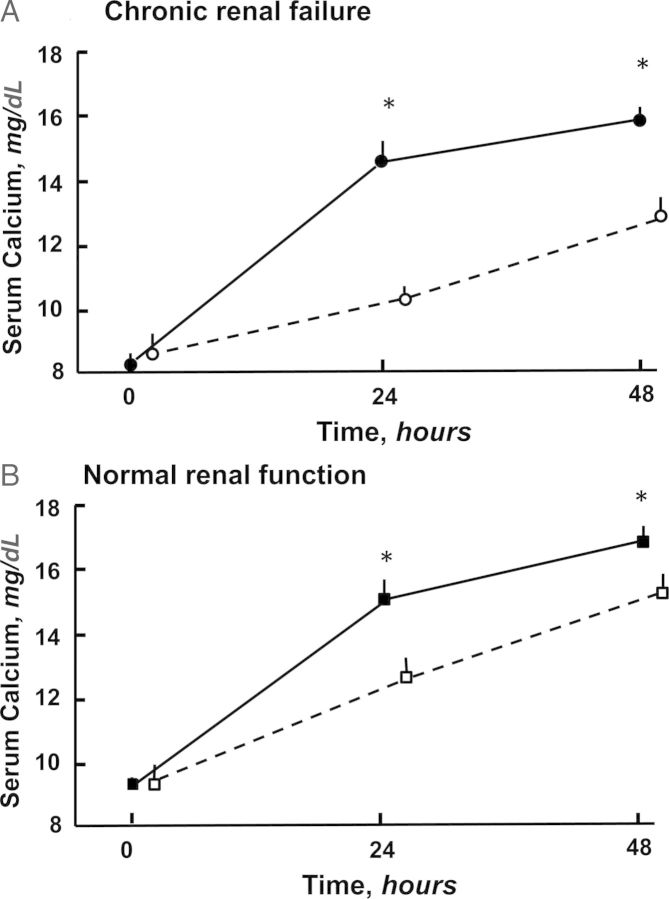
Fig. 1.
The effect of a PTH infusion on the serum calcium concentration was greater in the absence of calcitonin (CT) in rats both with (A) chronic renal failure (CRF) and (B) normal renal function (NRF). Solid circle is (CT−)CRF; open circle is (CT+)CRF; solid square is (CT−)NRF; and open square is (CT+)NRF [11]. Reprinted with permission from Kidney International.
Similar to PTH, CT secretion is modified by the CaSR. But while activation of the CaSR suppresses PTH secretion, CT secretion is stimulated. The mechanisms for the opposite effects downstream from the CaSR are still poorly understood. As with PTH, CT gene transcription is suppressed by 1,25D [14]. Both the CT and PTH receptors belong to the class II subclass of G-protein-coupled receptors [31]. The CT response to hypercalcemia is a sigmoidal curve opposite in direction to the sigmoidal curve of the PTH response to hypocalcemia [32–34]. In one animal study, a rapid induction of hypercalcemia resulted in a greater CT response than a slow induction of hypercalcemia of similar magnitude [35]. Also, as PTH secretion is suppressed by the induction of hypercalcemia, CT secretion in both control and CKD patients is suppressed by the induction of hypocalcemia [34]. In a study of normal and CKD subjects, a maximal CT response was seen after an increase in ionized calcium of 0.4 mM [34]. Many more studies of the PTH response to hypocalcemia have been performed than of the CT response to hypercalcemia. Interestingly, while every normal and azotemic subject in studies of PTH secretion has shown a sigmoidal response to hypocalcemia, studies in normal and azotemic humans and animals have shown that some patients and animals fail to increase CT secretion in response to hypercalcemia induced by a calcium infusion [34, 36]. In a study in cats, a strong correlation was found between the number of CT-positive cells in the thyroid gland and the plasma CT concentration induced by hypercalcemia [36]. Finally, both in azotemic patients and rats, baseline CT values have been shown to be increased compared with normal [32, 34, 37] and also to stimulate more during the induction of hypercalcemia [34].
Adaptation of calcitonin secretion to the existing serum calcium concentration
Similar to PTH secretion, CT secretion also appears to adapt to the ambient or existing serum calcium concentration [38]. In 1973 Deftos et al. studied the CT response to a calcium infusion in hypocalcemic patients with pseudohypoparathyroidism, idiopathic hypoparathyroidism and hypocalcemia from other causes, mostly malabsorption [23]. CT values were shown to increase before hypercalcemia developed. In a subsequent study in normal young male subjects, oral calcium loading that increased serum calcium to higher, but still normal values resulted in increases in serum CT that correlated with the increase in serum calcium [22]. In a study in normal, parathyroidectomized, and azotemic rats with the latter divided by a serum calcium less or greater than 8.5 mg/dL, it was shown that the two hypocalcemic groups, parathyroidectomized and azotemic rats, increased CT secretion in response to a PTH infusion before hypercalcemia was observed [32] (Figure 2). Finally, Messa et al. showed in normal subjects and CKD patients, a tight correlation between the set points for PTH and CT secretion with the latter greater than the former [34]. The correlation between the set points for PTH and CT secretion suggests that the PTH response to hypocalcemia and the CT response to hypercalcemia move together.
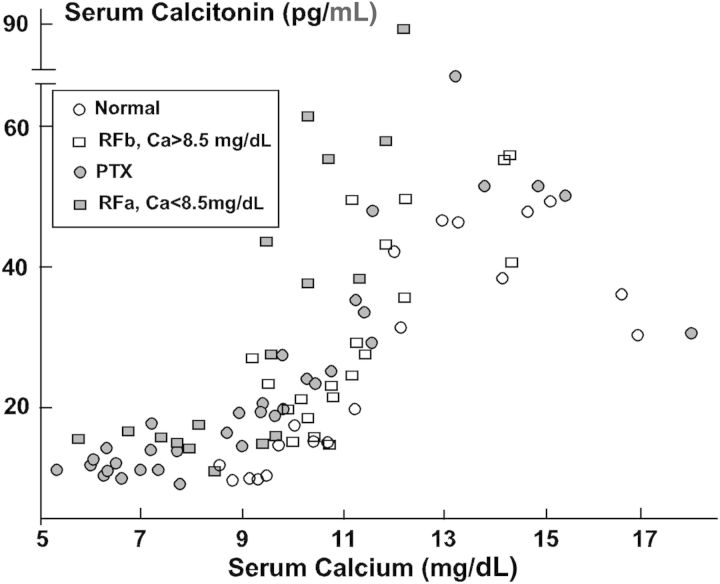
Fig. 2.
The sigmoidal relationship between serum calcium and calcitonin is shown in the four groups of rats: normal (N)—open circle; parathyroidectomized (PTX)—solid circle; renal failure with baseline serum calcium <8.5 mg/dL (RFa)—solid square; and renal failure with baseline serum calcium >8.5 mg/dL (RFb)—open square. When hypocalcemia was present (PTX and RFa), the calcitonin response to an increase in serum calcium began before hypercalcemia developed shifting the set point for calcitonin secretion to the left [32]. Reprinted with permission from Kidney International.
The adapting of PTH and CT secretion to the existing serum calcium concentration is an interesting phenomenon that may reflect a broader concept of physiologic adaptation. For example, adaptation to cold and hot weather as well as oxygen adaptation to high altitude in Himalayan mountain climbers is well known. However, adaptation of hormonal effects and secretion is less well appreciated. In type 1 diabetic patients, an unawareness of hypoglycemia is associated with prolonged insulin therapy and frequent episodes of hypoglycemia [39]. In a study of patients before and after removal of insulinomas (baseline serum glucose, 50 ± 7 mg/dL), symptomatology as well as the epinephrine, norepinephrine, glucagon, growth hormone, and cortisol response to a hypoglycemic clamp was greatly blunted [39]. After resection of the insulinoma, these same responses became similar to those in normal subjects.
Calcitonin secretion and chronic hypercalcemia: depletion of calcitonin versus adaptation
Besides adaptation to hypercalcemia, other causes for changes in CT secretion are also possible. Raue and associates have shown that chronic hypercalcemia resulted in a decrease in CT content of the thyroid gland and a diminished CT response to acute calcium stimulation while basal serum CT levels remained unchanged [40]. Also, it was shown that 1,25D-induced hypercalcemia failed to stimulate CT secretion [40] which may be related to the presence of 1,25D receptors on C cells [41] and a 1,25D decrease in CT gene transcription [14, 15, 42]. Interestingly, unlike hypocalcemia which stimulates PTH mRNA, calcium-induced hypercalcemia failed to affect CT mRNA [15, 42]. In an in vitro study with rat C cells, repetitive calcium stimulation led to a decline in CT release, but 2 h after reversing the calcium concentration in the media to basal values, the CT response to a calcium stimulus was restored [43]. In the cat, the CT response to hypercalcemia correlated with the number of CT-positive cells in the thyroid [36]. Also, it was shown that several cats failed to increase CT in response to hypercalcemia. Chronic hypocalcemia induced by parathyroidectomy in rats has resulted in increased thyroidal CT content after 50 days and longer, but interestingly not at 32 days [44, 45]. Such a finding could explain an enhanced CT response to calcium loading in chronic hypocalcemia [23]. However, in the study by Torres et al., the enhanced CT response to a calcium increase in hypocalcemic rats was observed in the absence of prolonged hypocalcemia [32].
Several studies have evaluated CT secretion in primary hyperparathyroidism which is characterized by chronic hypercalcemia. In one study, a gender difference was observed. Males, but not females had elevated baseline CT values and a further increase in CT values during a calcium infusion [46]. In a subsequent study, men and women with primary hyperparathyroidism had normal serum CT levels and the CT response to a calcium infusion was indistinguishable between men and women with primary hyperparathyroidism and normal men and women [47]. In another study performed in post-menopausal women with primary hyperparathyroidism, all the patients had normal serum CT levels and a blunted response to a calcium stimulus as compared with normal women [48]. In a study in horses, the rapid induction of hypercalcemia increased CT values by 6-fold, but CT values returned to baseline values before hypercalcemia resolved [49]. Thus, the question remains whether the lack of a CT response to hypercalcemia in primary hyperparathyroidism is due to a depletion of CT stores or a reset of the set point for CT secretion.
If the hormonal response of CT to hypercalcemia is indeed similar to that of the PTH response to hypocalcemia, CT secretion might have similar characteristics as PTH secretion. When dogs were subjected to hypocalcemia, repetitive cycles of the induction of and recovery from hypocalcemia done without pause, whether for 30 or 60 min, produced the same PTH response on the second as the first cycle (Figure 3A and B) [38]. Also, the PTH value for the same serum calcium concentration was greater during the induction of than the recovery from hypocalcemia (Figure 3C) and greater during the recovery from than the induction of hypercalcemia (not shown) [38]. This phenomenon, known as hysteresis, is not unique to PTH secretion, but is seen with other physiologic phenomenon [38]. But for PTH secretion, hysteresis may be important in preventing an overcorrection during the restoration of a normal serum calcium concentration [38]. Another interesting observation has been that an episodic versus a linear induction of hypocalcemia of the same magnitude over the same time period resulted in differences in PTH secretion [50]. Finally, it was shown that metabolic acidosis stimulates and metabolic alkalosis inhibits PTH secretion in the rat and dog [51–54]. Because the CaSR is sensitive to pH changes, it would not be surprising if CT secretion also responded to changes in pH. Moreover, because calcium suppresses PTH secretion and stimulates CT secretion, the effects of acidosis and alkalosis may be reversed from that of PTH with alkalosis stimulating and acidosis suppressing CT secretion.
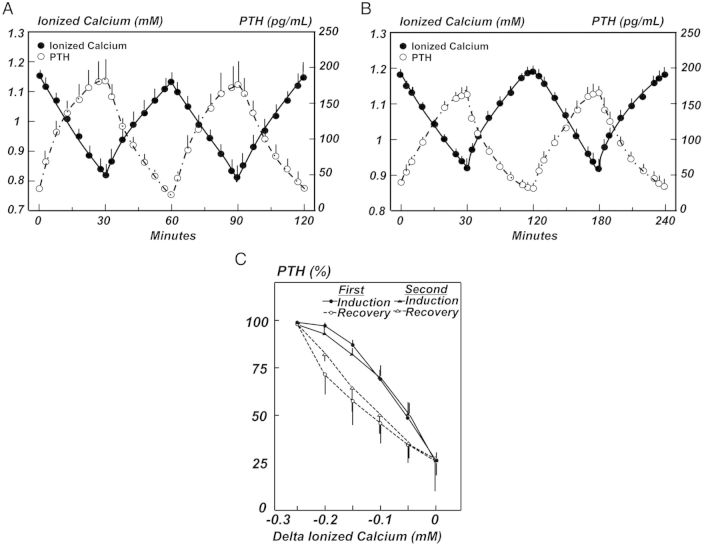
Fig. 3.
The PTH response to the sequential induction of and recovery from hypocalcemia is shown for 30 min (A) and 60 min (B) cycles in the dog. PTH values were similar during the first and second cycles both in the 30 and 60 min groups. (C) The lower PTH value for the same serum calcium concentration during the recovery from hypocalcemia than during the induction of hypocalcemia is shown and is known as hysteresis. Results of PTH hysteresis from dogs in the 60 min cycle were similar (data not shown). Data are the mean ± SE [38]. Reprinted with permission from the Clinical Journal of the American Society of Nephrology.
Calcitonin and gastrin
Besides being stimulated by calcium, CT secretion has been shown to be stimulated by gastrointestinal hormones such as gastrin. An infusion of pentagastrin, a synthetic peptide with gastrin-like effects, has been used to evaluate CT stimulation [55]. In one study of oral calcium loading, the increase in serum CT correlated with the increase in serum calcium, but not that of gastrin [22]. In a recent study, the peak CT response to a pentagastrin infusion in normal women and men was 26 and 38 pg/mL, respectively, while the response to a calcium infusion was 90 and 131 pg/mL, respectively [56]. Moreover, the CT response to pentagastrin was absent in 12 of 25 women and 4 of 25 men, while the CT response to calcium, was absent only in 2 of 18 women and none of the men. Because gastrin and other gastrointestinal hormones potentially stimulate CT secretion, interest developed in the concept that postprandial CT secretion acted to facilitate calcium deposition in bone and potentiate bone mass [21]. However, in studies in children with congenital hypothyroidism and in thyroidectomized patients, bone loss has been attributed to inadequate thyroid replacement and not to the absence of CT [24, 57]. Also, skeletal changes have not been observed in patients with elevated CT values in medullary thyroid carcinoma [31].
Potentially, there may be different challenges among species in defending against hypercalcemia. CT is an older hormone phylogenetically than PTH dating back to fish in the ocean with a need to protect against hypercalcemia while PTH developed in land-dwelling animals to protect against hypocalcemia. As such, this may be an explanation why salmon CT is more potent than CT from mammals [7, 58]. Not often discussed is that the need to protect against hypercalcemia may be different among land-dwelling animals. Humans and domesticated animals eat meals on a regular basis throughout the day. Conversely, carnivorous animals in the wild eat large meals consisting of a high percent of their body mass at irregular intervals. Besides a large protein load, the ingestion of calcium and phosphate is also great. In Burmese pythons fed meals of mice or rats once every 2 weeks with a combined mass of the meal averaging 25% of the snake's body mass, the minimum rate of oxygen consumption increased 17-fold 24 h after feeding [59]. Serum calcium did not change, but serum phosphorus increased from 6.7 to 11.4 mg/dL 3 days after eating. Serum bicarbonate increased from 11 mM fasting to 20 mM and remained significantly elevated for 6 days. Blood pH which was 7.37 fasting increased to 7.49 24 h after feeding before decreasing to 7.19 5 days after feeding. Whether similar physiologic adaptations occur in infrequently feeding carnivorous mammals in the wild and whether CT secretion plays a role and is possibly stimulated by gastrin secretion or the calcium load is an interesting question. Also, the possibility exists that post-prandial alkalemia could be an additional stimulus for CT secretion.
Calcitonin and gender/age
Gender and age differences in serum CT values have been reported. Deftos and associates reported that in normal adult subjects, basal CT values were similar between men and women, but basal CT values decreased with age in both groups and the response to a calcium or pentagastrin challenge was less in women than in men [25, 60]. A subsequent study by another group showed lower basal and stimulated CT values in women than in men, but no decrement in CT values with age [9]. The CT difference between women and men has been ascribed to a lower CT secretion rate in women [9]. In post-menopausal women, estrogen administration may increase CT levels [61]. Finally, infants and children may have higher serum CT levels than adults [62, 63].
Calcitonin and pregnancy/lactation
Pregnancy and lactation are unique because of the demand for transfer of maternal calcium to the fetus/infant. Serum CT and 1,25D values are increased during pregnancy and lactation [16, 17, 64]. Interestingly, CT has been shown to stimulate 1,25D production in the kidney in the proximal straight tubule while PTH and phosphorus stimulate 1,25D production in the proximal convoluted tubule [12, 13]. Clinical studies have shown that women lose ∼5–10% of trabecular bone mineral content during 6 months of lactation and fully regain it after weaning [18]. Similar changes are seen in mice and rats during lactation and weaning [18]. In a study in calcitonin/calcitonin gene-related-peptide-α-null mice (Ctcgrp), the decrease in bone mineral content was greater and the return to baseline bone mineral content took longer in Ctcgrp-null mice than in wildtype mice [64]. Administration of salmon CT in the null mice prevented the differences between null and wild-type mice. While CT is known to inhibit osteoclast activity [19, 65], the identification of CT receptors on osteocytes suggests that CT may also modify osteocyte function [31]. In summary, CT may have an important functional role in pregnancy and lactation.
Calcitonin and bone
Since the discovery of CT in the early 1960s, much interest has been focused on whether CT has an important role in normal bone health. It has been postulated that CT acts postprandially to use phosphate to store calcium in bone [21, 66]. But except perhaps for pregnancy and lactation, no specific CT deficiency or excess bone syndromes have been identified [66]. CT has been used to treat post-menopausal osteoporosis [67], but its benefit has not been dramatic. At least in ovariectomized and in normal beagle dogs in which bone histomorphometry and physical bone strength were evaluated, CT administration decreased osteoblast function, bone formation and physical bone strength [68, 69]. However, in a knockout mouse model in which the CT gene was deleted, no developmental defects were observed and procreation was normal [70]. Moreover, bone findings included an increase in trabecular bone volume and bone formation at 1 and 3 months of age while bone resorption was not changed. Baseline serum calcium values were unaffected but the administration of PTH resulted in greater hypercalcemia and urine deoxypyridinoline crosslinks excretion than in wild-type mice. Interestingly, the CT female knockout mouse appeared to be protected against ovariectomy-induced bone loss. While the effect of CT administration to treat hypercalcemia has been attributed to its inhibition of osteoclasts [19], an escape of osteoclasts from the effects of prolonged CT administration is well known [20, 71]. This result could possibly explain the failure to observe increased bone resorption in the absence of CT. The enhanced bone formation observed in the absence of CT is perhaps more difficult to explain. But CT has been shown to modify cell cultures of osteoblasts and osteocytes [72]. One intriguing hypothesis based on the finding of CT receptors on osteocytes, is that CT could potentially modify osteocyte products such as FGF23 or perhaps more importantly, sclerostin, a known regulator of bone formation [31].
Calcitonin and renal failure
The kidney is the primary site for metabolism of CT [8, 10]. As might be expected with decreased metabolism of CT in renal failure, CT values are increased in azotemic humans and animals [32, 34, 73, 74]. As in normal humans and animals, a sigmoidal CT response is seen during the induction of hypercalcemia in azotemic humans and animals [32–34]. The CT response to hypercalcemia has been shown to protect against the development of acute hypercalcemia in azotemic rats [11]. Also, a normal stimulatory response to pentagastrin has been reported in patients with chronic renal failure [74]. In a bone histomorphometric study, no correlation was found between bone activity and CT levels while correlations were observed with PTH [75]. Finally, several studies from many years ago showed that treatment with CT failed to improve renal osteodystrophy [76–78].
In summary, >50 years have transpired since the discovery of calcitonin. Strong evidence exists that the calcitonin response to an increase in the serum calcium concentration protects against the development of acute hypercalcemia. Calcitonin levels are increased during pregnancy and lactation and may play an important role in increasing 1,25D, which separately or in concert with calcitonin preserve and restore maternal bone mass during transfer of calcium to the fetus/infant. Unlike other hormones, no specific developmental or metabolic abnormalities have been associated with a deficiency or excess of calcitonin except for diarrhea in a few patients with medullary thyroid carcinoma. Unlike other hormones except for gender-specific hormones such as estrogen and androgens, distinct differences in the characteristics of calcitonin secretion appear to be present between males and females. Like the PTH response to hypocalcemia, the calcitonin response to hypercalcemia is a sigmoidal curve. Unlike PTH for which virtually all humans and animals respond to hypocalcemia with a PTH response, a number of humans and animals fail to produce a calcitonin response to hypercalcemia. Unlike PTH which only seems to respond to calcium perhaps modified by pH, calcitonin, besides responding to calcium, also responds to gastrointestinal hormones of which gastrin has been the best studied. The postprandial increase in gastrin and its association with calcitonin secretion has led to the hypothesis that calcitonin may have an important postprandial role in facilitating calcium and phosphate deposition in bone. Despite interest in this concept, no definite proof has been established. However, renewed interest in calcitonin may develop because of potential effects of calcitonin on the osteocyte, but bone abnormalities resulting from a deficiency or excess have not been shown so that any effect must be subtle or counterbalanced by other factors. Recent studies in the calcitonin gene knockout mouse model, have suggested that the absence of calcitonin increased bone mass through enhanced bone formation without having an effect on bone resorption which might have been predicted based on previous known effects of calcitonin on the osteoclast. Finally, whether increased calcitonin levels have any role in chronic kidney disease remains unknown. A summary of the known or possible effects or responses of calcitonin is shown in the Table 1. In conclusion, calcitonin, which is no longer even included as a chapter in the most recent edition of the ASBMR Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism [79], is a hormone preserved during evolution from ocean to land-based animals that continues to be an enigma more than 50 years after its discovery.
Table 1.
Known or possible effects or responses of calcitonin
| A. | Known effects or responses of calcitonin |
| No specific developmental or metabolic abnormalities from a deficiency or excess except for diarrhea in a few patients with medullary thyroid carcinoma | |
| Protects against the development of hypercalcemia | |
| Sigmoidal response to the development of hypercalcemia | |
| Secretion adapts to ambient serum calcium concentration | |
| Stimulated by calcium infusion and less predictably by pentagastrin infusion | |
| Immediate, but short-term inhibition of osteoclast activity | |
| Reciprocal relationship with 1,25 (OH)2 vitamin D (1,25D)—increased 1,25D production through 1-alpha hydroxylase in the straight proximal tubule and suppression of calcitonin mRNA by 1,25D | |
| Increases during pregnancy and lactation | |
| Gender differences with higher baseline values in males than in females; values may also decrease with age | |
| Elevated in chronic renal failure, but no proven effect | |
| B. | Possible, but unproven effects of calcitonin |
| Important factor during pregnancy and lactation in stimulating 1,25D thus preventing maternal bone loss and transferring calcium to the fetus/infant in conjunction with 1,25D | |
| Enhanced post-prandial deposition of calcium and phosphate in bone from stimulation of calcitonin by gastrointestinal hormones such as gastrin | |
| Modifier of osteocyte function and products | |
| Modifier of bone formation based on results in a calcitonin-gene knockout mouse model |
Conflict of interest statement
None of the authors have any conflicts of interest. The content of this review has not been published previously.
References
- 1.Copp DH, Cheney B. Calcitonin—a hormone from the parathyroid which lowers the calcium-level of the blood. Nature 1962; 193: 381–382 [DOI] [PubMed] [Google Scholar]
- 2.Copp D, Cameron EC, Cheney BA, et al. Evidence for calcitonin—a new hormone from the parathyroid gland that lowers blood calcium. Endocrinology 1962; 70: 638–649 [DOI] [PubMed] [Google Scholar]
- 3.Hirsch PF, Voelkel EF, Munson PL. Thyrocalcitonin: hypocalcemic, hypophosphatemic principle of the thyroid gland. Science 1964; 146: 412–413 [DOI] [PubMed] [Google Scholar]
- 4.Foster GV, Bagdiantz A, Kumar MA, et al. Thyroid origin of calcitonin. Nature 1964; 202: 1303–1305 [DOI] [PubMed] [Google Scholar]
- 5.Pearse AG. The cytochemistry of the thyroid C cells and their relationship to calcitonin. Proc R Soc London B Bio Sci 1966; 164: 478–487 [DOI] [PubMed] [Google Scholar]
- 6.Potts JT., Jr Chemistry of the calcitonins. Bone Miner 1992; 16: 169–173 [DOI] [PubMed] [Google Scholar]
- 7.Niall HD, Keutmann HT, Copp DH, et al. Amino acid sequence of salmon ultimobranchial calcitonin. Proc Natl Acad Sci USA 1969; 64: 771–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanis JA, Heynen G, Cundy T, et al. An estimate of the endogenous secretion rate of calcitonin in man. Clin Sci 1982; 63: 145–152 [DOI] [PubMed] [Google Scholar]
- 9.Tiegs RD, Body JJ, Barta JM, et al. Secretion and metabolism of monomeric human calcitonin: effects of age, sex, and thyroid damage. J Bone Miner Res 1986; 1: 339–349 [DOI] [PubMed] [Google Scholar]
- 10.Simmons RE, Hjelle JT, Mahoney C, et al. Renal metabolism of calcitonin. Am J Physiol Renal Physiol 1988; 254: F593–F600 [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez M, Felsenfeld AJ, Torres A, et al. Calcitonin, an important factor in the calcemic response to parathyroid hormone in the rat. Kidney Int 1991; 40: 219–225 [DOI] [PubMed] [Google Scholar]
- 12.Kawashima H, Torikai S, Kurokawa K. Calcitonin selectively stimulates 25-hydroxyvitamin D3-1 alpha- hydroxylase in proximal straight tubule of rat kidney. Nature 1981; 291: 327–329 [DOI] [PubMed] [Google Scholar]
- 13.Jaeger P, Jones W, Clemens TL, et al. Evidence that calcitonin stimulates 1,25-dihydroxyvitamin D production and intestinal absorption of calcium in vivo. J Clin Invest 1986; 78: 456–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naveh-Many T, Silver J. Regulation of calcitonin gene transcription by vitamin D metabolites in vivo in the rat. J Clin Invest 1988; 81: 270–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naveh-Many T, Raue F, Grauer A, et al. Regulation of calcitonin gene expression by hypocalcemia, hypercalcemia, and vitamin D in the rat. J Bone Miner Res 1992; 7: 1233–1237 [DOI] [PubMed] [Google Scholar]
- 16.Kumar R, Cohen WR, Silva P, et al. Elevated 1,25-dihydroxyvitamin D plasma levels in normal human pregnancy and lactation. J Clin Invest 1979; 63: 342–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevenson JC, Hillyard CJ, MacIntyre I, et al. A physiological role for calcitonin: Protection of the maternal skeleton. Lancet 1979; 314: 769–770 [DOI] [PubMed] [Google Scholar]
- 18.Kovacs CS, Kronenberg HM. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev 1997; 18: 832–872 [DOI] [PubMed] [Google Scholar]
- 19.Chambers TJ, Moore A. The sensitivity of isolated osteoclasts to morphological transformation by calcitonin. J Clin Endocrinol Metab 1983; 57: 819–824 [DOI] [PubMed] [Google Scholar]
- 20.Heersche JNM. Calcitonin effects on osteoclastic resorption: the ‘escape phenomenon’ revisited. Bone Miner 1992; 16: 174–177 [DOI] [PubMed] [Google Scholar]
- 21.Talmage RV, Grubb SA, Norimatsu H, et al. Evidence for an important physiological role for calcitonin. Proc Natl Acad Sci USA 1980; 77: 609–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Austin LA, Heath H, III, Go VLW. Regulation of calcitonin secretion in normal man by changes of serum calcium within the physiologic range. J Clin Invest 1979; 64: 1721–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deftos LJ, Powell D, Parthemore JG, et al. Secretion of calcitonin in hypocalcemic states in man. J Clin Invest 1973; 52: 3109–3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baran DT, Braverman LE. Thyroid hormones and bone mass. J Clin Endocrinol Metab 1991; 72: 1182–1183 [DOI] [PubMed] [Google Scholar]
- 25.Deftos LJ, Weisman MH, Williams GW, et al. Influence of age and sex on plasma calcitonin in human beings. N Engl J Med 1980; 302: 1351–1353 [DOI] [PubMed] [Google Scholar]
- 26.Costante G, Meringolo D, Durante C, et al. Predictive value of serum calcitonin levels for preoperative diagnosis of medullary thyroid carcinoma in a cohort of 5817 consecutive patients with thyroid nodules. J Clin Endocrinol Metab 2007; 92: 450–455 [DOI] [PubMed] [Google Scholar]
- 27.Sanderson PH, Marshall F, Wilson RE. Calcium and phosphorus homeostasis in the parathyroidectomized dog; an evaluation by means of ethylenediamine tetraacetate and calcium tolerance tests. J Clin Invest 1961; 39: 662–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kraut JA, Mishler DR, Kurokawa K. Effect of colchicine and calcitonin on calcemic response to metabolic acidosis. Kidney Int 1984; 25: 608–612 [DOI] [PubMed] [Google Scholar]
- 29.Davey RA, Turner AG, McManus JF, et al. Calcitonin receptor plays a physiological role to protect against hypercalcemia in mice. J Bone Miner Res 2008; 23: 1182–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kantham L, Quinn SJ, Egbuna OI, et al. The calcium-sensing receptor (CaSR) defends against hypercalcemia independently of its regulation of parathyroid hormone secretion. Am J Physiol Endocrinol Metab 2009; 297: E915–E923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davey RA, Findlay DM. Calcitonin: physiology or fantasy? J Bone Miner Res 2013; 28: 973–979 [DOI] [PubMed] [Google Scholar]
- 32.Torres A, Rodriguez M, Felsenfeld A, et al. Sigmoidal relationship between calcitonin and calcium: Studies in normal, parathyroidectomized, and azotemic rats. Kidney Int 1991; 40: 700–704 [DOI] [PubMed] [Google Scholar]
- 33.Felsenfeld AJ, Machado L, Rodriguez M. The relationship between serum calcitonin and calcium in the hemodialysis patient. Am J Kidney Dis 1993; 21: 292–299 [DOI] [PubMed] [Google Scholar]
- 34.Messa P, Mioni G, Turrin D, et al. The calcitonin-calcium relation curve and calcitonin secretory parameters in renal patients with variable degrees of renal function. Nephrol Dial Transplant 1995; 10: 2259–2265 [DOI] [PubMed] [Google Scholar]
- 35.Wang W, Lewin E, Olgaard K. Role of calcitonin in the rapid minute-to-minute regulation of plasma Ca2+ homeostasis in the rat. Eur J Clin Invest 2002; 32: 674–681 [DOI] [PubMed] [Google Scholar]
- 36.Pineda C, Aguilera-Tejero E, Raya AI, et al. Assessment of calcitonin response to experimentally induced hypercalcemia in cats. Am J Vet Res 2013; 74: 1514–1521 [DOI] [PubMed] [Google Scholar]
- 37.Martinez ME, Miguel JL, Gomez P, et al. Plasma calcitonin concentration in patients treated with chronic dialysis: differences between hemodialysis and CAPD. Clin Nephrol 1983; 19: 250–253 [PubMed] [Google Scholar]
- 38.Felsenfeld AJ, Rodriguez M, Aguilera-Tejero E. Dynamics of parathyroid hormone secretion in health and secondary hyperparathyroidism. Clin J Am Soc Nephrol 2007; 2: 1283–1305 [DOI] [PubMed] [Google Scholar]
- 39.Mitrakou A, Fanelli C, Veneman T, et al. Reversibility of unawareness of hypoglycemia in patients with insulinomas. N Engl J Med 1993; 329: 834–839 [DOI] [PubMed] [Google Scholar]
- 40.Raue F, Deutschle I, Kuntzel C, et al. Reversible diminished calcitonin secretion in the rat during chronic hypercalcemia. Endocrinology 1984; 115: 2362–2367 [DOI] [PubMed] [Google Scholar]
- 41.Freake HC, MacIntyre I. Specific binding of 1,25-dihydroxycholecalciferol in human medullary thyroid carcinoma. Biochem J 1982; 206: 181–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naveh-Many T, Friedlaender MM, Mayer H, et al. Calcium regulates parathyroid hormone messenger ribonucleic acid mRNA, but not calcitonin mRNA in vivo in the rat. Dominant role of 1,25-dihydroxyvitamin D. Endocrinology 1989; 125: 275–280 [DOI] [PubMed] [Google Scholar]
- 43.Scherubl H, Raue F, Zopf G, et al. Reversible desensitization of calcitonin secretion by repetitive stimulation with calcium. Mol Cell Endocrinol 1989; 63: 263–266 [DOI] [PubMed] [Google Scholar]
- 44.Peng T-C, Garner SC. Hypercalcitoninemia associated with return of serum calcium concentration toward normal in chronically parathyroidectomized rats. Endocrinology 1979; 104: 1624–1630 [DOI] [PubMed] [Google Scholar]
- 45.Raue F, Wieland U, Weiler C, et al. Enhanced calcitonin secretion in the rat after parathyroidectomy and during chronic calcium deprivation. Eur J Clin Invest 1988; 18: 284–289 [DOI] [PubMed] [Google Scholar]
- 46.Parthemore JG, Deftos LJ. Calcitonin secretion in primary hyperparathyroidism. J Clin Endocrinol Metab 1979; 49: 223–226 [DOI] [PubMed] [Google Scholar]
- 47.Tiegs RD, Body JJ, Barta J, et al. Plasma calcitonin in primary hyperparathyroidism: Failure of C-Cell response to sustained hypercalcemia. J Clin Endocrinol Metab 1986; 63: 785–788 [DOI] [PubMed] [Google Scholar]
- 48.Torring O, Bucht E, Sjoberg HE. Decreased plasma calcitonin response to a calcium clamp in primary hyperparathyroidism. Acta Endocrinol (Copenh) 1985; 108: 372–376 [DOI] [PubMed] [Google Scholar]
- 49.Rourke KM, Kohn CW, Levine AL, et al. Rapid calcitonin response to experimental hypercalcemia in healthy horses. Domest Anim Endocrinol 2009; 36: 197–201 [DOI] [PubMed] [Google Scholar]
- 50.Grant FD, Conlin PR, Brown EM. Rate and concentration dependence of parathyroid hormone dynamics during stepwise changes in serum ionized calcium in normal humans. J Clin Endocrinol Metab 1990; 71: 370–378 [DOI] [PubMed] [Google Scholar]
- 51.Bichara M, Mercier O, Borensztein P, et al. Acute metabolic acidosis enhances circulating parathyroid hormone, which contributes to the renal response against acidosis in the rat. J Clin Invest 1990; 86: 430–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopez I, Aguilera-Tejero E, Felsenfeld AJ, et al. Direct effect of acute metabolic and respiratory acidosis on parathyroid hormone secretion in the dog. J Bone Miner Res 2002; 17: 1691–1700 [DOI] [PubMed] [Google Scholar]
- 53.Lopez I, Rodriguez M, Felsenfeld AJ, et al. Direct suppressive effect of acute metabolic and respiratory alkalosis on parathyroid hormone secretion in the dog. J Bone Miner Res 2003; 18: 1478–1485 [DOI] [PubMed] [Google Scholar]
- 54.Lopez I, Aguilera-Tejero E, Estepa JC, et al. Role of acidosis-induced increases in calcium on PTH secretion in acute metabolic and respiratory acidosis in the dog. Am J Physiol Endocrinol Metab 2004; 286: E780–E785 [DOI] [PubMed] [Google Scholar]
- 55.Heath H, III, Sizemore GW. Plasma calcitonin in normal man: differences between men and women. J Clin Invest 1977; 60: 1135–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doyle P, Duren C, Nerlich K, et al. Potency and tolerance of calcitonin stimulation with high-dose calcium versus pentagastrin in normal adults. J Clin Endocrinol Metab 2009; 94: 2970–2974 [DOI] [PubMed] [Google Scholar]
- 57.Carey DE, Jones KL, Parthemore JG, et al. Calcitonin secretion in congenital nongoitrous cretinism. J Clin Invest 1980; 65: 892–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Copp DH. The discovery of calcitonin. Bone Miner 1992; 16: 157–159 [DOI] [PubMed] [Google Scholar]
- 59.Secor SM, Diamond J. Adaptive responses to feeding in Burmese pythons: pay before pumping. J Exp Biol 1995; 198: 1313–1325 [DOI] [PubMed] [Google Scholar]
- 60.Parthemore JG, Deftos LJ. Calcitonin secretion in normal human subjects. J Clin Endocrinol Metab 1978; 47: 184–188 [DOI] [PubMed] [Google Scholar]
- 61.Stevenson JC, Abeyasekera G, Hillyard CJ, et al. Calcitonin and calcium-regulating hormones in postmenopausal women: effect of oestrogens. Lancet 1981; 317: 693–695 [DOI] [PubMed] [Google Scholar]
- 62.Klein GL, Wadlington EL, Collins ED, et al. Calcitonin levels in sera of infants and children. Relations to age and periods. Calcif Tissue Int 1984; 36: 635–638 [DOI] [PubMed] [Google Scholar]
- 63.Body JJ, Chanoine JP, Dumon JC, et al. Circulating calcitonin levels in healthy children and subjects with congenital hypothyroidism from birth to adolescence. J Clin Endocrinol Metab 1993; 77: 565–567 [DOI] [PubMed] [Google Scholar]
- 64.Woodrow JP, Sharpe CJ, Fudge NJ, et al. Calcitonin plays a critical role in regulating skeletal mineral metabolism during lactation. Endocrinology 2006; 147: 4010–4021 [DOI] [PubMed] [Google Scholar]
- 65.Nicholson GC, Moseley JM, Sexton PM, et al. Abundant calcitonin receptors in isolated rat osteoclasts. Biochemical and autoradiographic characterization. J Clin Invest 1986; 78: 355–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirsch PF, Lester GE, Talmage RV. Calcitonin, an enigmatic hormone: does it have a function? J Musculoskelet Neuronal Interact 2001; 1: 299–305 [PubMed] [Google Scholar]
- 67.Gruber HE, Ivey JL, Baylink DJ, et al. Long-term calcitonin therapy in postmenopausal osteoporosis. Metabolism 1984; 33: 455–464 [DOI] [PubMed] [Google Scholar]
- 68.Monier-Faugere MC, Geng Z, Qi Q, et al. Calcitonin prevents bone loss, but decreases osteoblastic activity in ovariohysterectomized beagle dogs. J Bone Miner Res 1996; 11: 446–455 [DOI] [PubMed] [Google Scholar]
- 69.Pienkowski D, Doers TM, Monier-Faugere M-C, et al. Calcitonin alters bone quality in beagle dogs. J Bone Miner Res 1997; 12: 1936–1943 [DOI] [PubMed] [Google Scholar]
- 70.Hoff AO, Catala-Lehnen P, Thomas PM, et al. Increased bone mass is an unexpected phenotype associated with deletion of the calcitonin gene. J Clin Invest 2002; 110: 1849–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wada S, Udagawa N, Akatsu T, et al. Regulation by calcitonin and glucocorticoids of calcitonin receptor gene expression in mouse osteoclasts. Endocrinology 1997; 138: 521–529 [DOI] [PubMed] [Google Scholar]
- 72.Plotkin LI, Weinstein RS, Parfitt AM, et al. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest 1999; 104: 1363–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silva OL, Becker KL, Shalhoub RJ, et al. Calcitonin levels in chronic renal disease. Nephron 1977; 19: 12–18 [DOI] [PubMed] [Google Scholar]
- 74.Escalada J, Teruel JL, Pavon I, et al. Normal calcitonin response to pentagastrin stimulation in patients with chronic renal failure. Acta Endocrinol (Copenh) 1993; 129: 39–41 [DOI] [PubMed] [Google Scholar]
- 75.Malluche HH, Faugere M-C, Ritz E, et al. Endogenous calcitonin does not protect against hyperparathyroid bone disease in renal failure. Miner Electrolyte Metab 1986; 12: 113–118 [PubMed] [Google Scholar]
- 76.Delano BG, Baker R, Gardner B, et al. A trial of calcitonin therapy in renal osteodystrophy. Nephron 1973; 11: 287–293 [DOI] [PubMed] [Google Scholar]
- 77.Farrington K, Varghese Z, Moorhead JF. Human calcitonin in the treatment of renal osteodystrophy. J Lab Clin Med 1980; 96: 299–306 [PubMed] [Google Scholar]
- 78.Cundy T, Kanis JA, Heynen G, et al. Deterioration of renal bone disease in patients treated with salmon calcitonin. Clin Endocrinol (Oxf) 1982; 16: 29–37 [DOI] [PubMed] [Google Scholar]
- 79.Rosen CJ. (ed). Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Hoboken, NJ: Wiley-Blackwell, 2013 [Google Scholar]