Abstract
Potentially life-threatening enterocolitis is the most frequent complication in children with colonic aganglionosis (Hirschsprung disease, HSCR), and little is known about the mechanisms leading to enterocolitis. Splenic lymphopenia has been reported in the Endothelin Receptor B (Ednrb)-null mouse model of HSCR that develops enterocolitis. In this study, we sought to identify molecular mechanisms underlying this immune phenotype. We employed the Ednrb−/− mouse, and the knockout of its ligand, Edn3 (Edn3−/−). The major finding is that enterocolitis in the Ednrb−/− and Edn3−/− mice lead to thymic involution, splenic lymphopenia, and suppression of B lymphopoiesis as a consequence of colonic aganglionosis, not an intrinsic Edn3-Ednrb signaling defect directly affecting the lymphoid organs. We showed that adoptive transfer of Ednrb−/− marrow repopulated the RAG2-null mice marrow, thymus and spleen without development of enterocolitis. We identified the glucocorticoid corticosterone, as a potential mediator of the immune phenotype. This previously unrecognized pattern of immune abnormalities in mouse is nearly identical to lymphoid depletion in neonatal sepsis during severe physiological stress, suggesting that the mouse model used here could be also used for sepsis studies.
Keywords: Corticosterone, Endothelins, Enterocolitis, Glucocorticoids, Hirschsprung disease, HAEC, Lymphoid depletion
Introduction
Congenital aganglionic megacolon, more commonly known as Hirschsprung disease (HSCR), was first described by Härald Hirschsprung in 1887 [1]. The hallmark pathological feature of this condition is the absence of ganglion cells in the distal colon causing a functional bowel obstruction in newborns. Importantly on Hirschsprung’s original description, he also noted marked inflammation described as mucosal ulcerations and crypt abscess [1]. Later this inflammatory condition in HSCR patients became known as Hirschsprung-associated enterocolitis (HAEC) [2] characterized clinically by fever, abdominal distention, diarrhea, and sepsis [3]. Failure to recognize HSCR in the early perinatal period places children at greater risk of developing HAEC [4], with HAEC complicating 18–50% of these children in the preoperative period [5].
In the past decade more than 15 human genes have been identified to be involved in HSCR. While the RET proto-oncogene appears to be the dominant gene associated with HSCR pathogenesis, mutations in the Endothelin Receptor B and Endothelin 3 genes are the next most frequently identified, each accounting for approximately 5% of HSCR patients [6]. The endothelins (Edn1, Edn2, and Edn3, respectively) are a family of potent vasoactive local messengers that act via cell surface Endothelin Receptors A and B (EDNRA and EDNRB, respectively) on the vascular endothelium in postnatal life [7]. However, disruption of Edn3-Ednrb signaling during developmental stage E10.5–12.5 in the mouse embryo, leads to migratory disruption of the neural crest derived enteric nervous system and subsequent aganglionosis of the distal colon [8]. Rodent models with both naturally occurring and targeted mutations of the endothelin receptor B (Ednrb) gene are the most extensively studied animal models of Hirschsprung disease because they closely mimic the phenotypic characteristics of human Hirschsprung disease [9, 10].
To dissect the biological mechanisms contributing to HAEC, our group developed an animal model to study enterocolitis in “untreated” animals (without surgery) and “treated” animals after pull-through surgery [11, 12]. We employed the endothelin receptor B targeted-null mouse (Ednrb−/−), a well-known mouse model of HSCR that has aganglionosis of the rectum and distal colon as well as enterocolitis [9]. We extensively characterized the untreated animals and found that they developed enterocolitis that had histopathological similarities to children with HAEC. We then developed a novel single-stage microsurgical pull-through operation to resect the aganglionic segment of colon and establish fecal continuity in the Ednrb−/− mouse [13]. We showed that approximately 40% of the Ednrb−/− mice developed enterocolitis after surgery, closely resembling key features of HAEC after pull-through surgery in children [11, 12].
Recently we reported that the spleens of the untreated Ednrb−/− mice were markedly smaller than their wild-type littermates [14]. Further, the results from our initial study revealed a five to 20-fold reduction in total splenocytes. The splenic CD19+, CD4+, and CD8+ cells of the Ednrb−/− mice were dramatically decreased compared with wild-type littermates, and the extent of reduction strongly correlated with the severity of the enterocolitis.
In the current study, we sought to distinguish between two mechanistic explanations for the dramatic splenic lymphopenia we observed in the Ednrb−/− mice. One possibility is that Edn3-Ednrb signaling is required for normal development and functioning of the immune system in the adult animal, such that when disrupted, the lymphoid system fails to develop normally and lymphopenia ensues. Alternatively, the immune phenotype could be a secondary effect of colonic aganglionosis from Ednrb and Edn3 gene mutations in embryologic development.
We unexpectedly found that the immunological findings of the Edn3 and Ednrb knock out mice had remarkably similar features to lymphoid depletion in neonates and children with severe sepsis: including thymic involution, peripheral lymphopenia, lymph node involution, and splenic depletion [15–17]. Herein, we discuss mechanisms leading to HAEC and lymphoid depletion, and the implications for diagnosis and management of infants born with HSCR.
Results
Edn3−/− and Ednrb−/− mice have similar immune phenotype and enterocolitis
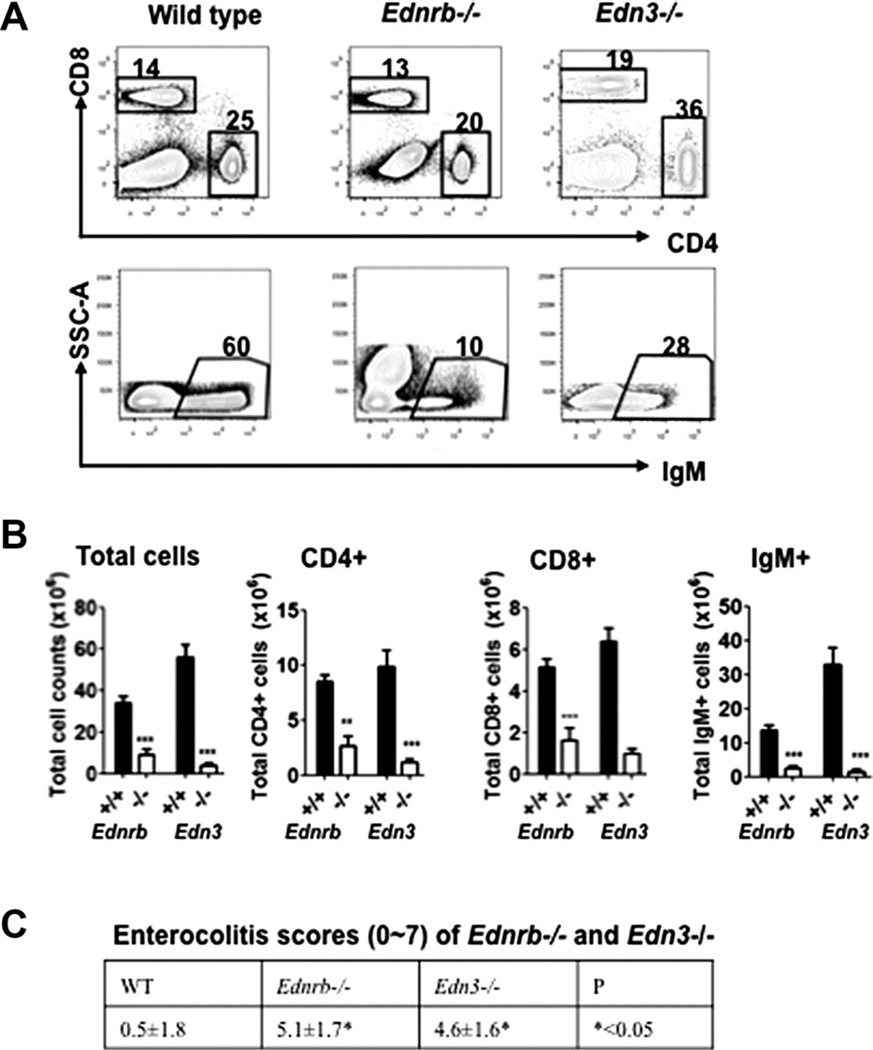
Previously, we reported that the Ednrb−/− mice with colonic aganglionosis have small spleens and splenic lymphopenia that most severely affected mature B cells, and to a lesser extent CD4+ and CD8+ T cells compared with wild-type littermate controls [14]. We also found that the Ednrb−/− mice developed enterocolitis, the severity of which inversely correlated with total splenic cell count and B cell count. We therefore hypothesized that Edn3-Ednrb signaling may be critical to immune system development. Hence, we also reasoned that the Edn3-null mice, carrying a null-mutation in the ligand for Ednrb that has a nearly identical colonic aganglionosis phenotype [18], might also have a similar immune phenotype to the Ednrb−/− mice. We performed FACS of splenocytes of Edn3−/− and Ednrb−/− mice and found the same striking reduction of mature B and T cells in the Edn3−/− mice that had an essentially identical pattern to the Ednrb−/− mice shown in Fig. 1A and B. Not surprisingly, the mean histopathologic enterocolitis scores of Ednrb−/− and Edn3−/− were 4.6 ± 1.6 and 5.1 ± 1.7 that were markedly elevated compared with WT of 0.5 ± 1.8 (p < 0.05) [11] (Fig. 1C). As anticipated, the Edn3−/− mice showed a nearly identical immune phenotype to the Ednrb−/− mice.
Figure 1.
Splenic lymphocyte composition and enterocolitis scores in Ednrb- and Edn3-null mice. (A) Representative FACS profiles and percentages of splenic CD4+, CD8+, and IgM+ cells of WT, Ednrb−/−, and Edn3−/− mice. (B) Total splenic cell numbers, numbers of splenic CD4+, CD8+, and IgM+ cells in Ednrb−/− and Edn3−/− mice. (C) Enterocolitis scores of wild type, Ednrb−/− and Edn3−/−. Data are presented as mean ± SEM of n = 5–6 mice/group. These data are representative of two separate experiments performed with both genotypes. *p < 0.05, **p < 0.01, and ***p < 0.001. Comparison between Ednrb−/− and +/+, Edn3−/− and +/+ was made using Student’s t-test.
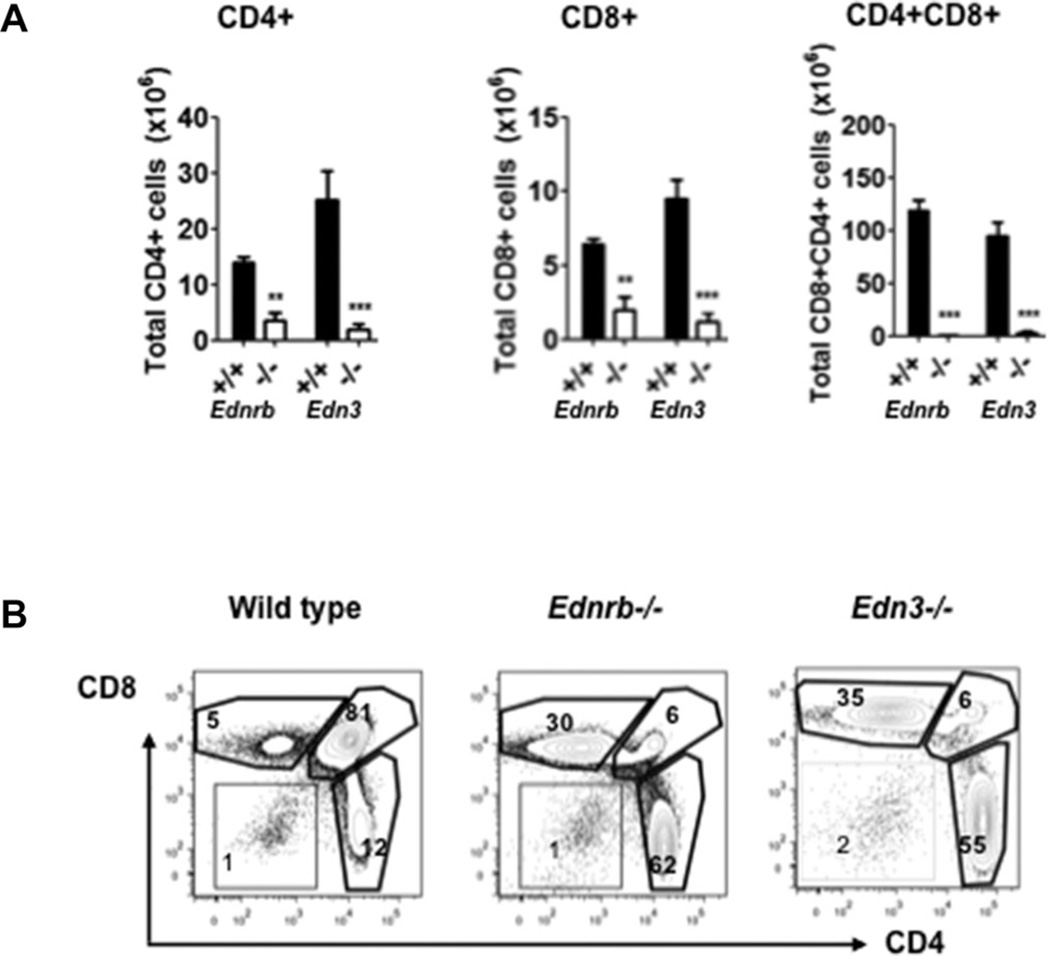
Suspecting that other immune organs may be affected as well, we performed FACS of thymocytes of the same genotypes. We found a marked shift from CD4+CD8+ double positive cells to single CD4+ and CD8+ cells (Fig. 2A) as well as dramatic reductions in the T-cell populations in the Ednrb−/− and Edn3−/− mice compared to WT controls (Fig. 2B).
Figure 2.
Thymocyte composition in Ednrb- and Edn3-null mice. (A) Summary of total numbers of CD4+, CD8+, and CD4+CD8+ cells in thymus. (B) Representative FACS profiles and percentages of CD4+, CD8+, and CD4+CD8+ cells in thymus. Data are shown as mean ± SEM of n = 5–6 mice/group, and are representative of two independent experiments performed with both genotypes. *p < 0.05, **p < 0.01, and ***p < 0.001. Comparisons between Ednrb−/− and +/+, Edn3−/−, and +/+ were performed using Student’s t-test.
B lineage lymphopoiesis is markedly reduced in Ednrb−/− and Edn3−/− mice
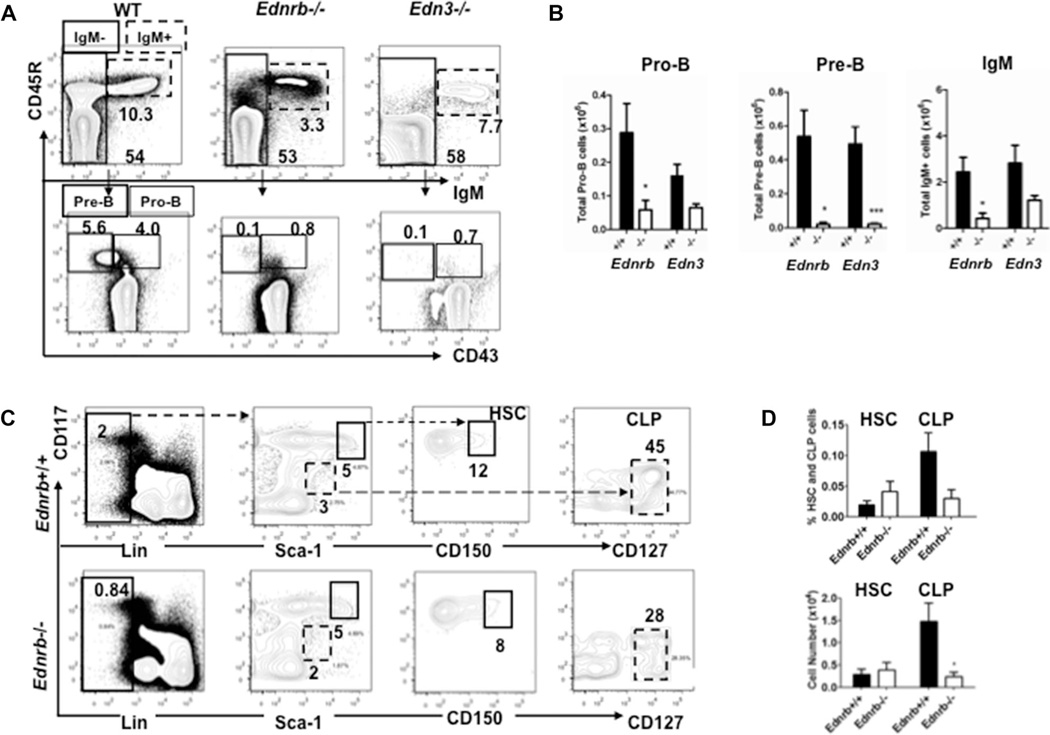
Given the reduction of B cells in the spleen, we wondered if it was due to loss of mature B cells or a decrease in B lymphopoiesis. So we performed FACS of bone marrow cells harvested from Ednrb−/− and Edn3−/− mice focusing on mature B cells, and the B cell precursors pro-B, pre-B as an initial step to assess B lymphopoiesis. We found marked reductions of the pro-B, pre-B, and mature B cell populations of the Ednrb−/− and Edn3−/− mice compared with WT (Fig. 3A and B). While the pre-B population was severely diminished 10 to 25-fold, the pro-B and IgM+ B cells were decreased two to fivefold.
Figure 3.
Bone marrow B lymphopoiesis in Ednrb- and Edn3-null mice at age P21. (A) Representative FACS profiles and percentages of pro-B and pre-B, and mature B cells of WT, Ednrb−/−, and Edn3−/− mice. Pro-B (CD45R+CD43+IgM−) and pre-B (CD45R+CD43−IgM−) cells were gated from CD45R+IgM− cells. IgM+ cells represent mature B cells. B, Number of pro-B, pre-B, and IgM+ cell numbers in bone marrow. These data are representative of two separate experiments performed with both genotypes. (C) Representative FACS histograms and percentages of HSCs[Lin−CD117 (c-kit)highSca-1highCD150+] and CLPs[Lin−CD117lowSca-1lowCD127+] cells in bone marrow. (D) Number and percentages of HSCs and CLPs in bone marrow of Ednrb−/− and their WT littermates. Data are presented as mean ± SEM of n = 5–6 mice/group. (C and D) Data shown are from a single experiment. *p < 0.05, **p < 0.01, and ***p < 0.001. Comparison between Ednrb−/− and +/+ was performed using Student’s t-test.
In light of these findings, we then assessed the hematopoietic stem cell (HSC) and common lymphocyte progenitor (CLP) populations in the Ednrb−/− mice only and found that CLP populations were also significantly reduced compared with wild type, but that the HSC’s were similar (Fig. 3C and D).
B lymphopoiesis is normal in young Ednrb−/− mice
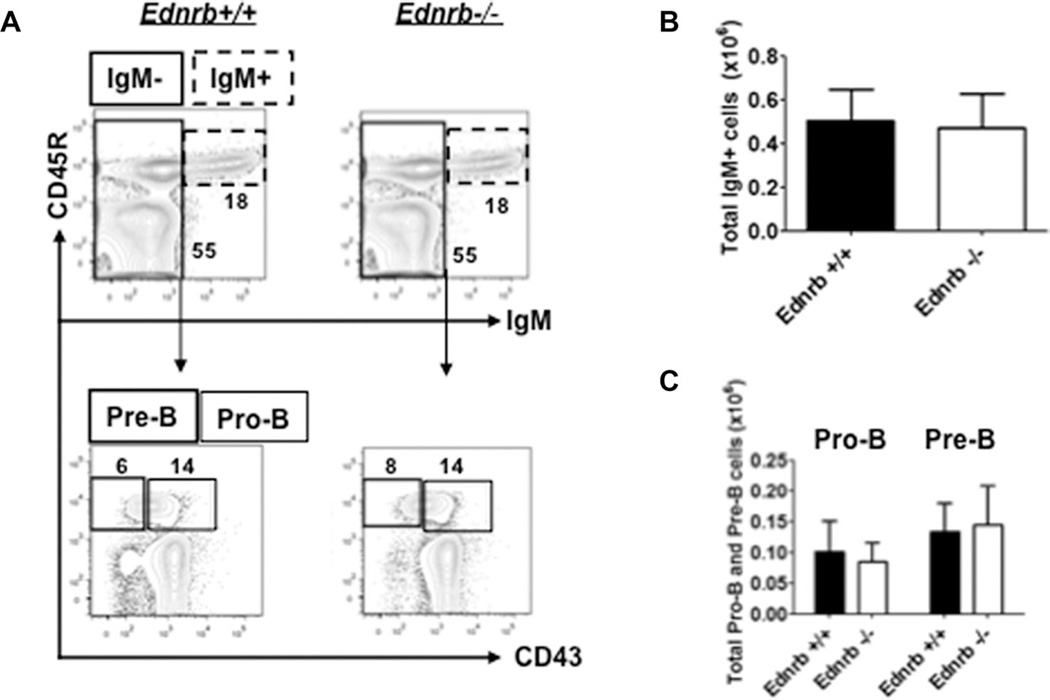
To approach the question of whether or not these findings were present at birth, we evaluated the pro-B, pre-B, and mature B-cell populations in age P10 Ednrb−/− mice before megacolon and enterocolitis developed. We found that B lymphopoiesis was essentially the same in Ednrb−/− mice compared with wild type (Fig. 4) indicating that the reduced B lymphopoiesis occurs in postnatal development, and that the Ednrb−/− mice are likely born with normal bone marrow B lymphopoiesis. We did not perform this analysis in the Edn3−/− mice due to breeding challenges with the strain.
Figure 4.
B lineage lymphopoiesis in neonatal (P10) Ednrb-null mice. (A) Representative FACS profiles and percentages of pro-B, pre-B, and mature B cells; Pro-B (CD45R+CD43−IgM−) and pre-B (CD45R+CD43+IgM−) cells were gated from CD45R+IgM− cells. (B) Number of IgM+ cells. (C) Number of pro-B and pre-B cells in bone marrow. These data are from a single experiment and presented as mean ± SEM of n = 5–6 mice/group). Comparison between Ednrb−/− and +/+ was made using Student’s t-test. None reached statistical significance.
Adoptive transfer of Ednrb−/− bone marrow to RAG2-null recipients does not result in enterocolitis
At this phase of our studies we suspected that Edn3-Ednrb signaling may play an important role in postnatal immune system development. However, we could not rule out the possibility that the profound immune phenotype was due to a secondary effect of aganglionosis leading to megacolon. To distinguish between these possibilities,
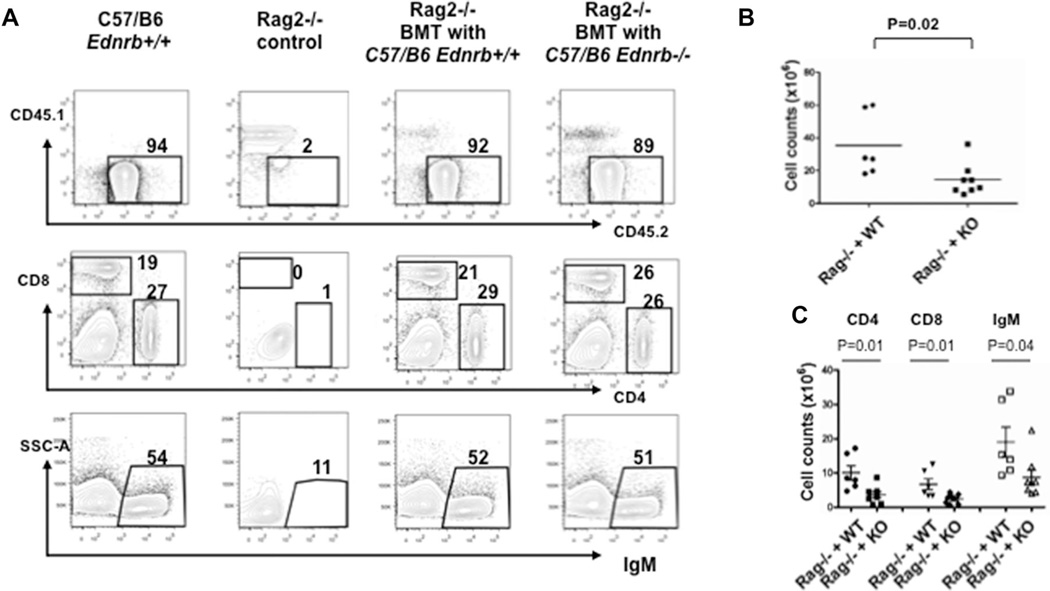
We performed adoptive transfer of Ednrb−/− marrow into RAG2-null mice with appropriate controls. The CD45.1 and CD45.2 system [19] was utilized to distinguish recipient from donor-derived stem cells, and also to track the proportion of donor and recipient derived cells to determine the success of bone marrow transplantation. The irradiated RAG2−/− mice expressing CD45.1 were recipients with BM cells from congenic C57/B6 Ednrb−/− mice expressing CD45.2. Recipients were euthanized at 8–10 weeks posttransplantation. Figure 5 shows a representative example of FACS analysis of splenocytes of the adoptive transfer experiment showing successful engraftment of the donor marrow along with appropriate controls. Approximately 50% of bone marrow, and more than 90% of splenocytes were repopulated by donor-derived CD45.2+ cells, in all recipients. The numbers and percentages of recipient thymic CD4+ and CD8+, and CD4+CD8+ cells was essentially the same between Ednrb−/− and Ednrb+/+ donors (data not shown).
Figure 5.
Reconstitution of splenic B and T lymphocytes in RAG2−/− recipients after adoptive transfer of Ednrb-null and wild type donor derived bone marrow. (A) Representative FACS profiles and percentages of CD4+, CD8+, and IgM+ cells in spleens of WT, RAG2−/− control, and RAG2−/− recipients with bone marrow transplantation of Ednrb+/+ and Ednrb−/− mice. (B) Summary of total lymphocyte numbers in spleens of RAG2−/− recipient mice. (C) Summary of total numbers of CD4+, CD8+, and IgM+ cells in spleens of RAG2−/− recipient mice. These data are from a single experiment and are presented as mean ± SEM of n = 6–8 mice/group. Comparison between groups was made using Student’s t-test.
The percentages of CD4+, CD8+, and IgM+ splenocytes between the recipients with donor Ednrb−/− and Ednrb+/+ BM cells did not show significant differences, and both recovered to the similar level (Fig. 5A, middle and lower panel). Although interestingly, the RAG2−/− mice transplanted with Ednrb−/− marrow had less than half the total splenic cell count than those from the Ednrb+/+ recipients (Fig. 5B and C).While we cannot fully explain this finding, we suspect that Ednrb may play a role in trafficking of both B and T lymphocytes to the spleen from the marrow and thymus.
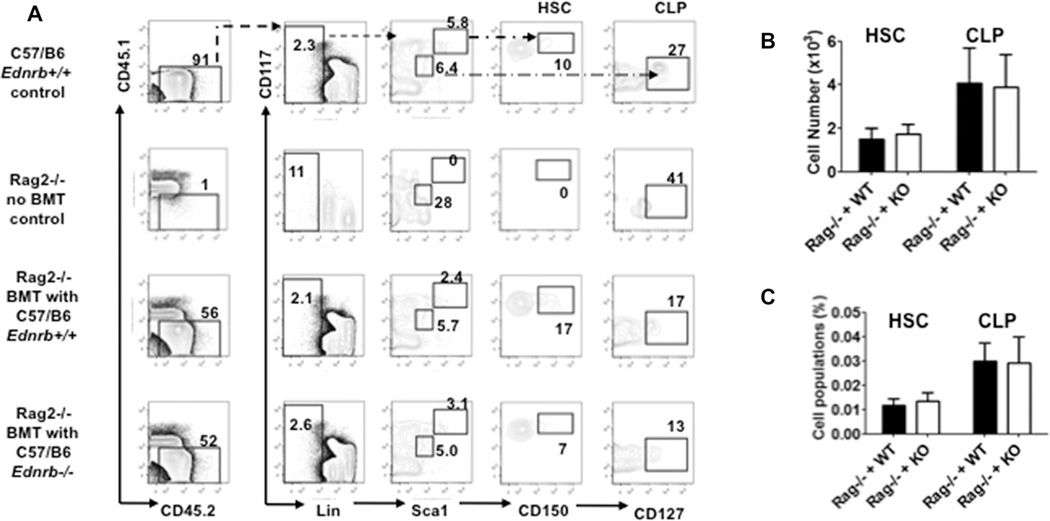
In the same animals, the HSCs and CLPs from the recipient mice of BM transplantation were determined and the results shown in Fig. 6. Donor BM cells both from C57/B6 Ednrb−/− and C57/B6 Ednrb+/+ engrafted and recovered to similar level in the RAG2−/− recipient mice. Pro-B, pre-B, and mature B (IgM+) populations were also determined but did not show any significant differences. Histopathological grading of the colon did not show any evidence of enterocolitis in recipients from either Ednrb−/− or Ednrb+/+ donors (data not shown). The results do not support an intrinsic Edn3-Ednrb signaling defect is the mechanism leading to the suppression of lymphopoiesis in the Ednrb and Edn3-null mice, but rather is likely a secondary effect of enterocolitis.
Figure 6.
Bone marrow reconstitution of HSCs and CLPs in RAG2−/− recipients after adoptive transfer of Ednrb-null and wild-type donor derived bone marrow. (A) Representative FACS profiles and percentages of HSCs and CLPs in bone marrow of WT, RAG2−/− control, and RAG2−/− recipients with bone marrow transplantation of Ednrb+/+ and Ednrb−/− mice. (B and C) Graphs show (B) the cell number and (C) the percentage of HSCs and CLPs. These data are from a single experiment and are presented as mean ± SEM of n = 6–8 mice/group. Comparison between groups was made using Student’s t-test. No significant differences were found.
Ednrb−/− and Edn3−/−mice have elevated serum corticosterone levels
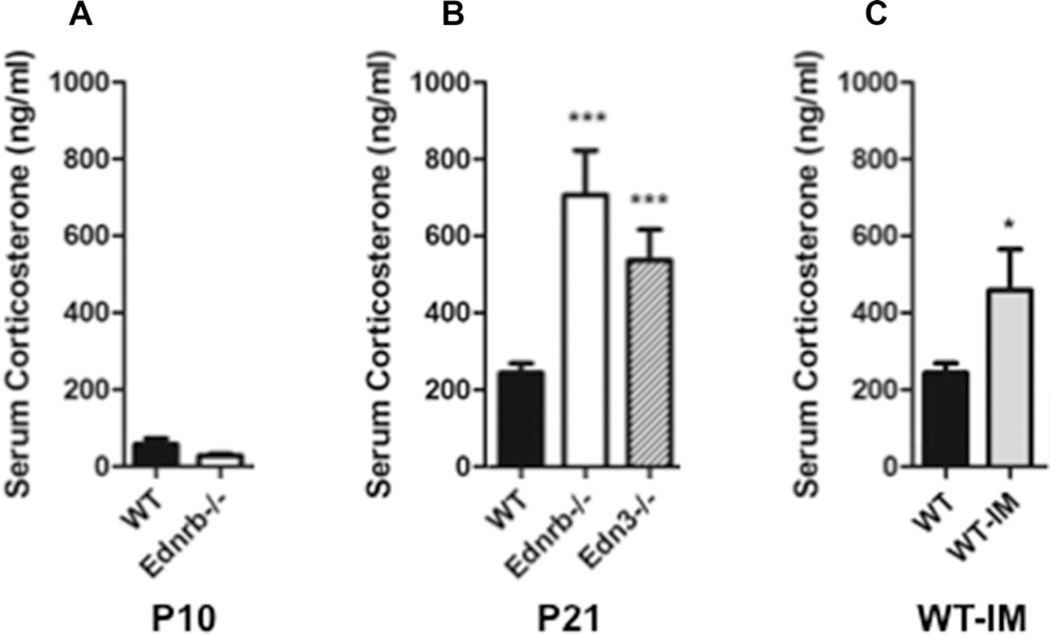
The observed pattern of immune suppression was similar to that seen in animals and humans with elevated levels of glucocorticoids [20, 21]. While not previously described, we reasoned that the Ednrb- and Edn3-null mice with megacolon and enterocolitis likely experienced significant physiological stress. We sought to investigate the hypothesis that enterocolitis led to elevated circulating glucocorticoids that was an important mediator causing the immune phenotype. To address this question, we determined serum corticosterone concentrations at age P10, before the Ednrb−/− mice had developed megacolon and enterocolitis. Both wild type and knockout showed similarly low levels (Fig. 7A). We then determined serum CS concentrations of Ednrb−/− and Edn3−/− mice at P21, a time when these mice develop enterocolitis and found two to threefold elevation compared with control (Fig. 7B).
Figure 7.
Serum corticosterone concentrations in neonatal Ednrb-null mice, weanling Ednrb- and Edn3-null mice, and wild-type mice with induced megacolon. Serum corticosterone concentrations (ng/mL) of: (A) age P10WTand Ednrb−/− mice. (B) Age P21 WT, Ednrb−/−, and Edn3−/− mice. (C) WT and wild type with induced megacolon (WT-IM). (A–C) Data are from a single experiment and are presented as mean ± SEM of n = 5–6 mice/group. *p < 0.05, **p < 0.01, and ***p < 0.001. Comparison between groups was made using Student’s t-test.
Induced megacolon mice have elevated serum corticosterone levels and suppressed B lymphopoiesis
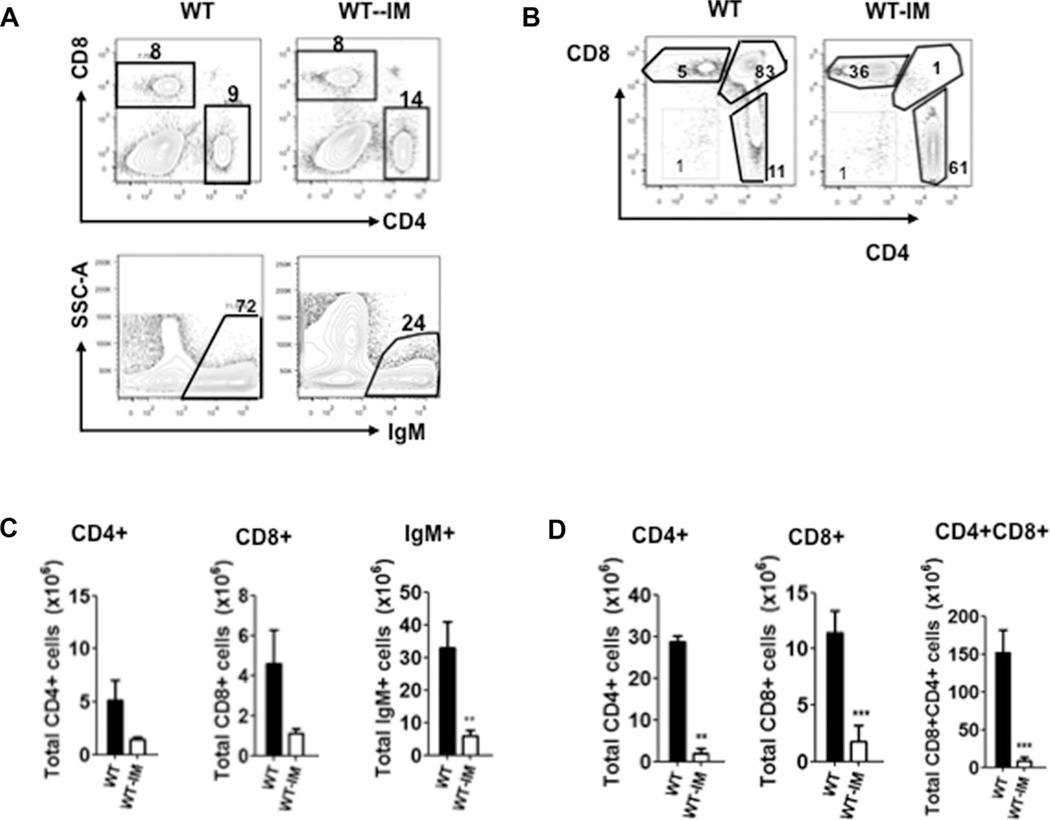
To exclude the possibility that Edn3-Ednrb signaling directly contributes to the elevated corticosterone in the Ednrb−/− and Edn3−/− mice, we induced megacolon in wild-type mice (WT-IM). We then measured serum CS concentrations of the WT-IM and littermate controls at P21 when obstruction has led to enterocolitis, and found markedly elevated levels in the WT-IM cohort, similar to the Ednrb−/− and Edn3−/− mice (Fig. 7C). When the spleen, thymus, and bone marrow lymphocyte populations were assessed, the spleen showed marked reduction of mature B cells, the thymus showed involution with dramatically reduced CD4+, CD8+, and CD4+CD8+ cells (Fig. 8). The marrow B lymphopoiesis was markedly reduced with pre-B cells most significantly affected (Fig. 9), as seen in the Ednrb and Edn3-null mice (Fig. 3B). These results show that inducing megacolon with development of enterocolitis in wild-type mice leads to elevated serum CS concentrations and the same immune phenotype seen in the Ednrb−/− and Edn3−/− mice, strongly suggesting that physiological stress from enterocolitis plays a significant role in the immune suppression. In addition, it nearly eliminates the possibility that postnatal Edn3-Ednrb signaling directly contributes to the immune phenotype.
Figure 8.
Splenic and thymic lymphocyte populations in wild-type mice with surgically induced megacolon (WT-IM). (A) Representative FACS profiles and percentages of CD4+, CD8+, and IgM+ cells in spleens of WT and WT-IM mice. (B) Representative FACS profiles and percentages of CD4+, CD8+, and CD4+CD8+ cells in thymus of WT and WT-IM mice. (C) Summary of mean number of CD4+, CD8+, and IgM+ cells in spleens of WT and WT-IM mice. (D) Summary of mean number of CD4+, CD8+, and CD4+CD8+ cells in thymus of WT and WT-IM mice. (A and B) Data are from a single experiment and are presented as mean ± SEM of n = 5–6 mice/group. *p < 0.05, **p < 0.01, and ***p < 0.001. Comparison between groups was made using Student’s t-test.
Figure 9.
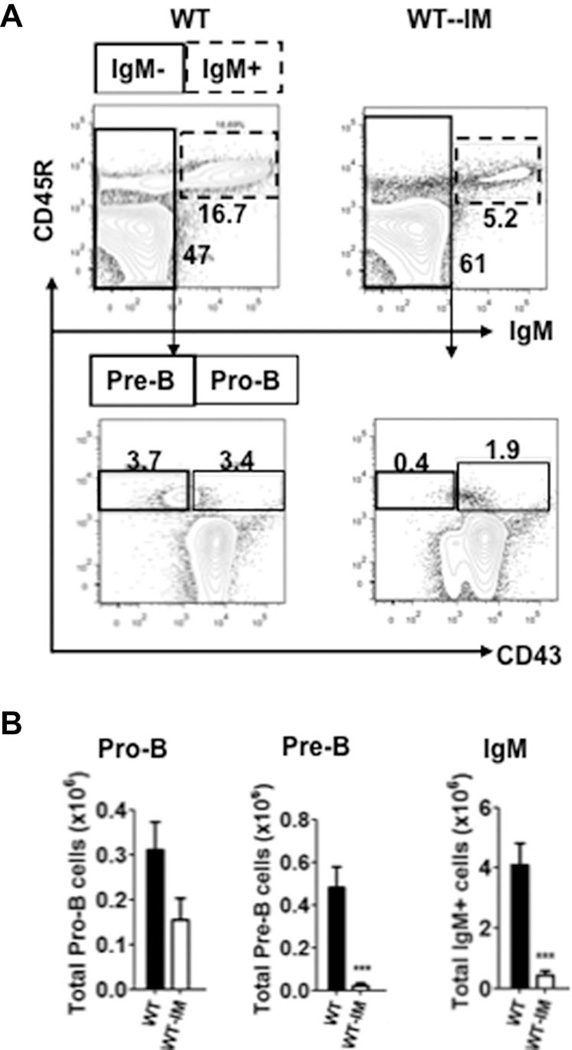
Pro-B, pre-B, and mature B-cell populations of wild-type mice with surgically induced megacolon. (A) Representative FACS profiles and percentages of pro-B, pre-B, and mature B cells; pro-B (CD45R+CD43−IgM−), and pre-B (CD45R+CD43+IgM−) cells were gated from CD45R+IgM− cells in bone marrow of WT and WT-IM mice; (B) Total numbers of pro-B, pre-B, and mature B cells in bone marrow of WT-IM with appropriate controls. Data are from a single experiment and are presented as mean ± SEM of n = 5–6 mice/group. *p < 0.05, **p < 0.01, and ***p < 0.001. Comparison between groups was made using Student’s t-test.
Discussion
The most striking finding of this study is that HAEC in the Ednrb- and Edn3-null mice causes lymphoid depletion as a consequence of colonic aganglionosis, not an intrinsic postnatal Edn3-Ednrb signaling defect directly affecting the lymphoid organs. We showed that adoptive transfer of Ednrb−/− donor bone marrow repopulated the RAG2-null mice bone marrow, thymus, and spleen without development of enterocolitis. Separately, when we induced megacolon in wild-type mice (IM-WT), they went on to develop enterocolitis and subsequently lymphoid depletion that recapitulated the immune phenotype of the Ednrb- and Edn3-null mice, further demonstrating that an Edn3-Ednrb signaling defect was not essential to reproduce the immune phenotype. We also found markedly elevated serum corticosterone levels in mice with enterocolitis, suggesting that corticosterone may be a major mediator of thymic involution, suppression of B lymphopoiesis, and splenic lymphoid depletion.
Interestingly, lymphoid depletion has been described in a number of clinical settings ranging from term and preterm infants who experience severe physiological stress [15, 17, 22, 23] to critically ill children with nosocomial sepsis and multiple organ failure [16]. Acute thymic involution has been observed in fetuses and neonates who succumbed from sepsis and chorioamnionitis. When Toti et al. performed detailed morphological and histological examination of thymuses of aborted fetuses and neonates with early onset sepsis (<48 h of birth) from mothers with documented chorioamnionitis, they found decreased thymic volume, reduced corticomedullary ratio, and severe reduction of thymocytes [23]. In the same group of subjects, Toti et al. subsequently reported reduced splenic volume and severe depletion of B and T lymphocytes only in the subgroup of neonates who developed sepsis [17].
In an autopsy study of 100 neonates, Glavina-Durdov et al. found a strong correlation between severe thymic involution (grade 4) and infectious causes of death [15]. They report that these findings were distinctly different from neonates who died of respiratory distress syndrome who had much less thymic involution. In addition, this study confirmed previous observations that the duration of illness plays a critical role in thymic involution [24] in that severe thymic involution was seen in 93% of neonates living greater than 72 h, compared with no involution in neonates living less than 12 h. Further, in neonates with severe thymic involution, they also noted peripheral lymphopenia within a day of their demise. Taken together, these neonatal studies strongly suggest that sepsis lasting greater than 3 days is most closely associated with high-grade thymic involution and splenic lymphoid depletion.
In a critical care setting, Felmet et al. described prolonged lymphopenia and lymphoid depletion in critically ill children with nosocomial sepsis and multiple organ failure [16]. They found that prolonged lymphopenia (absolute lymphocyte count <1000 for >7 days) occurred only in children with multiple organ failure and was associated independently with nosocomial infection, death, and splenic and lymph node hypocellularity. They concluded that prolonged lymphopenia and apoptosis-associated depletion of lymphoid organs play a role in nosocomial sepsis-related death in critically ill children.
Lymphoid depletion in critically ill neonates and children appears very similar to what we observed, in that the Ednrb−/−, Edn3−/−, and WT-IM mice with enterocolitis all of which showed markedly decreased thymic volume and similar reduction of T-cells, dramatic splenic depletion of CD4 and CD8 T cells and mature B cells consistent with severe physiological stress associated with sepsis in the neonatal period. We also found marked suppression of B lymphopoiesis primarily at the pro-B, pre-B stages, but not at the CLP. These findings are most consistent with glucocorticoid mediated lympholysis of pre-B and mature B cells via apoptosis mediated through the Bcl-2 pathway [16, 25–27].
Given our results showing reduced CD4+, CD8+, and IgM+ cell counts in only the spleen of RAG2-null recipients after adoptive transfer of Ednrb−/− compared with wild-type marrow (Fig. 5B and C), we cannot exclude the possibility that the splenic lymphopenia is the result of a combination of an Ednrb-dependent trafficking defect of lymphocytes to the spleen and the stress-mediated glucocorticoid effect. In the scenario in which both mechanisms are at work, the glucocorticoid effect may potentially exacerbate the trafficking defect, thereby contributing to the profound lymphopenia in the Ednrb−/− mice. Based on a recent study showing that Ednrb acting through ICAM-1 regulated T cell homing to tumor in an ovarian cancer model [28], we speculate that perhaps Ednrb may play a similar role on the endothelium of the spleen.
Recently, Dang et al. reported that the AGH-Ednrbsl/sl rat model of HSCR showed lymphoid depletion with markedly reduced spleen weight and total splenic cell count of less than 15% of wild-type AGH rats, with severe reduction of B cells, and CD4 and CD8 T cells [29]. The AGH-Ednrbsl/sl rat also had significantly reduced thymic weight and cell number, with no change in the CD4/CD8 ratio when studied at only 8 days of life, but otherwise very similar to the murine models in our study. In their study, the authors compared the AGH-Ednrbsl/sl rat to another congenic strain with the same Ednrb mutation the F344-Ednrbsl/sl that did not show these immune findings, leading the authors to propose that lymphopenia was likely developmental and was strongly modified by genetic background. On closer examination of these congenic rat models, there is a dramatic difference in length of aganglionosis between the congenic strains. The AGH-Ednrbsl/sl strain has long-segment aganglionosis extending into the small intestine where only 20% survived to weaning. Conversely, 100% of the F344-Ednrbsl/sl rats survived to weaning and 60% showed no symptoms of aganglionosis, healthy appearance with normal weight gain, and the remaining 40% with short-segment colonic aganglionosis die by P90 [30]. While the authors do not report an evaluation for histopathological evidence of enterocolitis in either rat strain, enterocolitis has been well described in spotting lethal rat strains, most notably those with long-segment aganglionosis [31] similar to the AGH-Ednrbsl/sl. Hence, the AGH-Ednrbsl/sl rat with long-segment aganglionosis very likely develops enterocolitis that contributes to its early demise, while the 40% F344-Ednrbsl/sl rats with short segment aganglionosis may develop enterocolitis much later but eventually succumb, and the remaining 60% of healthy F344-Ednrbsl/sl rats with no aganglionosis never develop enterocolitis. Viewed in the context of enterocolitis, the phenotype of the AGH-Ednrbsl/sl rat very much mirror our findings.
It has been well described that the neonate’s innate and adaptive immune systems are functionally impaired compared with adults [32, 33]. Some of these differences include decreased production of TH1 polarizing cytokines, type I interferons, and MHC class II expression on antigen presenting cells; an immature dendritic cell system; and decreased plasma concentrations of complement components as well as delayed and decreased antibody responses from B cells [32, 33]. The adaptive immune system in neonatal mice have decreased mature B cells in the splenic marginal zone, leading to the lack of functional marginal zones that trap bacteria in the first 1–2 weeks after birth. In addition, organized B cell follicles in the spleen, critical for T cell-B cell interactions, are first found 7–10 days after birth and splenic germinal centers are absent until 3 weeks in mice. Wynn et al. observed that peritoneal B1 cells, the main phagocytic cell in the adult peritoneal cavity that recognizes self- and common bacterial antigens, and secretes antibodies [32] are dramatically reduced in resting neonates [34]. This deficiency most likely contributes to the inability of neonates to control local peritoneal infection, such as that which develops in necrotizing enterocolitis (and possibly HAEC) or intestinal perforation and may lead to decompensation due to rapid systemic bacterial proliferation. They further demonstrated that RAG-1-deficient neonatal mice showed no difference in susceptibility to sepsis compared with wild type, indicating that the adaptive immune system does not participate significantly in the clearance of severe microbial infections in the peritoneum. They went on to show that neonates rely primarily on innate immunity for their protection from polymicrobial sepsis through TLR4 and TLR 7/8 signaling pathways. Additionally, maternal antibodies transferred to the neonate from its mother through the placenta and milk provide protection during the postnatal maturation of the immune system.
In light of the complex and dynamic maturation of the neonatal immune system in the first weeks of life, we noted that the appearance of lymphoid depletion is considerably later in the mouse models (14–21 days) than the human neonatal studies (less than 7 days after birth). One possible explanation for this later onset is due to bacterial colonization of the neonatal gut in the setting of functional colonic obstruction, with subsequent development of megacolon likely leading to both mechanical stress to the integrity of the mucosal lining and enterocolitis leading to bacterial translocation and eventually sepsis [35]. This sequence of steps is occurring simultaneously with immature, but rapidly developing adaptive immune defenses. We speculate that between 10 and 21 days in our model, that enterocolitis develops leading to bacteremia that is temporarily kept in check by the innate immune defenses and maternal passive immunity, but then eventually becomes overwhelmed leading to sepsis and lymphoid depletion prior to development of robust adaptive immunity.
To date there have been no published reports of lymphoid depletion and lymphopenia in neonates with HSCR. Since HSCR is a relatively rare disease and an even rarer cause of death in developed nations, it is conceivable that lymphoid depletion has not been investigated at autopsy, as no prior observation linking Hirschsprung-associated enterocolitis and lymphoid depletion has been reported. Alternatively, the Ednrb- and Edn3-null mouse models of HSCR may develop lymphoid depletion more readily than human neonates with HSCR, and the immune phenotype may not be present in humans with HSCR, although we believe this is not likely. Unlike mice with aganglionosis, most, if not all infants born with HSCR in developed countries will be treated with antibiotics, rectal washouts, and early surgical intervention [36]. Hence, they will have the aganglionic region of colon (obstructing segment) removed, thereby dramatically reducing the risk of sepsis and enterocolitis. However, the early diagnosis and prompt treatment scenario described above may not be possible in the resource poor areas of the world [37]. Unfortunately, this scenario may result in neonates with either undiagnosed, or a late diagnosis of HSCR with ensuing enterocolitis leading to sepsis and eventually death.
We suggest that the Ednrb- and Edn3-null mouse models of HSCR, enterocolitis and lymphoid depletion are most similar to those infants with a late diagnosis or untreated HSCR. We further speculate that lymphoid depletion occurs as a consequence of enterocolitis and sepsis, which then significantly impairs the adaptive immune function thereby, further increasing risk of mortality.
Materials and methods
Animals
The research protocol and all animal care procedures were approved by the Institutional Animal Care and Use Committee (IACUC protocol #2302) at Cedars-Sinai Medical Center. The following congenic mouse strains were used: Endothelin Receptor B-null Ednrbtm1Ywa/J on 129SvIm/J background and Ednrbtm1Ywa/J on C57/B6J background originally imported from Jackson Laboratory (Bar Harbor, ME, USA) this strain is available at The Jackson Laboratory Repository with the JAX Stock No. 021933; Endothelin 3-null Edn3tm1Ywa/J on 129SvEv background were a gift of Masashi Yanagisawa, University of Texas Southwestern Medical Center at Dallas. The breeding scheme, animal care and genotyping were performed as previously described [13]. The homozygotes (we refer to these as Ednrb−/− and Edn3−/− in this paper) are easily identified by the white coat color and progressively enlarging abdomens due to aganglionosis leading to megacolon. The wild-type and heterozygote littermates (we refer to these as Ednrb+/+ and Ednrb+/−, Edn3+/+ and Edn3+/−, respectively), are phenotypically normal. All studies were performed with congenic 129Sv Edn3 and Ednrb mice, except for bone marrow transplant studies that were performed with congenic B6 Ednrb mice. RAG2-null mice (RAG2−/−) (B6.SJL (129S6)-Ptprca/BoyCrTac-Rag2tm1Fwa N10) were purchased from Taconic.
Induction of megacolon in wild-type mice
Mice with surgically induced megacolon were created in wild-type animals by our lab using a previously described procedure [38]. We refer to these as WT-IM. Briefly, the neonatal mouse pups of Ednrb+/+ and Ednrb+/− at age P2–4 were given isoflurane anesthesia via a rodent anesthesia machine and the anal canal cauterized using electrocautery. Three to four weeks after the procedure the mice began showing clinical signs of megacolon and enterocolitis including infrequent stooling, abdominal distention, poor feeding with occasional diarrhea. The mice were euthanized and en bloc enterectomy, (including anus, colon, and small intestine) and splenectomy was performed for histopathological evaluation. The WT-IM were found to have megacolon and enterocolitis resulting from anal stenosis.
Preparation of cells
Ednrb−/−, Edn3−/−, WT-IM, and control animals were euthanized. Thymus and spleens were dissected free of fat, weighed, and single cell suspension was prepared using mechanical disruption as described [14, 39]. Bone marrow was flushed from femurs and tibias with Dulbecco’s PBS (free of Calcium and Magnesium) using a 23G needle. The marrow was processed into single cell suspension by repeatedly flushing and red blood cells were removed by RBC lysis buffer (Biolegend). Cells were counted using a hemocytometer by trypan blue staining and adjusted to 1 × 106/100 µL with PBS buffer.
Flow cytometry and data analysis
Single cell suspensions of the thymus, spleen, and BM were evaluated by BD LSRII fluorescence cytometry. Hundred microliters of cells (1 × 107 cells/mL) was plated in 96-well V-bottom culture plates and incubated with purified rat anti-mouse CD16/CD32 blocking antibody (BD Pharmingen) for 5 min. Cells from thymus and spleens were then incubated on ice for 30 min with an antibody panel for CD4 [clone RM4–5 FITC-labeled, 1/50), CD8 (clone 53–6.7; PE-labeled, 1/50), and CD11b (clone M1/70; PerCP-Cy5.5-labled, 1/50), and IgM (clone 11/41, APC-labeled, 1/10). Cells from BM were characterized by antibody panel of CD24 (clone M1/69, FITC-labeled, 1/50), CD45R (clone RA3–6B2, PerCP Cy5.5-labeled, 1/50), IgM (clone 11/41, APC-labeled, 1/10), CD43 (clone S7, PE-labeled, 1/50; BD Pharmingen, San Diego, CA). The cell-type specific Abs for HSCs and CLPs were Lin-FITC labeled lineage antibody cocktail (CD8a, CD3ε, TCRβ, TCRγδ, CD45R, and NK1.1, 1/50 for each; Gr1,Ter119, and IgM, 1/100 for each), CD150 (PE-labeled, 1/100), Sca-1 (PerCP Cy5.5-labeled, 4uL/106 cells), CD117(APC-labeled, 4 µL/106 cells), CD127(PECy7-labeled, 4 µL/106 cells). CD45.1 (e-Fluor450 conjugated anti-mouse CD45.1, 1/50) and CD45.2 (APC-eFluor780 conjugated anti-mouse CD45.1 (C57BL/6), 1/50) were added into each of above antibody panel for the evaluation of RAG2−/− recipient mice of bone marrow transplantation (BMT). A total of 2 × 106 cells (2 × 107 cells/mL) from bone marrow were used for the assessments of HSCs and CLPs. All antibodies unless otherwise stated were from eBiosciences (San Diego, CA). Cells were then washed twice with Dulbecco’s PBS (1×) and fixed in 2% paraformaldehyde in Dulbecco’s PBS overnight at 4°C. Data were acquired on BD LSRII fluorescence cytometry and analyzed with FlowJo 7.6 software. The cell numbers were calculated from the frequency of each positive staining against total cell number of spleen, thymus, and BM, respectively.
Bone marrow transplantation (BMT)
Congenic B6 Ednrb−/− and +/+ mice (CD 45.2) at P20–23 days were used as marrow donors. The 6–9 week-old RAG2−/− (CD45.1) were used as the recipients. Briefly, the femurs and tibias were dissected from C57/B6 Ednrb+/+ and Ednrb−/− mice and a single cell suspension of bone marrow was prepared as described above. A total of 5~7 × 106 Cells were injected intravenously into the RAG2−/− mice that were pre-irradiated with Gamacell GC40 irradiator at a dose of 0.85 gray/min for 4 min. Irradiated RAG2−/− without BMT will be used as irradiation control. Recipients were euthanized 8–10 weeks posttransplantation. RAG2−/− and C57/B6 Ednrb+/+ mice were euthanized for CD45.1 and CD45.2 positive controls, respectively. The spleen, thymus and femurs were harvested for preparation of single cell suspension. The populations of CD4+, CD8+, IgM+, pro-B, pre-B, HSC, and CLP cells of the recipients was analyzed by flow cytometry as described above. The colons were harvested for histopathological grading.
Assessment of serum corticosterone levels
For P21 and P10 WT, Ednrb−/−, Edn3−/−, and WT-IM mice, blood was collected via cardiac puncture using sterile technique under anesthesia. All blood was coagulated at 4°C for 2–4 h and centrifuged at 6000 rpm for 10 min; serum was removed and stored at −80°C until assayed. Total serum corticosterone was determined using a commercially available radioimmunoassay (RIA) kit according to the manufacturer’s instructions (MP Biomedical, OH, USA).
Histologic preparation of colon and histopathological scoring for enterocolitis
Colon tissue from all animals was prepared, sectioned, H&E, and histopathologically graded for both severity of inflammation (inflammatory infiltrates and presence/absence of necrosis (0–3); and depth of inflammation (none, mucosa, submucosa, muscularis propria, subserosa, and serosa, 0–4, respectively) are added together to determine an enterocolitis score (0–7) using the validated grading system described previously by Cheng et al. [11].
Statistical analysis
The data collected from the study animals were compared using Student’s t-test, unpaired, two-tailed, 95% confidence interval. Data are presented as means ± SEM, unless otherwise stated. Statistical analysis was performed using GraphPad Prism 5 software (GraphPad, Inc., San Diego, CA, USA).
Acknowledgments
We would like to thank Dr. Ken Dorshkind and his lab for kind assistance with analysis of FACS and for fruitful discussions throughout the study. We would also like to thank Dr. David Underhill and Dr. George Liu for critical reading of the manuscript. This work was supported by NIH grant 1K08DK090281 (PKF), and The Lippey Family Endowment and The Walter and Shirley Wang Endowed Chair in Pediatric Surgery at Cedars-Sinai Medical Center.
Abbreviations
- BMT
bone marrow transplantation
- CLP
common lymphocyte progenitor
- HAEC
Hirschsprung-associated enterocolitis
- HSC
hematopoietic stem cell
- HSCR
Hirschsprung disease
Footnotes
Conflict of interest
P.F. is a consultant for Karl Storz Endoscopy – America. The remaining authors declare that they have no conflict of interest.
References
- 1.Hirschsprung H. Stuhtragheit neugeborener infolge dilatationen und hypertrophie des colons. Jahruch Kinderheikunde. 1887;27:1. [Google Scholar]
- 2.Berry CL. Persistent changes in the large bowel following the enterocolitis associated with Hirschsprung’s disease. J. Pathol. 1969;97:731–732. doi: 10.1002/path.1710970421. [DOI] [PubMed] [Google Scholar]
- 3.Pastor AC, Osman F, Teitelbaum DH, Caty MG, Langer JC. Development of a standardized definition for Hirschsprung’s-associated enterocolitis: a Delphi analysis. J. Pediatr. Surg. 2009;44:251–256. doi: 10.1016/j.jpedsurg.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 4.Swenson O, Sherman JO, Fisher JH, Cohen E. The treatment and postoperative complications of congenital megacolon: a 25 year followup. Ann. Surg. 1975;182:266–273. doi: 10.1097/00000658-197509000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vieten D, Spicer R. Enterocolitis complicating Hirschsprung’s disease. Semin. Pediatr. Surg. 2004;13:263–272. doi: 10.1053/j.sempedsurg.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Kusafuka T, Wang Y, Puri P. Mutation analysis of the RET, the endothelin-B receptor, and the endothelin-3 genes in sporadic cases of Hirschsprung’s disease. J. Pediatr. Surg. 1997;32:501–504. doi: 10.1016/s0022-3468(97)90616-3. [DOI] [PubMed] [Google Scholar]
- 7.Kedzierski RM, Yanagisawa M. Endothelin system: the double-edged sword in health and disease. Annu. Rev. Pharmacol. Toxicol. 2001;41:851–876. doi: 10.1146/annurev.pharmtox.41.1.851. [DOI] [PubMed] [Google Scholar]
- 8.Druckenbrod NR, Epstein ML. Age-dependent changes in the gut environment restrict the invasion of the hindgut by enteric neural progenitors. Development. 2009;136:3195–3203. doi: 10.1242/dev.031302. [DOI] [PubMed] [Google Scholar]
- 9.Hosoda K, Hammer RE, Richardson JA, Baynash AG, Cheung JC, Giaid A, Yanagisawa M. Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell. 1994;79:1267–1276. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 10.Gariepy CE, Cass DT, Yanagisawa M. Null mutation of endothelin receptor type B gene in spotting lethal rats causes aganglionic megacolon and white coat color. Proc. Natl. Acad. Sci. USA. 1996;93:867–872. doi: 10.1073/pnas.93.2.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng Z, Dhall D, Zhao L, Wang HL, Doherty TM, Bresee C, Frykman PK. Murine model of Hirschsprung-associated enterocolitis. I: phenotypic characterization with development of a histopathologic grading system. J. Pediatr. Surg. 2010;45:475–482. doi: 10.1016/j.jpedsurg.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao L, Dhall D, Cheng Z, Wang HL, Doherty TM, Bresee C, Frykman PK. Murine model of Hirschsprung-associated enterocolitis II: surgical correction of aganglionosis does not eliminate enterocolitis. J. Pediatr. Surg. 2010;45:206–211. doi: 10.1016/j.jpedsurg.2009.10.035. discussion 211–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao L, Cheng Z, Dhall D, Doherty TM, Frykman PK. A novel corrective pullthrough surgery in a mouse model of Hirschsprung’s disease. J. Pediatr. Surg. 2009;44:759–766. doi: 10.1016/j.jpedsurg.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng Z, Wang X, Dhall D, Zhao L, Bresee C, Doherty TM, Frykman PK. Splenic lymphopenia in the endothelin receptor B-null mouse: implications for Hirschsprung associated enterocolitis. Pediatr. Surg. Int. 2011;27:145–150. doi: 10.1007/s00383-010-2787-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glavina-Durdov M, Springer O, Capkun V, Saratlija-Novakovic Z, Rozic D, Barle M. The grade of acute thymus involution in neonates correlates with the duration of acute illness and with the percentage of lymphocytes in peripheral blood smear. Pathological study. Biol. Neonate. 2003;83:229–234. doi: 10.1159/000069481. [DOI] [PubMed] [Google Scholar]
- 16.Felmet KA, Hall MW, Clark RS, Jaffe R, Carcillo JA. Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J. Immunol. 2005;174:3765–3772. doi: 10.4049/jimmunol.174.6.3765. [DOI] [PubMed] [Google Scholar]
- 17.Toti P, De Felice C, Occhini R, Schuerfeld K, Stumpo M, Epistolato MC, Vatti R, Buonocore G. Spleen depletion in neonatal sepsis and chorioamnionitis. Am. J. Clin. Pathol. 2004;122:765–771. doi: 10.1309/RV6E-9BMC-9954-A2WU. [DOI] [PubMed] [Google Scholar]
- 18.Baynash AG, Hosoda K, Giaid A, Richardson JA, Emoto N, Hammer RE, Yanagisawa M. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell. 1994;79:1277–1285. doi: 10.1016/0092-8674(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 19.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 20.Laakko T, Schwartz RC, Fraker PJ. IL-7-mediated protection of pro and pre-B cells from the adverse effects of corticosterone. Cell. Immunol. 2002;220:39–50. doi: 10.1016/s0008-8749(03)00023-6. [DOI] [PubMed] [Google Scholar]
- 21.Murray SE, Rosenzweig HL, Johnson M, Huising MO, Sawicki K, Stenzel-Poore MP. Overproduction of corticotropin-releasing hormone blocks germinal center formation: role of corticosterone and impaired follicular dendritic cell networks. J. Neuroimmunol. 2004;156:31–41. doi: 10.1016/j.jneuroim.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Gurevich P, Ben-Hur H, Czernobilsky B, Nyska A, Zuckerman A, Zusman I. Pathology of lymphoid organs in low birth weight infants subjected to antigen-related diseases: a morphological and morphometric study. Pathology. 1995;27:121–126. doi: 10.1080/00313029500169702. [DOI] [PubMed] [Google Scholar]
- 23.Toti P, De Felice C, Stumpo M, Schurfeld K, Di Leo L, Vatti R, Bianciardi G, Buonocore G, Seemayer TA, Luzi P. Acute thymic involution in fetuses and neonates with chorioamnionitis. Hum. Pathol. 2000;31:1121–1128. doi: 10.1053/hupa.2000.16676. [DOI] [PubMed] [Google Scholar]
- 24.van Baarlen J, Schuurman HJ, Reitsma R, Huber J. Acute thymus involution during infancy and childhood: immunohistology of the thymus and peripheral lymphoid tissues after acute illness. Pediatr. Pathol. 1989;9:261–275. doi: 10.3109/15513818909037731. [DOI] [PubMed] [Google Scholar]
- 25.Alnemri ES, Fernandes TF, Haldar S, Croce CM, Litwack G. Involvement of BCL-2 in glucocorticoid-induced apoptosis of human pre-B-leukemias. Cancer Res. 1992;52:491–495. [PubMed] [Google Scholar]
- 26.Lopez-Hoyos M, Carrio R, Merino J, Merino R. Regulation of B cell apoptosis by Bcl-2 and Bcl-XL and its role in the development of autoimmune diseases (Review) Int. J. Mol. Med. 1998;1:475–483. doi: 10.3892/ijmm.1.2.475. [DOI] [PubMed] [Google Scholar]
- 27.Jalalvand E, Javan M, Haeri-Rohani A, Ahmadiani A. Stress- and non-stress-mediated mechanisms are involved in pain-induced apoptosis in hippocampus and dorsal lumbar spinal cord in rats. Neuroscience. 2008;157:446–452. doi: 10.1016/j.neuroscience.2008.08.052. [DOI] [PubMed] [Google Scholar]
- 28.Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, Katsaros D, et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat. Med. 2008;14:28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- 29.Dang R, Sasaki N, Nishino T, Nakanishi M, Torigoe D, Agui T. Lymphopenia in Ednrb-deficient rat was strongly modified by genetic background. Biomed. Res. 2012;33:249–253. doi: 10.2220/biomedres.33.249. [DOI] [PubMed] [Google Scholar]
- 30.Dang R, Torigoe D, Suzuki S, Kikkawa Y, Moritoh K, Sasaki N, Agui T. Genetic background strongly modifies the severity of symptoms of Hirschsprung disease, but not hearing loss in rats carrying Ednrb(sl) mutations. PLoS One. 2011;6:e24086. doi: 10.1371/journal.pone.0024086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dembowski C, Hofmann P, Koch T, Kamrowski-Kruck H, Riedesel H, Krammer HJ, Kaup FJ, Ehrenreich H. Phenotype, intestinal morphology, and survival of homozygous and heterozygous endothelin B receptor–deficient (spotting lethal) rats. J. Pediatr. Surg. 2000;35:480–488. doi: 10.1016/s0022-3468(00)90218-5. [DOI] [PubMed] [Google Scholar]
- 32.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 33.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat. Rev. Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 34.Wynn JL, Scumpia PO, Winfield RD, Delano MJ, Kelly-Scumpia K, Barker T, Ungaro R, Levy O, Moldawer LL. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood. 2008;112:1750–1758. doi: 10.1182/blood-2008-01-130500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Austin KM. The pathogenesis of Hirschsprung’s disease-associated enterocolitis. Semin. Pediatr. Surg. 2012;21:319–327. doi: 10.1053/j.sempedsurg.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Frykman PK, Short SS. Hirschsprung-associated enterocolitis: prevention and therapy. Semin. Pediatr. Surg. 2012;21:328–335. doi: 10.1053/j.sempedsurg.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bandre E, Kabore RA, Ouedraogo I, Sore O, Tapsoba T, Bambara C, Wandaogo A. Hirschsprung’s disease: management problem in a developing country. Afr. J. Paediatr. Surg. 2010;7:166–168. doi: 10.4103/0189-6725.70418. [DOI] [PubMed] [Google Scholar]
- 38.Brann L, Furtado D, Migliazzo CV, Baxendale MJ, Wood JD. Secondary effects of aganglionosis in the piebald-lethal mouse model of Hirschsprung’s disease. Lab. Anim. Sci. 1977;27:946–954. [PubMed] [Google Scholar]
- 39.Peters W, Cyster JG, Mack M, Schlondorff D, Wolf AJ, Ernst JD, Charo IF. CCR2-dependent trafficking of F4/80dim macrophages and CD11cdim/intermediate dendritic cells is crucial for T cell recruitment to lungs infected with Mycobacterium tuberculosis. J. Immunol. 2004;172:7647–7653. doi: 10.4049/jimmunol.172.12.7647. [DOI] [PubMed] [Google Scholar]