Abstract
Background
Colonization and infection with vancomycin-resistant enterococci have been associated with exposure to antibiotics that are active against anaerobes. In mice that have intestinal colonization with vancomycin-resistant enterococci, these agents promote high-density colonization, whereas antibiotics with minimal antianaerobic activity do not.
Methods
We conducted a seven-month prospective study of 51 patients who were colonized with vancomycin-resistant enterococci, as evidenced by the presence of the bacteria in stool. We examined the density of vancomycin-resistant enterococci in stool during and after therapy with antibiotic regimens and compared the effect on this density of antianaerobic agents and agents with minimal antianaerobic activity. In a subgroup of 10 patients, cultures of environmental specimens (e.g., from bedding and clothing) were obtained.
Results
During treatment with 40 of 42 antianaerobic-antibiotic regimens (95 percent), high-density colonization with vancomycin-resistant enterococci was maintained (mean [±SD] number of organisms, 7.8± 1.5 log per gram of stool). The density of colonization decreased after these regimens were discontinued. Among patients who had not received antianaerobic antibiotics for at least one week, 10 of 13 patients who began such regimens had an increase in the number of organisms of more than 1.0 log per gram (mean increase, 2.2 log per gram), whereas among 10 patients who began regimens of antibiotics with minimal antianaerobic activity, there was a mean decrease in the number of enterococci of 0.6 log per gram (P= 0.006 for the difference between groups). When the density of vancomycin-resistant enterococci in stool was at least 4 log per gram, 10 of 12 sets of cultures of environmental specimens had at least one positive sample, as compared with 1 of 9 sets from patients with a mean number of organisms in stool of less than 4 log per gram (P=0.002).
Conclusions
For patients with vancomycin-resistant enterococci in stool, treatment with antianaerobic antibiotics promotes high-density colonization. Limiting the use of such agents in these patients may help decrease the spread of vancomycin-resistant enterococci.
In case–control studies, colonization and infection with vancomycin-resistant enterococci have been associated with exposure to vancomycin,1–6 third-generation cephalosporins,3–6 antibiotics that are active against anaerobes,3,6–8 ciprofloxacin,1 and aminoglycosides.4 The microbiologic basis for these associations is not well defined. If some antibiotics promote colonization and infection more than others that are equally effective, limiting the use of these agents may limit the spread of vancomycin-resistant enterococci.
We previously examined the effect of various antibiotics on intestinal colonization with vancomycin-resistant enterococci in mice.9 In mice with established colonization, as evidenced by the high density of vancomycin-resistant enterococci in stool, the administration of antibiotics with potent activity against anaerobes maintained this level of colonization, whereas the administration of antibiotics with less potent antianaerobic activity did not.9 We prospectively tested the hypothesis that antianaerobic antibiotics promote high-density colonization with vancomycin-resistant enterococci, whereas antibiotics with minimal anti-anaerobic activity do not. In a subgroup of patients, we tested the hypothesis that high-density colonization would result in increased shedding of the bacteria, increasing the likelihood of contamination of the environment.
METHODS
Study Design
We performed a seven-month prospective study of all inpatients at the Louis Stokes Cleveland Veterans Affairs Medical Center who had documented or newly diagnosed intestinal colonization with vancomycin-resistant enterococci. This institution includes an acute care facility and a nursing home facility that houses patients with colonization.
Stool samples were collected weekly from all patients with documented colonization to determine whether colonization was still present. The samples were refrigerated at 4°C and processed within one week. Patients who were colonized with Enterococcus gallinarum or E. casseliflavus, species that are intrinsically resistant to low concentrations of vancomycin, were excluded from the study. Information regarding the demographic characteristics of the patients, coexisting illnesses, and the use of antibiotic therapy was obtained through standardized chart review. Written informed consent was obtained from patients for whom environmental specimens (e.g., from bed linen, bedrails, clothes, and bedside tables) were obtained for culture. The hospital’s institutional review board approved the study protocol.
We analyzed the density of vancomycin-resistant enterococci in stool during and after therapy with all antianaerobic antibiotics and assessed the effect of initiating therapy with antianaerobic antibiotics as compared with antibiotics with minimal antianaerobic activity.
Assessment of the Density of Vancomycin-Resistant Enterococci in Stool
Antianaerobic antibiotics in the antimicrobial formulary included piperacillin–tazobactam, ampicillin–sulbactam, amoxicillin–clavulanate, cefoxitin, cefotetan, imipenem–cilastatin, meropenem, metronidazole, clindamycin, alatrofloxacin, vancomycin, and ceftriaxone.10,11 Vancomycin has potent in vitro activity only against gram-positive anaerobes, but oral administration of vancomycin results in marked inhibition of bacteroides species in humans.12 Ceftriaxone has less potent antianaerobic activity than cefoxitin or cefotetan, but it is excreted in bile and markedly decreases the levels of anaerobic flora in the stool in humans.13,14 Antibiotic regimens that included more than one antibiotic were classified as anti-anaerobic if they contained at least one antianaerobic antibiotic. Antibiotics with minimal antianaerobic activity on the formulary include ciprofloxacin, levofloxacin, dicloxacillin, nafcillin, cephalexin, cefepime, aztreonam, gentamicin, and trimethoprim–sulfamethoxazole.10
We assessed the density of vancomycin-resistant enterococci in stool during and after therapy with antianaerobic antibiotics. We compared oral and intravenous regimens, individual monotherapy regimens (when sufficient data were available), monotherapy regimens with regimens containing two or more antibiotics, and monotherapy with clindamycin or metronidazole with regimens with a broader spectrum of activity (i.e., activity against anaerobes and Enterobacteriaceae or vancomycin-susceptible enterococci).
Comparison of Antianaerobic-Antibiotic Regimens and Regimens of Antibiotics with Minimal Antianaerobic Activity
We compared the effect of initiating therapy with antianaerobic antibiotics and antibiotics with minimal antianaerobic activity on the density of vancomycin-resistant enterococci in stool. Patients were included in the analysis if the results of a base-line determination of density were available within two weeks before the initiation of the regimen, if an antianaerobic antibiotic had not been administered within seven days before the regimen was initiated, and if density was reassessed at least two days after the initiation of the regimen but no more than seven days after the completion of the antibiotic course. If multiple measurements had been made during the course of antibiotic therapy, the mean (±SD) was determined and used for analysis. Measurements of enterococci were analyzed on a logarithmic scale (base 10).
Environmental Cultures
One to three sets of environmental specimens were obtained from the gowns, linen, bedrails, and bedside tables of 10 patients with fecal incontinence. The density of vancomycin-resistant enterococci was quantified for a stool sample obtained within one week before the environmental specimens were obtained. Patients with diarrhea were excluded because diarrhea has previously been associated with environmental contamination.15 The specimens were obtained by swabbing 6 cm2 of each designated area with a premoistened swab. The swabs were placed in Todd–Hewitt broth, incubated overnight at 37°C, and inoculated onto agar plates (Enterococcosel, Becton Dickinson, Sparks, Md.), which contained 6 μg of vancomycin per milliliter. The plates were incubated for 48 hours at 37°C before being read.
Microbiologic Analysis and Molecular Typing
Species were identified according to standard methods. Selected isolates were inoculated onto plates of brain–heart infusion agar that contained 100 μg of ampicillin per milliliter, 10 or 100 μg of ciprofloxacin per milliliter, and 10 or 100 μg of levofloxacin per milliliter. The density of organisms per gram of stool in stool samples of approximately 75 mg was determined as previously described.9 If no vancomycin-resistant enterococci were detected, the lower limit of detection was assigned (approximately 2.8 log per gram). There was no significant difference in the results when four portions of a single stool specimen were analyzed or when four samples were analyzed after two weeks of refrigeration. Selected samples were plated on MacConkey agar to identify the number of aerobic and facultative gram-negative bacilli. In the case of six patients, pulsed-field gel electrophoresis was performed on stool and environmental isolates with the use of a commercial kit (CHEF genomic bacterial DNA plug kit, BioRad, Hercules, Calif.). The method used was a modification of the technique of Hoyen et al.16 The plugs were digested with SmaI for 16 hours (Promega, Madison, Wis.).
Statistical Analysis
Student’s unpaired t-test was used to compare continuous variables between groups. Student’s paired t-test was used to compare in individual patients the changes in density associated with treatment with antianaerobic-antibiotic regimens with the changes associated with regimens of antibiotics with minimal antianaerobic activity. All reported P values are two-sided. Analysis of variance was performed to compare the densities associated with therapy with different antianaerobic antibiotics. Fisher’s exact test was used to evaluate the association between high-density colonization (defined as ≥4 log per gram) and positive cultures of environmental specimens and to compare the number of regimens that contained two or more antibiotics in the antianaerobic-antibiotic group and in the group of antibiotics with minimal antianaerobic activity. Computations were performed with the use of Stata software (version 6.0, Stata, College Station, Tex.). Unless otherwise stated, mean values are given as means ±SD.
RESULTS
Characteristics of the Patients
A total of 61 inpatients had intestinal colonization with vancomycin-resistant enterococci during the study. Ten of these patients were excluded from the analysis for the following reasons: stool samples from six patients were unavailable, two patients were colonized with E. casseliflavus, and information about antimicrobial therapy was unavailable for two patients.
The base-line characteristics of the 51 patients and events that occurred during the study are shown in Table 1. Twenty-nine of 51 patients (57 percent) had at least three measurements of the density of vancomycin-resistant enterococci in stool; there was a total of 227 measurements. Eleven patients had paired stool samples collected one to three days apart, either when they were not receiving antibiotics or when they were receiving stable regimens of antibiotics. The mean difference in the number of organisms between these paired specimens was 0.4 log per gram of stool (range, 0.1 log to 0.7 log per gram). The eight patients from whom vancomycin-resistant enterococci was isolated from cultures of blood, urine, or a sacral wound had large numbers of organisms (≥6 log per gram) in stool specimens obtained concurrently.
Table 1.
Base-Line Characteristics of the 51 Patients and Events During the Study.
| Characteristic | Value |
|---|---|
| At base line | |
| Age — yr | |
| Mean | 67 |
| Range | 38–86 |
| Male sex — no. (%) | 49 (96) |
| Clinical conditions or interventions — no. (%) | |
| Diabetes mellitus | 20 (39) |
| Cancer | 10 (20) |
| End-stage renal disease | 6 (12) |
| Coronary bypass surgery within yr before study entry | 3 (6) |
| Percutaneous gastrostomy tube | 9 (18) |
| Cirrhosis | 4 (8) |
| Inflammatory bowel disease | 1 (2) |
| Human immunodeficiency virus infection | 2 (4) |
| Abdominal surgery within yr before study entry | 3 (6) |
| During the study — no. (%) | |
| Residence in nursing home facility | 25 (49) |
| Admission to intensive care unit | 15 (29) |
| Receipt of ≥3 different classes of antibiotics | 40 (78) |
| Clostridium difficile infection | 8 (16) |
| Vancomycin-resistant enterococci isolated from cultures of clinical specimens other than stool | 8 (16) |
| Blood | 5 |
| Urine | 2 |
| Sacral wound | 1 |
| Death during hospitalization | 8 (16) |
Density of Vancomycin-Resistant Enterococci during and after Therapy with Antianaerobic Antibiotics
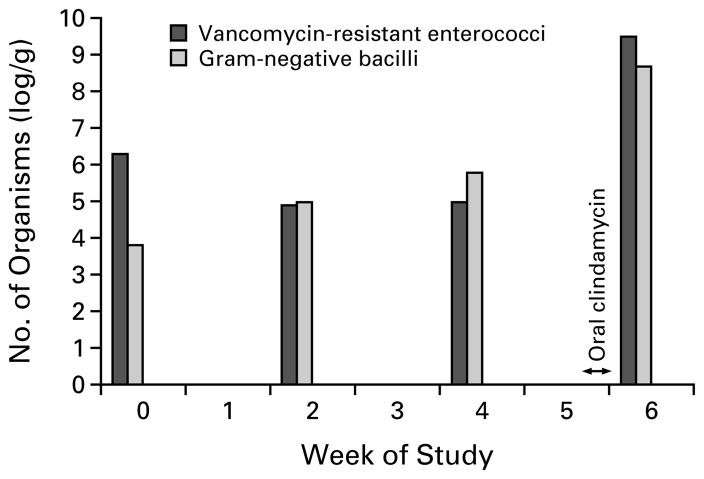
Thirty-three patients received a total of 42 regimens of antianaerobic antibiotics. During 40 of these 42 regimens (95 percent), the density of colonization remained high (mean number of organisms, 7.8±1.5 log per gram). Among the 78 measurements of stool samples obtained during antianaerobic-antibiotic therapy, the density exceeded 6 log per gram in 68 (87 percent). There were no significant differences in the mean number of organisms in stool samples between oral regimens and intravenous regimens (8.3±1.5 log and 7.7±1.5 log per gram, P=0.26), between regimens containing one antibiotic and those containing two or more antibiotics (7.8±1.6 log and 7.7 ±1.4 log per gram, P=0.88), between clindamycin or metronidazole monotherapy and regimens that had a broader spectrum of activity (8.2±1.9 log and 7.8 ±1.5 log per gram, P=0.54), or between individual monotherapy regimens, including vancomycin, piperacillin–tazobactam, amoxicillin–clavulanate, metronidazole, and clindamycin (P=0.6 for all comparisons). Figure 1 shows the increase in the density of vancomycin-resistant enterococci and aerobic and facultative gram-negative bacilli in stool that was associated with oral exposure to clindamycin in one patient.
Figure 1.
The Effect of Antibiotic Therapy on the Numbers of Vancomycin-Resistant Enterococci and the Total Number of Aerobic and Facultative Gram-Negative Bacilli in Stool Samples from a 57-Year-Old Man with Cirrhosis Resulting from Hepatitis C.
Six weeks before entering the study, the patient received a course of oral clindamycin; two weeks before entering the study, he received a course of oral ciprofloxacin. Five weeks after the study began, he received two days of oral clindamycin prophylactically before having teeth extracted at a time when he was doing well in the nursing home unit.
Orally administered antianaerobic antibiotics included metronidazole, amoxicillin–clavulanate, clindamycin, and vancomycin. Intravenously administered antianaerobic antibiotics included vancomycin, piperacillin–tazobactam, ampicillin–sulbactam, meropenem, imipenem–cilastatin, cefoxitin, ceftriaxone, alatrofloxacin, clindamycin, and metronidazole. The two patients in whom high-density colonization was not maintained during therapy with antianaerobic antibiotics were receiving piperacillin–tazobactam; isolates from these patients had a high level of resistance to piperacillin (minimal inhibitory concentration, 1056 μg per milliliter). In a previous report, piperacillin concentrations in stool ranged from undetectable to 276 mg per kilogram during therapy with intravenous piperacillin–tazobactam.17
When regimens of antianaerobic antibiotics were discontinued, the density of vancomycin-resistant enterococci in stool decreased in all 19 patients who had samples collected four or more weeks later. Nine patients were followed until one or more measurements showed that levels were undetectable (lower limit of detection, approximately 2.8 log per gram). The mean interval between the discontinuation of antibiotics and the finding of undetectable levels of vancomycin-resistant enterococci in stool was 17.4 weeks (range, 6 to 20).
Effect of Antianaerobic-Antibiotic Regimens and Regimens of Antibiotics without Antianaerobic Activity
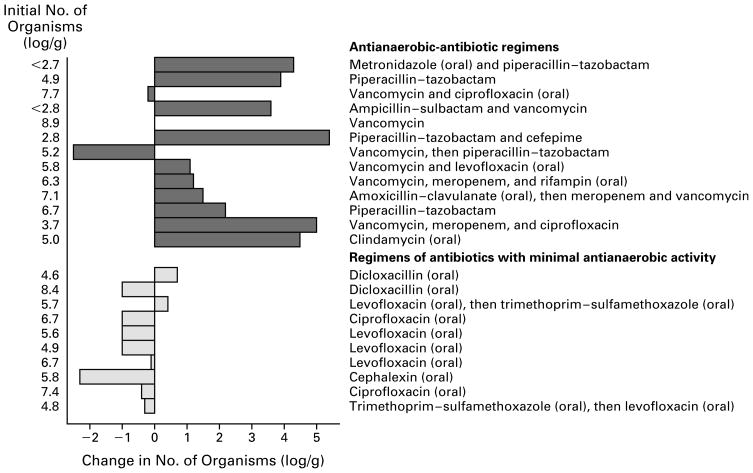
The effects of antianaerobic-antibiotic regimens and regimens of antibiotics with minimal antianaerobic activity on the density of vancomycin-resistant enterococci in stool are shown in Figure 2. Ten of 13 patients who received antianaerobic-antibiotic regimens had an increase in the number of organisms of more than 1.0 log per gram (mean increase, 2.2 log per gram), as compared with 0 of 10 patients who received regimens of antibiotics with minimal anti-anaerobic activity (P=0.006). The mean change in stool samples from these 10 patients was a decrease of 0.6 log per gram. Three patients received both types of regimens at different times; an increase in the number of organisms in stool of at least 1.0 log per gram occurred in all three during the antianaerobic-antibiotic regimens, but in none of the three during the other type of regimen (P=0.1). Figure 3 shows the changes in the density of vancomycin-resistant enterococci in stool associated with exposure to various antibiotic regimens in one patient.
Figure 2.
Effect on the Density of Vancomycin-Resistant Enterococci in Stool of Therapy with Antianaerobic-Antibiotic Regimens in 13 Patients and Regimens of Antibiotics with Minimal Antianaerobic Activity in 10 Patients.
A selected isolate from each study patient demonstrated a high level of resistance to vancomycin (minimal inhibitory concentration, >60 μg per milliliter) and ampicillin (minimal inhibitory concentration, >256 μg per milliliter). Isolates from patients who received ciprofloxacin or levofloxacin as part of a regimen of antibiotics with minimal antianaerobic activity had a high level of resistance to ciprofloxacin in vitro (minimal inhibitory concentration, >100 μg per milliliter) but not to levofloxacin (minimal inhibitory concentration, >10 μg but <100 μg per milliliter). Isolates from the two patients who received trimethoprim–sulfamethoxazole were resistant to this agent in vitro. All antibiotics were given intravenously unless the route is designated as oral.
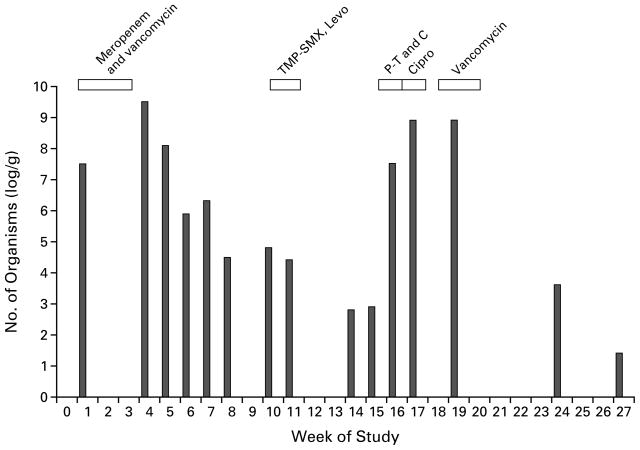
Figure 3.
Effect of Therapy with Antianaerobic-Antibiotic Regimens and Regimens of Antibiotics with Minimal Anti-anaerobic Activity on the Density of Vancomycin-Resistant Enterococci in Stool Samples from a 69-Year-Old Male Nursing Home Resident with a History of Cerebrovascular Accidents.
The patient initially received an antianaerobic-antibiotic regimen consisting of intravenous meropenem and vancomycin for 17 days, beginning in week 1. He then received a regimen of antibiotics with minimal antianaerobic activity: oral trimethoprim–sulfamethoxazole (TMP-SMX) for five days, beginning in week 10, followed by oral levofloxacin (Levo) for three days. The third regimen consisted of a combination of the two types of antibiotics: intravenous piperacillin–tazobactam (P-T) and cefepime (C) was given for 8 days, followed by oral ciprofloxacin (Cipro) for 10 days. The fourth regimen consisted of intravenous vancomycin — an antianaerobic antibiotic — for 14 days. Cultures of environmental specimens obtained from the patient’s bedside table and bed linen were positive during week 20, whereas cultures of specimens from the patient’s gown and bedrail were negative. Cultures of all four types of environmental specimens obtained during week 24 and week 27 were negative. The patterns on pulsed-field gel electrophoresis of three isolates obtained from stool cultures and an isolate obtained from the culture of an environmental specimen were identical. Vancomycin-resistant enterococci were detectable in all samples.
When the antianaerobic-antibiotic regimens were compared with the regimens of antibiotics with minimal antianaerobic activity, there were no significant differences in the duration of therapy before density was measured in stool samples (mean ±SE, 8.3±1.8 and 10.2±2.3 days; P=0.44), the total number of antibiotics received before the regimen being analyzed (mean ±SE, 4.0±0.49 and 3.8±0.53; P=0.8), or the patients’ Karnofsky performance scores (mean ±SD, 34.0±3.4 and 28.6±3.4; P=0.29). The changes in density associated with the two types of regimens did not differ significantly between the acute care facility and the nursing home facility (data not shown). The antianaerobic-antibiotic regimens were more likely to include at least two antibiotics than were the regimens of antibiotics with minimal antianaerobic activity (9 of 13 vs. 2 of 10 regimens, P=0.04). There was no significant difference, however, in the change in density between the use of a single antianaerobic antibiotic and the use of a regimen that included two or more such antibiotics (mean increase in the number of organisms, 2.7±2.0 log and 2.1±2.6 log per gram, respectively; P=0.64). A comparison of the 4 regimens that consisted of one antianaerobic antibiotic with the 10 regimens of antibiotics with minimal antianaerobic activity also revealed a significant difference in density between the groups (P=0.01).
Results of Environmental Cultures
Twenty-one sets of cultures of environmental specimens were obtained from 10 patients with fecal incontinence. Ten of 12 sets of cultures (83 percent) obtained from patients with a concurrent stool density of at least 4 log per gram had one or more positive samples, as compared with 1 of 9 sets (11 percent) obtained from patients with less than 4 log per gram (P=0.002). Three patients had positive cultures of environmental specimens when the density of vancomycin-resistant enterococci in stool was at least 4 log per gram, and negative cultures when the density was less than 4 log per gram. Isolates of vancomycin-resistant enterococci from stool samples from 6 of the 10 patients had five distinct patterns on pulsed-field gel electrophoresis. An isolate from cultures of environmental specimens from each of these six patients was clonally related to a corresponding isolate obtained from a stool sample.
DISCUSSION
In this prospective study of patients who were colonized with vancomycin-resistant enterococci, we found that the use of antianaerobic-antibiotic regimens perpetuated high-density intestinal colonization, whereas the use of antibiotics with minimal antianaerobic activity that we studied did not. In a subgroup of patients with fecal incontinence, those with high-density colonization were more likely to have positive cultures of environmental specimens than those with low-density colonization. These results suggest that limiting the use of antianaerobic antibiotics in patients colonized with vancomycin-resistant enterococci will minimize the level and duration of colonization and may reduce the rate of transmission of these organisms.
Several factors must be considered in evaluating the effect of antibiotics on intestinal vancomycin-resistant enterococci, including the spectrum of antimicrobial activity and the level of active antibiotic in the intestinal tract.18 An antibiotic may directly inhibit vancomycin-resistant enterococci if it is active against the colonizing strain, or it may inhibit bacteria that compete for nutrients or space and thus promote the overgrowth of vancomycin-resistant enterococci. The amount of active antibiotic in the intestinal tract depends on the extent of biliary or intestinal secretion and the degree of inactivation of the antibiotic.18 For example, imipenem–cilastatin has broad-spectrum antibacterial activity, but its level of excretion in bile is minimal, and in some studies, it produced only minor changes in intestinal flora.19 Therefore, our results should be considered in the context of other studies that have examined the effect of antibiotics on the intestinal flora of humans.
Our data are compatible with the hypothesis that antibiotics promote the overgrowth of vancomycin-resistant enterococci in the intestinal tract primarily through the inhibition of intestinal anaerobes. Antibiotic regimens with potent antianaerobic activity but minimal activity against other components of the intestinal flora (e.g., metronidazole and clindamycin) promoted high-density colonization. In contrast, antibiotic regimens with minimal antianaerobic activity but with activity against susceptible members of Enterobacteriaceae (e.g., ciprofloxacin, trimethoprim–sulfamethoxazole, and cephalexin) did not promote overgrowth. Several studies have demonstrated that these types of antibiotics may have similar effects on the density of vancomycin-susceptible enterococci in the stool of humans20–24 (Table 2). Clinical studies have also demonstrated an association between the use of antianaerobic antibiotics and colonization or infection with vancomycin-susceptible enterococci.26–31
Table 2.
Studies of the Effect of Antibiotics on the Stool Flora of Healthy Subjects and Patients.
| Type of Antibiotic | Type of Subjects | Change in Density of Stool Flora* | Reference | ||
|---|---|---|---|---|---|
| ENTEROCOCCI | ENTEROBACTERIACEAE | ANAEROBES | |||
| Minimal antianaerobic activity | |||||
| Cefepime | Healthy subjects | NC | Decreased | Increased | Bacher et al.25 |
| Levofloxacin | Healthy subjects | Decreased | Decreased | NC | Edlund et al.24 |
| Ciprofloxacin | Healthy subjects and patients | NC or decreased | Decreased | NC | Nord22 |
| Cefaclor | Patients | NC | Decreased | NC | Sakata et al.21 |
| Methicillin | Patients | NC | NC | NC or decreased | Sakata et al.21 |
| Trimethoprim–sulfamethoxazole | Healthy subjects | NC | Decreased | ND | Vollaard et al.20 |
| Potent antianaerobic activity | |||||
| Ceftriaxone | Patients | Increased | Decreased | Decreased | Arvidsson et al.,13 Bodey et al.14 |
| Cefoxitin | Healthy subjects | Increased | Decreased | Decreased | Nord et al.23 |
| Clindamycin | Healthy subjects | Increased | NC or increased | Decreased | Nord et al.23 |
NC denotes no significant change, and ND no data.
A direct inhibitory effect on vancomycin-resistant enterococci in the intestinal tract by the quinolone antibiotics with minimal antianaerobic activity cannot be excluded. For patients who received quinolones as part of regimens of antibiotics with minimal antianaerobic activity, selected isolates demonstrated a high level of resistance to ciprofloxacin but not to levofloxacin (Fig. 2). Quinolone antibiotics are present in high concentrations in stool (21 to 94 mg per kilogram of stool in the case of orally administered levofloxacin24 and 185 to 2200 mg per kilogram of stool in the case of orally administered ciprofloxacin32), but the activity of these antibiotics may be markedly reduced as a result of reversible binding to fecal matter.24,33 In humans, ciprofloxacin or levofloxacin minimally affects the anaerobic flora and either may decrease or have no effect on the density of enterococci.22,24 The fact that a high density of vancomycin-resistant enterococci (≥6.4 log colony-forming units per gram) was maintained in the stool of nine patients who received levofloxacin or ciprofloxacin as one component of an antianaerobic-antibiotic regimen suggests that any inhibitory effect of these antibiotics was outweighed by the effect of the other antibiotics (data not shown).
Data regarding the effect of other antibiotics with minimal antianaerobic activity on the density of vancomycin-resistant enterococci in stool are needed. In an earlier study, we demonstrated that subcutaneous cefepime or aztreonam did not promote the overgrowth of vancomycin-resistant enterococci in mice.9 Cefepime is an extended-spectrum cephalosporin that has relatively little antianaerobic activity, is excreted primarily by the kidneys, and causes minimal changes in the intestinal flora of humans.25 We have hypothesized that cephalosporin antibiotics promote the overgrowth of vancomycin-resistant enterococci to varying degrees, given the wide variation in their antianaerobic activity and degree of biliary excretion.9 Aztreonam is a monobactam antibiotic whose activity is limited to gram-negative aerobes.34 In humans, the administration of aztreonam has been associated with the overgrowth of stool enterococci in some studies,35,36 but not in others.34,37 Louie34 demonstrated that treatment of febrile patients with neutropenia with a combination of moxalactam (a potent anti-anaerobic antibiotic) and tobramycin, but not with a combination of aztreonam and tobramycin, resulted in suppression of fecal anaerobes, overgrowth of enterococci, and an increased frequency of fungal colonization.
The fact that the regimens of antibiotics with minimal antianaerobic activity included only orally administered single agents whereas the regimens of anti-anaerobic antibiotics were mostly given intravenously and included two or more agents is a potential confounding variable. Patients who required therapy with intravenous regimens that included multiple antibiotics might have been sicker than those who received oral therapy with a single antibiotic. An increased severity of illness has been associated with an increased likelihood of colonization or infection with vancomycin-resistant enterococci.38 Several findings argue against the possibility that these factors introduced statistically significant bias, however. The Karnofsky performance scores of patients who received antibiotics with minimal antianaerobic activity did not differ significantly from those of patients who received antianaerobic antibiotics; oral and single-drug regimens of antianaerobic antibiotics promoted high-density colonization as much as did intravenous and multiple-drug regimens; and in one patient who did not meet the criteria for analysis, low-density colonization was maintained during therapy with intravenous levofloxacin.
Our findings have important implications for the care of patients who are colonized with vancomycin-resistant enterococci. The use of antianaerobic antibiotics should be avoided in this group of patients. In clinical practice, antianaerobic antibiotics are often prescribed when their spectrum of activity is not required. The primary benefit of limiting the use of antianaerobic antibiotics in the population of patients we studied may be a reduction in the duration of colonization and, therefore, a reduction in the cost and inconvenience of the infection-control measures implemented. Since high-density colonization was more likely to be associated with positive cultures of environmental specimens, this measure may also reduce the rate of transmission of vancomycin-resistant enterococci. Since the use of antianaerobic antibiotics has been associated with bacteremia from vancomycin-resistant enterococci in patients with cancer,7,8 limiting the use of these antibiotics in higher-risk patients may reduce morbidity and mortality.
Acknowledgments
Supported by a Career Development Award grant (to Dr. Donskey); by the Office of Research and Development, the Department of Veterans Affairs; and in part by a training grant from the National Institutes of Health (AI 07024, to Drs. Donskey and Hoyen).
Footnotes
We are indebted to Lisa Parino, Kathy Willis, and members of the nursing and microbiology staffs at the Louis Stokes Cleveland Veterans Affairs Medical Center for their assistance; and to Rachel Meyers and Anna Gazos for assistance in the preparation of the manuscript.
References
- 1.Morris JG, Shay DK, Hebden JN, et al. Enterococci resistant to multiple antimicrobial agents, including vancomycin: establishment of endemicity in a university medical center. Ann Intern Med. 1995;123:250–9. doi: 10.7326/0003-4819-123-4-199508150-00002. [DOI] [PubMed] [Google Scholar]
- 2.Shay DK, Maloney SA, Montecalvo M, et al. Epidemiology and mortality risk of vancomycin-resistant enterococcal bloodstream infections. J Infect Dis. 1995;172:993–1000. doi: 10.1093/infdis/172.4.993. [DOI] [PubMed] [Google Scholar]
- 3.Beezhold DW, Slaughter S, Hayden MK, et al. Skin colonization with vancomycin-resistant enterococci among hospitalized patients with bacteremia. Clin Infect Dis. 1997;24:704–6. doi: 10.1093/clind/24.4.704. [DOI] [PubMed] [Google Scholar]
- 4.Handwerger S, Raucher B, Altarac D, et al. Nosocomial outbreak due to Enterococcus faecium highly resistant to vancomycin, penicillin, and gentamicin. Clin Infect Dis. 1993;16:750–5. doi: 10.1093/clind/16.6.750. [DOI] [PubMed] [Google Scholar]
- 5.Moreno F, Grota P, Crisp C, et al. Clinical and molecular epidemiology of vancomycin-resistant Enterococcus faecium during its emergence in a city in southern Texas. Clin Infect Dis. 1995;21:1234–7. doi: 10.1093/clinids/21.5.1234. [DOI] [PubMed] [Google Scholar]
- 6.Weinstein JW, Roe M, Towns M, et al. Resistant enterococci: a prospective study of prevalence, incidence, and factors associated with colonization in a university hospital. Infect Control Hosp Epidemiol. 1996;17:36–41. doi: 10.1086/647186. [DOI] [PubMed] [Google Scholar]
- 7.Edmond MB, Ober JF, Weinbaum DL, et al. Vancomycin-resistant Enterococcus faecium bacteremia: risk factors for infection. Clin Infect Dis. 1995;20:1126–33. doi: 10.1093/clinids/20.5.1126. [DOI] [PubMed] [Google Scholar]
- 8.Lucas GM, Lechtzin N, Puryear DW, Yau LL, Flexner CW, Moore RD. Vancomycin-resistant and vancomycin-susceptible enterococcal bacteremia: comparison of clinical features and outcomes. Clin Infect Dis. 1998;26:1127–33. doi: 10.1086/520311. [DOI] [PubMed] [Google Scholar]
- 9.Donskey CJ, Hanrahan JA, Hutton RA, Rice LB. Effect of parenteral antibiotic administration on persistence of vancomycin-resistant Enterococcus faecium in the mouse gastrointestinal tract. J Infect Dis. 1999;180:384–90. doi: 10.1086/314874. [DOI] [PubMed] [Google Scholar]
- 10.Lorber B. Bacteroides, Prevotella, and Fusobacterium species (and other medically important anaerobic gram-negative bacilli) In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas and Bennett’s principles and practice of infectious diseases. 4. Vol. 2. New York: Churchill Livingstone; 1995. pp. 2195–204. [Google Scholar]
- 11.Idem . Gas gangrene and other Clostridium-associated diseases. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas and Bennett’s principles and practice of infectious diseases. 4. Vol. 2. New York: Churchill Livingstone; 1995. pp. 2182–95. [Google Scholar]
- 12.Edlund C, Barkholt L, Olsson-Liljequist B, Nord CE. Effect of vancomycin on intestinal flora of patients who previously received antimicrobial therapy. Clin Infect Dis. 1997;25:729–32. doi: 10.1086/513755. [DOI] [PubMed] [Google Scholar]
- 13.Arvidsson A, Leijd B, Nord CE, Angelin B. Interindividual variability in biliary excretion of ceftriaxone: effects on biliary lipid metabolism and on intestinal microflora. Eur J Clin Invest. 1988;18:261–6. doi: 10.1111/j.1365-2362.1988.tb01256.x. [DOI] [PubMed] [Google Scholar]
- 14.Bodey GP, Fainstein V, Garcia I, Rosenbaum B, Wong Y. Effect of broad-spectrum cephalosporins on the microbial flora of recipients. J Infect Dis. 1983;148:892–7. doi: 10.1093/infdis/148.5.892. [DOI] [PubMed] [Google Scholar]
- 15.Boyce JM, Opal SM, Chow JW, et al. Outbreak of multidrug-resistant Enterococcus faecium with transferable vanB class vancomycin resistance. J Clin Microbiol. 1994;32:1148–53. doi: 10.1128/jcm.32.5.1148-1153.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoyen C, Rice LB, Conte S, Jacobs MR, Walsh-Sukys M, Toltzis P. Use of real time pulsed field gel electrophoresis to guide interventions during a nursery outbreak of Serratia marcescens infection. Pediatr Infect Dis J. 1999;18:357–60. doi: 10.1097/00006454-199904000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Nord CE, Brismar B, Kasholm-Tengve B, Tunevall G. Effect of piperacillin/tazobactam therapy on intestinal microflora. Scand J Infect Dis. 1992;24:209–13. doi: 10.3109/00365549209052614. [DOI] [PubMed] [Google Scholar]
- 18.Vollaard EJ, Clasener HAL. Colonization resistance. Antimicrob Agents Chemother. 1994;38:409–14. doi: 10.1128/aac.38.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borderon JC, Rastegar A, Laugier J, Gold F. The effect of imipenem/cilastatin on the aerobic faecal flora of children. J Antimicrob Chemother. 1986;18(Suppl E):121–5. doi: 10.1093/jac/18.supplement_e.121. [DOI] [PubMed] [Google Scholar]
- 20.Vollaard EJ, Clasener HA, Janssen AJ. Co-trimoxazole impairs colonization resistance in healthy volunteers. J Antimicrob Chemother. 1992;30:685–91. doi: 10.1093/jac/30.5.685. [DOI] [PubMed] [Google Scholar]
- 21.Sakata H, Fujita K, Yoshioka H. The effect of antimicrobial agents on fecal flora of children. Antimicrob Agents Chemother. 1996;29:225–9. doi: 10.1128/aac.29.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nord CE. Effect of the new quinolones on the human gastrointestinal microflora. Rev Infect Dis. 1988;10(Suppl 1):S193–S196. doi: 10.1093/clinids/10.supplement_1.s193. [DOI] [PubMed] [Google Scholar]
- 23.Nord CE, Heimdahl A, Kager L, Malmborg AS. The impact of different antimicrobial agents on the normal gastrointestinal microflora of humans. Rev Infect Dis. 1984;6(Suppl 1):S270–S275. doi: 10.1093/clinids/6.supplement_1.s270. [DOI] [PubMed] [Google Scholar]
- 24.Edlund C, Sjostedt S, Nord CE. Comparative effects of levofloxacin and ofloxacin on the normal oral and intestinal microflora. Scand J Infect Dis. 1997;29:383–6. doi: 10.3109/00365549709011835. [DOI] [PubMed] [Google Scholar]
- 25.Bacher K, Schaeffer M, Lode H, Nord CE, Borner K, Koeppe P. Multiple dose pharmacokinetics, safety, and effects on faecal microflora, of cefepime in healthy volunteers. J Antimicrob Chemother. 1992;30:365–75. doi: 10.1093/jac/30.3.365. [DOI] [PubMed] [Google Scholar]
- 26.Weigelt JA, Easley SM, Thal ER, Palmer LD, Newman VS. Abdominal surgical wound infection is lowered with improved perioperative enterococcus and bacteroides therapy. J Trauma. 1993;34:579–85. doi: 10.1097/00005373-199304000-00016. [DOI] [PubMed] [Google Scholar]
- 27.Sexton DJ, Harrell LJ, Thorpe JJ, Hunt DL, Reller LB. A case-control study of nosocomial ampicillin-resistant enterococcal infection and colonization at a university hospital. Infect Control Hosp Epidemiol. 1993;14:629–35. doi: 10.1086/646655. [DOI] [PubMed] [Google Scholar]
- 28.Yu VL. Enterococcal superinfection and colonization after therapy with moxalactam, a new broad-spectrum antibiotic. Ann Intern Med. 1981;94:784–5. doi: 10.7326/0003-4819-94-6-784. [DOI] [PubMed] [Google Scholar]
- 29.Moellering RC., Jr Enterococcal infections in patients treated with moxalactam. Rev Infect Dis. 1982;4(Suppl):S708–S711. doi: 10.1093/clinids/4.supplement_3.s708. [DOI] [PubMed] [Google Scholar]
- 30.Barrall DT, Kenney PR, Slotman GJ, Burchard KW. Enterococcal bacteremia in surgical patients. Arch Surg. 1985;120:57–63. doi: 10.1001/archsurg.1985.01390250049008. [DOI] [PubMed] [Google Scholar]
- 31.Boyce JM, Opal SM, Potter-Bynoe G, et al. Emergence and nosocomial transmission of ampicillin-resistant enterococci. Antimicrob Agents Chemother. 1992;36:1032–9. doi: 10.1128/aac.36.5.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krueger WA, Ruckdeschel G, Unertl K. Influence of intravenously administered ciprofloxacin on aerobic intestinal microflora and fecal drug levels when administered simultaneously with sucralfate. Antimicrob Agents Chemother. 1997;41:1725–30. doi: 10.1128/aac.41.8.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Saene JJM, van Saene HKF, Lerk CF. Inactivation of quinolone by feces. J Infect Dis. 1986;153:999–1000. doi: 10.1093/infdis/153.5.999. [DOI] [PubMed] [Google Scholar]
- 34.Louie TJ. Preservation of colonization resistance parameters during empiric therapy with aztreonam in the febrile neutropenic patient. Rev Infect Dis. 1985;7(Suppl 4):S747–S761. doi: 10.1093/clinids/7.supplement_4.s747. [DOI] [PubMed] [Google Scholar]
- 35.de Vries-Hospers HG, Welling GW, Swabb EA, van der Waaij D. Selective decontamination of the digestive tract with aztreonam: a study of 10 healthy volunteers. J Infect Dis. 1984;150:636–42. doi: 10.1093/infdis/150.5.636. [DOI] [PubMed] [Google Scholar]
- 36.Giuliano M, Barza M, Jacobus NV, Gorbach SL. Effect of broad-spectrum parenteral antibiotics on composition of intestinal microflora of humans. Antimicrob Agents Chemother. 1987;31:202–6. doi: 10.1128/aac.31.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones PG, Bodey GP, Swabb EA, Rosenbaum B. Effect of aztreonam on throat and stool flora of cancer patients. Antimicrob Agents Chemother. 1984;26:941–3. doi: 10.1128/aac.26.6.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edmond MB. Multidrug-resistant enterococci and the threat of vancomycin-resistant Staphylococcus aureus. In: Wenzel RP, editor. Prevention and control of nosocomial infections. 3. Baltimore: Williams & Wilkins; 1997. pp. 339–55. [Google Scholar]