Abstract
The EGF Receptor (EGFR) and its downstream signaling are implicated in lung cancer development. Therefore, much effort was spent in developing specific tyrosine kinase inhibitors (TKIs) that bind to the EGFR ATP-pocket, blocking EGFR phosphorylation/signaling. Clinical use of TKIs is effective in a subset of lung cancers with mutations in the EGFR kinase domain, rendering the receptor highly susceptible to TKIs. However, these benefits are limited, and emergence of additional EGFR mutations usually results in TKI resistance and disease progression.
Previously, we demonstrated one mechanism linking cigarette smoke (CS) to EGFR-driven lung cancer. Specifically, exposure of lung epithelial cells to CS-induced oxidative stress stimulates aberrant EGFR phosphorylation/activation with impaired receptor ubiquitination/degradation. The abnormal stabilization of the activated receptor leads to uncontrolled cell growth and tumorigenesis.
Here we describe for the first time a novel post-translational mechanism of EGFR resistance to TKIs. Exposure of airway epithelial cells to CS causes aberrant phosphorylation/activation of EGFR, resulting in a conformation that is different from that induced by the ligand EGF. Unlike EGF-activated EGFR, CS-activated EGFR binds c-Src and caveolin-1 and does not undergo canonical dimerization. Importantly, the CS-activated EGFR is not inhibited by TKIs (AG1478; Erlotinib; Gefitinib); in fact, the CS exposure induces TKI-resistance even in the TKI-sensitive EGFR mutants.
Our findings demonstrate that CS exposure stimulates not only aberrant EGFR phosphorylation impairing receptor degradation, but also induces a different EGFR conformation and signaling that are resistant to TKIs. Together, these findings offer new insights into CS-induced lung cancer development and TKI resistance.
Keywords: EGFR conformation, lung cancer, cigarette smoke, TKI resistance
Introduction
Smoking-related lung cancer is the leading cause of cancer deaths in both men and women in the United States. However, the molecular mechanisms underlying the induction of lung cancer by cigarette smoke (CS) are still poorly understood.
It is currently known that over-expression and deregulation of receptor tyrosine kinases (RTKs) are tightly connected to tumorigenesis. Of importance is the epidermal growth factor receptor (EGFR), a member of the ErbB family of RTKs, which also includes ErbB2, ErbB3, and ErbB4 (1). EGFR is implicated in a number of cancers including lung cancer (2–4). This receptor plays a role in normal cell processes, but the deregulation of its activation and downstream signaling leads to aberrant cell proliferation and cancer development (5–7).
The activation mechanism of EGFR in lung cancer has been a subject of intense studies, and a classical model of EGFR activation has been established wherein ligand binding induces receptor dimerization. This leads to activation of the EGFR intracellular tyrosine kinase domain and subsequent auto-phosphorylation of specific tyrosines on the C-terminal “tail” of the receptor, initiating a cascade of downstream signaling.
Over the last decade specific tyrosine kinase inhibitors (TKIs) have been developed to block EGFR activation / signaling. TKIs are small molecules that inhibit TK activity via binding reversibly to the EGFR ATP binding site; several TKIs are approved by the US Food and Drug Administration (FDA) for the treatment of metastatic lung cancer, including Erlotinib (Tarceva), while other TKIs are in preclinical trials (e.g. TKI AG1478) (8–11). Trials with TKIs indicated that tumor responses to the drugs were remarkable and sustainable in specific subsets of non-small cell lung cancer (NSCLC) patients that possess activating EGFR mutations (12–14). Intriguingly however, it has been noted anecdotally that patients responsive to TKI therapy developed TKI resistance if they began smoking (15).
Notably, molecular structure studies of the L858R mutant (MT) EGFR (16) demonstrated that the conformation of this TKI-sensitive MT differs from that of the wild type (WT) EGFR at the level of the kinase domain, which carries a constitutively “open” activating loop. This open loop turns the EGFR to be constitutively active. Moreover, such a conformation allows better access of TKIs to the EGFR pocket of ATP binding.
In the current study, we investigated whether smoking-related TKI resistance may be explained mechanistically by alterations in the EGFR conformation and signaling. Recently, we showed that EGFR activation induced by CS was in fact independent of ligand binding (5). Specifically, one of the major reactive oxidants in the gas phase of CS, hydrogen peroxide (H2O2), caused aberrant phosphorylation and activation of the EGFR (5) in human airway epithelial (HAE) cells. The abnormal phosphorylation of the receptor in cells exposed to H2O2-induced oxidative stress also acquired an aberrant activated conformation that impaired canonical dimerization of EGFR (17). This activated EGFR was neither ubiquitinated nor subsequently degraded due to its inability to bind the E3-lygase, c-Cbl. This allowed EGFR to remain active for a longer period at the plasma membrane, thereby causing prolonged survival signals that contributed to uncontrolled cell growth (5–6, 18).
Here we present evidence for a novel, active EGFR conformation caused by oxidative stress (ox-stress) from CS exposure. Unlike the canonical EGF-induced conformation, it does not dimerize, and it interacts strongly with c-Src, resulting in ligand-independent EGFR activation that is resistant to inhibition by TKIs.
In fact, both wild type (WT) and TKI-sensitive EGFR mutants (MTs) develop TKI resistance through this mechanism after CS exposure. These findings help to elucidate how cigarette smoke induces aberrant EGFR signaling which promotes lung cancer and therapy resistance.
Materials and Methods
Cell culture, treatments and reagents
A549 adenocarcinoma (ATCC), NCI-HCC827 (generous gifts from Dr. Philip Mack, University of California at Davis), and NIH-3T3 cells (generous gifts from Dr. Hamid Band, University of Nebraska Medical Center) (19) have been employed in this study. All the cell lines used in this study were previously characterized by others, as reported (19–21); no authentication was done by the authors. Details regarding the various media and source of reagents are provided in the online supplement.
Cigarette smoke exposure
Serum-starved cells were exposed to cigarette smoke as described before (5). Details regarding the exposure chamber are in the online supplement.
Immuno-precipitation (IP)
200–400 µg of total protein extracts were incubated for 3 h with 2–4 µg of antibodies (Abs): anti (α) 528 (against EGFR) or α4-2 (αactive-EGFR, kindly provided by Dr. K. Omi, Fujirebio Inc., Tokyo, Japan (22)). 50 µl of 50% protein A-agarose bead complexes (Repligen) were added to the samples and incubated for 90 min. Four washes (by sequential centrifugation and re-suspension) with the NP-40-lysis buffer were done prior to re-suspending the IPs in the loading dye for SDS-PAGE, as described before (5, 17).
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and immuno-blotting (IB)
5, 6, 8, 10 or 12% acrylamide gels were prepared and used in a two Cell system (BioRad) for 1–4 h at 100 V at room temperature (RT). 20–100 µg of total protein extracts or the IP samples were loaded into each well of the SDS-PAGE in the presence of a sodium dodecyl sulfate (SDS)/ dithiothreitol (DTT) reducing loading dye. After SDS-PAGE separation the proteins were transferred to a nitrocellulose membrane and “blocked” with 5% skim milk in Tris buffered saline with 0.05% tween-20 (TBST) for 120 min. or overnight, as described (17). Full details regarding the Abs used are provided in the online supplement.
Anchorage independent growth assay (soft agar/agarose assay)
5000 Single-suspended NIH-3T3 cells over-expressing the L858R EGFR MT were mixed in culture medium (38°C) containing 0.275% low melting point Agarose (Fermentas) and then seeded in “6-well plate” dishes (Falcon) on top of 0.6% Agar (BTS) layer, as previously described by others (19). Growing colonies (composed of at least 3 cells) were randomly counted by optical microscopy at 100× magnification.
Statistical analysis
Each treatment and experiment (IB, IP/IB or soft agar/agarose assay) was repeated at least three times. The plotted data are reported as mean ± Standard deviations (St-Devs). Statistical significance was determined by Student’s t-test and p-value < 0.05 was considered statistically significant.
Results
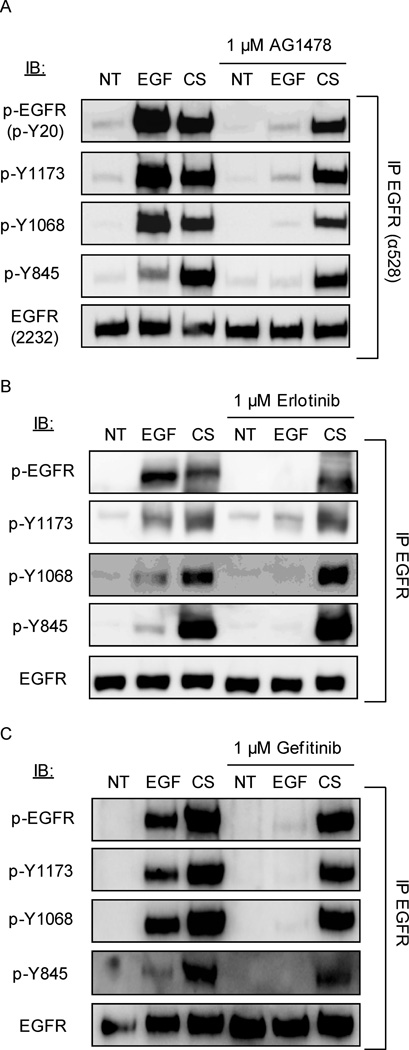
CS-induced phosphorylation of EGFR is not inhibited by TKIs, AG1478, Erlotinib and Gefitinib
Small-molecule tyrosine kinase inhibitors (TKIs), i.e. AG1478, Erlotinib and Gefitinib are used to target the EGFR, suppressing its kinase activity by reversibly blocking the ATP-binding site of the receptor. Thus, we tested the efficacy of these TKIs on CS-induced phosphorylation of EGFR.
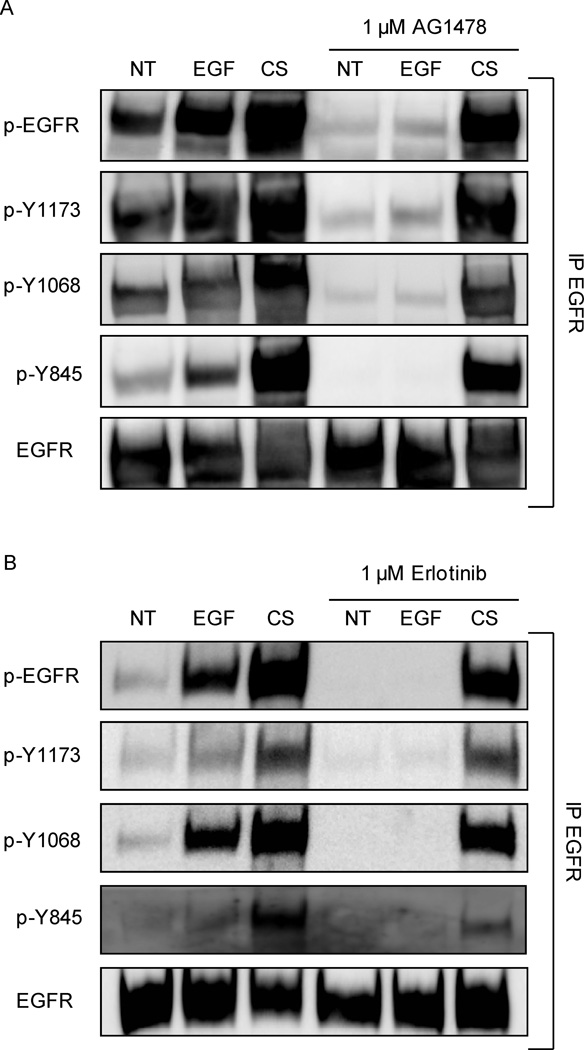
Serum-starved A549 cells were incubated (or not) with 1 µM AG1478 or Erlotinib or Gefitinib for 30 minutes and then treated (or not) with 100 ng/ml EGF or smoke from 1 cigarette for an additional 30 minutes. Cells were lysed, EGFR was immuno-precipitated (IPed) and immuno-blotted (IBed) for total receptor, total tyrosine (Tyr) phosphorylation, and specific Tyr (Y) phosphorylation sites, as indicated in Figure 1. Cells treated with EGF showed an increase of phosphorylation at the EGFR auto-phosphorylation sites Y1068 and Y1173 compared to untreated cells. Of the cells exposed to CS, activation at the auto-phosphorylation sites highly increased along with the c-Src-dependent trans-phosphorylation site (Y845) compared to control (we previously demonstrated that c-Src is highly activated in HAE cells exposed to CS (5)). With incubation of TKIs prior to treatment, AG1478 (Fig. 1A), Erlotinib (Fig. 1B) and Gefitinib (Fig. 1C) EGF-induced phosphorylation was inhibited as expected, highly decreasing the phosphorylation at sites Y1068 and Y1173. However, with CS treatment, the TKIs failed to suppress EGFR phosphorylation at all sites (Fig. 1A for AG1478, Fig. 1B for Erlotinib and Fig. 1C for Gefitinib).
Figure 1. Cigarette smoke (CS)-induced EGFR phosphorylation is not inhibited by Tyrosine Kinase Inhibitors (TKIs) AG1478, Erlotinib and Gefitinib.
Serum-starved A549 cells were incubated (or not) with A. 1 µM AG1478, B. 1 µM Erlotinib or C. 1 µM Gefitinib for 30 min. Cells were treated for 30 min. with 100 ng/ml EGF or exposed to smoke from 1 cigarette for 30 min. EGFR was IPed from the total cell lysates, separated by SDS-PAGE and IBed for total receptor, total tyrosine (Tyr-) phosphorylation level (p-EGFR) and specific Tyr- phosphorylation (Y845, Y1068, and Y1173). Each treatment and experiment/IP-IB was repeated three times. Sequential IBs of the immuno-precipitates were done by stripping the Abs off the membranes in between each IB. The images shown are representative of three independent experiments.
Cigarette smoke exposure leads to downstream survival and proliferation signaling that is not inhibited by TKIs
Extracellular signal-regulated kinases (ERK) 1/2 and Akt (also known as protein kinase B) are two well known mediators of cell proliferation and survival, and are known to be involved in cell transformation when persistently activated (23–24). We have shown previously (5) that exposure to smoke from one cigarette for 45 min activates downstream ERK 1/2 and Akt signaling. Furthermore, both EGFR and ERK1/2 phosphorylation persisted for up to two hours after the removal of the treatment medium exposed to CS, whereas removal of EGF returns phosphorylation to near baseline levels at these time points. This further demonstrated that these signals may be prolonged due to the inability of the EGFR to be degraded under CS-induced ox-stress.
Importantly, here we demonstrate, in the supplementary (Sup.) figure 1, that under CS exposure not only are the survival signals prolonged but also the TKI treatment cannot quench the CS-dependent phosphorylation/activation of ERK 1/2 and Akt/Protein kinase B.
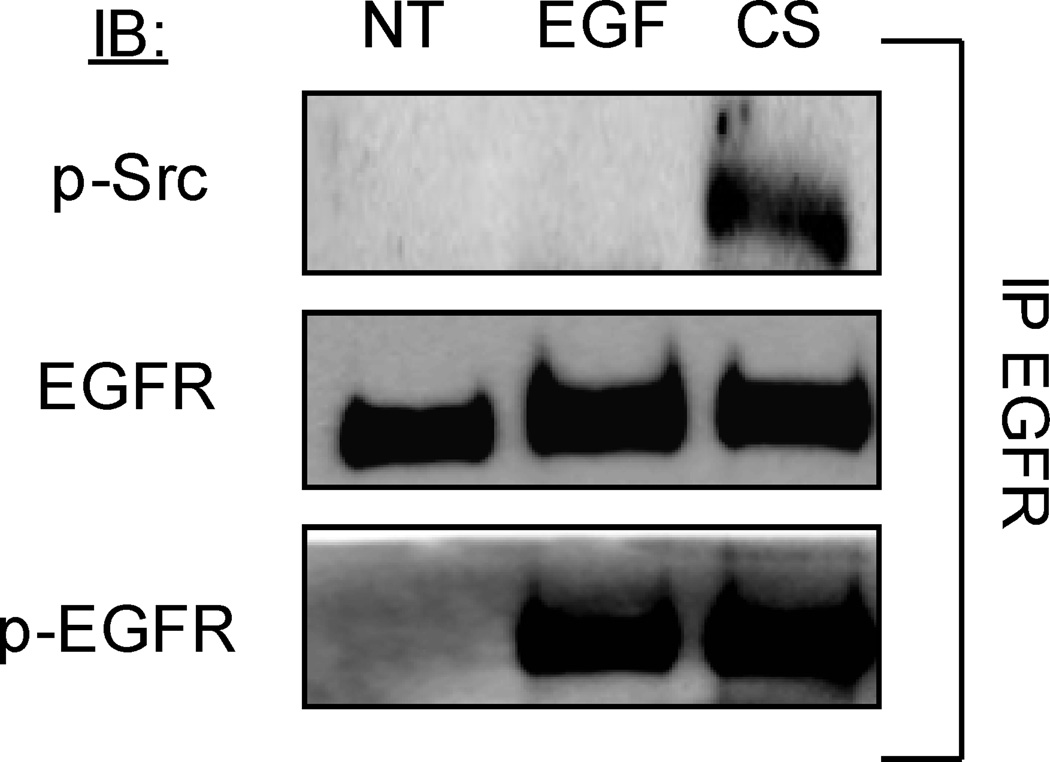
c-Src binds EGFR under CS exposure
Next we investigated whether CS exposure could induce the association of EGFR with c-Src.
Serum-starved A549 cells were treated (or not) with 100 ng/ml EGF for 15 min, or exposed to smoke from one cigarette for 30 min. Cells were lysed, EGFR was IPed and IBed for total EGFR and active (p-Y416) c-Src. As shown in figure 2, after CS exposure, c-Src (active c-Src) strongly associated with EGFR; whereas, upon EGF stimulation no differences in the interaction between c-Src and EGFR were observed in comparison to control (NT) conditions (Fig. 2). Since it has been shown that conformational changes in the intracellular domain of EGFR may lead to direct interaction between EGFR and c-Src (7, 19), such an observed interaction under CS exposure provides an indication for a novel conformation of the EGFR. This interaction also explains the strong level of phosphorylation observed at Y845 (Fig. 1).
Figure 2. EGFR directly interacts with c-Src (activated c-Src) under CS exposure.
Serum-starved A549 cells were exposed (or not) to 100 ng/ml EGF for 15 min. or to smoke from 1 cigarette for 30 min. EGFR was IPed from total cell lysates, separated by SDS PAGE, and IBed for total EGFR and Y-416 activated c-Src (p-Src). Each treatment and experiment/IP-IB was repeated three times. Sequential IBs of the immuno-precipitates were done by stripping the Abs off the membranes in between each IB. The images shown are representative of three independent experiments.
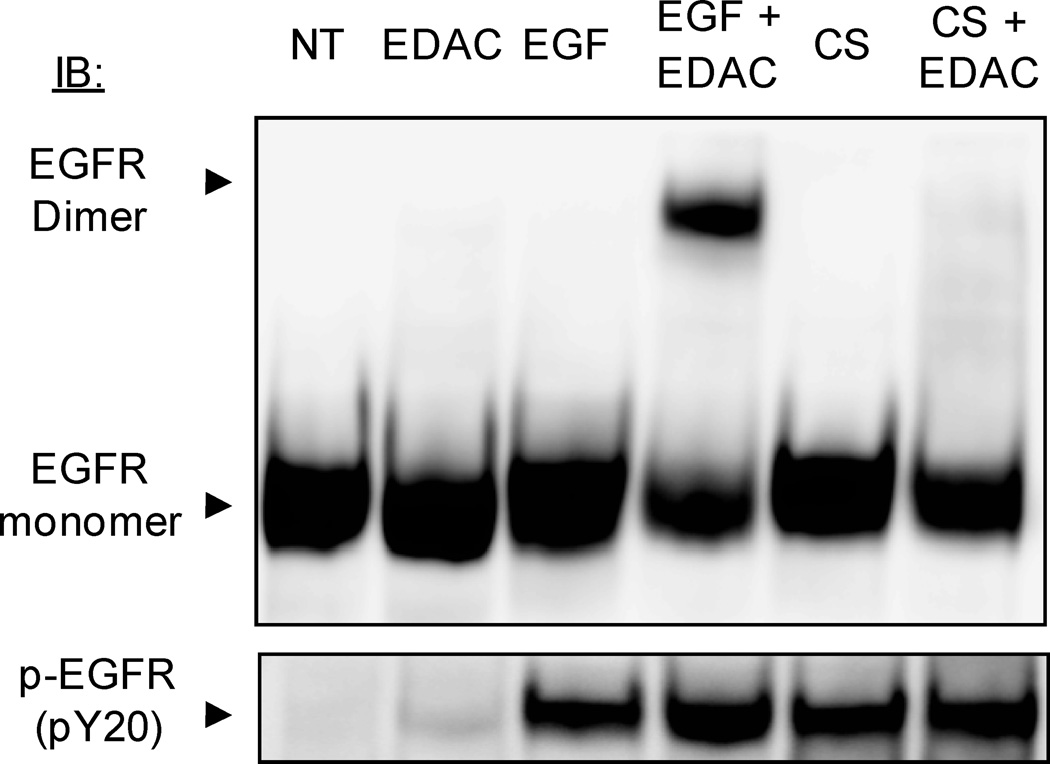
Activated EGFR does not dimerize under CS
We have shown before that H2O2-generated ox-stress did not induce formation of EGFR dimers in the presence of the cross linker EDAC both in airway epithelial cells and in NIH-3T3 cells over-expressing EGFR (17). Here we investigated the mechanism of EGFR dimerization under CS. Serum-starved A549 cells were initially exposed (or not) for 15 min. to 100 ng/ml EGF or to smoke from half a cigarette. Then, a cross-linking agent, EDAC (1 mM), was added (or not) for additional 15 min. (in the presence of EGF or CS, respectively). Cells were lysed and an equal amount of proteins (50 µg) were separated by SDS-PAGE and IBed for total and Tyr-phosphorylated EGFR (Fig. 3). As expected, treating with EGF and the cross linking agent resulted in the formation of a dimer with a band at ~340–360 kDa. On the other hand, CS induced the activation of EGFR, but no dimer was detected in the presence of EDAC (Fig. 3). This demonstrates that CS-induced activation of EGFR does not result in the “classical” dimerization of EGFR.
Figure 3. CS-induced activation of EGFR does not induce “classical” receptor dimerization.
Serum-starved A549 cells were exposed (or not) to 100 ng/ml EGF or exposed to smoke from ½ cigarette for 15 min. The cross linking reagent EDAC (1 mM) was added and incubation continued for an additional 15 min. 50 µg of total proteins from the cell lysates were separated by SDS-PAGE and IBed for total and Tyr-phosphorylated EGFR (p-EGFR). EGFR dimers and monomers are indicated. Each treatment and experiment/IP-IB was repeated three times. The images shown are representative of three independent experiments.
CS induces a novel active conformation of the EGFR intracellular domain
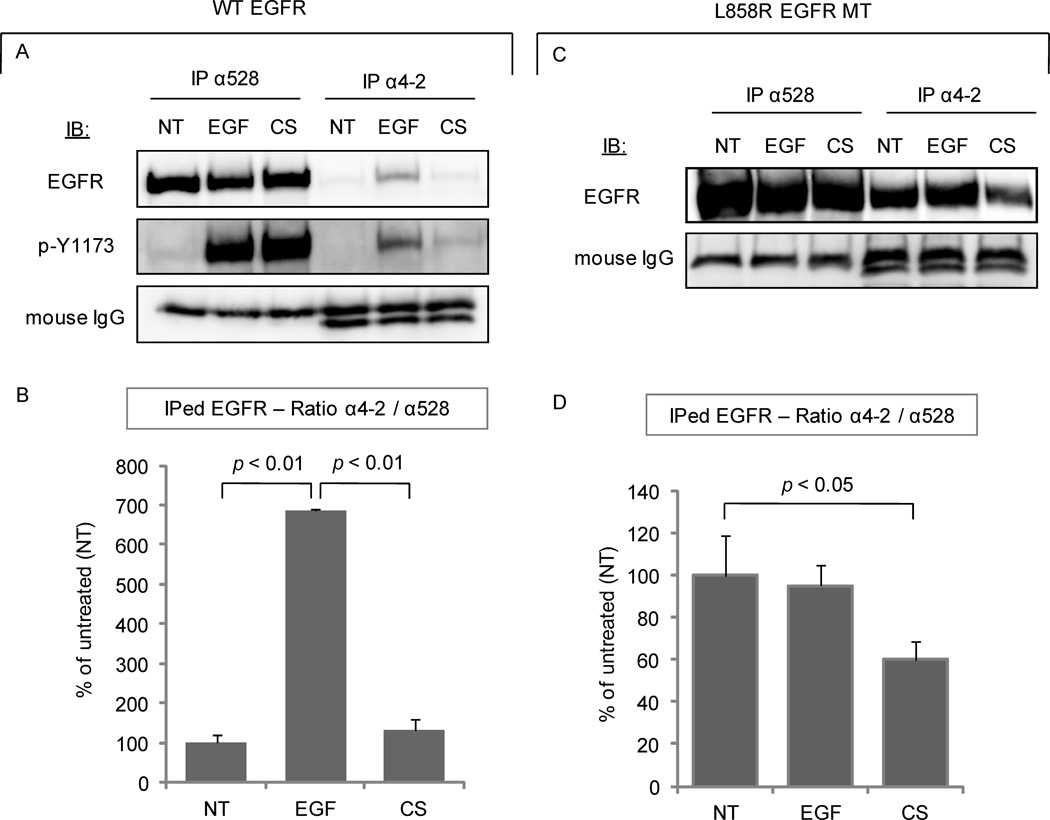
In order to substantiate our hypothesis that the EGFR acquires a new active conformation under CS exposure, we employed a novel “conformational change-sensitive” EGFR monoclonal antibody (mAb), anti- (α) 4-2 Ab (22). This antibody recognizes epitopes (amino acids 956–998) of EGFR in non-denaturing conditions. These epitopes are in the intracellular domain of EGFR and were found to be exposed only in the canonically ligand-activated WT EGFR. Additionally, the 4-2 mAb binds constitutively to the L858R EGFR MT since the same epitopes are constitutively exposed in that MT (22).
Serum-starved A549 cells were treated (or not) with EGF or CS as before. EGFR was IPed from 300 µg of total cell lysate using 3 µg of either α4-2 mAb or α528 mAb and then IBed for total EGFR (α2232) (Fig. 4A, B). The α528 mAb pulled down EGFR with the same affinity for all of the treatments, as expected (Fig. 4A). The α4-2 mAb bound significantly higher to the EGF-treated EGFR than to the untreated receptor. However, under CS exposure, although EGFR is activated (Fig. 1) as verified by strong blotting of its auto-phosphorylation sites (using αp-Y1173 Ab), its affinity to that special Ab, which marks a canonical activated conformation, did not change (Fig. 4A). The α4-2 mAb binds EGFR under EGF stimulation with an affinity ~700% greater than control conditions, but this does not happen under CS activation (Fig. 4B), indicating that the intracellular active conformation of EGFR under CS does not resemble that of the EGF-activated receptor.
Figure 4. CS exposure induces a novel conformation of EGFR.
A. A549 cells were treated (or not) with 100 ng/ml EGF for 15 min. or exposed to smoke from 1 cigarette for 30 min. EGFR was IPed from 300 µg of the total cell lysates using either α528 or α4-2 Ab and then IBed for total receptor or for Tyr-1173 phosphorylation. B. Histogram represents the average of three experiments for the ratio between the bands’ intensity of EGFR IPed with the α4-2 Ab and the total EGFR (IPed with α528 Ab). C. NIH-3T3 cells stably over-expressing L858R EGFR MT were treated and MT EGFR was IPed as above, in A. D. Histogram for the EGFR MT is built as above, in B.
Furthermore, we investigated the ability of the α4-2 mAb to bind the L858R EGFR MT. This was done by employing NIH-3T3 cells stably over-expressing this MT. Cells were treated with EGF or CS, as before, and EGFR was IPed from total cell lysates with either α4-2 or α528 mAbs, and IBed for total EGFR (α2232). Figures 4C and 4D confirm that the α4-2 mAb binds to the L858R EGFR MT with high affinity (Fig. 4C), as previously reported (22). However, its affinity is reduced upon CS exposure, even though the phosphorylation of the MT EGFR under EGF and CS exposure is comparable (data not shown). Indeed, there is ~40% reduction in the amount of MT EGFR pulled down by α4-2 mAb after CS exposure (Fig. 4C). This suggests that CS changes the conformation of both WT and MT L858R EGFR at the level of the intracellular domain, most likely at the level of the kinase domain. Therefore, this novel active conformation could be no longer inhibited by the TKIs, as described above.
The TKI-sensitive L858R somatic mutant EGFR acquires resistance to TKIs under CS
Since we observed that the TKIs were ineffective in inhibiting EGFR activation under CS, we tested their efficacy against the L858R EGFR MT upon CS exposure.
NIH-3T3 cells stably over-expressing the L858R EGFR MT were serum-starved and incubated (or not) with 1 µM AG1478 or Erlotinib for 30 minutes and then treated (or not) with 100 ng/ml EGF or smoke from 1 cigarette for an additional 30 minutes. Cells were lysed, EGFR was IPed and IBed for total receptor, total tyrosine phosphorylation, and specific tyrosine phosphorylation sites, as above.
As shown in figure 5, the L858R EGFR MT is constitutively active (as expected (7, 13, 25)), while its phosphorylation is slightly increased under both EGF and CS. The constitutive activation of the EGFR MT as well as the EGF-induced activation were inhibited by both TKIs, AG1478 (Fig. 5A) and Erlotinib (Fig. 5B), at the auto-phosphorylation sites Y1068 and Y1173 compared to untreated cells. However, this TKI-sensitive EGFR MT becomes resistant to TKI treatments upon exposure to CS (Fig. 5A relative to AG1478 and Fig. 5B relative to Erlotinib). Both the auto-phosphorylation sites Y1068 and Y1173 and the trans-phosphorylation site Y845 remained active under CS exposure even with the pre-incubation of AG1478 or Erlotinib. To further validate this response, another drug-sensitizing mutant EGFR with an in-frame deletion in exon 19 (EGFR Δ746–750) (which encodes part of the kinase domain) was subject to the same TKI treatments. As with the L858R EGFR MT, this deletion mutant acquired resistance to both AG1478 and Erlotinib under CS (data not shown).
Figure 5. TKI-sensitive L858R EGFR MT becomes resistant to TKIs under CS exposure.
NIH-3T3 cells stably over-expressing the L858R EGFR MT were serum-starved and then incubated (or not) with A. 1 µM AG1478 or B. 1 µM Erlotinib for 30 min. Then, the cells were treated for 30 min. with 100 ng/ml EGF or exposed to smoke from 1 cigarette for 30 min. EGFR was IPed from the total cell lysates, separated by SDS-PAGE and IBed for total receptor, total Tyr-phosphorylation level (p-EGFR) and specific Tyr-residue phosphorylation level (Y845, Y1068, and Y1173). Each treatment and experiment/IP-IB was repeated three times. The images shown are representative of three independent experiments.
Finally, we also repeated the CS/TKI treatments in the NSCLC cells, HCC827, which are harboring the TKI-sensitive EGFR mutant: Δ746–750 MT (26). Sup. figure 2 shows that exposure to CS of these TKI-sensitive NSCLC cells also causes EGFR resistance to TKIs.
Overall, our data suggest that CS induces a novel active conformation of EGFR which differs from the EGF-induced one and also is different from that of the L858R EGFR MT and other TKI-sensitive mutants. Such a novel acquired conformation could be the reason for the receptor’s acquired resistance to TKIs.
Collectively, all the above new data suggest that under CS exposure the conformational change of EGFR may no longer keep the kinase domain of the receptor fully open, so that the TKIs’ (AG1478, Erlotinib and Gefitinib) accessibility to the EGFR binding pocket and thus their ability to effectively inhibit EGFR phosphorylation/activation is reduced. This allows the EGFR and its downstream targets, ERK 1/2 and Akt to remain active.
CS exposure abolishes the TKI-dependent inhibition of anchorage independent growth of EGFR-transformed cells
Next, we assessed the effect of CS exposure on the anchorage-independent growth of EGFR-transformed cells in the presence or absence of TKI. We employed the NIH-3T3 cells stably over-expressing L858R EGFR, which were previously demonstrated to be very suitable for testing such transforming potential in soft agar/agarose colony assay (19).
Single-suspended cells were seeded inside a layer of 0.275% Agarose (in culture medium), and on top of a 0.6% Agar gel layer, as described in Material and Methods. Subsequently, the cells were fed daily with medium that was exposed, or not, to smoke from 1 cigarette for 30’ in the presence or absence of 1 µM gefitinib. Sup. figure 3 shows that incubation with 1 µM gefitinib could effectively inhibit the colony formation of the EGFR-transformed cells (in comparison to control-untreated (NT) cells). However, gefitinib treatment became ineffective upon CS exposure of those cells. This confirmed that CS exposure can overcome the TKI-sensitivity of EGFR-transformed cells, thus sustaining clonal growth of lung cancer even in the presence of TKI drugs.
Discussion
We define here the initial post-translational changes that occur in EGFR with cigarette smoke (CS) exposure of airway epithelial cells. These alterations in EGFR lead to aberrant signaling and cell proliferation, and ultimately constitute a molecular basis for CS-induced lung cancer initiation and promotion (5).
Given its frequent deregulation in non-small-cell lung cancer (NSCLC), EGFR became a logical target for therapy. The first targeted strategies employed a monoclonal antibody antagonist to the ligand binding domain (cetuximab) (27). However, more dramatic responses were observed with small molecule reversible inhibitors of the tyrosine kinase domain (TKIs, gefitinib or erlotinib). Though highly gratifying, these remarkable clinical responses were restricted to a subset of cases, typically adenocarcinoma occurring in female never-smokers of Japanese origin (27). Subsequently, these responders were found to possess mutations in the EGFR TK domain, which were thought to confer a selective growth advantage that could be neutralized by the TKIs (13, 25, 28–30). Most common, or “classic”, among these somatic mutations are the exon 19 deletion mutation (Δ746–750) and the exon 21 single-point substitution mutation (L858R) (31–32).
However, it has been recently observed that the same EGFR mutations are not limited to adenocarcinoma from female never-smokers. Indeed, a large number (43%) of EGFR mutations are actually found in adenocarcinoma tumor specimens from men and people who are smokers or former smokers (33). Yet, these smoking patient groups (harboring EGFR mutations, which are sensitive to TKIs) do not appear to benefit from the TKI treatment. This suggests that smoking could affect the TKI sensitivity of EGFR activating mutations.
Interestingly, it was recently shown that these somatic adenocarcinoma mutations induce aberrant downstream signaling that resembles that of the wild-type (WT) EGFR exposed to CS (5). Studies carried out by Dr. Yarden’s group (6) showed that the EGFR mutant L858R presented an impaired association with c-Cbl and ubiquitination as had been previously reported by our group for the WT EGFR exposed to CS-induced ox-stress (5, 34). Moreover, a recent publication by Dr. Band’s group (7) demonstrated that the mutant EGFR, but not the WT receptor, undergoes perinuclear accumulation and co-localization with recycling endosomal markers such as Rab 11, suggesting that mutant EGFRs display a different pattern of endocytosis, again similar to what had been previously described by our group for the WT EGFR exposed to CS (5, 18). Notably, the aberrant EGFR internalization has been proposed as one of the mechanisms that can generate TKI resistance with no additional somatic mutations (35–36). This may be further complemented by our previous finding (5) that under CS exposure EGFR is also capable of trafficking aberrantly to the perinuclear region.
Importantly, while the “classic” somatic mutations and CS exposure may result in similar downstream signaling phenotypes, in all likelihood they nonetheless represent distinct EGFR conformations and structure (16, 37).
This is most notably evidenced by the clinical findings that the sensitivity to TKIs is mostly observed in adenocarcinoma of non-smokers, whereas CS exposure is mostly associated with squamous cell carcinoma and adenocarcinoma that are not sensitive to TKIs. Perhaps for this reason, non-smoking adenocarcinoma patients who initially respond to TKIs develop resistance when they begin to smoke, while smoking patients (whose cancer developed in the setting of CS exposure) are resistant to TKI in the first place.
Therefore, here we propose that the aberrant mechanism of EGFR ligand-independent (5) activation in HAE cells exposed to CS is due to a novel and uncharacterized conformation of the intracellular domain of the receptor that leads to an active, yet stabilized, EGFR that is also resistant to TKI drugs. Therefore, CS-induced EGFR changes may contribute to both the initial disease pathogenesis in smokers and to emergence of TKI-resistance in nonsmokers who at first are sensitive to TKI.
To provide direct evidence for the conformational change of EGFR under CS, we used a novel “conformational change-sensitive” EGFR antibody (α4-2 mAb) (22), which we used before (17). This antibody was shown to bind epitopes of EGFR that are exposed only subsequent to EGFR canonical activation by its ligand, EGF, which induces a conformational change of the kinase domain (16). This antibody also binds constitutively to the L858R EGFR MT because the same epitopes are constitutively exposed in this mutant due to its open activating loop of the kinase domain (16, 22).
Interestingly, even though EGFR is highly activated by CS, the α4-2 mAb binds to the CS-stimulated EGFR with a much lower affinity than to that activated by EGF (Fig. 4A and B). Furthermore, we demonstrated that the high affinity of the α4-2 mAb for the L858R EGFR MT also dropped ~40% upon CS exposure. This was not the case upon EGF stimulation (Fig. 4C and D), indicating that CS exposure induces an active state of the EGFR that differs from that of the “conventional”/ EGF-stimulated EGFR.
Another indication for a unique conformational change of EGFR under CS-induced ox-stress was supported by the finding that EGFR was strongly associated with c-Src only upon CS (and not EGF) exposure of HAE cells (Fig. 2). We reported previously that highly phosphorylated Cav-1 is strongly bound to EGFR under CS-induced ox-stress (5, 18). Others reported that c-Src stably interacts with ErbB2, but not with WT EGFR, because of the difference in their kinase domains (38). This c-Src binding was shown to confer elevated transformation ability (39). Furthermore, the L858R EGFR MT could also bind c-Src (19).
Collectively, these findings suggest that CS exposure may induce TKI resistance solely through post-translational molecular changes without additional somatic mutations. These molecular alterations consist of an EGFR aberrant phosphorylation pattern caused by CS exposure (5) accompanied by an aberrant conformational change. A possible hypothesis is that this conformational change limits TKI accessibility to the ATP binding site of the receptor, thereby preventing the inhibitory effect of the drug and allowing continuous EGFR signaling and cell proliferation (see model in Fig. 6). Accordingly, we also provide evidence that CS exposure can abolish the TKI-dependent inhibition of ERK 1/2 and Akt activation (Sup. Fig. 1) and of the anchorage independent growth of EGFR-transformed cells in soft agar/agarose assay (Sup. Fig. 3).
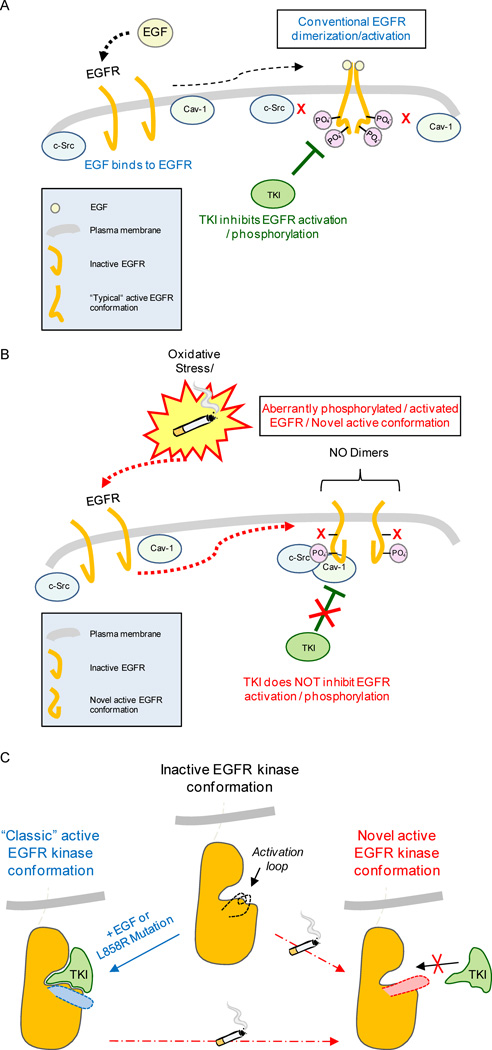
Figure 6. Proposed model of EGFR aberrant activation in lung epithelial cells exposed to cigarette smoke (CS).
A. Scheme of the conventional mechanism of activation/dimerization and phosphorylation of EGFR upon stimulation by the ligand EGF; activation/phosphorylation at specific tyrosine residues can be inhibited by TKI drugs. B. CS aberrantly activates EGFR, inducing aberrant phosphorylation* accompanied by a novel active conformation of the receptor that is bound by active c-Src and caveolin-1 (Cav-1)*. Such aberrantly activated EGFR does not dimerize “conventionally” and becomes resistant to TKI drugs. *Please note: aberrant phosphorylation of EGFR and Cav-1 binding to EGFR under CS-induced ox-stress were demonstrated by our group before (5, 18). C. Modeling EGFR structure/function alterations and change in conformation that may lead to TKI resistance following CS exposure.
Of note is that even an irreversible EGFR inhibitor, such as CL387785, turned to be only 50% efficient in inhibiting EGFR activation (phosphorylation) if the cells were simultaneously exposed to the inhibitor and to CS (data not shown). Importantly, we have also carried out control experiments addressing whether the TK inhibitors may be “damaged” under CS exposure. We found that it was not the case. The effectiveness of the CS-exposed TKIs was evaluated in EGF-stimulated cells and was shown to be as potent as before the TKIs were exposed to CS (data not shown).
Taken together, we show herein that EGF-stimulated WT EGFR, L858R and Δ746–750 EGFR MTs (with or without EGF stimulation) have dramatic inhibition of activation/phosphorylation in the presence of the TKIs AG1478, Erlotinib or Gefitinib (Fig. 1, 5 and Sup. Fig. 2). In contrast, upon CS exposure, both the WT and those EGFR MTs become resistant to TKIs, showing a strong phosphorylation at the auto-phosphorylation sites Y1173 and Y1068. The receptors’ acquired TKI-resistance supports once again a CS-induced conformational change in the intracellular kinase domain of EGFR, which could reduce TKIs’ accessibility to their binding site. These findings may explain the clinical observation, reported anecdotally, that lung cancer patients who resume smoking while receiving TKI treatment rapidly develop resistance and 85,disease progression (15).
Therefore, we conclude that CS-induced post-translational changes in EGFR could provide an important mechanism of disease pathogenesis underlying TKI-resistance in smokers. Additional studies are required to elucidate the exact conformation of EGFR under CS-induced ox-stress; a direction that may lead to the development of more effective TKIs capable of inhibiting EGFR signaling even in the presence of CS or other forms of ox-stress.
Supplementary Material
Acknowledgements
We thank Dr. K. Omi, Fujirebio Inc., Tokyo (Japan), for providing the EGFR mAb 4-2. We thank Dr. H. Band (University of Nebraska Medical Center) for providing the NIH-3T3 cell line stably over expressing EGFR (WT or MTs). We thank Dr. Philip Mack (University of California at Davis) for providing the NCI-HCC827 NSCLC cells. We thank Dr. David S. Baston (University of California at Davis) for technical assistance.
Grant support
This work was supported by grants from the National Institutes of Health (HL-66189 to T.G.) and from the Tobacco-Related Disease Research Program (TRDRP) (17RT-0131 to T.G. and 20FT-0087 to S.F.).
Abbreviations used are
- ~
about
- α
anti-
- Δ
delta (deletion)
- Ab or Abs
antibody or antibodies
- Cav-1
caveolin-1
- CS
cigarette smoke
- EDAC
1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- H2O2
hydrogen peroxide
- HAE
human airway epithelial
- HRP
horseradish peroxidase
- IB, or IBed
immuno-blotting or immuno-blotted
- IP or IPed
immuno-precipitation or immuno-precipitated
- m
monoclonal
- min.
minutes
- MT or MTs
mutant or mutants
- NSCLC
non-small cell lung cancer
- NT
non treated or untreated
- ox-stress
oxidative stress
- p-
phosphorylated
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- St-Devs
standard deviations
- TKI or TKIs
tyrosine kinase inhibitor(s)
- Y or Tyr-
tyrosine
- WT
wild type
Footnotes
The authors declare no conflict of interest.
References
- 1.Goldkorn T, Filosto S. Lung injury and cancer: Mechanistic insights into ceramide and EGFR signaling under cigarette smoke. Am J Respir Cell Mol Biol. 2010;43:259–268. doi: 10.1165/rcmb.2010-0220RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 3.Lu Z, Jiang G, Blume-Jensen P, Hunter T. Epidermal growth factor-induced tumor cell invasion and metastasis initiated by dephosphorylation and downregulation of focal adhesion kinase. Mol Cell Biol. 2001;21:4016–4031. doi: 10.1128/MCB.21.12.4016-4031.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell. 2002;110:669–672. doi: 10.1016/s0092-8674(02)00966-2. [DOI] [PubMed] [Google Scholar]
- 5.Khan EM, Lanir R, Danielson AR, Goldkorn T. EGF receptor exposed to cigarette smoke is aberrantly activated and undergoes perinuclear trafficking. FASEB J. 2008;22:910–917. doi: 10.1096/fj.06-7729com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shtiegman K, Kochupurakkal BS, Zwang Y, Pines G, Starr A, Vexler A, et al. Defective ubiquitinylation of EGFR mutants of lung cancer confers prolonged signaling. Oncogene. 2007;26:6968–6978. doi: 10.1038/sj.onc.1210503. [DOI] [PubMed] [Google Scholar]
- 7.Chung BM, Raja SM, Clubb RJ, Tu C, George M, Band V, et al. Aberrant trafficking of NSCLC-associated EGFR mutants through the endocytic recycling pathway promotes interaction with Src. BMC Cell Biol. 2009;10:84. doi: 10.1186/1471-2121-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis AG, Doherty MM, Walker F, Weinstock J, Nerrie M, Vitali A, et al. Preclinical analysis of the analinoquinazoline AG1478, a specific small molecule inhibitor of EGF receptor tyrosine kinase. Biochem Pharmacol. 2006;71:1422–1434. doi: 10.1016/j.bcp.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe S, Tanaka J, Ota T, Kondo R, Tanaka H, Kagamu H, et al. Clinical responses to EGFR-tyrosine kinase inhibitor retreatment in non-small cell lung cancer patients who benefited from prior effective gefitinib therapy: a retrospective analysis. BMC Cancer. 2011;11:1. doi: 10.1186/1471-2407-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu JY, Wu SG, Yang CH, Chang YL, Chang YC, Hsu YC, et al. Comparison of gefitinib and erlotinib in advanced NSCLC and the effect of EGFR mutations. Lung Cancer. 2011;72:205–212. doi: 10.1016/j.lungcan.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Gridelli C, Maione P, Bareschino MA, Schettino C, Sacco PC, Ambrosio R, et al. Erlotinib in the treatment of non-small cell lung cancer: current status and future developments. Anticancer Res. 2010;30:1301–1310. [PubMed] [Google Scholar]
- 12.Fukuoka MYS, Giaccone G, Tamura T, Nakagawa K, Douillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S, Rischin D, Eek R, Horai T, Noda K, Takata I, Smit E, Averbuch S, Macleod A, Feyereislova A, Dong RP, Baselga J. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected] J Clin Oncol. 2003 Jun 15;21:2237–2246. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 13.Shigematsu H, Gazdar AF. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int J Cancer. 2006;118:257–262. doi: 10.1002/ijc.21496. [DOI] [PubMed] [Google Scholar]
- 14.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 15.Lara P. (UC Davis School of Medicine) Personal anecdotal communication [Google Scholar]
- 16.Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006;125:1137–1149. doi: 10.1016/j.cell.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Filosto S, Khan E, Tognon E, Becker C, Ashfaq M, Ravid T, et al. EGF receptor exposed to oxidative stress acquires abnormal phosphorylation and aberrant activated conformation that impairs canonical dimerization. PLoS ONE. 2011;6:e23240. doi: 10.1371/journal.pone.0023240. Epub 2011 Aug 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan E, Heidinger J, Levy M, Lisanti M, Ravid T, Goldkorn T. EGF receptor exposed to oxidative stress undergoes Src- and caveolin-1-dependent perinuclear trafficking. J Biol Chem. 2006;281:14486–14493. doi: 10.1074/jbc.M509332200. [DOI] [PubMed] [Google Scholar]
- 19.Chung BM, Dimri M, George M, Reddi AL, Chen G, Band V, et al. The role of cooperativity with Src in oncogenic transformation mediated by non-small cell lung cancer-associated EGF receptor mutants. Oncogene. 2009;28:1821–1832. doi: 10.1038/onc.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girard L, Zochbauer-Muller S, Virmani AK, Gazdar AF, Minna JD. Genome-wide allelotyping of lung cancer identifies new regions of allelic loss, differences between small cell lung cancer and non-small cell lung cancer, and loci clustering. Cancer Res. 2000;60:4894–4906. [PubMed] [Google Scholar]
- 21.Giard DJ, Aaronson SA, Todaro GJ, Arnstein P, Kersey JH, Dosik H, et al. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973;51:1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- 22.Ise N, Omi K, Miwa K, Honda H, Higashiyama S, Goishi K. Novel monoclonal antibodies recognizing the active conformation of epidermal growth factor receptor. Biochem Biophys Res Commun. 2010;394:685–690. doi: 10.1016/j.bbrc.2010.03.052. [DOI] [PubMed] [Google Scholar]
- 23.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 24.Vicent S, Lopez-Picazo JM, Toledo G, Lozano MD, Torre W, Garcia-Corchon C, et al. ERK1/2 is activated in non-small-cell lung cancer and associated with advanced tumours. Br J Cancer. 2004;90:1047–1052. doi: 10.1038/sj.bjc.6601644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 26.Guo A, Villen J, Kornhauser J, Lee KA, Stokes MP, Rikova K, et al. Signaling networks assembled by oncogenic EGFR and c-Met. Proc Natl Acad Sci U S A. 2008;105:692–697. doi: 10.1073/pnas.0707270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yatabe Y. EGFR mutations and the terminal respiratory unit. Cancer Metastasis Rev. 29:23–36. doi: 10.1007/s10555-010-9205-8. [DOI] [PubMed] [Google Scholar]
- 28.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 29.Minna JD, Gazdar AF, Sprang SR, Herz J. Cancer. A bull's eye for targeted lung cancer therapy. Science. 2004;304:1458–1461. doi: 10.1126/science.1099578. [DOI] [PubMed] [Google Scholar]
- 30.Weinstein IB. Cancer. Addiction to oncogenes--the Achilles heal of cancer. Science. 2002;297:63–64. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 31.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 32.Mountzios G, Fouret P, Soria JC. Mechanisms of Disease: signal transduction in lung carcinogenesis -- a comparison of smokers and never-smokers. Nat Clin Pract Oncol. 2008;5:610–618. doi: 10.1038/ncponc1181. [DOI] [PubMed] [Google Scholar]
- 33.D'Angelo SP, Pietanza MC, Johnson ML, Riely GJ, Miller VA, Sima CS, et al. Incidence of EGFR exon 19 deletions and L858R in tumor specimens from men and cigarette smokers with lung adenocarcinomas. J Clin Oncol. 2011;29:2066–2070. doi: 10.1200/JCO.2010.32.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ravid T, Sweeney C, Gee P, Carraway KRK, Goldkorn T. EGF receptor activation under oxidative stress fails to promote c-Cbl mediated down regulation. J Biol Chem. 2002;12:12. doi: 10.1074/jbc.M204677200. [DOI] [PubMed] [Google Scholar]
- 35.Kwak EL, Sordella R, Bell DW, Godin-Heymann N, Okimoto RA, Brannigan BW, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci U S A. 2005;102:7665–7670. doi: 10.1073/pnas.0502860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang WC, Chen YJ, Li LY, Wei YL, Hsu SC, Tsai SL, et al. Nuclear translocation of epidermal growth factor receptor by Akt-dependent phosphorylation enhances breast cancer-resistant protein expression in gefitinib-resistant cells. J Biol Chem. 2011;286:20558–20568. doi: 10.1074/jbc.M111.240796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jura N, Endres NF, Engel K, Deindl S, Das R, Lamers MH, et al. Mechanism for activation of the EGF receptor catalytic domain by the juxtamembrane segment. Cell. 2009;137:1293–1307. doi: 10.1016/j.cell.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim H, Chan R, Dankort DL, Zuo D, Najoukas M, Park M, et al. The c-Src tyrosine kinase associates with the catalytic domain of ErbB-2: implications for ErbB-2 mediated signaling and transformation. Oncogene. 2005;24:7599–7607. doi: 10.1038/sj.onc.1208898. [DOI] [PubMed] [Google Scholar]
- 39.Marcotte R, Zhou L, Kim H, Roskelly CD, Muller WJ. c-Src associates with ErbB2 through an interaction between catalytic domains and confers enhanced transforming potential. Mol Cell Biol. 2009;29:5858–5871. doi: 10.1128/MCB.01731-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.