Abstract
Lipoxygenases (LOXs) form a heterogeneous class of lipid peroxidizing enzymes, which have been implicated in cell proliferation and differentiation but also in the pathogenesis of various diseases with major public health relevance. As other fatty acid dioxygenases LOX oxidize polyunsaturated fatty acids to their corresponding hydroperoxy derivatives, which are further transformed to bioactive lipid mediators (eicosanoids and related substances). On the other hand, lipoxygenases are key players in regulation of the cellular redox homeostasis, which is an important element in gene expression regulation. Although the first mammalian lipoxygenases were discovered 40 years ago and although the enzymes have been well characterized with respect to their structural and functional properties the biological roles of the different lipoxygenase isoforms are not completely understood. This review is aimed at summarizing the current knowledge on the physiological roles of different mammalian LOX-isoforms and their patho-physiological function in inflammatory, metabolic, hyperproliferative, neurodegenerative and infectious disorders.
Keywords: eicosanoids, inflammation, atherosclerosis, cancer, brain, stroke, infection
1. Introduction
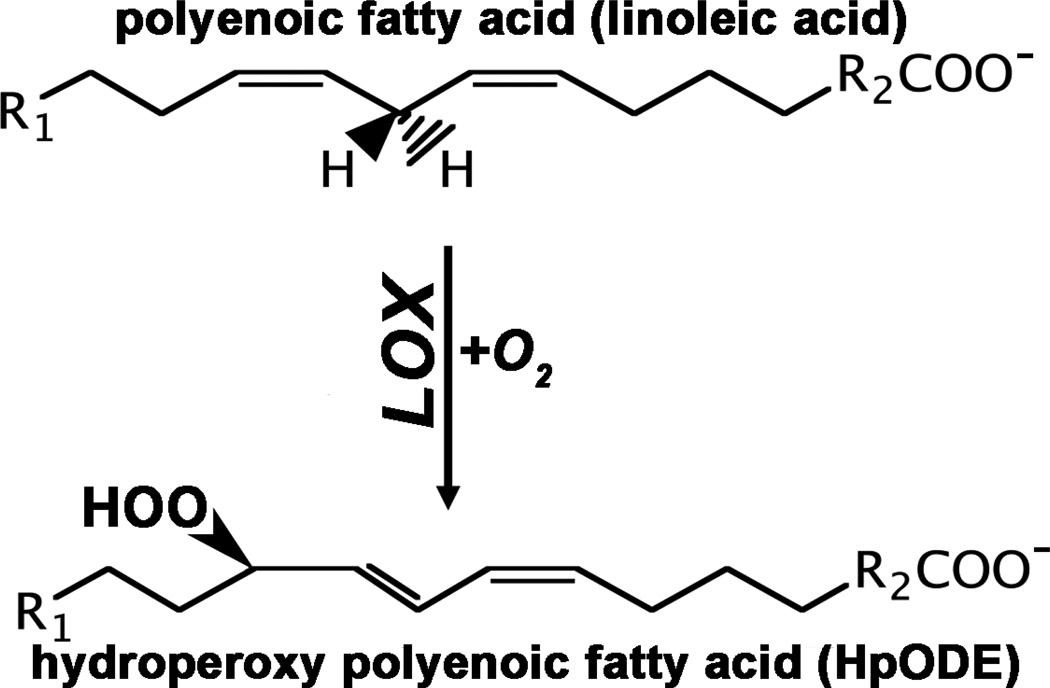
Lipoxygenases (LOXs) are non-heme iron-containing dioxygenases [1, 2] that catalyze dioxygenation of polyunsaturated fatty acids containing at least two isolated cis-double bonds (Fig. 1). In mammalian cells linoleic acid (C18:Δ2, n-6) and arachidonic acid (C20:Δ4, n-6) are the most abundant polyenoic fatty acids that serve as substrates for the different mammalian LOX-isoforms. In general, mammalian LOXs prefer free fatty acids as substrate over polyenoic fatty acid containing ester lipids but the cellular concentration of free fatty acids is rather low. Thus, an active LOX pathway requires liberation of substrate fatty acids from the ester lipids localized in the cellular membranes. After hydrolytic cleavage of the membrane ester lipids catalyzed by cytosolic phospholipase A2 [3] the liberated fatty acids [mainly arachidonic acid (AA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)] are alternatively oxygenated by cyclooxygenases (COX) to G-prostaglandins (PGG2 in case of AA, PGG3 in case of EPA, PGG4 in case of DHA) or by LOX isoforms to various hydroperoxy derivatives of the substrate fatty acids [2]. The primary products of the LOX pathway are subsequently converted to a large array of bioactive lipid mediators, which include leukotrienes [4], lipoxins [5], hepoxilins [6], eoxins [7], resolvins [8], protectins [9] and others. However, the classical concept of the arachidonic acid cascade may not be the only way, by which LOXs exhibit their bioactivity. There are at least two alternative scenarios (Fig. 2): i) Some LOX isoforms are capable of oxygenating polyenoic fatty acids if they are constituents of phospholipids [10] or cholesterol esters [11]. The introduction of a hydrophilic peroxide group into the hydrophobic tail of a fatty acid changes the physico-chemical properties of the ester lipids. Clustering of oxidized lipids within the lipid bilayer of a biomembrane leads to the formation of “hydrophilic pores”. By this mechanism the barrier function of the membrane is impaired which may lead to cellular dysfunction. ii) The cellular redox state is of major cell physiological relevance. It impacts the gene expression pattern of a given cell population [12] on transcriptional and post-transcriptional levels and thus determines the cellular phenotype. In each cell the redox homeostasis is maintained by the balanced equilibrium of pro- and anti-oxidative processes and LOXs constitute some of the key pro-oxidative players in the redox homeostasis. LOX-catalyzed formation of hydroperoxy lipids impacts the activity of redox-dependent transcription and/or translation factors [13], which in turn leads to up- and/or down-regulation of the expression of redox sensitive genes.
Fig. 1. Simplified scheme of the lipoxygenase reaction.
LOXs convert polyenoic fatty acids containing at least one 1,4-pentadiene system to their corresponding hydroperoxy derivatives. Atmospheric oxygen serves as second substrate.
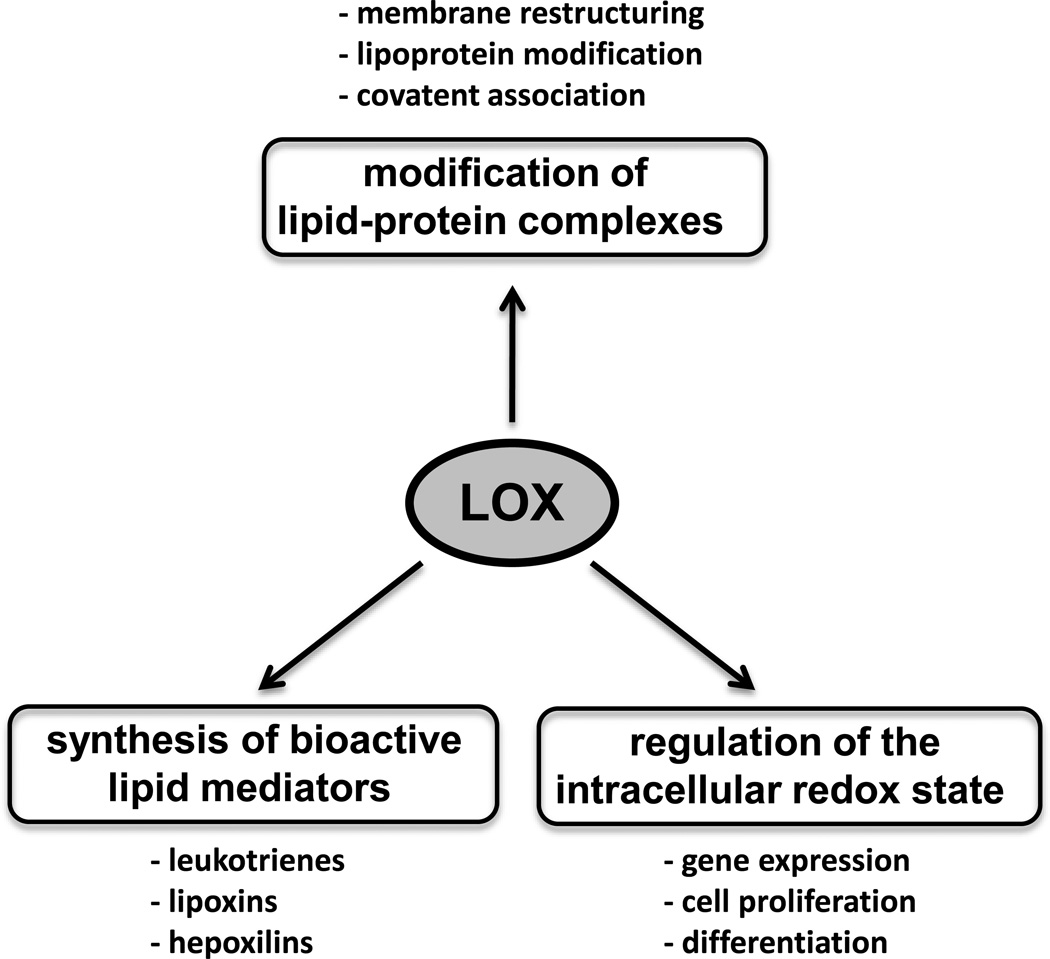
Fig. 2. Biological function of lipoxygenase.
Lipoxygenases may exhibit their biological functionality via three different mechanistic scenarios. i) Formation of bioactive lipid mediators, ii) Structural modification of complex lipid-protein assemblies. iii) Modification of the cellular redox homeostasis, which alters the gene expression pattern.
The molecular details of how the different LOX-isoforms exhibit their bioactivity have been explored for many years and a large number of reports employing various loss-of-function (siRNA-mediated expression knockdown, knockout mice) as well as gain-of-function (cellular transfection studies, transgenic animals) strategies have provided a deeper insight into the biological importance of LOXs in health and disease. Nonetheless, our knowledge of the biological role of various LOX-isoforms, in particular for ALOX15, ALOX15B, and ALOX12 is still somewhat limited. This review is aimed at summarizing and critically evaluating the experimental data characterizing the physiological and patho-physiological roles of various LOX-isoforms in mammals. Of course, LOXs have been the topic of previous reviews and a PubMed search with the key words “lipoxygenase and review” yielded some 1700 hits. However, most of these reviews cover selected areas of LOX research such as LOX enzymology [1], ALOX5 pathway and leukotriene signaling [2] or LOX in bone disease [14]. To the best of our knowledge there is no recent review paper summarizing the current knowledge of the biological role of mammalian LOX isoforms in health and disease.
During the past decades LOX research has developed rapidly and a PubMed search with the keyword “lipoxygenase” gave some 15,600 hits. Since 2003 about 500 articles have been published annually and because of space limitations it was not possible to reference here even 10% of these reports. Thus, although we tried to make a balanced selection we might have overlooked important articles and we apologize to those distinguished colleagues whose work we have not had sufficient space to reference.
2. Lipoxygenase distribution, classification and properties
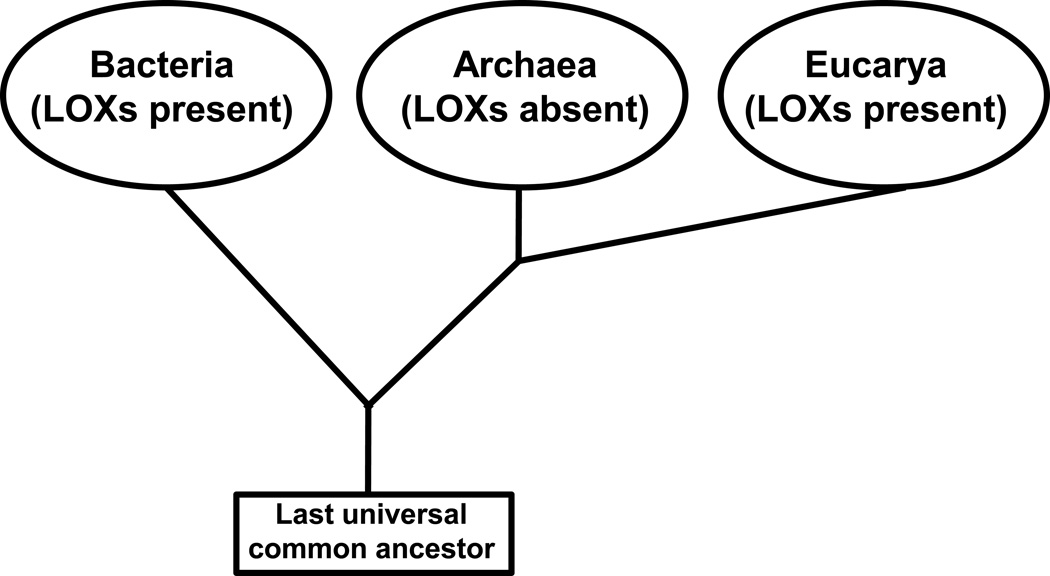
LOX occur in two (bacteria, eukarya) of the three domains of terrestrial life [1, 15] but their occurrence in archaea remains unclear (Fig. 3). The genomic sequences of selected archeae (Methanococcus voltae, Halorubrum kocurii) also contain LOX-like sequences but in the absence of any functional data it remains unclear if these sequences encode for a functional LOX-isoform. When we performed multiple amino acid alignments of these putative LOX sequences with the primary structure of well-characterized pro- and eukaryotic LOXs we observed only low (<25%) degrees of amino acid conservation. Moreover, we did not find conservation of the iron liganding residues suggesting that the sequences of interest may not encode for functional LOXs. The occurrence of LOX in single cell organisms, in plants and lower metazoa [15–17] has been reviewed before but distribution of LOX isoforms in multicellular vertebrates has not been summarized systematically.
Fig. 3. Distribution of lipoxygenases in the kingdoms of terrestrial life.
Lipoxygenase genes have been detected in two (bacteria, eukarya) of the three kingdoms of terrestrial life. Although LOX-like sequences have also been described in archaea, no functional LOX enzyme has been reported to occur.
2.1. Classification of mammalian lipoxygenases and LOX genes
The human genome involves six functional LOX genes (ALOX15, ALOX15B, ALOX12, ALOX12B, ALOXE3, ALOX5), which encode for six different LOX-isoforms [18]. Except for the ALOX5 gene, which was mapped to chromosome 10, all other LOX genes are localized in a joint gene cluster on chromosome 17. The corresponding mouse genes [18] were detected in syntenic regions on chromosome 6 (alox5) and 11 (other LOX-isoforms). Originally, the human LOX isoforms were classified with respect to their specificity of arachidonic acid oxygenation but this nomenclature turned out to be misleading and caused confusion among scientists not working in the LOX field [1]. These days the gene nomenclature is frequently employed to define the LOX isoenzyme and Table 1 summarizes human and murine LOX-isoforms and assigns names of the genes to the different isoenzymes. For this review we will use the names of the genes also when we talk about the corresponding enzymes. To differentiate between genes and proteins we employ italic letters when referring to enzymes but use normal letters when referring to the genes.
Table 1.
Human ALOX genes and major expression sites of the corresponding LOX-isoforms
| Human gene | Former name | Mouse gene | Former name | Major expression | References |
|---|---|---|---|---|---|
| ALOX15 | 12/15-LOX | alox15 | Lc12-LOX* | eosinophils, bronchial epithelium | [19–21, 78] |
| ALOX15B | 15-LOX2 | alox15b | 8-LOX | hair roots, skin, prostate | [22, 23] |
| ALOX12 | pl12-LOX** | alox12 | pl12-LOX | thrombocytes, skin | [24, 25, 92] |
| ALOX12B | 12R-LOX | alox12b | 12R-LOX | skin, | [26, 27] |
| ALOXE3 | eLOX3 | aloxe3 | eLOX3 | Skin | [28–30] |
| ALOX5 | 5-LOX | alox5 | alox5 | leukocytes, macrophages, dendritic cells | [114, 120, 138, 417] |
| pseudogene | alox12e | elox12 | Skin | [18] | |
lc – leukocyte-type,
pl – platelet-type
The ALOX15 gene encodes for the 12/15-LOX, which is expressed at high levels in eosinophils [19], broncho-alveolar epithelial cells [20] and interleukin 4 treated monocytes [21]. The ALOX15B gene encodes for 15-LOX2, which is highly expressed in epithelial cells [22, 23]. The ALOX12 gene encodes for the platelet-type 12-LOX (pl12-LOX), which is expressed at high levels in blood platelets [24] but also occurs in the skin [25]. The ALOX12B gene [26, 27], which encodes for a 12R-lipoxygenating enzyme and the ALOXE3 gene [28, 29] encode for two distinct epidermis-type LOX isoforms, which are co-expressed in the skin. These enzymes have been implicated in epidermal differentiation [30] and appear to be important for the development of the epidermal water barrier [31, 32]. The ALOX5 gene encodes for a 5-lipoxygenating enzyme, which plays a major role in leukotriene biosynthesis [2, 33].
In mice the situation is somewhat different (Table 1). Mouse Alox12, Alox12b, Aloxe3 and Alox5 share high degrees of amino acid conservations with their human orthologs and exhibit similar enzymatic properties. However, this is not the case for mouse Alox15 and mouse Alox15b. In fact, mouse Alox15 is a 12-lipoxygenating enzyme converting arachidonic acid mainly to 12S-HpETE [34]. In contrast, the human ortholog exhibits a 15-lipoxygenating activity [35]. Because of its reaction specificity and its high-level expression in murine leukocytes mouse Alox15 has previously been named leukocyte-type 12-LOX but this nomenclature should not be used any more. In general, LOXs, which have previously been named leukocyte-type 12-LOXs [mice [34], rats [36], pigs [37] cattle [38], macaca [39] and other mammals), should be classified as 12-lipoxygenating ALOX15 isoforms. Analysis of the completely sequenced genomes of these and additional mammalian species did not provide any evidence for the simultaneous existence of separate ALOX15 and leukocyte-type 12-LOX genes in a single mammalian species. Even in rabbits, where 15- and 12-lipoxygenating ALOX15 variants are expressed [40], only a single copy ALOX15 gene exists. For the time being it remains unclear how a single ALOX15 gene is able to encode in a tissue specific manner [40] for two functionally distinct enzyme species, but post-translational mRNA modification [41] might be involved. It should explicitly be stressed here that in humans there is a single copy ALOX15 gene but there is no additional gene encoding for a leukocyte type 12-LOX. On the other hand, mice, rats, pigs, cattle, macaca and others express 12-lipoxygenating ALOX15 isoforms. The molecular basis for the variable reaction specificity of ALOX15 orthologs from different species has been explored in detail [39, 42] and multiple mutagenesis studies have indicated that single amino acid exchanges at critical positions convert the 15-lipoxygenating human ALOX15 into a 12-lipoxygenating isoform [1, 43]. Inversely, the 12-lipoxygenating mouse Alox15 (formerly called mouse leukocyte-type 12-LOX) can easily be converted into a 15-lipoxygenating enzyme by L353F exchange [44].
Human ALOX15B converts arachidonic acid almost completely to 15S-HpETE [22]. In contrast, the mouse ortholog, which shares a high degree of overall amino acid conservation with the human enzyme, exhibits an arachidonic acid 8S-lipoxygenating activity [23]. Site directed mutagenesis of Tyr603 and His604 of human ALOX15B to the corresponding residues present at these positions in murine Alox15b (Tyr603Asp+His604Val) leads to a complete shift in the positional specificity of arachidonic acid oxygenation from 15S-HpETE to 8S-HpETE formation [45]. The inverse mutagenesis strategy starting with human ALOX15B leads to partial alterations in the reaction specificity [45]. When we compared (data not shown) the ALOX15B amino acid sequences of different mammals (man, chimpanzee, gorilla, orangutan, macaca, baboon, cattle, pigs, rat) we found that all of them share the human motif (Asp-Val or Asp-Ile). Only mice have a Tyr-His combination at these positions. Thus, among mammals mice are somewhat unique and although not tested for other mammals arachidonic acid 15-lipoxygenation may be predicted for other (chimpanzee, gorilla, orangutan, macaca, baboon, cattle, pigs, rat) mammalian ALOX15B orthologs. It would be of mechanistic interest to experimentally test this prediction and explore in more detail the biological background of this unusual reaction specificity of mouse Alox15b.
2.2. Enzymatic properties of mammalian lipoxygenases
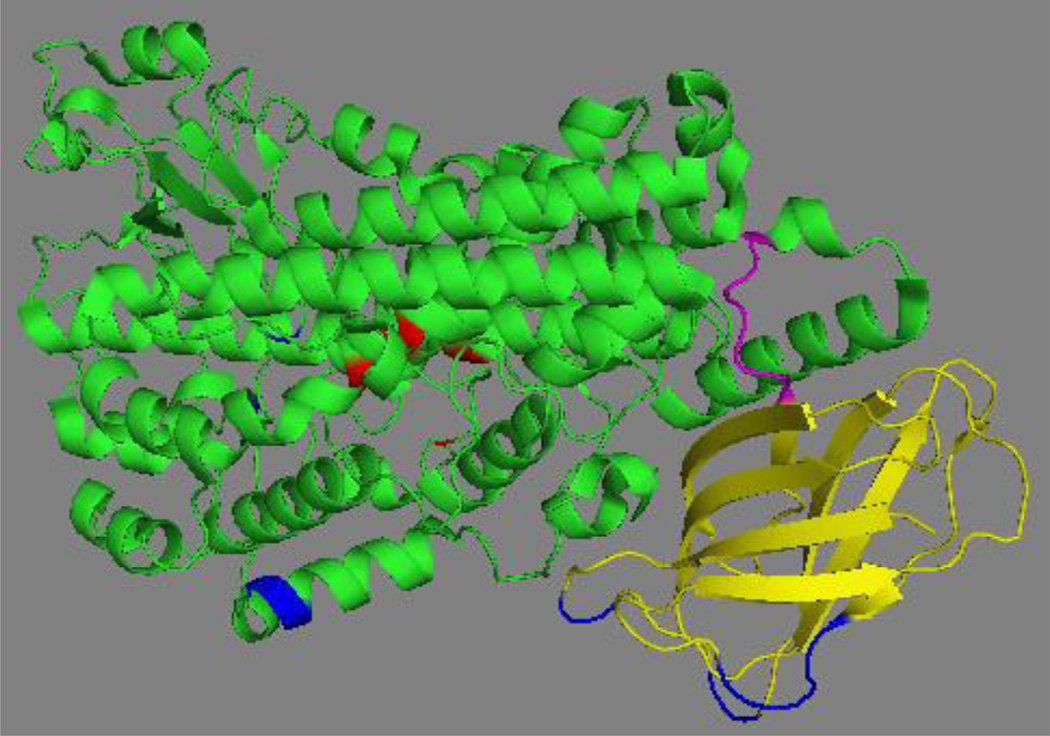
Mammalian LOXs are single polypeptide chain proteins that fold into a two-domain structure (Fig. 4). The small (about 15 kDa) N-terminal domain consists of several parallel and anti-parallel β-sheets and has been implicated in activity regulation and membrane binding. The C-terminal catalytic domain consists of several helices and contains the catalytic nonheme iron localized in the putative substrate-binding pocket. For mammalian LOXs complete crystal structures are currently available for rabbit ALOX15 [46, 47], which serves as a suitable model for the human ortholog; for a stabilized version of the human ALOX5 [48]; for the catalytic domain of porcine ALOX15 [49]; and for human ALOX15B [50]. In addition, X-ray data have been published for a phosphorylation-mimicking mutant (Ser663Asp) of the stabilized version of human ALOX5 [51]. However, these data need to be interpreted with care since the functional consequences of the phosphorylation mimicking mutations (Ser663Asp exchange converts the reaction specificity of the stabilized human ALOX5 from 5- to 15-lipoxygenation) could not be confirmed for native ALOX5 orthologs of man, mice and zebrafish [52].
Fig. 4. Crystal structure of the stabilized version of human ALOX5.
The N-terminal β-barrel domain is shown in yellow, the flexible inter-domain linker (D113-L118) in magenta, the C-terminal catalytic domain in green and the iron liganding residues in red. The residues mutated in wild-type ALOX5 to get the stabilized version of the enzyme suitable for crystallization are indicated in blue. The image was constructed from the X-ray diffraction data using the PyMol software package.
In aqueous solutions the structure of proteins is less rigid than in crystals. To compare the degree of motional flexibility of rabbit ALOX15 and soybean-LOX1, small angle X-ray scattering (SAXS), dynamic fluorescence, and fluorescence resonance energy transfer measurements were carried out. The results suggest that rabbit ALOX15 is more susceptible to temperature-induced structural alterations and exhibits a higher degree of global conformational flexibility [53]. There are several processes contributing to global structural flexibility of rabbit ALOX15: i) Interdomain movement: Comparative SAXS measurements on aqueous solutions of recombinant rabbit ALOX15 and its catalytically active N-terminal truncation mutant (no N-terminal β-barrel domain) suggested the possibility of interdomain movement [54]. Such interdomain movement was not confirmed for the soybean enzyme [55]. Although SAXS data in general can be interpreted in different ways [56] more recent molecular investigations into the dimerization behavior of rabbit ALOX15 [57] and molecular dynamics simulations [58] confirmed the possibility of interdomain movement for this enzyme. For the solution structure of human ALOX12 a similar interdomain movement has been suggested [59]. ii) Alternative conformers: Reevaluation [47] of the X-ray data set obtained for the crystallized rabbit ALOX15 [46] suggested that the enzyme undergoes conformational changes when binding an inhibitor at the active site. Helices surrounding the catalytic center appear to relocate upon ligand binding. However, it remains unclear whether these structural rearrangements are peculiar to the active site probe (inhibitor) used for crystallization or whether binding of substrate fatty acids at the active site also induces similar structural alterations. iii) Allosteric properties: Human ALOX5 [60], ALOX15 and ALOX15B [61, 62] appear to exhibit allosteric properties. Although the binding sites for allosteric effectors have not been identified for ALOX15 and ALOX15B kinetic data suggest the existence of different enzyme conformers. Interestingly, the allosteric regulators of human ALOX15 do not affect the catalytic activity of the less flexible soybean LOX1 [63]. iv) Enzyme dimerization: For a long time LOXs have been suggested to function as monomeric enzymes. However, more recent data on recombinant human ALOX15 [64], human ALOX12 [59] and human ALOX5 [65] suggests the existence of LOX dimers in aqueous solutions. Human ALOX15 may undergo ligand-induced dimerization and molecular dynamics simulations suggested that LOX dimers are surprisingly stable in the presence of substrate fatty acids. Introduction of negatively charged residues (Trp181Glu, His585Glu, LeuL183Glu, Leu192Glu) at the protein surface disturbs monomer interactions compromising the catalytic activity of the mutants [64]. In addition, the rabbit ALOX15 forms oligomers upon membrane binding [66].
The catalytic cycle of the LOX reaction involves four consecutive elementary reactions [1, 2]: i) hydrogen abstraction from a bisallylic methylene forming a carbon centered fatty acid radical, ii) rearrangement of the fatty acid radical, iii) introduction of molecular dioxygen forming a oxygen-centered peroxy radical, iv) reduction of the hydroperoxy radical to the corresponding anion. Hydrogen abstraction appears to be the rate-limiting step of the catalytic cycle and this elementary reaction involves hydrogen tunneling [67, 68]. Thus, LOXs may be considered quantum chemical enzymes and some aspects of the reaction mechanisms cannot adequately be described employing traditional thermodynamics.
3. Biological function of mammalian LOX isoforms
Following the classical concept of the arachidonic acid cascade LOXs exert their bioactivity via the formation of lipid mediators that regulate the functional phenotype of a given cell population (Fig. 2). However, at least two alternative concepts have been introduced to explain LOX functionality: i) Several LOX isoforms are capable of oxidizing complex ester lipids and even lipid-protein assemblies (biomembranes, lipoproteins) modifying their structural and functional parameters. This concept is at least in part applicable for the roles of different LOX isoforms in erythropoiesis, epidermal differentiation and atherogenesis (see 3.1.1., 3.1.2., 3.3.2.2.). ii) LOXs are lipid peroxidizing enzymes and by forming lipid peroxides they modify the cellular redox state. Since the cellular redox equilibrium is an important regulator of cell proliferation and gene expression (3.1.3.) intracellular LOX-activity may impact cell functionality. Of course, LOXs are not the only enzymes modifiying the cellular redox state since a large number of pro- and anti-oxidative enzymes exist in mammalian cells. However, the catalytic activity of LOX clearly contributes to cellular redox homeostasis.
3.1. Lipoxygenases in cell development and proliferation
3.1.1. ALOX15 in erythropoiesis
Normal erythrocytes and their immediate precursors (reticulocytes) do not contain sizable amounts of ALOX15. However, when erythropoiesis is challenged in rabbits [69] by either repeated bleeding or forced hemolysis (phenylhydrazine injection) reticulocytes express large amounts of ALOX15. In fact, rabbit reticulocytes are the richest natural source of ALOX15 and model calculations suggested that up to 4 mg of ALOX15 protein is present in 1 ml of packed reticulocytes [70]. Interestingly, the enzyme is almost undetectable in young stress reticulocytes but during in vitro maturation of these cells expression of the enzyme parallels the maturational decline of cellular respiration [71]. These anti-parallel biological dynamics (increase in ALOX15 expression vs. decrease in cellular respiration) and the observation that isolated ALOX15 in vitro induces structural decomposition of rat liver mitochondria [72] implicated ALOX15 in maturational breakdown of mitochondria during late erythopoiesis. Consistent with this hypothesis, oxidation products formed by ALOX15 were found in reticulocyte membranes [73]. In vitro studies with the isolated rabbit ALOX15 showed that the enzyme does not just bind to mitochondrial and other organelle membranes and oxidize the membrane lipids [74], but also directly permeabilizes them, forming pores in the membrane [66]. Freshly isolated reticulocytes matured in vitro degrade their mitochondria more slowly in the presence of a LOX inhibitor [75–77]. However, functional inactivation of the Alox15 gene in mice did not lead to major functional defects in erythropoiesis [78], and we (Kühn, unpublished results) did not find significant differences in the standard hematological parameters (erythrocyte count, Hb, HK, MCHC, MCV) of Alox15-deficient mice when compared with Alox15-sufficient controls. In addition to intracellular degradation initiated by ALOX15 [70], there are two competing hypotheses for how mitochondrial degradation occurs in erythroid cells [79]: i) engulfment and digestion within autophagic vacuoles [80]; and ii) exocytosis of mitochondria within exosomes [81]. There is experimental support for all three scenarios, but none of the proposed mechanisms appears to provide a complete answer [82]. It may simply be that inhibition of one pathway can be compensated for by one or both of the others. Indeed, ALOX15 inhibition leads to an increase in autophagic vacuoles in cultured cells, and in the livers of Alox15 knockout mice in vivo [83]. Furthermore, exosome formation by in vitro matured reticulocytes is impaired by addition of a LOX inhibitor [83], suggesting there is considerable crosstalk between these pathways. Further studies of erythropoiesis under both stressed and non-stressed conditions are needed to investigate the relative contribution of each of the three mitochondrial degradation pathways.
3.1.2. Lipoxygenases in epidermal differentiation and skin development
ALOX12B (12R-LOX) and ALOXE3 (eLOX-3) have been implicated in late epidermal differentiation, particularly in maintenance of the Stratum corneum [84, 85]. Mammalian skin is composed of three principal layers (epidermis, dermis, subcutis) and the Stratum corneum constitutes the outermost layer of the epidermis. Its major function is to protect the organism from infection, irritants, and from loss of water. The Stratum corneum consists of specialized cells (corneocytes), which are according to the brick and mortar model [86] imbedded in a compact extracellular matrix consisting of cross-linked proteins and special extracellular lipids. Like red blood cells, corneocytes are anucleated cells, which do not contain intracellular organelles [87]. They originate from interfollicular epidermal stem cells localized in the Stratum basale of the epidermis and mature via keratinocytes into corneocytes. During this maturation process the cells migrate perpendicularly through the epidermis and their journey towards the surface of the skin takes approximately 14 days. In more basal layers of the Stratum corneum corneocytes are bridged together through specialized junctions (corneodesmosomes) but these junctions disintegrate as the cells mature resulting in desquamation. Corneocytes are characterized by the cornified envelope, which is formed beneath the plasma membrane [88, 89]. It consists of a 10 nm thick layer of highly crosslinked insoluble proteins and a 5 nm thick layer of ceramide lipids that are covalently bound to the proteins. Ceramides also occur in the extracellular space and here they organize the extracellular lipids into orderly lamellae. Together, the cornified envelope and extracellular lipid lamellae, are essential for effective physical and water barrier function in the skin [90].
In normal mouse skin five different LOX-isoforms (Alox15b, Alox12, Alox12b, Aloxe3, Aloxe12) are expressed. Targeted inactivation of the Alox12 [91], Alox12b [84] and Aloxe3 [85] genes led to an impaired water barrier function of the skin. Alox12 knockout mice are viable and reproduce normally [92] and thus, defective function of this enzyme is likely to be compensated by the other LOX-isoforms. In contrast, Alox12b and Aloxe3 knockout mice die shortly after birth because of rapid dehydration [84, 85].
In humans autosomal recessive congenital ichthyosis (ARCI) is a group of skin diseases, which is characterized by intense scaling [93]. Naturally occurring mutations in the ALOX12B and ALOXE3 genes have frequently been detected in ichthyosis patients [94, 95]. In a large group of 250 ARCI patients [96] 11 previously unidentified mutations have been described in the two LOX genes in 21 ARCI patients from 19 unrelated families. These data indicated that mutations in the two genes are the second most common cause for ARCI in this patient cohort. More detailed analysis of the sequence data revealed a high allelic heterogeneity for the ALOX12B gene, and two mutational hotspots in the ALOXE3 gene have been identified. Functional characterization of these mutations indicated a loss of catalytic activity suggesting a causal relation between the loss-of-function mutations and pathogenesis [96]. Unfortunately, the frequency of functionally deficient ALOX12B and ALOXE3 mutants in the average population (not ARCI patients) has not been determined. Of course, ALOX12B and ALOXE3 deficiencies are not the only reason for ARCI and mutations in other genes such as transglutaminase-1 do also contribute [97].
The question why defective ALOX12B and ALOXE3 expression in man and mice induces impaired formation of the water barrier of the skin is still a matter of discussion. The current understanding of the molecular mechanisms [30, 31, 98] is that under normal conditions ALOX12B catalyzes oxygenation of skin specific ceramides to their corresponding hydroperoxídes. These hydroperoxy lipids are subsequently converted by the hydroperoxide isomerase activity of ALOXE3 [99] to hepoxilin-like secondary lipid peroxidation products [6]. This oxidative modification triggers preferential removal of the oxidized linoleate moieties from the ceramides resulting in the formation free ω-hydroxyceramides. These reactive lipids may subsequently be linked covalently to proteins contributing to the formation of the cornified envelope [88, 89]. Although there are still some mechanistic caveats (e.g. which lipid hydrolyzing enzymes prefer hepoxilin-containing ceramides over the non-oxidized counterparts) this pathogenetic scenario is supported by several lines of experimental observations [98]: i) Murine epidermis contains oxidized linoleate containing ceramides, which are almost absent in the skin of Alox12b knockout mice. ii) The oxidized linoleate residues in the ceramide lipids are chiral suggesting their enzymatic origin. iii) Covalently cross-linked ceramides in the epidermis of Alox12b knockout mice are severely reduced in the epidermis of these animals. iv) Aloxe3 knockout mice show a less severe phenotype when compared with Alox12b-deficient animals, which was associated with a reduction in covalently bound ceramides.
3.1.3. Lipoxygenases in cell proliferation and carcinogenesis
The role of LOX isoforms in cell proliferation and carcinogenesis appears to be very complex and the observed effects are sometimes controversial. ALOX5 metabolites, such as 5-HETE, its oxidation product 5-oxo-ETE and peptido leukotrienes, stimulate cell proliferation and thus, may act as pro-carcinogenics [100]. 12-HETE, the major arachidonic acid oxygenation product of ALOX12 and ALOX12B, which is also formed in smaller amounts by ALOX15 orthologs, also exhibits pro-carcinogenic activities stimulating cell adhesion, metastasis and neoangiogenesis [101]. These data are consistent with the observation that overexpression of ALOX15 in human prostate cancer cells increases tumorgenesis [102]. In contrast, the major ALOX15 and ALOX15B metabolite of linoleic acid oxygenation (13-HODE) induces apoptosis in human colorectal cancer and thus, exhibits anti-carcinogenic properties [103]. Expression of ALOX15B is reduced in human prostate, esophageal and skin carcinoma [104–106] and the enzyme was suggested as tumor suppressor protein [107]. It should, however, be stressed that there is no uniform expression regulation of different LOX isoforms in all types of malignancies. In contrast, expression regulation strongly depends on the kind of tumor and perhaps on its developmental stage. For instance, the enzymatic activity of ALOX15 is down-regulated in colorectal carcinoma [108] but up-regulation was observed in prostate cancer [109].
If one reviews the relevant LOX literature four (ALOX15, ALOX15B, ALOX12, ALOX5) of the six human LOX isoforms have been implicated in regulation of cell proliferation and carcinogenesis. There are a number of reports exploring the roles of these LOX isoforms in different types of cancer [110–115], but the majority of the reports implicate LOXs in colorectal [116] and prostate carcinoma [117]. Thus, for this review we will focus on these two types of cancer. Carcinogenesis is a complex process that involves increased cell proliferation, reduced apoptosis, tumor associated neoangiogenesis, up-regulation of cellular adhesion and invasiveness (metastasis) as well as down-regulation or circumvention of immunological defense reactions. In all of these processes LOX isoenzymes have been implicated [118] but there is no unifying concept for the biological roles of the different LOX isoforms.
3.1.3.1. Lipoxygenases in colorectal cancer
Comparison of the steady state concentrations of LOX metabolites in normal, polyp and colorectal cancer mucosa did not identify significant differences in 12-HETE, 15-HETE and leukotriene B4 levels [119]. However, the tissue concentrations of 13S-HODE declined across this progressive sequence. In a separate study a strong correlation between ALOX5 expression and increased polyp size as well as higher tumor grade suggested a role for this enzyme in early stages of colon cancer [120]. These data are consistent with the overexpression of ALOX5 in colon polyps and carcinoma tissue described in another study [121]. Summarizing these results it was concluded that ALOX5 expression is an early event in the mechanistic sequence leading to colon cancer, with increased expression in adenoma, while ALOX12 expression appears to be a later event, possibly mediating invasion and metastasis. The products of the ALOX5 pathway (leukotrienes) have been suggested to induce their biological effects as endocrine or paracrine mediators via binding at cell surface receptors of surrounding cells. For instance, leukotriene B4 (LTB4) regulates colon cancer growth via binding at the BLT1 receptor. A BLT1 receptor antagonist, and siRNA-induced expression silencing of this protein suppressed LTB4-induced cell proliferation [122]. On the other hand, autocrine signaling mechanisms, such as activation of endogenous transcription factors, have also been described for various LOX products [123, 124].
The role of ALOX15 in colorectal carcinoma has been a matter of discussion for several years and still the picture is not clear. Transfection of HCT116 colon carcinoma cells with ALOX15 induced activation of the ERK protein kinase led to increased rates of cell proliferation. These data suggest a pro-carcinogenic activity of the enzyme [125]. Treatment of these cells with NDGA, a non-specific LOX inhibitor with antioxidant properties, appeared to block ERK activation, which is consistent with the pro-carcinogenic activity of ALOX15 [125]. The underlying mechanisms have not been explored in detail but it might well be that NDGA-induced cell cycle arrest is related to an off-target effect of this compound. NDGA is a potent antioxidant and interferes with the redox state of the cells. Since cell cycle regulation is impacted by the redox equilibrium [126] the observed anti-proliferative effect might not directly be related to ALOX15 inhibition. In similar cellular models of colorectal carcinoma (HCT116, HT29) ALOX15 exhibited anti-carcinogenic properties, which was related to inhibition of the anti-apoptotic effect of the inflammatory transcription factor nuclear factor kappa B [127]. Here again, the molecular basis for the observed anti-carcinogenic affect is not completely understood but overexpression of ALOX15 inhibited the degradation of the inhibitor of kappa B, impaired nuclear translocation of p65 and p50, decreased DNA binding in the nucleus and reduced the transcriptional activity of NF-κB [128]. Unresolved chronic inflammation is a key process in tumor progression and thus, pro-resolving lipid mediators (eicosanoids and related metabolites of other polyenoic fatty acids) such as lipoxins [129], resolvins [130] and maresins [131] need to be discussed as regulators of carcinogenesis [132]. Resolving eicosanoids are generally believed to exhibit anti-tumor activities [133]. Chronic inflammation of colonic mucosa creates a pro-carcinogenic milieu and patients suffering from ulcerative colitis exhibit defective lipoxin biosynthesis [134]. Thus, the lack of pro-resolving mediators may drive carcinogenic transformation of normal epithelial cells during chronic inflammation. On the other hand, under certain conditions these mediators may also act in a pro-carcinogenic manner. For instance, depletion of regulatory T cells induced by cyclophosphamide treatment of patients with large established tumors caused significant tumor progression and this effect was suggested to be mediated by an increase in lipoxin A4 levels [135].
3.1.3.2.Lipoxygenases in prostate cancer
Four LOX isoforms (ALOX5, ALOX15, ALOX15B, ALOX12) have been implicated in the pathogenesis of prostate cancer and pro- as well as anti-carcinogenic effects have been reported. As pro-inflammatory enzyme ALOX5 was suggested to be pro-carcinogenic. The enzyme is overexpressed in prostate adenocarcinoma [136] but the molecular mechanisms for the pro-carcinogenic effects are not completely understood. Inhibition of the enzyme triggers apoptosis in different types of prostate cancer [137–139] and pharmacological interference with other constituents of leukotriene signaling induced similar effects [140]. For instance, the cysteinyl leukotriene receptor 1 (cysLTR1) is overexpressed in prostate cancer and cysLTR1 antagonists inhibit prostate cancer cell growth by up-regulating apoptotic cell death [141]. Moreover, downregulation of the OXE receptor for 5-oxo-ETE reduced prostate cancer cell survival [142].
ALOX15 is expressed at variable levels in different prostate carcinoma cell lines [143] and formation of 13-HODE suggested functional activity of the enzyme [144]. Forced overexpression of ALOX15 in cultured human prostate cancer cells augmented the rate of cell proliferation and subcutaneous transplantation of ALOX15-transfected PC3 cells into athymic nude mice increased the frequency of tumor formation and tumor size [102]. Similarly, conditional expression of human ALOX15 in mouse prostate induces prostatic intraepithelial neoplasia [145]. Taken together, these data suggest a pro-carcinogenic character of this enzyme. On the other hand, ALOX15-mediated metabolism of docosahexaenoic acid is required for apoptosis in prostate cancer cells [146] and ALOX15 metabolites of docosahexaenoic acid inhibit prostate cancer cell proliferation and cell survival [147]. Hence, in the presence of DHA the enzyme might exhibit anti-carcinogenic properties.
ALOX15B is expressed in normal human adult prostate and its expression is impaired in prostate intraepithelial neoplasia and in prostate cancer [148]. In normal prostate cells the enzyme has been identified as negative cell cycle regulator and consequently, a function of ALOX15B as tumor suppressor has been suggested in prostate carcinoma [149]. Although the molecular basis for the tumor suppressive activity is not completely understood some mechanistic scenarios have been suggested. 15S-HETE inhibits proliferation in PC3 prostate carcinoma cells and this effect involves activation of peroxisome proliferator-activated receptor gamma by the ALOX15 product 15S-HETE [150]. Since 15-HETE can also be formed by ALOX15 these two enzymes may contribute to this protective effect. Expression of ALOX15B is cell-autonomously up-regulated in cultured prostate cells, and induction of enzyme expression was associated with cell senescence [151]. Moreover, transgenic expression of human ALOX15B in mouse prostate leads to hyperplasia and cell senescence [152]. It should be stressed at this point that the product specificity of mouse and human ALOX15B are different. For the human enzyme 15-HETE is the exclusive arachidonic acid oxygenation product [22], whereas mouse alox15b makes 8-HETE [23]. Thus, induction of cell senescence by ALOX15B overexpression may not be related to the formation of arachidonic acid oxygenation products. Expression of ALOX15B is tightly regulated in prostate cancer and several mechanisms have been described. The reaction product of human ALOX15B catalyzed arachidonic acid oxygenation (15-HETE) activates enzyme expression via activation of peroxisome proliferator activated receptor gamma (PPARγ) [153]. However, it remains unclear if similar observations can be made in murine experimental setups. Because of the different reaction specificities of human and mouse ALOX15B 8-HETE should be the preferred activator of murine PPARγ. Unfortunately, to the best of our knowledge such experimental data are currently not available. In addition, the tumor suppressive effects of ALOX15B may be related to the down-regulation of vascular endothelial growth factor in prostate carcinoma and to induction of tumor dormancy by the enzyme [154]. In other words, loss of ALOX15B functionality was suggested to represent a key step for prostate cancer cells to exit from dormancy and embark on malignant progression [154]. In prostate cancer expression of ALOX15B is strongly down-regulated but the underlying molecular mechanisms are not completely understood. Recently, it has been reported that glucocorticoid signaling may be involved in ALOX15B expression regulation and these data might be relevant for prostate cancer [155].
ALOX12 exhibits pro-carcinogenic activities in the prostate. It stimulates tumor growth and neoangiogenesis [101], increases the metastatic potential [156] and promotes tumor cell survival [157]. Although the mechanistic reasons for the tumor-promoting effects have not been studied in detail this activity might involve NFκB signaling [158, 159].
Most naturally occurring polyenoic fatty acids serve as LOX substrates and the biological activity of the products formed from a certain fatty acid may differ from that formed by the same LOX isoform from a different fatty acid. For instance, the major ALOX15 product of arachidonic acid metabolism (15-HETE) might exhibit different biological effects than the major ALOX15 product of docosahexaenoic acid (17-HDHE). Thus, the alimentary supply of polyenoic fatty acids might modify the overall character of LOX isoforms in prostate cancer [160–162].
3.2. Lipoxygenases in inflammation
According to the classical concept of the arachidonic acid cascade LOXs are key enzymes in the biosynthesis of linear eicosanoids and related mediators originating from other polyenoic fatty acids (leukotrienes, lipoxins, resolvins, maresins, hepoxilins, eoxins etc.) and these compounds [6–11] been implicated as promoting and/or protecting against pathogenic inflammation. However, the patho-physiological role of LOX may not be restricted to the formation of signaling lipids. LOXs are lipid peroxidizing enzymes and their catalytic activity may impact the cellular redox state. Since the redox state is an important regulator of the cellular gene expression pattern the catalytic activity of the enzyme may alter the functional phenotype of mammalian cells [163]. In fact, transfection-induced overexpression of ALOX15 in U937 cells alters the gene expression pattern (GSE8173) but the physiological consequences of these expression alterations have not been characterized in detail.
3.2.1. Pro-inflammatory properties of lipoxygenases
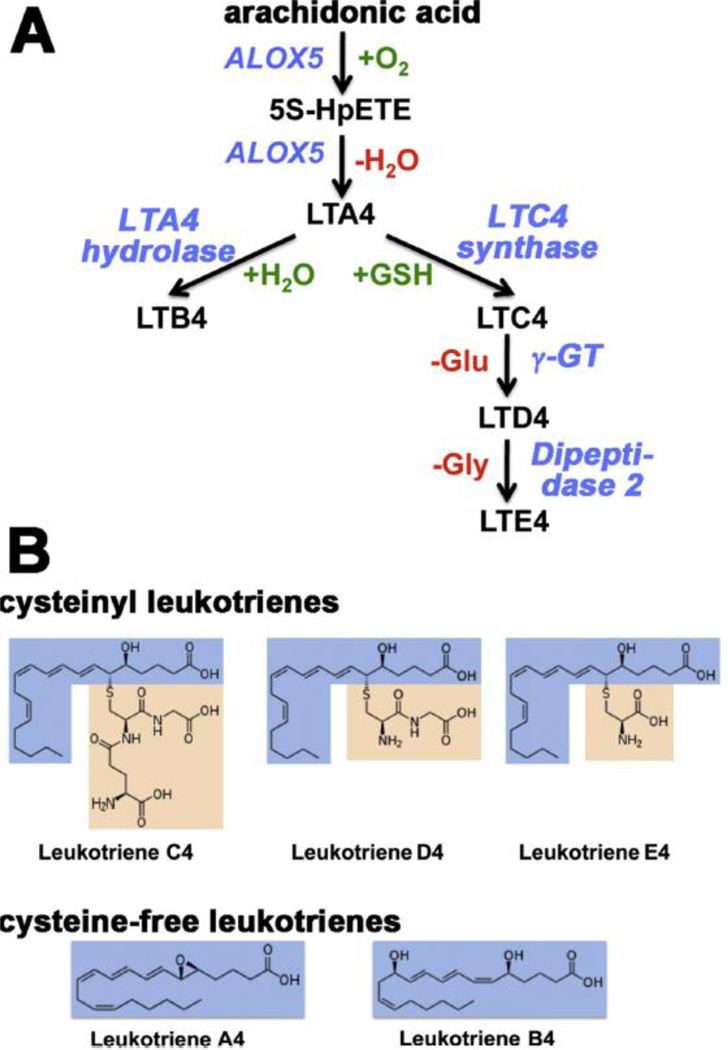
Inflammation is a protective response of the organism aimed at fighting inflammation inducers. This fight requires a balanced activity of various cellular and humoral constituents of the adaptive and innate immune system, and inflammatory mediators have coordinating functions. There is a large array of lipid and non-lipid mediators regulating acute inflammation as well as its inflammatory resolution. Leukotrienes (LT) are classical pro-inflammatory mediators originating from the ALOX5 pathway [2, 164]. They are biosynthesized in different types of leukocytes and other immune competent cells from free arachidonic acid. The key enzyme in the biosynthetic cascade is ALOX5 [2, 165], which catalyzes the first two steps of leukotriene biosynthesis (oxygenation of arachidonic acid to 5S-HpETE and conversion of 5S-HpETE to LTA4). The leukotriene biosynthetic cascade has extensively been reviewed before [33, 166] and most enzymes and regulators involved in this pathway have been well characterized with respect to their structural and functional properties [2]. There are two principal classes of LTs (peptido LTs and peptide-free LTs) and additional non-LT ALOX5 products such as 5-HETE, and 5-oxo-ETE [167]. The various LTs (Fig. 5) exhibit different bioactivities and preferentially act on different cells types:
Peptido-LTs: The cysteinyl LTs (LTC4, LTD4, LTE4) are constituents of the slow-reacting substance of anaphylaxis and play an important role for the pathogenesis of allergic diseases, such as bronchial asthma [168], rhinitis [169] and allergic eye disease [170]. On the molar basis cysLTs are at least 1000-times more effective as bronchoconstrictor than histamine [171], and nanomolar concentrations of cys-LTs cause plasma leakage and cell adherence in postcapillary venules leading to bronchial edema [172]. In addition, cysteinyl LTs induce mucus secretion in vitro and in vivo [173, 174] and may also alter mucus viscosity [174]. Taken together, these effects implicated leukotrienes in the pathogenesis of pulmonary dysfunction, and after leukotriene synthesis inhibitors [175] and leukotriene receptor antagonists [176, 177] became available as drugs, anti-leukotriene therapy has been employed in the clinic to supplement glucocorticoid-base therapeutic schemes [178]. Nevertheless, as monotherapy, inhaled corticosteroids display superior efficacy to anti-leukotrienes in patients with persistent asthma and this superiority is particularly evident in patients with moderate airway obstruction [179]. Asthma patients who continue to experience symptoms despite being on regular inhaled corticosteroids represent a management challenge, and long-acting beta(2)-agonists or anti-leukotrienes are two treatment options that could be considered as add-on strategies. Direct comparison of these two therapeutic approaches suggest that long-acting beta(2)-agonist treatment might be superior to anti-leukotriene therapy in reducing oral steroid treated exacerbations [180]. However, the differences in lung function and quality of life scores were rather moderate but there was evidence of increased risk of serious adverse events under long-acting beta(2)-agonist treatment. In general, the beneficial effects of anti-leukotriene therapy have recently been challenged. On one hand, this therapeutic concept brings remarkable ease of anti-inflammatory treatment, administration and symptom improvement with minimal side effects to the management of adult asthma [181]. On the other hand, it was suggested to limit anti-leukotriene therapy to asthmatics, who are refractory to inhaled corticosteroids or cannot use inhalant devices. Considering the low incidence of these circumstances oral anti-leukotrienes should be more carefully considered for treating asthma in the clinical environment but several clinically relevant issues (effects of anti-leukotriene medication on peripheral airways and on airway remodeling, alternative administration concepts) remain to be clarified before anti-leukotriene therapy could serve as a more effective strategy in the treatment of bronchial asthma in adults [177]. In children the beneficial effects of anti-leukotriene therapy for bronchial asthma are even more difficult to evaluate because the number and the size of clinical trials carried out so far is rather limited [182]. The currently available data of randomized studies suggest that there is no firm evidence supporting the suggestion that adding leukotriene receptor antagonists (montelukast) to inhaled corticosteroid therapy is safe and effective to reduce the occurrence of moderate or severe asthma attacks in children taking low-dose inhaled corticosteroids [183]. After being on the market for more than 10 years, the limited number of available studies testing anti-leukotrienes in children, the absence of data on preschoolers, and the inconsistency of available trials reporting of efficacy and safety of clinical outcomes is disappointing and limit general conclusions [183]. However, considering the pro-inflammatory effects that leukotrienes have in experimental setups, it is rather surprising that the outcome of anti-leukotriene treatment are not better and that in some clinical trials only a minority of patients could be classified as full responders. This discrepancy might be explained by additional LT receptors that are not affected by the current drugs. In addition, there may be different phenotypes of bronchial asthma and some of them might involve LT to a lesser degree [182, 183].
Peptide-free LTs: Leukotriene B4, the major bioactive peptide-free leukotriene, is a strong endogenous stimulator of the innate immune response [184]. It is released from polymorphonuclear leukocytes, monocytes and macrophages and induces cell aggregation and increases vascular permeability [185]. It stimulates chemotaxis and adherence of neutrophils to the vascular wall [186, 187]. It also binds to two major types of cell surface receptors (BLT1, BLT2) and induces G-protein dependent intracellular signaling cascades leading to activation of inflammatory cells [188, 189]. Although LTB4 is one of the most powerful pro-inflammatory mediators, neither LTB4 synthesis inhibitors (inhibitors of ALOX5 or LTA4 hydrolase) nor BLT1/BLT2 receptor antagonist turned out to be effective anti-inflammatory drugs.
Fig. 5. Classification and structure of leukotrienes.
A) Leukotriene biosynthesis, B) Structure of leukotrienes.
3.2.2. Anti-inflammatory properties of lipoxygenases
Termination of acute inflammation was previously considered a passive process, which became possible because of the decay of pro-inflammatory signals. However, during the past decade there has been a change in this paradigm. Today we consider inflammatory resolution an active process, which proceeds according to a biological program, aimed at reestablishing normal tissue homeostasis [190]. Inflammatory resolution is initiated by alterations of the cellular composition in the inflamed tissue (neutrophils and pro-inflammatory M1 macrophages are replaced by anti-inflammatory M2 macrophages that clean up the battle field) and by a switch in inflammatory signaling. For instance, formation of pro-inflammatory lipid mediators (prostaglandins, leukotrienes) is down-regulated whereas biosynthesis of anti-inflammatory (proresolution) mediators is switched on. Endogenous pro-resolving lipid mediators include a number of LOX products such as lipoxins [7, 191], resolvins [192], protectins [11], maresins [193], and others. The specific interaction of the pro-resolution mediators with G protein-coupled receptors (GPCR 32, ALX, BLT1) on the surface of immune cells induces a number of pro-resolution processes. For instance, leukocyte migration is reduced [194], vascular permeability returns to normal [195], pro-inflammatory neutrophils undergo apoptosis [196] and M2 macrophages phagocytose apoptotic neutrophils, bacterial remnants and necrotic debris [197]. While adequate inflammatory resolution prevents tissue injury leading to restitutio ad integrum, inadequate resolution results in chronic inflammation.
The proresolving eicosanoids and docosanoids are multiple oxygenation products of three major polyenoic fatty acids (arachidonic acid, eicosapentaenoic acid, docosahexaenoic acid) that are biosynthesized by a concerted activity of various LOX isoforms with different positional specificity (ALOX12, ALOX15, ALOX15B, ALOX5). Since aspirin-treated COX2 exhibits a 15R-LOX activity [198] it also participates in the biosynthesis of these mediators [199] and the anti-inflammatory effect of aspirin may partly be related to this mechanism. In vivo, lipoxins are formed from arachidonic acid via transcellular biosynthetic mechanisms involving 5-, 12- and 15-lipoxygenating LOX isoforms [200]. However, LxB4 can also formed by double oxygenation of 15S-HETE methyl ester by purified rabbit ALOX15 alone [201]. Resolvins and protectins are biosynthesized from the omega-3 fatty acids mainly from eicosapentaenoic and docosahexaenoic acid. These fatty acids occur in high concentrations in marine organisms (fish oil) and their anti-inflammatory properties [202, 203] are well known. Lipoxins, they are biosynthesized via multiple oxygenations of arachidonic acid by aspirin treated COX-2 and/or several LOX-isoforms (ALOX12, ALOX15, ALOX15B). Protectins, previously called neuroprotectins since they were discovered in the brain [204], have been implicated in resolution of neuroinflammation. However, they also occur in peripheral tissues. Maresins are macrophage-derived mediators of inflammatory resolution [205], which are mainly formed from docosahexaenoic acid. Their name is an acronym for macrophage mediator in resolving inflammation, and as resolvins they exhibit potent anti-inflammatory properties. For instance, they prevent infiltration of pro-inflammatory neutrophils into inflamed tissues and stimulate phagocytosis of apoptotic neutrophils and cell debris by M2 macrophages [205]. In two different experimental models of arthritis systemic functional silencing of the ALOX15 gene induced uncontrolled inflammation and tissue damage. These data are consistent with an anti-inflammatory and tissue-protective role of the enzyme [206]. Although peritoneal macrophages of these animals produced significantly reduced levels of lipoxin A4 it remains unclear whether the formation of these pro-resolving mediators is the major reason for the anti-inflammatory effect. Alternatively, it was suggested that ALOX15 may play an important role in development of osteoclasts but here again the molecular mechanisms are not well understood [207].
The anti-inflammatory properties of certain LOX isoforms (ALOX12, ALOX15, ALOX15B) should not be limited to their involvement in the biosynthesis of pro-resolving eicosanoids since alternative concepts may also be applicable. The primary products of linoleic acid and arachidonic oxygenation by ALOX15 and ALOX15B (13S-HpODE, 15S-HpETE) exhibit anti-inflammatory activities in various inflammation models [208]. Moreover, LOX metabolites may activate PPAR signaling [209, 210] stimulating anti-inflammation via this pathway [211]. Oxidized phospholipids, which may be formed by ALOX15 catalyzed oxygenation of membrane lipids [74], are capable of preventing the binding of agonists to toll-like receptors and thus, prevent activation of the innate immune response [212]. Unilateral somatic gene transfer of ALOX15 in an experimental model of glomerulonephritis suppresses inflammation and preserved kidney function in the transfected kidney [213]. Although the mechanism of this effect has not been explored in detail the data are consistent with an anti-inflammatory effect of ALOX15.
3.3. Lipoxygenases in the cardio-vascular system
3.3.1. Lipoxygenases in blood pressure regulation and hypertension
15-lipoxygenating LOX-isoforms (ALOX15, ALOX15B) have been implicated in regulation of vascular tone and thus, may play a role in blood pressure regulation and hypertension [214–216]. More than 20 years ago it was reported that arachidonic acid induces endothelium-dependent relaxation of rabbit aorta [217]. Since this effect was not seen in the presence of the unspecific LOX inhibitor nordihydroguaiaretic acid LOX metabolites have been suggested as molecular inducers of vasorelaxation. Similar effects have been reported for bovine coronary arteries [218] and later on these metabolites have been identified as 11,14,15- and 11,12,15-trihydroxyeicosatrienoic acids [219]. Although the source of these metabolites has not been identified at this stage of research [219] later expression silencing studies [220] and adenovirus mediated somatic gene transfer [221, 222] suggested the involvement of ALOX15. Interestingly, chronic hypoxia and hypercholesterolemia enhanced ALOX15 mediated vasorelaxation in rabbit arteries [223, 224]. More direct evidence for the in vivo relevance of ALOX15 in blood pressure regulation was recently provided by experiments with ALOX15-deficient mice [225]. Although systolic blood pressures did not differ between these mice and wild-type controls Alox15−/−-mice exhibited higher resistance towards L-NAME- and high-salt-induced hypertension than corresponding controls. The ALOX15 inhibitor nordihydroguaiaretic acid attenuated this resistance suggesting the involvement of lipid peroxidation. The molecular basis for this effect has not been explored and it remains unclear whether or not it is related to the vasomotor properties of ALOX15 products. Interestingly, injection of wild-type peritoneal macrophages, which are a major source of ALOX15 in mice, into ALOX15-deficient animals abolished their resistance toward L-NAME-induced hypertension. Inversely, wildtype mice acquired resistance to L-NAME-induced hypertension after depletion of macrophages by clodronate injection [225].
3.3.2. Lipoxygenases in atherogenesis
Three different LOX-isoforms (ALOX5, ALOX15, ALOX15B) have been implicated in the pathogenesis of atherosclerosis [226–228]. ALOX5 and ALOX15B are expressed at high levels in advanced atherosclerotic lesions whereas ALOX15 mRNA was only present in small amounts [229]. However, low levels of lesional expression of ALOX15 in advanced human plaques do not necessarily exclude involvement of the enzyme in atherogenesis. If ALOX15 is involved in maturation and differentiation of macrophages it might contribute to atherogenesis without being expressed in the lesion: i) If the enzyme is involved in early stage of hematopoietic differentiation (monocyte/macrophage maturation), which proceeds in the bone marrow, functionally different macrophages are likely to be generated and thus, foam cell formation may be impacted. ii) If the enzyme is expressed in cells not present in the lesions it might contribute to systemic (not local) LDL oxidation, which is considered a risk factor for atherogenesis. iii) If ALOX15 is only involved in early stages of lesion formation [230, 231] it may be absent in advanced lesions but still might contribute to early stages of lesion development. In all these cases expression silencing and pharmacological intervention with ALOX15 pathway may impact lesion formation without lesional expression of the enzyme.
3.3.2.1. ALOX5, leukotriene signaling and vascular inflammation
Already 25 years ago the formation of LTB4 in human atherosclerotic lesions was demonstrated [232]. Moreover, in human atherosclerotic coronary arteries key enzymes of leukotriene biosynthesis (ALOX5, ALOX5AP, LTA4H) have been detected and the arteries exhibited a contractile response when challenged with LTC4 and LTD4 [233]. More recently, high levels of expression of all enzymes of the leukotriene biosynthetic cascade were found in human atherosclerotic plaques and the expression levels of ALOX5 and LTA4H correlated with symptoms of lesional instability [234].
In different murine atherosclerosis models variable and inconclusive data were obtained with respect to the patho-physiological relevance of leukotriene signaling. For instance, when fed a lipid rich diet ALOX5-deficient mice are not protected from lipid deposition in the vessel wall but show an increased tendency for the development of aortic aneurysms [235]. These data link the leukotriene pathway to inflammatory disturbance of vessel wall remodeling [236] rather than to lipid homeostasis However, in another mouse model of aneurysm formation (angiotensin II treatment) genetic and pharmacological interference with leukotriene biosynthesis did not show significant effects [237]. Deletion of the BLT1 receptor reduced lesion formation during early stages of plaque development, but was without any effect at more advanced disease stages [238]. On the other hand, BLT1 deficient mice were protected from aortic aneurysm formation in the angiotensin II model [239] and a selective BLT1 antagonist protected against the early phase aneurysm development [240]. For the time being it remains unclear what might be the reasons for the inconsistent effects observed in humans and mouse atherosclerosis models. It is well known that hyperlipidemic mouse atherosclerosis models do not adequately mirror all aspects of human atherosclerosis. Thus, more relevant animal atherosclerosis models (non-human primates) and more detailed clinical trials are required to assess the therapeutic potential of anti-leukotrienes therapy in treatment and prevention of human atherosclerosis, aortic aneurysms and myocardial infarction.
3.3.2.2. ALOX15 and lipoprotein modification
In the early 1990s the LDL oxidation hypothesis was introduced [241, 242] and its refined version was more recently critically reviewed [243]. This hypothesis suggested that oxidized LDL exhibits strong pro-atherogenic activities because it is rapidly taken up by macrophages via scavenger receptor mediated pathways. Since these pathways are not feedback-controlled excessive intercellular lipid deposition may occur and macrophages develop into lipid-laden foam cells. These cells then accumulate in the subendothelial space of the arteries to form fatty streaks which are considered early atherosclerotic lesions [244]. Since ALOX15 is capable of modifying LDL [245] and other lipoproteins [246] by oxidizing their ester lipids the enzyme has been implicated in atherogenesis. In atherosclerotic lesions of rabbits [231] and humans [230, 247] esterified specific LOX-products (mainly 13S-HODE) have been detected but the biosynthetic origins of these products have not been explored in detail. In particular, the question whether they are formed by ALOX15, ALOX15B, or alternative biosynthetic pathways has not been answered conclusively. Several studies employing ALOX15 deficient mice supported a pro-atheorgenic role of ALOX15 [248–253]. On the other hand, overexpression of ALOX15 in two rabbit and one mouse atherosclerosis models suggested anti-atherogenic effects [254–256]. In one of these studies it was suggested that ALOX15 activity in the local milieu afforded atheroprotection via the formation of proresolving lipid mediators [256] and this was later on suggested as more general paradigm [257]. Taken together, as discussed for the ALOX5 pathway the role of ALOX15 in atherosclerosis is controversial [258, 259] and this may be related to mechanistic differences of the various animal atherosclerosis models.
3.3.2.3. ALOX15B in atherogenesis
As indicated above gene expression studies in advanced human atherosclerotic lesions suggested high-level expression of ALOX15B [229, 260] and these data suggested a role of this LOX isoform in atherogenesis. Macrophage expression of ALOX15B has been reported under hypoxic conditions [261] and hypoxia inducible factor (HIF) has been implicated [260]. Catalytic activity of this enzyme was related to chemokine release [262] and the enzyme has been suggested as tissue marker for atherosclerotic carotid artery [263]. More recently, polymorphisms in the ALOX15B gene have been associated with coronary artery disease [228] but the underlying molecular mechanisms remain unclear. Functional characterization of the mutant enzymes did not reveal major defects and thus, ALOX15B products might not be involved [228]. Nevertheless, lesional expression of ALOX15B, which accepts arachidonic acid, linoleic acid and other polyenoic fatty acid as substrate [264, 265], may contribute to the formation of specific LOX products detected in the lesion lipids [230, 231, 247].
3.3.3. Lipoxygenase in platelet function and atherothrombosis
Two LOX isoforms (ALOX12, ALOX15B) have been suggested to impact platelet function and atherothrombosis. The first LOX-isoforms detected in animals was the ALOX12, which is present in large amounts in human platelets [266]. Unfortunately, the precise role of this enzyme for blood platelet physiology is still a matter of discussion since pro- and antithrombotic activities have been reported. Blood platelets of ALOX12-deficient mice exhibited an increased sensitivity for ADP-induced aggregation suggesting the ALOX12 pathway as down-regulator for platelet aggregation (anti-thrombotic effect) [92]. In contrast, more recent studies suggested that ALOX12-derived 12-HETE [267] and possibly other oxylipins [268] may play an important role as prothrombotic mediators in atherothrombosis. Although the molecular basis for the anti-thrombotic effect of the ALOX12 pathway has not been studied in detail at this time more recent data suggest the involvement of protein kinase C. To determine the functional interaction between protein kinase C and ALOX12 during platelet activation pharmacological interventions studies were carried out using specific modulators of the two pathways [269]. Separate inhibition of ALOX12 and PKC resulted in impaired secretion of dense granule and in attenuation of both aggregation and αIIbβ(3) activation. However, activation of PKC downstream of ALOX12 inhibition rescued agonist-induced aggregation and integrin activation. Inhibition of ALOX12 had no effect on PKC-mediated aggregation indicating that ALOX12 is localized upstream of PKC in the signaling cascade. Taken together these studies support an essential role for PKC downstream of ALOX12 activation in human platelets and suggest ALOX12 as a possible target for antiplatelet therapy [269]. In a similar study pharmacological interference with ALOX12 activity resulted in attenuation of platelet aggregation, selective inhibition of dense granule secretion, and inhibition of platelet adhesion [270]. ALOX12-deficient mice showed attenuated integrin activity. These data confirm the role of ALOX12 in regulating platelet function and thrombosis and provide the basis for the development of innovative strategies for the therapy of thrombosis [270, 271]. Moreover, dihydroxylated metabolites derived from alpha-linoleic acid inhibit platelet function [272] and a geometric isomer of protectin D also prevented platelet aggregation at submicromolar concentrations when induced by either collagen, arachidonic acid or thromboxane [273].
Although ALOX15B is not expressed in human platelets this enzyme has recently been implicated in the regulation of platelet functionality [274]. Impedance aggregometry indicated that the major oxygenation products of arachidonic acid conversion by ALOX15B (15-HETE, 15-HpETE) stimulated platelet aggregation. Moreover, platelet aggregation was augmented by the addition of cell lysates of ischemic human macrophages, which express large amounts of ALOX15B, whereas platelet aggregation was reduced when lysates of ALOX15B siRNA treated macrophages were used. These data suggest that ALOX15B expression in human plaques may be involved in thrombus formation [274].
3.4. Lipoxygenases in the central nervous system
3.4.1. Physiological roles of lipoxygenases in the CNS
3.4.1.1. Lipoxygenase expression in the CNS
ALOX15 is the major LOX isoform in both rat [275] and canine brain [276]. Various cell types in the brain express ALOX15 [276], but the expression levels under normal conditions are rather low. Depending on disease state and type of oxidative stress, it can also be up-regulated in different cell types (see below). In contrast to ALOX15, ALOX12 does not appear to be expressed in significant amounts in rat brain [275, 277]. The mouse ortholog of human ALOX12B was reported to be expressed in adult brain cortex, however its possible function there remains elusive [278]. In zebrafish, an atypical 12-lipoxygenating enzyme species was found to be essential for normal brain development, but it is at present unclear which mammalian LOX-isoform this enzyme corresponds to [279].
3.4.1.2. 12-HETE and 12-HpETE as second messengers of semaphorin signaling: interactions with the actin cytoskeleton and growth cone turning/collapse
Both 12-HETE and 12-HpETE have been implicated separately as signaling mediators in axon guidance, indicating a direct function of 12-lipoxygenating LOX isoforms in brain development [280–283]. These eicosanoids function as classic second messengers [284], relaying and amplifying the signal produced by external stimuli including the axon guidance molecule semaphorin 3A (Sema3A) [280]. We recently showed that both 12-HETE and 12-HpETE can function as potent messengers in the Sema3A pathway, with 12-HpETE being the more efficient [285]. 5-HETE was not able to replace 12-HETE and 12-HpETE in this assay. 12(R)-HETE and 15-HETE have not been tested. At present, the further components downstream of 12-HETE/12-HpETE are not clear; both direct binding of 12-HETE to the actin cytoskeleton [286], and the involvement of the monooxygenase MICAL (Molecule Interacting with CasL) as mediator [287] have been documented. A separate line of evidence suggests the involvement of a protein kinase C (PKCε and MARCKS [288].
In addition to these effects on neuronal architecture, ALOX15 influences synaptic signaling by its effects on long-term depression [289–291] and long-term potentiation [292, 293], which are core elements of interneuronal communication. The latter effect was mediated by 12-HpETE acting on L-type calcium channels [290]. Nonetheless, ALOX15 knockout mice do not show any overt behavioral defects, suggesting that either these effects can be bypassed, or the knockouts have found a way to compensate, for example by up-regulating one of the other 12-lipoxygenating isoforms. In line with this latter possibility, the brains of ALOX15(−/−) mice still generate 12-HETE, albeit at much reduced levels [291]. Residual 12-HETE appears to be the (S)-isomer, according to our findings using a stereospecific enzyme immunoassay (Pekcec and van Leyen, unpublished results).
3.4.2. Patho-physiological roles of lipoxygenases in the CNS (ischemia, neurodegeneration)
Neurons are especially vulnerable to oxidative stress, and oxidative stress-related pathology is a hallmark of several CNS diseases, including stroke, Parkinson’s, and Alzheimer’s Disease. LOXs are both activated by and contribute to oxidative stress, and are thus likely to be major players in these pathologies. ALOX15 has been linked to apoptotic cell death in cultured primary neurons [22, 294–296] as well as several brain-derived cell lines, including the human neuroblastoma cell line SH-SY5Y [297, 298] and the mouse hippocampal cell line HT22 [22, 299, 300]. The mechanism is apoptotic, but likely mediated by mitochondrial damage and apoptosis-inducing factor (AIF), rather than by caspase activation [299, 301, 302]. Similar damaging effects can be elicited in vivo by direct injection of arachidonic acid into the brain, which causes edema [303, 304]. Injecting glutathione disulfide, which is the oxidized version of glutathione, likewise induces brain damage via 12/15-LOX [305].
3.4.2.1. Oxidative stress and ALOX15 in the developing brain: periventricular leukomalacia
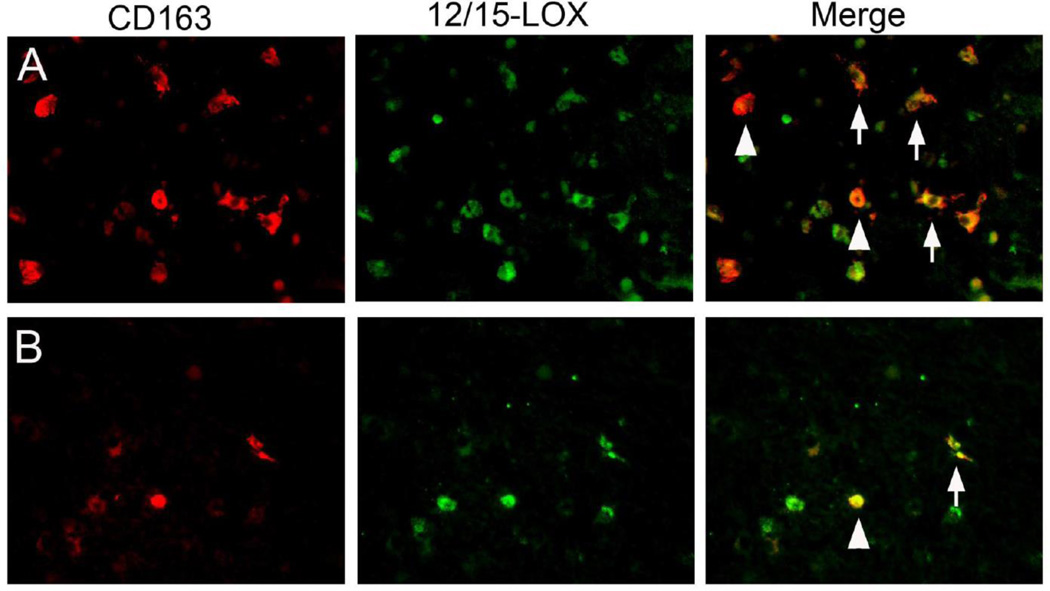
Periventricular leukomalacia (PVL) is a white matter injury in infants that is the dominant pathological factor in determining long-term cognitive and motor deficits in premature infants. It is characterized by necrotic lesions, and a diffuse type of injury involving microglia. In a recent study, we showed that ALOX15 expression is increased in the brains of PVL infants [306]. Several cell types were affected, including microglia (Fig. 6) and oligodendrocyte precursor cells. Importantly, some of these cells were TUNEL-positive, indicating these were injured cells and suggesting that ALOX15 contributed to disease pathology. This hypothesis is supported by cell culture studies, where oligodendrocyte precursors are vulnerable to an ALOX15-dependent form of cell death when cultured in the absence of cysteine [307, 308]. Similarly, LOX inhibitors protect oligodendrocytes against hyperoxia [309]. The mechanism is likely similar to that in neurons, with AIF translocation to the nucleus as apoptotic effector [310]. It will be interesting to see if LOX inhibitors are protective in animal models of PVL.
Fig. 6. Co-localization of ALOX15 with the microglial/macrophage marker CD163.
Depending on disease state, various cell types can show increased ALOX15. Shown here in A and B are two examples of brain tissue from infants with periventricular leukomalacia, where ALOX15 (12/15-LOX) co-localizes with the microglial/macrophage marker CD163, suggesting a role in disease pathology. ALOX15 in these brains was also increased in oligodendrocytes (courtesy of Dr. Robin Haynes, Children’s Hospital Boston)
3.4.2.2. Genetic associations with stroke
In 2004 polymorphisms in the gene encoding ALOX5 activating protein (ALOX5AP) were linked to an increased risk for ischemic stroke [311]. The protein encoded by ALOX5AP is required for ALOX5 activity [312]. In the aftermath, numerous replication studies were carried out in several different ethnic populations, with variable outcomes. For example, the findings originally made in the Icelandic cohort were replicated in Scottish and Spanish [313], but not in a Swedish [314] population. One U.S.-based study did not confirm this connection [315], while another found an association for Americans of European, but not of African descent [316]. Similarly, studies of Chinese populations in some cases confirmed the original results, in others did not; but a recent meta-analysis concluded that the link could be confirmed in the Chinese population [317]. A genetic study of English and German patients reported significant associations for several genes of the leukotriene pathway [318]. It should be emphasized that increased risk of stroke does not imply an increased severity of strokes as well. Those types of study, which could uncover target genes relevant for treatment of stroke, are very difficult to carry out, and none have so far been reported.
3.4.2.3. ALOX15 in stroke
The strongest evidence for any LOX isoform causing injury to the CNS exists in stroke. Early studies in the 1970s showed an increase in free fatty acids including arachidonic acid in a rat model of ischemia [319], an indication of phospholipase activity that suggested proteins of the arachidonic acid cascade might contribute to ischemic injury. In 1984, Moskowitz and colleagues reported increased levels of leukotrienes, as well as the Alox15 metabolite 12-HETE, in the brains of gerbils subjected to forebrain ischemia [320]. A large body of evidence has since then accumulated to demonstrate the involvement of ALOX15 in causing brain injury following stroke [321]. Several events converge to favor the activation of ALOX15. Arachidonic acid liberated from phospholipids by cytosolic phospholipase A2 (cPLA2) provides additional substrate and, together with increased levels of reactive oxygen species (ROS) activates ALOX15 [322]. Glutathione levels drop, removing one of the antioxidant pathways that serve to keep ALOX15 activity in balance [323]. Conversely, intracellular calcium rises, favoring membrane binding of the activated enzyme [324]. In addition, the protein levels of ALOX15 increase specifically in the penumbra region surrounding the core infarct, the brain region which is vulnerable to delayed cell death [325]. The factors leading to transcriptional up-regulation in the ischemic brain have not been determined yet, but may include members of the STAT family of transcriptional activators, which regulate ALOX15 expression in several other cell types [326–328].
Increased ALOX15 in the ischemic cortex is accompanied by increased pro-apoptotic AIF, in both human stroke patients [329], as well as mouse models of stroke [301]. ALOX15 also co-localizes with MDA2, an antibody that recognizes malonedialdehyde-modified lysisne residues, indicative of phospholipid oxidation [329]. Taken together, these findings document that ALOX15 is part of a major cell death pathway that is activated in the ischemic brain. Consistent with these observations, ALOX15 gene knockout protects mice against stroke [325, 330], and also reduces leakage of the blood-brain barrier and edema formation [331]. Importantly, these protective effects could be replicated by pre-treatment with LOX inhibitors. While those early inhibitors also had strong antioxidant activity, we have since introduced newer inhibitors with low antioxidant activity [308, 332], and found those to be protective even when given four hours after onset of the experimental stroke [329]. These compounds may be clinically useful in treating stroke.
3.4.2.4. ALOX5 in stroke
Early reports of LOX activity in animal models of stroke emphasized ALOX5 products over those of ALOX15, although the latter were also detected [320]. The increased leukotrienes were later shown to be blood-derived, rather than being formed in the brain parenchyma [333]. Several ALOX5 inhibitors were reported to be neuroprotective, but these were typically strong antioxidants, which lack major isoform-specificity [334]. A later study showed that in two different ischemia models, ALOX5 knockout mice had an equal amount of injury compared to wild-type mice [335]. Since then, the focus has shifted more to ALOX15 and its effects on stroke severity. Nonetheless, it is not unlikely that ALOX5 and its products are involved in specific subsets of stroke pathology, especially those featuring an increased inflammatory component.
3.4.2.5. Neuroprotection through lipoxygenase metabolites in stroke
In contrast to the damaging effects of LOX activity, a separate line of investigations is exploring the restorative potential of LOX-derived mediators including lipoxins and protectins. For example, neuroprotective effects of rosiglitazone were related to ALOX5-dependent formation of lipoxin A4 [336]. In line with these findings, an agonist of the lipoxin A4 receptor provided neurovascular protection in a rat model of ischemic stroke [337]. This was accompanied by a reduction of the matrix metalloproteinase MMP-9, which was also diminished in another study by administration of lipoxin A4 methyl ester [338].
An extensive body of work has accumulated to demonstrate the potential of protectins, specifically neuroprotectin D1 (NPD1), to reduce injury in animal models of stroke [339]. The underlying principle is the conversion of docosahexaenoic acid to NPD1 by a succession of LOX-mediated oxidation reactions, and infusion of DHA has been shown to be protective in experimental stroke [340]. Intriguingly, a closely related isomer termed AT-NPD1 can be generated in situ in the brain by administration of aspirin with docosahexaenoic acid, and this AT-NPD1 also has equivalent neuroprotective properties [341].
3.4.2.6. Lipoxygenase involvement in Alzheimer’s and other neurodegenerative diseases
In Alzheimer's, both ALOX5 and ALOX15 have been implicated, but their precise roles are far from clear. ALOX15 expression is increased in the brains of Alzheimer's patients [342], along with increased levels of 12- and 15-HETE in the cerebrospinal fluid of patients with Alzheimer's or mild cognitive impairment pathology [343]. Consistent with a damaging function of ALOX15 in Alzheimer's, degenerative defects in the transgenic Alzheimer's mouse model tg2576 were reduced when ALOX15 was absent [344]. Conversely, in another study ALOX15 expression was reduced in the hippocampus of Alzheimer's patients [345] and this effect was paralleled by reduced neuroprotectin D1 levels. In contrast, ALOX5 was increased in hippocampus and cortex of Alzheimer's patients [346]. Rao et al. found elevated levels of ALOX12 and ALOX15, but no increase in ALOX5 when comparing Alzheimer’s brains to those with no pathology [347]. There are several possible reasons for these discrepancies, which may be related to the complexity of disease progression. Other factors may include small sample size, differences in brain regions and different techniques used for analysis. Further studies are needed to get a clearer picture of differential LOX expression and its consequences in Alzheimer's brains.
In cell culture models, an amyloid beta-derived peptide was found to cause cell death in primary neurons. This effect was blocked by inhibition of ALOX15 and ALOX12 with baicalein, and similar protective effects were observed when an antisense oligonucleotide targeting ALOX15 expression was employed [348, 349]. Again somewhat at odds with these findings, another line of experiments suggested that miRNA125b, a micro-RNA that binds at the 3'-UTR of ALOX15 mRNA and down-regulates its translation, was increased in Alzheimer's patients. In primary neuronal-glial cells treated with IL1β and amyloid β1–42, an increase of miRNA125b led to down-regulation of ALOX15 [350]. An antagomir to miRNA125b that restored ALOX15 protected these cells. In both of these cases, a direct causative link to Alzheimer's pathology remains to be established.
In other CNS Diseases, much remains to be studied about possible LOX involvement in the respective pathology. Somewhat surprisingly, in a mouse model of multiple sclerosis, experimental allergic encephalomyelitis, knockout mice deficient in either ALOX5 or ALOX15 actually do worse [351]. After spinal cord injury, ALOX15 was increased 25-fold in rats, compared to a 1.7-fold up-regulation detected for COX-2 [352]. But whether or not this increase contributes to the injury is presently unknown.
3.5. Lipoxygenases in metabolic disorders
3.5.1. Diabetes
A number of LOX metabolites and several LOX isoforms have been implicated in the pathogenesis of diabetes. Both ALOX5 generated leukotriene B4, and 12-HETE generated by either ALOX15 or ALOX12 was elevated in diabetic patients with severe cardiac ischemia [353]. Especially for ALOX15 a substantial body of evidence has accumulated to link the enzyme to the pathogenesis of diabetes [354–362]. Since LOXs are pro-oxidative enzymes producing hydroperoxy lipids, LOX-induced oxidative stress and subsequent mitochondrial dysfunction might account for much of the increased pathology detected in diabetic cardiomyopathy and other vascular diseases [363]. Increased levels of 12S-HETE were linked to coronary artery disease in type 2 diabetic patients [364]. More generally, ALOX15 has been shown to be up-regulated in both cell culture and animal models of diabetes [365].
Insulin secretion of cultured human islet cells was reduced by nanomolar concentrations of 12(S)-HETE and 12HpETE. These data suggest that 12(S)-HETE reduces insulin secretion in human islets but it remains unclear, which LOX-isoforms contribute to the in vivo production of 12S-HETE [354]. Hepoxilin A3, generated from 12-HpETE by hepoxilin A3 synthase, can induce insulin secretion in pancreatic beta cells and islets [366]. Furthermore, HXA3 protects the rat insulinoma cell line RINm5F against oxidative stress-induced cell death, although the mechanism needs further study [367].
3.5.1.1. LOX in Type 1 diabetes
There is only limited information currently available on the potential role of LOX isoforms in type-1 diabetes. Female nonobese diabetic (NOD) mice are a suitable model for this disease [368]. Remarkably, the NOD-ALOX15null strain, in which ALOX15 is absent, is almost completely protected [369]. These results suggest ALOX15 contributes to the pathology, and may be related to effects of ALOX15 on islet cell and/or macrophage functionality [370]. Similarly, Alox15(−/−) mice are resistant to induction of Type 1 diabetes by streptozotocin [357]. In addition, increased levels of ALOX5 metabolites have been detected in diabetic rats [371], but the biological relevance of this observation remains unclear. To the best of our knowledge there is no detailed study currently available characterizing the role of any LOX-isoform in type-1 diabetes in humans.
3.5.1.2. LOX in Type 2 diabetes
Nadler and colleagues investigated the role of ALOX15 in adipocytes in vivo and in cultured cells [372]. The enzyme is induced in white epididymal adipocytes in mice fed a high-fat diet. On a cellular level, a similar up-regulation can be seen when 3T3-L1 adipocytes, which are treated with palmitate. The ALOX15 products 12-HETE and 12-HpETE diminish the response of 3T3-L1 adipocytes to insulin, consistent with the resistence to insulin characteristic of type 2 diabetes [372].
3.5.2. Lipoxygenase isoforms in adipocyte maturation, obesity and metabolic syndrome
Adipocytes, the major cell type of adipose tissue, differentiate from mesenchymal stem cells via fibroblasts. A complex regulatory network controls adipogenesis, and PPARγ is a decisive factor in this process. 3T3-L1 cells are frequently employed as cellular model of adipocyte differentiation [373] and the LOX inhibitors nordihydroguaiaretic acid and baicalein inhibit adipocyte maturation of 3T3-L1 cells in vitro [374]. The inhibitory effect of baicalein was prevented by administration of the PPARγ agonist rosiglitazone implicating LOX-isoforms and PPARγ in adipocyte maturation in this in vitro model [374]. Gene expression profiles indicated the presence of Alox15, Alox12 and Aloxe3 in white and brown adipose tissue, but among them only Aloxe3 is expressed at high levels in 3T3-L1 cells. Forced expression of this LOX isoform or addition of ALOXE3 products (hepoxilins) stimulated adipogenesis and RNAi-mediated expression knockdown prevented adipocyte differentiation [374]. Although these data need to be confirmed for in vivo development of adipocytes the results suggested that ALOXE3 might constitute an important player in adipogenesis and that specific ALOXE3 inhibitors might be useful to interfere with this process.
In addition of ALOXE3 other LOX-isoforms, such as ALOX15, ALOX12 and ALOX5 have been implicated in adipogenesis [375]. 15-HETE, the major arachidonic acid oxygenation product of ALOX15 and ALOX15B, induced angiogenesis in adipose tissue and thus, has been implicated in growth of adipose tissue [376]. In a limited (1215 subjects) Chinese genetic correlation study a polymorphism in the ALOX12 gene (rs2073438) was significantly associated with total and percentage fat mass (p=0.007 and p=0.012, respectively) suggesting that ALOX12 might contribute to the variation of obesity phenotypes in young Chinese men [377]. Unfortunately, the underlying molecular basis has not been explored and it remains to be clarified whether there is a causal relation between ALOX12 expression and body fat mass. Since adipositas is currently considered a chronic inflammation of the adipose tissue [378] the ALOX5 pathway has also been implicated in the pathogenesis of this disorder. Blockade of leukotriene signaling by treatment with LOX inhibitors and leukotriene B4 antagonists as well as RNAi-induced expression silencing of leukotriene B4 receptors in human and mouse preadipocytes isolated from native adipose tissues showed acceleration of differentiation into mature adipocytes [379]. From these data the authors concluded that leukotriene B4 signaling may negatively regulate preadipocyte differentiation via induction of transforming growth factor expression [379].
Nonalcoholic fatty liver disease (NAFLD) is a major hepatic consequence of the metabolic syndrome. ALOX15 mRNA is up-regulated in ApoE(−/−) mice, which are frequently employed as model for NAFLD [380]. These days adipositas is considered a chronic low grade inflammatory disorder of the adipose tissue and the inflammatory activities of ALOX12 and ALOX15 in the adipose tissue have recently been reviewed [375]. Consistent with the inflammation hypothesis increased levels of ALOX5 products have been interpreted as signs of low-grade inflammation in adipose tissue, which could contribute to pre-diabetic pathology [381]. Adipose tissue and adipocytes from obese Zucker rats, a model for metabolic syndrome, featured both increased ALOX15 and ALOX5 levels, as well as their eicosanoid products [382].
An important question is how the ALOX15 expression is up-regulated in these low-grade inflammatory models. It is known that in several cell types including macrophages, IL-4 and IL-13 regulate ALOX15 levels [326, 383]. The transcription factor PPARγ might be involved in expression regulation of ALOX15. Since this transcription factor is activated by the ALOX15 metabolites 12- and 15-HETE there may exist a damaging feed forward mechanism [384].
Catalytic activity of ALOX15 is tightly regulated on transcriptional, translational, and post-translational levels and anti-oxidative enzymes such as glutathione peroxidases GPX-1 and -4 have been implicated [385]. In the absence of GPX4 the activity of ALOX15 is increased and may lead to cell death in a cellular model system [323]. However, it remains to be shown whether this is also the case in vivo. In any case, these findings along with loss of the glutathione substrate may be an important factor in the oxidative tissue damage found in metabolic syndrome.
4. Lipoxygenase isoforms in infectious diseases
When entering the body pathogenes induce an inflammatory host response and LOX products have been implicated as signaling molecules in this protective reaction complex [2, 33, 166]. In principle, the severity of the inflammatory response depends on the balance of pro- and anti-inflammatory mediators and both, hosts and pathogens may contribute to this equilibrium of signaling molecules. For the time being little is known about the role of different LOX-isoforms in infectious diseases and only scattered experimental data are currently available for viral, bacterial and parasite infections as well as for mycosis.
4.1. Lipoxygenase isoforms in viral infections
The human immune system responds to viral infections with an activation of inflammatory cells, and leukotrienes as classical inflammatory mediators [2, 33] have been implicated in this defense reaction. Although no functional LOX sequences have currently been described in major pathogenic viruses eicosanoid production of hosts cells might impact the infection process. The Epstein-Barr Virus (EBV) up-regulates the formation of pro-inflammatory leukotrienes in human peripheral mononuclear cells [386]. Moreover, EBV infection triggers malignant transformation of lymphocytes to Burkitt’s lymphoma cells, which are characterized by an increased resistance to apoptosis [387]. Such apoptosis-resistant cells overexpressed ALOX5 and ALOX15 and Inhibitor studies as well as cell incubation in the presence of 5-HETE suggest that ALOX5 and to a lesser extent other LOX-isoforms might be involved in EBV-mediated lymphoma progression [387].
A further example for the involvement of the ALOX5 pathway in the pathogenesis of virus infection is the human cytomegalovirus (HCMV). In vitro, HCMV infection of human vascular smooth muscle cells strongly increased the expression of ALOX5 mRNA (170-fold) and protein [388]. In vivo, HCMV-infected vascular smooth muscle cells express the ALOX5 protein and these data suggest that leukotriene signaling might be upregulated during HCMV infections. However, it remains unclear whether ALOX5 expression in vivo is directly induced by the virus or whether ALOX5 expression is just a consequence of the accompanying inflammatory response.
When human neutrophils are infected in vitro with the dengue virus (DENV-2), ALOX5 expression is upregulated and the cells biosynthesize significantly more leukotriene B4 [389]. In the presence of MK886 (ALOX5 activating protein antagonist) the increase in leukotriene B4 biosynthesis is reduced indicating the relevance of the ALOX5 pathway in dengue virus infection. Consistent with this conclusion is the observation that in vivo plasma levels of leukotriene B4 were significantly elevated during the febrile stages of dengue infections [389].
The respiratory syncytial virus (RSV) induces bronchiolitis [390] and the resolution of this respiratory disorder is mediated by alternatively activated M2-macrophages. RSV infection of ALOX5 and ALOX15 deficient macrophages and mice failed to elicit differentiation of M2 macrophages. Interestingly, treatment of Alox5-deficient macrophages with lipoxin A4 and resolvin E1, but not with leukotriene B4 or leukotriene D4, restored the expression of M2-macrophage markers. From these data the authors concluded that patients with RSV infections might benefit from treatment with proresolving eicosanoids.
4.2. Lipoxygenase isoforms in bacterial infections
LOXs occur in selected bacteria [15] but other bacterial pathogens do not contain functional LOX sequences. For those bacteria carrying functional LOX genes [391] the corresponding enzymes may contribute to systemic eicosanoid formation during host-pathogen interaction. Puerperal sepsis is the leading cause of maternal mortality worldwide and Streptococcus pyogenes is the major etiologic agent of severe postpartum sepsis [392]. Mice lacking the Alox5 pathway were significantly more vulnerable to puerperal sepsis when compared with corresponding wild-type controls [393]. Although the mechanistic basis of this deleterious effect of Alox5 expression silencing has not been explored the failure of Alox5-deficient mice to synthesize pro-inflammatory leukotrienes may contribute to the increased vulnerability.
Acute pulmonary infection by Streptococcus pneumoniae is characterized by high bacterial numbers in the lung and a robust alveolar influx of polymorphonuclear leukocytes. S. pneumoniae infection induced expression of 12-lipoxygenating LOX isoforms in cultured pulmonary epithelium and in the lungs of infected mice [394]. Pharmacological (inhibitors) and genetic (Alox15 knockout mice) interference with the Alox15 pathway reduced lung inflammation in vivo [394] and mechansitic studies suggest that pneumococcal pulmonary inflammation is paralleled by disruption of the lung epithelium via Alox15 dependent hepoxilin A3 production. In the absence of Alox15 lower amounts of hepoxilins were detected, which was suggested to contributed to reduced pathology [394]. In pneumococcus-induced otitis media Alox5 appears to be of major patho-physiological relevance [395]. In an in vivo rat model of this disease Alox5 expression was strongly upregulated and this effect was paralleled by an increase in middle ear fluid. Here again, the mechansitic details have not been explored but the data relate alox5 expression to the pathogenesis of pneumococcus induced otitis media.
Anti-inflammatory lipoxins, which are biosynthesized in vivo by a concerted activity of various LOX-isoforms (Alox5, Alox12, Alox15), are key mediators in the resistance of host cells to M. tuberculosis infection [396]. High levels of lipoxin A4 were detected in the blood of infected wild-type mice but significantly lower levels were quantified in Alox5 deficient animals. Bacterial burdens in Alox5 deficient lungs were significantly lower than in the organs of wildtype controls and the increased the resistance of Alox5−/− mice was counteracted by administration of a stable lipoxin A4 analog. From these data the authors concluded that the anti-inflammatory lipoxins negatively regulate the protective Th1 response against mycobacterial infection and suggested that inhibition of lipoxin biosynthesis could serve as a strategy for enhancing host resistance towards M. tuberculosis [396].
4.3. Lipoxygenase isoforms in parasite infections and mycosis
Toxoplasmosis is a parasitic disease caused by the protozoan Toxoplasma gondii and up to one third of the world's human population is estimated to carry a Toxoplasma infection [397]. The human ALOX12 gene has susceptibility alleles for human congenital toxoplasmosis and RNAi-mediated expression knockdown of this gene attenuated progression of T. gondii infection [398]. These data implicate ALOX12 in host defense against T. gondii but the underlying molecular mechanisms remain unclear.
Schistosoma are parasitic flatworms. They induce Schistosomiasis, which is considered by the WHO the second most socioeconomically devastating parasitic disease (after malaria) with hundreds of millions infected people worldwide [399]. Periovular granuloma formation during S. mansoni infection is a complex immunologic response and LOX inhibitors reduced granuloma formation [400]. At the acute stage of infection, when granuloma formation is usually maximal, Alox5 deficient mice developed smaller granulomas around liver-deposited schistosome eggs compared with wild type and Alox15 deficient mice. These data suggest that Alox5 but not Alox15 may play a role in the host responses to schistosomiasis.
Paracoccidioidomycosis is a systemic mycosis caused by the thermodimorphic fungus Paracoccidioides brasiliensis. When Alox5 deficient mice and corresponding wild-type controls were intravenously inoculated with P. brasiliensis they exhibited an increased survival rate [401]. The disease resistance was associated with augmented nitric oxide production, reduced number of CD4(+)-CD25(+) regulatory T cells and higher levels of gamma interferon as well as interleukin-12 levels in the lungs. These results suggest that expression of Alox5 increased the susceptibility of mice for P. brasiliensis suggesting that this pathway might constitute a potential target for therapeutic intervention.
Candida albicans is an opportunistic fungal pathogen that resides commensally on epithelial surfaces, but can cause severe inflammation in immunocompromized patients [402]. C. albicans is capable of biosynthesizing anti-inflammatory resolvins (RvE1) but in contrast to human cells there is not transcellular biosynthesis [403]. Although the biosynthetic mechanisms have not been clarified RvE1 in vitro enhanced phagocytosis of C. albicans by human neutrophils and augmented intracellular ROS generation and killing. Moreover, in a mouse model of systemic candidiasis RvE1 stimulated clearance of the fungus from circulating blood suggesting a possible role for RvE1 and its biosynthesizing machinery in C. albicans infections.
5. Lipoxygenase inhibitors as potential drugs
Since LOX isoforms have been implicated in the pathogenesis of major human diseases LOX inhibitors are of medical interest. According to their inhibitor mechanisms LOX inhibitors can be classified into 5 principal groups (Fig. 7): i) Redox inhibitors interfering with the valency change of the nonheme iron during the catalytic cycle. ii) Iron chelators complexing the iron ion at the active site. iii) Active site probes competing with substrate fatty acids in the substrate binding pocket, iv) Suicide substrates leading to irreversible inactivation of the enzyme; v) Allosteric inhibitors that bind to a site other than the substrate binding pocket. Although a number of pharmaceutical companies including Abbott, Merck, Wellcome and ICI initiated LOX inhibitor programs the only LOX inhibitor that has made it to the clinics is the ALOX5 inhibitor zileuton [175]. This drug has been approved as anti-asthmatic but owing to its unfavorable pharmacokinetics and its side effects it has not reached wide spread acceptance. Originally, zileuton was available in two formulations under the brand names ZYFLO and ZYFLO CR. The immediate-release formulation ZYFLO is given at a dosage of 600 mg four times per day. The extended-release formulation (ZYFLO CR) is taken twice daily. Although there have been a number of promising LOX-inhibiting compounds other than zileuton their therapeutic potential was disappointing. Moreover, evaluation of the recent patent activities revealed only compounds with moderate inhibitory potency [404]. The most promising advances in drug development have been made for FLAP (ALOX5 activating protein) antagonists and it might well be that such compounds will enter the market as anti-asthmatics [404]. There are a number of recent reviews summarizing the current knowledge in the field of LOX inhibitors [2, 404–406] and there is no need for re-reviewing them. However, we would like to briefly discuss three problems that might have contributed to the fact that LOX inhibitors have performed rather weakly in the clinics despite their promising experimental potential.
Fig. 7. Principal modes of action of lipoxygenase inhibitors.
NDGA - nordihydroguaiaretic acid, HODE - hydroxy octadecadienoic acid, ETYA - 5,8,11,14-eicosatetraynoic acid, ODYA - 9,12-octadecadiynoic acid, OPP - 4-(2-oxapentadeca-4-yne)phenylpropanoic acid,
5.1. Isoform-specific LOX inhibitors
As indicated in Table 1 six functional LOX isoforms exist in humans. In the murine genome there are seven functional LOX genes. Although except for the ALOXE3 the different human LOX isoforms catalyzed the same principal reaction (oxygenation of polyenoic fatty acids) they have been implicated in different physiological and patho-physiological processes. Consequently, inhibitors that impact the catalytic activity of several LOX isozymes are likely to have unwanted side effects. For instance, an ALOX5 inhibitor, aimed at developing an anti-asthmatic drug [404], should not significantly impact the activity of ALOX12B. Otherwise problems with skin development may occur [84, 85]. This functional multiplicity of LOXs requires the development of isoform-specific inhibitors. Most experts working in this field are well aware of this problem and test the isoform-specificity of their compounds in different assay systems. However, the assay systems employed in the past are not strictly comparable and thus, the data might be misleading: i) The purified rabbit ALOX15 has frequently been employed as model enzyme for the corresponding human ortholog but it still remains unclear whether the two enzymes have similar inhibitor sensitivities. The two ALOX15 orthologs share a high degree of amino acid conservation (>80%) but there are amino acid differences that might impact inhibitor sensitivity. ii) In the past, impure enzyme preparations (cell lysates of different cells) were employed as enzyme sources for activity assays. For instance, lysates of rat basophilic leukemia cells were used as ALOX5 source and platelet lysates were employed as source for human ALOX12 [407]. Unfortunately, these assay systems are not strictly comparable since foreign proteins may bind the inhibitors, which impacts IC50 values. To circumvent these problems inhibitor studies should always be carried out with purified recombinant human enzyme preparations [408, 409]. Unfortunately, some human LOX-isoforms (ALOXE3, ALOX12B) are not well expressed as recombinant proteins making this strategy difficult to follow. Alternatively, all LOX isoforms should be expressed in a single eukaryotic overexpression system (such as COS or HEK cells) and the cellular lysate may be employed as enzyme source. Recently, a systematic study was carried out, in which all rat 12-lipoxygenating LOX isoforms were overexpressed in HEK cells and an array of commercially available LOX inhibitors was tested using the cell lysates [410]. The data obtained indicate that the commercial LOX-inhibitors (NDGA, CDC, AA861, baicalein, PD146176) only exhibited a low degree of isoform specificity although some of them have previously suggested as isoform-specific LOX inhibitors. Most surprisingly, PD146176, which has been employed in experimental strategies as ALOX15 specific inhibitor [323, 407, 411], did not at all inhibit rat ALOX15 [410]. For the time being, it remains unclear why this compound effectively inhibits rabbit and human ALOX15 [407] and not the rat ortholog [410] but it may be related to the fact that rat ALOX15 is a 12-lipoxygenating enzyme [34] whereas rabbit and human orthologs are 15-lipoxygenating [35]. However, to avoid misinterpretations of experimental data it is most important to make sure that human ALOX15-inhibitors, which are scheduled to be employed as mechanistic probes in murine systems, have been tested for LOX inhibition in these experimental setups (rat, mouse or other species). It might well be that an inhibitor that effectively inhibits human ALOX15 does not at all inhibit the orthologous enzymes of other species. Thus, effects of such compounds in mouse or rat experimental systems are probably due to off-target effects of the compounds although they might be interpreted as consequence of ALOX15 inhibition.
5.2. Species-specific differences of LOX orthologs
To explore the patho-physiological roles of different LOX-isoforms murine disease models are required. Unfortunately, some murine LOX isoforms have different properties than their human ortholog and these differences may impact inhibitor sensitivity. For instance, murine Alox15 isoforms (mouse, rat) are 12-lipoxygenating enzymes [34] whereas the human ortholog is 15-lipoxygenating [35, 412]. The molecular basis for this functional difference has previously been explored [44] and appears to be related to the volume of the substrate-binding pocket [1]. If this hypothesis is correct it may explain the differences in inhibitor sensitivity of rat [410] and human ALOX15 orthologs [407]. There is a second species-specific difference between mouse and human LOX orthologs. Human ALOX15B converts arachidonic acid almost exclusively to 15-HpETE whereas the mouse ortholog is 8-lipoxygenating [45]. Here again, mutagenesis studies indicated that two critical amino acids make the functional difference between 15S- and 8S-lipoxygenation. Although there are currently no detailed experimental data comparing the inhibitor sensitivities of mouse and human ALOX15B it might be speculated that the differences in reaction specificity may also impact this enzyme property.
5.3. Off-target effects of LOX-inhibitors
Some LOX inhibitors, such as NDGA or propylgallate contain catechol structures and thus, exhibit anti-oxidative properties. Thus, in addition to being LOX inhibitors they may directly impact the redox homeostasis in biological systems. Since the cellular redox homeostasis is important for regulating the gene expression pattern on genetic [413] and epigenetic [12, 414] levels, it is difficult to discriminate which of the two functions (LOX inhibition vs. redox activity) is the major reason for a biological effect. Consequently, results obtained with these types of LOX inhibitors need to be interpreted with care and to avoid misinterpretation inhibitor studies should always be confirmed by alternative loss-of-function strategies such as siRNA induced expression silencing and/or the use of genetic knock-out models.
6. Concluding remarks and perspectives
Because of the proposed biological roles of LOXs and their implication in the pathogenesis of public health-relevant human diseases these enzymes have received significant attention over the past decades. Although most human LOX isoforms can be expressed as recombinant proteins and have been well characterized with respect to their structural and functional properties there are a number of caveats that need to be addressed in the future. If one considers rabbit ALOX15 as suitable structural model for the human ortholog, crystal data are now available for four (ALOX15, ALOX12, ALOX5, ALOX15B) of the six human LOX isoforms. Although there are subtle differences between the different isoforms the general fold of the enzymes is similar and on the basis of the current structural data reliable models may be constructed for the remaining LOX isoforms (ALOX12B, ALOXE3). When compared with other LOX isoforms the oxygenase activity of ALOXE3 is limited and thus, the enzyme has been suggested to function as fatty acid peroxide isomerase. Although some studies have been performed to explore the molecular reasons for the lacking oxygenase activity of ALOXE3 [415, 416] the structural basis remains unclear. Crystal data for ALOXE3 might shed some light on this mechanistic detail. Furthermore, although dimerization of various LOX isoforms has recently suggested [64, 65] the mechanistic and biological consequences of this effect have rarely been studied. It might well be that enzyme dimerization may contribute to the allosteric properties that have been described for various LOX isoforms [62, 265]. Another problem in molecular LOX research is lacking direct structural information on LOX-substrate complexes. The currently available structural data on enzyme-ligand complexes [46–51] do not conclusively answer the questions on the structural basis for the reaction specificity of the different LOX-isoforms.
Although knockout mice are currently available for 5 LOX isoforms (Alox5, Alox15, Alox12, Alox12b, Aloxe3) the biological roles of ALOX15 and ALOX12 are not well understood. ALOX12 and ALOX15 knockout mice are viable and although irregular epididymal maturation has been reported for ALOX15-deficient sperms the animals breed well and corresponding mouse colonies can be established easily. On the other hand, challenging experiments suggest the involvement of the two enzymes in physiological processes. It might well be that the mild phenotypes of Alox15, Alox12 and Alox5 knockout mice may in part be related to the fact that no conditional knockouts are currently available for these two enzymes. One problem with non-conditional knockout mice is that their creation is somewhat selective (only embryonic stem cells that survived the genetic manipulation were selected for blastocyst injection) and that compensatory mechanisms during early embryogenesis cannot be ruled out. To overcome these problems inducible knockout systems should be established but such experiments are time consuming and quite expensive.
One of the most serious problems in LOX research in the past decade has been the lack of sensitive (in vivo IC50 in the lower nanomolar range) isoform-specific inhibitors that could be employed in animal disease models or in experimental clinical studies in humans. There are a large number of LOX inhibitor structures in the chemical databases but for most of them isoform-specificity has not been explored in strictly comparable assay systems. Only recently this problem was partly overcome [408, 409], but the new compounds have not yet reached widespread acceptance. In addition, the different functional characteristics of LOX orthologs in different mammalian species (12-lipoxygenating Alox15 in mice vs. 15-lipoxygenating ALOX15 in humans) make the situation even more complex. A broad experimental screening program for isoform-specific LOX inhibitors employing high throughput systems, which are based on purified recombinant human LOX isoforms, would help to approach these problems. Of course, such experiments and the following refinement strategies are time consuming and expensive and for the time being the pharmaceutical industry appears to be not interested in such strategies because of the controversial reports on the bioactivity of various LOX isoforms. However, the recent experimental data on the involvement of certain LOX isoforms in neurological disorders (especially ALOX5 and ALOX15) and the potential use of ALOX15 inhibitors for anti-stroke therapy [332] might change this situation.
Acknowledgement
Financial support for this work was provided by GRK1673/1 granted by Deutsche Forschungsgemeinschaft (GRK 1673/1 to H.K.) and by the U.S. National Institutes of Health (R01 NS49430 and R01 NS069939 to K.v.L.).
List of abbreviations
- LOX
lipoxygenase
- COX
cyclooxygenase
- LT
leukotrienes
- HETE
hydroxy eicosatetraenoic acid
- PPAR
peroxysome proliferator activated receptor
- MICAL
Molecule Interacting with CasL
- ARCI
autosomal recessive congenital ichthyosis
- GPCR
G-protein coupled receptors
- NOD
nonobese diabetes
- RSV
respiratory syncytial virus
- NDGA
nordihydroguaiaretic acid
- ALX
lipoxin receptor
- OXE
oxo-eicosanoid receptor
References
- 1.Ivanov I, Heydeck D, Hofheinz K, Roffeis J, O'Donnell VB, Kuhn H, Walther M. Molecular enzymology of lipoxygenases. Archives of biochemistry and biophysics. 2010;503:161–174. doi: 10.1016/j.abb.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Haeggstrom JZ, Funk CD. Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chemical reviews. 2011;111:5866–5898. doi: 10.1021/cr200246d. [DOI] [PubMed] [Google Scholar]
- 3.Mancini AD, Di Battista JA. The cardinal role of the phospholipase A(2)/cyclooxygenase-2/prostaglandin E synthase/prostaglandin E(2) (PCPP) axis in inflammostasis. Inflammation research : official journal of the European Histamine Research Society … [et al.] 2011;60:1083–1092. doi: 10.1007/s00011-011-0385-7. [DOI] [PubMed] [Google Scholar]
- 4.Savari S, Vinnakota K, Zhang Y, Sjolander A. Cysteinyl leukotrienes and their receptors: bridging inflammation and colorectal cancer. World journal of gastroenterology : WJG. 2014;20:968–977. doi: 10.3748/wjg.v20.i4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romano M. Lipoxin and aspirin-triggered lipoxins. The Scientific World Journal. 2010;10:1048–1064. doi: 10.1100/tsw.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pace-Asciak CR. The hepoxilins and some analogues: a review of their biology. British journal of pharmacology. 2009;158:972–981. doi: 10.1111/j.1476-5381.2009.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sachs-Olsen C, Sanak M, Lang AM, Gielicz A, Mowinckel P, Lodrup Carlsen KC, Carlsen KH, Szczeklik A. Eoxins: a new inflammatory pathway in childhood asthma. The Journal of allergy and clinical immunology. 2010;126:859–867. e859. doi: 10.1016/j.jaci.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Yoo S, Lim JY, Hwang SW. Resolvins: Endogenously-Generated Potent Painkilling Substances and their Therapeutic Perspectives. Current neuropharmacology. 2013;11:664–676. doi: 10.2174/1570159X11311060009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chemical reviews. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schewe T, Halangk W, Hiebsch C, Rapoport SM. A lipoxygenase in rabbit reticulocytes which attacks phospholipids and intact mitochondria. FEBS letters. 1975;60:149–152. doi: 10.1016/0014-5793(75)80439-x. [DOI] [PubMed] [Google Scholar]
- 11.Belkner J, Wiesner R, Kuhn H, Lankin VZ. The oxygenation of cholesterol esters by the reticulocyte lipoxygenase. FEBS letters. 1991;279:110–114. doi: 10.1016/0014-5793(91)80263-3. [DOI] [PubMed] [Google Scholar]
- 12.Kim GH, Ryan JJ, Archer SL. The role of redox signaling in epigenetics and cardiovascular disease. Antioxidants & redox signaling. 2013;18:1920–1936. doi: 10.1089/ars.2012.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buelna-Chontal M, Zazueta C. Redox activation of Nrf2 & NF-kappaB: a double end sword? Cellular signalling. 2013;25:2548–2557. doi: 10.1016/j.cellsig.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Baldock PA, Eisman JA. Genetic determinants of bone mass. Current opinion in rheumatology. 2004;16:450–456. doi: 10.1097/01.moo.0000127828.34643.b4. [DOI] [PubMed] [Google Scholar]
- 15.Hansen J, Garreta A, Benincasa M, Fuste MC, Busquets M, Manresa A. Bacterial lipoxygenases, a new subfamily of enzymes? A phylogenetic approach. Applied microbiology and biotechnology. 2013;97:4737–4747. doi: 10.1007/s00253-013-4887-9. [DOI] [PubMed] [Google Scholar]
- 16.Andreou A, Brodhun F, Feussner I. Biosynthesis of oxylipins in non-mammals. Progress in lipid research. 2009;48:148–170. doi: 10.1016/j.plipres.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Yuan D, Zou Q, Yu T, Song C, Huang S, Chen S, Ren Z, Xu A. Ancestral genetic complexity of arachidonic acid metabolism in Metazoa. Biochimica et biophysica acta. 2014 doi: 10.1016/j.bbalip.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Funk CD, Chen XS, Johnson EN, Zhao L. Lipoxygenase genes and their targeted disruption. Prostaglandins & other lipid mediators. 2002;68–69:303–312. doi: 10.1016/s0090-6980(02)00036-9. [DOI] [PubMed] [Google Scholar]
- 19.Sigal E, Grunberger D, Cashman JR, Craik CS, Caughey GH, Nadel JA. Arachidonate 15-lipoxygenase from human eosinophil-enriched leukocytes: partial purification and properties. Biochemical and biophysical research communications. 1988;150:376–383. doi: 10.1016/0006-291x(88)90531-1. [DOI] [PubMed] [Google Scholar]
- 20.Sigal E, Dicharry S, Highland E, Finkbeiner WE. Cloning of human airway 15-lipoxygenase: identity to the reticulocyte enzyme and expression in epithelium. The American journal of physiology. 1992;262:L392–L398. doi: 10.1152/ajplung.1992.262.4.L392. [DOI] [PubMed] [Google Scholar]
- 21.Conrad DJ, Kuhn H, Mulkins M, Highland E, Sigal E. Specific inflammatory cytokines regulate the expression of human monocyte 15-lipoxygenase. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:217–221. doi: 10.1073/pnas.89.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brash AR, Boeglin WE, Chang MS. Discovery of a second 15S-lipoxygenase in humans. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:6148–6152. doi: 10.1073/pnas.94.12.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jisaka M, Kim RB, Boeglin WE, Nanney LB, Brash AR. Molecular cloning and functional expression of a phorbol ester-inducible 8S-lipoxygenase from mouse skin. The Journal of biological chemistry. 1997;272:24410–24416. doi: 10.1074/jbc.272.39.24410. [DOI] [PubMed] [Google Scholar]
- 24.Funk CD, Furci L, FitzGerald GA. Molecular cloning, primary structure, and expression of the human platelet/erythroleukemia cell 12-lipoxygenase. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:5638–5642. doi: 10.1073/pnas.87.15.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Virmani J, Johnson EN, Klein-Szanto AJ, Funk CD. Role of 'platelet-type' 12-lipoxygenase in skin carcinogenesis. Cancer letters. 2001;162:161–165. doi: 10.1016/s0304-3835(00)00634-0. [DOI] [PubMed] [Google Scholar]
- 26.Boeglin WE, Kim RB, Brash AR. A 12R-lipoxygenase in human skin: mechanistic evidence, molecular cloning, and expression. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:6744–6749. doi: 10.1073/pnas.95.12.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meruvu S, Walther M, Ivanov I, Hammarstrom S, Furstenberger G, Krieg P, Reddanna P, Kuhn H. Sequence determinants for the reaction specificity of murine (12R)-lipoxygenase: targeted substrate modification and site-directed mutagenesis. The Journal of biological chemistry. 2005;280:36633–36641. doi: 10.1074/jbc.M508260200. [DOI] [PubMed] [Google Scholar]
- 28.Kinzig A, Heidt M, Furstenberger G, Marks F, Krieg P. cDNA cloning, genomic structure, and chromosomal localization of a novel murine epidermis-type lipoxygenase. Genomics. 1999;58:158–164. doi: 10.1006/geno.1999.5816. [DOI] [PubMed] [Google Scholar]
- 29.Yu Z, Schneider C, Boeglin WE, Brash AR. Human and mouse eLOX3 have distinct substrate specificities: implications for their linkage with lipoxygenases in skin. Archives of biochemistry and biophysics. 2006;455:188–196. doi: 10.1016/j.abb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krieg P, Furstenberger G. The role of lipoxygenases in epidermis. Biochimica et biophysica acta. 2014;1841:390–400. doi: 10.1016/j.bbalip.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Zheng Y, Yin H, Boeglin WE, Elias PM, Crumrine D, Beier DR, Brash AR. Lipoxygenases mediate the effect of essential fatty acid in skin barrier formation: a proposed role in releasing omega-hydroxyceramide for construction of the corneocyte lipid envelope. The Journal of biological chemistry. 2011;286:24046–24056. doi: 10.1074/jbc.M111.251496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brash AR, Yu Z, Boeglin WE, Schneider C. The hepoxilin connection in the epidermis. The FEBS journal. 2007;274:3494–3502. doi: 10.1111/j.1742-4658.2007.05909.x. [DOI] [PubMed] [Google Scholar]
- 33.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 34.Chen XS, Kurre U, Jenkins NA, Copeland NG, Funk CD. cDNA cloning, expression, mutagenesis of C-terminal isoleucine, genomic structure, and chromosomal localizations of murine 12-lipoxygenases. The Journal of biological chemistry. 1994;269:13979–13987. [PubMed] [Google Scholar]
- 35.Kuhn H, Barnett J, Grunberger D, Baecker P, Chow J, Nguyen B, Bursztyn-Pettegrew H, Chan H, Sigal E. Overexpression, purification and characterization of human recombinant 15-lipoxygenase. Biochimica et biophysica acta. 1993;1169:80–89. doi: 10.1016/0005-2760(93)90085-n. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe T, Haeggstrom JZ. Rat 12-lipoxygenase: mutations of amino acids implicated in the positional specificity of 15- and 12-lipoxygenases. Biochemical and biophysical research communications. 1993;192:1023–1029. doi: 10.1006/bbrc.1993.1519. [DOI] [PubMed] [Google Scholar]
- 37.Yoshimoto T, Suzuki H, Yamamoto S, Takai T, Yokoyama C, Tanabe T. Cloning and sequence analysis of the cDNA for arachidonate 12-lipoxygenase of porcine leukocytes. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:2142–2146. doi: 10.1073/pnas.87.6.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Marzo N, Sloane DL, Dicharry S, Highland E, Sigal E. Cloning and expression of an airway epithelial 12-lipoxygenase. The American journal of physiology. 1992;262:L198–L207. doi: 10.1152/ajplung.1992.262.2.L198. [DOI] [PubMed] [Google Scholar]
- 39.Vogel R, Jansen C, Roffeis J, Reddanna P, Forsell P, Claesson HE, Kuhn H, Walther M. Applicability of the triad concept for the positional specificity of mammalian lipoxygenases. The Journal of biological chemistry. 2010;285:5369–5376. doi: 10.1074/jbc.M109.057802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berger M, Schwarz K, Thiele H, Reimann I, Huth A, Borngraber S, Kuhn H, Thiele BJ. Simultaneous expression of leukocyte-type 12-lipoxygenase and reticulocyte-type 15-lipoxygenase in rabbits. Journal of molecular biology. 1998;278:935–948. doi: 10.1006/jmbi.1998.1737. [DOI] [PubMed] [Google Scholar]
- 41.Baranov PV, Gesteland RF, Atkins JF. Recoding: translational bifurcations in gene expression. Gene. 2002;286:187–201. doi: 10.1016/s0378-1119(02)00423-7. [DOI] [PubMed] [Google Scholar]
- 42.Borngraber S, Browner M, Gillmor S, Gerth C, Anton M, Fletterick R, Kuhn H. Shape and specificity in mammalian 15-lipoxygenase active site. The functional interplay of sequence determinants for the reaction specificity. The Journal of biological chemistry. 1999;274:37345–37350. doi: 10.1074/jbc.274.52.37345. [DOI] [PubMed] [Google Scholar]
- 43.Sloane DL, Leung R, Craik CS, Sigal E. A primary determinant for lipoxygenase positional specificity. Nature. 1991;354:149–152. doi: 10.1038/354149a0. [DOI] [PubMed] [Google Scholar]
- 44.Borngraber S, Kuban RJ, Anton M, Kuhn H. Phenylalanine 353 is a primary determinant for the positional specificity of mammalian 15-lipoxygenases. Journal of molecular biology. 1996;264:1145–1153. doi: 10.1006/jmbi.1996.0702. [DOI] [PubMed] [Google Scholar]
- 45.Jisaka M, Kim RB, Boeglin WE, Brash AR. Identification of amino acid determinants of the positional specificity of mouse 8S-lipoxygenase and human 15S-lipoxygenase-2. The Journal of biological chemistry. 2000;275:1287–1293. doi: 10.1074/jbc.275.2.1287. [DOI] [PubMed] [Google Scholar]
- 46.Gillmor SA, Villasenor A, Fletterick R, Sigal E, Browner MF. The structure of mammalian 15-lipoxygenase reveals similarity to the lipases and the determinants of substrate specificity. Nature structural biology. 1997;4:1003–1009. doi: 10.1038/nsb1297-1003. [DOI] [PubMed] [Google Scholar]
- 47.Choi J, Chon JK, Kim S, Shin W. Conformational flexibility in mammalian 15S-lipoxygenase: Reinterpretation of the crystallographic data. Proteins. 2008;70:1023–1032. doi: 10.1002/prot.21590. [DOI] [PubMed] [Google Scholar]
- 48.Gilbert NC, Bartlett SG, Waight MT, Neau DB, Boeglin WE, Brash AR, Newcomer ME. The structure of human 5-lipoxygenase. Science. 2011;331:217–219. doi: 10.1126/science.1197203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu S, Mueser TC, Marnett LJ, Funk MO., Jr Crystal structure of 12-lipoxygenase catalytic-domain-inhibitor complex identifies a substrate-binding channel for catalysis. Structure. 2012;20:1490–1497. doi: 10.1016/j.str.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kobe MJ, Neau DB, Mitchell CE, Bartlett SG, Newcomer ME. The structure of human 15-lipoxygenase-2 with a substrate mimic. The Journal of biological chemistry. 2014;289:8562–8569. doi: 10.1074/jbc.M113.543777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gilbert NC, Rui Z, Neau DB, Waight MT, Bartlett SG, Boeglin WE, Brash AR, Newcomer ME. Conversion of human 5-lipoxygenase to a 15-lipoxygenase by a point mutation to mimic phosphorylation at Serine-663. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:3222–3229. doi: 10.1096/fj.12-205286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.S A. 2014 unpublished results, in. [Google Scholar]
- 53.Mei G, Di Venere A, Nicolai E, Angelucci CB, Ivanov I, Sabatucci A, Dainese E, Kuhn H, Maccarrone M. Structural properties of plant and mammalian lipoxygenases. Temperature-dependent conformational alterations and membrane binding ability. Biochemistry. 2008;47:9234–9242. doi: 10.1021/bi800638v. [DOI] [PubMed] [Google Scholar]
- 54.Hammel M, Walther M, Prassl R, Kuhn H. Structural flexibility of the N-terminal beta-barrel domain of 15-lipoxygenase-1 probed by small angle X-ray scattering. Functional consequences for activity regulation and membrane binding. Journal of molecular biology. 2004;343:917–929. doi: 10.1016/j.jmb.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 55.Dainese E, Sabatucci A, van Zadelhoff G, Angelucci CB, Vachette P, Veldink GA, Agro AF, Maccarrone M. Structural stability of soybean lipoxygenase-1 in solution as probed by small angle X-ray scattering. Journal of molecular biology. 2005;349:143–152. doi: 10.1016/j.jmb.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 56.Hammel M. Validation of macromolecular flexibility in solution by small-angle X-ray scattering (SAXS) European biophysics journal : EBJ. 2012;41:789–799. doi: 10.1007/s00249-012-0820-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shang W, Ivanov I, Svergun DI, Borbulevych OY, Aleem AM, Stehling S, Jankun J, Kuhn H, Skrzypczak-Jankun E. Probing dimerization and structural flexibility of mammalian lipoxygenases by small-angle X-ray scattering. Journal of molecular biology. 2011;409:654–668. doi: 10.1016/j.jmb.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 58.Moin ST, Hofer TS, Sattar R, Ul-Haq Z. Molecular dynamics simulation of mammalian 15S-lipoxygenase with AMBER force field. European biophysics journal : EBJ. 2011;40:715–726. doi: 10.1007/s00249-011-0684-5. [DOI] [PubMed] [Google Scholar]
- 59.Aleem AM, Jankun J, Dignam JD, Walther M, Kuhn H, Svergun DI, Skrzypczak-Jankun E. Human platelet 12-lipoxygenase, new findings about its activity, membrane binding and low-resolution structure. Journal of molecular biology. 2008;376:193–209. doi: 10.1016/j.jmb.2007.11.086. [DOI] [PubMed] [Google Scholar]
- 60.Falgueyret JP, Denis D, Macdonald D, Hutchinson JH, Riendeau D. Characterization of the arachidonate and ATP binding sites of human 5-lipoxygenase using photoaffinity labeling and enzyme immobilization. Biochemistry. 1995;34:13603–13611. doi: 10.1021/bi00041a040. [DOI] [PubMed] [Google Scholar]
- 61.Wecksler AT, Kenyon V, Deschamps JD, Holman TR. Substrate specificity changes for human reticulocyte and epithelial 15-lipoxygenases reveal allosteric product regulation. Biochemistry. 2008;47:7364–7375. doi: 10.1021/bi800550n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wecksler AT, Jacquot C, van der Donk WA, Holman TR. Mechanistic investigations of human reticulocyte 15- and platelet 12-lipoxygenases with arachidonic acid. Biochemistry. 2009;48:6259–6267. doi: 10.1021/bi802332j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wecksler AT, Garcia NK, Holman TR. Substrate specificity effects of lipoxygenase products and inhibitors on soybean lipoxygenase-1. Bioorganic & medicinal chemistry. 2009;17:6534–6539. doi: 10.1016/j.bmc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ivanov I, Shang W, Toledo L, Masgrau L, Svergun DI, Stehling S, Gomez H, Di Venere A, Mei G, Lluch JM, Skrzypczak-Jankun E, Gonzalez-Lafont A, Kuhn H. Ligand-induced formation of transient dimers of mammalian 12/15-lipoxygenase: a key to allosteric behavior of this class of enzymes? Proteins. 2012;80:703–712. doi: 10.1002/prot.23227. [DOI] [PubMed] [Google Scholar]
- 65.Hafner AK, Cernescu M, Hofmann B, Ermisch M, Hornig M, Metzner J, Schneider G, Brutschy B, Steinhilber D. Dimerization of human 5-lipoxygenase. Biological chemistry. 2011;392:1097–1111. doi: 10.1515/BC.2011.200. [DOI] [PubMed] [Google Scholar]
- 66.van Leyen K, Duvoisin RM, Engelhardt H, Wiedmann M. A function for lipoxygenase in programmed organelle degradation. Nature. 1998;395:392–395. doi: 10.1038/26500. [DOI] [PubMed] [Google Scholar]
- 67.Klinman JP. Importance of protein dynamics during enzymatic C–H bond cleavage catalysis. Biochemistry. 2013;52:2068–2077. doi: 10.1021/bi301504m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hatcher E, Soudackov AV, Hammes-Schiffer S. Proton-coupled electron transfer in soybean lipoxygenase: dynamical behavior and temperature dependence of kinetic isotope effects. Journal of the American Chemical Society. 2007;129:187–196. doi: 10.1021/ja0667211. [DOI] [PubMed] [Google Scholar]
- 69.Rapoport SM, Schewe T, Wiesner R, Halangk W, Ludwig P, Janicke-Hohne M, Tannert C, Hiebsch C, Klatt D. The lipoxygenase of reticulocytes. Purification, characterization and biological dynamics of the lipoxygenase; its identity with the respiratory inhibitors of the reticulocyte. European journal of biochemistry / FEBS. 1979;96:545–561. doi: 10.1111/j.1432-1033.1979.tb13068.x. [DOI] [PubMed] [Google Scholar]
- 70.Schewe T, Wiesner R, Rapoport SM. Lipoxygenase from rabbit reticulocytes. Methods in enzymology. 1981;71(Pt C):430–441. doi: 10.1016/0076-6879(81)71054-1. [DOI] [PubMed] [Google Scholar]
- 71.Hohne M, Bayer D, Prehn S, Schewe T, Rapoport SM. In vitro maturation of rabbit reticulocytes. III. Response of lipoxygenase. Biomedica biochimica acta. 1983;42:1129–1134. [PubMed] [Google Scholar]
- 72.Schewe T, Halangk W, Hiebsch C, Rapoport S. Degradation of mitochondria by cytosolic factors in reticulocytes. Acta biologica et medica Germanica. 1977;36:563–572. [PubMed] [Google Scholar]
- 73.Kuhn H, Brash AR. Occurrence of lipoxygenase products in membranes of rabbit reticulocytes. Evidence for a role of the reticulocyte lipoxygenase in the maturation of red cells. The Journal of biological chemistry. 1990;265:1454–1458. [PubMed] [Google Scholar]
- 74.Kuhn H, Belkner J, Wiesner R, Brash AR. Oxygenation of biological membranes by the pure reticulocyte lipoxygenase. The Journal of biological chemistry. 1990;265:18351–18361. [PubMed] [Google Scholar]
- 75.Rapoport SM, Schewe T. The maturational breakdown of mitochondria in reticulocytes. Biochimica et biophysica acta. 1986;864:471–495. doi: 10.1016/0304-4157(86)90006-7. [DOI] [PubMed] [Google Scholar]
- 76.Grullich C, Duvoisin RM, Wiedmann M, van Leyen K. Inhibition of 15-lipoxygenase leads to delayed organelle degradation in the reticulocyte. FEBS letters. 2001;489:51–54. doi: 10.1016/s0014-5793(01)02080-4. [DOI] [PubMed] [Google Scholar]
- 77.Blanc L, Barres C, Bette-Bobillo P, Vidal M. Reticulocyte-secreted exosomes bind natural IgM antibodies: involvement of a ROS-activatable endosomal phospholipase iPLA2. Blood. 2007;110:3407–3416. doi: 10.1182/blood-2007-04-085845. [DOI] [PubMed] [Google Scholar]
- 78.Sun D, Funk CD. Disruption of 12/15-lipoxygenase expression in peritoneal macrophages. Enhanced utilization of the 5-lipoxygenase pathway and diminished oxidation of low density lipoprotein. The Journal of biological chemistry. 1996;271:24055–24062. [PubMed] [Google Scholar]
- 79.Gronowicz G, Swift H, Steck TL. Maturation of the reticulocyte in vitro. Journal of cell science. 1984;71:177–197. doi: 10.1242/jcs.71.1.177. [DOI] [PubMed] [Google Scholar]
- 80.Kent G, Minick OT, Volini FI, Orfei E. Autophagic vacuoles in human red cells. The American journal of pathology. 1966;48:831–857. [PMC free article] [PubMed] [Google Scholar]
- 81.Griffiths RE, Kupzig S, Cogan N, Mankelow TJ, Betin VM, Trakarnsanga K, Massey EJ, Parsons SF, Anstee DJ, Lane JD. The ins and outs of human reticulocyte maturation: autophagy and the endosome/exosome pathway. Autophagy. 2012;8:1150–1151. doi: 10.4161/auto.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mortensen M, Ferguson DJ, Simon AK. Mitochondrial clearance by autophagy in developing erythrocytes: clearly important, but just how much so? Cell cycle. 2010;9:1901–1906. doi: 10.4161/cc.9.10.11603. [DOI] [PubMed] [Google Scholar]
- 83.Jang I, Park S, Cho JW, Yigitkanli K, van Leyen K, Roth J. Genetic ablation and short-duration inhibition of lipoxygenase results in increased macroautophagy. Experimental cell research. 2014;321:276–287. doi: 10.1016/j.yexcr.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 84.Krieg P, Rosenberger S, de Juanes S, Latzko S, Hou J, Dick A, Kloz U, van der Hoeven F, Hausser I, Esposito I, Rauh M, Schneider H. Aloxe3 knockout mice reveal a function of epidermal lipoxygenase-3 as hepoxilin synthase and its pivotal role in barrier formation. The Journal of investigative dermatology. 2013;133:172–180. doi: 10.1038/jid.2012.250. [DOI] [PubMed] [Google Scholar]
- 85.Epp N, Furstenberger G, Muller K, de Juanes S, Leitges M, Hausser I, Thieme F, Liebisch G, Schmitz G, Krieg P. 12R-lipoxygenase deficiency disrupts epidermal barrier function. The Journal of cell biology. 2007;177:173–182. doi: 10.1083/jcb.200612116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nemes Z, Steinert PM. Bricks and mortar of the epidermal barrier. Experimental & molecular medicine. 1999;31:5–19. doi: 10.1038/emm.1999.2. [DOI] [PubMed] [Google Scholar]
- 87.Eckhart L, Lippens S, Tschachler E, Declercq W. Cell death by cornification. Biochimica et biophysica acta. 2013;1833:3471–3480. doi: 10.1016/j.bbamcr.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 88.Kypriotou M, Huber M, Hohl D. The human epidermal differentiation complex: cornified envelope precursors, S100 proteins and the 'fused genes' family. Experimental dermatology. 2012;21:643–649. doi: 10.1111/j.1600-0625.2012.01472.x. [DOI] [PubMed] [Google Scholar]
- 89.Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nature reviews. Molecular cell biology. 2005;6:328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- 90.Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Experimental dermatology. 2008;17:1063–1072. doi: 10.1111/j.1600-0625.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 91.Johnson EN, Nanney LB, Virmani J, Lawson JA, Funk CD. Basal transepidermal water loss is increased in platelet-type 12-lipoxygenase deficient mice. The Journal of investigative dermatology. 1999;112:861–865. doi: 10.1046/j.1523-1747.1999.00595.x. [DOI] [PubMed] [Google Scholar]
- 92.Johnson EN, Brass LF, Funk CD. Increased platelet sensitivity to ADP in mice lacking platelet-type 12-lipoxygenase. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3100–3105. doi: 10.1073/pnas.95.6.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Akiyama M. Harlequin ichthyosis and other autosomal recessive congenital ichthyoses: the underlying genetic defects and pathomechanisms. Journal of dermatological science. 2006;42:83–89. doi: 10.1016/j.jdermsci.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 94.Eckl KM, Krieg P, Kuster W, Traupe H, Andre F, Wittstruck N, Furstenberger G, Hennies HC. Mutation spectrum and functional analysis of epidermis-type lipoxygenases in patients with autosomal recessive congenital ichthyosis. Human mutation. 2005;26:351–361. doi: 10.1002/humu.20236. [DOI] [PubMed] [Google Scholar]
- 95.Vahlquist A, Bygum A, Ganemo A, Virtanen M, Hellstrom-Pigg M, Strauss G, Brandrup F, Fischer J. Genotypic and clinical spectrum of self-improving collodion ichthyosis: ALOX12B, ALOXE3, and TGM1 mutations in Scandinavian patients. The Journal of investigative dermatology. 2010;130:438–443. doi: 10.1038/jid.2009.346. [DOI] [PubMed] [Google Scholar]
- 96.Eckl KM, de Juanes S, Kurtenbach J, Natebus M, Lugassy J, Oji V, Traupe H, Preil ML, Martinez F, Smolle J, Harel A, Krieg P, Sprecher E, Hennies HC. Molecular analysis of 250 patients with autosomal recessive congenital ichthyosis: evidence for mutation hotspots in ALOXE3 and allelic heterogeneity in ALOX12B. The Journal of investigative dermatology. 2009;129:1421–1428. doi: 10.1038/jid.2008.409. [DOI] [PubMed] [Google Scholar]
- 97.Terrinoni A, Serra V, Codispoti A, Talamonti E, Bui L, Palombo R, Sette M, Campione E, Didona B, Annicchiarico-Petruzzelli M, Zambruno G, Melino G, Candi E. Novel transglutaminase 1 mutations in patients affected by lamellar ichthyosis. Cell death & disease. 2012;3:e416. doi: 10.1038/cddis.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Munoz-Garcia A, Thomas CP, Keeney DS, Zheng Y, Brash AR. The importance of the lipoxygenase-hepoxilin pathway in the mammalian epidermal barrier. Biochimica et biophysica acta. 2014;1841:401–408. doi: 10.1016/j.bbalip.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu Z, Schneider C, Boeglin WE, Marnett LJ, Brash AR. The lipoxygenase gene ALOXE3 implicated in skin differentiation encodes a hydroperoxide isomerase. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9162–9167. doi: 10.1073/pnas.1633612100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.DuBois RN. Leukotriene A4 signaling, inflammation, and cancer. Journal of the National Cancer Institute. 2003;95:1028–1029. doi: 10.1093/jnci/95.14.1028. [DOI] [PubMed] [Google Scholar]
- 101.Nie D, Hillman GG, Geddes T, Tang K, Pierson C, Grignon DJ, Honn KV. Platelet-type 12-lipoxygenase in a human prostate carcinoma stimulates angiogenesis and tumor growth. Cancer research. 1998;58:4047–4051. [PubMed] [Google Scholar]
- 102.Kelavkar UP, Nixon JB, Cohen C, Dillehay D, Eling TE, Badr KF. Overexpression of 15-lipoxygenase-1 in PC-3 human prostate cancer cells increases tumorigenesis. Carcinogenesis. 2001;22:1765–1773. doi: 10.1093/carcin/22.11.1765. [DOI] [PubMed] [Google Scholar]
- 103.Shureiqi I, Jiang W, Zuo X, Wu Y, Stimmel JB, Leesnitzer LM, Morris JS, Fan HZ, Fischer SM, Lippman SM. The 15-lipoxygenase-1 product 13-S-hydroxyoctadecadienoic acid down-regulates PPAR-delta to induce apoptosis in colorectal cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9968–9973. doi: 10.1073/pnas.1631086100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shappell SB, Boeglin WE, Olson SJ, Kasper S, Brash AR. 15-lipoxygenase-2 (15-LOX-2) is expressed in benign prostatic epithelium and reduced in prostate adenocarcinoma. The American journal of pathology. 1999;155:235–245. doi: 10.1016/S0002-9440(10)65117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hsi LC, Wilson LC, Eling TE. Opposing effects of 15-lipoxygenase-1 and-2 metabolites on MAPK signaling in prostate. Alteration in peroxisome proliferator-activated receptor gamma. The Journal of biological chemistry. 2002;277:40549–40556. doi: 10.1074/jbc.M203522200. [DOI] [PubMed] [Google Scholar]
- 106.Xu XC, Shappell SB, Liang Z, Song S, Menter D, Subbarayan V, Iyengar S, Tang DG, Lippman SM. Reduced 15S-lipoxygenase-2 expression in esophageal cancer specimens and cells and upregulation in vitro by the cyclooxygenase-2 inhibitor, NS398. Neoplasia. 2003;5:121–127. doi: 10.1016/s1476-5586(03)80003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bhatia B, Maldonado CJ, Tang S, Chandra D, Klein RD, Chopra D, Shappell SB, Yang P, Newman RA, Tang DG. Subcellular localization and tumor-suppressive functions of 15-lipoxygenase 2 (15-LOX2) and its splice variants. The Journal of biological chemistry. 2003;278:25091–25100. doi: 10.1074/jbc.M301920200. [DOI] [PubMed] [Google Scholar]
- 108.Wu Y, Fang B, Yang XQ, Wang L, Chen D, Krasnykh V, Carter BZ, Morris JS, Shureiqi I. Therapeutic molecular targeting of 15-lipoxygenase-1 in colon cancer. Molecular therapy : the journal of the American Society of Gene Therapy. 2008;16:886–892. doi: 10.1038/mt.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kelavkar U, Cohen C, Eling T, Badr K. 15-lipoxygenase-1 overexpression in prostate adenocarcinoma. Advances in experimental medicine and biology. 2002;507:133–145. doi: 10.1007/978-1-4615-0193-0_22. [DOI] [PubMed] [Google Scholar]
- 110.Tavakoli-Yaraki M, Karami-Tehrani F, Salimi V, Sirati-Sabet M. Induction of apoptosis by Trichostatin A in human breast cancer cell lines: involvement of 15-Lox-1. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2013;34:241–249. doi: 10.1007/s13277-012-0544-7. [DOI] [PubMed] [Google Scholar]
- 111.Wen Z, Liu H, Li M, Li B, Gao W, Shao Q, Fan B, Zhao F, Wang Q, Xie Q, Yang Y, Yu J, Qu X. Increased metabolites of 5-lipoxygenase from hypoxic ovarian cancer cells promote tumor-associated macrophage infiltration. Oncogene. 2014 doi: 10.1038/onc.2014.85. [DOI] [PubMed] [Google Scholar]
- 112.Roffeis J, Hornung D, Kuhn H, Walther M. 15-Lipoxygenase-2 is differentially expressed in normal and neoplastic ovary. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation. 2007;16:568–575. doi: 10.1097/CEJ.0b013e32801023c4. [DOI] [PubMed] [Google Scholar]
- 113.Celenk F, Bayramoglu I, Yilmaz A, Menevse A, Bayazit Y. Expression of cyclooxygenase-2, 12-lipoxygenase, and inducible nitric oxide synthase in head and neck squamous cell carcinoma. The Journal of craniofacial surgery. 2013;24:1114–1117. doi: 10.1097/SCS.0b013e31828f2491. [DOI] [PubMed] [Google Scholar]
- 114.Faronato M, Muzzonigro G, Milanese G, Menna C, Bonfigli AR, Catalano A, Procopio A. Increased expression of 5-lipoxygenase is common in clear cell renal cell carcinoma. Histology and histopathology. 2007;22:1109–1118. doi: 10.14670/HH-22.1109. [DOI] [PubMed] [Google Scholar]
- 115.Hayashi T, Nishiyama K, Shirahama T. Inhibition of 5-lipoxygenase pathway suppresses the growth of bladder cancer cells. International journal of urology : official journal of the Japanese Urological Association. 2006;13:1086–1091. doi: 10.1111/j.1442-2042.2006.01485.x. [DOI] [PubMed] [Google Scholar]
- 116.Cathcart MC, Lysaght J, Pidgeon GP. Eicosanoid signalling pathways in the development and progression of colorectal cancer: novel approaches for prevention/intervention. Cancer metastasis reviews. 2011;30:363–385. doi: 10.1007/s10555-011-9324-x. [DOI] [PubMed] [Google Scholar]
- 117.Matsuyama M, Yoshimura R. The target of arachidonic acid pathway is a new anticancer strategy for human prostate cancer. Biologics : targets & therapy. 2008;2:725–732. doi: 10.2147/btt.s3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schneider C, Pozzi A. Cyclooxygenases and lipoxygenases in cancer. Cancer metastasis reviews. 2011;30:277–294. doi: 10.1007/s10555-011-9310-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shureiqi I, Chen D, Day RS, Zuo X, Hochman FL, Ross WA, Cole RA, Moy O, Morris JS, Xiao L, Newman RA, Yang P, Lippman SM. Profiling lipoxygenase metabolism in specific steps of colorectal tumorigenesis. Cancer prevention research. 2010;3:829–838. doi: 10.1158/1940-6207.CAPR-09-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wasilewicz MP, Kolodziej B, Bojulko T, Kaczmarczyk M, Sulzyc-Bielicka V, Bielicki D, Ciepiela K. Overexpression of 5-lipoxygenase in sporadic colonic adenomas and a possible new aspect of colon carcinogenesis. International journal of colorectal disease. 2010;25:1079–1085. doi: 10.1007/s00384-010-0980-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Melstrom LG, Bentrem DJ, Salabat MR, Kennedy TJ, Ding XZ, Strouch M, Rao SM, Witt RC, Ternent CA, Talamonti MS, Bell RH, Adrian TA. Overexpression of 5-lipoxygenase in colon polyps and cancer and the effect of 5-LOX inhibitors in vitro and in a murine model. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:6525–6530. doi: 10.1158/1078-0432.CCR-07-4631. [DOI] [PubMed] [Google Scholar]
- 122.Ihara A, Wada K, Yoneda M, Fujisawa N, Takahashi H, Nakajima A. Blockade of leukotriene B4 signaling pathway induces apoptosis and suppresses cell proliferation in colon cancer. Journal of pharmacological sciences. 2007;103:24–32. doi: 10.1254/jphs.fp0060651. [DOI] [PubMed] [Google Scholar]
- 123.Ye YN, Liu ES, Shin VY, Wu WK, Cho CH. The modulating role of nuclear factor-kappaB in the action of alpha7-nicotinic acetylcholine receptor and cross-talk between 5-lipoxygenase and cyclooxygenase-2 in colon cancer growth induced by 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone. The Journal of pharmacology and experimental therapeutics. 2004;311:123–130. doi: 10.1124/jpet.104.068031. [DOI] [PubMed] [Google Scholar]
- 124.Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: complex stories. Nature reviews. Cancer. 2004;4:61–70. doi: 10.1038/nrc1254. [DOI] [PubMed] [Google Scholar]
- 125.Yoshinaga M, Buchanan FG, DuBois RN. 15-LOX-1 inhibits p21 (Cip/WAF 1) expression by enhancing MEK-ERK 1/2 signaling in colon carcinoma cells. Prostaglandins & other lipid mediators. 2004;73:111–122. doi: 10.1016/j.prostaglandins.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 126.Chiu J, Dawes IW. Redox control of cell proliferation. Trends in cell biology. 2012;22:592–601. doi: 10.1016/j.tcb.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 127.Cimen I, Tuncay S, Banerjee S. 15-Lipoxygenase-1 expression suppresses the invasive properties of colorectal carcinoma cell lines HCT-116 and HT-29. Cancer science. 2009;100:2283–2291. doi: 10.1111/j.1349-7006.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cimen I, Astarci E, Banerjee S. 15-lipoxygenase-1 exerts its tumor suppressive role by inhibiting nuclear factor-kappa B via activation of PPAR gamma. Journal of cellular biochemistry. 2011;112:2490–2501. doi: 10.1002/jcb.23174. [DOI] [PubMed] [Google Scholar]
- 129.Ryan A, Godson C. Lipoxins: regulators of resolution. Current opinion in pharmacology. 2010;10:166–172. doi: 10.1016/j.coph.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 130.Lee HN, Surh YJ. Therapeutic potential of resolvins in the prevention and treatment of inflammatory disorders. Biochemical pharmacology. 2012;84:1340–1350. doi: 10.1016/j.bcp.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 131.Dalli J, Colas RA, Serhan CN. Novel n-3 immunoresolvents: structures and actions. Scientific reports. 2013;3:1940. doi: 10.1038/srep01940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Janakiram NB, Mohammed A, Rao CV. Role of lipoxins, resolvins, and other bioactive lipids in colon and pancreatic cancer. Cancer metastasis reviews. 2011;30:507–523. doi: 10.1007/s10555-011-9311-2. [DOI] [PubMed] [Google Scholar]
- 133.Wendel M, Heller AR. Anticancer actions of omega-3 fatty acids--current state and future perspectives. Anti-cancer agents in medicinal chemistry. 2009;9:457–470. doi: 10.2174/1871520610909040457. [DOI] [PubMed] [Google Scholar]
- 134.Mangino MJ, Brounts L, Harms B, Heise C. Lipoxin biosynthesis in inflammatory bowel disease. Prostaglandins & other lipid mediators. 2006;79:84–92. doi: 10.1016/j.prostaglandins.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 135.Zhang B, Jia H, Liu J, Yang Z, Jiang T, Tang K, Li D, Huang C, Ma J, Shen GX, Ye D, Huang B. Depletion of regulatory T cells facilitates growth of established tumors: a mechanism involving the regulation of myeloid-derived suppressor cells by lipoxin A4. Journal of immunology. 2010;185:7199–7206. doi: 10.4049/jimmunol.1001876. [DOI] [PubMed] [Google Scholar]
- 136.Gupta S, Srivastava M, Ahmad N, Sakamoto K, Bostwick DG, Mukhtar H. Lipoxygenase-5 is overexpressed in prostate adenocarcinoma. Cancer. 2001;91:737–743. doi: 10.1002/1097-0142(20010215)91:4<737::aid-cncr1059>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 137.Sarveswaran S, Thamilselvan V, Brodie C, Ghosh J. Inhibition of 5-lipoxygenase triggers apoptosis in prostate cancer cells via down-regulation of protein kinase C-epsilon. Biochimica et biophysica acta. 2011;1813:2108–2117. doi: 10.1016/j.bbamcr.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ghosh J. Inhibition of arachidonate 5-lipoxygenase triggers prostate cancer cell death through rapid activation of c-Jun N-terminal kinase. Biochemical and biophysical research communications. 2003;307:342–349. doi: 10.1016/s0006-291x(03)01201-4. [DOI] [PubMed] [Google Scholar]
- 139.Ghosh J, Myers CE. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13182–13187. doi: 10.1073/pnas.95.22.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sarveswaran S, Myers CE, Ghosh J. MK591, a leukotriene biosynthesis inhibitor, induces apoptosis in prostate cancer cells: synergistic action with LY294002, an inhibitor of phosphatidylinositol 3'-kinase. Cancer letters. 2010;291:167–176. doi: 10.1016/j.canlet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 141.Matsuyama M, Hayama T, Funao K, Kawahito Y, Sano H, Takemoto Y, Nakatani T, Yoshimura R. Overexpression of cysteinyl LT1 receptor in prostate cancer and CysLT1R antagonist inhibits prostate cancer cell growth through apoptosis. Oncology reports. 2007;18:99–104. [PubMed] [Google Scholar]
- 142.Bishayee K, Khuda-Bukhsh AR. 5-lipoxygenase antagonist therapy: a new approach towards targeted cancer chemotherapy. Acta biochimica et biophysica Sinica. 2013;45:709–719. doi: 10.1093/abbs/gmt064. [DOI] [PubMed] [Google Scholar]
- 143.Hong SH, Avis I, Vos MD, Martinez A, Treston AM, Mulshine JL. Relationship of arachidonic acid metabolizing enzyme expression in epithelial cancer cell lines to the growth effect of selective biochemical inhibitors. Cancer research. 1999;59:2223–2228. [PubMed] [Google Scholar]
- 144.Spindler SA, Sarkar FH, Sakr WA, Blackburn ML, Bull AW, LaGattuta M, Reddy RG. Production of 13-hydroxyoctadecadienoic acid (13-HODE) by prostate tumors and cell lines. Biochemical and biophysical research communications. 1997;239:775–781. doi: 10.1006/bbrc.1997.7471. [DOI] [PubMed] [Google Scholar]
- 145.Kelavkar UP, Parwani AV, Shappell SB, Martin WD. Conditional expression of human 15-lipoxygenase-1 in mouse prostate induces prostatic intraepithelial neoplasia: the FLiMP mouse model. Neoplasia. 2006;8:510–522. doi: 10.1593/neo.06202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hu Y, Sun H, O'Flaherty JT, Edwards IJ. 15-Lipoxygenase-1-mediated metabolism of docosahexaenoic acid is required for syndecan-1 signaling and apoptosis in prostate cancer cells. Carcinogenesis. 2013;34:176–182. doi: 10.1093/carcin/bgs324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.O'Flaherty JT, Hu Y, Wooten RE, Horita DA, Samuel MP, Thomas MJ, Sun H, Edwards IJ. 15-lipoxygenase metabolites of docosahexaenoic acid inhibit prostate cancer cell proliferation and survival. PloS one. 2012;7:e45480. doi: 10.1371/journal.pone.0045480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tang DG, Bhatia B, Tang S, Schneider-Broussard R. 15-lipoxygenase 2 (15-LOX2) is a functional tumor suppressor that regulates human prostate epithelial cell differentiation, senescence, and growth (size) Prostaglandins & other lipid mediators. 2007;82:135–146. doi: 10.1016/j.prostaglandins.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 149.Tang S, Bhatia B, Zhou J, Maldonado CJ, Chandra D, Kim E, Fischer SM, Butler AP, Friedman SL, Tang DG. Evidence that Sp1 positively and Sp3 negatively regulate and androgen does not directly regulate functional tumor suppressor 15-lipoxygenase 2 (15-LOX2) gene expression in normal human prostate epithelial cells. Oncogene. 2004;23:6942–6953. doi: 10.1038/sj.onc.1207913. [DOI] [PubMed] [Google Scholar]
- 150.Shappell SB, Gupta RA, Manning S, Whitehead R, Boeglin WE, Schneider C, Case T, Price J, Jack GS, Wheeler TM, Matusik RJ, Brash AR, Dubois RN. 15S-Hydroxyeicosatetraenoic acid activates peroxisome proliferator-activated receptor gamma and inhibits proliferation in PC3 prostate carcinoma cells. Cancer research. 2001;61:497–503. [PubMed] [Google Scholar]
- 151.Bhatia B, Tang S, Yang P, Doll A, Aumueller G, Newman RA, Tang DG. Cell-autonomous induction of functional tumor suppressor 15-lipoxygenase 2 (15-LOX2) contributes to replicative senescence of human prostate progenitor cells. Oncogene. 2005;24:3583–3595. doi: 10.1038/sj.onc.1208406. [DOI] [PubMed] [Google Scholar]
- 152.Suraneni MV, Schneider-Broussard R, Moore JR, Davis TC, Maldonado CJ, Li H, Newman RA, Kusewitt D, Hu J, Yang P, Tang DG. Transgenic expression of 15-lipoxygenase 2 (15-LOX2) in mouse prostate leads to hyperplasia and cell senescence. Oncogene. 2010;29:4261–4275. doi: 10.1038/onc.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Subbarayan V, Krieg P, Hsi LC, Kim J, Yang P, Sabichi AL, Llansa N, Mendoza G, Logothetis CJ, Newman RA, Lippman SM, Menter DG. 15-Lipoxygenase-2 gene regulation by its product 15-(S)-hydroxyeicosatetraenoic acid through a negative feedback mechanism that involves peroxisome proliferator-activated receptor gamma. Oncogene. 2006;25:6015–6025. doi: 10.1038/sj.onc.1209617. [DOI] [PubMed] [Google Scholar]
- 154.Tang Y, Wang MT, Chen Y, Yang D, Che M, Honn KV, Akers GD, Johnson SR, Nie D. Downregulation of vascular endothelial growth factor and induction of tumor dormancy by 15-lipoxygenase-2 in prostate cancer. International journal of cancer. Journal international du cancer. 2009;124:1545–1551. doi: 10.1002/ijc.24118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Feng Y, Bai X, Yang Q, Wu H, Wang D. Downregulation of 15-lipoxygenase 2 by glucocorticoid receptor in prostate cancer cells. International journal of oncology. 2010;36:1541–1549. doi: 10.3892/ijo_00000641. [DOI] [PubMed] [Google Scholar]
- 156.Nie D, Nemeth J, Qiao Y, Zacharek A, Li L, Hanna K, Tang K, Hillman GG, Cher ML, Grignon DJ, Honn KV. Increased metastatic potential in human prostate carcinoma cells by overexpression of arachidonate 12-lipoxygenase. Clinical & experimental metastasis. 2003;20:657–663. doi: 10.1023/a:1027302408187. [DOI] [PubMed] [Google Scholar]
- 157.Pidgeon GP, Tang K, Cai YL, Piasentin E, Honn KV. Overexpression of platelet-type 12-lipoxygenase promotes tumor cell survival by enhancing alpha(v)beta(3) and alpha(v)beta(5) integrin expression. Cancer research. 2003;63:4258–4267. [PubMed] [Google Scholar]
- 158.Kandouz M, Nie D, Pidgeon GP, Krishnamoorthy S, Maddipati KR, Honn KV. Platelet-type 12-lipoxygenase activates NF-kappaB in prostate cancer cells. Prostaglandins & other lipid mediators. 2003;71:189–204. doi: 10.1016/s1098-8823(03)00042-x. [DOI] [PubMed] [Google Scholar]
- 159.Jain G, Cronauer MV, Schrader M, Moller P, Marienfeld RB. NF-kappaB signaling in prostate cancer: a promising therapeutic target? World journal of urology. 2012;30:303–310. doi: 10.1007/s00345-011-0792-y. [DOI] [PubMed] [Google Scholar]
- 160.Kelavkar UP, Hutzley J, McHugh K, Allen KG, Parwani A. Prostate tumor growth can be modulated by dietarily targeting the 15-lipoxygenase-1 and cyclooxygenase-2 enzymes. Neoplasia. 2009;11:692–699. doi: 10.1593/neo.09334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Stohs SJ, Goldberg K. What You Should Know About Omega-3 Fatty Acids and Prostate Cancer. Journal of dietary supplements. 2014 doi: 10.3109/19390211.2014.902003. [DOI] [PubMed] [Google Scholar]
- 162.Gu Z, Suburu J, Chen H, Chen YQ. Mechanisms of omega-3 polyunsaturated fatty acids in prostate cancer prevention. BioMed research international. 2013;2013:824563. doi: 10.1155/2013/824563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Forman HJ, Ursini F, Maiorino M. An overview of mechanisms of redox signaling. Journal of molecular and cellular cardiology. 2014 doi: 10.1016/j.yjmcc.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Radmark O, Samuelsson B. Regulation of the activity of 5-lipoxygenase, a key enzyme in leukotriene biosynthesis. Biochemical and biophysical research communications. 2010;396:105–110. doi: 10.1016/j.bbrc.2010.02.173. [DOI] [PubMed] [Google Scholar]
- 165.Radmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase: regulation of expression and enzyme activity. Trends in biochemical sciences. 2007;32:332–341. doi: 10.1016/j.tibs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 166.Singh RK, Gupta S, Dastidar S, Ray A. Cysteinyl leukotrienes and their receptors: molecular and functional characteristics. Pharmacology. 2010;85:336–349. doi: 10.1159/000312669. [DOI] [PubMed] [Google Scholar]
- 167.Powell WS, Rokach J. The eosinophil chemoattractant 5-oxo-ETE and the OXE receptor. Progress in lipid research. 2013;52:651–665. doi: 10.1016/j.plipres.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Singh RK, Tandon R, Dastidar SG, Ray A. A review on leukotrienes and their receptors with reference to asthma. The Journal of asthma : official journal of the Association for the Care of Asthma. 2013;50:922–931. doi: 10.3109/02770903.2013.823447. [DOI] [PubMed] [Google Scholar]
- 169.Cobanoglu B, Toskala E, Ural A, Cingi C. Role of leukotriene antagonists and antihistamines in the treatment of allergic rhinitis. Current allergy and asthma reports. 2013;13:203–208. doi: 10.1007/s11882-013-0341-4. [DOI] [PubMed] [Google Scholar]
- 170.Gane J, Buckley R. Leukotriene receptor antagonists in allergic eye disease: a systematic review and meta-analysis. The journal of allergy and clinical immunology. In practice. 2013;1:65–74. doi: 10.1016/j.jaip.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 171.Dahlen SE, Hedqvist P, Hammarstrom S, Samuelsson B. Leukotrienes are potent constrictors of human bronchi. Nature. 1980;288:484–486. doi: 10.1038/288484a0. [DOI] [PubMed] [Google Scholar]
- 172.Dahlen SE, Bjork J, Hedqvist P, Arfors KE, Hammarstrom S, Lindgren JA, Samuelsson B. Leukotrienes promote plasma leakage and leukocyte adhesion in postcapillary venules: in vivo effects with relevance to the acute inflammatory response. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:3887–3891. doi: 10.1073/pnas.78.6.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Peatfield AC, Piper PJ, Richardson PS. The effect of leukotriene C4 on mucin release into the cat trachea in vivo and in vitro. British journal of pharmacology. 1982;77:391–393. doi: 10.1111/j.1476-5381.1982.tb09310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kaliner M, Shelhamer JH, Borson B, Nadel J, Patow C, Marom Z. Human respiratory mucus. The American review of respiratory disease. 1986;134:612–621. doi: 10.1164/arrd.1986.134.3.612. [DOI] [PubMed] [Google Scholar]
- 175.Berger W, De Chandt MT, Cairns CB. Zileuton: clinical implications of 5-Lipoxygenase inhibition in severe airway disease. International journal of clinical practice. 2007;61:663–676. doi: 10.1111/j.1742-1241.2007.01320.x. [DOI] [PubMed] [Google Scholar]
- 176.Poff CD, Balazy M. Drugs that target lipoxygenases and leukotrienes as emerging therapies for asthma and cancer. Current drug targets. Inflammation and allergy. 2004;3:19–33. doi: 10.2174/1568010043483917. [DOI] [PubMed] [Google Scholar]
- 177.Matsuse H, Kohno S. Leukotriene receptor antagonists pranlukast and montelukast for treating asthma. Expert opinion on pharmacotherapy. 2014;15:353–363. doi: 10.1517/14656566.2014.872241. [DOI] [PubMed] [Google Scholar]
- 178.Laidlaw TM, Boyce JA. Cysteinyl leukotriene receptors, old and new; implications for asthma. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2012;42:1313–1320. doi: 10.1111/j.1365-2222.2012.03982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. The Cochrane database of systematic reviews. 2012;5:CD002314. doi: 10.1002/14651858.CD002314.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ducharme FM, Lasserson TJ, Cates CJ. Addition to inhaled corticosteroids of long-acting beta2-agonists versus anti-leukotrienes for chronic asthma. The Cochrane database of systematic reviews. 2011:CD003137. doi: 10.1002/14651858.CD003137.pub4. [DOI] [PubMed] [Google Scholar]
- 181.Amlani S, Nadarajah T, McIvor RA. Montelukast for the treatment of asthma in the adult population. Expert opinion on pharmacotherapy. 2011;12:2119–2128. doi: 10.1517/14656566.2011.600689. [DOI] [PubMed] [Google Scholar]
- 182.Dumitru C, Chan SM, Turcanu V. Role of leukotriene receptor antagonists in the management of pediatric asthma: an update. Paediatric drugs. 2012;14:317–330. doi: 10.2165/11599930-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 183.Chauhan BF, Ben Salah R, Ducharme FM. Addition of anti-leukotriene agents to inhaled corticosteroids in children with persistent asthma. The Cochrane database of systematic reviews. 2013;10:CD009585. doi: 10.1002/14651858.CD009585.pub2. [DOI] [PubMed] [Google Scholar]
- 184.Le Bel M, Brunet A, Gosselin J. Leukotriene B4, an endogenous stimulator of the innate immune response against pathogens. Journal of innate immunity. 2014;6:159–168. doi: 10.1159/000353694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Smith MJ. Biological activities of leukotriene B4. Agents and actions. 1981;11:571–572. doi: 10.1007/BF01978745. [DOI] [PubMed] [Google Scholar]
- 186.Palmblad J, Malmsten CL, Uden AM, Radmark O, Engstedt L, Samuelsson B. Leukotriene B4 is a potent and stereospecific stimulator of neutrophil chemotaxis and adherence. Blood. 1981;58:658–661. [PubMed] [Google Scholar]
- 187.Afonso PV, Janka-Junttila M, Lee YJ, McCann CP, Oliver CM, Aamer KA, Losert W, Cicerone MT, Parent CA. LTB4 is a signal-relay molecule during neutrophil chemotaxis. Developmental cell. 2012;22:1079–1091. doi: 10.1016/j.devcel.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yokomizo T. Leukotriene B4 receptors: novel roles in immunological regulations. Advances in enzyme regulation. 2011;51:59–64. doi: 10.1016/j.advenzreg.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 189.Hicks A, Monkarsh SP, Hoffman AF, Goodnow R., Jr Leukotriene B4 receptor antagonists as therapeutics for inflammatory disease: preclinical and clinical developments. Expert opinion on investigational drugs. 2007;16:1909–1920. doi: 10.1517/13543784.16.12.1909. [DOI] [PubMed] [Google Scholar]
- 190.Freire MO, Van Dyke TE. Natural resolution of inflammation. Periodontology 2000. 2013;63:149–164. doi: 10.1111/prd.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hu S, Mao-Ying QL, Wang J, Wang ZF, Mi WL, Wang XW, Jiang JW, Huang YL, Wu GC, Wang YQ. Lipoxins and aspirin-triggered lipoxin alleviate bone cancer pain in association with suppressing expression of spinal proinflammatory cytokines. Journal of neuroinflammation. 2012;9:278. doi: 10.1186/1742-2094-9-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Spite M, Claria J, Serhan CN. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell metabolism. 2014;19:21–36. doi: 10.1016/j.cmet.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:1755–1765. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fierro IM, Colgan SP, Bernasconi G, Petasis NA, Clish CB, Arita M, Serhan CN. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 inhibit human neutrophil migration: comparisons between synthetic 15 epimers in chemotaxis and transmigration with microvessel endothelial cells and epithelial cells. Journal of immunology. 2003;170:2688–2694. doi: 10.4049/jimmunol.170.5.2688. [DOI] [PubMed] [Google Scholar]
- 195.Ereso AQ, Cureton EL, Cripps MW, Sadjadi J, Dua MM, Curran B, Victorino GP. Lipoxin a(4) attenuates microvascular fluid leak during inflammation. The Journal of surgical research. 2009;156:183–188. doi: 10.1016/j.jss.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.El Kebir D, Filep JG. Modulation of Neutrophil Apoptosis and the Resolution of Inflammation through beta2 Integrins. Frontiers in immunology. 2013;4:60. doi: 10.3389/fimmu.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. The Journal of biological chemistry. 2010;285:3451–3461. doi: 10.1074/jbc.M109.044131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Meade EA, Smith WL, DeWitt DL. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. The Journal of biological chemistry. 1993;268:6610–6614. [PubMed] [Google Scholar]
- 199.Romano M. Lipid mediators: lipoxin and aspirin-triggered 15-epi-lipoxins. Inflammation & allergy drug targets. 2006;5:81–90. doi: 10.2174/187152806776383152. [DOI] [PubMed] [Google Scholar]
- 200.Sala A, Folco G, Murphy RC. Transcellular biosynthesis of eicosanoids. Pharmacological reports : PR. 2010;62:503–510. doi: 10.1016/s1734-1140(10)70306-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kuhn H, Wiesner R, Alder L, Fitzsimmons BJ, Rokach J, Brash AR. Formation of lipoxin B by the pure reticulocyte lipoxygenase via sequential oxygenation of the substrate. European journal of biochemistry / FEBS. 1987;169:593–601. doi: 10.1111/j.1432-1033.1987.tb13650.x. [DOI] [PubMed] [Google Scholar]
- 202.Salmon JA, Terano T. Supplementation of the diet with eicosapentaenoic acid: a possible approach to the treatment of thrombosis and inflammation. The Proceedings of the Nutrition Society. 1985;44:385–389. doi: 10.1079/pns19850063. [DOI] [PubMed] [Google Scholar]
- 203.Horrocks LA, Yeo YK. Health benefits of docosahexaenoic acid (DHA) Pharmacological research : the official journal of the Italian Pharmacological Society. 1999;40:211–225. doi: 10.1006/phrs.1999.0495. [DOI] [PubMed] [Google Scholar]
- 204.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. The Journal of biological chemistry. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 205.Serhan CN, Chiang N. Resolution phase lipid mediators of inflammation: agonists of resolution. Current opinion in pharmacology. 2013;13:632–640. doi: 10.1016/j.coph.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kronke G, Katzenbeisser J, Uderhardt S, Zaiss MM, Scholtysek C, Schabbauer G, Zarbock A, Koenders MI, Axmann R, Zwerina J, Baenckler HW, van den Berg W, Voll RE, Kuhn H, Joosten LA, Schett G. 12/15-lipoxygenase counteracts inflammation and tissue damage in arthritis. Journal of immunology. 2009;183:3383–3389. doi: 10.4049/jimmunol.0900327. [DOI] [PubMed] [Google Scholar]
- 207.Kronke G, Uderhardt S, Katzenbeisser J, Schett G. The 12/15-lipoxygenase pathway promotes osteoclast development and differentiation. Autoimmunity. 2009;42:383–385. doi: 10.1080/08916930902832488. [DOI] [PubMed] [Google Scholar]
- 208.Kuhn H. Biosynthesis, metabolization and biological importance of the primary 15-lipoxygenase metabolites 15-hydro(pero)XY-5Z,8Z,11Z,13E-eicosatetraenoic acid and 13-hydro(pero)XY-9Z,11E-octadecadienoic acid. Progress in lipid research. 1996;35:203–226. doi: 10.1016/s0163-7827(96)00008-2. [DOI] [PubMed] [Google Scholar]
- 209.Limor R, Sharon O, Knoll E, Many A, Weisinger G, Stern N. Lipoxygenase-derived metabolites are regulators of peroxisome proliferator-activated receptor gamma-2 expression in human vascular smooth muscle cells. American journal of hypertension. 2008;21:219–223. doi: 10.1038/ajh.2007.39. [DOI] [PubMed] [Google Scholar]
- 210.Altmann R, Hausmann M, Spottl T, Gruber M, Bull AW, Menzel K, Vogl D, Herfarth H, Scholmerich J, Falk W, Rogler G. 13-Oxo-ODE is an endogenous ligand for PPARgamma in human colonic epithelial cells. Biochemical pharmacology. 2007;74:612–622. doi: 10.1016/j.bcp.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 211.Martin H. Role of PPAR-gamma in inflammation. Prospects for therapeutic intervention by food components. Mutation research. 2010;690:57–63. doi: 10.1016/j.mrfmmm.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 212.Oskolkova OV, Afonyushkin T, Preinerstorfer B, Bicker W, von Schlieffen E, Hainzl E, Demyanets S, Schabbauer G, Lindner W, Tselepis AD, Wojta J, Binder BR, Bochkov VN. Oxidized phospholipids are more potent antagonists of lipopolysaccharide than inducers of inflammation. Journal of immunology. 2010;185:7706–7712. doi: 10.4049/jimmunol.0903594. [DOI] [PubMed] [Google Scholar]
- 213.Munger KA, Montero A, Fukunaga M, Uda S, Yura T, Imai E, Kaneda Y, Valdivielso JM, Badr KF. Transfection of rat kidney with human 15-lipoxygenase suppresses inflammation and preserves function in experimental glomerulonephritis. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13375–13380. doi: 10.1073/pnas.96.23.13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chawengsub Y, Gauthier KM, Campbell WB. Role of arachidonic acid lipoxygenase metabolites in the regulation of vascular tone. American journal of physiology. Heart and circulatory physiology. 2009;297:H495–H507. doi: 10.1152/ajpheart.00349.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Nasjletti A. Arthur C. Corcoran Memorial Lecture. The role of eicosanoids in angiotensin-dependent hypertension. Hypertension. 1998;31:194–200. doi: 10.1161/01.hyp.31.1.194. [DOI] [PubMed] [Google Scholar]
- 216.Zhu D, Ran Y. Role of 15-lipoxygenase/15-hydroxyeicosatetraenoic acid in hypoxia-induced pulmonary hypertension. The journal of physiological sciences : JPS. 2012;62:163–172. doi: 10.1007/s12576-012-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pfister SL, Campbell WB. Arachidonic acid- and acetylcholine-induced relaxations of rabbit aorta. Hypertension. 1992;20:682–689. doi: 10.1161/01.hyp.20.5.682. [DOI] [PubMed] [Google Scholar]
- 218.Rosolowsky M, Campbell WB. Role of PGI2 and epoxyeicosatrienoic acids in relaxation of bovine coronary arteries to arachidonic acid. The American journal of physiology. 1993;264:H327–H335. doi: 10.1152/ajpheart.1993.264.2.H327. [DOI] [PubMed] [Google Scholar]
- 219.Pfister SL, Spitzbarth N, Nithipatikom K, Edgemond WS, Falck JR, Campbell WB. Identification of the 11,14,15- and 11,12, 15-trihydroxyeicosatrienoic acids as endothelium-derived relaxing factors of rabbit aorta. The Journal of biological chemistry. 1998;273:30879–30887. doi: 10.1074/jbc.273.47.30879. [DOI] [PubMed] [Google Scholar]
- 220.Tang X, Holmes BB, Nithipatikom K, Hillard CJ, Kuhn H, Campbell WB. Reticulocyte 15-lipoxygenase-I is important in acetylcholine-induced endothelium-dependent vasorelaxation in rabbit aorta. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:78–84. doi: 10.1161/01.ATV.0000191640.73313.ad. [DOI] [PubMed] [Google Scholar]
- 221.Aggarwal NT, Holmes BB, Cui L, Viita H, Yla-Herttuala S, Campbell WB. Adenoviral expression of 15-lipoxygenase-1 in rabbit aortic endothelium: role in arachidonic acid-induced relaxation. American journal of physiology. Heart and circulatory physiology. 2007;292:H1033–H1041. doi: 10.1152/ajpheart.00624.2006. [DOI] [PubMed] [Google Scholar]
- 222.Aggarwal NT, Chawengsub Y, Gauthier KM, Viita H, Yla-Herttuala S, Campbell WB. Endothelial 15-lipoxygenase-1 overexpression increases acetylcholine-induced hypotension and vasorelaxation in rabbits. Hypertension. 2008;51:246–251. doi: 10.1161/HYPERTENSIONAHA.107.104125. [DOI] [PubMed] [Google Scholar]
- 223.Aggarwal NT, Pfister SL, Gauthier KM, Chawengsub Y, Baker JE, Campbell WB. Chronic hypoxia enhances 15-lipoxygenase-mediated vasorelaxation in rabbit arteries. American journal of physiology. Heart and circulatory physiology. 2009;296:H678–H688. doi: 10.1152/ajpheart.00777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 224.Aggarwal NT, Pfister SL, Campbell WB. Hypercholesterolemia enhances 15-lipoxygenase-mediated vasorelaxation and acetylcholine-induced hypotension. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:2209–2215. doi: 10.1161/ATVBAHA.108.177113. [DOI] [PubMed] [Google Scholar]
- 225.Kriska T, Cepura C, Magier D, Siangjong L, Gauthier KM, Campbell WB. Mice lacking macrophage 12/15-lipoxygenase are resistant to experimental hypertension. American journal of physiology. Heart and circulatory physiology. 2012;302:H2428–H2438. doi: 10.1152/ajpheart.01120.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhao L, Funk CD. Lipoxygenase pathways in atherogenesis. Trends in cardiovascular medicine. 2004;14:191–195. doi: 10.1016/j.tcm.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 227.Poeckel D, Funk CD. The 5-lipoxygenase/leukotriene pathway in preclinical models of cardiovascular disease. Cardiovascular research. 2010;86:243–253. doi: 10.1093/cvr/cvq016. [DOI] [PubMed] [Google Scholar]
- 228.Wuest SJ, Horn T, Marti-Jaun J, Kuhn H, Hersberger M. Association of polymorphisms in the ALOX15B gene with coronary artery disease. Clinical biochemistry. 2014;47:349–355. doi: 10.1016/j.clinbiochem.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 229.Gertow K, Nobili E, Folkersen L, Newman JW, Pedersen TL, Ekstrand J, Swedenborg J, Kuhn H, Wheelock CE, Hansson GK, Hedin U, Haeggstrom JZ, Gabrielsen A. 12- and 15-lipoxygenases in human carotid atherosclerotic lesions: associations with cerebrovascular symptoms. Atherosclerosis. 2011;215:411–416. doi: 10.1016/j.atherosclerosis.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 230.Kuhn H, Heydeck D, Hugou I, Gniwotta C. In vivo action of 15-lipoxygenase in early stages of human atherogenesis. The Journal of clinical investigation. 1997;99:888–893. doi: 10.1172/JCI119253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kuhn H, Belkner J, Zaiss S, Fahrenklemper T, Wohlfeil S. Involvement of 15-lipoxygenase in early stages of atherogenesis. The Journal of experimental medicine. 1994;179:1903–1911. doi: 10.1084/jem.179.6.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.De Caterina R, Mazzone A, Giannessi D, Sicari R, Pelosi W, Lazzerini G, Azzara A, Forder R, Carey F, Caruso D, et al. Leukotriene B4 production in human atherosclerotic plaques. Biomedica biochimica acta. 1988;47:S182–S185. [PubMed] [Google Scholar]
- 233.Allen S, Dashwood M, Morrison K, Yacoub M. Differential leukotriene constrictor responses in human atherosclerotic coronary arteries. Circulation. 1998;97:2406–2413. doi: 10.1161/01.cir.97.24.2406. [DOI] [PubMed] [Google Scholar]
- 234.Qiu H, Gabrielsen A, Agardh HE, Wan M, Wetterholm A, Wong CH, Hedin U, Swedenborg J, Hansson GK, Samuelsson B, Paulsson-Berne G, Haeggstrom JZ. Expression of 5-lipoxygenase and leukotriene A4 hydrolase in human atherosclerotic lesions correlates with symptoms of plaque instability. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8161–8166. doi: 10.1073/pnas.0602414103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhao L, Moos MP, Grabner R, Pedrono F, Fan J, Kaiser B, John N, Schmidt S, Spanbroek R, Lotzer K, Huang L, Cui J, Rader DJ, Evans JF, Habenicht AJ, Funk CD. The 5-lipoxygenase pathway promotes pathogenesis of hyperlipidemia-dependent aortic aneurysm. Nature medicine. 2004;10:966–973. doi: 10.1038/nm1099. [DOI] [PubMed] [Google Scholar]
- 236.Kuhn H, Chaitidis P, Roffeis J, Walther M. Arachidonic Acid metabolites in the cardiovascular system: the role of lipoxygenase isoforms in atherogenesis with particular emphasis on vascular remodeling. Journal of cardiovascular pharmacology. 2007;50:609–620. doi: 10.1097/FJC.0b013e318159f177. [DOI] [PubMed] [Google Scholar]
- 237.Cao RY, Adams MA, Habenicht AJ, Funk CD. Angiotensin II-induced abdominal aortic aneurysm occurs independently of the 5-lipoxygenase pathway in apolipoprotein E-deficient mice. Prostaglandins & other lipid mediators. 2007;84:34–42. doi: 10.1016/j.prostaglandins.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 238.Subbarao K, Jala VR, Mathis S, Suttles J, Zacharias W, Ahamed J, Ali H, Tseng MT, Haribabu B. Role of leukotriene B4 receptors in the development of atherosclerosis: potential mechanisms. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:369–375. doi: 10.1161/01.ATV.0000110503.16605.15. [DOI] [PubMed] [Google Scholar]
- 239.Ahluwalia N, Lin AY, Tager AM, Pruitt IE, Anderson TJ, Kristo F, Shen D, Cruz AR, Aikawa M, Luster AD, Gerszten RE. Inhibited aortic aneurysm formation in BLT1-deficient mice. Journal of immunology. 2007;179:691–697. doi: 10.4049/jimmunol.179.1.691. [DOI] [PubMed] [Google Scholar]
- 240.Kristo F, Hardy GJ, Anderson TJ, Sinha S, Ahluwalia N, Lin AY, Passeri J, Scherrer-Crosbie M, Gerszten RE. Pharmacological inhibition of BLT1 diminishes early abdominal aneurysm formation. Atherosclerosis. 2010;210:107–113. doi: 10.1016/j.atherosclerosis.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. The Journal of clinical investigation. 1991;88:1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Chisolm GM, Steinberg D. The oxidative modification hypothesis of atherogenesis: an overview. Free radical biology & medicine. 2000;28:1815–1826. doi: 10.1016/s0891-5849(00)00344-0. [DOI] [PubMed] [Google Scholar]
- 243.Steinberg D. The LDL modification hypothesis of atherogenesis: an update. Journal of lipid research. 2009;50(Suppl):S376–S381. doi: 10.1194/jlr.R800087-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Perrotta I. Ultrastructural features of human atherosclerosis. Ultrastructural pathology. 2013;37:43–51. doi: 10.3109/01913123.2011.592721. [DOI] [PubMed] [Google Scholar]
- 245.Belkner J, Stender H, Kuhn H. The rabbit 15-lipoxygenase preferentially oxygenates LDL cholesterol esters, and this reaction does not require vitamin E. The Journal of biological chemistry. 1998;273:23225–23232. doi: 10.1074/jbc.273.36.23225. [DOI] [PubMed] [Google Scholar]
- 246.Pirillo A, Uboldi P, Kuhn H, Catapano AL. 15-Lipoxygenase-mediated modification of high-density lipoproteins impairs SR-BI- and ABCA1-dependent cholesterol efflux from macrophages. Biochimica et biophysica acta. 2006;1761:292–300. doi: 10.1016/j.bbalip.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 247.Folcik VA, Nivar-Aristy RA, Krajewski LP, Cathcart MK. Lipoxygenase contributes to the oxidation of lipids in human atherosclerotic plaques. The Journal of clinical investigation. 1995;96:504–510. doi: 10.1172/JCI118062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Cyrus T, Witztum JL, Rader DJ, Tangirala R, Fazio S, Linton MF, Funk CD. Disruption of the 12/15-lipoxygenase gene diminishes atherosclerosis in apo E-deficient mice. The Journal of clinical investigation. 1999;103:1597–1604. doi: 10.1172/JCI5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Cyrus T, Pratico D, Zhao L, Witztum JL, Rader DJ, Rokach J, FitzGerald GA, Funk CD. Absence of 12/15-lipoxygenase expression decreases lipid peroxidation and atherogenesis in apolipoprotein e-deficient mice. Circulation. 2001;103:2277–2282. doi: 10.1161/01.cir.103.18.2277. [DOI] [PubMed] [Google Scholar]
- 250.George J, Afek A, Shaish A, Levkovitz H, Bloom N, Cyrus T, Zhao L, Funk CD, Sigal E, Harats D. 12/15-Lipoxygenase gene disruption attenuates atherogenesis in LDL receptor-deficient mice. Circulation. 2001;104:1646–1650. doi: 10.1161/hc3901.095772. [DOI] [PubMed] [Google Scholar]
- 251.Huo Y, Zhao L, Hyman MC, Shashkin P, Harry BL, Burcin T, Forlow SB, Stark MA, Smith DF, Clarke S, Srinivasan S, Hedrick CC, Pratico D, Witztum JL, Nadler JL, Funk CD, Ley K. Critical role of macrophage 12/15-lipoxygenase for atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;110:2024–2031. doi: 10.1161/01.CIR.0000143628.37680.F6. [DOI] [PubMed] [Google Scholar]
- 252.Poeckel D, Zemski Berry KA, Murphy RC, Funk CD. Dual 12/15- and 5-lipoxygenase deficiency in macrophages alters arachidonic acid metabolism and attenuates peritonitis and atherosclerosis in ApoE knock-out mice. The Journal of biological chemistry. 2009;284:21077–21089. doi: 10.1074/jbc.M109.000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zhao L, Pratico D, Rader DJ, Funk CD. 12/15-Lipoxygenase gene disruption and vitamin E administration diminish atherosclerosis and oxidative stress in apolipoprotein E deficient mice through a final common pathway. Prostaglandins & other lipid mediators. 2005;78:185–193. doi: 10.1016/j.prostaglandins.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 254.Shen J, Herderick E, Cornhill JF, Zsigmond E, Kim HS, Kuhn H, Guevara NV, Chan L. Macrophage-mediated 15-lipoxygenase expression protects against atherosclerosis development. The Journal of clinical investigation. 1996;98:2201–2208. doi: 10.1172/JCI119029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Trebus F, Heydeck D, Schimke I, Gerth C, Kuhn H. Transient experimental anemia in cholesterol-fed rabbits induces systemic overexpression of the reticulocyte-type 15-lipoxygenase and protects from aortic lipid deposition, Prostaglandins. leukotrienes, and essential fatty acids. 2002;67:419–428. doi: 10.1054/plef.2002.0452. [DOI] [PubMed] [Google Scholar]
- 256.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22:3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Hersberger M. Potential role of the lipoxygenase derived lipid mediators in atherosclerosis: leukotrienes, lipoxins and resolvins. Clinical chemistry and laboratory medicine : CCLM / FESCC. 2010;48:1063–1073. doi: 10.1515/CCLM.2010.212. [DOI] [PubMed] [Google Scholar]
- 258.Kuhn H, Romisch I, Belkner J. The role of lipoxygenase-isoforms in atherogenesis. Molecular nutrition & food research. 2005;49:1014–1029. doi: 10.1002/mnfr.200500131. [DOI] [PubMed] [Google Scholar]
- 259.Wittwer J, Hersberger M. The two faces of the 15-lipoxygenase in atherosclerosis. Prostaglandins, leukotrienes, and essential fatty acids. 2007;77:67–77. doi: 10.1016/j.plefa.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 260.Hulten LM, Olson FJ, Aberg H, Carlsson J, Karlstrom L, Boren J, Fagerberg B, Wiklund O. 15-Lipoxygenase-2 is expressed in macrophages in human carotid plaques and regulated by hypoxia-inducible factor-1alpha. European journal of clinical investigation. 2010;40:11–17. doi: 10.1111/j.1365-2362.2009.02223.x. [DOI] [PubMed] [Google Scholar]
- 261.Rydberg EK, Krettek A, Ullstrom C, Ekstrom K, Svensson PA, Carlsson LM, Jonsson-Rylander AC, Hansson GI, McPheat W, Wiklund O, Ohlsson BG, Hulten LM. Hypoxia increases LDL oxidation and expression of 15-lipoxygenase-2 in human macrophages. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:2040–2045. doi: 10.1161/01.ATV.0000144951.08072.0b. [DOI] [PubMed] [Google Scholar]
- 262.Danielsson KN, Rydberg EK, Ingelsten M, Akyurek LM, Jirholt P, Ullstrom C, Forsberg GB, Boren J, Wiklund O, Hulten LM. 15-Lipoxygenase-2 expression in human macrophages induces chemokine secretion and T cell migration. Atherosclerosis. 2008;199:34–40. doi: 10.1016/j.atherosclerosis.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 263.Moller MJ, Qin Z, Toursarkissian B. Tissue markers in human atherosclerotic carotid artery plaque. Annals of vascular surgery. 2012;26:1160–1165. doi: 10.1016/j.avsg.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 264.Joshi N, Hoobler EK, Perry S, Diaz G, Fox B, Holman TR. Kinetic and structural investigations into the allosteric and pH effect on the substrate specificity of human epithelial 15-lipoxygenase-2. Biochemistry. 2013;52:8026–8035. doi: 10.1021/bi4010649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wecksler AT, Kenyon V, Garcia NK, Deschamps JD, van der Donk WA, Holman TR. Kinetic and structural investigations of the allosteric site in human epithelial 15-lipoxygenase-2. Biochemistry. 2009;48:8721–8730. doi: 10.1021/bi9009242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Hamberg M, Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proceedings of the National Academy of Sciences of the United States of America. 1974;71:3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Katoh A, Ikeda H, Murohara T, Haramaki N, Ito H, Imaizumi T. Platelet-derived 12-hydroxyeicosatetraenoic acid plays an important role in mediating canine coronary thrombosis by regulating platelet glycoprotein IIb/IIIa activation. Circulation. 1998;98:2891–2898. doi: 10.1161/01.cir.98.25.2891. [DOI] [PubMed] [Google Scholar]
- 268.Tourdot BE, Ahmed I, Holinstat M. The emerging role of oxylipins in thrombosis and diabetes. Frontiers in pharmacology. 2014;4:176. doi: 10.3389/fphar.2013.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Yeung J, Apopa PL, Vesci J, Kenyon V, Rai G, Jadhav A, Simeonov A, Holman TR, Maloney DJ, Boutaud O, Holinstat M. Protein kinase C regulation of 12-lipoxygenase-mediated human platelet activation. Molecular pharmacology. 2012;81:420–430. doi: 10.1124/mol.111.075630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Yeung J, Apopa PL, Vesci J, Stolla M, Rai G, Simeonov A, Jadhav A, Fernandez-Perez P, Maloney DJ, Boutaud O, Holman TR, Holinstat M. 12-lipoxygenase activity plays an important role in PAR4 and GPVI-mediated platelet reactivity. Thrombosis and haemostasis. 2013;110:569–581. doi: 10.1160/TH13-01-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Yeung J, Holinstat M. 12-lipoxygenase: a potential target for novel anti-platelet therapeutics. Cardiovascular & hematological agents in medicinal chemistry. 2011;9:154–164. doi: 10.2174/187152511797037619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Chen P, Vericel E, Lagarde M, Guichardant M. Poxytrins, a class of oxygenated products from polyunsaturated fatty acids, potently inhibit blood platelet aggregation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:382–388. doi: 10.1096/fj.10-161836. [DOI] [PubMed] [Google Scholar]
- 273.Liu M, Chen P, Vericel E, Lelli M, Beguin L, Lagarde M, Guichardant M. Characterization and biological effects of di-hydroxylated compounds deriving from the lipoxygenation of ALA. Journal of lipid research. 2013;54:2083–2094. doi: 10.1194/jlr.M035139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Vijil C, Hermansson C, Jeppsson A, Bergstrom G, Hulten LM. Arachidonate 15-lipoxygenase enzyme products increase platelet aggregation and thrombin generation. PloS one. 2014;9:e88546. doi: 10.1371/journal.pone.0088546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Watanabe T, Medina JF, Haeggstrom JZ, Radmark O, Samuelsson B. Molecular cloning of a 12-lipoxygenase cDNA from rat brain. European journal of biochemistry / FEBS. 1993;212:605–612. doi: 10.1111/j.1432-1033.1993.tb17699.x. [DOI] [PubMed] [Google Scholar]
- 276.Nishiyama M, Okamoto H, Watanabe T, Hori T, Hada T, Ueda N, Yamamoto S, Tsukamoto H, Watanabe K, Kirino T. Localization of arachidonate 12-lipoxygenase in canine brain tissues. Journal of neurochemistry. 1992;58:1395–1400. doi: 10.1111/j.1471-4159.1992.tb11355.x. [DOI] [PubMed] [Google Scholar]
- 277.Hada T, Hagiya H, Suzuki H, Arakawa T, Nakamura M, Matsuda S, Yoshimoto T, Yamamoto S, Azekawa T, Morita Y, et al. Arachidonate 12-lipoxygenase of rat pineal glands: catalytic properties and primary structure deduced from its cDNA. Biochimica et biophysica acta. 1994;1211:221–228. doi: 10.1016/0005-2760(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 278.Sun D, McDonnell M, Chen XS, Lakkis MM, Li H, Isaacs SN, Elsea SH, Patel PI, Funk CD. Human 12(R)-lipoxygenase and the mouse ortholog. Molecular cloning, expression, and gene chromosomal assignment. The Journal of biological chemistry. 1998;273:33540–33547. doi: 10.1074/jbc.273.50.33540. [DOI] [PubMed] [Google Scholar]
- 279.Haas U, Raschperger E, Hamberg M, Samuelsson B, Tryggvason K, Haeggstrom JZ. Targeted knock-down of a structurally atypical zebrafish 12S-lipoxygenase leads to severe impairment of embryonic development. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:20479–20484. doi: 10.1073/pnas.1117094108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Mikule K, Gatlin JC, de la Houssaye BA, Pfenninger KH. Growth cone collapse induced by semaphorin 3A requires 12/15-lipoxygenase. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:4932–4941. doi: 10.1523/JNEUROSCI.22-12-04932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Nishiyama M, Hoshino A, Tsai L, Henley JR, Goshima Y, Tessier-Lavigne M, Poo MM, Hong K. Cyclic AMP/GMP-dependent modulation of Ca(2+) channels sets the polarity of nerve growth-cone turning. Nature. 2003;423:990–995. doi: 10.1038/nature01751. [DOI] [PubMed] [Google Scholar]
- 282.de La Houssaye BA, Mikule K, Nikolic D, Pfenninger KH. Thrombin-induced growth cone collapse: involvement of phospholipase A(2) and eicosanoid generation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:10843–10855. doi: 10.1523/JNEUROSCI.19-24-10843.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Ross S, Essary B, de la Houssaye BA, Pan Z, Mikule K, Mubarak O, Pfenninger KH. Thrombin causes pseudopod detachment via a pathway involving cytosolic phospholipase A2 and 12/15-lipoxygenase products. Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research. 2000;11:19–30. [PubMed] [Google Scholar]
- 284.Piomelli D. Metabolism of arachidonic acid in nervous system of marine mollusk Aplysia californica. The American journal of physiology. 1991;260:R844–R848. doi: 10.1152/ajpregu.1991.260.5.R844. [DOI] [PubMed] [Google Scholar]
- 285.Pekcec A, Yigitkanli K, Jung JE, Pallast S, Xing C, Antipenko A, Minchenko M, Nikolov DB, Holman TR, Lo EH, van Leyen K. Following experimental stroke, the recovering brain is vulnerable to lipoxygenase-dependent semaphorin signaling. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27:437–445. doi: 10.1096/fj.12-206896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Kang LT, Vanderhoek JY. Mono (S) hydroxy fatty acids: novel ligands for cytosolic actin. Journal of lipid research. 1998;39:1476–1482. [PubMed] [Google Scholar]
- 287.Hung RJ, Yazdani U, Yoon J, Wu H, Yang T, Gupta N, Huang Z, van Berkel WJ, Terman JR. Mical links semaphorins to F-actin disassembly. Nature. 2010;463:823–827. doi: 10.1038/nature08724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Estrada-Bernal A, Gatlin JC, Sunpaweravong S, Pfenninger KH. Dynamic adhesions and MARCKS in melanoma cells. Journal of cell science. 2009;122:2300–2310. doi: 10.1242/jcs.047860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Normandin M, Gagne J, Bernard J, Elie R, Miceli D, Baudry M, Massicotte G. Involvement of the 12-lipoxygenase pathway of arachidonic acid metabolism in homosynaptic long-term depression of the rat hippocampus. Brain research. 1996;730:40–46. doi: 10.1016/0006-8993(96)00428-3. [DOI] [PubMed] [Google Scholar]
- 290.DeCostanzo AJ, Voloshyna I, Rosen ZB, Feinmark SJ, Siegelbaum SA. 12-Lipoxygenase regulates hippocampal long-term potentiation by modulating L-type Ca2+ channels. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:1822–1831. doi: 10.1523/JNEUROSCI.2168-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Feinmark SJ, Begum R, Tsvetkov E, Goussakov I, Funk CD, Siegelbaum SA, Bolshakov VY. 12-lipoxygenase metabolites of arachidonic acid mediate metabotropic glutamate receptor-dependent long-term depression at hippocampal CA3-CA1 synapses. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:11427–11435. doi: 10.1523/JNEUROSCI.23-36-11427.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Piomelli D, Volterra A, Dale N, Siegelbaum SA, Kandel ER, Schwartz JH, Belardetti F. Lipoxygenase metabolites of arachidonic acid as second messengers for presynaptic inhibition of Aplysia sensory cells. Nature. 1987;328:38–43. doi: 10.1038/328038a0. [DOI] [PubMed] [Google Scholar]
- 293.Piomelli D, Shapiro E, Feinmark SJ, Schwartz JH. Metabolites of arachidonic acid in the nervous system of Aplysia: possible mediators of synaptic modulation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1987;7:3675–3686. doi: 10.1523/JNEUROSCI.07-11-03675.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Canals S, Casarejos MJ, de Bernardo S, Rodriguez-Martin E, Mena MA. Nitric oxide triggers the toxicity due to glutathione depletion in midbrain cultures through 12-lipoxygenase. The Journal of biological chemistry. 2003;278:21542–21549. doi: 10.1074/jbc.M213174200. [DOI] [PubMed] [Google Scholar]
- 295.Khanna S, Roy S, Ryu H, Bahadduri P, Swaan PW, Ratan RR, Sen CK. Molecular basis of vitamin E action. Tocotrienol modulates 12-lipoxygenase, a key mediator of glutamate-induced neurodegeneration. J Biol Chem. 2003 doi: 10.1074/jbc.M307075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Zhang Y, Wang H, Li J, Jimenez DA, Levitan ES, Aizenman E, Rosenberg PA. Peroxynitrite-induced neuronal apoptosis is mediated by intracellular zinc release and 12-lipoxygenase activation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:10616–10627. doi: 10.1523/JNEUROSCI.2469-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Lovat PE, Oliverio S, Ranalli M, Corazzari M, Rodolfo C, Bernassola F, Aughton K, Maccarrone M, Hewson QD, Pearson AD, Melino G, Piacentini M, Redfern CP. GADD153 and 12-lipoxygenase mediate fenretinide-induced apoptosis of neuroblastoma. Cancer research. 2002;62:5158–5167. [PubMed] [Google Scholar]
- 298.Lovat PE, Ranalli M, Corazzari M, Raffaghello L, Pearson AD, Ponzoni M, Piacentini M, Melino G, Redfern CP. Mechanisms of free-radical induction in relation to fenretinide-induced apoptosis of neuroblastoma. Journal of cellular biochemistry. 2003;89:698–708. doi: 10.1002/jcb.10551. [DOI] [PubMed] [Google Scholar]
- 299.Pallast S, Arai K, Wang X, Lo EH, van Leyen K. 12/15-Lipoxygenase targets neuronal mitochondria under oxidative stress. Journal of neurochemistry. 2009;111:882–889. doi: 10.1111/j.1471-4159.2009.06379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.van Leyen K, Siddiq A, Ratan RR, Lo EH. Proteasome inhibition protects HT22 neuronal cells from oxidative glutamate toxicity. Journal of neurochemistry. 2005;92:824–830. doi: 10.1111/j.1471-4159.2004.02915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Pallast S, Arai K, Pekcec A, Yigitkanli K, Yu Z, Wang X, Lo EH, van Leyen K. Increased nuclear apoptosis-inducing factor after transient focal ischemia: a 12/15-lipoxygenase-dependent organelle damage pathway. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30:1157–1167. doi: 10.1038/jcbfm.2009.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Tobaben S, Grohm J, Seiler A, Conrad M, Plesnila N, Culmsee C. Bid-mediated mitochondrial damage is a key mechanism in glutamate-induced oxidative stress and AIF-dependent cell death in immortalized HT-22 hippocampal neurons. Cell death and differentiation. 2011;18:282–292. doi: 10.1038/cdd.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Chan PH, Fishman RA. Brain edema: induction in cortical slices by polyunsaturated fatty acids. Science. 1978;201:358–360. doi: 10.1126/science.663662. [DOI] [PubMed] [Google Scholar]
- 304.Chan PH, Fishman RA, Caronna J, Schmidley JW, Prioleau G, Lee J. Induction of brain edema following intracerebral injection of arachidonic acid. Ann Neurol. 1983;13:625–632. doi: 10.1002/ana.410130608. [DOI] [PubMed] [Google Scholar]
- 305.Park HA, Khanna S, Rink C, Gnyawali S, Roy S, Sen CK. Glutathione disulfide induces neural cell death via a 12-lipoxygenase pathway. Cell death and differentiation. 2009;16:1167–1179. doi: 10.1038/cdd.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Haynes RL, van Leyen K. 12/15-lipoxygenase expression is increased in oligodendrocytes and microglia of periventricular leukomalacia. Developmental neuroscience. 2013;35:140–154. doi: 10.1159/000350230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Wang H, Li J, Follett PL, Zhang Y, Cotanche DA, Jensen FE, Volpe JJ, Rosenberg PA. 12-Lipoxygenase plays a key role in cell death caused by glutathione depletion and arachidonic acid in rat oligodendrocytes. The European journal of neuroscience. 2004;20:2049–2058. doi: 10.1111/j.1460-9568.2004.03650.x. [DOI] [PubMed] [Google Scholar]
- 308.van Leyen K, Arai K, Jin G, Kenyon V, Gerstner B, Rosenberg PA, Holman TR, Lo EH. Novel lipoxygenase inhibitors as neuroprotective reagents. Journal of neuroscience research. 2008;86:904–909. doi: 10.1002/jnr.21543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Gerstner B, DeSilva TM, Genz K, Armstrong A, Brehmer F, Neve RL, Felderhoff-Mueser U, Volpe JJ, Rosenberg PA. Hyperoxia causes maturation-dependent cell death in the developing white matter. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:1236–1245. doi: 10.1523/JNEUROSCI.3213-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Baud O, Li J, Zhang Y, Neve RL, Volpe JJ, Rosenberg PA. Nitric oxide-induced cell death in developing oligodendrocytes is associated with mitochondrial dysfunction and apoptosis-inducing factor translocation. The European journal of neuroscience. 2004;20:1713–1726. doi: 10.1111/j.1460-9568.2004.03616.x. [DOI] [PubMed] [Google Scholar]
- 311.Helgadottir A, Manolescu A, Thorleifsson G, Gretarsdottir S, Jonsdottir H, Thorsteinsdottir U, Samani NJ, Gudmundsson G, Grant SF, Thorgeirsson G, Sveinbjornsdottir S, Valdimarsson EM, Matthiasson SE, Johannsson H, Gudmundsdottir O, Gurney ME, Sainz J, Thorhallsdottir M, Andresdottir M, Frigge ML, Topol EJ, Kong A, Gudnason V, Hakonarson H, Gulcher JR, Stefansson K. The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nature genetics. 2004;36:233–239. doi: 10.1038/ng1311. [DOI] [PubMed] [Google Scholar]
- 312.Evans JF, Ferguson AD, Mosley RT, Hutchinson JH. What's all the FLAP about?: 5-lipoxygenase-activating protein inhibitors for inflammatory diseases. Trends in pharmacological sciences. 2008;29:72–78. doi: 10.1016/j.tips.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 313.Domingues-Montanari S, Fernandez-Cadenas I, del Rio-Espinola A, Corbeto N, Krug T, Manso H, Gouveia L, Sobral J, Mendioroz M, Fernandez-Morales J, Alvarez-Sabin J, Ribo M, Rubiera M, Obach V, Marti-Fabregas J, Freijo M, Serena J, Ferro JM, Vicente AM, Oliveira SA, Montaner J. Association of a genetic variant in the ALOX5AP with higher risk of ischemic stroke: a case-control, meta-analysis and functional study. Cerebrovascular diseases. 2010;29:528–537. doi: 10.1159/000302738. [DOI] [PubMed] [Google Scholar]
- 314.Kostulas K, Gretarsdottir S, Kostulas V, Manolescu A, Helgadottir A, Thorleifsson G, Gudmundsson LJ, Thorsteinsdottir U, Gulcher JR, Stefansson K, Hillert J. PDE4D and ALOX5AP genetic variants and risk for Ischemic Cerebrovascular Disease in Sweden. Journal of the neurological sciences. 2007;263:113–117. doi: 10.1016/j.jns.2007.06.042. [DOI] [PubMed] [Google Scholar]
- 315.Barral S, Fernandez-Cadenas I, Bis JC, Montaner J, Ikram AM, Launer LJ, Fornage M, Schmidt H, Brickman AM, Seshadri S, Mayeux R. No association of ALOX5AP polymorphisms with risk of MRI-defined brain infarcts. Neurobiology of aging. 2012;33:629, e621–e623. doi: 10.1016/j.neurobiolaging.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Kaushal R, Pal P, Alwell K, Haverbusch M, Flaherty M, Moomaw C, Sekar P, Kissela B, Kleindorfer D, Chakraborty R, Broderick J, Deka R, Woo D. Association of ALOX5AP with ischemic stroke: a population-based case-control study. Human genetics. 2007;121:601–607. doi: 10.1007/s00439-007-0338-y. [DOI] [PubMed] [Google Scholar]
- 317.Wang G, Liu R, Zhang J. The arachidonate 5-lipoxygenase-activating protein (ALOX5AP) gene SG13S114 polymorphism and ischemic stroke in Chinese population: a meta-analysis. Gene. 2014;533:461–468. doi: 10.1016/j.gene.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 318.Bevan S, Dichgans M, Wiechmann HE, Gschwendtner A, Meitinger T, Markus HS. Genetic variation in members of the leukotriene biosynthesis pathway confer an increased risk of ischemic stroke: a replication study in two independent populations. Stroke; a journal of cerebral circulation. 2008;39:1109–1114. doi: 10.1161/STROKEAHA.107.491969. [DOI] [PubMed] [Google Scholar]
- 319.Bazan NG., Jr Effects of ischemia and electroconvulsive shock on free fatty acid pool in the brain. Biochimica et biophysica acta. 1970;218:1–10. doi: 10.1016/0005-2760(70)90086-x. [DOI] [PubMed] [Google Scholar]
- 320.Moskowitz MA, Kiwak KJ, Hekimian K, Levine L. Synthesis of compounds with properties of leukotrienes C4 and D4 in gerbil brains after ischemia and reperfusion. Science. 1984;224:886–889. doi: 10.1126/science.6719118. [DOI] [PubMed] [Google Scholar]
- 321.van Leyen K. Lipoxygenase: an emerging target for stroke therapy. CNS & neurological disorders drug targets. 2013;12:191–199. doi: 10.2174/18715273112119990053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Schewe T, Rapoport SM, Kuhn H. Enzymology and physiology of reticulocyte lipoxygenase: comparison with other lipoxygenases. Advances in enzymology and related areas of molecular biology. 1986;58:191–272. doi: 10.1002/9780470123041.ch6. [DOI] [PubMed] [Google Scholar]
- 323.Seiler A, Schneider M, Forster H, Roth S, Wirth EK, Culmsee C, Plesnila N, Kremmer E, Radmark O, Wurst W, Bornkamm GW, Schweizer U, Conrad M. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell metabolism. 2008;8:237–248. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 324.Brinckmann R, Schnurr K, Heydeck D, Rosenbach T, Kolde G, Kuhn H. Membrane translocation of 15-lipoxygenase in hematopoietic cells is calcium-dependent and activates the oxygenase activity of the enzyme. Blood. 1998;91:64–74. [PubMed] [Google Scholar]
- 325.van Leyen K, Kim HY, Lee SR, Jin G, Arai K, Lo EH. Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke; a journal of cerebral circulation. 2006;37:3014–3018. doi: 10.1161/01.STR.0000249004.25444.a5. [DOI] [PubMed] [Google Scholar]
- 326.Heydeck D, Thomas L, Schnurr K, Trebus F, Thierfelder WE, Ihle JN, Kuhn H. Interleukin-4 and -13 induce upregulation of the murine macrophage 12/15-lipoxygenase activity: evidence for the involvement of transcription factor STAT6. Blood. 1998;92:2503–2510. [PubMed] [Google Scholar]
- 327.Kuhn H, Heydeck D, Brinckman R, Trebus F. Regulation of cellular 15-lipoxygenase activity on pretranslational, translational, and posttranslational levels. Lipids. 1999;34(Suppl):S273–S279. doi: 10.1007/BF02562317. [DOI] [PubMed] [Google Scholar]
- 328.Conrad DJ, Lu M. Regulation of human 12/15-lipoxygenase by Stat6-dependent transcription. American journal of respiratory cell and molecular biology. 2000;22:226–234. doi: 10.1165/ajrcmb.22.2.3786. [DOI] [PubMed] [Google Scholar]
- 329.Yigitkanli K, Pekcec A, Karatas H, Pallast S, Mandeville E, Joshi N, Smirnova N, Gazaryan I, Ratan RR, Witztum JL, Montaner J, Holman TR, Lo EH, van Leyen K. Inhibition of 12/15-lipoxygenase as therapeutic strategy to treat stroke. Annals of neurology. 2013;73:129–135. doi: 10.1002/ana.23734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Khanna S, Roy S, Slivka A, Craft TK, Chaki S, Rink C, Notestine MA, DeVries AC, Parinandi NL, Sen CK. Neuroprotective properties of the natural vitamin E alpha-tocotrienol. Stroke; a journal of cerebral circulation. 2005;36:2258–2264. doi: 10.1161/01.STR.0000181082.70763.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Jin G, Arai K, Murata Y, Wang S, Stins MF, Lo EH, van Leyen K. Protecting against cerebrovascular injury: contributions of 12/15-lipoxygenase to edema formation after transient focal ischemia. Stroke; a journal of cerebral circulation. 2008;39:2538–2543. doi: 10.1161/STROKEAHA.108.514927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Rai G, Joshi N, Jung JE, Liu Y, Schultz L, Yasgar A, Perry S, Diaz G, Zhang Q, Kenyon V, Jadhav A, Simeonov A, Lo EH, van Leyen K, Maloney DJ, Holman TR. Potent and selective inhibitors of human reticulocyte 12/15-lipoxygenase as anti-stroke therapies. Journal of medicinal chemistry. 2014;57:4035–4048. doi: 10.1021/jm401915r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Saito K, Levine L, Moskowitz MA. Blood components contribute to rise in gerbil brain levels of leukotriene-like immunoreactivity after ischemia and reperfusion. Stroke; a journal of cerebral circulation. 1988;19:1395–1398. doi: 10.1161/01.str.19.11.1395. [DOI] [PubMed] [Google Scholar]
- 334.Ban M, Tonai T, Kohno T, Matsumoto K, Horie T, Yamamoto S, Moskowitz MA, Levine L. A flavonoid inhibitor of 5-lipoxygenase inhibits leukotriene production following ischemia in gerbil brain. Stroke; a journal of cerebral circulation. 1989;20:248–252. doi: 10.1161/01.str.20.2.248. [DOI] [PubMed] [Google Scholar]
- 335.Kitagawa K, Matsumoto M, Hori M. Cerebral ischemia in 5-lipoxygenase knockout mice. Brain research. 2004;1004:198–202. doi: 10.1016/j.brainres.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 336.Sobrado M, Pereira MP, Ballesteros I, Hurtado O, Fernandez-Lopez D, Pradillo JM, Caso JR, Vivancos J, Nombela F, Serena J, Lizasoain I, Moro MA. Synthesis of lipoxin A4 by 5-lipoxygenase mediates PPARgamma-dependent, neuroprotective effects of rosiglitazone in experimental stroke. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:3875–3884. doi: 10.1523/JNEUROSCI.5529-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Hawkins KE, DeMars KM, Singh J, Yang C, Cho HS, Frankowski JC, Dore S, Candelario-Jalil E. Neurovascular protection by post-ischemic intravenous injections of the lipoxin A4 receptor agonist, BML-111, in a rat model of ischemic stroke. Journal of neurochemistry. 2014;129:130–142. doi: 10.1111/jnc.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Wu Y, Wang YP, Guo P, Ye XH, Wang J, Yuan SY, Yao SL, Shang Y. A lipoxin A4 analog ameliorates blood-brain barrier dysfunction and reduces MMP-9 expression in a rat model of focal cerebral ischemia-reperfusion injury. Journal of molecular neuroscience : MN. 2012;46:483–491. doi: 10.1007/s12031-011-9620-5. [DOI] [PubMed] [Google Scholar]
- 339.Bazan NG. Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and Alzheimer's disease. Journal of lipid research. 2009;50(Suppl):S400–S405. doi: 10.1194/jlr.R800068-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Belayev L, Khoutorova L, Atkins KD, Eady TN, Hong S, Lu Y, Obenaus A, Bazan NG. Docosahexaenoic Acid therapy of experimental ischemic stroke. Translational stroke research. 2011;2:33–41. doi: 10.1007/s12975-010-0046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Bazan NG, Eady TN, Khoutorova L, Atkins KD, Hong S, Lu Y, Zhang C, Jun B, Obenaus A, Fredman G, Zhu M, Winkler JW, Petasis NA, Serhan CN, Belayev L. Novel aspirin-triggered neuroprotectin D1 attenuates cerebral ischemic injury after experimental stroke. Experimental neurology. 2012;236:122–130. doi: 10.1016/j.expneurol.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Pratico D, Zhukareva V, Yao Y, Uryu K, Funk CD, Lawson JA, Trojanowski JQ, Lee VM. 12/15-lipoxygenase is increased in Alzheimer's disease: possible involvement in brain oxidative stress. The American journal of pathology. 2004;164:1655–1662. doi: 10.1016/S0002-9440(10)63724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Yao Y, Clark CM, Trojanowski JQ, Lee VM, Pratico D. Elevation of 12/15 lipoxygenase products in AD and mild cognitive impairment. Annals of neurology. 2005;58:623–626. doi: 10.1002/ana.20558. [DOI] [PubMed] [Google Scholar]
- 344.Yang H, Zhuo JM, Chu J, Chinnici C, Pratico D. Amelioration of the Alzheimer's disease phenotype by absence of 12/15-lipoxygenase. Biological psychiatry. 2010;68:922–929. doi: 10.1016/j.biopsych.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 345.Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. The Journal of clinical investigation. 2005;115:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Firuzi O, Zhuo J, Chinnici CM, Wisniewski T, Pratico D. 5-Lipoxygenase gene disruption reduces amyloid-beta pathology in a mouse model of Alzheimer's disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22:1169–1178. doi: 10.1096/fj.07-9131.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Rao JS, Rapoport SI, Kim HW. Altered neuroinflammatory, arachidonic acid cascade and synaptic markers in postmortem Alzheimer's disease brain. Translational psychiatry. 2011;1:e31. doi: 10.1038/tp.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 348.Lebeau A, Terro F, Rostene W, Pelaprat D. Blockade of 12-lipoxygenase expression protects cortical neurons from apoptosis induced by beta-amyloid peptide. Cell death and differentiation. 2004;11:875–884. doi: 10.1038/sj.cdd.4401395. [DOI] [PubMed] [Google Scholar]
- 349.Lebeau A, Esclaire F, Rostene W, Pelaprat D. Baicalein protects cortical neurons from beta-amyloid (25–35) induced toxicity. Neuroreport. 2001;12:2199–2202. doi: 10.1097/00001756-200107200-00031. [DOI] [PubMed] [Google Scholar]
- 350.Zhao Y, Bhattacharjee S, Jones BM, Hill J, Dua P, Lukiw WJ. Regulation of Neurotropic Signaling by the Inducible, NF-kB-Sensitive miRNA-125b in Alzheimer's Disease (AD) and in Primary Human Neuronal-Glial (HNG) Cells. Molecular neurobiology. 2013 doi: 10.1007/s12035-013-8595-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Emerson MR, LeVine SM. Experimental allergic encephalomyelitis is exacerbated in mice deficient for 12/15-lipoxygenase or 5-lipoxygenase. Brain research. 2004;1021:140–145. doi: 10.1016/j.brainres.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 352.Di Giovanni S, Knoblach SM, Brandoli C, Aden SA, Hoffman EP, Faden AI. Gene profiling in spinal cord injury shows role of cell cycle in neuronal death. Annals of neurology. 2003;53:454–468. doi: 10.1002/ana.10472. [DOI] [PubMed] [Google Scholar]
- 353.Issan Y, Hochhauser E, Guo A, Gotlinger KH, Kornowski R, Leshem-Lev D, Lev E, Porat E, Snir E, Thompson CI, Abraham NG, Laniado-Schwartzman M. Elevated level of pro-inflammatory eicosanoids and EPC dysfunction in diabetic patients with cardiac ischemia. Prostaglandins & other lipid mediators. 2013;100–101:15–21. doi: 10.1016/j.prostaglandins.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Ma K, Nunemaker CS, Wu R, Chakrabarti SK, Taylor-Fishwick DA, Nadler JL. 12-Lipoxygenase Products Reduce Insulin Secretion and {beta}-Cell Viability in Human Islets. The Journal of clinical endocrinology and metabolism. 2010;95:887–893. doi: 10.1210/jc.2009-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Nunemaker CS, Chen M, Pei H, Kimble SD, Keller SR, Carter JD, Yang Z, Smith KM, Wu R, Bevard MH, Garmey JC, Nadler JL. 12-Lipoxygenase-knockout mice are resistant to inflammatory effects of obesity induced by Western diet. American journal of physiology. Endocrinology and metabolism. 2008;295:E1065–E1075. doi: 10.1152/ajpendo.90371.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Chen M, Yang ZD, Smith KM, Carter JD, Nadler JL. Activation of 12-lipoxygenase in proinflammatory cytokine-mediated beta cell toxicity. Diabetologia. 2005;48:486–495. doi: 10.1007/s00125-005-1673-y. [DOI] [PubMed] [Google Scholar]
- 357.Bleich D, Chen S, Zipser B, Sun D, Funk CD, Nadler JL. Resistance to type 1 diabetes induction in 12-lipoxygenase knockout mice. The Journal of clinical investigation. 1999;103:1431–1436. doi: 10.1172/JCI5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Bleich D, Chen S, Gu JL, Nadler JL. The role of 12-lipoxygenase in pancreatic -cells (Review) International journal of molecular medicine. 1998;1:265–272. [PubMed] [Google Scholar]
- 359.Sears DD, Miles PD, Chapman J, Ofrecio JM, Almazan F, Thapar D, Miller YI. 12/15-lipoxygenase is required for the early onset of high fat diet-induced adipose tissue inflammation and insulin resistance in mice. PloS one. 2009;4:e7250. doi: 10.1371/journal.pone.0007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Laybutt DR, Sharma A, Sgroi DC, Gaudet J, Bonner-Weir S, Weir GC. Genetic regulation of metabolic pathways in beta-cells disrupted by hyperglycemia. The Journal of biological chemistry. 2002;277:10912–10921. doi: 10.1074/jbc.M111751200. [DOI] [PubMed] [Google Scholar]
- 361.Natarajan R, Gu JL, Rossi J, Gonzales N, Lanting L, Xu L, Nadler J. Elevated glucose and angiotensin II increase 12-lipoxygenase activity and expression in porcine aortic smooth muscle cells. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:4947–4951. doi: 10.1073/pnas.90.11.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Marutani E, Kosugi S, Tokuda K, Khatri A, Nguyen R, Atochin DN, Kida K, Van Leyen K, Arai K, Ichinose F. A novel hydrogen sulfide-releasing N-methyl-D-aspartate receptor antagonist prevents ischemic neuronal death. The Journal of biological chemistry. 2012;287:32124–32135. doi: 10.1074/jbc.M112.374124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 364.Zhang HJ, Sun CH, Kuang HY, Jiang XY, Liu HL, Hua WF, Liu ZJ, Zhou H, Sui H, Qi R. 12(S)-hydroxyeicosatetraenoic acid levels link to coronary artery disease in type 2 diabetic patients. J Endocrinol Invest. 2012 doi: 10.3275/8654. In Press. [DOI] [PubMed] [Google Scholar]
- 365.Dobrian AD, Lieb DC, Cole BK, Taylor-Fishwick DA, Chakrabarti SK, Nadler JL. Functional and pathological roles of the 12- and 15-lipoxygenases. Progress in lipid research. 2011;50:115–131. doi: 10.1016/j.plipres.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Nigam S, Zafiriou MP, Deva R, Ciccoli R, Roux-Van der Merwe R. Structure, biochemistry and biology of hepoxilins: an update. The FEBS journal. 2007;274:3503–3512. doi: 10.1111/j.1742-4658.2007.05910.x. [DOI] [PubMed] [Google Scholar]
- 367.Zafiriou MP, Zelarayan LC, Noack C, Renger A, Nigam S, Siafaka-Kapadai A. Hepoxilin A(3) protects beta-cells from apoptosis in contrast to its precursor, 12-hydroperoxyeicosatetraenoic acid. Biochimica et biophysica acta. 2011;1811:361–369. doi: 10.1016/j.bbalip.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 368.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annual review of immunology. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 369.McDuffie M, Maybee NA, Keller SR, Stevens BK, Garmey JC, Morris MA, Kropf E, Rival C, Ma K, Carter JD, Tersey SA, Nunemaker CS, Nadler JL. Nonobese diabetic (NOD) mice congenic for a targeted deletion of 12/15-lipoxygenase are protected from autoimmune diabetes. Diabetes. 2008;57:199–208. doi: 10.2337/db07-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Green-Mitchell SM, Tersey SA, Cole BK, Ma K, Kuhn NS, Cunningham TD, Maybee NA, Chakrabarti SK, McDuffie M, Taylor-Fishwick DA, Mirmira RG, Nadler JL, Morris MA. Deletion of 12/15-lipoxygenase alters macrophage and islet function in NOD-Alox15(null) mice, leading to protection against type 1 diabetes development. PloS one. 2013;8:e56763. doi: 10.1371/journal.pone.0056763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Zhang RL, Lu CZ, Ren HM, Xiao BG. Metabolic changes of arachidonic acid after cerebral ischemia-reperfusion in diabetic rats. Experimental neurology. 2003;184:746–752. doi: 10.1016/S0014-4886(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 372.Chakrabarti SK, Cole BK, Wen Y, Keller SR, Nadler JL. 12/15-lipoxygenase products induce inflammation and impair insulin signaling in 3T3-L1 adipocytes. Obesity. 2009;17:1657–1663. doi: 10.1038/oby.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Madsen L, Petersen RK, Sorensen MB, Jorgensen C, Hallenborg P, Pridal L, Fleckner J, Amri EZ, Krieg P, Furstenberger G, Berge RK, Kristiansen K. Adipocyte differentiation of 3T3-L1 preadipocytes is dependent on lipoxygenase activity during the initial stages of the differentiation process. The Biochemical journal. 2003;375:539–549. doi: 10.1042/bj20030503. [DOI] [PubMed] [Google Scholar]
- 374.Hallenborg P, Jorgensen C, Petersen RK, Feddersen S, Araujo P, Markt P, Langer T, Furstenberger G, Krieg P, Koppen A, Kalkhoven E, Madsen L, Kristiansen K. Epidermis-type lipoxygenase 3 regulates adipocyte differentiation and peroxisome proliferator-activated receptor gamma activity. Molecular and cellular biology. 2010;30:4077–4091. doi: 10.1128/MCB.01806-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Cole BK, Lieb DC, Dobrian AD, Nadler JL. 12- and 15-lipoxygenases in adipose tissue inflammation. Prostaglandins & other lipid mediators. 2013;104–105:84–92. doi: 10.1016/j.prostaglandins.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Soumya SJ, Binu S, Helen A, Reddanna P, Sudhakaran PR. 15(S)-HETE-induced angiogenesis in adipose tissue is mediated through activation of PI3K/Akt/mTOR signaling pathway. Biochemistry and cell biology = Biochimie et biologie cellulaire. 2013;91:498–505. doi: 10.1139/bcb-2013-0037. [DOI] [PubMed] [Google Scholar]
- 377.Xiao WJ, He JW, Zhang H, Hu WW, Gu JM, Yue H, Gao G, Yu JB, Wang C, Ke YH, Fu WZ, Zhang ZL. ALOX12 polymorphisms are associated with fat mass but not peak bone mineral density in Chinese nuclear families. International journal of obesity. 2011;35:378–386. doi: 10.1038/ijo.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Iyer A, Fairlie DP, Prins JB, Hammock BD, Brown L. Inflammatory lipid mediators in adipocyte function and obesity. Nature reviews. Endocrinology. 2010;6:71–82. doi: 10.1038/nrendo.2009.264. [DOI] [PubMed] [Google Scholar]
- 379.Hirata K, Katayama K, Nakajima A, Takada K, Kamisaki Y, Wada K. Role of leukotriene B(4) receptor signaling in human preadipocyte differentiation. Biochemical and biophysical research communications. 2012;429:197–203. doi: 10.1016/j.bbrc.2012.10.110. [DOI] [PubMed] [Google Scholar]
- 380.Ferre N, Martinez-Clemente M, Lopez-Parra M, Gonzalez-Periz A, Horrillo R, Planaguma A, Camps J, Joven J, Tres A, Guardiola F, Bataller R, Arroyo V, Claria J. Increased susceptibility to exacerbated liver injury in hypercholesterolemic ApoE-deficient mice: potential involvement of oxysterols. American journal of physiology. Gastrointestinal and liver physiology. 2009;296:G553–G562. doi: 10.1152/ajpgi.00547.2007. [DOI] [PubMed] [Google Scholar]
- 381.Neels JG. A role for 5-lipoxygenase products in obesity-associated inflammation and insulin resistance. Adipocyte. 2013;2:262–265. doi: 10.4161/adip.24835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Chakrabarti SK, Wen Y, Dobrian AD, Cole BK, Ma Q, Pei H, Williams MD, Bevard MH, Vandenhoff GE, Keller SR, Gu J, Nadler JL. Evidence for activation of inflammatory lipoxygenase pathways in visceral adipose tissue of obese Zucker rats. American journal of physiology. Endocrinology and metabolism. 2011;300:E175–E187. doi: 10.1152/ajpendo.00203.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Schnurr K, Borchert A, Kuhn H. Inverse regulation of lipid-peroxidizing and hydroperoxyl lipid-reducing enzymes by interleukins 4 and 13. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1999;13:143–154. doi: 10.1096/fasebj.13.1.143. [DOI] [PubMed] [Google Scholar]
- 384.Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C, Witztum JL, Funk CD, Conrad D, Glass CK. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400:378–382. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 385.Borchert A, Schnurr K, Thiele BJ, Kuhn H. Cloning of the mouse phospholipid hydroperoxide glutathione peroxidase gene. FEBS letters. 1999;446:223–227. doi: 10.1016/s0014-5793(99)00221-5. [DOI] [PubMed] [Google Scholar]
- 386.Belfiore MC, Natoni A, Barzellotti R, Merendino N, Pessina G, Ghibelli L, Gualandi G. Involvement of 5-lipoxygenase in survival of Epstein-Barr virus (EBV)-converted B lymphoma cells. Cancer letters. 2007;254:236–243. doi: 10.1016/j.canlet.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 387.Gosselin J, Borgeat P. Epstein-Barr virus modulates 5-lipoxygenase product synthesis in human peripheral blood mononuclear cells. Blood. 1997;89:2122–2130. [PubMed] [Google Scholar]
- 388.Qiu H, Straat K, Rahbar A, Wan M, Soderberg-Naucler C, Haeggstrom JZ. Human CMV infection induces 5-lipoxygenase expression and leukotriene B4 production in vascular smooth muscle cells. The Journal of experimental medicine. 2008;205:19–24. doi: 10.1084/jem.20070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Loke WM, Chow AY, Lam Mok Sing K, Lee CY, Halliwell B, Lim EC, Quek AM, Ooi EE, Seet RC. Augmentation of 5-lipoxygenase activity and expression during dengue serotype-2 infection. Virology journal. 2013;10:322. doi: 10.1186/1743-422X-10-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Shirey KA, Lai W, Pletneva LM, Karp CL, Divanovic S, Blanco JC, Vogel SN. Role of the lipoxygenase pathway in RSV-induced alternatively activated macrophages leading to resolution of lung pathology. Mucosal immunology. 2014;7:549–557. doi: 10.1038/mi.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Garreta A, Val-Moraes SP, Garcia-Fernandez Q, Busquets M, Juan C, Oliver A, Ortiz A, Gaffney BJ, Fita I, Manresa A, Carpena X. Structure and interaction with phospholipids of a prokaryotic lipoxygenase from Pseudomonas aeruginosa. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27:4811–4821. doi: 10.1096/fj.13-235952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Arulkumaran N, Singer M. Puerperal sepsis. Best practice & research. Clinical obstetrics & gynaecology. 2013;27:893–902. doi: 10.1016/j.bpobgyn.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 393.Soares EM, Mason KL, Rogers LM, Serezani CH, Faccioli LH, Aronoff DM. Leukotriene B4 enhances innate immune defense against the puerperal sepsis agent Streptococcus pyogenes. Journal of immunology. 2013;190:1614–1622. doi: 10.4049/jimmunol.1202932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Bhowmick R, Tin Maung NH, Hurley BP, Ghanem EB, Gronert K, McCormick BA, Leong JM. Systemic disease during Streptococcus pneumoniae acute lung infection requires 12-lipoxygenase-dependent inflammation. Journal of immunology. 2013;191:5115–5123. doi: 10.4049/jimmunol.1300522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Lin J, Vambutas A, Haruta A, Paparella MM, Giebink GS, Kim Y. Pneumococcus activation of the 5-lipoxygenase pathway and production of glycoproteins in the middle ear of rats. The Journal of infectious diseases. 1999;179:1145–1151. doi: 10.1086/314714. [DOI] [PubMed] [Google Scholar]
- 396.Bafica A, Scanga CA, Serhan C, Machado F, White S, Sher A, Aliberti J. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. The Journal of clinical investigation. 2005;115:1601–1606. doi: 10.1172/JCI23949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 398.Witola WH, Liu SR, Montpetit A, Welti R, Hypolite M, Roth M, Zhou Y, Mui E, Cesbron-Delauw MF, Fournie GJ, Cavailles P, Bisanz C, Boyer K, Withers S, Noble AG, Swisher CN, Heydemann PT, Rabiah P, McLeod R. ALOX12 In Human Toxoplasmosis. Infection and immunity. 2014 doi: 10.1128/IAI.01505-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014 doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Secor WE, Powell MR, Morgan J, Wynn TA, Funk CD. Mice deficient for 5-lipoxygenase, but not leukocyte-type 12-lipoxygenase, display altered immune responses during infection with Schistosoma mansoni. Prostaglandins & other lipid mediators. 1998;56:291–304. doi: 10.1016/s0090-6980(98)00059-8. [DOI] [PubMed] [Google Scholar]
- 401.Tristao FS, Rocha FA, Moreira AP, Cunha FQ, Rossi MA, Silva JS. 5-Lipoxygenase activity increases susceptibility to experimental Paracoccidioides brasiliensis infection. Infection and immunity. 2013;81:1256–1266. doi: 10.1128/IAI.01209-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Yapar N. Epidemiology and risk factors for invasive candidiasis. Therapeutics and clinical risk management. 2014;10:95–105. doi: 10.2147/TCRM.S40160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Haas-Stapleton EJ, Lu Y, Hong S, Arita M, Favoreto S, Nigam S, Serhan CN, Agabian N. Candida albicans modulates host defense by biosynthesizing the pro-resolving mediator resolvin E1. PloS one. 2007;2:e1316. doi: 10.1371/journal.pone.0001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Hofmann B, Steinhilber D. 5-Lipoxygenase inhibitors: a review of recent patents (2010–2012) Expert opinion on therapeutic patents. 2013;23:895–909. doi: 10.1517/13543776.2013.791678. [DOI] [PubMed] [Google Scholar]
- 405.Aparoy P, Reddy KK, Reddanna P. Structure and ligand based drug design strategies in the development of novel 5- LOX inhibitors. Current medicinal chemistry. 2012;19:3763–3778. doi: 10.2174/092986712801661112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Capra V, Back M, Barbieri SS, Camera M, Tremoli E, Rovati GE. Eicosanoids and their drugs in cardiovascular diseases: focus on atherosclerosis and stroke. Medicinal research reviews. 2013;33:364–438. doi: 10.1002/med.21251. [DOI] [PubMed] [Google Scholar]
- 407.Sendobry SM, Cornicelli JA, Welch K, Bocan T, Tait B, Trivedi BK, Colbry N, Dyer RD, Feinmark SJ, Daugherty A. Attenuation of diet-induced atherosclerosis in rabbits with a highly selective 15-lipoxygenase inhibitor lacking significant antioxidant properties. British journal of pharmacology. 1997;120:1199–1206. doi: 10.1038/sj.bjp.0701007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Kenyon V, Rai G, Jadhav A, Schultz L, Armstrong M, Jameson JB, 2nd, Perry S, Joshi N, Bougie JM, Leister W, Taylor-Fishwick DA, Nadler JL, Holinstat M, Simeonov A, Maloney DJ, Holman TR. Discovery of potent and selective inhibitors of human platelet-type 12- lipoxygenase. Journal of medicinal chemistry. 2011;54:5485–5497. doi: 10.1021/jm2005089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Rai G, Kenyon V, Jadhav A, Schultz L, Armstrong M, Jameson JB, Hoobler E, Leister W, Simeonov A, Holman TR, Maloney DJ. Discovery of potent and selective inhibitors of human reticulocyte 15-lipoxygenase-1. Journal of medicinal chemistry. 2010;53:7392–7404. doi: 10.1021/jm1008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Gregus AM, Dumlao DS, Wei SC, Norris PC, Catella LC, Meyerstein FG, Buczynski MW, Steinauer JJ, Fitzsimmons BL, Yaksh TL, Dennis EA. Systematic analysis of rat 12/15-lipoxygenase enzymes reveals critical role for spinal eLOX3 hepoxilin synthase activity in inflammatory hyperalgesia. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27:1939–1949. doi: 10.1096/fj.12-217414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Conrad M, Sandin A, Forster H, Seiler A, Frijhoff J, Dagnell M, Bornkamm GW, Radmark O, Hooft van Huijsduijnen R, Aspenstrom P, Bohmer F, Ostman A. 12/15-lipoxygenase-derived lipid peroxides control receptor tyrosine kinase signaling through oxidation of protein tyrosine phosphatases. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15774–15779. doi: 10.1073/pnas.1007909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Sloane DL, Dixon RA, Craik CS, Sigal E. Expression of cloned human 15-lipoxygenase in eukaryotic and prokaryotic systems. Advances in prostaglandin, thromboxane, and leukotriene research. 1991;21A:25–28. [PubMed] [Google Scholar]
- 413.Brune B, Dehne N, Grossmann N, Jung M, Namgaladze D, Schmid T, von Knethen A, Weigert A. Redox control of inflammation in macrophages. Antioxidants & redox signaling. 2013;19:595–637. doi: 10.1089/ars.2012.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Goswami SK. Cellular redox, epigenetics and diseases. Sub-cellular biochemistry. 2013;61:527–542. doi: 10.1007/978-94-007-4525-4_23. [DOI] [PubMed] [Google Scholar]
- 415.Zheng Y, Brash AR. On the role of molecular oxygen in lipoxygenase activation: comparison and contrast of epidermal lipoxygenase-3 with soybean lipoxygenase-1. The Journal of biological chemistry. 2010;285:39876–39887. doi: 10.1074/jbc.M110.180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Zheng Y, Brash AR. Dioxygenase activity of epidermal lipoxygenase-3 unveiled: typical and atypical features of its catalytic activity with natural and synthetic polyunsaturated fatty acids. The Journal of biological chemistry. 2010;285:39866–39875. doi: 10.1074/jbc.M110.155374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Funk CD, Hoshiko S, Matsumoto T, Radmark O, Samuelsson B. Characterization of the human 5-lipoxygenase gene. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:2587–2591. doi: 10.1073/pnas.86.8.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]