Abstract
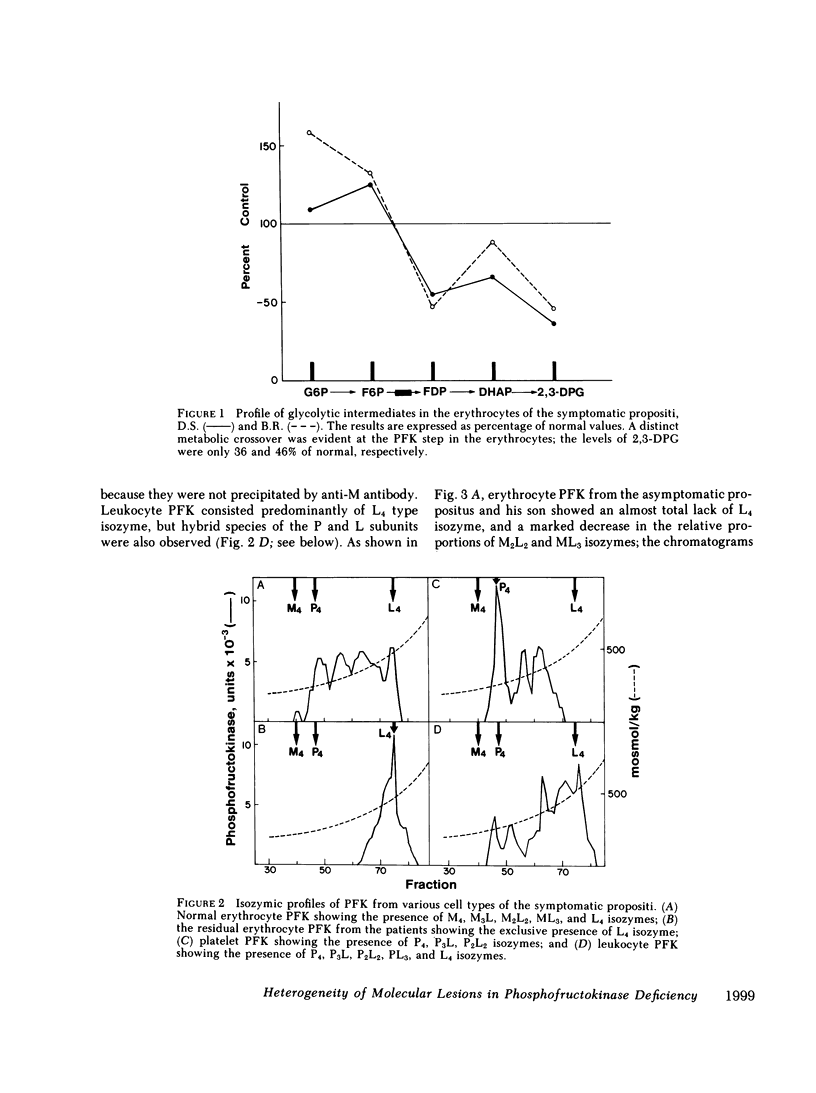
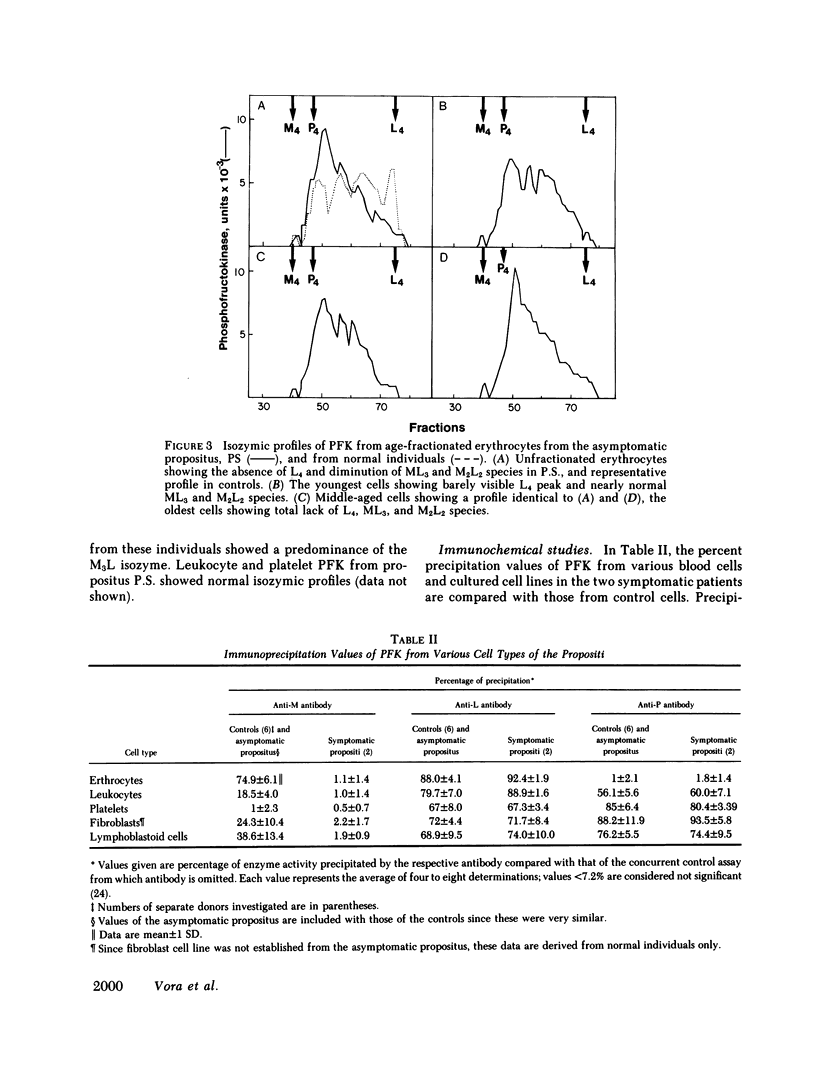
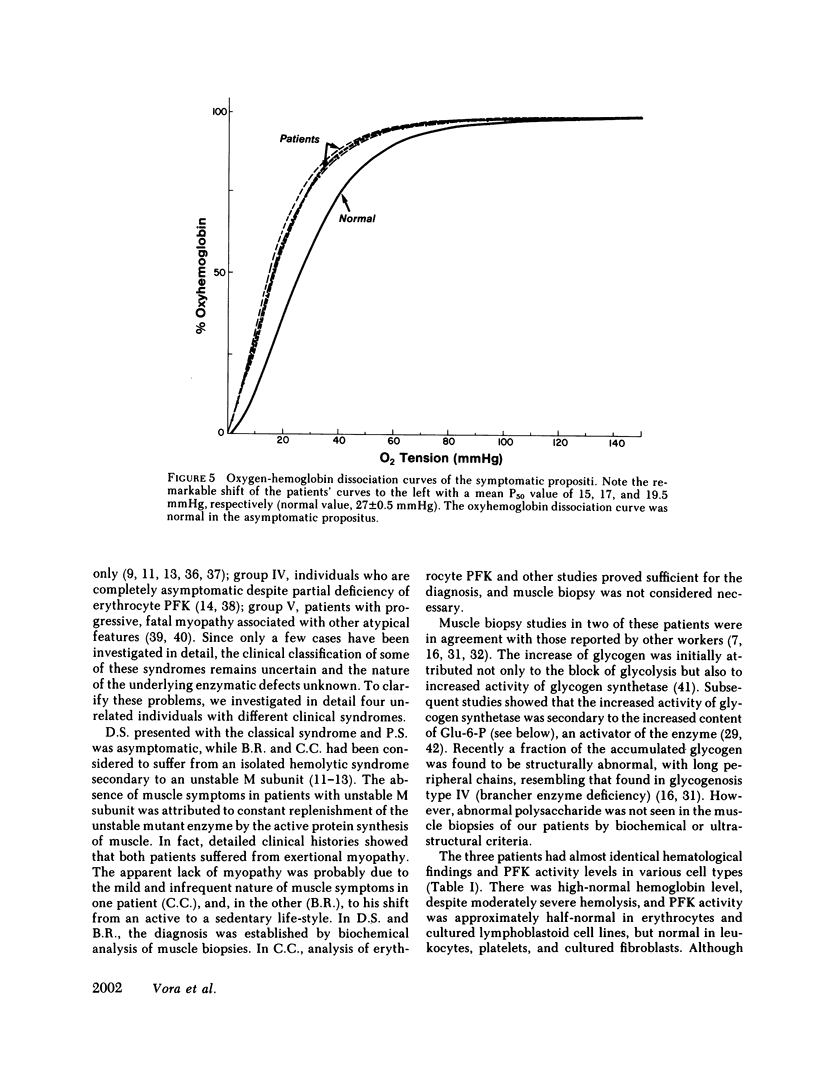
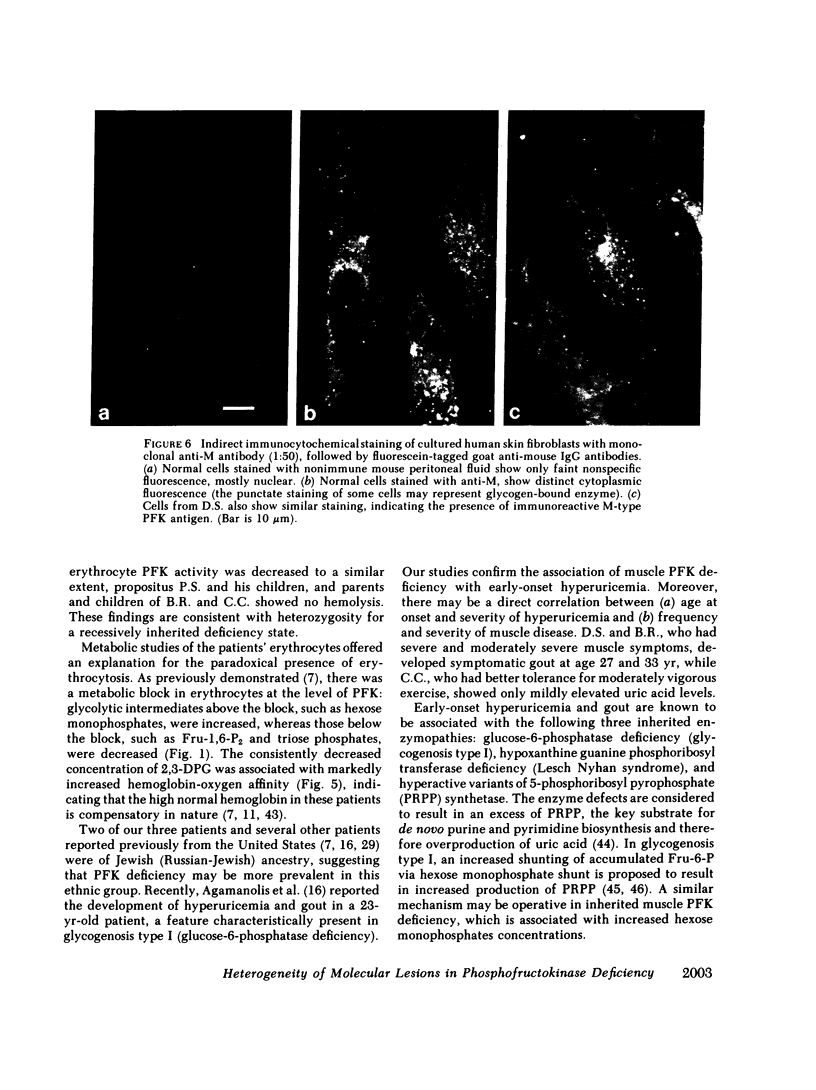
Human phosphofructokinase (PFK; EC 2.7.1.11) exists in tetrameric isozymic forms. Muscle and liver contain the homotetramers M4 and L4, whereas erythrocytes contain five isozymes composed of M (muscle) and L (liver) subunits, i.e., M4, M3L, M2L2, ML3, and L4. Inherited defects of erythrocyte PFK are usually partial and are described in association with heterogeneous clinical syndromes. To define the molecular basis and pathogenesis of this enzymopathy, we investigated four unrelated individuals manifesting myopathy and hemolysis (glycogenosis type VII), isolated hemolysis, or no symptoms at all. The three symptomatic patients showed high-normal hemoglobin levels, despite hemolysis and early-onset hyperuricemia. They showed total lack of muscle-type PFK and suffered from exertional myopathy of varying severity. In the erythrocytes, a metabolic crossover was evident at the PFK step: the levels of hexose monophosphates were elevated and those of 2,3-diphosphoglycerate (2,3-DPG) were depressed, causing strikingly increased hemoglobin-oxygen affinity. In all cases, the residual erythrocyte PFK consisted exclusively of L4 isozyme, indicating homozygosity for the deficiency of the catalytically active M subunit. However, presence of immunoreactive M subunit was shown in cultured fibroblasts by indirect immunofluorescence with monoclonal anti-M antibody. The fourth individual was completely asymptomatic, had normal erythrocyte metabolism, and had no evidence of hemolysis. His residual erythrocyte PFK showed a striking decrease of the L4, ML3, and M2L2 isozymes, secondary to a mutant unstable L subunit. Identical alterations of erythrocyte PFK were found in his asymptomatic son, indicating heterozygosity for the mutant unstable L subunit in this kindred. These studies show that, except for the varying severity of the myopathic symptoms, glycogenosis type VII has highly uniform clinical and biochemical features and results from homozygosity for mutant inactive M subunit(s). The absence of anemia despite hemolysis may be explained by the low 2,3-DPG levels. The hyperuricemia may result from hyperactivity of the hexose monophosphate shunt. In contrast, the clinically silent carrier state results from heterozygosity for mutant M or L subunit. Of the two, the M subunit appears to be more critical for adequate glycolytic flux in the erythrocyte, since its absence is correlated with hemolysis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agamanolis D. P., Askari A. D., Di Mauro S., Hays A., Kumar K., Lipton M., Raynor A. Muscle phosphofructokinase deficiency: two cases with unusual polysaccharide accumulation and immunologically active enzyme protein. Muscle Nerve. 1980 Nov-Dec;3(6):456–467. doi: 10.1002/mus.880030602. [DOI] [PubMed] [Google Scholar]
- Boulard M. R., Meienhofer M. C., Bois M., Reviron M., Najean Y. Letter: Red-cell phosphofructokinase deficiency. N Engl J Med. 1974 Oct 31;291(18):978–979. doi: 10.1056/nejm197410312911819. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Kimball H. R. Defective granulocyte chemotaxis in the Chediak-Higashi syndrome. J Clin Invest. 1971 Dec;50(12):2645–2652. doi: 10.1172/JCI106765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corash L. M., Piomelli S., Chen H. C., Seaman C., Gross E. Separation of erythrocytes according to age on a simplified density gradient. J Lab Clin Med. 1974 Jul;84(1):147–151. [PubMed] [Google Scholar]
- Corash L., Shafer B., Perlow M. Heterogeneity of human whole blood platelet subpopulations. II. Use of a subhuman primate model to analyze the relationship between density and platelet age. Blood. 1978 Oct;52(4):726–734. [PubMed] [Google Scholar]
- Davidson M., Miranda A. F., Bender A. N., DiMauro S., Vora S. Muscle phosphofructokinase deficiency. Biochemical and immunological studies of phosphofructokinase isozymes in muscle culture. J Clin Invest. 1983 Aug;72(2):545–550. doi: 10.1172/JCI111002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMauro S., Rowland L. P., DiMauro P. M. Control of glycogen metabolism in human muscle. Evidence from glycogen storage diseases. Arch Neurol. 1970 Dec;23(6):534–540. doi: 10.1001/archneur.1970.00480300056007. [DOI] [PubMed] [Google Scholar]
- Dupond J. L., Robert M., Carbillet J. P., Leconte des Floris R. Glycogénose musculaire et anémie hémolytique par déficit enzymatique chez deux germains. Forme familiale de maladie de Tarui, par déficit en phosphofructokinase musculaire et érythrocytaire. Nouv Presse Med. 1977 Sep 17;6(30):2665–2668. [PubMed] [Google Scholar]
- Etiemble J., Kahn A., Boivin P., Bernard J. F., Goudemand M. Hereditary hemolytic anemia with erythrocyte phosphofructokinase deficiency: studies of some properties of erythrocyte and muscle enzyme. Hum Genet. 1976 Jan 28;31(1):83–91. doi: 10.1007/BF00270403. [DOI] [PubMed] [Google Scholar]
- Etiemble J., Picat C., Siméon J., Blatrix C., Boivin P. Inherited erythrocyte phosphofructokinase deficiency: molecular mechanism. Hum Genet. 1980;55(3):383–390. doi: 10.1007/BF00290222. [DOI] [PubMed] [Google Scholar]
- Guibaud P., Carrier H., Mathieu M., Dorche C., Parchoux B., Béthenod M., Larbre F. Observation familiale de dystrophie musculaire congénitale par déficit en phosphofructokinase. Arch Fr Pediatr. 1978 Dec;35(10):1105–1115. [PubMed] [Google Scholar]
- Hays A. P., Hallett M., Delfs J., Morris J., Sotrel A., Shevchuk M. M., DiMauro S. Muscle phosphofructokinase deficiency: abnormal polysaccharide in a case of late-onset myopathy. Neurology. 1981 Sep;31(9):1077–1086. doi: 10.1212/wnl.31.9.1077. [DOI] [PubMed] [Google Scholar]
- Howell R. R. Hyperuricemia in childhood. Fed Proc. 1968 Jul-Aug;27(4):1078–1082. [PubMed] [Google Scholar]
- Kahn A., Etiemble J., Meienhofer M. C., Bovin P. Erythrocyte phosphofructokinase deficiency associated with an unstable variant of muscle phosphofructokinase. Clin Chim Acta. 1975 Jun 20;61(3):415–419. doi: 10.1016/0009-8981(75)90434-9. [DOI] [PubMed] [Google Scholar]
- Kahn A., Meienhofer M. C., Cottreau D., Lagrange J. L., Dreyfus J. C. Phosphofructokinase (PFK) isozymes in man. I. Studies of adult human tissues. Hum Genet. 1979 Apr 17;48(1):93–108. doi: 10.1007/BF00273280. [DOI] [PubMed] [Google Scholar]
- Kahn A., Weil D., Cottreau D., Dreyfus J. C. Muscle phosphofructokinase deficiency in man: expression of the defect in blood cells and cultured fibroblasts. Ann Hum Genet. 1981 Feb;45(Pt 1):5–14. doi: 10.1111/j.1469-1809.1981.tb00300.x. [DOI] [PubMed] [Google Scholar]
- Kelley W. N., Rosenbloom F. M., Seegmiller J. E., Howell R. R. Excessive production of uric acid in type I glycogen storage disease. J Pediatr. 1968 Apr;72(4):488–496. doi: 10.1016/s0022-3476(68)80339-7. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- Layzer R. B., Rowland L. P., Ranney H. M. Muscle phosphofructokinase deficiency. Arch Neurol. 1967 Nov;17(5):512–523. doi: 10.1001/archneur.1967.00470290066009. [DOI] [PubMed] [Google Scholar]
- Meienhofer M. C., Lagrange J. L., Cottreau D., Lenoir G., Dreyfus J. C., Kahn A. Phosphofructokinase in human blood cells. Blood. 1979 Aug;54(2):389–400. [PubMed] [Google Scholar]
- Miwa S., Sato T., Murao H., Kozuru M., Ibayashi H. A new type of phosphofructokinase deficiency hereditary nonspherocytic hemolytic anemia. Nihon Ketsueki Gakkai Zasshi. 1972 Feb;35(1):113–118. [PubMed] [Google Scholar]
- Piomelli S., Corash L. M., Davenport D. D., Miraglia J., Amorosi E. L. In vivo lability of glucose-6-phosphate dehydrogenase in GdA- and GdMediterranean deficiency. J Clin Invest. 1968 Apr;47(4):940–948. doi: 10.1172/JCI105786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman C., Wyss S., Piomelli S. The decline in energetic metabolism with aging of the erythrocyte and its relationship to cell death. Am J Hematol. 1980;8(1):31–42. doi: 10.1002/ajh.2830080105. [DOI] [PubMed] [Google Scholar]
- Serratrice G., Monges A., Roux H., Aquaron R., Gambarelli D. Forme myopathique du déficit en phosphofroctokinase. Rev Neurol (Paris) 1969 Apr;120(4):271–277. [PubMed] [Google Scholar]
- TARUI S., OKUNO G., IKURA Y., TANAKA T., SUDA M., NISHIKAWA M. PHOSPHOFRUCTOKINASE DEFICIENCY IN SKELETAL MUSCLE. A NEW TYPE OF GLYCOGENOSIS. Biochem Biophys Res Commun. 1965 May 3;19:517–523. doi: 10.1016/0006-291x(65)90156-7. [DOI] [PubMed] [Google Scholar]
- Tarlow M. J., Ellis D. A., Pearce G. W., Anderson M. Muscle phosphofructokinase deficiency (Tarui's disease). Proc Nutr Soc. 1979 Dec;38(3):110A–110A. [PubMed] [Google Scholar]
- Tarui S., Kono N., Kuwajima M., Ikura Y. Type VII glycogenosis (muscle and erythrocyte phosphofructokinase deficiency). Monogr Hum Genet. 1978;9:42–47. doi: 10.1159/000401609. [DOI] [PubMed] [Google Scholar]
- Tobin W. E., Huijing F., Porro R. S., Salzman R. T. Muscle phosphofructokinase deficiency. Arch Neurol. 1973 Feb;28(2):128–130. doi: 10.1001/archneur.1973.00490200076011. [DOI] [PubMed] [Google Scholar]
- Vora S., Corash L., Engel W. K., Durham S., Seaman C., Piomelli S. The molecular mechanism of the inherited phosphofructokinase deficiency associated with hemolysis and myopathy. Blood. 1980 Apr;55(4):629–635. [PubMed] [Google Scholar]
- Vora S., Durham S., de Martinville B., George D. L., Francke U. Assignment of the human gene for muscle-type phosphofructokinase (PFKM) to chromosome 1 (region cen leads to q32) using somatic cell hybrids and monoclonal anti-M antibody. Somatic Cell Genet. 1982 Jan;8(1):95–104. doi: 10.1007/BF01538653. [DOI] [PubMed] [Google Scholar]
- Vora S. Isozymes of human phosphofructokinase in blood cells and cultured cell lines: molecular and genetic evidence for a trigenic system. Blood. 1981 Apr;57(4):724–732. [PubMed] [Google Scholar]
- Vora S., Miranda A. F., Hernandez E., Francke U. Regional assignment of the human gene for platelet-type phosphofructokinase (PFKP) to chromosome 10p: novel use of polyspecific rodent antisera to localize human enzyme genes. Hum Genet. 1983;63(4):374–379. doi: 10.1007/BF00274765. [DOI] [PubMed] [Google Scholar]
- Vora S., Seaman C., Durham S., Piomelli S. Isozymes of human phosphofructokinase: identification and subunit structural characterization of a new system. Proc Natl Acad Sci U S A. 1980 Jan;77(1):62–66. doi: 10.1073/pnas.77.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora S., Wims L. A., Durham S., Morrison S. L. Production and characterization of monoclonal antibodies to the subunits of human phosphofructokinase: new tools for the immunochemical and genetic analyses of isozymes. Blood. 1981 Oct;58(4):823–829. [PubMed] [Google Scholar]
- Waterbury L., Frenkel E. P. Hereditary nonspherocytic hemolysis with erythrocyte phosphofructokinase deficiency. Blood. 1972 Mar;39(3):415–425. [PubMed] [Google Scholar]
- Zenella A., Mariani M., Meola G., Fagnani F., Sirchia G. Phosphofructokinase (PFK) deficiency due to a catalytically inactive mutant M-type subunit. Am J Hematol. 1982 May;12(3):215–225. doi: 10.1002/ajh.2830120303. [DOI] [PubMed] [Google Scholar]





