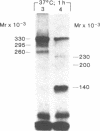
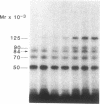
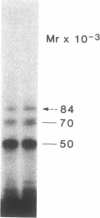
Abstract
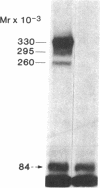
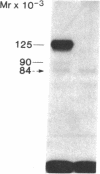
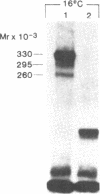
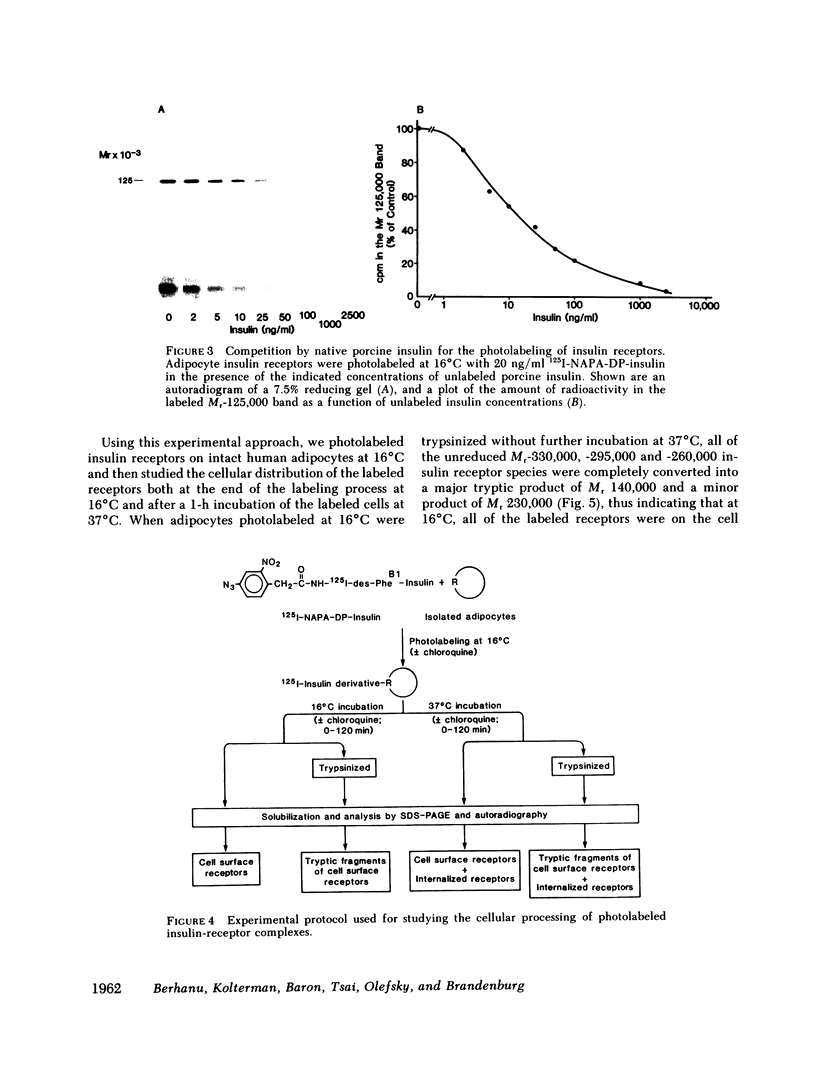
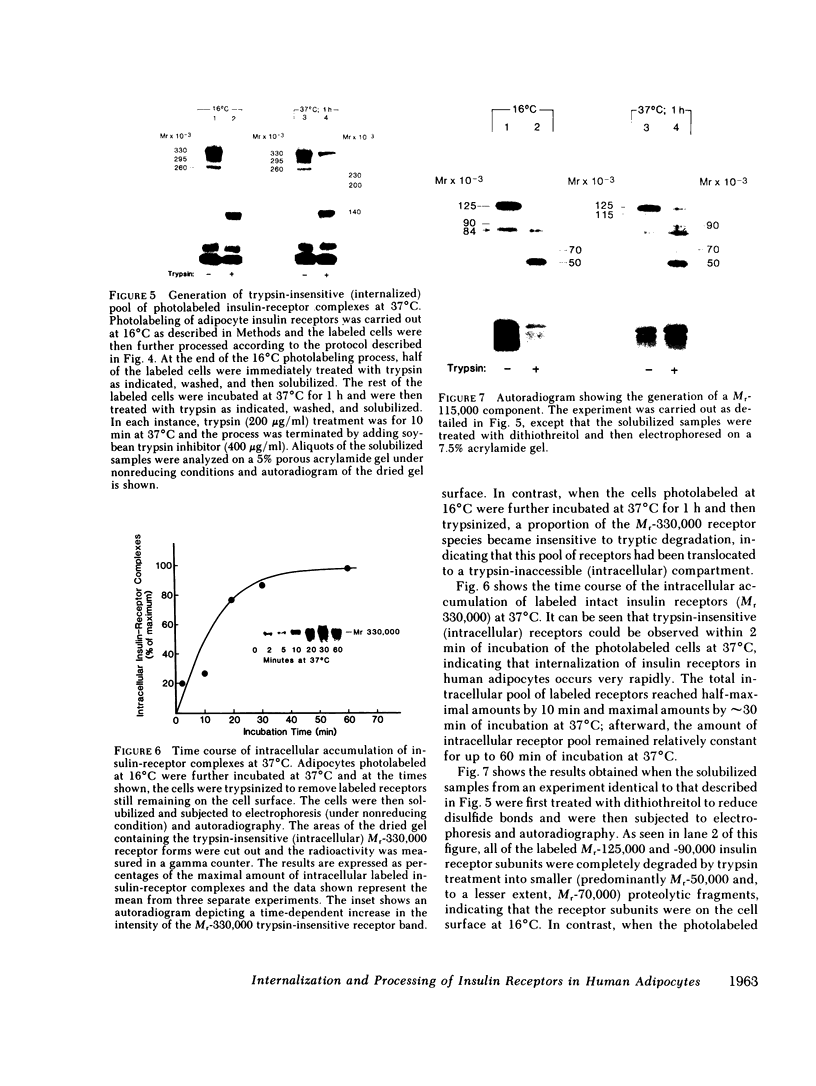
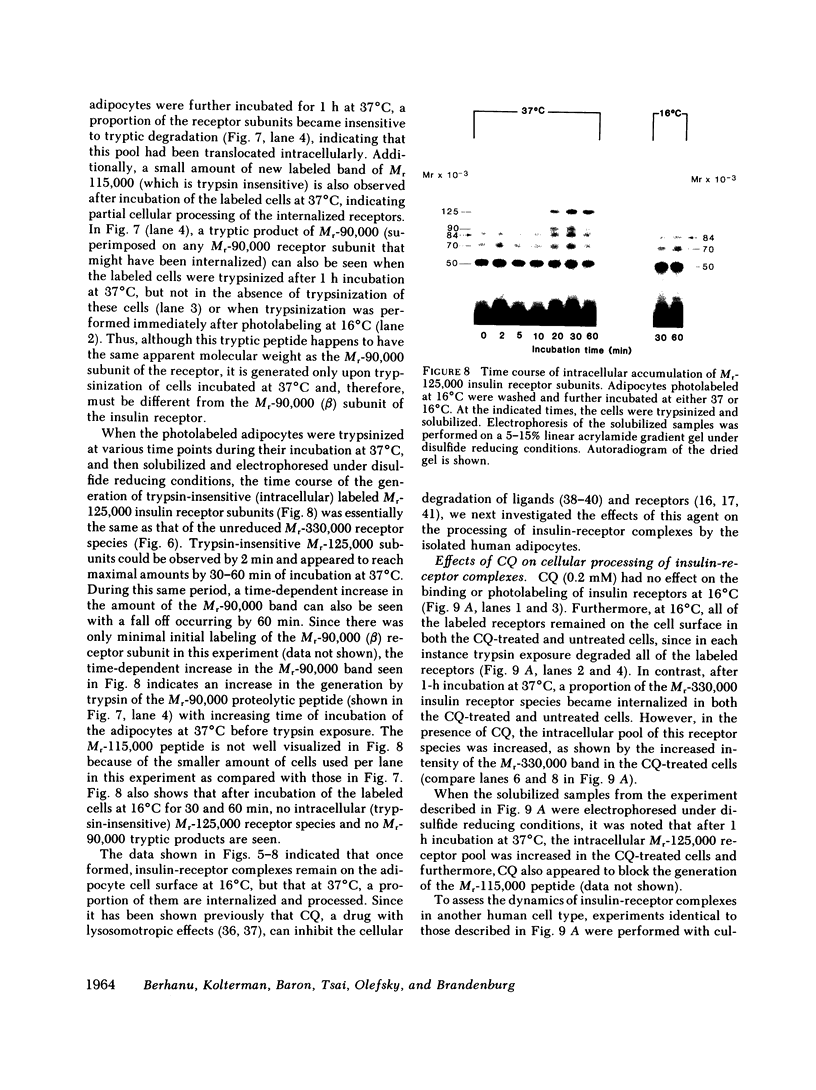
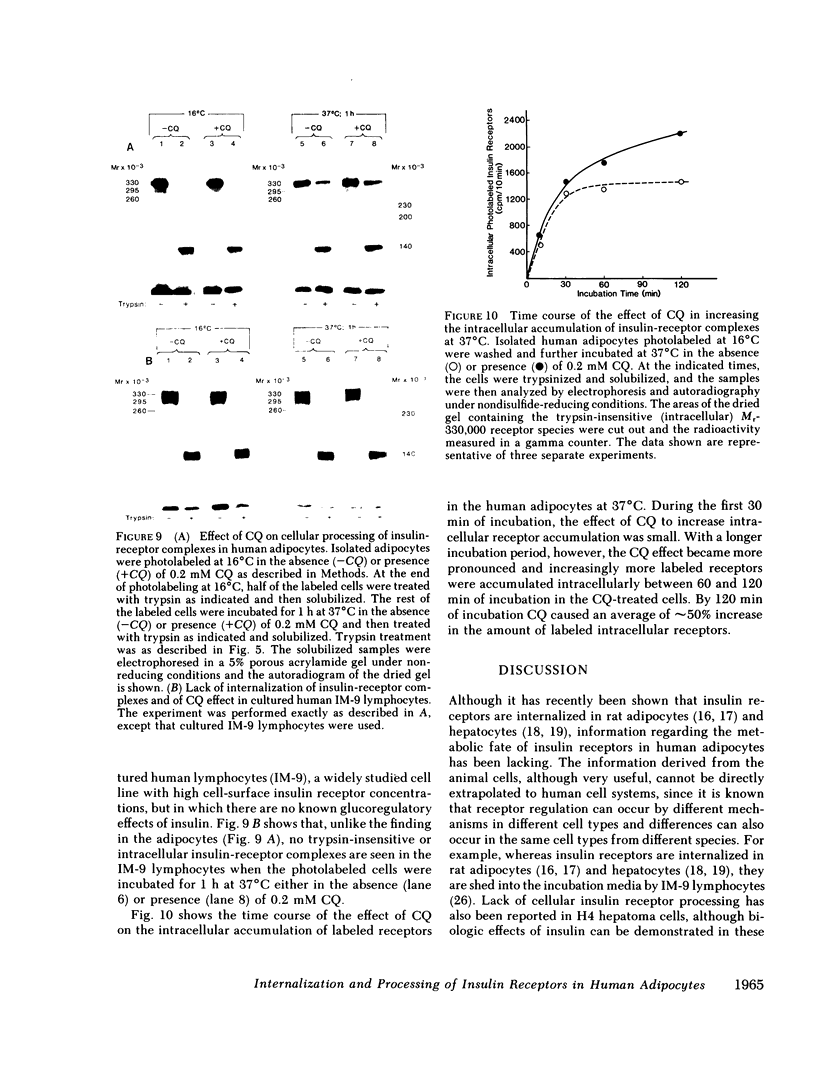
We photolabeled and characterized insulin receptors in isolated adipocytes from normal human subjects and then studied the cellular fate of the labeled insulin-receptor complexes at physiologic temperatures. The biologically active photosensitive insulin derivative, B2(2-nitro-4-azidophenylacetyl)des-PheB1-insulin (NAPA-DP-insulin) was used to photoaffinity label the insulin receptors, and the specifically labeled cellular proteins were identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography. At saturating concentrations, the binding of 125I-NAPA-DP-insulin to the isolated adipocytes at 16 degrees C was rapid (half-maximal in approximately 1 min and maximal in approximately 10 min) and approximately 25% of the specifically bound ligand was covalently linked to the cells by a 3-min exposure to long-wave (366 nm) ultraviolet light. Analysis of the photolabeled cellular proteins by PAGE in the absence of disulfide reductants revealed the specific labeling of a major protein band of Mr 330,000 and two less intense bands of Mr 295,000 and 260,000. Upon reduction of disulfide bonds with dithiothreitol, all three unreduced forms of the insulin receptor were converted into a major labeled Mr-125,000 band and a less intensely labeled Mr-90,000 band. The labeling of the Mr-125,000 receptor subunit was saturable and native porcine insulin effectively inhibited (half-maximal inhibition at 12 ng/ml) the photolabeling of this binding subunit by NAPA-DP insulin. When intact adipocytes photolabeled at 16 degrees C (a temperature that inhibits endocytosis) were immediately trypsinized, all of the labeled receptor bands were converted into small molecular weight tryptic fragments, indicating that at 16 degrees C all of the labeled insulin-receptor complexes remained on the cell surface. However, when the photolabeled cells were further incubated at 37 degrees C and then trypsinized, a proportion of the labeled receptors became trypsin insensitive, indicating that this fraction has been translocated to the cell interior and thus was inaccessible to the trypsin in the incubation medium. The intracellular translocation of the labeled receptors was observed within 2 min, became half-maximal by 10 min, and maximal by approximately 30 min of incubation at 37 degrees C. Cellular processing of the internalized insulin-receptor complexes also occurred, since incubation at 37 degrees C (but not 16 degrees C) resulted in the generation of a Mr-115,000 component from the labeled receptors. Inclusion of chloroquine, a drug with lysosomotropic properties, in the incubation media caused a time-dependent increase (maximal increase of 50% above control by 2 h at 37 degrees C) in the intracellular pool of labeled receptors. In contrast to these findings in human adipocytes, no appreciable internalization of insulin-receptor complexes and no chloroquine effect was observed in cultures human IM-9 lymphocytes during a 1-h incubation at 37 degrees C. We concluded that in isolated human adipocytes: (a) the subunit structure of insulin receptors is the same as that reported for several other tissues, (b) insulin-receptor complexes are rapidly internalized and processed at physiologic temperatures, and (c) the cellular processing of insulin-receptor complexes occurs at one or more chloroquine-sensitive intracellular site(s).
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer J. A., Gorden P., Roth J. Defect in insulin binding to receptors in obese man. Amelioration with calorie restriction. J Clin Invest. 1975 Jan;55(1):166–174. doi: 10.1172/JCI107907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avruch J., Nemenoff R. A., Blackshear P. J., Pierce M. W., Osathanondh R. Insulin-stimulated tyrosine phosphorylation of the insulin receptor in detergent extracts of human placental membranes. Comparison to epidermal growth factor-stimulated phosphorylation. J Biol Chem. 1982 Dec 25;257(24):15162–15166. [PubMed] [Google Scholar]
- Bar R. S., Gorden P., Roth J., Kahn C. R., De Meyts P. Fluctuations in the affinity and concentration of insulin receptors on circulating monocytes of obese patients: effects of starvation, refeeding, and dieting. J Clin Invest. 1976 Nov;58(5):1123–1135. doi: 10.1172/JCI108565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck-Nielsen H. The pathogenic role of an insulin-receptor defect in diabetes mellitus of the obese. Diabetes. 1978 Dec;27(12):1175–1181. doi: 10.2337/diab.27.12.1175. [DOI] [PubMed] [Google Scholar]
- Berhanu P., Olefsky J. M. Photoaffinity labeling of insulin receptors in viable cultured human lymphocytes. Demonstration of receptor shedding and degradation. Diabetes. 1982 May;31(5 Pt 1):410–417. doi: 10.2337/diab.31.5.410. [DOI] [PubMed] [Google Scholar]
- Berhanu P., Olefsky J. M., Tsai P., Thamm P., Saunders D., Brandenburg D. Internalization and molecular processing of insulin receptors in isolated rat adipocytes. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4069–4073. doi: 10.1073/pnas.79.13.4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackard W. G., Guzelian P. S., Small M. E. Down regulation of insulin receptors in primary cultures of adult rat hepatocytes in monolayer. Endocrinology. 1978 Aug;103(2):548–553. doi: 10.1210/endo-103-2-548. [DOI] [PubMed] [Google Scholar]
- Bolinder J., Kager L., Ostman J., Arner P. Differences at the receptor and postreceptor levels between human omental and subcutaneous adipose tissue in the action of insulin on lipolysis. Diabetes. 1983 Feb;32(2):117–123. doi: 10.2337/diab.32.2.117. [DOI] [PubMed] [Google Scholar]
- Brandenburg D., Diaconescu C., Saunders D., Thamm P. Covalent linking of photoreactive insulin to adipocytes produces a prolonged signal. Nature. 1980 Aug 21;286(5775):821–822. doi: 10.1038/286821a0. [DOI] [PubMed] [Google Scholar]
- Ciaraldi T. P., Kolterman O. G., Olefsky J. M. Mechanism of the postreceptor defect in insulin action in human obesity. Decrease in glucose transport system activity. J Clin Invest. 1981 Oct;68(4):875–880. doi: 10.1172/JCI110342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M., Fox C. F. Molecular mechanism of mitogen action: processing of receptor induced by epidermal growth factor. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2644–2648. doi: 10.1073/pnas.75.6.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R., Deibert D., Hendler R., Felig P., Soman V. Insulin sensitivity and insulin binding to monocytes in maturity-onset diabetes. J Clin Invest. 1979 May;63(5):939–946. doi: 10.1172/JCI109394. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Fehlmann M., Carpentier J. L., Le Cam A., Thamm P., Saunders D., Brandenburg D., Orci L., Freychet P. Biochemical and morphological evidence that the insulin receptor is internalized with insulin in hepatocytes. J Cell Biol. 1982 Apr;93(1):82–87. doi: 10.1083/jcb.93.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried S. K., Lavau M., Pi-Sunyer F. X. Variations of glucose metabolism by fat cells from three adipose depots of the rat. Metabolism. 1982 Sep;31(9):876–883. doi: 10.1016/0026-0495(82)90176-7. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brunschede G. Y., Brown M. S. Inhibition of proteolytic degradation of low density lipoprotein in human fibroblasts by chloroquine, concanavalin A, and Triton WR 1339. J Biol Chem. 1975 Oct 10;250(19):7854–7862. [PubMed] [Google Scholar]
- Gorden P., Carpentier J. L., Moule M. L., Yip C. C., Orci L. Direct demonstration of insulin receptor internalization. A quantitative electron microscopic study of covalently bound 125I-photoreactive insulin incubated with isolated hepatocytes. Diabetes. 1982 Jul;31(7):659–662. doi: 10.2337/diab.31.7.659. [DOI] [PubMed] [Google Scholar]
- Green A., Olefsky J. M. Evidence for insulin-induced internalization and degradation of insulin receptors in rat adipocytes. Proc Natl Acad Sci U S A. 1982 Jan;79(2):427–431. doi: 10.1073/pnas.79.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammons G. T., Jarett L. Lysosomal degradation of receptor-bound 125I-labeled insulin by rat adipocytes: its characterization and dissociation from the short-term biologic effects of insulin. Diabetes. 1980 Jun;29(6):475–486. doi: 10.2337/diab.29.6.475. [DOI] [PubMed] [Google Scholar]
- Hirsch J., Gallian E. Methods for the determination of adipose cell size in man and animals. J Lipid Res. 1968 Jan;9(1):110–119. [PubMed] [Google Scholar]
- Hofmann C., Ji T. H., Miller B., Steiner D. F. Photoaffinity labeling of the insulin receptor in H4 hepatoma cells: lack of cellular receptor processing. J Supramol Struct Cell Biochem. 1981;15(1):1–13. doi: 10.1002/jsscb.1981.380150102. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Hazum E., Shechter Y., Cuatrecasas P. Insulin receptor: covalent labeling and identification of subunits. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4918–4921. doi: 10.1073/pnas.76.10.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Karlsson F. A., Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science. 1982 Jan 8;215(4529):185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blithe D. L., Crettaz M., Kahn C. R. Insulin stimulates tyrosine phosphorylation of the insulin receptor in a cell-free system. Nature. 1982 Aug 12;298(5875):667–669. doi: 10.1038/298667a0. [DOI] [PubMed] [Google Scholar]
- Kolterman O. G., Gray R. S., Griffin J., Burstein P., Insel J., Scarlett J. A., Olefsky J. M. Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus. J Clin Invest. 1981 Oct;68(4):957–969. doi: 10.1172/JCI110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolterman O. G., Insel J., Saekow M., Olefsky J. M. Mechanisms of insulin resistance in human obesity: evidence for receptor and postreceptor defects. J Clin Invest. 1980 Jun;65(6):1272–1284. doi: 10.1172/JCI109790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono T., Barham F. W. The relationship between the insulin-binding capacity of fat cells and the cellular response to insulin. Studies with intact and trypsin-treated fat cells. J Biol Chem. 1971 Oct 25;246(20):6210–6216. [PubMed] [Google Scholar]
- Kuehn L., Meyer H., Rutschmann M., Thamm P. Identification of photoaffinity labeled insulin receptor proteins by linear polyacrylamide gradient gel electrophoresis under non-denaturing conditions. FEBS Lett. 1980 May 5;113(2):189–192. doi: 10.1016/0014-5793(80)80588-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Mullikin D., Williams L. T. A desensitized state of the beta adrenergic receptor not associated with high-affinity agonist occupancy. Mol Pharmacol. 1978 Mar;14(2):376–380. [PubMed] [Google Scholar]
- Lie S. O., Schofield B. Inactivation of lysosomal function in normal cultured human fibroblasts by chloroquine. Biochem Pharmacol. 1973 Dec 1;22(23):3109–3114. doi: 10.1016/0006-2952(73)90197-4. [DOI] [PubMed] [Google Scholar]
- Livingston J. N., Purvis B. J., Lockwood D. H. Insulin induced changes in insulin binding and insulin-sensitivity of adipocytes. Metabolism. 1978 Dec;27(12 Suppl 2):2009–2014. doi: 10.1016/s0026-0495(78)80017-1. [DOI] [PubMed] [Google Scholar]
- Livingston J. N., Purvis B. J., Lockwood D. H. Insulin-dependent regulation of the insulin-sensitivity of adipocytes. Nature. 1978 Jun 1;273(5661):394–396. doi: 10.1038/273394a0. [DOI] [PubMed] [Google Scholar]
- Marshall S., Olefsky J. M. Effects of insulin incubation on insulin binding, glucose transport, and insulin degradation by isolated rat adipocytes. Evidence for hormone-induced desensitization at the receptor and postreceptor level. J Clin Invest. 1980 Oct;66(4):763–772. doi: 10.1172/JCI109914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall S., Olefsky J. M. Effects of lysosomotropic agents on insulin interactions with adipocytes. Evidence for a lysosomal pathway for insulin processing and degradation. J Biol Chem. 1979 Oct 25;254(20):10153–10160. [PubMed] [Google Scholar]
- Mott D. M., Howard B. V., Bennett P. H. Stoichiometric binding and regulation of insulin receptors on human diploid fibroblasts using physiologic insulin levels. J Biol Chem. 1979 Sep 25;254(18):8762–8767. [PubMed] [Google Scholar]
- Olefsky J. M. Decreased insulin binding to adipocytes and circulating monocytes from obese subjects. J Clin Invest. 1976 May;57(5):1165–1172. doi: 10.1172/JCI108384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M. LIlly lecture 1980. Insulin resistance and insulin action. An in vitro and in vivo perspective. Diabetes. 1981 Feb;30(2):148–162. doi: 10.2337/diab.30.2.148. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Marshall S., Berhanu P., Saekow M., Heidenreich K., Green A. Internalization and intracellular processing of insulin and insulin receptors in adipocytes. Metabolism. 1982 Jul;31(7):670–690. doi: 10.1016/0026-0495(82)90197-4. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Reaven G. M. Insulin binding in diabetes. Relationships with plasma insulin levels and insulin sensitivity. Diabetes. 1977 Jul;26(7):680–688. doi: 10.2337/diab.26.7.680. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. The insulin receptor: its role in insulin resistance of obesity and diabetes. Diabetes. 1976 Dec;25(12):1154–1162. doi: 10.2337/diab.25.12.1154. [DOI] [PubMed] [Google Scholar]
- Pedersen O., Hjøllund E. Insulin receptor binding to fat and blood cells and insulin action in fat cells from insulin-dependent diabetics. Diabetes. 1982 Aug;31(8 Pt 1):706–715. doi: 10.2337/diab.31.8.706. [DOI] [PubMed] [Google Scholar]
- Petruzzelli L. M., Ganguly S., Smith C. J., Cobb M. H., Rubin C. S., Rosen O. M. Insulin activates a tyrosine-specific protein kinase in extracts of 3T3-L1 adipocytes and human placenta. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6792–6796. doi: 10.1073/pnas.79.22.6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilch P. F., Axelrod J. D., Colello J., Czech M. P. Unimpaired signal transduction by the adipocyte insulin receptor following its partial proteolytic fragmentation. J Biol Chem. 1981 Feb 25;256(4):1570–1575. [PubMed] [Google Scholar]
- Posner B. I., Patel B. A., Khan M. N., Bergeron J. J. Effect of chloroquine on the internalization of 125I-insulin into subcellular fractions of rat liver. Evidence for an effect of chloroquine on Golgi elements. J Biol Chem. 1982 May 25;257(10):5789–5799. [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Roth R. A., Cassell D. J. Insulin receptor: evidence that it is a protein kinase. Science. 1983 Jan 21;219(4582):299–301. doi: 10.1126/science.6849137. [DOI] [PubMed] [Google Scholar]
- Siegel T. W., Ganguly S., Jacobs S., Rosen O. M., Rubin C. S. Purification and properties of the human placental insulin receptor. J Biol Chem. 1981 Sep 10;256(17):9266–9273. [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Stadel J. M., De Lean A., Mullikin-Kilpatrick D., Sawyer D. D., Lefkowitz R. J. Catecholamine-induced desensitization in turkey erythrocytes: cAMP mediated impairment of high affinity agonist binding without alteration in receptor number. J Cyclic Nucleotide Res. 1981;7(1):37–47. [PubMed] [Google Scholar]
- Van Obberghen E., Ksauga M., Le Cam A., Hedo J. A., Itin A., Harrison L. C. Biosynthetic labeling of insulin receptor: studies of subunits in cultured human IM-9 lymphocytes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1052–1056. doi: 10.1073/pnas.78.2.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip C. C., Moule M. L., Yeung C. W. Characterization of insulin receptor subunits in brain and other tissues by photoaffinity labeling. Biochem Biophys Res Commun. 1980 Oct 31;96(4):1671–1678. doi: 10.1016/0006-291x(80)91366-2. [DOI] [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]