Abstract
IMPORTANCE
Foods that have similar carbohydrate content can differ in the amount they raise blood glucose. The effects of this property, called the glycemic index, on risk factors for cardiovascular disease and diabetes are not well understood.
OBJECTIVE
To determine the effect of glycemic index and amount of total dietary carbohydrate on risk factors for cardiovascular disease and diabetes.
DESIGN, SETTING, AND PARTICIPANTS
Randomized crossover-controlled feeding trial conducted in research units in academic medical centers, in which 163 overweight adults (systolic blood pressure, 120–159 mm Hg) were given 4 complete diets that contained all of their meals, snacks, and calorie-containing beverages, each for 5 weeks, and completed at least 2 study diets. The first participant was enrolled April 1, 2008; the last participant finished December 22, 2010. For any pair of the 4 diets, there were 135 to 150 participants contributing at least 1 primary outcome measure.
INTERVENTIONS
(1) A high–glycemic index (65% on the glucose scale), high-carbohydrate diet (58% energy); (2) a low–glycemic index (40%), high-carbohydrate diet; (3) a high–glycemic index, low-carbohydrate diet (40% energy); and (4) a low–glycemic index, low-carbohydrate diet. Each diet was based on a healthful DASH-type diet.
MAIN OUTCOMES AND MEASURES
The 5 primary outcomes were insulin sensitivity, determined from the areas under the curves of glucose and insulin levels during an oral glucose tolerance test; levels of low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides; and systolic blood pressure.
RESULTS
At high dietary carbohydrate content, the low– compared with high–glycemic index level decreased insulin sensitivity from 8.9 to 7.1 units (−20%, P = .002); increased LDL cholesterol from 139 to 147 mg/dL (6%, P ≤ .001); and did not affect levels of HDL cholesterol, triglycerides, or blood pressure. At low carbohydrate content, the low– compared with high–glycemic index level did not affect the outcomes except for decreasing triglycerides from 91 to 86 mg/dL (−5%, P = .02). In the primary diet contrast, the low–glycemic index, low-carbohydrate diet, compared with the high–glycemic index, high-carbohydrate diet, did not affect insulin sensitivity, systolic blood pressure, LDL cholesterol, or HDL cholesterol but did lower triglycerides from 111 to 86 mg/dL (−23%, P ≤ .001).
CONCLUSIONS AND RELEVANCE
In this 5-week controlled feeding study, diets with low glycemic index of dietary carbohydrate, compared with high glycemic index of dietary carbohydrate, did not result in improvements in insulin sensitivity, lipid levels, or systolic blood pressure. In the context of an overall DASH-type diet, using glycemic index to select specific foods may not improve cardiovascular risk factors or insulin resistance.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT00608049
The health effects of dietary carbohydrate (both type and amount) are of substantial interest to health professionals, the general public, and policymakers. Some carbohydrate-rich foods have less effect than others to increase blood glucose. This property of individual foods, called the “glycemic index,” is quantified according to the amount 50 g of its carbohydrate compared with 50 g of glucose increases blood glucose during 2 hours.1,2 For example, a banana increases blood glucose more than an apple that has the same amount of carbohydrate. Boiled sweet potato increases blood glucose more than boiled carrot. Meals or complete diets may be designed using these tables to have a desired overall glycemic index.3–5 Glycemic index is but 1 attribute of carbohydrate-containing foods. Further, nutrients often cluster. Hence, the effects of glycemic index, if any, might actually result from other nutrients, such as fiber, potassium, and polyphenols, which favorably affect health. Even though some nutrition policies advocate consumption of low–glycemic index foods and even promote food labeling with glycemic index values, the independent benefits of glycemic index are uncertain, especially when persons are already consuming a healthful diet rich in whole grains, vegetables, and fruits.
Clinical trials that studied the effect of lowering glycemic index on insulin sensitivity and cardiovascular disease (CVD) risk factors reported diverse results that may be related to concomitant changes in content of total carbohydrate and fiber, concomitant weight loss, and presence of and use of treatments for diabetes.6 Using the controlled feeding technique, we studied diets that had a large contrast in glycemic index, while at the same time we controlled intake of total carbohydrates and other key nutrients such as fatty acids, potassium, and sodium and maintained baseline bodyweight. The background diets in which we manipulated glycemic index were healthful dietary patterns established in the Dietary Approaches to Stop Hypertension (DASH)7 and Optimal Macronutrient Intake to Prevent Heart Disease (OmniHeart)8 studies that are being recommended in dietary guidelines to prevent CVD. We studied the effect of glycemic index when total carbohydrate is high (58%) as it is in the DASH diet7 or low (40%) as in the OmniHeart unsaturated fat diet8 or Mediterranean diets.9,10 We hypothesized that a low compared with a high glycemic index, especially of a high-carbohydrate diet, would cause modest though potentially important improvements in insulin sensitivity and CVD risk factors.
Methods
The trial was conducted at Johns Hopkins Medical Institutions, Baltimore, Maryland, and Brigham and Women’s Hospital, Boston, Massachusetts, and was approved by their institutional review boards. Each participant gave written informed consent. A full description of the methods is in the trial protocol in Supplement 1.
Participants
Eligibility criteria were age 30 years or older; systolic blood pressure 120 to 159 mm Hg and diastolic, 70 to 99 mm Hg; and body mass index (BMI) 25 or higher (calculated as weight in kilograms divided by height in meters squared). The enrollment targets were 50% women and 50% black. Participants self-identified their race or ethnicity using the choices provided and required by the National Institutes of Health. We oversampled black individuals because of their disproportionate burden of insulin resistance and other risk factors that result in high rates of diabetes and cardiovascular disease.
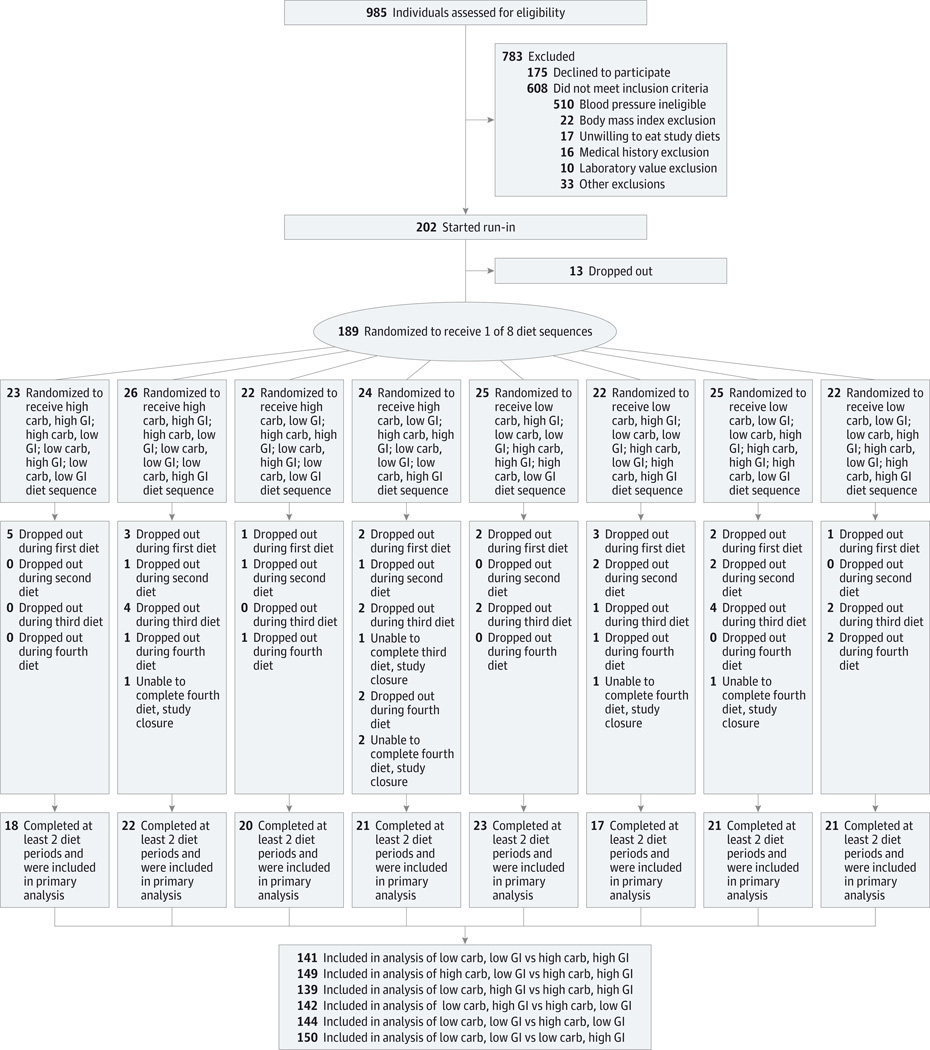
Exclusion criteria included having cardiovascular disease, diabetes, or chronic kidney disease; taking medication that lowers blood pressure or lipids; and having a fasting blood glucose level of 125 mg/dL or greater (to convert glucose to mmol/L, multiply by 0.0555) (Figure 1). The trial protocol has a complete list of exclusions (Supplement 1). The goal was 160 participants finishing at least the first 2 of 4 diet periods. The primary recruitment strategy was mass mailing of brochures, flyers, and coupons. The primary sources of mailing lists were commercial vendors and local governments for lists of registered voters or drivers.
Figure 1.
Participant Screening, Enrollment, and Follow-up in the OmniCarb Study
Carb indicates carbohydrate; GI, glycemic index.
Controlled Diet Intervention
Eligible participants began an 8-day run-in phase during which each study diet was given for 2 days. During run-in and the 4 diet periods, participants were provided all of their meals, snacks, and calorie-containing beverages. After the run-in, the participants were randomized to a sequence of the 4 study diets. For a crossover study with 4 diets to be administered, there are 24 possible sequences, of which we used 8. We wanted to ensure that the high– and low–glycemic index diet components were each used in the first 2 periods for all participants. With this constraint, high- and low-carbohydrate components could be chosen in any order, leading to 4 distinct sequences for the first 2 diets. Once the first 2 diets had been determined, the remaining 2 could be assigned in any order, leading to a total of 8 distinct diet sequences. Thirteen blocks of random permutations of the 8 permissible sequences were established for each site, to support up to 104 sequential randomizations per site. Permutations were developed using the sample() function of R version 2.0 on March 14, 2008. The data center (directed by V.J.C.) was the holder of the diet sequences, and requests for a participant to be randomized were provided to each sites’ dietary staff once the participant had successfully completed the run-in.
Each diet was given for 5 weeks separated by a break of at least 2 weeks during which study participants ate their self-selected diet. Calorie intake was adjusted to maintain initial body weight. Participants completed a daily food diary for each day on the controlled diets. They recorded any foods that they did not eat and any additional items eaten. Their on-site, weekday meal attendance was recorded and meal consumption was monitored by trained staff. During the daily on-site meals monitored by study staff, participants had to consume the entire meal on-site. Participants were observed while eating and trays were cleared with staff present to ensure no food was discarded.
Study Diets
Two diets had a high carbohydrate composition (58%of daily energy), one with a high glycemic index (≥65 on the glucose scale) and the other with a low glycemic index (≤45 on the glucose scale). Another 2 diets had a low carbohydrate composition (40%of daily energy) also with either a high (≥65) or a low (≤45) glycemic index. The glycemic index cut points corresponded approximately to the first and fifth quintiles of US population-based intake.11 Table 1 displays the targets and estimated content of nutrients of the 4 study diets as determined from food analysis software (ESHA Food Processor SQL, version 10.2, ESHA Research).
Table 1.
Nutrient Targets and Content of the 4 Study Dietsa
| Nutrients | Targets for Low-Carbohydrate Diets |
Menu Calculations | Targets for High-Carbohydrate Diets |
Menu Calculations | ||
|---|---|---|---|---|---|---|
| Low Carbohydrate, High Glycemic Index |
Low Carbohydrate, Low Glycemic Index |
High Carbohydrate, High Glycemic Index |
High Carbohydrate, Low Glycemic Index |
|||
| Energy, kcal | 2000 | 2011 | 1993 | 2000 | 2011 | 1998 |
| Protein, % | 23 | 23 | 23 | 15 | 16 | 16 |
| Carbohydrate, % | 40 | 41 | 40 | 58 | 58 | 57 |
| Fat, % | 37 | 37 | 37 | 27 | 27 | 27 |
| Saturated fat, % | 6 | 7 | 7 | 6 | 6 | 6 |
| Monounsaturated fat, % | 20 | 18 | 19 | 13 | 12 | 13 |
| Polyunsaturated fat, % | 11 | 10 | 10 | 8 | 7 | 8 |
| Fiber, gb | 26–31b | 29 | 33 | 26–31b | 32 | 37 |
| Cholesterol, mg | 150 | 170 | 163 | 150 | 90 | 89 |
| Calcium, mg | 1000 | 993 | 995 | 1000 | 1032 | 1051 |
| Potassium, mg | 4100 | 3949 | 4026 | 4100 | 3963 | 4103 |
| Sodium, mg | 2300 | 2305 | 2199 | 2300 | 2245 | 2211 |
| Magnesium, mg | 400 | 468 | 440 | 400 | 462 | 429 |
| Glycemic index | ||||||
| Low | ≤45 | NA | 40 | ≤45 | NA | 41 |
| High | ≥65 | 65 | NA | ≥65 | 66 | NA |
| Glycemic load | NA | 112 | 64 | NA | 172 | 104 |
Abbreviation: NA, not applicable.
Seven-day average.
There were 2 targets for dietary fiber, the daily amount (26–31 g) and the maximal difference of 5 g between high– and low–glycemic index diets.
The glycemic index values of individual foods were calculated primarily using published tables.2 High– and low–glycemic index meals were constructed around similar categories of foods that have different glycemic index to achieve the target contrast between high–and low–glycemic index diets (eg, instant potatoes vs pasta; instant oatmeal vs steel cut oats; white bread vs whole kernel bread; bananas vs apples) (eTable 1 in Supplement 2). The glycemic index values of the breads were measured directly, in vivo.1 Fiber intakes averaged 31 g in the high–glycemic index and 35 g in the low–glycemic index diets. The diets also provided similar amounts of other nutrients that might affect trial outcomes.
Measurements
The 5 primary outcomes were insulin sensitivity; systolic blood pressure; and low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride levels. Secondary outcomes included diastolic blood pressure, fasting and 2-hour blood glucose and insulin, and other lipoprotein parameters.
Blood pressure was measured by trained and certified staff using a validated automated oscillometric OMRON 907 device12 at the clinic on 3 days during screening for eligibility; on 1 day during run-in; and on 1 day during the first, second, and third weeks and on 5 days in the final fourth and fifth weeks, during each of the 4 diet periods. On each occasion, the blood pressure was measured 3 times. The measurements during the last 2 weeks were averaged and constituted the outcome variable for blood pressure, as done previously.8
At baseline and in the final 10 days of each of the 4 diet periods, blood was collected after an overnight 8- to 18-hour fast (mean, 13 hours), and plasma and serum were separated, aliquoted, and stored at −70°C until analysis.
Plasma was separated by ultracentrifugation into very-low-density lipoproteins (VLDL) (density ≤1.006 g/mL), LDL (1.006≤density ≤1.063 g/mL), and HDL(density ≥1.063). Plasma total and lipoprotein cholesterol, triglycerides, and apolipoproteins B, C-III, and E were measured using enzymatic kits or enzyme-linked immunosorbent assay. HDL cholesterol was measured in the supernatant of plasma after the precipitation of apolipoprotein B–containing lipoproteins with dextran sulfate, 50 000 molecular weight.
Insulin sensitivity was measured by an oral glucose tolerance test, 75 g, during screening and the final 10 days of each diet period. Blood was sampled at 0, 10, 20, 30, 60, 90, and 120 minutes. Insulin sensitivity was calculated by the index of Matsuda and DeFronzo that uses blood glucose and serum insulin levels at 0, 30, 60, 90, and 120 minutes.13
In a self-selected subcohort of 35% (n = 61), a 12-hour meal test was conducted in the final 2 weeks of each diet period. On that day, participants were given the same diet type for that diet period for breakfast, lunch, and dinner, which had a mean 486, 610, 618 kcal, respectively, for a typical 2000-kcal diet, the same as in the other days of the controlled diet. Blood was sampled at fixed intervals just before eating breakfast; 30, 60, and 90 minutes after starting breakfast; and hourly thereafter through 12 hours.14 Incremental area under the curve (AUCi) was calculated by summing the time intervals 30 to 210 minutes, 270 to 510 minutes, and 570 to 690 minutes. This 12-hour meal test is a process variable that determines the differences in blood glucose caused by the differences among the diets in glycemic index and amount of carbohydrate. Diets with higher glycemic index and higher amount of carbohydrate are expected to increase the 12-hour blood glucose AUCi.
Urine collections (24-hour) were obtained once during screening and once during the last 2 weeks of each diet period. Data collection personnel were blinded to diet sequence. Information on serious adverse events was collected from participants and their medical records and reported to the institutional review board as required.
Analysis Plan
The diet contrasts pertaining to the effect of glycemic index were high glycemic index vs low glycemic index in the setting of high total carbohydrate intake and separately in the setting of low total carbohydrate intake. The trial design also allowed a test of the effects of lowering total dietary carbohydrate, separately in the setting of high–glycemic index and low–glycemic index foods. Although this 4-period study could be analyzed as a factorial design, combining the high- and low-carbohydrate periods to test glycemic index, and combining the high– and low–glycemic index diets to test level of carbohydrate, we considered it likely that glycemic index has a stronger effect when the total carbohydrate intake is high and that carbohydrate level has a stronger effect when the glycemic index is high. Therefore, a factorial analysis was considered inappropriate. We provide point estimates and nominal 95% confidence intervals for the effects of these diet contrasts on all outcomes.
In the protocol-specified analytical plan, the primary analysis is a comparison of the high-carbohydrate, high–glycemic index diet and the low-carbohydrate, low–glycemic index diet, representing a single integrated measure of the hypothesized maximal effect on the 5 primary outcomes of manipulating dietary carbohydrate by reducing its amount and glycemic index. To achieve approximate nominal 95%coverage for the resulting group of 5 confidence intervals for the 5 primary outcomes, we tested mean within-person changes for statistical significance at the .01 level and used 99% confidence intervals.15 For nonprimary outcomes, a 5% significance level was specified in the protocol as a guide. Because some participants did not provide measures on all outcomes for all diets, multiple imputation analysis was performed for the 5 primary outcomes. There was no qualitative effect of multiple imputation compared with complete case analysis. Full details are given in the online appendix (eFigure 1 in Supplement 2).
The distribution of within-person differences in response variables for pairs of diets was analyzed using the t.test function of R version 3.1.1. This provides estimates of average effect, standard error of the estimate, and limits of confidence intervals for selected confidence coefficients. Statistical visualization and additional analyses such as multiple imputation sensitivity analysis and tests for carryover effects were also performed using R. We used standard assessments of carryover effects in crossover designs based on the comparison of distributions of sums of outcomes between groups of participants receiving treatments in different orders.16 All P values are 2-sided.
A sample size of 160 participants provided at least 80% power to investigate the dietary effects on blood pressure, lipids, and insulin sensitivity. Minimal detectable differences for the effect of glycemic index at a significance level of 5% were systolic blood pressure, 1.4 mm Hg; HDL cholesterol, 1.6 mg/dL (to convert HDL and LDL cholesterol to mmol/L, multiply by 0.0259); LDL cholesterol, 3.9 mg/dL; triglycerides, 11 mg/dL (to convert triglycerides to mmol/L, multiply by 0.0113); and insulin sensitivity, 2.9 (Matsuda index units).
Results
One hundred sixty-three participants completed at least 2 diets and were included in the analysis of outcomes (Figure 1). For any pair of diets, there were 135 to 150 participants. The trial ended when at least 160 participants completed at least 2 diets, as planned.
Women comprised 52%of the participants; 51%were black (Table 2). Hypertension was present in 26% of participants; obesity (BMI ≥30) in 56%; LDL cholesterol of 130 mg/dL or greater in 68%; triglycerides of 150 mg/dL or greater in 17%; and fasting blood glucose of 100 mg/dL or greater in 30%.
Table 2.
Baseline Characteristics of Participants (N=163)
| Characteristic | Baseline Value |
|---|---|
| Age, mean (SD), y | 53 (11) |
| Female sex, No. (%) | 85 (52) |
| Race/ethnicity, No. (%) | |
| Black | 83 (51) |
| Non-Hispanic white | 66 (40) |
| Hispanic white | 5 (3) |
| Asian | 4 (2) |
| Other | 5 (3) |
| Body mass indexa | |
| Mean (SD) | 32 (6) |
| ≥30, No. (%) | 92 (56) |
| Waist circumference, mean (SD), cm | 104 (14) |
| Hypertension, No. (%) | 43 (26) |
| Smoking status, No. (%) | |
| Current | 26 (16) |
| Former | 33 (20) |
| Never | 104 (64) |
| Marital status, No. (%) | |
| Married | 47 (29) |
| Divorced or separated | 38 (23) |
| Widowed | 9 (6) |
| Never married | 69 (42) |
| Education level, No. (%) | |
| High school or less | 30 (18) |
| Some college | 63 (39) |
| College graduate or beyond | 70 (43) |
| Household income, annual, No. (%), $ | |
| <30 000 | 54 (33) |
| 30 000–59 999 | 58 (36) |
| ≥60 000 | 44 (27) |
| Did not give information | 7 (4) |
| Alcoholic beverage drinking, none | 90 (55) |
| Drinks among drinkers, mean (SD), No./wk | 4 (3) |
| Menopausal (women), No. (%) | 49 (58) |
| Metabolic syndrome, No. (%) | 33 (20) |
| Dietary intake per day (FFQ), mean (SD) | |
| Energy, kcal | 2175 (1131) |
| Carbohydrate, % | 52 (10) |
| Protein, % | 15 (4) |
| Fat, % | 33 (7) |
| Saturated fat, % | 10 (3) |
| Monosaturated fat, % | 13 (3) |
| Polyunsaturated fat, % | 10 (2) |
| Cholesterol, mean (SD), mg | 236 (149) |
| Dietary fiber, mean (SD), g | 22 (13) |
Abbreviation: FFQ, Food Frequency Questionnaire.
Calculated as weight in kilograms divided by height in meters squared.
Measures of Adherence and Process Variables
According to participant self-reports and observation at the daily lunch or dinner, adherence was high; all study food was consumed and no nonstudy foods were eaten on 96%of person-days on each diet. Participants lost an average of 1 kg of body weight from baseline to the end of each diet period, the same for each diet type. Alcoholic beverages were consumed on 10% to 11% of all person-days across the 4 diet periods.
Urinary nitrogen and creatinine levels, biomarkers of dietary protein, were higher on the low-carbohydrate diets (that provided 23% kcal of energy from protein) than the high-carbohydrate diets (that provided 15%kcal of energy from protein) (eTable 2 in Supplement 2). Urinary sodium and potassium excretion were similar during each diet period.
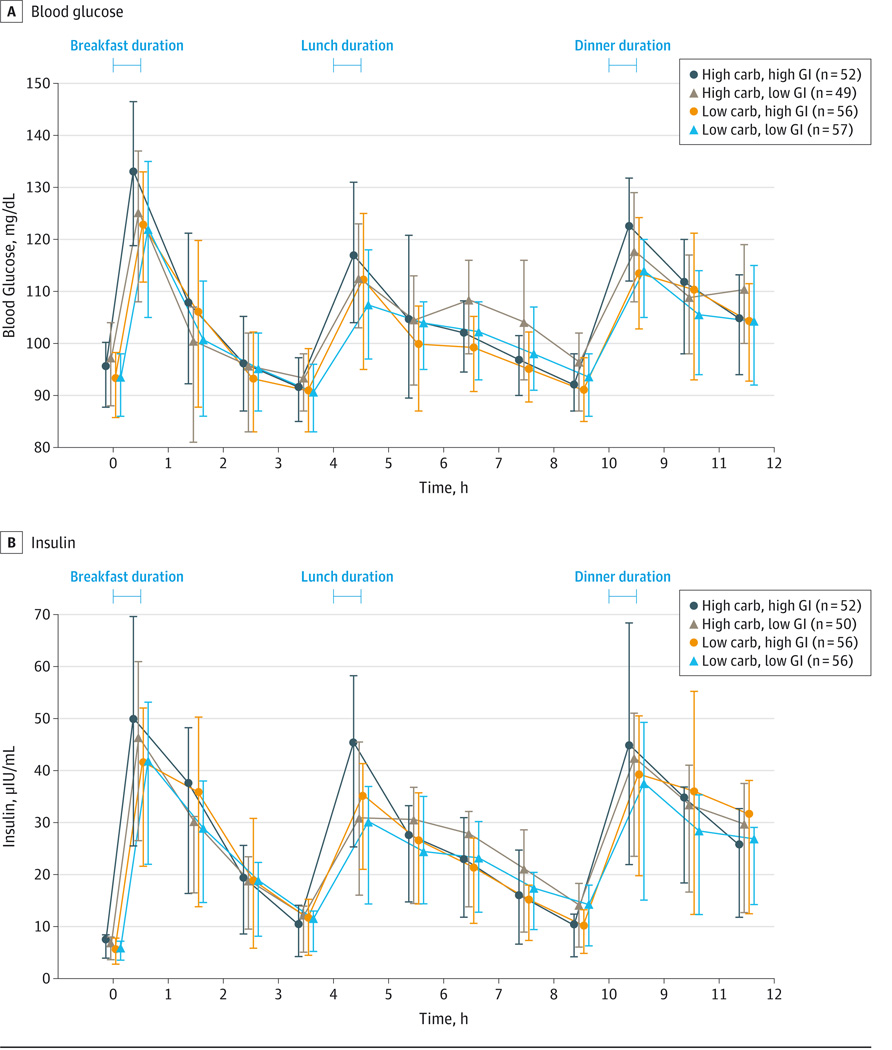
At the high carbohydrate content, the low– compared with the high–glycemic index level reduced the 12-hour blood glucose AUCi by 17%(P = .06); however, at the low carbohydrate content, the low–comparedwith the high–glycemic index level did not reduce blood glucose AUCi (Figure 2 and eTable 3 in Supplement 2). Reduced carbohydrate content of the high–glycemic index level lowered 12-hour blood glucose AUCi by 19% (P = .009); however, reduced carbohydrate content of the low–glycemic index level did not reduce blood glucose AUCi. The low carbohydrate content and the low–glycemic index level together lowered it by 20%, compared with high carbohydrate content, high–glycemic index level.
Figure 2.
Effect of the Study Diets on Blood Glucose and Insulin Levels Over 12 Hours
In the morning after a 10- to 12-hour fast and during the fourth or fifth week of each dietary period, the participants were given breakfast, lunch, and dinner that had the food and nutrient composition of the assigned diet period. Blood was sampled before breakfast, usually at 8:00 am, 8:30 am, 9:00 am, and hourly, ending at approximately 7:30 pm. See eTable 3 in Supplement 2 for data on glucose and insulin area under the curve and statistical testing. A self-selected subgroup of participants were included. Carb indicates carbohydrate; GI, glycemic index. To convert glucose to mmol/L, multiply by 0.0555; insulin to pmol/L, multiply by 6.945.
Effect of Low Compared With High Glycemic Index of Dietary Carbohydrate
At the high dietary carbohydrate content, the low– compared with the high–glycemic index level significantly reduced insulin sensitivity from8.9 to 7.1 units (−20%, P = .002) (Figure 3 and Table 3). At the low carbohydrate content, the low– compared with the high–glycemic index level did not affect insulin sensitivity but increased fasting blood glucose level by 2.5 mg/dL (P = .007) (eTable 13 in Supplement 2). Mean glucose and insulin levels during the oral glucose tolerance test are shown in eFigure 2 in Supplement 2.
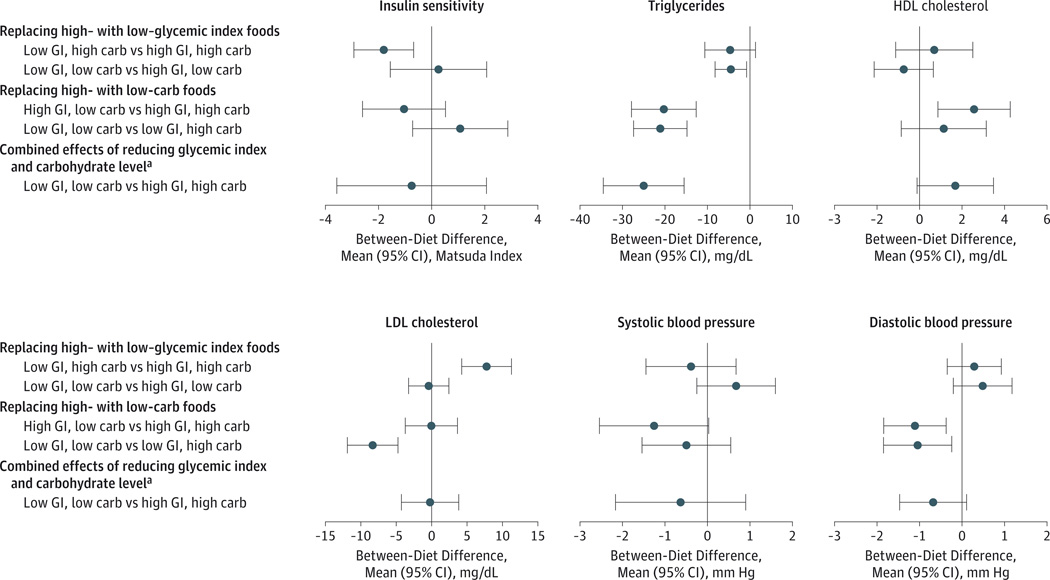
Figure 3.
Effect of Study Diets on Main Outcomes
The primary outcomes were systolic blood pressure, insulin sensitivity, and levels of low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides. Diastolic blood pressure was a secondary outcome. Additional data related to these outcomes are presented in Table 3 and eTable 3 in Supplement 2. Apolipoproteins and other lipid outcomes are in eTable 4. Carb indicates carbohydrate; GI, glycemic index. To convert cholesterol to mmol/L, multiply by 0.0259; triglycerides to mmol/L, multiply by 0.0113.
aFor the 5 primary outcomes on the primary diet contrast (insulin sensitivity, triglycerides, HDL cholesterol, LDL cholesterol, and systolic blood pressure), we plot and tabulate 99% CI to achieve nominal 95% coverage.
Table 3.
Primary Outcomes at Baseline and at the End of Feeding on Each Dieta
| Measure | Baselineb | Mean (SD) at End of Each Feeding Period | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| High-Carbohydrate, High–Glycemic Index Diet |
High-Carbohydrate, Low–Glycemic Index Diet |
Low-Carbohydrate, High–Glycemic Index Diet |
Low-Carbohydrate, Low–Glycemic Index Diet |
|||||||
| No. of Participants |
Mean (SD) | No. of Participants |
Mean (SD) | No. of Participants |
Mean (SD) | No. of Participants |
Mean (SD) | No. of Participants |
Mean (SD) | |
| Insulin sensitivityc | 162 | 7.3 (5.8) | 147 | 8.9 (9.5) | 153 | 7 (6.4)d | 151 | 7.9 (7.4) | 151 | 8.1 (10.3) |
| Triglycerides, mg/dL | 163 | 104.6 (67.1) | 150 | 110.8 (65.7) | 153 | 107.4 (59.2) | 151 | 90.4 (48.1)d,e | 153 | 86.4f (48.1)d,e |
| LDL cholesterol, mg/dL | 163 | 153 (42.1) | 150 | 138.6 (36.6) | 152 | 146.2 (37.2)d | 151 | 138 (36.1)e | 153 | 138 (36.8)e |
| HDL cholesterol, mg/dL | 163 | 58.3 (16) | 150 | 56.9 (15.1) | 153 | 57.3 (17.2) | 151 | 59 (17.5)d | 153 | 58.2 (15.7) |
| Systolic blood pressure, mm Hg | 163 | 132 (9.1) | 150 | 123.9 (11.5) | 153 | 123.8 (10.5) | 152 | 122.6 (10.3) | 154 | 123.4 (10.1) |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein.
SI conversion factors: To convert cholesterol to mmol/L, multiply by 0.0259; triglycerides to mmol/L, multiply by 0.0113.
Data reflect the distributions of outcome measures for each diet, while statistics and tests reported in the “Results” section reflect pairwise within-person differences estimated according to the crossover design. Therefore, mean differences reported in the text do not exactly match the differences between means in this table. Number providing outcome measures for each pair of diets: High carbohydrate, high glycemic index and high carbohydrate, low glycemic index: insulin sensitivity, 146; triglycerides, 149; LDL cholesterol, 148; HDL cholesterol, 149; systolic blood pressure, 149. High carbohydrate, high glycemic index and low carbohydrate, high glycemic index: insulin sensitivity, 135; triglycerides, 138; LDL cholesterol, 138; HDL cholesterol, 138; systolic blood pressure, 139. High carbohydrate, high glycemic index and low carbohydrate, low glycemic index: insulin sensitivity, 136; triglycerides, 140; LDL cholesterol, 140; HDL cholesterol, 140; systolic blood pressure, 141. High carbohydrate, low glycemic index and low carbohydrate, low glycemic index: insulin sensitivity, 142; triglycerides, 143; LDL cholesterol, 142; HDL cholesterol, 143; systolic blood pressure, 144. Low carbohydrate, high glycemic index and low carbohydrate, low glycemic index: insulin sensitivity, 147; triglycerides, 149; LDL cholesterol, 149; HDL cholesterol, 149; systolic blood pressure, 150.
Baseline measurements taken during screening visits while participants were eating their own diets.
Insulin sensitivity determined from blood glucose and insulin levels during a 2-hour 7-time point oral glucose tolerance test by the method of Matsuda and DeFronzo.13
P ≤ .01 in comparison with high-carbohydrate, high–glycemic index diet.
P ≤ .01 in comparison with high-carbohydrate, low–glycemic index diet.
P = .02 in comparison with low-carbohydrate, high–glycemic index diet.
At the high carbohydrate content, the low– compared with the high–glycemic index level significantly increased LDL cholesterol from 139 to 147 mg/dL (6%, P ≤ .001) (Figure 3 and Table 3) and LDL apolipoprotein B from 71 to 75 mg/dL (5%, P = .008) (eTable 4 in Supplement 2). At the low carbohydrate content, the low– compared with the high–glycemic index level decreased plasma total triglyceride level from 91 to 86 mg/dL (−5%, P = .02). Glycemic index level did not affect HDL cholesterol level or systolic blood pressure or diastolic blood pressure.
Effect of Amount of Carbohydrate
A low compared with a high dietary carbohydrate content did not affect insulin sensitivity at either the high– or the low–glycemic index level (Figure 3 and Table 3). The oral glucose tolerance test increased serum glucose at the 1-hour peak more during the low carbohydrate than during the high carbohydrate periods (P = .05) (eFigure 2 and eTable 3 in Supplement 2).
A low compared with a high dietary carbohydrate content significantly lowered plasma total triglycerides at both high– and the low–glycemic index levels. Specifically, the low-carbohydrate, high–glycemic index diet compared with the high-carbohydrate, high–glycemic index diet lowered triglycerides from 111 to 90 mg/dL (−18%, P ≤ .001); and the low-carbohydrate, low–glycemic index diet, compared with the high-carbohydrate, low–glycemic index diet, lowered triglycerides from 108 to 87 mg/dL (−20%, P ≤ .001) (Figure 3 and Table 3). A low compared with a high dietary carbohydrate content, at both high– or low–glycemic index levels, also lowered VLDL apolipoprotein B, VLDL cholesterol, VLDL triglycerides (all P ≤ .001), and LDL triglycerides (P ≤ .002) (eTable 4 in Supplement 2). A low compared with a high dietary carbohydrate content at the high–glycemic index level increased HDL cholesterol from 57 to 60 mg/dL (4%, P = .003) and additionally decreased VLDL apolipoprotein C-III (P = .02). A low compared with a high dietary carbohydrate content lowered diastolic blood pressure by about 1 mm Hg (P ≤ .01) at both high– or low–glycemic index levels.
Combined Effects of Dietary Content of Carbohydrate and the Glycemic Index of the Carbohydrate
The low–glycemic index, low-carbohydrate diet compared with the high–glycemic index, high-carbohydrate diet lowered mean plasma triglycerides from111 to 86mg/dL (−23%, P ≤ .001) but did not significantly affect mean levels of insulin sensitivity, systolic blood pressure, LDL cholesterol, or HDL cholesterol (Figure 3 and Table 3). There was no evidence of additive effects of glycemic index level and dietary carbohydrate content on any of the outcomes.
Other Analyses
No evidence was found of carryover between high– and low–glycemic index diet periods (lowest Wilcoxon P = .44), or between high- and low-carbohydrate diet periods (lowest P = .25) for the primary outcomes. A sensitivity analysis restricted to the 135 participants who completed all 4 diets yielded results similar to the primary analyses (eTable 5 in Supplement 2). The effects of the diets were uniform across subgroups defined by sex, race/ethnicity (black and nonblack), metabolic syndrome, baseline level of outcome variables, and BMI (25–29.9 vs ≥30) (eTables 6–16 in Supplement 2).
Serious adverse events occurred in 7 participants: injuries from automobile crashes (3 participants), kidney stone (1), acute asthma (1), osteomyelitis (1), and pneumonia (1). None were judged to be related to the study procedures. There were no unintended or unanticipated effects.
Changes From Baseline
All 4 study diets were associated with lower systolic blood pressure by 7 to 9 mm Hg (Table 3) and diastolic blood pressure by 4 to 6 mm Hg (eTable 3 in Supplement 2). Compared with baseline, the high-carbohydrate, high–glycemic index diet; the low-carbohydrate, high–glycemic index diet; and the low-carbohydrate, low–glycemic index diets were associated with lower LDL cholesterol by 9% to 10%, and the high-carbohydrate, low–glycemic index diet by 4%.
Discussion
In this trial, which enrolled overweight and obese participants with prehypertension or stage 1 hypertension, composing a healthful diet with low–glycemic index carbohydrate-containing foods rather than high–glycemic index foods did not improve insulin sensitivity, HDL cholesterol levels, LDL cholesterol levels, or systolic blood pressure but did reduce plasma triglyceride levels slightly by 4 to 5 mg/dL. Paradoxically, the low–glycemic index, high-carbohydrate diet compared with the high–glycemic index, high-carbohydrate diet decreased insulin sensitivity and increased LDL cholesterol and LDL apolipoprotein B levels while other dietary factors that affect LDL levels such as saturated fat, cholesterol, and fiber were held constant. These findings are contrary to our hypotheses on glycemic index.
As we found previously in the OmniHeart trial,8 the beneficial effects of the DASH diet can be improved modestly by reducing its carbohydrate content. Lowering the carbohydrate content and compensating the reduced calories with unsaturated fat and protein substantially lowered triglycerides and VLDL levels and slightly lowered diastolic blood pressure, confirming previously established findings.8,17 It is also meaningful that every DASH-type diet studied in previous trials and this trial7,8,18 lowered blood pressure and LDL cholesterol levels of the participants from baseline when they were eating their usual diets. Thus, the new information in the present study is that composing a DASH-type diet with low–glycemic index foods compared with high–glycemic index foods does not improve CVD risk factors and may in fact reduce insulin sensitivity and increase LDL cholesterol.
We found that a low compared with a high glycemic index of a high-carbohydrate diet decreased insulin sensitivity measured by an oral glucose tolerance test. Fasting glucose level was higher on low–glycemic index than high–glycemic index dietary carbohydrate as previously reported.3,19–21 Perhaps the low–glycemic index diet induced more morning insulin resistance to maintain an adequate blood glucose level. However, a low–glycemic index diet did not affect insulin sensitivity in other studies in which body weight either remained constant during the trial or decreased by a similar amount in the high– and low–glycemic index groups.19,21–29
We chose a 5-week duration of the intervention feeding periods based on results of previous studies, which suggested that 5 weeks was sufficient to detect changes in our outcomes (trial protocol in Supplement 1). A recent meta-analysis of 14 trials that had durations of at least 6 months found no effect of lowering glycemic index on lipids or fasting glucose, although fasting insulin was reduced.30 Lowering glycemic index did not affect insulin sensitivity22,29,31,32 or blood pressure in trials of at least 6-month duration.3,22,29,31–33 These results do not suggest that the effects of lowering glycemic index become apparent only after a longer duration of intake.
This trial did not address the effect of glycemic index in a typical US diet. Rather we studied a low compared with a high glycemic index in a DASH-type diet. However, we do not attribute the null findings on glycemic index to the healthfulness or specific content of the DASH diet. For example, in several European studies19,23,31,32 and one in Brazil,22 the researchers gave or prescribed selected foods to the participants to use in their own diets instead of providing complete diets that differed from their usual diets. In these studies, lowering glycemic index did not increase insulin sensitivity or improve blood pressure, HDL cholesterol level, or triglyceride level; LDL cholesterol level decreased in one of these studies19 but did not change in the others.22,23,31,32
We showed in a subsample of the participants that the glycemic index values of individual foods computed from dietary tables, when assembled into meals, produced expected differences in blood glucose AUCi over 12 hours, a process variable, thus confirming previous results.3–5 Compared with the high–glycemic index, high-carbohydrate diet, either a lower–glycemic index level or a lower carbohydrate content lowered glucose AUCi with no further benefit from the diet with both lower glycemic index and lower carbohydrate levels. These results suggest that lowering glycemic index or lowering carbohydrates for breakfast, lunch, and dinner reduces blood glucose during 12 hours without any further reduction from lowering both together. Thus, the effects of these 2 changes in dietary carbohydrate were not additive, suggesting a plateau effect, as also found in a similar study.27 This finding could have policy implications because a lower day-long glucose concentration, as indicated by lower hemoglobin A1c level, predicts lower risk of diabetes and CVD according to results of cohort studies.34 Diets high in glycemic index or glycemic load were associated with a higher incidence of diabetes according to meta-analyses.35,36 Our results suggest that at carbohydrate levels as in the lower-carbohydrate diets in this study and in Mediterranean diets, glycemic index levels between 40 and 65 spanning the range in the US diet may not affect blood glucose levels throughout the day.
After we started this trial, reports of trials that involved glycemic index have accumulated. A meta-analysis of 28 trials found that lowering glycemic index did not affect HDL cholesterol or triglyceride levels and lowered LDL cholesterol level only if fiber content was also increased.37 The LDL cholesterol–lowering effect of dietary fiber, per se, is well-established.38 In our study, LDL cholesterol levels and other components of LDL such as apolipoprotein B and apolipoprotein C-III were higher during the low–glycemic index, high-carbohydrate diet than during the other 3 diets. There were no increases in foods or nutrients in the low–glycemic index, high-carbohydrate diet that have known effects to raise LDL levels. In fact, the low–glycemic index, high-carbohydrate diet contained slightly less dietary cholesterol and more fiber than the other diets, but these differences would have lowered not raised LDL levels. Low–glycemic index diets did not lower blood pressure.3,19,21,22,31–33 Other studies in participants without diabetes found that high vs low glycemic index did not affect glycemia or insulin resistance.21,23,25,27,28,32 We caution that we did not study lowering glycemic index in people with type 2 diabetes to control their hyperglycemia; 2 meta-analyses reported benefits6,39 and our findings should not be extended to type 2 diabetes. We also did not study the influence of glycemic index on weight loss. Lowering glycemic index may improve weight loss6 or maintenance40,41 according to a meta-analysis6 and some more recent clinical trials,40,41 although others did not find an advantage of low–glycemic index diets.3,22,27,29,31,42
This trial oversampled black individuals because of their greater burden of type 2 diabetes and CVD that could be modifiable by dietary change. The results were similar in black and white participants. The main dietary contrast of interest, high vs low glycemic index, included 163 participants, exceeding the goal of 160. However, the number of participants for each dietary contrast ranged from 135 to 150. Still, the precision of estimation of effects, as shown by the confidence intervals, was adequate for clinically relevant inference on the risk factors of interest.
Conclusions
In this 5-week controlled feeding study, diets with low glycemic index of dietary carbohydrate, compared with high glycemic index of dietary carbohydrate, did not result in improvements in insulin sensitivity, lipid levels, or systolic blood pressure. In the context of an overall DASH-type diet, using glycemic index to select specific foods may not improve cardiovascular risk factors or insulin resistance.
Supplementary Material
Acknowledgments
Funding/Support: The study was supported by an investigator-initiated grant from the National Heart, Lung, and Blood Institute (R01HL084568) and received additional support from National Institute of Diabetes and Digestive and Kidney Diseases (R21 DK098720-02); the Harvard Clinical and Translational Science Center (8UL1TR0001750-05) from the National Center for Advancing Translational Science; and the general clinical research center at Brigham and Women’s Hospital (M01-02635).
Role of the Funders/Sponsors: The investigators were responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication. The funding agency provided critical review of the research grant application and the protocol and monitored the progress of the study. The study received donations of some foods that were used in the study diets from Almond Board of California, Kraft Foods, Cabot Cheese, Rudolph’s Bakery of Toronto Canada, and McCormick Seasonings. These companies were not involved in the design, execution, analysis, interpretation, or manuscript writing or critique.
Footnotes
Supplemental content at jama.com
Author Contributions: Dr Sacks had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sacks, Carey, Miller, Swain, White, Yee, Appel.
Acquisition, analysis, or interpretation of data: Sacks, Carey, Anderson, Miller, Copeland, Charleston, Harshfield, Laranjo, McCarron, Yee, Appel.
Drafting of the manuscript: Sacks, Carey, Anderson, Copeland, Laranjo, Swain.
Critical revision of the manuscript for important intellectual content: Sacks, Carey, Anderson, Miller, Charleston, Harshfield, McCarron, White, Yee, Appel.
Statistical analysis: Carey, Harshfield, Laranjo.
Obtained funding: Sacks, Carey, Appel.
Administrative, technical, or material support: Carey, Miller, Copeland, Harshfield, Laranjo, Swain, Appel.
Study supervision: Sacks, Carey, Anderson, Miller, McCarron, Appel.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Sacks was an expert witness in litigation involving POM Wonderful, Hershey, Unilever, and Keebler. No other disclosures were reported.
Additional Contributions: We thank David S. Ludwig, MD, PhD, Children’s Hospital, Boston, for consultation on the design and execution of this study and for critical reading and comments on the manuscript for which he received compensation. We thank Catherine Loria, PhD, the program officer at the National Heart, Lung, and Blood Institute, who monitored the progress of the study; and the data and safety monitoring committee (Janet King, PhD, Chair, Children’s Hospital of Oakland Research Institute; Barry Davis, MD, University of Texas School of Public Health, Houston; Henry Ginsberg, MD, Columbia University College of Physicians and Surgeons, New York; Alice Lichtenstein, DSc, Tufts University, Boston; and Edward Horton, MD, Joslin Diabetes Center, Boston); these colleagues did not receive compensation. We also thank the Frederick Church of the Brethren, Frederick, Maryland, which provided space for distribution of food to study participants.
REFERENCES
- 1.Wolever TM, Jenkins DJ, Jenkins AL, Josse RG. The glycemic index: methodology and clinical implications. Am J Clin Nutr. 1991;54(5):846–854. doi: 10.1093/ajcn/54.5.846. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care. 2008;31(12):2281–2283. doi: 10.2337/dc08-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolever TM, Gibbs AL, Mehling C, et al. The Canadian Trial of Carbohydrates in Diabetes (CCD), a 1-y controlled trial of low-glycemic-index dietary carbohydrate in type 2 diabetes: no effect on glycated hemoglobin but reduction in C-reactive protein. Am J Clin Nutr. 2008;87(1):114–125. doi: 10.1093/ajcn/87.1.114. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds RC, Stockmann KS, Atkinson FS, Denyer GS, Brand-Miller JC. Effect of the glycemic index of carbohydrates on day-long (10 h) profiles of plasma glucose, insulin, cholecystokinin and ghrelin. Eur J Clin Nutr. 2009;63(7):872–878. doi: 10.1038/ejcn.2008.52. [DOI] [PubMed] [Google Scholar]
- 5.Fabricatore AN, Ebbeling CB, Wadden TA, Ludwig DS. Continuous glucose monitoring to assess the ecologic validity of dietary glycemic index and glycemic load. Am J Clin Nutr. 2011;94(6):1519–1524. doi: 10.3945/ajcn.111.020354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Livesey G, Taylor R, Hulshof T, Howlett J. Glycemic response and health: a systematic review and meta-analysis: relations between dietary glycemic properties and health outcomes. Am J Clin Nutr. 2008;87(1) suppl:258S–268S. doi: 10.1093/ajcn/87.1.258S. [DOI] [PubMed] [Google Scholar]
- 7.Appel LJ, Moore TJ, Obarzanek E, et al. DASH Collaborative Research Group. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336(16):1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 8.Appel LJ, Sacks FM, Carey VJ, et al. OmniHeart Collaborative Research Group. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294(19):2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 9.de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99(6):779–785. doi: 10.1161/01.cir.99.6.779. [DOI] [PubMed] [Google Scholar]
- 10.Estruch R, Ros E, Salas-Salvadó J, et al. PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368(14):1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 11.Michaud DS, Fuchs CS, Liu S, Willett WC, Colditz GA, Giovannucci E. Dietary glycemic load, carbohydrate, sugar, and colorectal cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2005;14(1):138–147. [PubMed] [Google Scholar]
- 12.White WB, Anwar YA. Evaluation of the overall efficacy of the Omron office digital blood pressure HEM-907 monitor in adults. Blood Press Monit. 2001;6(2):107–110. doi: 10.1097/00126097-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 14.Gannon MC, Nuttall FQ, Westphal SA, Fang S, Ercan-Fang N. Acute metabolic response to high-carbohydrate, high-starch meals compared with moderate-carbohydrate, low-starch meals in subjects with type 2 diabetes. Diabetes Care. 1998;21(10):1619–1626. doi: 10.2337/diacare.21.10.1619. [DOI] [PubMed] [Google Scholar]
- 15.Ludbrook J. Multiple inferences using confidence intervals. Clin Exp Pharmacol Physiol. 2000;27(3):212–215. doi: 10.1046/j.1440-1681.2000.03223.x. [DOI] [PubMed] [Google Scholar]
- 16.Jones G, Kenward MG. Design and Analysis of Cross-Over Trials. Second Ed. Boca Raton, FL: CRC Press; 2003. [Google Scholar]
- 17.Mensink RP, Katan MB. Effect of dietary fatty acids on serum lipids and lipoproteins: a meta-analysis of 27 trials. Arterioscler Thromb. 1992;12(8):911–919. doi: 10.1161/01.atv.12.8.911. [DOI] [PubMed] [Google Scholar]
- 18.Obarzanek E, Sacks FM, Vollmer WM, et al. DASH Research Group. Effects on blood lipids of a blood pressure-lowering diet: the Dietary Approaches to Stop Hypertension (DASH) Trial. Am J Clin Nutr. 2001;74(1):80–89. doi: 10.1093/ajcn/74.1.80. [DOI] [PubMed] [Google Scholar]
- 19.Sloth B, Krog-Mikkelson I, Flint A, et al. No difference in body weight decrease between a low-glycemic-index and a high-glycemic-index diet but reduced LDL cholesterol after 10-wk ad libitum intake of the low-glycemic-index diet. Am J Clin Nutr. 2004;80(2):337–347. doi: 10.1093/ajcn/80.2.337. [DOI] [PubMed] [Google Scholar]
- 20.Hartman TJ, Albert PS, Zhang Z, et al. Consumption of a legume-enriched, low-glycemic index diet is associated with biomarkers of insulin resistance and inflammation among men at risk for colorectal cancer. J Nutr. 2010;140(1):60–67. doi: 10.3945/jn.109.114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Runchey SS, Pollak MN, Valsta LM, et al. Glycemic load effect on fasting and post-prandial serum glucose, insulin, IGF-1 and IGFBP-3 in a randomized, controlled feeding study. Eur J Clin Nutr. 2012;66(10):1146–1152. doi: 10.1038/ejcn.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sichieri R, Moura AS, Genelhu V, Hu F, Willett WC. An 18-mo randomized trial of a low-glycemic-index diet and weight change in Brazilian women. Am J Clin Nutr. 2007;86(3):707–713. doi: 10.1093/ajcn/86.3.707. [DOI] [PubMed] [Google Scholar]
- 23.Vrolix R, Mensink RP. Effects of glycemic load on metabolic risk markers in subjects at increased risk of developing metabolic syndrome. Am J Clin Nutr. 2010;92(2):366–374. doi: 10.3945/ajcn.2009.28339. [DOI] [PubMed] [Google Scholar]
- 24.Järvi AE, Karlström BE, Granfeldt YE, Björck IE, Asp NG, Vessby BO. Improved glycemic control and lipid profile and normalized fibrinolytic activity on a low-glycemic index diet in type 2 diabetic patients. Diabetes Care. 1999;22(1):10–18. doi: 10.2337/diacare.22.1.10. [DOI] [PubMed] [Google Scholar]
- 25.Shikany JM, Phadke RP, Redden DT, Gower BA. Effects of low- and high-glycemic index/glycemic load diets on coronary heart disease risk factors in overweight/obese men. Metabolism. 2009;58(12):1793–1801. doi: 10.1016/j.metabol.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raatz SK, Torkelson CJ, Redmon JB, et al. Reduced glycemic index and glycemic load diets do not increase the effects of energy restriction on weight loss and insulin sensitivity in obese men and women. J Nutr. 2005;135(10):2387–2391. doi: 10.1093/jn/135.10.2387. [DOI] [PubMed] [Google Scholar]
- 27.McMillan-Price J, Petocz P, Atkinson F, et al. Comparison of 4 diets of varying glycemic load on weight loss and cardiovascular risk reduction in overweight and obese young adults: a randomized controlled trial. Arch Intern Med. 2006;166(14):1466–1475. doi: 10.1001/archinte.166.14.1466. [DOI] [PubMed] [Google Scholar]
- 28.de Rougemont A, Normand S, Nazare JA, et al. Beneficial effects of a 5-week low-glycaemic index regimen on weight control and cardiovascular risk factors in overweight non-diabetic subjects. Br J Nutr. 2007;98(6):1288–1298. doi: 10.1017/S0007114507778674. [DOI] [PubMed] [Google Scholar]
- 29.Fabricatore AN, Wadden TA, Ebbeling CB, et al. Targeting dietary fat or glycemic load in the treatment of obesity and type 2 diabetes: a randomized controlled trial. Diabetes Res Clin Pract. 2011;92(1):37–45. doi: 10.1016/j.diabres.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwingshackl L, Hoffmann G. Long-term effects of low glycemic index/load vs high glycemic index/load diets on parameters of obesity and obesity-associated risks: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis. 2013;23(8):699–706. doi: 10.1016/j.numecd.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Jebb SA, Lovegrove JA, Griffin BA, et al. RISCK Study Group. Effect of changing the amount and type of fat and carbohydrate on insulin sensitivity and cardiovascular risk: the RISCK (Reading, Imperial, Surrey, Cambridge, and Kings) trial. Am J Clin Nutr. 2010;92(4):748–758. doi: 10.3945/ajcn.2009.29096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gögebakan O, Kohl A, Osterhoff MA, et al. DiOGenes. Effects of weight loss and long-term weight maintenance with diets varying in protein and glycemic index on cardiovascular risk factors: the diet, obesity, and genes (DiOGenes) study: a randomized, controlled trial. Circulation. 2011;124(25):2829–2838. doi: 10.1161/CIRCULATIONAHA.111.033274. [DOI] [PubMed] [Google Scholar]
- 33.Jenkins DJ, Kendall CW, McKeown-Eyssen G, et al. Effect of a low-glycemic index or a high-cereal fiber diet on type 2 diabetes: a randomized trial. JAMA. 2008;300(23):2742–2753. doi: 10.1001/jama.2008.808. [DOI] [PubMed] [Google Scholar]
- 34.Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362(9):800–811. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mirrahimi A, de Souza RJ, Chiavaroli L, et al. Associations of glycemic index and load with coronary heart disease events: a systematic review and meta-analysis of prospective cohorts. J Am Heart Assoc. 2012;1(5):e000752. doi: 10.1161/JAHA.112.000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong JY, Zhang L, Zhang YH, Qin LQ. Dietary glycaemic index and glycaemic load in relation to the risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Br J Nutr. 2011;106(11):1649–1654. doi: 10.1017/S000711451100540X. [DOI] [PubMed] [Google Scholar]
- 37.Goff LM, Cowland DE, Hooper L, Frost GS. Low glycaemic index diets and blood lipids: a systematic review and meta-analysis of randomised controlled trials. Nutr Metab Cardiovasc Dis. 2013;23(1):1–10. doi: 10.1016/j.numecd.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr. 1999;69(1):30–42. doi: 10.1093/ajcn/69.1.30. [DOI] [PubMed] [Google Scholar]
- 39.Thomas DE, Elliott EJ. The use of low-glycaemic index diets in diabetes control. Br J Nutr. 2010;104(6):797–802. doi: 10.1017/S0007114510001534. [DOI] [PubMed] [Google Scholar]
- 40.Ebbeling CB, Swain JF, Feldman HA, et al. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA. 2012;307(24):2627–2634. doi: 10.1001/jama.2012.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larsen TM, Dalskov SM, van Baak M, et al. Diet, Obesity, and Genes (Diogenes) Project. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363(22):2102–2113. doi: 10.1056/NEJMoa1007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heilbronn LK, Noakes M, Clifton PM. The effect of high- and low-glycemic index energy restricted diets on plasma lipid and glucose profiles in type 2 diabetic subjects with varying glycemic control. J Am Coll Nutr. 2002;21(2):120–127. doi: 10.1080/07315724.2002.10719204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.