Abstract
The emotional responses of schizophrenic, depressed, and normal subjects and whether differences in the emotional responding of these groups depended on how emotional responses were elicited or measured were examined. Twenty-three blunted and 20 nonblunted schizophrenics, 17 unipolar depressed subjects, and 20 normal subjects were exposed to a series of affect-eliciting stimuli. The stimuli varied in valence (positive vs. negative) and in level of cognitive demand. Subjects reported their subjective experiences, and their facial expressions were videotaped. Blunted schizophrenics were the least facially expressive, although their reported subjective experiences did not differ from those of the other groups. The nonblunted schizophrenics were more responsive than the depressed subjects to the positive stimuli, although the two groups did not differ in their clinical ratings of affective flatness.
Historically, psychopathologists have placed a great deal of emphasis on the role of emotional disturbance in schizophrenia. Whereas both Bleuler (1911/1950) and Kraepelin (1919/1971) considered affective flatness to be a universal symptom of schizophrenia, the International Pilot Study of Schizophrenia (World Health Organization, 1973) revealed that only 66% of the schizophrenic patients studied exhibited flat affect. People with major depression have also been found to have flat affect (e.g., Andreasen, 1979; Pogue-Geile & Harrow, 1984). Although blunted affect may not be a universal symptom of schizophrenia and is not specific to the disorder, it does appear to have prognostic importance (Carpenter, Bartko, Strauss, & Hawk, 1978; Knight, Roff, Barmen, & Moss, 1979).
Most of what is known about affective flatness has come from studies in which psychiatric patients were observed during clinical interviews and received unidimensional ratings of emotional expressiveness. Unfortunately, in such studies there is a risk of overlooking several important factors. For example, it is essential to specify which facets of the emotional response are blunted. Neurological evidence indicates that the systems controlling facial expressions and subjective experience can act independently (e.g., Ross & Mesulam, 1979). This suggests the need to systematically examine both the subjective experience and the outward expression of emotion. Another important factor that has generally been overlooked is the distinction between positive and negative emotions. Knowledge concerning how groups of psychiatric patients differ from each other and from normal persons, such as whether the differences are restricted to a particular facet of emotional responding or are limited to positive or negative emotions, has implications beyond its potential diagnostic utility. The answers to these questions can provide clues to the origins of affective disturbances in psychopathologic disorder.
The mechanism that mediates schizophrenics’ affective disturbance is unknown. One possible explanation is that emotional disturbances may be secondary to cognitive deficits. This hypothesis was raised by Bleuler (1911/1950), who wrote, “When concepts and ideas are only thought of in fragments, when thinking always loses itself in side issues and irrelevances, when entirely incorrect associational pathways are utilized, then certainly the emotional expressions (taking the normal as standard) cannot be adequate” (p. 365). Cognitive deficits could lead to affective flatness if they interfered with the processing of stimuli that elicit affect. If this is the case, the likelihood that schizophrenics would express emotions would be expected to vary as a function of the amount of reflection or cognitive processing that is required to experience an emotional response to a stimulus. On the other hand, blunted affect may not be a mere epiphenomenon produced by cognitive impairment. For example, blunted affect may be caused by a disturbance in the mechanism responsible for the outward expression associated with emotional responses.
Systematic study of the emotional expression of schizophrenics has only recently begun. Oltmanns, Strauss, Heinrichs, and Driesen (1988) studied the facial action of schizophrenic and normal subjects while the subjects were viewing excerpts from film clips that were expected to elicit emotional reactions. The schizophrenic subjects exhibited fewer emotional reactions than did the normal subjects in response to both amusing and frightening film clips. Oltmanns et al. also found that the schizophrenic subjects who were rated as most emotionally blunted during a clinical interview were the least emotionally responsive in the laboratory situation.
The goal of our study was to replicate and extend the findings of Oltmanns et al. (1988). First, we wanted to know whether the impairment in emotional expression that was seen in the laboratory paradigm was specific to schizophrenia or whether similar deficits in emotional responsivity would also be found among persons with major depression. Second, we wanted to examine whether differences in the emotional responding of normal and different psychopathological groups would depend on how the emotional responses were elicited or measured. In particular, we wished to explore the role of the valence and cognitive demand of the stimuli used to elicit emotional responses. Third, we wanted to explore the relation between deficits in emotional expression and other facets of emotional disturbance such as anhedonia and depressed mood.
Method
Subjects
Four groups of subjects participated in the study: 23 schizophrenics with blunted affect (“blunted schizophrenics”); 20 schizophrenics with nonblunted affect (“nonblunted schizophrenics”); 17 patients with unipolar major depression; and 20 normal control subjects. All of the psychiatric patients were receiving outpatient treatment at the time of the study. Schizophrenics were placed in the blunted group if the staff research psychologist noted that the patient exhibited blunted or flat affect as defined in the Diagnostic and Statistical Manual of Mental Disorders (3rd ed.; DSM-III; American Psychiatric Association, 1980), and the presence of affective flatness was corroborated by the staff psychiatrist. Schizophrenics who did not meet this criterion were placed in the nonblunted group.
As a further check on the division of schizophrenic patients into blunted and nonblunted groups, the investigator (Howard Berenbaum), who was blind to the subjects’ group assignments, gave each subject a global rating of blunted affect after a brief structured interview designed to obtain basic sociodemographic information. The ratings of blunted affect were made on a 7-point scale ranging from not at all blunted (1) to extremely blunted (7). The investigator focused primarily on facial expressions and vocal inflections when making his blunting rating. Group means are reported in Table 1. A Group × Sex analysis of variance (ANOVA) revealed a significant group main effect, F(3, 72) = 38.83, p < .0001. There was no significant main effect for sex, nor was there a significant Group × Sex interaction. A Tukey Honestly Significant Difference (HSD) post hoc test (p < .05) indicated that the blunted schizophrenics had significantly more blunted affect than did all three of the other groups, none of which differed significantly from one another. The assignments of schizophrenics to blunted and nonblunted groups were not changed on the basis of the investigator’s bluntness ratings. Reclassifying the schizophrenics according to the investigator’s blunting ratings did not lead to any changes in the results of the statistical analyses.
Table 1.
Sociodemographic Characteristics, Blunting, IQ, and Medication Status
| Item | Blunted schizophrenics |
Nonblunted schizophrenics |
Depressives | Normal subjects |
|---|---|---|---|---|
| Age | ||||
| M | 34.4 | 32.4 | 38.9 | 36.1 |
| SD | 10.7 | 9.5 | 11.3 | 10.8 |
| Education | ||||
| M | 11.0 | 11.3 | 11.6 | 11.5 |
| SD | 2.4 | 3.7 | 1.2 | 1.6 |
| Race (% Black) | 43.5 | 55.0 | 23.5 | 40.5 |
| Sex (% female) | 47.8 | 50.0 | 64.7 | 50.0 |
| Blunting ratings | ||||
| M | 5.1 | 2.1 | 2.1 | 1.3 |
| SD | 1.7 | 1.4 | 1.2 | 0.4 |
| PPVT score | ||||
| M | 87.7 | 94.8 | 106.4 | 105.6 |
| SD | 23.4 | 20.1 | 21.9 | 16.3 |
| Medications taken | ||||
| Neuroleptic (%) | 100 | 100 | 35 | 0 |
| Antidepressant (%) | 4 | 15 | 76 | 0 |
| Lithium (%) | 13 | 10 | 6 | 0 |
| Antiparkinsonian (%) | 61 | 55 | 6 | 0 |
| No. of hospitalizations | ||||
| M | 3.4 | 4.8 | 2.1 | 0.00 |
| SD | 2.5 | 5.4 | 2.7 | 0.00 |
Note. PPVT = Peabody Picture Vocabulary Test.
Lifetime diagnoses were made by a staff research psychologist according to DSM-III criteria. The diagnoses were made after a semistructured diagnostic interview that was conducted for research purposes along with a review of each patient’s clinical records. All diagnoses were corroborated by a staff psychiatrist.
The diagnoses of 23 of the 60 psychiatric patients (9 nonblunted schizophrenics, 7 blunted schizophrenics, and 7 depressed patients) were randomly selected to be checked by the investigator after the patients had already completed the experimental procedures and before he had been told the patients’ diagnoses. Diagnoses were made after the investigator reviewed the patient’s records and conducted a structured diagnostic interview with the sections of the Diagnostic Interview Schedule (Robins, Helzer, Croughan, & Ratcliff, 1981) that pertained to affective disorders and schizophrenia. Two of the 23 diagnoses made by the investigator disagreed with those of the staff psychiatrist and psychologist: Of the 16 patients given a staff diagnosis of blunted schizophrenia, 1 was given a diagnosis of schizoaffective disorder by the investigator, as was 1 of the 7 patients given a diagnosis of major depression by the staff. Interrater reliability between the investigator’s diagnoses and those of the clinical staff, measured by means of kappa, was .81. The 2 patients diagnosed as schizoaffective by the investigator were classified according to the original staff diagnoses in the statistical analyses. The results of the statistical analyses did not change when the data from these 2 patients were excluded.
Normal control subjects were recruited from a variety of sources, including posters placed in laundromats, university nonprofessional staff, and hospital nonprofessional staff. An attempt was made to match subjects in all groups on the bases of age, sex, race, and level of education. Normal control subjects were screened with the sections of the Diagnostic Interview Schedule (Robins et al., 1981) that pertained to affective disorders and schizophrenia. Potential subjects for the normal control group who received lifetime diagnoses of schizophrenia, schizoaffective disorder, or any form of major affective disorder were excluded from the study.
The sociodemographic characteristics of each of the four groups are presented in Table 1. ANOVAs did not reveal any significant group differences in age or education. Chi-square tests did not reveal any significant group differences in sex or race. A Group × Sex ANOVA (excluding normal subjects) did not reveal any significant main effects or interactions in the number of hospitalizations, which are also reported in Table 1.
The Peabody Picture Vocabulary Test (PPVT; Dunn, 1959) was used as a measure of verbal intelligence. Group means on the PPVT are reported in Table 1. A Group × Sex ANOVA revealed a significant group main effect, F(3, 72) = 4.60, p < .01. The highest PPVT scores were obtained by depressed subjects, followed by the normal, the nonblunted schizophrenic, and the blunted schizophrenic subjects. A Tukey HSD post hoc test indicated that the blunted schizophrenics had significantly (p < .05) lower IQ scores than did both the depressed subjects and the normal subjects.
The percentages of subjects in each of the psychiatric groups who were receiving different types of medication are also reported in Table 1. All of the schizophrenics were receiving neuroleptic medication.1 Forty-three percent of the blunted schizophrenics and 55% of the nonblunted schizophrenics were receiving fluphenazine decanoate. The average dosages of fluphenazine decanoate were 14.4 (mg per week) (SD = 9.4) among the blunted schizophrenics and 15.1 mg per week (SD = 6.9) among the nonblunted schizophrenics, t (17) = 0.18 (ns). The schizophrenics not receiving fluphenazine decanoate were receiving a variety of orally administered neuroleptic agents. The average daily dosages, calculated according to milligrams of chlorpromazine equivalents (Davis, 1976), were 484 (SD =332) among blunted schizophrenics and 429 (SD = 267) among nonblunted schizophrenics, t(19)=0.42 (ns). None of the psychiatric patients showed signs of tardive dyskinesia.
Stimulus Materials
Subjects were presented with a series of stimuli intended to elicit emotional responses. The affect-evoking stimuli varied along two dimensions: (a) positive (e.g., happiness) versus negative (e.g., disgust and contempt) and (b) the level of cognitive demand or reflection that presumably was required on the part of the subject in order to respond emotionally to the stimulus. The stimuli used in this study were selected after pilot testing with college students and were not intended to elicit reactions differing in intensity.
The stimuli involving low cognitive demand were different-tasting drinks. Tastes are capable of eliciting facial responses in anencephalic neonates (Steiner, 1974) and presumably do not require reflection. The drink that was intended to elicit negative responses was composed of one part distilled white vinegar (reduced with water to 4% acidity) and two parts water. The drink that was intended to elicit positive responses was composed of one part extra fine white sugar and five parts water by volume. Artificial food coloring was added to both drinks so that they had the same appearance.
In addition to tasting drinks, subjects were shown brief film clips lasting between 2 min 47 s and 3 min 32 s. The film clips were expected to require greater amounts of reflection and cognitive processing than were the drinks in order to elicit emotional responses. One of the two film clips that was intended to elicit a negative emotional response began with a scene taken from the film Chinatown and ended with a scene from the film Marathon Man; the other was composed of two scenes taken from the film The Godfather. One of the two film clips that was intended to elicit a positive emotional response was taken from the film Bill Cosby: Himself, in which Bill Cosby performs a stand-up comedy routine, and the audiological portion was edited so that the audience’s laughter was deleted; the other was taken from the cartoon Alt Baba Bunny featuring Bugs Bunny and Daffy Duck.
The stimuli intended to elicit negative responses were expected to elicit primarily disgust, contempt, and fear and were not expected to elicit sadness. Sadness-eliciting film clips were not included because in previous work in our laboratory (e.g., Berenbaum, Snowhite, & Oltmanns, 1987) they had been unsuccessful in eliciting facial expressions of sadness.
Procedure
Subjects first completed the Scales for Physical and Social Anhedonia (Chapman, Chapman, & Raulin, 1976) and the Beck Depression Inventory2 (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961). Subjects then performed a facial mimicry task so that we could determine whether groups differed in the ability to move facial muscles, such as pulling up the corners of the lips in order to form a smile. It was uncommon for subjects to be unable to perform a facial movement, and the success rates of the groups did not differ significantly.
Finally, subjects were shown the four film clips and tasted the drinks. The order of the stimulus presentations was random, except that subjects were always shown at least one film clip between drinks. Subjects viewed the film clips and tasted the drinks while seated alone in a well-lit room. They were not permitted to eat, drink, or smoke during the entire procedure, and they were asked to try to sit still while viewing the film clips. The film clips were shown on a 19-in. (48.3-cm) color video monitor approximately 7 feet (2.13 m) in front of the subject. The drinks were presented to the subjects in clear 7-oz (207-ml) glasses that were approximately one-fourth full. Subjects were asked to take one sip of the drink, using a straw. They were told that they could drink as little or as much as they wished, with the provision that they drink at least enough to know what the drink tasted like. The subjects were videotaped from approximately the shoulders up while they watched the film clips and tasted the drinks.3 The camera was behind a one-way mirror so that the subjects could not see it, although they had been told when informed consent was obtained that they would be videotaped. Immediately after each film clip and drink, the subjects were asked to fill out a form to indicate how happy or disgusted the movie and the drink had made them feel. The ratings were made on 7-point scales (1 = not at all; 3 = slightly; 5 = moderately; 7 = extremely).
Scoring and Interrater Reliability of Facial Measures
Facial expressions were rated by a Facial Action Coding System (FACS; Ekman & Friesen, 1978) accredited rater according to the Emotion Facial Action Coding System (EMFACS) (Friesen, 1986), which is a special version of the FACS. EMFACS ratings provide information concerning which parts of the face are moving as well as which emotion or blend of emotions a person is most likely to be experiencing. The rater, whose only role in the project was to make EMFACS ratings, was blind to the subjects’ group memberships. In addition, the rater was unaware of which drinks the subjects were consuming and which films they were watching.
Rather than obtaining EMFACS ratings of the entire video records of each subject watching each film clip, we rated only a preselected portion of each subject’s video record. We selected four segments from each film clip for which to obtain EMFACS ratings. The segments were selected after we observed pilot subjects’ responses to the film clips. We selected segments that elicited facial expressions from some but not all pilot subjects. The average length of the segments was 11.5 s, and each segment consisted of material from only a single scene. The segment of the drink tasting that was selected to be rated began as soon as the subject started drinking and ended 5 s after the subject stopped drinking.
The EMFACS action unit ratings were converted, through the use of the EMFACS dictionary, into predictions of emotion. The most frequently rated emotion was happiness, and contempt was the second most frequent. Several other emotions were rated, including disgust, anger, and surprise, but none of these were rated very frequently. We developed a new category, the sum of all rated emotions other than happiness, and refer to it as nonhappy.
The video records of 15 randomly selected subjects were rated by a second rater, also blind to subjects’ groups, in order to assess interrater reliability. The only two emotions rated with reasonably high interrater reliability were happiness (κ = .68) and contempt (κ = .64). Interrater reliability for the nonhappy category was also acceptable (κ = .55). Interrater reliability (measured with kappa) of the other specific emotions, such as surprise, were low because of the very low base rates.
Because only a portion of the video record was rated, group differences in the EMFACS ratings could arise for reasons other than differences in emotional expressiveness. In particular, it seemed plausible that the schizophrenics would respond to portions of the film clips other than those that elicited responses from the pilot subjects. Group differences could also arise if the schizophrenics responded to the same parts of the film clips as did the normal subjects but the responses were delayed long enough to prevent their being included in the rated video segments. In order to rule out the possibility that group differences on the EMFACS ratings were an artifact of rating only a portion of the video record, we obtained additional facial ratings. A research assistant, who was blind to subjects’ group memberships, recorded the number of facial expressions and the length of time during each complete film clip during which 10 randomly selected blunted schizophrenics and 10 randomly selected normal control subjects exhibited facial expressions of emotion. Both groups were divided evenly by sex. In order to examine interrater reliability, 6 of these 20 subjects were randomly selected to be rated in a similar way by a second rater. Interrater reliability, measured with the intraclass correlation (in which raters were treated as random effects and a single rater's ratings was treated as the unit of reliability), was .87.
The number of segments (summed across all four film clips) during which EMFACS ratings of facial expressions were recorded was significantly correlated with the number of expressions recorded during the four complete film clips (r = .89, p < .001). This suggests that the number of segments during which expressions were recorded (which is what we used as our primary dependent variable for facial expressiveness) is meaningfully related to how often subjects exhibited facial expressions throughout the entire film clip. Thus even though our primary dependent variable for facial expressiveness is based on ratings of only a portion of the video material, it appears to provide an excellent estimate of how expressive the subjects appeared throughout the entire video record.
Results
To limit the number of variables that we examined, we chose a single subjective emotion score and a single facial expression score for each type of stimulus. We combined scores for the two positive film clips and scores for the two negative film clips. Because it was rare for subjects in both the pilot and the present studies to exhibit or report happiness in response to the negative stimuli or to exhibit or report negative emotions in response to the positive stimuli, we restricted our analyses to examining positive emotional responses to the positive stimuli and negative emotional responses to the negative stimuli. For positive stimuli, we examined the reported and the observed happiness scores;4 for negative stimuli, we examined reported disgust and the observed nonhappy scores.
The observed emotion scores for the film clips were based on the number of segments during which an emotional expression was exhibited. For example, a subject’s observed emotion score for the positive films was based on the number of segments in the two positive film clips during which the subject smiled. Subjects received observed emotion scores of 0 or 1 for each drink, depending on whether they exhibited an emotional expression. For example, subjects who exhibited a nonhappy response to the negative drink received an observed emotion score of 1. Z scores based on the means and standard deviations of the entire sample, rather than on the original raw scores, were used in all ANOVAs in which more than a single dependent variable was examined. This was necessary because of the different ranges and mean scores for the different dependent variables.
Responses to Affect-Eliciting Stimuli
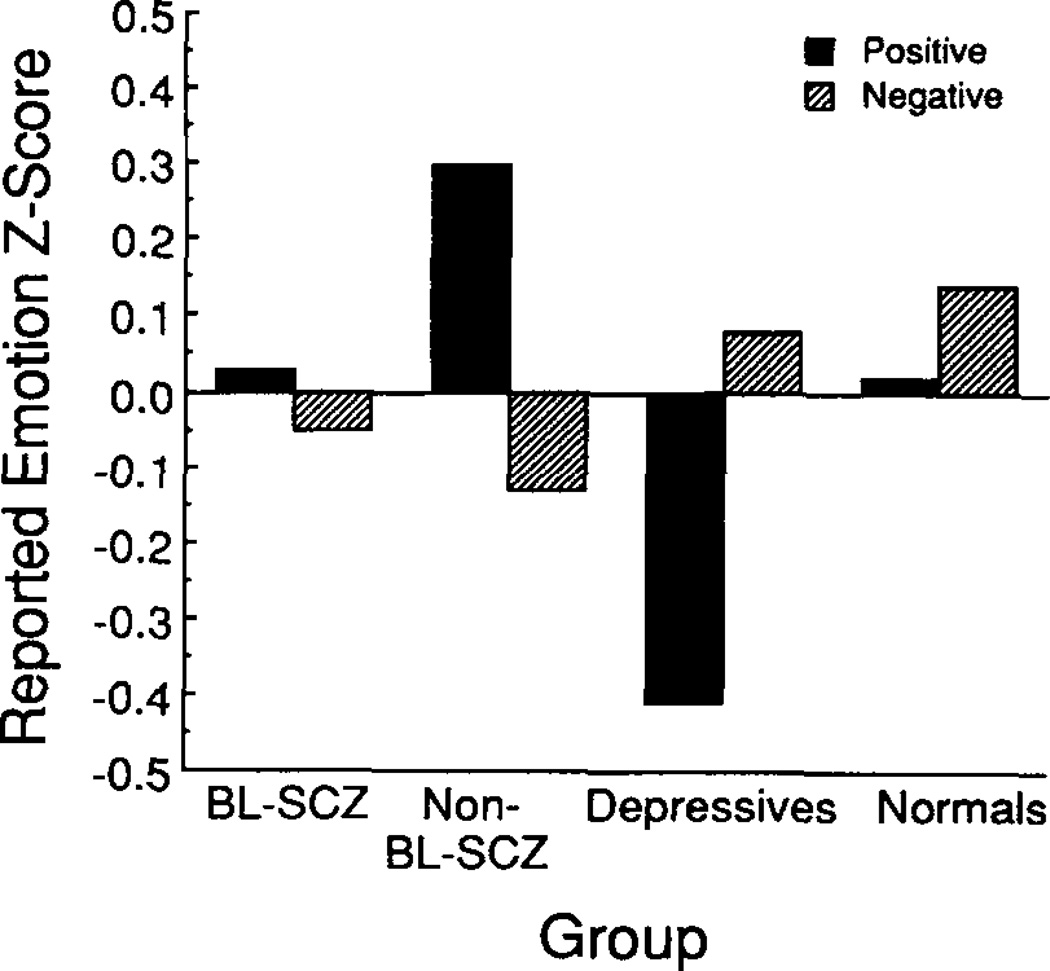
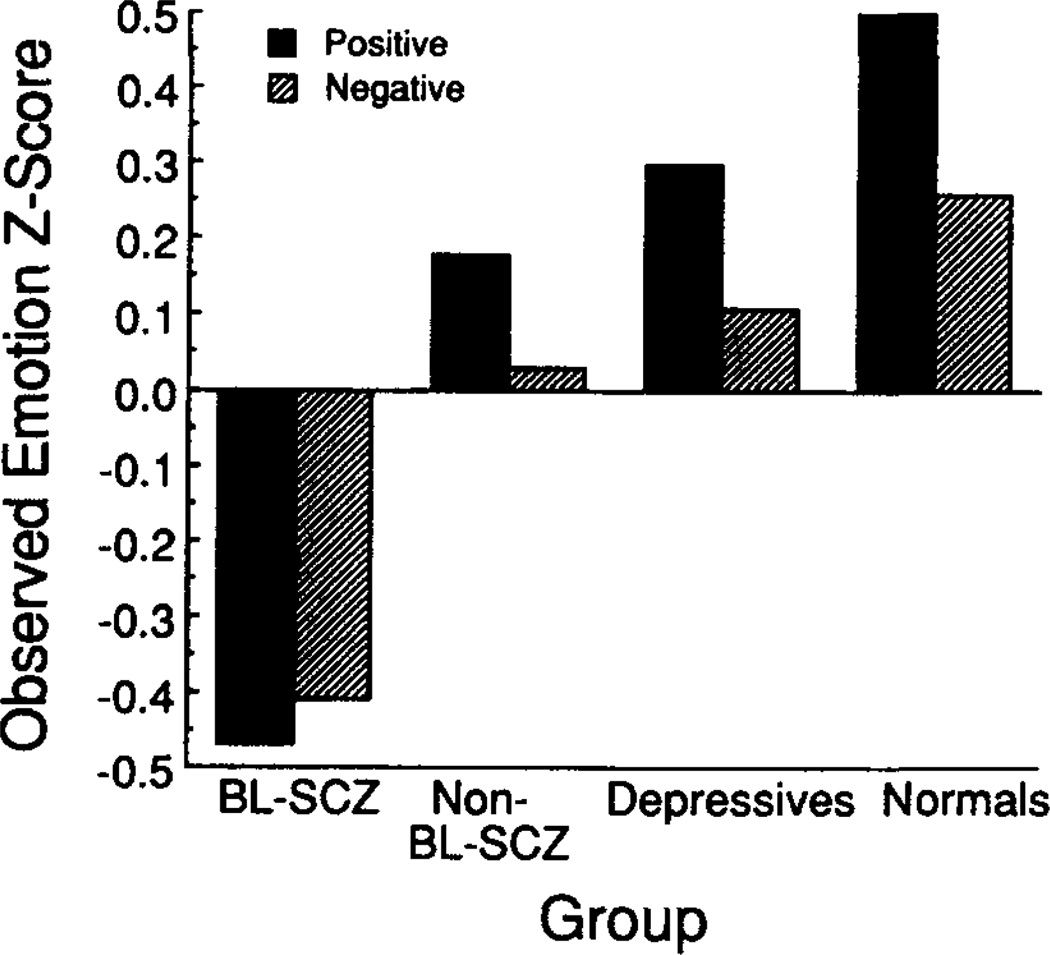
The nonstandardized group means, collapsed across sex, are reported in Table 2. Standardized group means, averaged across sex and level of cognitive demand, are illustrated in Figures 1 and 2. The size of the bars in Figures 1 and 2 reveal the degree to which group means deviated from the total sample mean. Bars that rise above 0 indicate that the group mean was larger than the total sample mean; bars that fall below 0 indicate that the group mean was smaller than the total sample mean.
Table 2.
Emotional Responses to Affect-Eliciting Stimuli
| Stimuli/ response |
Blunted schizophrenics |
Nonblunted schizophrenics |
Depressives | Normal subjects |
||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | |
| Positive drink | ||||||||
| Subjective experiencea | 3.0 | 2.4 | 3.9 | 2.3 | 1.8 | 1.7 | 2.5 | 1.6 |
| Facial expressionb | 0.6 | 0.5 | 0.2 | 0.4 | 0.4 | 0.5 | 0.1 | 0.3 |
| Positive film clips | ||||||||
| Subjective experiencec | 8.0 | 4.3 | 8.6 | 3.9 | 6.4 | 2.9 | 8.4 | 2.8 |
| Facial expressiond | 1.0 | 1.3 | 3.4 | 2.1 | 1.9 | 1.8 | 3.9 | 2.6 |
| Negative drink | ||||||||
| Subjective experiencec | 3.5 | 2.8 | 4.1 | 2.5 | 5.3 | 1.8 | 5.0 | 2.1 |
| Facial expressiond | 0.6 | 0.5 | 0.7 | 0.5 | 0.9 | 0.3 | 0.9 | 0.4 |
| Negative film clips | ||||||||
| Subjective experiencec | 9.2 | 3.6 | 7.5 | 5.0 | 8.6 | 4.3 | 9.1 | 3.6 |
| Facial expressiond | 0.8 | 1.2 | 2.3 | 2.5 | 2.2 | 2.4 | 2.6 | 2.2 |
Scores could range from 1 to 7.
Scores could be either 0 or 1.
Scores could range from 2 to 14.
Scores could range from 0 to 8.
Figure 1.
Reported emotion z scores, averaged across sex and level of cognitive demand. (BL-SCZ = blunted schizophrenics; Non-BL-SCZ = nonblunted schizophrenics.)
Figure 2.
Observed emotion z scores, averaged across sex and level of cognitive demand. (BL-SCZ = blunted schizophrenics; Non-BL-SCZ = nonblunted schizophrenics.)
The goal of the first analysis was to examine (a) whether there were group differences in emotional responding and (b) whether such differences depended on how emotional responses were elicited or measured. We addressed these questions by conducting a 4 (group) × 2 (sex) × 2 (stimulus valence: positive vs. negative affect-eliciting stimuli) × 2 (level of cognitive demand) × 2 (measurement: reported vs. observed emotions) ANOYA, in which group and sex were the two between-subjects factors and stimulus valence, cognitive demand, and measurement were the three within-subject factors.5
Significant main effects for the within-subject factors were impossible because this analysis was conducted with z scores and the entire sample. Our interest in the within-subject factors was limited to whether they interacted with group. A Group × Measure interaction would indicate that the extent of group differences depended on whether emotional responses were measured with self-report or facial expressions. A Group × Valence interaction would indicate that the groups responded differently to the stimuli, depending on whether the stimuli elicited positive or negative responses. A Group × Cognitive Demand interaction would indicate that group differences varied as a function of the cognitive demand of the stimuli.
The initial ANOVA revealed a significant main effect for group, F(3, 68) = 6.08, p < .005.6 In addition to the main effect for group, there were significant interactions between group and measure,7 F(3, 68) = 4.63, p < .01, and between group and valence, F(3, 68) = 3.96, p < .05, indicating that group differences were influenced by how emotional responses were elicited and measured. This analysis did not reveal a significant interaction between group and cognitive demand. Thus it appears that although the groups differed in their responses to the affect-eliciting stimuli, these differences did not depend on whether the subjects were responding to drinks or to film clips.
Follow-up ANOVAs conducted separately for observed and reported emotions revealed a significant group main effect for observed emotions, F(3, 68) = 12.00, p < .001, whereas the group main effect for reported emotions was not statistically significant. One-way ANOVAs revealed significant group main effects for observed responses to both positive and negative stimuli (in which the sum of z scores for positive and negative stimuli was the dependent variable). Tukey HSD post hoc tests indicated that both the normal and the nonblunted schizophrenic subjects exhibited significantly (p < .05) more facial expressions in response to the positive stimuli than did either the blunted schizophrenic or the depressed subjects and that the normal subjects exhibited significantly (p < .05) more facial expressions than did the blunted schizophrenics in response to the negative stimuli.
The facial ratings made on the complete film clips indicated that the difference between the normal and the blunted schizophrenic subjects was not merely an artifact of our choice of film clip segments for which to obtain EMFACS ratings. The sample of normal subjects for whom we obtained ratings of the complete film clips exhibited facial expressions of emotion during an average of 22.3 s per film clip, which was significantly longer than the average of 2.7 s exhibited by the blunted schizophrenics, r(18) = 2.27, p < .05. The groups also differed in the number of facial expressions that they exhibited. The normal subjects had an average of 3.0 expressions per film clip, which was significantly more than the average of 0.4 exhibited by the blunted schizophrenics, t(18) = 2.43, p < .05.
The interaction between group and valence appeared to be attributable primarily to the responsiveness of nonblunted schizophrenic and depressed subjects. A separate ANOVA conducted with only these two groups revealed a significant interaction between group and valence, F(1, 30) = 10.14, p < .005. The nonblunted schizophrenics were significantly more responsive than the depressed subjects to the positive stimuli (responses summed across the positive emotional response z scores), t(33) = 3.29, p < .005. Although the depressed subjects had higher scores than did the nonblunted schizophrenics in response to the negative stimuli, the difference was not statistically significant.
As noted earlier, there was a significant group effect for scores on the PPVT. Consequently, we conducted a Group × Sex × Stimulus Valence × Cognitive Demand × Measurement analysis of covariance, using IQ as the covariate. As before, there was a significant main effect for group, F(3, 67) = 6.13, p < .005, and there were significant interactions between group and valence, F(3, 68) = 3.96, p < .05, and between group and measure, F(3, 68) = 4.63, p < .01. Although the results of such an analysis must be interpreted with extreme caution (Chapman & Chapman, 1973, pp. 82–83), they are consistent with the hypothesis that the significant effects noted earlier are not artifacts of group differences in intelligence.
Questionnaire Measures
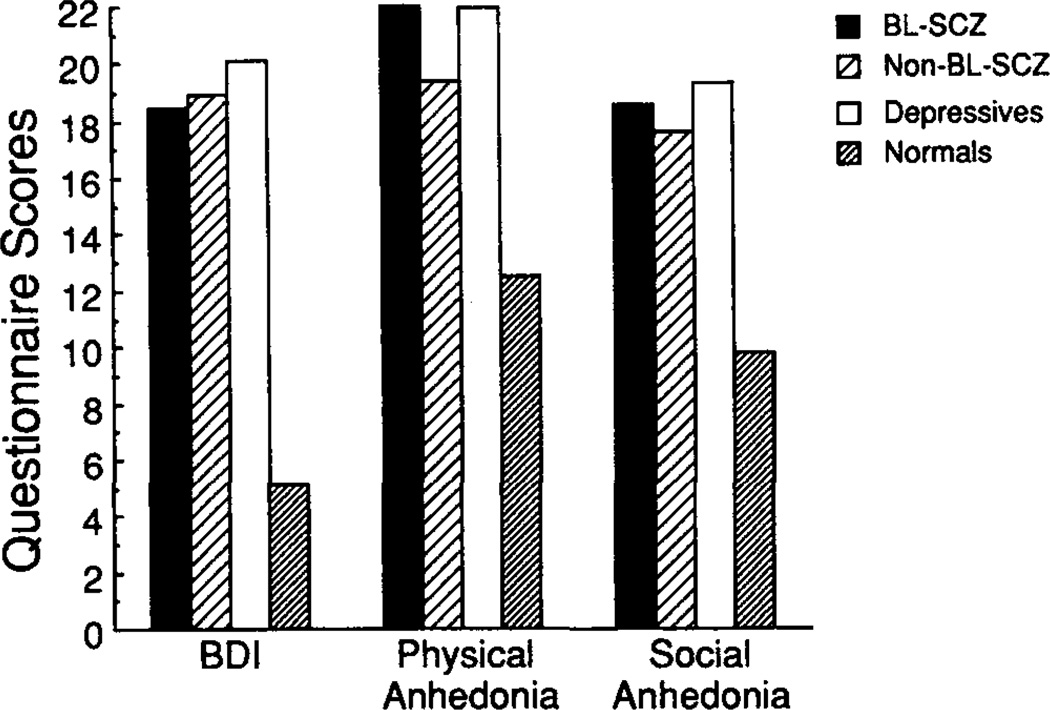
Group means, averaged across sex, on the Beck Depression Inventory and the Scales for Physical and Social Anhedonia are illustrated in Figure 3. Group × Sex ANOVAs revealed significant group effects8 for the Beck Depression Inventory, F(3, 71) = 7.69, p < .001; the Scale for Social Anhedonia, F(3, 72) = 5.89, p <.01; and the Scale for Physical Anhedonia, F(3,72) = 4.60, p < .01. There was a significant sex difference on the Beck Depression Inventory, F(1, 71) = 4.12), p < .05: Female subjects had higher scores. There were no significant Group × Sex interactions. Tukey HSD post hoc tests (p < .05) revealed that all three of the psychiatric groups differed from the normal subjects, but not from one another, on the Beck Depression Inventory and the Scale for Social Anhedonia. The blunted schizophrenic and the depressed subjects differed significantly from the normal subjects on the Scale for Physical Anhedonia.
Figure 3.
Depression and anhedonia questionnaire scores. (BL-SCZ = blunted schizophrenics; Non-BL-SCZ = nonblunted schizophrenics; BDI = Beck Depression Inventory)
Discussion
The blunted schizophrenics were least facially expressive in response to the affect-eliciting stimuli. This finding was consistent with our expectations and suggests that our laboratory procedure tapped some aspect of clinician-rated blunted affect. The results of this study point out the potential utility of laboratory procedures for studying emotional disturbance in schizophrenia and depression.
Perhaps the most interesting result of this study was the significant interaction between group and method of measurement. The blunted schizophrenics differed significantly from other groups in their facial expressions of emotion but not in their reported emotional experiences. These findings suggest that blunted affect in schizophrenia may be primarily a disturbance of expression and not of the ability to feel, or at least report, emotional experiences. This is further supported by the three psychiatric groups’ similarities in their self-reports of depression and anhedonia.
In this study we were able to examine several potential explanatory mechanisms for schizophrenics’ reduced emotional expressiveness. The stimuli were intentionally chosen so that they would elicit emotional responses in markedly different ways. We hypothesized that our manipulation of the stimulus dimension that we called cognitive demand (e.g., drinks vs. films) would have different effects on the different groups. We expected this because of the different ways in which stimuli at the different levels of this dimension would elicit emotional responses. The absence of an interaction between group and cognitive demand is not consistent with the hypothesis that the emotional deficit observed in schizophrenia is a simple consequence of cognitive deficit. Of course, our results do not prove that affective flatness in schizophrenia is unrelated to cognitive deficits. In the absence of data validating the cognitive demand manipulations, we cannot be certain of its relation to information processing. Even if the cognitive demand manipulation succeeded in doing what it was intended to, there may be other cognitive deficits that were not tapped in this study. In addition, the stimuli may have differed along dimensions other than cognitive demand, such as a social-nonsocial dimension. Despite these caveats, our results suggest that if a causal relation between cognitive deficits and affective flattening does exist, it is probably more complex than we had originally anticipated.
Although it has been suggested that affective flattening may be caused by neuroleptic agents (e.g., Sommers, 1985), a simple relation between medication and emotional expression was not found in this study. The blunted and nonblunted schizophrenics were receiving equivalent types and amounts of medication, which indicates that the administration of neuroleptic agents is not sufficient to cause affective flatness. Although this study has helped to clarify the nature of the emotional disturbance in schizophrenia, additional research is necessary to elucidate the mechanism responsible for the reduction in emotional expressiveness.
Another important finding was the significant Group × Valence interaction that emerged because the nonblunted schizophrenic and depressed subjects differed in their pattern of responses to the affect-eliciting stimuli. The depressed subjects were significantly less responsive than the nonblunted schizophrenics to the positive stimuli. It is possible that the depressed subjects would have differed significantly from the other groups in response to the negative stimuli if we had used negative stimuli that elicited sadness.
The difference between the depressed and the nonblunted schizophrenic subjects is particularly interesting because the two groups did not differ from each other or from normal subjects on ratings of blunted affect made during a clinical interview. Thus although global ratings of blunted affect can provide an indication of emotional expressiveness, they are not capable of elucidating differences in the types of emotional dysfunctions seen in groups with different psychopathological disorders.
In order to expand the understanding of emotional disturbances, it is important to continue conducting multidimensional assessments of emotional reactivity. In such studies, researchers should examine which specific emotions, such as sadness, anger, and fear, are exaggerated or diminished in different psychopathological disorders. As our findings indicate, measures of emotional reactivity that are based on standard clinical interviews are probably not sufficient for discovering the many ways in which psychiatric groups differ from normal persons and from each other. It is also essential to examine the different facets of emotional responding, such as subjective experience and facial expression. Such a strategy will enable researchers to determine which specific aspects of emotional functioning are disturbed among persons with different psychiatric disorders.
The difference between the depressed subjects’ and nonblunted schizophrenics’ responses to the affect-eliciting stimuli is also interesting because the levels of depression reported by the two groups did not differ. Our results raise questions about the meaning of reported depression among schizophrenics. The results suggest that the depression reported by schizophrenics may be qualitatively different from the depression experienced by patients with primary diagnoses of major depression. At the very least, our results show that even when schizophrenics report being depressed, they do not share the same pattern of emotional responding exhibited by persons with major depression.
The three psychiatric groups exhibited different patterns of responding to the affect-eliciting stimuli. The blunted schizophrenics were least facially expressive. The nonblunted schizophrenics differed significantly from the depressed subjects in responses to the positive affect-eliciting stimuli. Corresponding differences on the measures of depression and anhedonia were not found, although all three groups differed quite markedly from the normal subjects. These results suggest that schizophrenics and depressed subjects exhibit a variety of disturbances in emotional functioning, each of which may be influenced independently of one another.
Acknowledgments
Most of the data and analyses presented in this article are based on a dissertation submitted by Howard Berenbaum, under the supervision of Thomas Oltmanns, to the graduate school of Indiana University. The study was funded by an Indiana University doctoral student grant in aid of research and a University of Illinois Research Board grant awarded to Howard Berenbaum and by Grant MH31536 awarded by the National Institute of Mental Health to Thomas Oltmanns.
The contributions of the following dissertation committee members are gratefully acknowledged: Alexander Buchwald, John Junginger, Robert Levenson, and George Rebec. We also thank the helpful staff at the Indiana University Medical Center’s affiliated clinics, especially the Wishard Hospital Day Treatment program and the Community Support Center.
Footnotes
Portions of this article were presented at the Third Annual Meeting of the Society for Research in Psychopathology, Cambridge, Massachusetts, November 1988.
The correlations between medication dosage and measures of emotional disturbance were weak. The average correlation between dosage and reported emotions was .02. The average correlation with observed emotional expressions was −.04. Schizophrenics receiving antiparkinsonian medication did not differ significantly from those who were not. Finally, depressives who were receiving antipsychotic medication did not differ significantly from those who were not. However, calculation of these correlations is not a strong way to evaluate drug effects (Neale & Oltmanns, 1980).
One of the subjects (a male nonblunted schizophrenic) omitted one page of the Beck Depression Inventory, and a score was therefore not calculated.
Because of mechanical failure, human error, or both, the response of 1 subject (a male nonblunted schizophrenic) to the Chinatown film clip was not recorded, as were the complete video records of 2 additional subjects (a depressed female subject and a female blunted schizophrenic). In addition, 1 of the subjects (a depressed female subject) exhibited a continuous chewing movement while viewing the film clips (but not at any other time), and therefore her observed responses to the film clips were treated as missing data.
Ekman and Friesen (1982) distinguished between felt and unfelt smiles, the former including action of both the zygomatic major and the orbicularis oculi muscles. Both felt and unfelt smiles were combined to form the observed happiness scores. Separate analyses with only the felt smiles resulted in findings similar to those obtained with the combined smile scores.
The dependent variables for this analysis were (a) reported and observed happiness in response to the positive stimuli and (b) reported disgust and observed nonhappy responses to the negative stimuli. It is unconventional to use different dependent measures for different conditions in the same analysis. The analysis was carried out this way because, as mentioned earlier, it was uncommon for subjects to exhibit or report positive emotions in response to the negative stimuli or negative emotions in response to the positive stimuli. Thus although different measures were used for the different conditions, all of the dependent variables were measures of the anticipated emotional responses.
When we conducted a parallel set of data analyses in which schizophrenics were divided into blunted and nonblunted groups on the basis of a median split of the experimeter’s blunting ratings, the results were identical to those obtained when the original division of schizophrenics into blunted and nonblunted groups was used.
Method of measurement (reported vs. observed) was treated as a within-subject factor in the analysis of variance, rather than as multiple dependent measures. The idea of treating different measures as within-subject factors in an analysis of variance was proposed by Block, Levine, and McNemar (1951) as a means of conducting a univariate profile analysis. Because this procedure is somewhat unorthodox, a second analysis was conducted in order to assess the Group × Measurement interaction. Difference scores based on the difference between the reported and observed z scores were computed for each subject in response to each stimulus. A Group × Sex multivariate analysis of variance was then conducted with the six difference scores (one difference score per stimulus) as dependent measures. A main effect for group, F (18, 178.68) = 2.05, p < 0.01, was significant; this indicates that the differences between reported and observed scores differed significantly across groups and is consistent with the significant Group × Measure interaction obtained in the univariate analysis of variance.
Similar results were obtained when subjects with infrequency scores of 3 or higher were excluded from the analyses.
Contributor Information
Howard Berenbaum, University of Illinois.
Thomas F. Oltmanns, University of Virginia
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed. Washington, DC: Author; 1980. [Google Scholar]
- Andreasen NC. Affective flattening and the criteria for schizophrenia. American Journal of Psychiatry. 1979;136:944–947. doi: 10.1176/ajp.136.7.944. [DOI] [PubMed] [Google Scholar]
- Beck AX, Ward CH, Mendelson M, Mock JE, Erbaugh JK. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berenbaum H, Snowhite R, Oltmanns TF. Anhedonia and emotional responses to affect evoking stimuli. Psychological Medicine. 1987;17:677–684. doi: 10.1017/s0033291700025915. [DOI] [PubMed] [Google Scholar]
- Bleuler E. In: Dementia praecox or the group of schizophrenias. Zinkin J, translator. New York: International Universities Press; 1950. (Original work published 1911) [Google Scholar]
- Block J, Levine L, McNemar Q. Testing for the existence of psychometric patterns. Journal of Abnormal and Social Psychology. 1951;46:356–359. doi: 10.1037/h0060492. [DOI] [PubMed] [Google Scholar]
- Carpenter WT, Jr, Bartko JJ, Strauss JS, Hawk AB. Signs and symptoms as predictors of outcome: A report from the international pilot study of schizophrenia. American Journal of Psychiatry. 1978;135:940–945. doi: 10.1176/ajp.135.8.940. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. Disordered thought in schizophrenia. New York: Appleton-Century-Crofts; 1973. [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. Journal of Abnormal Psychology. 1976;85:374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- Davis JM. Comparative doses and costs of antipsychotic medication. Archives of General Psychiatry. 1976;33:858–861. doi: 10.1001/archpsyc.1976.01770070088010. [DOI] [PubMed] [Google Scholar]
- Dunn LM. Peabody Picture Vocabulary Test (manual) Nashville, TN: American Guidance Service; 1959. [Google Scholar]
- Ekman P, Friesen WV. The facial action coding system (FACS): A technique for the measurement of facial action. Palo Alto, CA: Consulting Psychologists Press; 1978. [Google Scholar]
- Ekman P, Friesen WV. Felt, false, and miserable smiles. Journal of Nonverbal Behavior. 1982;6:238–252. [Google Scholar]
- Friesen W. Recent developments in FACS-EMFACS. Face Value: Facial Measurement Newsletter. 1986;1:1–2. [Google Scholar]
- Knight RA, Roff JD, Barrnett J, Moss JL. Concurrent and predictive validity of thought disorder and affectivity: A 22 year follow-up of acute schizophrenics. Journal of Abnormal Psychology. 1979;88:1–12. doi: 10.1037//0021-843x.88.1.1. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. In: Dementia praecox and paraphrenia. Barclay RM, translator. Huntingdon, NY: Krieger; 1971. (Original work published 1919) [Google Scholar]
- Neale JM, Oltmanns TF. Schizophrenia. New York: Wiley; 1980. [Google Scholar]
- Oltmanns TF, Strauss ME, Heinrichs DW, Driesen N. Social facilitation of emotional expression in schizophrenia; Paper presented at the meeting of the Society for Research in Psychopathology; Cambridge, MA. 1988. Nov, [Google Scholar]
- Pogue-Geile MF, Harrow M. Negative and positive symptoms in schizophrenia and depression: A followup. Schizophrenia Bulletin. 1984;10:371–387. doi: 10.1093/schbul/10.3.371. [DOI] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Croughan J, Ratcliff KS. The NIMH Diagnostic Interview Schedule: Its history, characteristics, and validity. Archives of General Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Ross ED, Mesulam MM. Dominant language functions of the right hemisphere? Prosody and emotional gesturing. Archives of Neurology. 1979;36:144–148. doi: 10.1001/archneur.1979.00500390062006. [DOI] [PubMed] [Google Scholar]
- Sommers AE. Negative symptoms: Conceptual and methodological problems. Schizophrenia Bulletin. 1985;11:364–379. doi: 10.1093/schbul/11.3.364. [DOI] [PubMed] [Google Scholar]
- Steiner JE. Innate discriminative human facial expressions to taste and smell stimulation. Annals of the New York Academy of Science. 1974;237:229–233. doi: 10.1111/j.1749-6632.1974.tb49858.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The international pilot study of schizophrenia. Geneva: Author; 1973. [Google Scholar]