Abstract
Purpose
The assessment of cortisol in hair has gained popularity as a means to measure retrospective hypothalamic-pituitary-adrenal activity in a number of species; however, cortisol levels from human hair subjected to typical chemicals for cosmetic or hygienic purposes may be altered by the chemicals used. The purposed of this study was to determine if exposure of hair to chemical processing or shampooing impacts cortisol values.
Methods
Human hair not exposed to prior chemical processing was cut from the posterior vertex region of the head of 106 human subjects as close to the scalp as possible. The hair sample was divided into 4-6 full-length clusters depending on quantity of hair available. Each hair sample was processed for baseline (native) cortisol and remaining clusters were exposed to five standard chemical hair treatments (Experiment 1) or were shampooed 15 or 30 times (Experiment 2). Hair was ground and cortisol levels were determined by enzyme immunoassay (EIA). Comparisons were made between native hair and processed hair using paired t-tests and Pearson correlation.
Results
Hair cortisol as assessed by EIA was significantly altered by chemical processing but in somewhat different ways. Exposure to bleach (harshest exposure), demi-perm (least exposure) or 15-30 shampoos resulted in a significant decrease in cortisol level while exposure to varying percentages of peroxides increased cortisol measured. There were no differences in cortisol levels associated with sex, age or tobacco use in the native hair for this particular group.
Conclusion
Chemical processing and frequent shampooing affect cortisol levels measured in hair. Chemically processed or excessively shampooed hair should be avoided when recruiting subjects for hair cortisol studies.
Cortisol reflects hypothalamic pituitary adrenal (HPA) activity in both humans and animals [1, 2]. Despite its ubiquitous application as a marker of HPA activation, the ideal matrix from which to obtain cortisol estimates depends on the study question and population. While cortisol levels may be obtained from blood, urine, saliva, feces or hair, [3-6] cortisol levels may fluctuate rapidly over the course of the day in response to multiple daily-life experiences as well as circadian rhythms. Other assessments of cortisol status, such as the cortisol awakening response or salivary cortisol diurnal slope, [7] provide differing information regarding HPA activation and are subject to participant error in collection or non-compliance with collection protocols [8-9]. Hair cortisol is easily collected by investigators in a controlled setting and is less subject to daily intra-individual fluctuations [10].
Integration of hair cortisol assessment into stress research may obviate previously reported methodological problems associated with cortisol collection using plasma, urine or saliva since hair cortisol can chronicle HPA activity over several months [11-12]. While this method of cortisol assessment has been validated for use in numerous species, many questions remain regarding the use of hair cortisol in humans [13]. Humans vary widely in hair colour, texture, hair hygiene habits, ethnicity, body mass index and health status. Furthermore, psychological state and trait status may affect measured levels [14].
Approximately 75% of American women report colouring their hair [15]. As our study focus is frequently on women of childbearing age, this becomes an issue. In order to permanently alter the colour of hair, the hair cuticle, or outer layer of hair, must be opened so that native hair colour is removed prior to depositing chemical hair colour in the cortex of the hair shaft. [16-17] Three main chemicals are involved in this process: 1) hydrogen peroxide, which is an oxidizing agent or developer, 2) ammonia, which is an alkaline agent that allows lightening by catalyzing the reaction between the native hair colour and the peroxide, and 3) persulfate, which is a bleach that removes the pigment from the hair [16]. The ammonia separates the hair cuticle and allows the new hair colour to penetrate the cortex of the hair [17]. As part of this process, sulfur is removed which causes hair to harden [15].
Recognizing that the cortex of hair is exposed during chemical hair processing, and that hair cortex surrounds the hair shaft medulla where cortisol is thought to enter hair from the blood, the reliability of cortisol levels that are obtained from humans with chemically processed hair is questionable [1]. The objective of this study was to determine if cortisol levels measured from human hair are significantly altered by common methods of cosmetic and hygienic hair processing: by exposure to peroxides, ammonia or persulfate or by repeated washing with commercial shampoo products.
Methods
After obtaining institutional review board approval and subject consent, human hair was cut from non-pregnant and pregnant females, and from males. All subjects denied any type of chemical processing of their hair in the past year. Subject demographics included age, gender, race/ethnicity, medication and tobacco use and hair hygiene (frequency of shampooing, use of heat treatments).
Hair was cut from the posterior vertex region as close to the scalp as possible using scissors or thinning shears. Hair length was recorded and proximal ends of the hair samples were marked. Hair samples were stored in aluminum foil for protection as recommended [18]. All hair samples were divided into a minimum of two clusters to a maximum of six clusters of full-length hair. If hair was longer than 5 cm, the strands were divided at 5 cm from the scalp and only the proximal segment used for analysis. For experiment 1, clusters of hair were exposed to various common products used for chemical processing. One cluster was set aside from each subject and used as the “native” hair cluster, to which all other clusters were compared. The other clusters were exposed to one or more of seven hair treatments (depending on the number of available hair clusters): potassium persulfate (bleach), <2% peroxide (H2O2), (demi-perm or semi-permanent colour), 6% H2O2, 9% H2O2 or 12% H2O2. All peroxide treatments also contained ammonia. A licensed cosmetologist (AG) instructed the first author (CH) on standard salon procedures using Wella Koleston Perfect© and Wella Color Touch© (Wella Professionals, Procter & Gamble, USA) products prior to processing hair samples.
For Experiment 2, a variety of common commercially available shampoos, to represent the assortment of shampoos by cost, hair texture, and popularity used commonly by our subjects, were used to wash hair samples from untreated clusters described previously. Hair samples were placed in individual 15 ml conical centrifuge tubes with shampoo diluted 5-fold. Each tube was vigorously shaken for 5-10 seconds. The hair was removed from the tube and placed under a running stream of deionized water for 5 seconds to rinse the hair after each wash step. Hair samples were washed either 15 or 30 times with the same hair product for comparison to native hair.
Following either chemical treatment or shampoo, each cluster from Experiments 1 and 2 was washed three times in isopropanol and dried as previously described [3]. After washing, drying, and weighing, the hair was ground in 2 ml cryovials (Wheaton, Millville, NJ, USA) using a ball mill (Retsch, Haan, Germany) that held the cryovial and sample as well as a 3/16 inch stainless steel ball bearing in specially milled aluminum cassettes holding three cryovials. The cassette containing the cryotubes was immersed in liquid nitrogen to snap freeze the hair prior to grinding to facilitate the process. Samples were ground for approximately 5 min. The powdered hair (5-10 mg) was extracted in the same cryovial in HPLC grade methanol at room temperature for 24 hours with slow shaking. Following extraction, the cryovial was spun for 120 s in a microcentrifuge at 20000 g and 200 μl supernatant was removed, placed into a second microcentrifuge tube and dried for 24 hr under a hood. The dried extract was then reconstituted in 133 μl of assay buffer and cortisol levels determined using a commercial high sensitivity EIA kit (Salimetrics LLC, State College, PA, USA) according to manufacturer's directions. A pooled control consisting of previously ground hair was extracted and run on each EIA plate for determination of inter-assay coefficients of variation. Inter-assay coefficient of variation (CV) for the control hair pool was 11.6% and intra-assay CV was 1.9%.
Statistical analyses applied paired t-tests comparing native to chemically processed and shampooed hair from the same individual. Pearson correlations evaluated unprocessed hair cortisol levels versus the matching chemically processed hair cortisol levels. Age, sex, race/ethnicity and tobacco status were also assessed using ANOVA. All analyses were performed using Prism (GraphPad Software, Inc., San Diego, CA).
Results
Table 1 provides descriptive information on participants. Hair cortisol from 88 healthy women and 18 men, ages 7-60 was evaluated. Subjects were primarily female, white, non-Hispanic, non-smoking, and washed their hair anywhere from 2-7 times per week (mean± standard deviation: 5.04±1.7 times/week).
TABLE 1.
Characteristics of subjects (n=106)
| Age: years (Mean± SD) | 35.0±8.7 |
| Female: n(%) | 88(83) |
| Smoking: n(%) | 14(13) |
| Race/ethnicity: n(%) | |
| White non-Hispanic | 90(84.5) |
| Black non-Hispanic | 5(4.7) |
| Hispanic | 11(10.3) |
| Native American | 2(1.9) |
| Asian | 1(<1) |
| Washes per week (Mean±SD) | 5.4±1.6 |
| Native hair cortisol (pg/mg, 3cm from scalp (Mean±SD) | 9.61±10.15 |
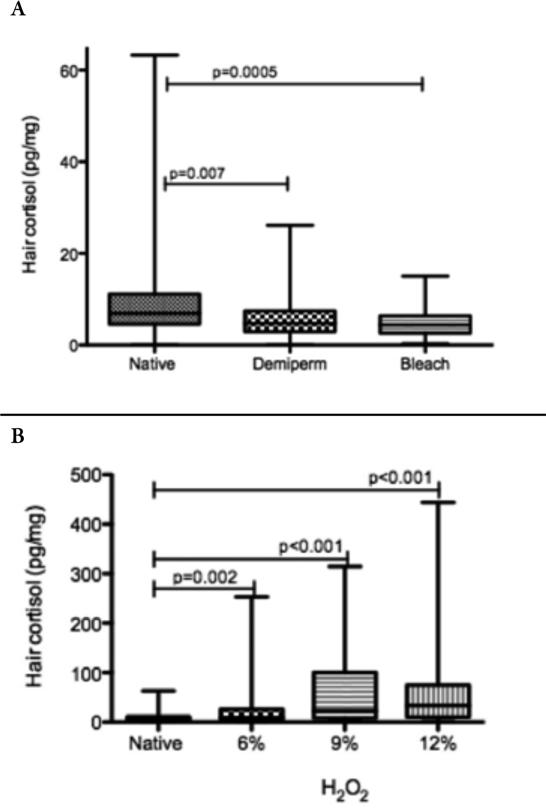
Significant differences were noted between native hair cortisol and processed hair cortisol values following all chemical treatments (Figure 1a, 1b). While the greatest and least chemical exposures, bleach and demi-perm, significantly decreased hair cortisol levels, exposure to varying percentages of peroxides significantly increased cortisol levels.
FIGURE 1.
Native hair cortisol levels, pg/mg, (Mean± standard deviation in box, whiskers represent maximum and minimum values) vs. processed hair cortisol using: a. bleach and demi-permanent color (<2% peroxide) and b. 6% peroxide, 9% peroxide and 12% peroxide (H2O2).
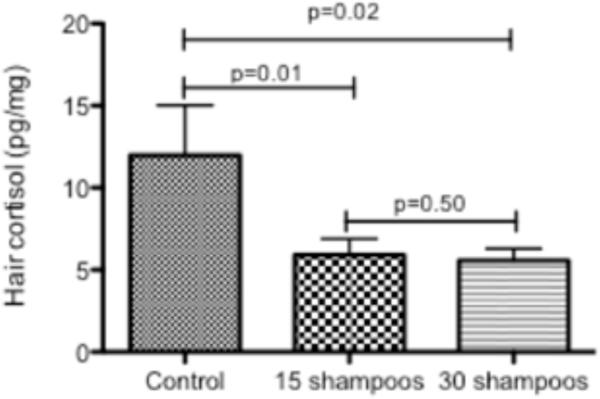
Significant reductions in hair cortisol level were also noted when hair was shampooed 15 and 30 times compared with native hair cortisol (Figure 2). No significant differences were noted by type of shampoo. It is worth mentioning that hair that began the washing process appearing healthy and shiny became dry and easily broken following the repeated washing regardless of the type of shampoo.
FIGURE 2.
Mean and standard deviation native hair cortisol (pg/mg) levels vs, hair shampooed 15 or 30 times.
Native hair cortisol values were evaluated among subjects of different sex, age groups smoking status and heat treatments. No significant differences were noted (data not shown). When stratified by race/ethnicity no significant differences were noted. A trend towards elevated mean native cortisol levels was observed in the hair of five African American subjects (mean ± standard deviation: 36.85±46.0 pg/mg) and two Native American subjects (mean ± standard deviation: 40.5±32.2 pg/mg).
Discussion
As the use of hair cortisol as a proxy measure of HPA activation becomes more common, new questions arise regarding how factors that impact cortisol may affect study outcomes. In this study, it has been demonstrated that professional salon colouring with persulfates and peroxides, as well as frequent shampooing, significantly impacts cortisol levels when compared with unprocessed hair from the same individual. Although this has been tacitly assumed, it is necessary to demonstrate directly.
In the study by Dettenborn et al., no difference was seen in hair cortisol levels amongst humans with different natural hair colours, taking various medications and with or without tobacco use [13]. Higher hair cortisol levels were found in young children and older adults, and men showed higher hair cortisol levels than women [19]. Species-specific sex differences in hair cortisol vary. For example, nonhuman primate males show lower hair cortisol levels than females, but show similar developmental patterns [20]. In the present study, no difference was seen in hair cortisol between male and females or in cortisol levels over a more restricted age range. There may be variable differences in cortisol values when comparing diverse populations, sex and age.
The influence of individual hair hygiene habits in humans remains poorly understood. Data from Sauvé et al. comparing the effect of “dyeing” on human hair suggested no difference in cortisol levels when compared to native hair or number of times the same strands were dyed in vitro [21]. Conversely, lower hair cortisol levels were noted in hair that was dyed in vivo. Their data also suggest that shampoo may leach cortisol from the hair shaft leading to lower levels in distal hair segments [21]. These mixed results do not take into consideration the amount of persulfate (bleach) or peroxides present in the drugstore brand of hair dye used. Our findings mirror the decreased cortisol levels in cosmetically treated hair exposed to peroxides.
Exposure of human hair to shampoo solution, hot water immersion or ultraviolet irradiation led to significant cortisol loss in hair [22]. Similarly, this study showed that exposure of hair to excessive shampooing (both 15 and 30 times) decreased cortisol levels. Hair cortisol may also be lost by long-term exposure to external environments and ultraviolet sun exposure [22]. Thus, cortisol obtained from hair samples closest to the scalp in the posterior vertex is most likely to accurately reflect retrospective HPA activity [13]. Stability of cortisol in hair more generally has been demonstrated in studies of hair from archeological samples over 2,000 years old, where cortisol levels approximated levels observed in “fresh” samples [23]. This is an important observation as it suggests that archival hair collections can be assayed regardless of age.
It has been shown previously that repeated washing of nonhuman primate hair 20 times with water alone and 20 times with shampoo and water were both associated with depletion of measured cortisol much like we observed for human hair [24]. The current study is, however, the first to expose native human hair to common professional chemical hair processing treatments concordant with the spectrum of hair treatments that a man or woman might experience in the salon.
The elevated cortisol levels noted with peroxides are most likely related to assay interference by altering the pH, interfering with binding properties of the assay or antibody structure, or affecting the substrate reactions related to the assay chromagen. Although colour changes in the pH indicator of the assay were not noted, the methanol extraction fluid was seen to change colour when a subject has used hair dye products. This type of colour change is always noted in our assay notes and this subject is eliminated from the data set at the time of data analysis.
Limitations of this study include a small percentage of African- and Native American subjects. African hair differs from Caucasian hair in several notable ways: hair density, rate of hair growth and telogen counts (an approximation of percentage of growing hairs) [25]. This must be taken into consideration when comparing cortisol levels between subjects of different race/ethnicities in general. Exposure of African American hair in the present study to chemicals did not alter hair cortisol in our study but other products such as hair straighteners were not tested.
Our data indicate that cosmetic chemical processing and multiple shampoos significantly alter hair cortisol measurements. Depending on the chemical used, cortisol levels may be markedly decreased or markedly increased. Based on our present observations, reliable cortisol levels may be obtained from subjects with chemically processed hair only if unprocessed segments of hair can be obtained from as close to the scalp as possible. The site most commonly recommended for hair collection is the posterior vertex of the head. For women who use highlights, this is rarely an area that is coloured. By thinking creatively, one is likely to identify sites from which native hair can be more easily collected; however the take home message from the present study is that harsh chemical treatment associated with most hair colouring processes will have significant impacts on hair cortisol and should be avoided in study design when possible.
Acknowledgments
The authors would like to acknowledge grants received from NICHD 2K12HD001271-11 (CH) and NCI CA126971 (MLL) and a contract from Patient Centered Outcomes Research Institute CE-1304-6208 (MLL).
References
- 1.Raul JS, et al. Detection of physiological concentrations of cortisol and cortisone in human hair. Clin Biochem. 2004;37(12):1105–11. doi: 10.1016/j.clinbiochem.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Gozansky WS, et al. Salivary cortisol determined by enzyme immunoassay is preferable to serum total cortisol for assessment of dynamic hypothalamic--pituitary--adrenal axis activity. Clin Endocrinol (Oxf ) 2005;63(3):336–41. doi: 10.1111/j.1365-2265.2005.02349.x. [DOI] [PubMed] [Google Scholar]
- 3.D'Anna-Hernandez KL, et al. Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: comparison to salivary cortisol. Physiol Behav. 2011;104(2):348–53. doi: 10.1016/j.physbeh.2011.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanaelst B, et al. Intercorrelations between serum, salivary, and hair cortisol and child-reported estimates of stress in elementary school girls. Psychophysiology. 2012;49(8):1072–81. doi: 10.1111/j.1469-8986.2012.01396.x. [DOI] [PubMed] [Google Scholar]
- 5.Behie AM, Pavelka MS, Chapman CA. Sources of variation in fecal cortisol levels in howler monkeys in Belize. Am J Primatol. 2010;72(7):600–6. doi: 10.1002/ajp.20813. [DOI] [PubMed] [Google Scholar]
- 6.Aardal E, Holm AC. Cortisol in saliva--reference ranges and relation to cortisol in serum. Eur J Clin Chem Clin Biochem. 1995;33(12):927–32. doi: 10.1515/cclm.1995.33.12.927. [DOI] [PubMed] [Google Scholar]
- 7.Clow A, et al. The cortisol awakening response: more than a measure of HPA axis function. Neurosci Biobehav Rev. 2010;35(1):97–103. doi: 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Hellhammer J, et al. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: state- and trait components. Psychoneuroendocrinology. 2007;32(1):80–6. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34(10):1423–36. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Staufenbiel SM, et al. Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology. 2013;38(8):1220–35. doi: 10.1016/j.psyneuen.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Fairbanks LA, et al. Heritability and genetic correlation of hair cortisol in vervet monkeys in low and higher stress environments. Psychoneuroendocrinology. 2011;36(8):1201–8. doi: 10.1016/j.psyneuen.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirschbaum C, et al. Hair as a retrospective calendar of cortisol production-Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 2009;34(1):32–7. doi: 10.1016/j.psyneuen.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 13.Dettenborn L, et al. The assessment of cortisol in human hair: associations with sociodemographic variables and potential confounders. Stress. 2012;15(6):578–88. doi: 10.3109/10253890.2012.654479. [DOI] [PubMed] [Google Scholar]
- 14.Dettenborn L, et al. Higher cortisol content in hair among long-term unemployed individuals compared to controls. Psychoneuroendocrinology. 2010;35(9):1404–9. doi: 10.1016/j.psyneuen.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Nohynek GJ, et al. Toxicity and human health risk of hair dyes. Food Chem Toxicol. 2004;42(4):517–43. doi: 10.1016/j.fct.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Kojima T, et al. Dyeing regions of oxidative hair dyes in human hair investigated by nanoscale secondary ion mass spectrometry. Colloids Surf B Biointerfaces. 2013;106:140–4. doi: 10.1016/j.colsurfb.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 17.Skopp G, Potsch L, Moeller MR. On cosmetically treated hair-aspects and pitfalls of interpretation. Forensic Sci Int. 1997;84(1-3):43–52. doi: 10.1016/s0379-0738(96)02047-6. [DOI] [PubMed] [Google Scholar]
- 18.Wennig R. Potential problems with the interpretation of hair analysis results. Forensic Sci Int. 2000;107(1-3):5–12. doi: 10.1016/s0379-0738(99)00146-2. [DOI] [PubMed] [Google Scholar]
- 19.Stalder T, et al. Cortisol in hair, body mass index and stress-related measures. Biol Psychol. 2012;90(3):218–23. doi: 10.1016/j.biopsycho.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Laudenslager ML, Jorgensen MJ, Fairbanks LA. Developmental patterns of hair cortisol in male and female nonhuman primates: lower hair cortisol levels in vervet males emerge at puberty. Psychoneuroendocrinology. 2012;37(10):1736–9. doi: 10.1016/j.psyneuen.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sauve B, et al. Measurement of cortisol in human hair as a bio-marker of systemic exposure. Clin Invest Med. 2007;30(5):E183–91. doi: 10.25011/cim.v30i5.2894. [DOI] [PubMed] [Google Scholar]
- 22.Li J, et al. Time course of cortisol loss in hair segments under immersion in hot water. Clin Chim Acta. 2012;413(3-4):434–40. doi: 10.1016/j.cca.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 23.Webb E, T.S., Nelson A, White C, Koren G, Rieder M, Van Uum S. Assessing individual systemic stress through cortisol analysis of archaeological hair. Journal of Archaeological Science. 2010;37(4):807–812. [Google Scholar]
- 24.Hamel AF, et al. Effects of shampoo and water washing on hair cortisol concentrations. Clin Chim Acta. 2011;412(3-4):382–5. doi: 10.1016/j.cca.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loussouarn G. African hair growth parameters. Br J Dermatol. 2001;145(2):294–7. doi: 10.1046/j.1365-2133.2001.04350.x. [DOI] [PubMed] [Google Scholar]