Antibodies to native glycosylated myelin oligodendrocyte glycoprotein (MOG), measured by cell-based assays, have been reported in adults with acute disseminated encephalomyelitis (ADEM) and in children with demyelinating diseases, including multiple sclerosis (MS) and ADEM. More recently, antibodies to both truncated1 and full-length2 MOG have been identified in a few adults with aquaporin-4 (AQP4) antibody–negative neuromyelitis optica spectrum disorder (NMOSD). We report a patient with a rapidly worsening longitudinally extensive transverse myelitis (LETM) whose serum antibodies were subsequently found to bind the full-length version of MOG. Aggressive and early immunomodulation correlated closely with reduction in MOG titer and clinical improvement.
Case report.
A 41-year-old woman was transferred to our center with a 3-day history of rapidly ascending sensorimotor disturbance. She described initial sensory difficulties in the left leg that progressed over 48 hours to involve both lower limbs, followed by weakness and urinary retention. Over the first 12 hours of admission, her sensory symptoms progressed to involve the thorax. Within 72 hours of onset, she developed a flaccid paralysis below T1. She became drowsy and had a weak cough.
Examination revealed mild encephalopathy, subtle weakness in the right upper limb, and normal (not brisk) upper limb reflexes. She had a flaccid paralysis in the lower limbs with absent reflexes. There was loss of pinprick, temperature, and light touch below the T2 dermatome and hyperalgesia at T1. Proprioception was intact.
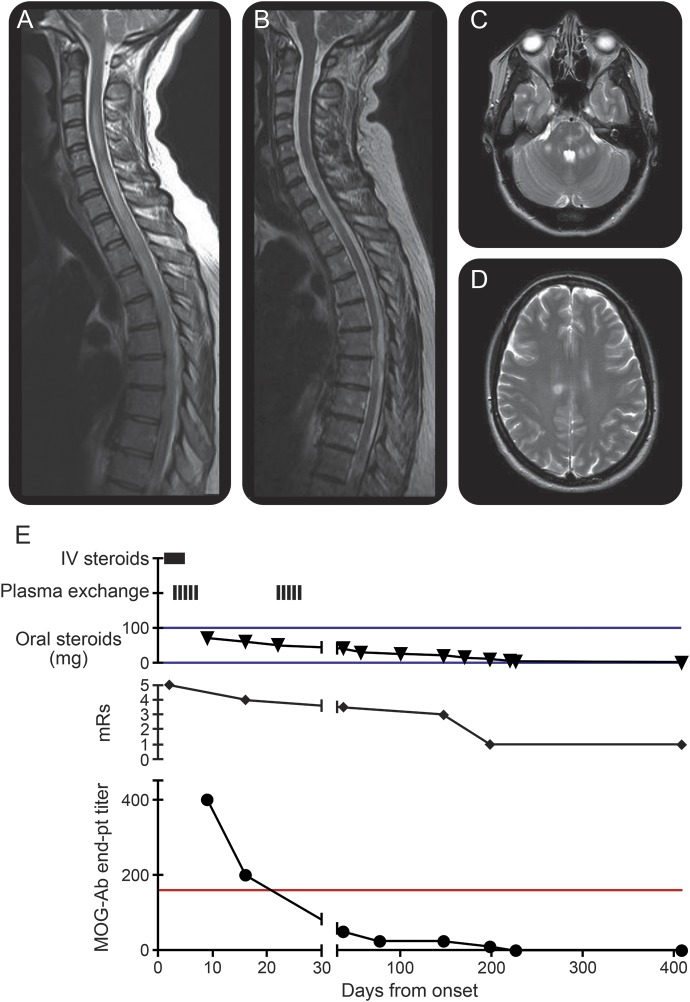
Complete blood count, erythrocyte sedimentation rate, biochemical profile, HIV serology, antinuclear antibodies, rheumatoid factor, and serum angiotensin-converting enzyme were all normal or negative. MRI on admission revealed extensive spinal cord hyperintensity extending from the high cervical cord to the midthoracic cord, and white matter changes in posterior fossa and cerebral hemispheres, which appeared to be inflammatory (figure, A). CSF analysis revealed a white cell count of 32 × 109/L (all lymphocytes), with paired oligoclonal bands in serum and CSF. A diagnosis of NMOSD was made.
Figure. Clinical and radiologic course.
(A) The T2 contrast-enhanced sequence on day 3 shows an extensive central cord lesion extending from C2 to T7. The cord is swollen. Multiple areas of contrast enhancement were present throughout the cord (not shown). (B) Day 438: The cord edema has resolved and there is no longer contrast enhancement (not shown). Posterior fossa (C) and supratentorial (D) white matter changes were evident on a T2-weighted scan performed 3 days after symptom onset. These changes resolved on follow-up imaging (not shown). (E) The myelin oligodendrocyte glycoprotein antibody (MOG-Ab) titer fell rapidly following the commencement of immunotherapy (reference range < 1:160; red line). There was a steady improvement in modified Rankin Scale (mRS) score over 200 days (mRS = 1).
Methylprednisolone 1 g was given daily for 5 days, followed by high-dose oral prednisolone (70 mg/day). Five days of plasma exchange were performed, beginning on day 3 of symptoms. There was rapid improvement in encephalopathy. The sensory level descended to T4 and there was subtle lower limb improvement. After 3 weeks, plasma exchange was repeated, with further significant improvement in lower limb function and return of bladder control. Labile hypotension during plasma exchange was the only significant manifestation of autonomic dysfunction.
Cell-based assays for antibodies against AQP4 and the C-terminal–truncated human MOG were both negative (serum 1:20). However, subsequently the patient's serum was found to bind to full-length untagged human MOG (endpoint titer: 1:400). Titers of antibody to full-length untagged human MOG decreased in response to plasma exchange and weaning of oral steroids, paralleling the clinical progress (figure, C). Full-length untagged human MOG antibodies were negative by 6 months after presentation and remain negative more than 200 days after cessation of steroids (on day 180). No other immunosuppression was used. At 12 months after symptom onset, the patient has ongoing spasticity and mild sensory change but has returned to work as a nurse in primary care.
Discussion.
In our patient, a diagnosis of NMOSD was favored over ADEM because the patient's presentation was of severe LETM with only mild encephalopathy. We acknowledge, however, that there is much overlap between the clinical-radiologic features of NMOSD and limited forms of ADEM, and the presence or absence of autoantibodies to MOG or AQP4 are probably the markers that distinguish them in terms of pathogenic mechanisms and outcome.
This case illustrates that MOG antibodies may be missed using cell-based assays employing the short form of MOG3 and that testing for antibodies against full-length MOG is necessary in patients with LETM who are negative for antibodies to AQP4.1,2,4 The extracellular domain of MOG is common to both forms of the antigen. It is unclear why deletion of the cytosolic domain, which defines the short form, affects binding of antibodies to the extracellular domain, but it may be that this cytosolic domain affects surface expression of the protein or quaternary structure. This case demonstrates that using the full-length form of MOG provides a more sensitive assay.
This case also exemplifies the need to consider aggressive immunotherapy in a patient in whom antibody-mediated disease is suspected, even if proof of a positive antibody is not immediately forthcoming. Furthermore, although MOG-associated disease has been suggested as having a good outcome, if acute treatment is delayed, complications of illness and long-term disability may occur.
Footnotes
Author contributions: Dr. Morris: concept and design, acquisition of data, analysis and interpretation of results, care of patient. Dr. Waters: acquisition of data, analysis and interpretation of results, critical revision of the manuscript. Dr. Woodhall: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Kuker: acquisition of data, analysis and interpretation of results. Prof. Vincent: critical revision of the manuscript for important intellectual content. Dr. Leite: concept and design, analysis and interpretation of results, care of patient. Dr. Sen: concept and design, analysis and interpretation of results, critical revision of the manuscript for important intellectual content, care of patient, overall supervision.
Study funding: Oxford NIHR Biomedical Research Centre.
Disclosure: K.A. Morris reports no disclosures. P. Waters has received speaker honoraria from Biogen Idec Japan and Euroimmun AG; has been a review editor for Frontiers in Molecular Innate Immunity; and holds a patent for assays for the detection of antibodies to lgi, Caspr2, and tag-1. M.R. Woodhall reports no disclosures. W. Kuker is on the editorial board for Neuroradiology. A. Vincent has received travel funding and speaker honoraria from Baxter International Inc and Biogen Inc; is on the editorial board for Neurology; was an Associate Editor for Brain; holds patents with Oxford University for LGI1/CASPR2 antibodies, licensed to Euroimmun AG, and for GABAAR antibodies, in negotiation with Euroimmun AG; receives publishing royalties from Athena Diagnostics, Euroimmun AG, Blackwell Publishing, and Mac Keith Press; has consulted for Athena Diagnostics; and received research support from NIHR. M.I. Leite is supported by NHS National Specialised Commissioning Group for Neuromyelitis optica, UK and NIHR Oxford Biomedical Research Centre; has received speaking honoraria from Biogen Idec and travel and educational grants from Biogen Idec and UK; and is on the editorial board for Neuromuscular Disorders. A. Sen has received speaker honoraria and/or travel funding from UCB Pharma and Esai; is supported by the NIHR Oxford Biomedical Research Centre; and has received research support from Oxford Health Services Charities, John Radcliffe Hospital Oxford. Go to Neurology.org/nn for full disclosure forms. The Article Processing Charge was paid by the authors.
References
- 1.Kitley J, Waters P, Woodhall M, et al. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study. JAMA Neurol 2014;71:276–283 doi: 10.1001/jamaneurol.2013.5857. [DOI] [PubMed] [Google Scholar]
- 2.Sato DK, Callegaro D, Lana-Peixoto MA, et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology 2014;82:474–481 doi: 10.1212/WNL.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Connor KC, McLaughlin KA, De Jager PL, et al. Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat Med 2007;13:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reindl M, Di Pauli F, Rostásy K, Berger T. The spectrum of MOG autoantibody-associated demyelinating diseases. Nat Rev Neurol 2013;9:455–461 doi: 10.1038/nrneurol.2013.118. [DOI] [PubMed] [Google Scholar]