Abstract
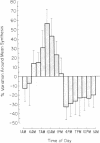
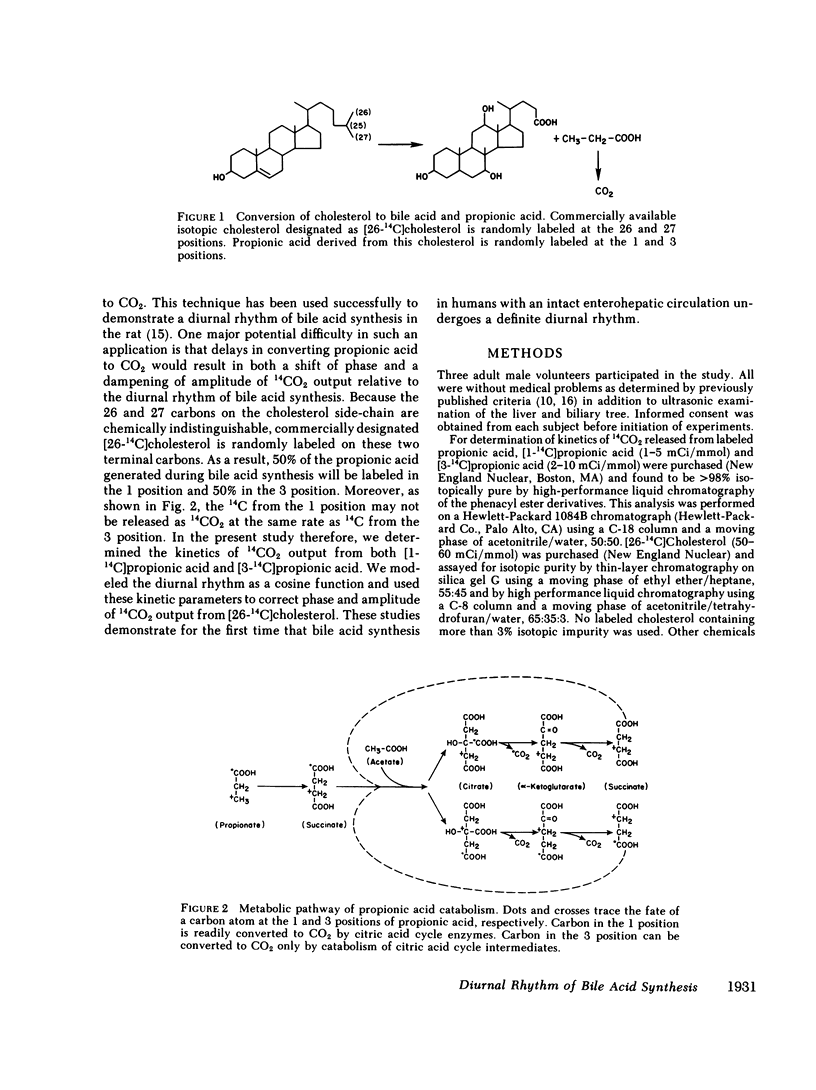
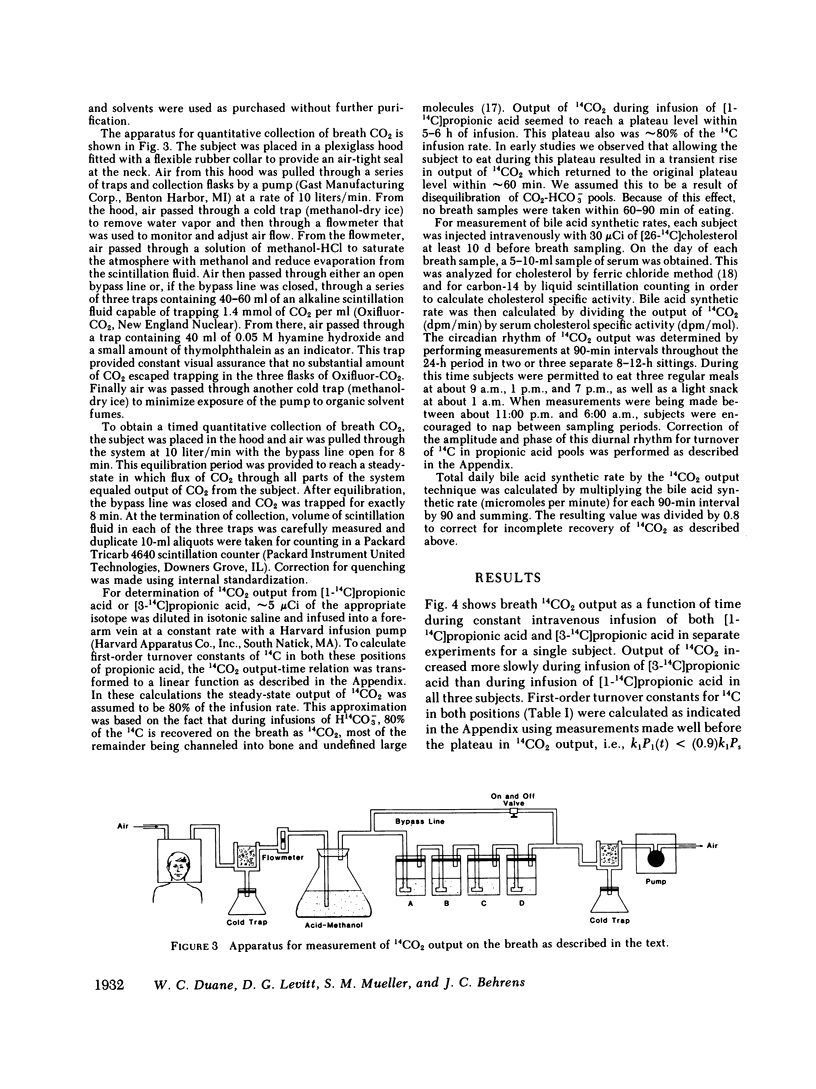
Regulation of bile acid synthesis in man is incompletely understood, in part because of difficulty in making measurements over short time periods when the enterohepatic circulation is intact. We investigated the possibility of a diurnal rhythm of bile acid synthesis in three human subjects given [26-14C]cholesterol. When this isotope of cholesterol, which is randomly labeled in the 26 and 27 positions, is converted to bile acid, the 14C is released as propionic acid randomly labeled in the 1 and 3 positions. The labeled propionic acid is then oxidized to 14CO2, output of which is a function of bile acid synthesis. However, delays in transit of the 14C through propionic acid and CO2-HCO-3 pools would shift the phase and dampen the amplitude of 14CO2 output relative to an existing diurnal rhythm of bile acid synthesis. Therefore, using constant infusion methods, we determined the turnover constants for conversion to 14CO2 of [1-14C]propionic acid and [3-14C]propionic acid to be 0.36-0.59 h-1 and 0.14-0.16 h-1, respectively. Using these constants and modeling the diurnal rhythm as a cosine function, we determined that amplitude of 14CO2 output from [26-14C]cholesterol was reduced 35% and acrophase was delayed 2.4-3.0 h relative to the diurnal rhythm of bile acid synthesis. None of the diurnal rhythm in 14CO2 output from [26-14C]cholesterol resulted from diurnal variation in propionic acid or CO2-HCO-3 metabolism since constant infusion of [1-14C]propionic acid and [3-14C]propionic acid for 30 h revealed no diurnal variation in output of 14CO2. These studies demonstrate for the first time that humans with an intact enterohepatic circulation have a diurnal rhythm of bile acid synthesis with an amplitude of +/- 35-55% around mean synthesis, and an acrophase at about 9 a.m.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Admirand W. H., Small D. M. The physicochemical basis of cholesterol gallstone formation in man. J Clin Invest. 1968 May;47(5):1043–1052. doi: 10.1172/JCI105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlberg J., Angelin B., Björkhem I., Einarsson K., Leijd B. Hepatic cholesterol metabolism in normo- and hyperlipidemic patients with cholesterol gallstones. J Lipid Res. 1979 Jan;20(1):107–115. [PubMed] [Google Scholar]
- Björkhem I., Oftebro H., Skrede S., Pedersen J. I. Assay of intermediates in bile acid biosynthesis using isotope dilution--mass spectrometry: hepatic levels in the normal state and in cerebrotendinous xanthomatosis. J Lipid Res. 1981 Feb;22(2):191–200. [PubMed] [Google Scholar]
- Boyar R. M., Rosenfeld R. S., Kapen S., Finkelstein J. W., Roffwarg H. P., Weitzman E. D., Hellman L. Human puberty. Simultaneous augmented secretion of luteinizing hormone and testosterone during sleep. J Clin Invest. 1974 Sep;54(3):609–618. doi: 10.1172/JCI107798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carulli N., Ponz De Leon M., Zironi F., Pinetti A., Smerieri A., Iori R., Loria P. Hepatic cholesterol and bile acid metabolism in subjects with gallstones: comparative effects of short erm feeding of chenodeoxycholic and ursodeoxycholic acid. J Lipid Res. 1980 Jan;21(1):35–43. [PubMed] [Google Scholar]
- Danzinger R. C., Hofmann A. F., Thistle J. L., Schoenfield L. J. Effect of oral chenodeoxycholic acid on bile acid kinetics and biliary lipid composition in women with cholelithiasis. J Clin Invest. 1973 Nov;52(11):2809–2821. doi: 10.1172/JCI107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M., Wilson J. D. Regulation of cholesterol metabolism. 3. N Engl J Med. 1970 May 28;282(22):1241–1249. doi: 10.1056/NEJM197005282822206. [DOI] [PubMed] [Google Scholar]
- Duane W. C., Adler R. D., Bennion L. J., Ginsberg R. L. Determination of bile acid pool size in man: a simplified method with advantages of increases precision, shortened analysis time, and decreased isotope exposure. J Lipid Res. 1975 Mar;16(2):155–158. [PubMed] [Google Scholar]
- Duane W. C., Gilberstadt M. L., Wiegand D. M. Diurnal rhythms of bile acid production in the rat. Am J Physiol. 1979 Mar;236(3):R175–R179. doi: 10.1152/ajpregu.1979.236.3.R175. [DOI] [PubMed] [Google Scholar]
- Duane W. C., Ginsberg R. L., Bennion L. J. Effects of fasting on bile acid metabolism and biliary lipid composition in man. J Lipid Res. 1976 May;17(3):211–219. [PubMed] [Google Scholar]
- Duane W. C., Holloway D. E., Hutton S. W., Corcoran P. J., Haas N. A. Comparison of bile acid synthesis determined by isotope dilution versus fecal acidic sterol output in human subjects. Lipids. 1982 May;17(5):345–348. doi: 10.1007/BF02535192. [DOI] [PubMed] [Google Scholar]
- Duane W. C. Simulation of the defect of bile acid metabolism associated with cholesterol cholelithiasis by sorbitol ingestion in man. J Lab Clin Med. 1978 Jun;91(6):969–978. [PubMed] [Google Scholar]
- Einarsson K., Hellström K., Kallner M. Bile acid kinetics in relation to sex, serum lipids, body weights, and gallbladder disease in patients with various types of hyperlipoproteinemia;. J Clin Invest. 1974 Dec;54(6):1301–1311. doi: 10.1172/JCI107876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg F. Implications of biologic rhythms for clinical practice. Hosp Pract. 1977 Jan;12(1):139–149. doi: 10.1080/21548331.1977.11707067. [DOI] [PubMed] [Google Scholar]
- Hanson R. F., Staples A. B., Williams G. C. Metabolism of 5 beta-cholestane-3 alpha, 7 alpha, 12 alpha, 26-tetrol and 5 beta-cholestane-3 alpha, 7 alpha, 12 alpha, 25-tetrol into cholic acid in normal human subjects. J Lipid Res. 1979 May;20(4):489–493. [PubMed] [Google Scholar]
- Hanson R. F., Szczepanik-Van Leeuwen P., Williams G. C. Stereochemistry of the side chain oxidation of 5 beta-cholestane-3 alpha,7 alpha,12 alpha-triol in man. J Biol Chem. 1980 Feb 25;255(4):1483–1485. [PubMed] [Google Scholar]
- LaRusso N. F., Hoffman N. E., Hofmann A. F., Northfield T. C., Thistle J. L. Effect of primary bile acid ingestion on bile acid metabolism and biliary lipid secretion in gallstone patients. Gastroenterology. 1975 Dec;69(6):1301–1314. [PubMed] [Google Scholar]
- Mathé D., Chevallier F. Effects of single ingestion of several bile acids or cholestyramine on 14CO2 output in [26 14C] cholesterol-fed rats. Biochimie. 1976;58(10):1293–1295. doi: 10.1016/s0300-9084(76)80132-0. [DOI] [PubMed] [Google Scholar]
- Myant N. B., Lewis B. Estimation of the rate of breakdown of cholesterol in man by measurement of 14CO-2 excretion after intravenous [26-14C]cholesterol. Clin Sci. 1966 Feb;30(1):117–127. [PubMed] [Google Scholar]
- Myant N. B., Mitropoulos K. A. Cholesterol 7 alpha-hydroxylase. J Lipid Res. 1977 Mar;18(2):135–153. [PubMed] [Google Scholar]
- Needleman P. The synthesis and function of prostaglandins in the heart. Fed Proc. 1976 Oct;35(12):2376–2381. [PubMed] [Google Scholar]
- Northfield T. C., LaRusso N. F., Hofmann A. F., Thistle J. L. Biliary lipid output during three meals and an overnight fast. II. Effect of chenodeoxycholic acid treatment in gallstone subjects. Gut. 1975 Jan;16(1):12–17. doi: 10.1136/gut.16.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarfordt S. H., Greenfield M. F. Estimation of cholesterol and bile acid turnover in man by kinetic analysis. J Clin Invest. 1973 Aug;52(8):1937–1945. doi: 10.1172/JCI107378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redinger R. N., Chow L., Grace D. M. Cholesterol oxidation in primates by simultaneous sterol balance and breath analysis. Am J Physiol. 1978 Jul;235(1):R55–R63. doi: 10.1152/ajpregu.1978.235.1.R55. [DOI] [PubMed] [Google Scholar]
- Reichard G. A., Jr, Haff A. C., Skutches C. L., Paul P., Holroyde C. P., Owen O. E. Plasma acetone metabolism in the fasting human. J Clin Invest. 1979 Apr;63(4):619–626. doi: 10.1172/JCI109344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salen G., Nicolau G., Shefer S., Mosbach E. H. Hepatic cholesterol metabolism in patients with gallstones. Gastroenterology. 1975 Sep;69(3):676–684. [PubMed] [Google Scholar]
- Schwartz C. C., Vlahcevic Z. R., Halloran L. G., Gregory D. H., Meek J. B., Swell L. Evidence for the existence of definitive hepatic cholesterol precursor compartments for bile acids and biliary cholesterol in man. Gastroenterology. 1975 Dec;69(6):1379–1382. [PubMed] [Google Scholar]
- Shefer S., Cheng F. W., Batta A. K., Dayal B., Tint G. S., Salen G. Biosynthesis of chenodeoxycholic acid in man: stereospecific side-chain hydroxylations of 5beta-cholestane-3alpha,7alpha-diol. J Clin Invest. 1978 Sep;62(3):539–545. doi: 10.1172/JCI109158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer S., Hauser S., Bekersky I., Mosbach E. H. Feedback regulation of bile acid biosynthesis in the rat. J Lipid Res. 1969 Nov;10(6):646–655. [PubMed] [Google Scholar]
- Winchell H. S., Stahelin H., Kusubov N., Slanger B., Fish M., Pollycove M., Lawrence J. H. Kinetics of CO2-HCO3 minus in normal adult males. J Nucl Med. 1970 Dec;11(12):711–715. [PubMed] [Google Scholar]
- ZLATKIS A., ZAK B., BOYLE A. J. A new method for the direct determination of serum cholesterol. J Lab Clin Med. 1953 Mar;41(3):486–492. [PubMed] [Google Scholar]