Abstract
Renal and gastrointestinal diseases affect a significant portion of the general population. The process of decision making regarding surgical clearance and pre-operative management of the various complexities and medical conditions associated with these diseases hence becomes crucial. To optimize postoperative outcomes, the considerations for the care of this patient population revolve around effective management of hemostasis and electrolyte status. This subset of conditions is uniquely important with regard to the negative impact of improper administration of medications and perioperative care on patients’ prognoses. A thorough understanding and knowledge of standards of care and treatment guidelines for patients with renal dysfunction and gastrointestinal disease assures comprehensive preoperative planning and surgical clearance. This may ultimately lead to improvement of surgical outcomes and potential decrease in postoperative morbidity and mortality.
Keywords: Renal disease, gastrointestinal disease, preoperative, medical clearance, joint replacement
Introduction
Renal diseases as well as gastro-intestinal disease affect a large portion of orthopedics patients, requiring additional understanding of the intricacies involved in their care. Chronic kidney disease (CKD) is a progressive disorder that typically results from glomerulonephritis, diabetes mellitus and hypertension, with nearly 75% of CKD diagnoses caused by these conditions1–4. Acute kidney injury (AKI), a milder decrease in renal function, is often linked to iatrogenic causes such as perioperative anesthesia and medications. Estimates of prevalence for AKI suggest that it may affect 1% of all hospitalized patients, and it is a well-documented independent predictor of poor health outcomes5–7. Gastrointestinal diseases encompass a wide spectrum of conditions. Liver cirrhosis affects up to 1% of the U.S. population while annual incidence of irritable bowel disease (IBD) reaches 29/100,000 per year8–10. Consideration and knowledge of the major co-morbidities associated with the disease processes of kidney dysfunction and gastrointestinal disease and their respective medical treatments are key components of pre-operative medical clearance of orthopaedic patients.
An ample knowledge of the disease manifestation and treatment guidelines for patients with gastrointestinal and renal pathologies would yield a substantial decrease in postoperative morbidity and mortality. Both conditions, in particular the renal system, have a preponderance of established literature detailing the disease origins, variations, and modalities of care. Vigilant evaluation of markers for disease management and prudent perioperative management of medication dosing are paramount. In this review we provide recommendations and considerations for the care of patients with renal and gastrointestinal conditions.
Renal Considerations
Chronic Conditions
CKD is a progressive disorder defined as a glomerular filtration rate (GFR) <60 mL/min per 1.73 m2, which represents a loss of half or more of the normal adult renal function level. The National Kidney Foundation (NKF) provides thorough recommendations for both proper disease evaluation and classification – details of which exceed the scope of this review 11,12. Progression of CKD leads to kidney failure and end stage renal disease (ESRD), defined by the NKF as CKD with a GFR of less than 15 mL/min per 1.73 m2 11. Both CKD and ESRD are predictive of prolonged hospital stay and increased all-cause mortality following surgery13–20. This population is predisposed to multiple possible peri- and post-operative complications, due to the disease process itself, its treatment and the associated comorbidities. These include endothelial dysfunction and hypercoagulability, elevated serum homocysteine, microalbuminuria, and accelerated vascular calcification due to deficient mineral metabolism16,18,19.
Advanced stage renal disease is an independent risk factor for increased post-operative cardiovascular mortality and morbidity15. A longstanding correlation exists between patients treated with prolonged renal replacement therapy and cardiovascular complications21,22. Patients with CKD (all stages) with superimposed cardiovascular disease (CVD) are up to 10 times more likely to die before even reaching a classification of ESRD and can expect their CVD to progress at twice the normal rate when compared to patients with CVD only 23.
Several cardiovascular considerations should be highlighted in CKD patients. Atherosclerosis is a common form of arterial vascular disease seen more extensively in patients with renal dysfunction. It may manifest as ischemic heart disease (angina), myocardial infarction, cerebrovascular disease, peripheral vascular disease, or congestive heart failure24. In 2011, the prevalence of CHF reached up to 31.2% among all Medicare CKD patients4.
The prevalence of atrial fibrillation (AFIB) in the CKD population is as high as 25%4. Uremia, by interfering with the autonomic nervous system and affecting baroreceptor function, predisposes to a higher risk for the development of arrhythmias and AFIB 25. This leads eventually to an increased risk of thromboembolic events.
CKD patients are noted to have a high incidence of concomitant hypertension. In 2011, as many as 63% of these patients were receiving angiotensin converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB) and up to 47% received beta blockers, as a pharmacotherapy to control their blood pressure and renal disease progression 26. Many anaesthetic agents induce peripheral vasodilation and cardiac depression. In patients maintained on ACEI or ARB perioperatively, this will accentuate the risks for hypotension and subsequent renal hypoperfusion, leading to a further potential intraoperative renal insult27. Craig et al. indicate that perioperative development of hypotension relative to preoperative levels is a risk for intolerable further renal damage 28. They recommend individualized treatment of hypotension as opposed to the absolute stringent measure of <90 mm Hg 28.
The renal system plays a pivotal role in hematopoiesis. Through the synthesis of erythropoietin, the kidneys drive differentiation and proliferation of erythrocyte progenitor cells and thus control erythrocyte concentration 29,30. It is estimated that up to 18% of stage 3 patients (GFR <30 mL/min/1.73 m2) and nearly 60% of those in stages 4–5 (GFR <30 mL/min/1.73 m2) are anemic31. Epoetin alfa, approved in 1989, was the first erythropoiesis-stimulating agent (ESA) developed for the treatment of anemia in CKD patients 32. Anemia is common in the setting of orthopaedic surgery, and the condition confers increased risk of blood transfusion and peri-and postoperative morbidity and mortality 33. Anemia in CKD (but not CKD alone) was associated with a higher risk of blood transfusion, increased length of hospital stay, periprosthetic joint infection, and increased incidence of 30-day readmission in orthopaedic patients 34–37. A significant risk for infection is associated with blood transfusion38. Of particular interest, more restrictive transfusion strategies in orthopaedic patients had significantly reduced risk ratios for the development of healthcare-associated infections38–44.The Kidney Disease Outcomes Quality Initiative (KDOQI) and Network for Advancement of Transfusion Alternatives (NATA) both recommend treatment of anemia with ESA33. This algorithm proved to reduce the transfusion requirements in CKD patients. However, the safety and efficacy of this medication is being questioned in recent trials. The CHOIR study showed that treatment of anemic non-dialysis CKD patients to Hb of 13.5 g/dL greatly increased the risk of cardiovascular complications and death compared with Hb levels of 11.3 g/dL45. In another study, Pfeffer et al. demonstrated that ESA use significantly raised incidence of stroke46. In addition, a rise in risks of cardiovascular accidents has also been shown in more recent trials47,48. The risks and benefits of administration of these drugs for patients with CKD-associated anemia should be individualized and further investigated32,45–50.
Dialysis and transplantation are the final recourse when renal failure has progressed beyond the point of independent functionality. Dialysis-dependent patients have longer hospital stays, higher surgical and postoperative complications, and mortality rates when compared to those not requiring intervention4,15,51–56. Dialysis patients with superimposed DM and hypertension are predisposed to even higher risks52. Of particular significance, ESRD patients on dialysis or those who received kidney transplant are at a greater risk for complications following total hip arthroplasty (THA) or total knee arthroplasty (TKA)57–62. Renal impairment is proposed as an independent risk factor for both periprosthetic joint infection (PJI) in TKA and elevated 90-day readmission risk in THA63,64.
Frequent complications are reported among patients on chronic hemodialysis undergoing hip fracture repair. In this patient population, Karaeminogullari et al. reported a significant correlation between length of hemodialysis and postoperative mortality rates54. The recommended treatment for these patients with intra-trochanteric and non-displaced femoral neck fractures is osteosynthesis, while hemiarthroplasty is indicated for those with displaced femoral neck fracture54. THA for transplant patients and those on dialysis are associated with high mortality rates, and should be reserved for those who demonstrate a considerable potential for positive post-operative outcomes58,65.
The current literature indicates that patients undergoing hemodialysis are at a greater risk for perioperative complications when compared to those receiving peritoneal dialysis. Patients on hemodialysis have a 31% higher incidence of hip fracture compared to those receiving peritoneal dialysis.56 When comparing bone lesions between these two patients population, it is noted that those on peritoneal dialysis present with low-turnover lesions, while those on hemodialysis regimen have high-turnover lesions 66. This observation can be in part explained by the significantly higher parathyroid hormone (PTH) levels in hemodialysis patients, representing increased risk of bone depletion and resorption66. In addition to osteodystrophy, steroid use in chronic hemodialysis patients may pose a further burden on bone deterioration67.
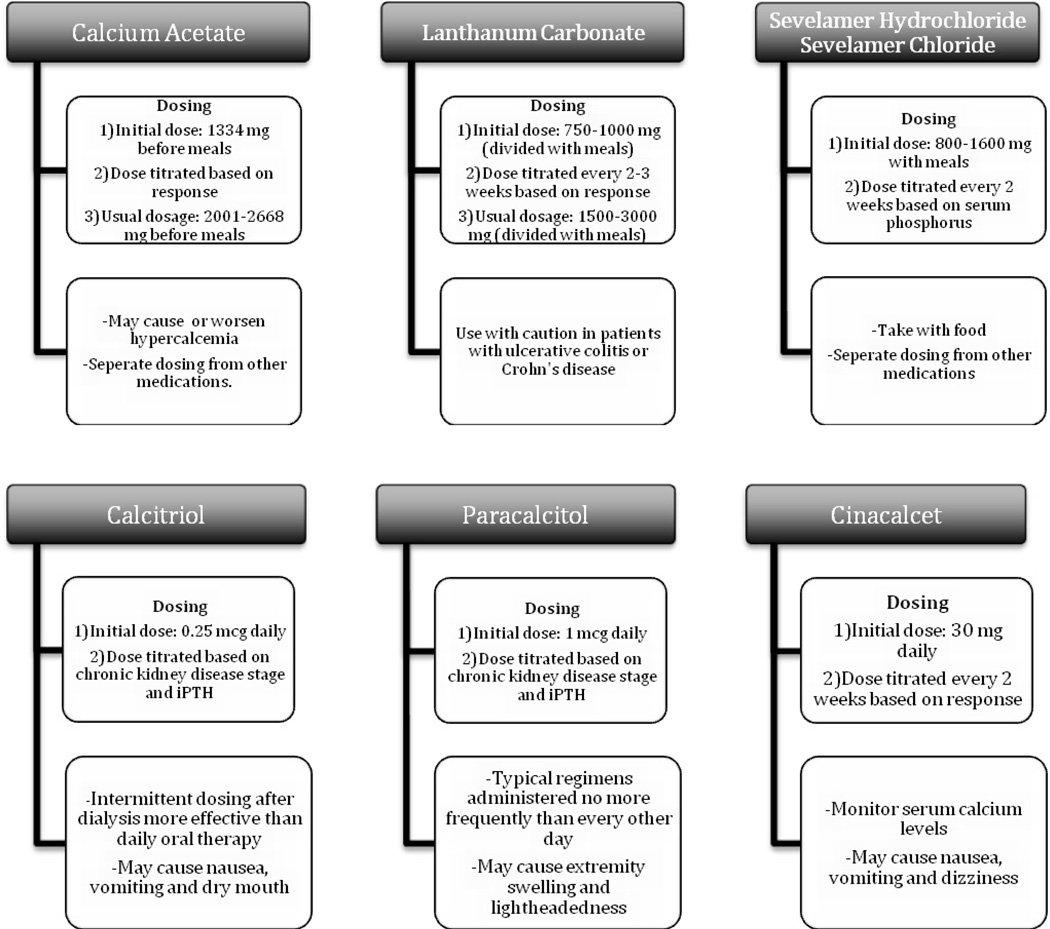
The management of bone mineral disturbances in these patients involves maintaining physiologic levels of PTH and stimulating vitamin D receptors68. The pharmacologic classes of available treatment options include phosphate binders, vitamin D analogs, and calcimimetics68 (Figure 1).
Figure 1.
Pharmacologic management of CKD-mineral bone disorder.
There is no reliable evidence that agents such as dopamine, diuretics, calcium channel blockers, ACEIs, N-acetyl cysteine (NAC), atrial natriuretic peptide (ANP), sodium bicarbonate, antioxidants, ESA, and selected hydration fluids exert any renal protective influence during surgery69. Unexplained preoperative decline in renal function merits postponement of the procedure and meticulous investigations towards identification of any possible insult reason and the condition rectified70. Maintaining the patient’s hydration and fluid balance remains the most effective method for preventing further renal damage71–73. The proper adjustment of drug dosages, taking into consideration the patient’s impaired renal function, must be considered in all stages of the surgical process74.
The Acute Setting
Acute kidney injury (AKI) is the rapid loss of renal function leading to a decrease in GFR and urine output, and an accompanying derangement of normal serum electrolyte levels. Several methods have been developed to classify acute kidney injury based on serum creatinine (SCr) levels, GFR, and urine output. The two most commonly used systems are the RIFLE and the Acute Kidney Injury Network (AKIN) classifications75. The RIFLE classification defines five stages of acute kidney injury (listed in Table 1). The AKIN criteria define AKI as a rapid (within 48 hours) decline in kidney function, and classify it into three stages (listed in Table 2).
Table 1.
RIFLE classification stages and criteria for defining AKI75
| Stage | GFR and SCr Criteria | Urine Output Criteria |
|---|---|---|
| Risk | SCr 1.5x baseline or GFR decrease >25% | <0.5 mL/kg/hr for 6 hours |
| Injury | SCr 2x baseline or GFR decrease >50% | <0.5 mL/kg/hr for 12 hours |
| Failure | SCr 3x baseline or GFR decrease >75% or SCr level ≥4 mg/dL | <0.3 mL/kg/hr for 24 hours or anuria for 12 hours |
| Loss | Complete loss of kidney function > 4 weeks | |
| End Stage Renal Disease | Complete loss of kidney function > 3 months | |
Table 2.
AKIN stages and criteria for defining AKI75
| Stage | SCr Criteria | Urine Output Criteria |
|---|---|---|
| Stage 1 | Increase in SCr >0.3 mg/dL or SCr 1.5–2x baseline | <0.5 mL/kg/hr for 6 hours |
| Stage 2 | SCr 2–3x baseline | <0.5 mL/kg/hr for 12 hours |
| Stage 3 | SCr >3x baseline, or SCr >4 mg/dL with an acute rise of >0.5 mg/dL, or receiving renal replacement therapy |
<0.3 mL/kg/hr for 24 hours or anuria for 12 hours |
The incidence of AKI reaches up to 5% of all hospitalized patients with approximately 30–40% of all cases occurring in surgical patients5,76. This incidence varies with the type of surgery, with the highest being associated with cardiac surgery, vascular surgery, and liver transplantation7,27,75,77. A wide range of values have been reported for the incidence of AKI following orthopedic surgery, from as low as 0.55% following hip and knee arthroplasty, to as high as 16% following hip fracture surgery78,79. The average incidence of developing AKI following all orthopedic procedures has been reported to be approximately 9%7.
The causes of AKI can be classified as pre-renal, intrinsic, or post-renal. Pre-renal AKI is the result of hypoperfusion of the kidney, intrinsic AKI is the result of direct damage to the renal parenchyma, and post-renal AKI results from kidney outflow obstruction80. Multiple studies have identified risk factors for the development of AKI following orthopedic surgery, including, but not limited to, pre-existing kidney disease, heart disease, vascular disease, diabetes, and perioperative dehydration7,78,79,81. Another commonly identified risk factor contributing to the development of AKI is the use of nephrotoxic medications, such as non-steroidal anti-inflammatories (NSAIDs), ACEIs, ARBs, aminoglycoside antibiotics, and IV contrast dye7,27,75–77,79,80. Through the inhibition of prostaglandin synthesis, NSAIDs cause vasoconstriction of the glomerular afferent arteriole, resulting in hypoperfusion of the kidney75. Contrast material can cause renal vasoconstriction, similarly leading to hypoperfusion and AKI75. ACE inhibitors and ARBs decrease blood pressure and inhibit the kidney’s autoregulatory mechanisms, leading to decreased perfusion and potential renal injury75,82. The rates of AKI in orthopedic patients have been found to be significantly higher on ACE inhibitors or ARBs82. Certain medications, such as aminoglycosides, cause direct nephrotoxicity in large concentrations. This explains the higher rates of AKI witnessed with the use of gentamicin, an aminoglycoside, when compared to cephalosporins as surgical prophylaxis for orthopedic patients 75,83.
Acute kidney injury is often a self-limited disease, with reports of up to 82.5% of patients that developed AKI following orthopedic procedures returning to baseline pre-operative renal function. However, patients that returned to baseline renal function post AKI didn’t show significant difference in mortality rates when compared those with sustained renal injury, reflecting possible serious sequelae associated with the of AKI 7. The development of AKI following hip fracture was associated with a significantly increased cost, longer hospital stay, higher rate of acute, 30-day, and 120-day mortality, and post-operative complications7,79,81. A similar association is seen in patients developing AKI following total joint arthroplasty78. While not specific to orthopedic procedures, several other studies assessed the effects of AKI on the outcomes of non-cardiac surgical procedures. The consensus of these studies is that AKI leads to significantly higher rates of mortality, morbidity, and length of hospitalization following surgical procedures5,27,75–77,84. Thus, identification of patients at risk for AKI pre-operatively is critically important for ensuring optimal surgical outcomes.
When considering a patient for orthopedic surgery, the surgeon should identify patients at risk for developing AKI and minimize it by optimizing pre-, intra-, and post-operative conditions. The RIFLE and AKIN criteria define AKI using GFR, SCr, and urine output, so monitoring of these parameters post-operatively is important for early disease recognition. Serum creatinine, though, may take up to 48 hours to rise when at least 50% of a patient’s nephrons have been damaged 75,81. Several biomarkers are proposed as potential early indicators of AKI, such as cystatin C and neutrophil gelatinase-associated lipocalin (NGAL)27,75,76,81. However those are not routinely used, and a decrease in urine output, either intra- or post-operatively, remains the most useful early marker for AKI27,75,76,81. Several factors should be considered when identifying patients at risk for the development of AKI. First the recognition of risk factors for AKI development is important for identifying patients in need of close monitoring post-operatively79. Second, the type of orthopedic procedure appears to influence the risk of developing AKI, with the highest post-operative incidences generally reported following hip fracture surgery79,81. These procedures are associated with severe hemorrhage, and there is often a delay in fluid administration, which exacerbates the dehydration and might accelerate the decline in renal function85. The maintenance of adequate volume status and hydration perioperatively ensures a proper renal perfusion, which in turn decreases the risk of developing AKI7,27,75,77,84,85. Similarly, optimization of cardiac output, hemoglobin levels, and intra-operative blood pressure are other important factors involved in maintenance of renal perfusion and oxygenation7,27,75,77. Another measure to minimize the risk of renal damage is the discontinuation of any potentially nephrotoxic medications27,75,84. ACE inhibitors and ARBs should be discontinued on the day of surgery and not restarted until the postoperative renal function stabilizes at the baseline levels75. The use of NSAIDs should be cautious in high-risk patients, and avoided in hypovolemic ones75. The administration of IV contrast with proper hydration with an isotonic fluid minimizes the risk of damage to the kidneys75.
Urinary Tract Infections
A urinary tract infection is defined by the presence of urinary urgency, frequency, and dysuria in the presence of bacteria in the urine86. Asymptomatic bacteriuria, on the other hand, is the presence of bacteria in the urine, generally defined as more than 100,000 colony forming units (CFU)/mL, without any symptoms86. UTIs are the most common nosocomial complication after total joint arthroplasty87. The incidence of UTIs is reported to be between 0.7–2.4% following total hip and knee arthroplasties, and reaches up to be 15% in patients with severe urinary retention88. The incidence of UTIs following all orthopedic procedures is approximately 10%87.
The single most important risk factor for the development of a UTI is the presence of a urinary catheter87. The risk increases by 5–10% for every day of catheterization after 48 hours, and the median time to onset of a UTI is 6.5 days87,89. Patients with a urinary catheter for more than two days following surgery are substantially more likely to develop a UTI than those with a catheter for less than two days, and policies to minimize the length of post-operative catheterization following orthopedic procedures have been shown to lead to significantly lower rates of UTIs87,89. Urinary retention is also a major risk factor for the development of UTIs, and the use of an indwelling catheter for a short duration actually leads to fewer UTIs when compared to a straight catheter on an as-needed basis or not treating the retention86.
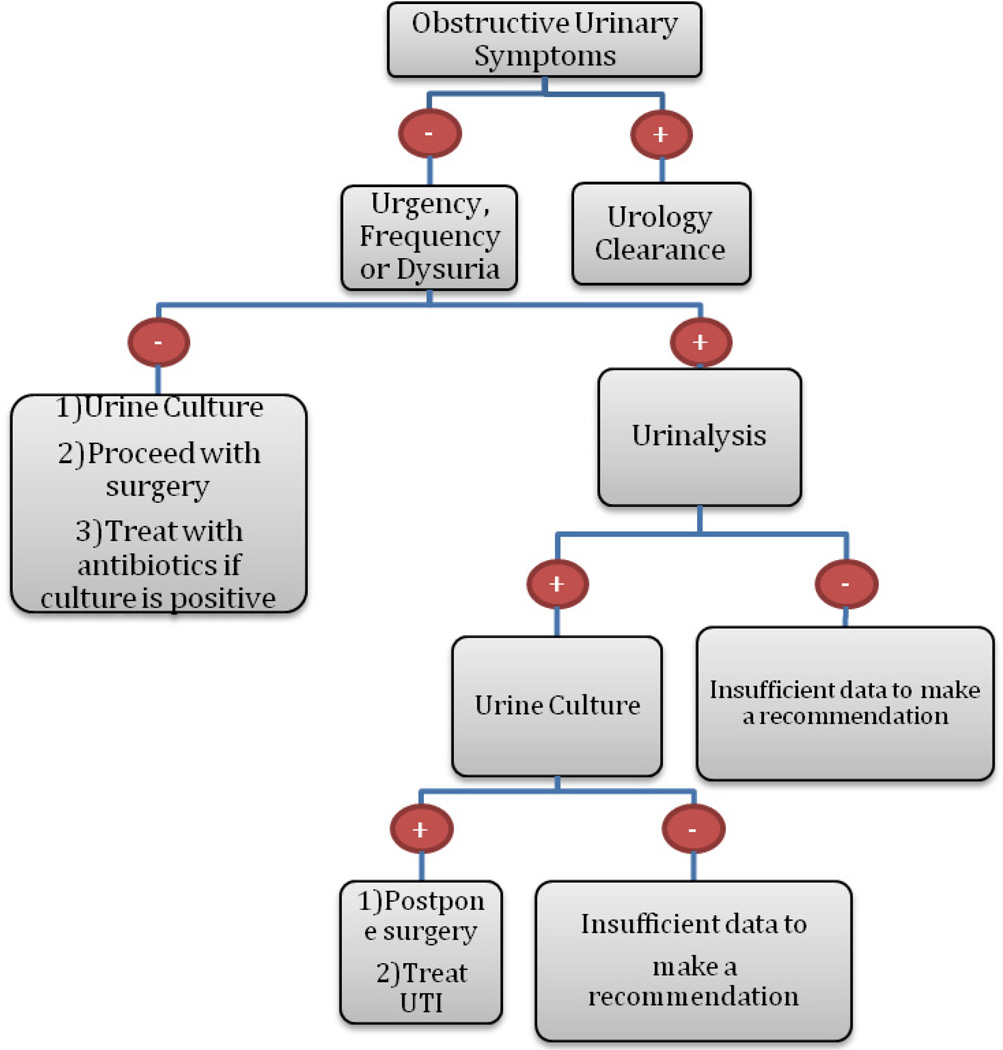
Currently, no consensus exists on the need to treat UTIs prior to orthopedic surgery, or on the relationship of perioperative UTIs to complications following orthopedic procedures90. Several studies have concluded that UTIs are associated with the development of postoperative complications, such as wound infections, delayed wound healing, joint infection following total joint arthroplasty, and the need for earlier revisions following arthroplasty86,91,92. Other studies, however, have found no relationship between perioperative UTIs and the risk of wound infection following arthroplasty90. The testing for and treatment of asymptomatic bacteriuria is unwarranted, due to the lack of association with post-operative complications 88,93. An algorithm has been developed for pre-operative clearance of patients with urinary symptoms for orthopedic surgery (Figure 2)86. However, given more recent data calling into question the association of UTIs with post-operative complications and the unnecessary need to treat asymptomatic bacteriuria, the proposed algorithm requires further investigation to determine its validity.
Figure 2.
Algorithm for pre-operative clearance for orthopedic surgery for patients with urinary symptoms
Gastrointestinal Considerations
Inflammatory Bowel Disease
The two main subtypes of inflammatory bowel diseases (IBD) are ulcerative colitis (UC) and Crohn's disease (CD), both chronic and relapsing 94,95. These conditions cause chronic debilitating pain and fatigue without noticeably affecting the mortality rates of the affected individuals9,10,96. The annual incidence of IBD in the United States and Northern Europe reaches up to 19/100,000 per year and 29/100,000 per year, respectively9,10.
In UC, inflammation of the gastrointestinal tract (GIT) involves the mucosal layer in a continuous pattern and is localized most commonly in the colon, cecum, and rectum94. Patients typically complain of abdominal cramping and bloody diarrhea mixed with pus or mucus94. CD is notorious for affecting the GIT in a skipping, non-continuous fashion, with transmural lesions that might involve any part of the tract.
The extra-intestinal manifestations of IBD, such as arthritis and hemostatic disturbances, contribute to significantly higher postoperative complications rates 97. Peripheral joint involvement constitutes the major arthritic complaint in IBD patients and is present in 4.5% of patients at time of initial IBD diagnosis, 12% at six years after diagnosis, and 30% at twenty years follow up98,99. While this is a non-erosive or deforming arthritis, up to 6% of IBD patients develop ankylosing spondylitis that progressively leads to spinal fusion99. These are important considerations for surgical clearance as they may impede the postoperative rehabilitation course.
The chronic blood loss from GI bleeding, with a superimposed chronic inflammatory state, predisposes IBD patients to anemia that might be severe enough to require blood transfusions95,100,101. It is important to note that CD patients may be even at a higher risk of anemia due to duodenal inflammation and the subsequent iron malabsorption and deficiency102. Preoperative autologous blood transfusions, although associated with a lower transfusion-induced red cell alloimmunization, have been shown to decrease preoperative hemoglobin levels in patients donating 4 weeks prior to surgery 103–105. Hence, providing parenteral iron replacement prior to orthopaedic procedures may remain the intervention of choice to minimize anemia-associated complications102,106. The administration of 900 mg intravenous iron sucrose through a 2–3 weeks period prior to surgical procedures shows an optimal increase of hemoglobin levels in these anemic patients106.
The potential hemostatic instability is further pronounced in IBD patients by the increased platelet activation due to the inflammation-induced hyper-coagulable state, predisposing patients to a higher risk of thromboembolic complications107,108. This occurs in roughly one-third of IBD patients and increases their likelihood of acquiring a deep venous thrombosis (DVT) postoperatively107–109. While NSAIDs are typically contraindicated in these patients due to the preexisting GI damage, heparin and low molecular weight heparin can be safely administered as a DVT prophylactic measure110.
The higher post-operative failure rates, witnessed in IBD patients undergoing orthopedic procedures, might be explained by the interference of the disease process with osseous integration and subsequent weakening of the implant-integrity area 97. The chronic inflammatory state associated with the disease, may trigger osteoclasts and osteoblasts, through cytokine-mediated pathways, and possibly affect bone resorbtion 97,111–114. Nutritional and hormonal deficits might also considerably contribute to these complications114. The noticeable increase in GI nutritional losses and decrease in absorptive potential can lead to multiple vitamins, minerals, proteins, and fat deficiencies115–119. The predominance of a suboptimal nutritional status among IBD patients might lead to an increase in post-operative infection rate, a decreased wound and bone healing, and eventually affecting surgical outcomes and morbidity114,120–123.
In addition to the disease process itself, the pharmacologic management of IBD may also contribute to these complications124. TNF-α is a macrophage-derived cytokine that mediates inflammation, making it an ideal target in the treatment of autoimmune disorders such as IBD125. TNF-α inhibitors are an effective treatment for both CD and UC126. They induce remission of the disease, and hence improve quality of life and reduce IBD related hospitalizations126. Since TNF-α plays a protective role in the physiologic immune response, patients on TNF-α inhibitors regimen have a higher risk of developing serious and opportunistic infections127–132. Some studies have shown no increase in postoperative complication rates in UC or CD patients treated with this class of medications 125. However, due to the potential increased risk of infections, The Club Rheumatismes et Inflammation (CTI) presented perioperative guidelines for TNF-α inhibitor administration based on the pharmacological half-lives133. Etanercept should be discontinued one week prior to surgery, while a duration of four weeks between surgery and the last dose of adalimumab and infliximab is recommended133. Since TNF- α inhibitors slow the wound healing process, they should only be resumed after two weeks of appropriate wound healing134,135.
The treatment with glucocorticoids is a common option for IBD patients125,136. However, prolonged use of glucocorticoids compromises the immune system, decreases bone quality, increases the risk of osteonecrosis, and impairs wound healing137–144. To avoid the acute adrenal insufficiency associated with the abrupt discontinuation of chronic glucocorticoids treatment, and concurrently minimize the associated postoperative complications, patients should be maintained on a physiologic dose of glucocorticoids and then taper up to preoperative doses following the procedure125,141,145.
Other notable medications used for IBD treatment are methotrexate (MTX) and azathioprine 125. MTX administration in the perioperative period is not associated with a higher risk of complications, and hence can be safely continued in IBD patients undergoing surgical procedures125,146–148. However, MTX should be withheld 1 week preoperatively and at least 1–2 weeks postoperatively in patients with compromised renal function, due to their higher risks of toxicity149–151. Azathioprine and 6-mercaptopurine are widely used as glucocorticoid sparing agents in IBD, with no significant effects on surgical outcomes or morbidity, and thus, can be only held on the day of surgery and resumed within 36 hours as long as renal function is not impaired125.
Liver Cirrhosis
Cirrhosis of the liver is the result of various chronic liver diseases that start with the damage and necrosis of hepatic cells, causing regenerative nodules which become surrounded by diffuse fibrous bands, leading to portal hypertension and end stage liver disease8,152. The prevalence of cirrhosis in the United States is estimated to be 0.15% or 400,000; however, a more reasonable estimate is approximately 1% of the population due to the undiagnosed viral hepatitis and undetected cirrhosis8. Chronic viral infections such as the hepatitis B virus (HBV) and hepatitis C virus (HCV), alongside with excessive alcohol consumption, play the major role in causing cirrhosis 153,154.
With a wide spectrum of metabolic disturbances, liver cirrhosis compromises multiple physiologic processes. The disease is associated with a state of impaired immunity, coagulopathy, malnutrition, and metabolic bone disease155–161. With a decreased neutrophil mobilization and phagocytic activity, and lower levels of IgM, IgG, and IgA antibodies, cirrhotic patients have a lower bacterial opsonization capacity and are predisposed to more overwhelming infections 162–164. Secondary to vitamin D and calcium malabsorption, these patients have a reduced bone mineral density and new bone formation, and hence are more prone to develop osteomalacia and osteoporosis 165. Thus, screening all cirrhosis patients with bone scans may be necessary to provide insight and prevent further bone disease161.
Patients with liver cirrhosis are at an increased risk of perioperative complications with high morbidity and mortality rates seen in abdominal and non-abdominal surgeries166–174. These patients have an increased rate of bacterial infection that may be due to impaired phagocytic function and other immune deficiencies160,175,176. The Child-Turcotte-Pugh (CTP) score has been the standard for assessing operative risks in cirrhosis patients for years, utilizing patient albumin and bilirubin serum levels, pro-thrombin time, and severity of ascites and encephalopathy levels177,178. This assessment tool places a patient in a class based on the severity of the cirrhosis with A being lowest and C being most severe178. Hsieh et al. reported a 26.7% complication rate in patients with cirrhosis undergoing total hip arthroscopies (THA) 155. The patients found to be in class B or C of the CTP test were significantly more likely to have complications (52.9%) than those in class A(10.2%)155. This study found that advanced age, elevated creatinine, decreased albumin, increased operative blood loss, decreased platelet count, encephalopathy and ascites were associated with a higher perioperative morbidity, consistent with the variables tested with the CTP test155. Similarly, total knee arthroplasty (TKA), total hip arthroplasty (THA) and lumbar surgery in class B and C cirrhosis patients were at a significantly higher morbidity, mortality, and complication rates179–182. While orthopedic procedures may be safely performed in CTP class A cirrhosis patients, cautious evaluation and determination of potential risks versus benefits of surgical intervention should be sought in those with more severe levels of cirrhosis (B and C).
Conclusion
With high prevalence and incidence rates within the general population, renal and gastrointestinal diseases affect a significant portion of orthopedic patients. The acute and chronic kidney function deteriorations, inflammatory bowel disease and liver cirrhosis impose hemostatic and electrolyte imbalances that require specific management. When clearing those patients for surgical procedures, the outcome-affecting factors related to the disease process itself and the medications implicated in these conditions treatment should be thoroughly evaluated and revisited.
A comprehensive understanding of these conditions and their associated comorbidities will lead to considerable improvements in perioperative medical management and postoperative outcomes. These interventions have the potential to substantially decrease surgery-associated morbidity and mortality.
Footnotes
Conflict of interest: No conflicts declared.
References
- 1.Kuo HW, Tsai SS, Tiao MM, Liu YC, Lee IM, Yang CY. Analgesic use and the risk for progression of chronic kidney disease. Pharmacoepidemiology and drug safety. 2010 Jul;19(7):745–751. doi: 10.1002/pds.1962. [DOI] [PubMed] [Google Scholar]
- 2.Lin JL, Lin-Tan DT, Hsu KH, Yu CC. Environmental lead exposure and progression of chronic renal diseases in patients without diabetes. The New England journal of medicine. 2003 Jan 23;348(4):277–286. doi: 10.1056/NEJMoa021672. [DOI] [PubMed] [Google Scholar]
- 3.Collins AJ, Foley RN, Chavers B, et al. 'United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012 Jan;59(1 Suppl 1):A7, e1–e420. doi: 10.1053/j.ajkd.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Renal Data System UADRAoCKDaE-SRDitUS. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2013. [Google Scholar]
- 5.Kheterpal S, Tremper KK, Englesbe MJ, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology. 2007 Dec;107(6):892–902. doi: 10.1097/01.anes.0000290588.29668.38. [DOI] [PubMed] [Google Scholar]
- 6.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. Journal of the American Society of Nephrology : JASN. 2005 Nov;16(11):3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 7.Kateros K, Doulgerakis C, Galanakos SP, Sakellariou VI, Papadakis SA, Macheras GA. Analysis of kidney dysfunction in orthopaedic patients. BMC nephrology. 2012;13:101. doi: 10.1186/1471-2369-13-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008 Mar 8;371(9615):838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ponder A, Long MD. A clinical review of recent findings in the epidemiology of inflammatory bowel disease. Clinical epidemiology. 2013;5:237–247. doi: 10.2147/CLEP.S33961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004 May;126(6):1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 11.Andrew S, MD PhD, Levey MJC, Ethan Balk, MD PhD, Annamaria T. Kausz, MD MS, Adeera Levin, MD, Michael WSteffes, MD PhD, Ronald J. Hogg, MD, Ronald D. Perrone, MD, Joseph Lau, MD, Garabed Eknoyan., MD National Kidney Foundation Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. Annals of Internal Medicine. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 12.National Kidney Foundation. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 update. American Journal of Kidney Diseases. 2012;60(5):850–886. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Shadman R, Allison MA, Criqui MH. Glomerular filtration rate and N-terminal pro-brain natriuretic peptide as predictors of cardiovascular mortality in vascular patients. Journal of the American College of Cardiology. 2007 Jun 5;49(22):2172–2181. doi: 10.1016/j.jacc.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 14.Damman K, Navis G, Voors AA, et al. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. Journal of cardiac failure. 2007 Oct;13(8):599–608. doi: 10.1016/j.cardfail.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Mathew A, Devereaux PJ, O'Hare A, et al. Chronic kidney disease and postoperative mortality: a systematic review and meta-analysis. Kidney international. 2008 May;73(9):1069–1081. doi: 10.1038/ki.2008.29. [DOI] [PubMed] [Google Scholar]
- 16.Adams MJ, Irish AB, Watts GF, Oostryck R, Dogra GK. Hypercoagulability in chronic kidney disease is associated with coagulation activation but not endothelial function. Thrombosis research. 2008;123(2):374–380. doi: 10.1016/j.thromres.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 17.Wannamethee SG, Shaper AG, Lowe GD, Lennon L, Rumley A, Whincup PH. Renal function and cardiovascular mortality in elderly men: the role of inflammatory, procoagulant, and endothelial biomarkers. European heart journal. 2006 Dec;27(24):2975–2981. doi: 10.1093/eurheartj/ehl402. [DOI] [PubMed] [Google Scholar]
- 18.Jager A, Kostense PJ, Nijpels G, et al. Serum homocysteine levels are associated with the development of (micro)albuminuria: the Hoorn study. Arteriosclerosis, thrombosis, and vascular biology. 2001 Jan;21(1):74–81. doi: 10.1161/01.atv.21.1.74. [DOI] [PubMed] [Google Scholar]
- 19.Raggi P, Boulay A, Chasan-Taber S, et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? Journal of the American College of Cardiology. 2002 Feb 20;39(4):695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 20.Ackland GL, Moran N, Cone S, Grocott MP, Mythen MG. Chronic kidney disease and postoperative morbidity after elective orthopedic surgery. Anesthesia and analgesia. 2011 Jun;112(6):1375–1381. doi: 10.1213/ANE.0b013e3181ee8456. [DOI] [PubMed] [Google Scholar]
- 21.Lindner A, Charra B, Sherrard DJ, Scribner BH. Accelerated atherosclerosis in prolonged maintenance hemodialysis. The New England journal of medicine. 1974 Mar 28;290(13):697–701. doi: 10.1056/NEJM197403282901301. [DOI] [PubMed] [Google Scholar]
- 22.Ryan TP, Fisher SG, Elder JL, et al. Increased cardiovascular risk associated with reduced kidney function. American journal of nephrology. 2009;29(6):620–625. doi: 10.1159/000194455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins AJ, Li S, Gilbertson DT, Liu J, Chen SC, Herzog CA. Chronic kidney disease and cardiovascular disease in the Medicare population. Kidney international. Supplement. 2003 Nov;(87):S24–S31. doi: 10.1046/j.1523-1755.64.s87.5.x. [DOI] [PubMed] [Google Scholar]
- 24.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003 Oct 28;108(17):2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 25.Savica V, Musolino R, Di Leo R, Santoro D, Vita G, Bellinghieri G. Autonomic dysfunction in uremia. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2001 Oct;38(4 Suppl 1):S118–S121. doi: 10.1053/ajkd.2001.27418. [DOI] [PubMed] [Google Scholar]
- 26.Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern? Archives of internal medicine. 2000 Mar 13;160(5):685–693. doi: 10.1001/archinte.160.5.685. [DOI] [PubMed] [Google Scholar]
- 27.Sear JW. Kidney dysfunction in the postoperative period. British journal of anaesthesia. 2005 Jul;95(1):20–32. doi: 10.1093/bja/aei018. [DOI] [PubMed] [Google Scholar]
- 28.Craig RG, Hunter JM. Recent developments in the perioperative management of adult patients with chronic kidney disease. British journal of anaesthesia. 2008 Sep;101(3):296–310. doi: 10.1093/bja/aen203. [DOI] [PubMed] [Google Scholar]
- 29.Krantz SB. Erythropoietin. Blood. 1991 Feb 1;77(3):419–434. [PubMed] [Google Scholar]
- 30.Valliant A, Hofmann RM. Managing dialysis patients who develop anemia caused by chronic kidney disease: focus on peginesatide. International journal of nanomedicine. 2013;8:3297–3307. doi: 10.2147/IJN.S44944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foley RN, Wang C, Collins AJ. Cardiovascular risk factor profiles and kidney function stage in the US general population: the NHANES III study. Mayo Clinic proceedings. 2005 Oct;80(10):1270–1277. doi: 10.4065/80.10.1270. [DOI] [PubMed] [Google Scholar]
- 32.Coyne DW. Anemia in chronic kidney disease treating the numbers, not the patients. JAMA internal medicine. 2014 May;174(5):708–709. doi: 10.1001/jamainternmed.2013.13305. [DOI] [PubMed] [Google Scholar]
- 33.Goodnough LT, Maniatis A, Earnshaw P, et al. Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. British journal of anaesthesia. 2011 Jan;106(1):13–22. doi: 10.1093/bja/aeq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graves A, Yates P, Hofmann AO, Farmer S, Ferrari P. Predictors of perioperative blood transfusions in patients with chronic kidney disease undergoing elective knee and hip arthroplasty. Nephrology. 2014 Jul;19(7):404–409. doi: 10.1111/nep.12239. [DOI] [PubMed] [Google Scholar]
- 35.Kendoff D, Tomeczkowski J, Fritze J, Gombotz H, von Heymann C. [Preoperative anemia in orthopedic surgery: clinical impact, diagnostics and treatment] Der Orthopade. 2011 Nov;40(11):1018–1020. 1023–1015, 1027–1018. doi: 10.1007/s00132-011-1789-3. [DOI] [PubMed] [Google Scholar]
- 36.Greenky M, Gandhi K, Pulido L, Restrepo C, Parvizi J. Preoperative anemia in total joint arthroplasty: is it associated with periprosthetic joint infection? Clinical orthopaedics and related research. 2012 Oct;470(10):2695–2701. doi: 10.1007/s11999-012-2435-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seicean A, Alan N, Seicean S, Neuhauser D, Weil RJ. The effect of blood transfusion on short-term, perioperative outcomes in elective spine surgery. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2014 Sep;21(9):1579–1585. doi: 10.1016/j.jocn.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Rohde JM, Dimcheff DE, Blumberg N, et al. Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA : the journal of the American Medical Association. 2014 Apr 2;311(13):1317–1326. doi: 10.1001/jama.2014.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carson JL, Terrin ML, Barton FB, et al. A pilot randomized trial comparing symptomatic vs. hemoglobin-level-driven red blood cell transfusions following hip fracture. Transfusion. 1998 Jun;38(6):522–529. doi: 10.1046/j.1537-2995.1998.38698326331.x. [DOI] [PubMed] [Google Scholar]
- 40.Grover M, Talwalkar S, Casbard A, et al. Silent myocardial ischaemia and haemoglobin concentration: a randomized controlled trial of transfusion strategy in lower limb arthroplasty. Vox sanguinis. 2006 Feb;90(2):105–112. doi: 10.1111/j.1423-0410.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- 41.Foss NB, Kristensen MT, Jensen PS, Palm H, Krasheninnikoff M, Kehlet H. The effects of liberal versus restrictive transfusion thresholds on ambulation after hip fracture surgery. Transfusion. 2009 Feb;49(2):227–234. doi: 10.1111/j.1537-2995.2008.01967.x. [DOI] [PubMed] [Google Scholar]
- 42.So-Osman C, Nelissen R, Te Slaa R, Coene L, Brand R, Brand A. A randomized comparison of transfusion triggers in elective orthopaedic surgery using leucocyte-depleted red blood cells. Vox sanguinis. 2010 Jan;98(1):56–64. doi: 10.1111/j.1423-0410.2009.01225.x. [DOI] [PubMed] [Google Scholar]
- 43.Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. The New England journal of medicine. 2011 Dec 29;365(26):2453–2462. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carson JL, Altman DG, Duff A, et al. Risk of bacterial infection associated with allogeneic blood transfusion among patients undergoing hip fracture repair. Transfusion. 1999 Jul;39(7):694–700. doi: 10.1046/j.1537-2995.1999.39070694.x. [DOI] [PubMed] [Google Scholar]
- 45.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. The New England journal of medicine. 2006 Nov 16;355(20):2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 46.Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. The New England journal of medicine. 2009 Nov 19;361(21):2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 47.Briet M, Barhoumi T, Mian MO, et al. Effects of recombinant human erythropoietin on resistance artery endothelial function in stage 4 chronic kidney disease. Journal of the American Heart Association. 2013 Apr;2(2):e000128. doi: 10.1161/JAHA.113.000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCullough PA, Barnhart HX, Inrig JK, et al. Cardiovascular toxicity of epoetin-alfa in patients with chronic kidney disease. American journal of nephrology. 2013;37(6):549–558. doi: 10.1159/000351175. [DOI] [PubMed] [Google Scholar]
- 49.Coyne DW. It’s time to compare anemia management strategies in hemodialysis. Clinical journal of the American Society of Nephrology : CJASN. 2010 Apr;5(4):740–742. doi: 10.2215/CJN.02490409. [DOI] [PubMed] [Google Scholar]
- 50.Coyne DW. The health-related quality of life was not improved by targeting higher hemoglobin in the Normal Hematocrit Trial. Kidney international. 2012 Jul;82(2):235–241. doi: 10.1038/ki.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Jager DJ, Grootendorst DC, Jager KJ, et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA : the journal of the American Medical Association. 2009 Oct 28;302(16):1782–1789. doi: 10.1001/jama.2009.1488. [DOI] [PubMed] [Google Scholar]
- 52.Cherng YG, Liao CC, Chen TH, Xiao D, Wu CH, Chen TL. Are non-cardiac surgeries safe for dialysis patients? - A population-based retrospective cohort study. PloS one. 2013;8(3):e58942. doi: 10.1371/journal.pone.0058942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gajdos C, Hawn MT, Kile D, Robinson TN, Henderson WG. Risk of major nonemergent inpatient general surgical procedures in patients on long-term dialysis. JAMA surgery. 2013 Feb;148(2):137–143. doi: 10.1001/2013.jamasurg.347. [DOI] [PubMed] [Google Scholar]
- 54.Karaeminogullari O, Demirors H, Sahin O, Ozalay M, Ozdemir N, Tandogan RN. Analysis of outcomes for surgically treated hip fractures in patients undergoing chronic hemodialysis. The Journal of bone and joint surgery. American volume. 2007 Feb;89(2):324–331. doi: 10.2106/JBJS.E.01320. [DOI] [PubMed] [Google Scholar]
- 55.McCleery MA, Leach WJ, Norwood T. Rates of infection and revision in patients with renal disease undergoing total knee replacement in Scotland. The Journal of bone and joint surgery. British volume. 2010 Nov;92(11):1535–1539. doi: 10.1302/0301-620X.92B11.23870. [DOI] [PubMed] [Google Scholar]
- 56.Lin ZZ, Wang JJ, Chung CR, et al. Epidemiology and mortality of hip fracture among patients on dialysis: Taiwan National Cohort Study. Bone. 2014 Jul;64:235–239. doi: 10.1016/j.bone.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 57.Lieberman JR, Fuchs MD, Haas SB, et al. Hip arthroplasty in patients with chronic renal failure. The Journal of arthroplasty. 1995 Apr;10(2):191–195. doi: 10.1016/s0883-5403(05)80126-3. [DOI] [PubMed] [Google Scholar]
- 58.Sakalkale DP, Hozack WJ, Rothman RH. Total hip arthroplasty in patients on long-term renal dialysis. The Journal of arthroplasty. 1999 Aug;14(5):571–575. doi: 10.1016/s0883-5403(99)90079-7. [DOI] [PubMed] [Google Scholar]
- 59.Shrader MW, Schall D, Parvizi J, McCarthy JT, Lewallen DG. Total hip arthroplasty in patients with renal failure: a comparison between transplant and dialysis patients. The Journal of arthroplasty. 2006 Apr;21(3):324–329. doi: 10.1016/j.arth.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 60.Sunday JM, Guille JT, Torg JS. Complications of joint arthroplasty in patients with end-stage renal disease on hemodialysis. Clinical orthopaedics and related research. 2002 Apr;(397):350–355. doi: 10.1097/00003086-200204000-00040. [DOI] [PubMed] [Google Scholar]
- 61.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. The New England journal of medicine. 2004 Sep 23;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 62.Deegan BF, Richard RD, Bowen TR, Perkins RM, Graham JH, Foltzer MA. Impact of Chronic Kidney Disease Stage on Lower-extremity Arthroplasty. Orthopedics. 2014 Jul 1;37(7):e613–e618. doi: 10.3928/01477447-20140626-51. [DOI] [PubMed] [Google Scholar]
- 63.Bozic KJ, Lau E, Kurtz S, Ong K, Berry DJ. Patient-related risk factors for postoperative mortality and periprosthetic joint infection in medicare patients undergoing TKA. Clinical orthopaedics and related research. 2012 Jan;470(1):130–137. doi: 10.1007/s11999-011-2043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bozic KJ, Lau E, Kurtz S, et al. Patient-related risk factors for periprosthetic joint infection and postoperative mortality following total hip arthroplasty in Medicare patients. The Journal of bone and joint surgery. American volume. 2012 May 2;94(9):794–800. doi: 10.2106/JBJS.K.00072. [DOI] [PubMed] [Google Scholar]
- 65.Garcia-Ramiro S, Cofan F, Esteban PL, et al. Total hip arthroplasty in hemodialysis and renal transplant patients. Hip international : the journal of clinical and experimental research on hip pathology and therapy. 2008 Jan-Mar;18(1):51–57. doi: 10.1177/112070000801800110. [DOI] [PubMed] [Google Scholar]
- 66.Sherrard DJ, Hercz G, Pei Y, et al. The spectrum of bone disease in end-stage renal failure--an evolving disorder. Kidney international. 1993 Feb;43(2):436–442. doi: 10.1038/ki.1993.64. [DOI] [PubMed] [Google Scholar]
- 67.Nagoya S, Nagao M, Takada J, Kuwabara H, Kaya M, Yamashita T. Efficacy of cementless total hip arthroplasty in patients on long-term hemodialysis. The Journal of arthroplasty. 2005 Jan;20(1):66–71. doi: 10.1016/j.arth.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 68.Ruf KM, Clifford T. Management of chronic kidney disease-mineral bone disorder. Orthopedics. 2010 Feb;33(2):98–101. doi: 10.3928/01477447-20100104-20. [DOI] [PubMed] [Google Scholar]
- 69.Zacharias M, Mugawar M, Herbison GP, et al. Interventions for protecting renal function in the perioperative period. The Cochrane database of systematic reviews. 2013;9:CD003590. doi: 10.1002/14651858.CD003590.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kalamas AG, Niemann CU. Patients with chronic kidney disease. The Medical clinics of North America. 2013 Nov;97(6):1109–1122. doi: 10.1016/j.mcna.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 71.Brienza N, Giglio MT, Marucci M, Fiore T. Does perioperative hemodynamic optimization protect renal function in surgical patients? A meta-analytic study. Critical care medicine. 2009 Jun;37(6):2079–2090. doi: 10.1097/CCM.0b013e3181a00a43. [DOI] [PubMed] [Google Scholar]
- 72.Jarnberg PO. Renal protection strategies in the perioperative period. Best practice & research. Clinical anaesthesiology. 2004 Dec;18(4):645–660. doi: 10.1016/j.bpa.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 73.Tang IY, Murray PT. Prevention of perioperative acute renal failure: what works? Best practice & research. Clinical anaesthesiology. 2004 Mar;18(1):91–111. doi: 10.1016/j.bpa.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 74.Sakabe T, Imai R, Murata H, et al. Life expectancy and functional prognosis after femoral neck fractures in hemodialysis patients. Journal of orthopaedic trauma. 2006 May;20(5):330–336. doi: 10.1097/00005131-200605000-00006. [DOI] [PubMed] [Google Scholar]
- 75.Borthwick E, Ferguson A. Perioperative acute kidney injury: risk factors, recognition, management, and outcomes. BMJ. 2010;341:c3365. doi: 10.1136/bmj.c3365. [DOI] [PubMed] [Google Scholar]
- 76.Thakar CV. Perioperative acute kidney injury. Adv Chronic Kidney Dis. 2013 Jan;20(1):67–75. doi: 10.1053/j.ackd.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 77.Josephs SA, Thakar CV. Perioperative risk assessment, prevention, and treatment of acute kidney injury. Int Anesthesiol Clin. 2009;47(4):89–105. doi: 10.1097/AIA.0b013e3181b47e98. [DOI] [PubMed] [Google Scholar]
- 78.Jafari SM, Huang R, Joshi A, Parvizi J, Hozack WJ. Renal impairment following total joint arthroplasty: who is at risk? The Journal of arthroplasty. 2010 Sep;25(6 Suppl):49–53. 53, e41–e42. doi: 10.1016/j.arth.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 79.Bennet SJ, Berry OM, Goddard J, Keating JF. Acute renal dysfunction following hip fracture. Injury. 2010 Apr;41(4):335–338. doi: 10.1016/j.injury.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 80.Bushnell BD, Horton JK, McDonald MF, Robertson PG. Perioperative medical comorbidities in the orthopaedic patient. J Am Acad Orthop Surg. 2008 Apr;16(4):216–227. doi: 10.5435/00124635-200804000-00005. [DOI] [PubMed] [Google Scholar]
- 81.Ulucay C, Eren Z, Kaspar EC, et al. Risk factors for acute kidney injury after hip fracture surgery in the elderly individuals. Geriatr Orthop Surg Rehabil. 2012 Dec;3(4):150–156. doi: 10.1177/2151458512473827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med. 2014 May;9(5):283–288. doi: 10.1002/jhm.2155. [DOI] [PubMed] [Google Scholar]
- 83.Bell S, Davey P, Nathwani D, et al. Risk of AKI with Gentamicin as Surgical Prophylaxis. J Am Soc Nephrol. 2014 May; doi: 10.1681/ASN.2014010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jones DR, Lee HT. Surgery in the patient with renal dysfunction. Anesthesiol Clin. 2009 Dec;27(4):739–749. doi: 10.1016/j.anclin.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 85.Lewis JR, Hassan SK, Wenn RT, Moran CG. Mortality and serum urea and electrolytes on admission for hip fracture patients. Injury. 2006 Aug;37(8):698–704. doi: 10.1016/j.injury.2006.04.121. [DOI] [PubMed] [Google Scholar]
- 86.David TS, Vrahas MS. Perioperative lower urinary tract infections and deep sepsis in patients undergoing total joint arthroplasty. J Am Acad Orthop Surg. 2000 Jan-Feb;8(1):66–74. doi: 10.5435/00124635-200001000-00007. 2000. [DOI] [PubMed] [Google Scholar]
- 87.Stéphan F, Sax H, Wachsmuth M, Hoffmeyer P, Clergue F, Pittet D. Reduction of urinary tract infection and antibiotic use after surgery: a controlled, prospective, before-after intervention study. Clin Infect Dis. 2006 Jun;42(11):1544–1551. doi: 10.1086/503837. [DOI] [PubMed] [Google Scholar]
- 88.Bouvet C, Lübbeke A, Bandi C, et al. Is there any benefit in pre-operative urinary analysis before elective total joint replacement? Bone Joint J. 2014 Mar;96-B(3):390–394. doi: 10.1302/0301-620X.96B3.32620. [DOI] [PubMed] [Google Scholar]
- 89.Wald HL, Ma A, Bratzler DW, Kramer AM. Indwelling urinary catheter use in the postoperative period: analysis of the national surgical infection prevention project data. Arch Surg. 2008 Jun;143(6):551–557. doi: 10.1001/archsurg.143.6.551. [DOI] [PubMed] [Google Scholar]
- 90.Koulouvaris P, Sculco P, Finerty E, Sculco T, Sharrock NE. Relationship between perioperative urinary tract infection and deep infection after joint arthroplasty. Clin Orthop Relat Res. 2009 Jul;467(7):1859–1867. doi: 10.1007/s11999-008-0614-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ollivere BJ, Ellahee N, Logan K, Miller-Jones JC, Allen PW. Asymptomatic urinary tract colonisation predisposes to superficial wound infection in elective orthopaedic surgery. Int Orthop. 2009 Jun;33(3):847–850. doi: 10.1007/s00264-008-0573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bozic KJ, Lau E, Ong K, et al. Risk factors for early revision after primary total hip arthroplasty in Medicare patients. Clin Orthop Relat Res. 2014 Feb;472(2):449–454. doi: 10.1007/s11999-013-3081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cordero-Ampuero J, González-Fernández E, Martínez-Vélez D, Esteban J. Are antibiotics necessary in hip arthroplasty with asymptomatic bacteriuria? Seeding risk with/without treatment. Clin Orthop Relat Res. 2013 Dec;471(12):3822–3829. doi: 10.1007/s11999-013-2868-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007 May 12;369(9573):1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 95.Kornbluth A, Sachar DB. Practice Parameters Committee of the American College of G. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. The American journal of gastroenterology. 2010 Mar;105(3):501–523. doi: 10.1038/ajg.2009.727. quiz 524. [DOI] [PubMed] [Google Scholar]
- 96.Matricon J, Barnich N, Ardid D. Immunopathogenesis of inflammatory bowel disease. Self/nonself. 2010 Oct;1(4):299–309. doi: 10.4161/self.1.4.13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kapadia BH, Issa K, Nagrare N, Pivec R, Banerjee S, Mont MA. Higher revision and complication rates following total hip arthroplasty in patients with inflammatory bowel disease. The Journal of arthroplasty. 2014 Mar;29(3):596–600. doi: 10.1016/j.arth.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 98.Palm O, Moum B, Jahnsen J, Gran JT. The prevalence and incidence of peripheral arthritis in patients with inflammatory bowel disease, a prospective population-based study (the IBSEN study) Rheumatology (Oxford) 2001 Nov;40(11):1256–1261. doi: 10.1093/rheumatology/40.11.1256. [DOI] [PubMed] [Google Scholar]
- 99.Orchard TR. Management of arthritis in patients with inflammatory bowel disease. Gastroenterology & hepatology. 2012 May;8(5):327–329. [PMC free article] [PubMed] [Google Scholar]
- 100.Kornbluth A, Sachar DB. Practice Parameters Committee of the American College of G. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. The American journal of gastroenterology. 2004 Jul;99(7):1371–1385. doi: 10.1111/j.1572-0241.2004.40036.x. [DOI] [PubMed] [Google Scholar]
- 101.Carter MJ, Lobo AJ, Travis SP. Ibd Section BSoG. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004 Sep;53 Suppl 5:V1–V16. doi: 10.1136/gut.2004.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Garcia-Erce JA, Gomollon F, Munoz M. Blood transfusion for the treatment of acute anaemia in inflammatory bowel disease and other digestive diseases. World journal of gastroenterology : WJG. 2009 Oct 7;15(37):4686–4694. doi: 10.3748/wjg.15.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Papay P, Hackner K, Vogelsang H, et al. High risk of transfusion-induced alloimmunization of patients with inflammatory bowel disease. The American journal of medicine. 2012 Jul;125(7):717, e711–e718. doi: 10.1016/j.amjmed.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 104.Lee GC, Cushner FD. The effects of preoperative autologous donations on perioperative blood levels. The journal of knee surgery. 2007 Jul;20(3):205–209. doi: 10.1055/s-0030-1248044. [DOI] [PubMed] [Google Scholar]
- 105.Cushner FD, Hawes T, Kessler D, Hill K, Scuderi GR. Orthopaedic-induced anemia: the fallacy of autologous donation programs. Clinical orthopaedics and related research. 2005 Feb;(431):145–149. [PubMed] [Google Scholar]
- 106.Theusinger OM, Leyvraz PF, Schanz U, Seifert B, Spahn DR. Treatment of iron deficiency anemia in orthopedic surgery with intravenous iron: efficacy and limits: a prospective study. Anesthesiology. 2007 Dec;107(6):923–927. doi: 10.1097/01.anes.0000291441.10704.82. [DOI] [PubMed] [Google Scholar]
- 107.Singh S, Kumar N, Loftus EV, Jr, Kane SV. Neurologic complications in patients with inflammatory bowel disease: increasing relevance in the era of biologics. Inflammatory bowel diseases. 2013 Mar-Apr;19(4):864–872. doi: 10.1002/ibd.23011. [DOI] [PubMed] [Google Scholar]
- 108.Yoshida H, Granger DN. Inflammatory bowel disease: a paradigm for the link between coagulation and inflammation. Inflammatory bowel diseases. 2009 Aug;15(8):1245–1255. doi: 10.1002/ibd.20896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Scarpa M, Pilon F, Pengo V, et al. Deep venous thrombosis after surgery for inflammatory bowel disease: is standard dose low molecular weight heparin prophylaxis enough? World journal of surgery. 2010 Jul;34(7):1629–1636. doi: 10.1007/s00268-010-0490-8. [DOI] [PubMed] [Google Scholar]
- 110.Chande N. Prevention of venous thromboembolism in hospitalized patients with inflammatory bowel disease. Inflammatory bowel diseases. 2013 Mar;19(3):669–671. doi: 10.1097/MIB.0b013e31827e7a0f. [DOI] [PubMed] [Google Scholar]
- 111.Robinson RJ, al-Azzawi F, Iqbal SJ, et al. Osteoporosis and determinants of bone density in patients with Crohn's disease. Digestive diseases and sciences. 1998 Nov;43(11):2500–2506. doi: 10.1023/a:1026650719552. [DOI] [PubMed] [Google Scholar]
- 112.Schett G. Effects of inflammatory and anti-inflammatory cytokines on the bone. European journal of clinical investigation. 2011 Dec;41(12):1361–1366. doi: 10.1111/j.1365-2362.2011.02545.x. [DOI] [PubMed] [Google Scholar]
- 113.Weitzmann MN. The Role of Inflammatory Cytokines, the RANKL/OPG Axis, and the Immunoskeletal Interface in Physiological Bone Turnover and Osteoporosis. Scientifica. 2013;2013:125705. doi: 10.1155/2013/125705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jahnsen J, Falch JA, Mowinckel P, Aadland E. Vitamin D status, parathyroid hormone and bone mineral density in patients with inflammatory bowel disease. Scandinavian journal of gastroenterology. 2002 Feb;37(2):192–199. doi: 10.1080/003655202753416876. [DOI] [PubMed] [Google Scholar]
- 115.Schneeweiss B, Lochs H, Zauner C, et al. Energy and substrate metabolism in patients with active Crohn's disease. The Journal of nutrition. 1999 Apr;129(4):844–848. doi: 10.1093/jn/129.4.844. [DOI] [PubMed] [Google Scholar]
- 116.Doherty CP, Crofton PM, Sarkar MA, et al. Malnutrition, zinc supplementation and catch-up growth: changes in insulin-like growth factor I, its binding proteins, bone formation and collagen turnover. Clinical endocrinology. 2002 Sep;57(3):391–399. doi: 10.1046/j.1365-2265.2002.01622.x. [DOI] [PubMed] [Google Scholar]
- 117.Imes S, Pinchbeck BR, Dinwoodie A, Walker K, Thomson AB. Iron, folate, vitamin B-12, zinc, and copper status in outpatients with Crohn's disease: effect of diet counseling. Journal of the American Dietetic Association. 1987 Jul;87(7):928–930. [PubMed] [Google Scholar]
- 118.Fagerlund TH, Dahl DG. [Zinc deficiency as a complication of Crohn disease] Tidsskrift for den Norske laegeforening : tidsskrift for praktisk medicin, ny raekke. 1983 Apr 30;103(12):999–1000. [PubMed] [Google Scholar]
- 119.Tiomny E, Horwitz C, Graff E, Rozen P, Gilat T. Serum zinc and taste acuity in Tel-Aviv patients with inflammatory bowel disease. The American journal of gastroenterology. 1982 Feb;77(2):101–104. [PubMed] [Google Scholar]
- 120.Stechmiller JK. Understanding the role of nutrition and wound healing. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition. 2010 Feb;25(1):61–68. doi: 10.1177/0884533609358997. [DOI] [PubMed] [Google Scholar]
- 121.Guarniero R, de Barros Filho TE, Tannuri U, Rodrigues CJ, Rossi JD. Study of fracture healing in protein malnutrition. Revista paulista de medicina. 1992 Mar-Apr;110(2):63–68. [PubMed] [Google Scholar]
- 122.Young ME. Malnutrition and wound healing. Heart & lung : the journal of critical care. 1988 Jan;17(1):60–67. [PubMed] [Google Scholar]
- 123.Day SM, DeHeer DH. Reversal of the detrimental effects of chronic protein malnutrition on long bone fracture healing. Journal of orthopaedic trauma. 2001 Jan;15(1):47–53. doi: 10.1097/00005131-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 124.Jahnsen J, Falch JA, Mowinckel P, Aadland E. Bone mineral density in patients with inflammatory bowel disease: a population-based prospective two-year follow-up study. Scandinavian journal of gastroenterology. 2004 Feb;39(2):145–153. doi: 10.1080/00365520310007873. [DOI] [PubMed] [Google Scholar]
- 125.Kumar A, Auron M, Aneja A, Mohr F, Jain A, Shen B. Inflammatory bowel disease: perioperative pharmacological considerations. Mayo Clinic proceedings. 2011 Aug;86(8):748–757. doi: 10.4065/mcp.2011.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Peyrin-Biroulet L. Anti-TNF therapy in inflammatory bowel diseases: a huge review. Minerva gastroenterologica e dietologica. 2010 Jun;56(2):233–243. [PubMed] [Google Scholar]
- 127.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA : the journal of the American Medical Association. 2006 May 17;295(19):2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 128.Bernatsky S, Habel Y, Rahme E. Observational studies of infections in rheumatoid arthritis: a metaanalysis of tumor necrosis factor antagonists. The Journal of rheumatology. 2010 May;37(5):928–931. doi: 10.3899/jrheum.091107. [DOI] [PubMed] [Google Scholar]
- 129.Giles JT, Bartlett SJ, Gelber AC, et al. Tumor necrosis factor inhibitor therapy and risk of serious postoperative orthopedic infection in rheumatoid arthritis. Arthritis and rheumatism. 2006 Apr 15;55(2):333–337. doi: 10.1002/art.21841. [DOI] [PubMed] [Google Scholar]
- 130.Tubach F, Salmon D, Ravaud P, et al. Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: The three-year prospective French Research Axed on Tolerance of Biotherapies registry. Arthritis and rheumatism. 2009 Jul;60(7):1884–1894. doi: 10.1002/art.24632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dixon WG, Hyrich KL, Watson KD, et al. Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR) Annals of the rheumatic diseases. 2010 Mar;69(3):522–528. doi: 10.1136/ard.2009.118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dao KH, Herbert M, Habal N, Cush JJ. Nonserious infections: should there be cause for serious concerns? Rheumatic diseases clinics of North America. 2012 Nov;38(4):707–725. doi: 10.1016/j.rdc.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 133.Strand V, Smolen JS, van Vollenhoven RF, et al. Certolizumab pegol plus methotrexate provides broad relief from the burden of rheumatoid arthritis: analysis of patient-reported outcomes from the RAPID 2 trial. Annals of the rheumatic diseases. 2011 Jun;70(6):996–1002. doi: 10.1136/ard.2010.143586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mooney DP, O'Reilly M, Gamelli RL. Tumor necrosis factor and wound healing. Annals of surgery. 1990 Feb;211(2):124–129. doi: 10.1097/00000658-199002000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Goh L, Jewell T, Laversuch C, Samanta A. Should anti-TNF therapy be discontinued in rheumatoid arthritis patients undergoing elective orthopaedic surgery? A systematic review of the evidence. Rheumatology international. 2012 Jan;32(1):5–13. doi: 10.1007/s00296-011-2040-6. [DOI] [PubMed] [Google Scholar]
- 136.Carmona L, Cross M, Williams B, Lassere M, March L. Rheumatoid arthritis. Best practice & research. Clinical rheumatology. 2010 Dec;24(6):733–745. doi: 10.1016/j.berh.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 137.Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. The Journal of the American Academy of Orthopaedic Surgeons. 2006 Sep;14(9):544–551. doi: 10.5435/00124635-200609000-00004. [DOI] [PubMed] [Google Scholar]
- 138.Chmell MJ, Scott RD. Total knee arthroplasty in patients with rheumatoid arthritis. An overview. Clinical orthopaedics and related research. 1999 Sep;(366):54–60. doi: 10.1097/00003086-199909000-00008. [DOI] [PubMed] [Google Scholar]
- 139.Luessenhop CP, Higgins LD, Brause BD, Ranawat CS. Multiple prosthetic infections after total joint arthroplasty. Risk factor analysis. The Journal of arthroplasty. 1996 Oct;11(7):862–868. doi: 10.1016/s0883-5403(96)80189-6. [DOI] [PubMed] [Google Scholar]
- 140.Anstead GM. Steroids, retinoids, and wound healing. Advances in wound care : the journal for prevention and healing. 1998 Oct;11(6):277–285. [PubMed] [Google Scholar]
- 141.Pieringer H, Stuby U, Biesenbach G. Patients with rheumatoid arthritis undergoing surgery: how should we deal with antirheumatic treatment? Seminars in arthritis and rheumatism. 2007 Apr;36(5):278–286. doi: 10.1016/j.semarthrit.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 142.Madsen PV, Andersen G. Multifocal osteonecrosis related to steroid treatment in a patient with ulcerative colitis. Gut. 1994 Jan;35(1):132–134. doi: 10.1136/gut.35.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rutgeerts PJ. Review article: the limitations of corticosteroid therapy in Crohn’s disease. Alimentary pharmacology & therapeutics. 2001 Oct;15(10):1515–1525. doi: 10.1046/j.1365-2036.2001.01060.x. [DOI] [PubMed] [Google Scholar]
- 144.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn's disease: TREAT registry. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2006 May;4(5):621–630. doi: 10.1016/j.cgh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 145.Shaw M, Mandell BF. Perioperative management of selected problems in patients with rheumatic diseases. Rheumatic diseases clinics of North America. 1999 Aug;25(3):623–638. ix. doi: 10.1016/s0889-857x(05)70089-2. [DOI] [PubMed] [Google Scholar]
- 146.Grennan DM, Gray J, Loudon J, Fear S. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Annals of the rheumatic diseases. 2001 Mar;60(3):214–217. doi: 10.1136/ard.60.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Murata K, Yasuda T, Ito H, Yoshida M, Shimizu M, Nakamura T. Lack of increase in postoperative complications with low-dose methotrexate therapy in patients with rheumatoid arthritis undergoing elective orthopedic surgery. Modern rheumatology / the Japan Rheumatism Association. 2006;16(1):14–19. doi: 10.1007/s10165-005-0444-4. [DOI] [PubMed] [Google Scholar]
- 148.Pieringer H, Stuby U, Biesenbach G. The place of methotrexate perioperatively in elective orthopedic surgeries in patients with rheumatoid arthritis. Clinical rheumatology. 2008 Oct;27(10):1217–1220. doi: 10.1007/s10067-008-0888-y. [DOI] [PubMed] [Google Scholar]
- 149.Morgacheva O, Furst DE. Use of MTX in the elderly and in patients with compromised renal function. Clinical and experimental rheumatology. 2010 Sep-Oct;28(5 Suppl 61):S85–S94. [PubMed] [Google Scholar]
- 150.Danoff JR, Moss G, Liabaud B, Geller JA. Total knee arthroplasty considerations in rheumatoid arthritis. Autoimmune diseases. 2013;2013:185340. doi: 10.1155/2013/185340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rosandich PA, Kelley JT, 3rd, Conn DL. Perioperative management of patients with rheumatoid arthritis in the era of biologic response modifiers. Current opinion in rheumatology. 2004 May;16(3):192–198. doi: 10.1097/00002281-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 152.Pramoolsinsup C. Management of viral hepatitis B. Journal of gastroenterology and hepatology. 2002 Feb;17 Suppl:S125–S145. doi: 10.1046/j.1440-1746.17.s1.3.x. [DOI] [PubMed] [Google Scholar]
- 153.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. Journal of hepatology. 2006 Oct;45(4):529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 154.Mendenhall CL, Seeff L, Diehl AM, et al. Antibodies to hepatitis B virus and hepatitis C virus in alcoholic hepatitis and cirrhosis: their prevalence and clinical relevance. The VA Cooperative Study Group (No. 119) Hepatology. 1991 Oct;14(4 Pt 1):581–589. doi: 10.1016/0270-9139(91)90042-t. [DOI] [PubMed] [Google Scholar]
- 155.Hsieh PH, Chen LH, Lee MS, Chen CH, Yang WE, Shih CH. Hip arthroplasty in patients with cirrhosis of the liver. The Journal of bone and joint surgery. British volume. 2003 Aug;85(6):818–821. [PubMed] [Google Scholar]
- 156.Donaghy AJ, Delhanty PJ, Ho KK, Williams R, Baxter RC. Regulation of the growth hormone receptor/binding protein, insulin-like growth factor ternary complex system in human cirrhosis. Journal of hepatology. 2002 Jun;36(6):751–758. doi: 10.1016/s0168-8278(02)00049-1. [DOI] [PubMed] [Google Scholar]
- 157.Idilman R, de Maria N, Uzunalimoglu O, van Thiel DH. Hepatic osteodystrophy: a review. Hepatogastroenterology. 1997 Mar-Apr;44(14):574–581. [PubMed] [Google Scholar]
- 158.Kelly DA, Tuddenham EG. Haemostatic problems in liver disease. Gut. 1986 Mar;27(3):339–349. doi: 10.1136/gut.27.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Blake JC, Sprengers D, Grech P, McCormick PA, McIntyre N, Burroughs AK. Bleeding time in patients with hepatic cirrhosis. Bmj. 1990 Jul 7;301(6742):12–15. doi: 10.1136/bmj.301.6742.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gomez F, Ruiz P, Schreiber AD. Impaired function of macrophage Fc gamma receptors and bacterial infection in alcoholic cirrhosis. The New England journal of medicine. 1994 Oct 27;331(17):1122–1128. doi: 10.1056/NEJM199410273311704. [DOI] [PubMed] [Google Scholar]
- 161.Goral V, Simsek M, Mete N. Hepatic osteodystrophy and liver cirrhosis. World journal of gastroenterology : WJG. 2010 Apr 7;16(13):1639–1643. doi: 10.3748/wjg.v16.i13.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ono Y, Watanabe T, Matsumoto K, Ito T, Kunii O, Goldstein E. Opsonophagocytic dysfunction in patients with liver cirrhosis and low responses to tumor necrosis factor-alpha and lipopolysaccharide in patients' blood. Journal of infection and chemotherapy : official journal of the Japan Society of Chemotherapy. 2004 Aug;10(4):200–207. doi: 10.1007/s10156-004-0321-7. [DOI] [PubMed] [Google Scholar]
- 163.Fiuza C, Salcedo M, Clemente G, Tellado JM. In vivo neutrophil dysfunction in cirrhotic patients with advanced liver disease. The Journal of infectious diseases. 2000 Aug;182(2):526–533. doi: 10.1086/315742. [DOI] [PubMed] [Google Scholar]
- 164.Christou L, Pappas G, Falagas ME. Bacterial infection-related morbidity and mortality in cirrhosis. The American journal of gastroenterology. 2007 Jul;102(7):1510–1517. doi: 10.1111/j.1572-0241.2007.01286.x. [DOI] [PubMed] [Google Scholar]
- 165.Carey E, Balan V. Metabolic bone disease in patients with liver disease. Current gastroenterology reports. 2003 Feb;5(1):71–77. doi: 10.1007/s11894-003-0012-z. [DOI] [PubMed] [Google Scholar]
- 166.Mansour A, Watson W, Shayani V, Pickleman J. Abdominal operations in patients with cirrhosis: still a major surgical challenge. Surgery. 1997 Oct;122(4):730–735. doi: 10.1016/s0039-6060(97)90080-5. discussion 735–736. [DOI] [PubMed] [Google Scholar]
- 167.Doberneck RC, Sterling WA, Jr, Allison DC. Morbidity and mortality after operation in nonbleeding cirrhotic patients. American journal of surgery. 1983 Sep;146(3):306–309. doi: 10.1016/0002-9610(83)90402-6. [DOI] [PubMed] [Google Scholar]
- 168.Aranha GV, Greenlee HB. Intra-abdominal surgery in patients with advanced cirrhosis. Arch Surg. 1986 Mar;121(3):275–277. doi: 10.1001/archsurg.1986.01400030029003. [DOI] [PubMed] [Google Scholar]
- 169.Schwartz SI. Biliary tract surgery and cirrhosis: a critical combination. Surgery. 1981 Oct;90(4):577–583. [PubMed] [Google Scholar]
- 170.Ueda H, Iwasaki A, Kusano T, Shirakusa T. Thoracotomy in patients with liver cirrhosis. Scandinavian journal of thoracic and cardiovascular surgery. 1994;28(1):37–41. doi: 10.3109/14017439409098708. [DOI] [PubMed] [Google Scholar]
- 171.Tinkoff G, Rhodes M, Diamond D, Lucke J. Cirrhosis in the trauma victim. Effect on mortality rates. Annals of surgery. 1990 Feb;211(2):172–177. doi: 10.1097/00000658-199002000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lopez-Delgado JC, Esteve F, Javierre C, et al. Predictors of long-term mortality in patients with cirrhosis undergoing cardiac surgery. The Journal of cardiovascular surgery. 2014 Mar 18; [PubMed] [Google Scholar]
- 173.Mosko JD, Nguyen GC. Increased perioperative mortality following bariatric surgery among patients with cirrhosis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011 Oct;9(10):897–901. doi: 10.1016/j.cgh.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 174.Neeff HP, Streule GC, Drognitz O, et al. Early mortality and long-term survival after abdominal surgery in patients with liver cirrhosis. Surgery. 2014 Apr;155(4):623–632. doi: 10.1016/j.surg.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 175.Sipeki N, Antal-Szalmas P, Lakatos PL, Papp M. Immune dysfunction in cirrhosis. World journal of gastroenterology : WJG. 2014 Mar 14;20(10):2564–2577. doi: 10.3748/wjg.v20.i10.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Navasa M, Rimola A, Rodes J. Bacterial infections in liver disease. Seminars in liver disease. 1997;17(4):323–333. doi: 10.1055/s-2007-1007209. [DOI] [PubMed] [Google Scholar]
- 177.O’Leary JG, Friedman LS. Predicting surgical risk in patients with cirrhosis: from art to science. Gastroenterology. 2007 Apr;132(4):1609–1611. doi: 10.1053/j.gastro.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 178.Friedman LS. The risk of surgery in patients with liver disease. Hepatology. 1999 Jun;29(6):1617–1623. doi: 10.1002/hep.510290639. [DOI] [PubMed] [Google Scholar]
- 179.Liao JC, Chen WJ, Chen LH, et al. Complications associated with instrumented lumbar surgery in patients with liver cirrhosis: a matched cohort analysis. The spine journal : official journal of the North American Spine Society. 2013 Aug;13(8):908–913. doi: 10.1016/j.spinee.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 180.Lin TY, Liao JC, Chen WJ, et al. Surgical risks and perioperative complications of instrumented lumbar surgery in patients with liver cirrhosis. Biomedical journal. 2014 Jan-Feb;37(1):18–23. doi: 10.4103/2319-4170.113376. [DOI] [PubMed] [Google Scholar]
- 181.Shih LY, Cheng CY, Chang CH, Hsu KY, Hsu RW, Shih HN. Total knee arthroplasty in patients with liver cirrhosis. The Journal of bone and joint surgery. American volume. 2004 Feb;86-A(2):335–341. doi: 10.2106/00004623-200402000-00017. [DOI] [PubMed] [Google Scholar]
- 182.Cohen SM, Te HS, Levitsky J. Operative risk of total hip and knee arthroplasty in cirrhotic patients. The Journal of arthroplasty. 2005;20(4):460–466. doi: 10.1016/j.arth.2004.05.004. [DOI] [PubMed] [Google Scholar]