Abstract
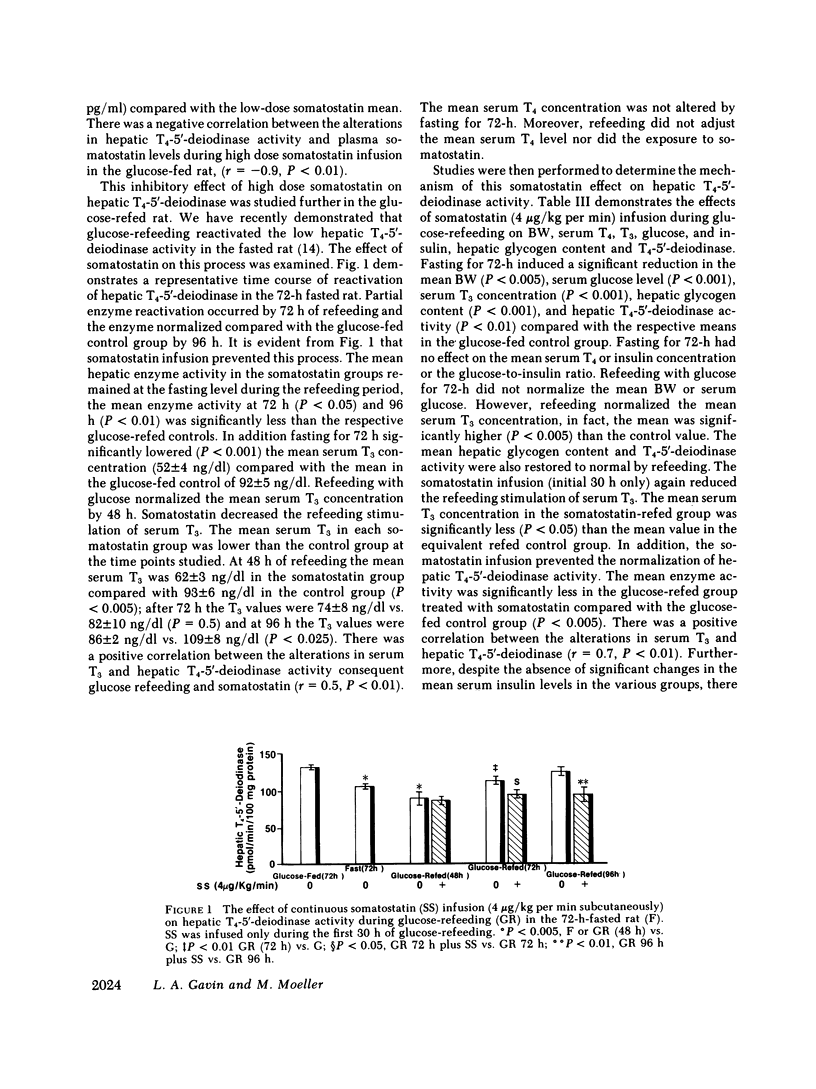
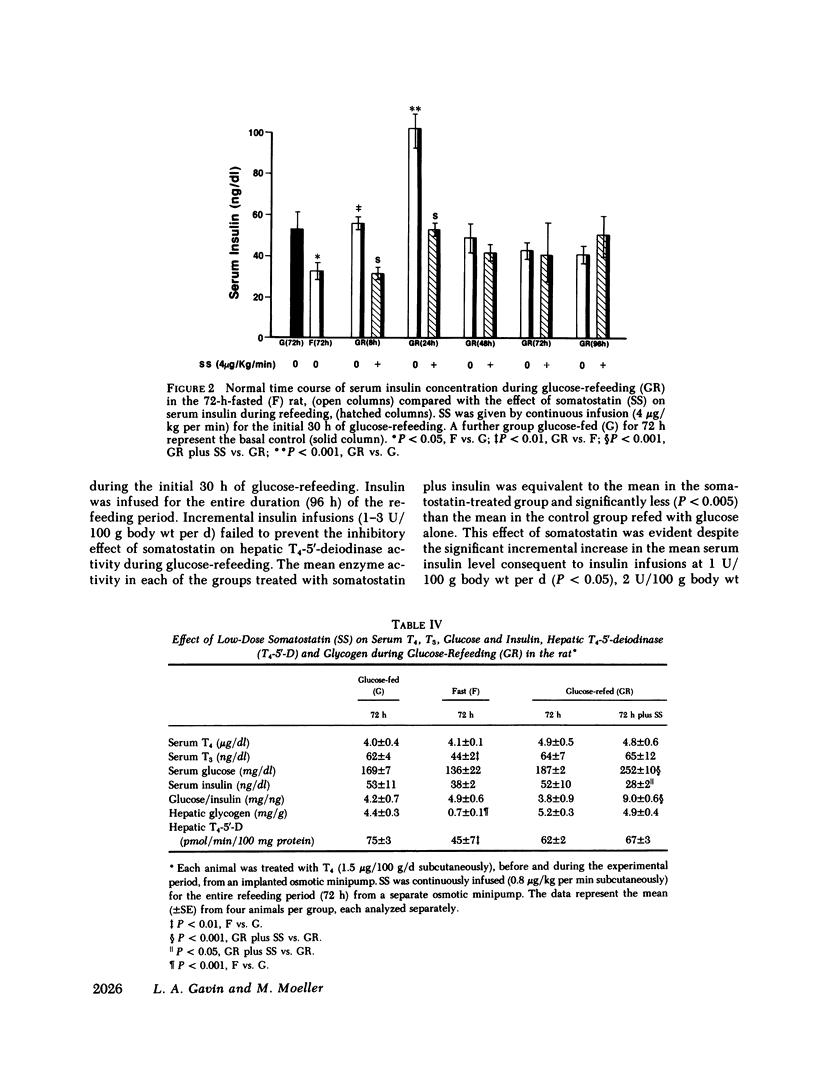
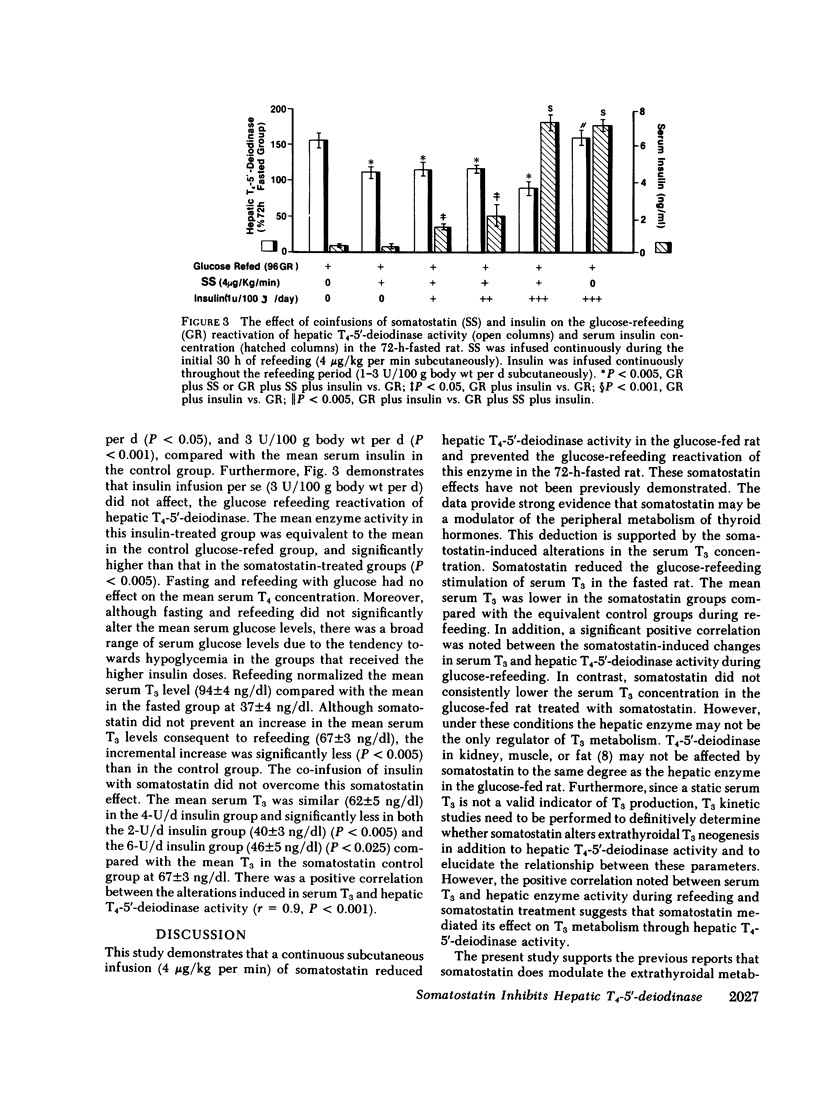
Somatostatin decreases the serum 3,5,3'-triiodothyronine (T3) concentration in athyreotic subjects treated with L-thyroxine (T4). The present study was performed to determine the effect of somatostatin on T4-5'-deiodinase activity in rat tissue homogenate preparations. This enzyme is an important regulator of T3 production. Continuous somatostatin infusion at high dose (4 micrograms/kg per min subcutaneously) and low dose (0.8 micrograms/kg per min subcutaneously) for 48-72 h significantly increased (P less than 0.001) the mean aorta plasma somatostatin-like immunoreactivity concentration to 786 +/- 65 and 448 +/- 58 pg/ml, respectively compared with the normal mean of 69 +/- 17 pg/ml in the carbohydrate-fed rat (20% glucose in water ad lib.). The mean hepatic T4-5'-deiodinase activity at both 48 h (100 +/- 5 pmol/min per 100 mg protein) and 72 h (90 +/- 7 pmol/min per 100 mg protein) was significantly reduced in the high-dose group (P less than 0.005), compared with the mean enzyme activity in the glucose-fed control group (138 +/- 6 pmol/min per 100 mg protein). There was a negative correlation (r = -0.9, P less than 0.01) between the alterations in the peripheral plasma somatostatin-like immunoreactivity concentration and hepatic T4-5'-deiodinase activity. High-dose somatostatin did not consistently lower the serum T3 concentration in the glucose-fed rat. Somatostatin had no effect on pituitary T4-5'-deiodinase activity in the glucose-fed rat. High-dose somatostatin also significantly inhibited (P less than 0.01) the glucose-refeeding reactivation of hepatic T4-5'-deiodinase in the 72-h-fasted rat. The mean enzyme activity after 96 h was 96 +/- 8 pmol/min per 100 mg protein compared with 127 +/- 4 pmol/min per 100 mg protein in the refed control group. Somatostatin had a similar inhibitory effect on serum T3. There was a positive correlation (r = 0.5, P less than 0.01) between the somatostatin-induced alterations in serum T3 and hepatic T4-5'-deiodinase during refeeding. A significant positive correlation (r = 07, P less than 0.005) was noted between the somatostatin effect on hepatic T4-5'-deiodinase activity and the induced hypoinsulinemia in the fed group. In addition, a significant negative correlation (r = -0.9, P less than 0.001) was noted between the suppressed enzyme activity and the serum glucose/insulin ratio in the refed group. However, although low-dose somatostatin also induced the same degree of hypoinsulinemia (P less than 0.05) in the fed and refed groups it had no effect on hepatic T4-5'-deiodinase activity. Furthermore, despite the induction of hyperinsulinemia during refeeding, the high dose somatostatin inhibitory effect on enzyme activity persisted. Thus, somatostatin inhibited hepatic T4-5'-deiodinase activity in the carbohydrate-fed rat and prevented the carbohydrate-refeeding normalization of enzyme activity in the 72-h-fasted rat. The effect of somatostatin on enzyme activity was independent of the associated hypoinsulinemia. In the carbohydrate-fed animal the somatostatin effect was selective, as the hormone had no effect on pituitary T4-5'-deiodinase activity. These data suggest that somatostatin could play a role in the peripheral metabolism of thyroid hormones.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berelowitz M., Kronheim S., Pimstone B., Shapiro B. Somatostatin-like immunoreactivity in rat blood. Characterization, regional differences, and responses to oral and intravenous glucose. J Clin Invest. 1978 May;61(5):1410–1414. doi: 10.1172/JCI109059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berelowitz M., Maeda K., Harris S., Frohman L. A. The effect of alterations in the pituitary-thyroid axis on hypothalamic content and in vitro release of somatostatin-like immunoreactivity. Endocrinology. 1980 Jul;107(1):24–29. doi: 10.1210/endo-107-1-24. [DOI] [PubMed] [Google Scholar]
- Cardell R. R., Larner J., Babcock M. B. Correlation between structure and glycogen content of livers from rats on a controlled feeding schedule. Anat Rec. 1973 Sep;177(1):23–37. doi: 10.1002/ar.1091770104. [DOI] [PubMed] [Google Scholar]
- Cavalieri R. R. Impaired peripheral conversion of thyroxine to triiodothyronine,. Annu Rev Med. 1977;28:57–65. doi: 10.1146/annurev.me.28.020177.000421. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Caldwell M. D., Dietz M. R., Exton J. H., Crofford O. B. The effect of somatostatin on glucose uptake and production by rat tissues in vitro. Diabetes. 1977 Aug;26(8):740–748. doi: 10.2337/diab.26.8.740. [DOI] [PubMed] [Google Scholar]
- Dhillon A. P., Rode J., Leathem A., Papadaki L. Somatostatin: a paracrine contribution to hypothyroidism in Hashimoto's thyroiditis. J Clin Pathol. 1982 Jul;35(7):764–770. doi: 10.1136/jcp.35.7.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel B. J., Grodsky G. M. Effect of continuous low-dose insulin treatment on subsequent incidence of diabetes in genetically prediabetic Chinese hamsters. Diabetes. 1979 Jun;28(6):544–547. doi: 10.2337/diab.28.6.544. [DOI] [PubMed] [Google Scholar]
- Gavin L. A., Bui F., McMahon F., Cavalieri R. R. Sequential deiodination of thyroxine to 3,3'-diiodothyronine via 3,5,3'-triiodothyronine and 3,3',5'-triiodothyronine in rat liver homogenate. The effects of fasting versus glucose feeding. J Biol Chem. 1980 Jan 10;255(1):49–54. [PubMed] [Google Scholar]
- Gavin L. A., McMahon F. A., Moeller M. Dietary modification of thyroxine deiodination in rat liver is not mediated by hepatic sulfhydryls. J Clin Invest. 1980 Apr;65(4):943–946. doi: 10.1172/JCI109751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin L. A., McMahon F. A., Moeller M. The mechanism of impaired T3 production from T4 in diabetes. Diabetes. 1981 Aug;30(8):694–699. doi: 10.2337/diab.30.8.694. [DOI] [PubMed] [Google Scholar]
- Gavin L. A., Moeller M. Glucagon does not modulate the alterations in T3 metabolism consequent to dietary manipulation and diabetes. Diabetes. 1983 Sep;32(9):798–803. doi: 10.2337/diab.32.9.798. [DOI] [PubMed] [Google Scholar]
- Gavin L. A., Moeller M. The mechanism of recovery of hepatic T4-5'-deiodinase during glucose-refeeding: role of glucagon and insulin. Metabolism. 1983 Jun;32(6):543–551. doi: 10.1016/0026-0495(83)90023-9. [DOI] [PubMed] [Google Scholar]
- Harris A. R., Fang S. L., Hinerfeld L., Braverman L. E., Vagenakis A. G. The role of sulfhydryl groups on the impaired hepatic 3',3,5-triiodothyronine generation from thyroxine in the hypothyroid, starved, fetal, and neonatal rodent. J Clin Invest. 1979 Mar;63(3):516–524. doi: 10.1172/JCI109330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. R., Fang S. L., Vagenakis A. G., Braverman L. E. Effect of starvation, nutriment replacement, and hypothyroidism on in vitro hepatic T4 to T3 conversion in the rat. Metabolism. 1978 Nov;27(11):1680–1690. doi: 10.1016/0026-0495(78)90290-1. [DOI] [PubMed] [Google Scholar]
- Hermansen K. Secretion of somatostatin from the normal and diabetic pancreas. Studies in vitro. Diabetologia. 1980;19(6):492–504. doi: 10.1007/BF00253175. [DOI] [PubMed] [Google Scholar]
- Hirooka Y., Hollander C. S., Suzuki S., Ferdinand P., Juan S. I. Somatostatin inhibits release of thyrotropin releasing factor from organ cultures of rat hypothalamus. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4509–4513. doi: 10.1073/pnas.75.9.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. M. Thyroxine 5'-monodeiodination in rat anterior pituitary homogenates. Endocrinology. 1980 Feb;106(2):567–576. doi: 10.1210/endo-106-2-567. [DOI] [PubMed] [Google Scholar]
- Kazumi T., Utsumi M., Yoshino G., Ishihara K., Hirose Y., Makimura H., Baba S. Somatostatin concentration responds to arginine in portal plasma: effects of fasting, streptozotocin diabetes, and insulin administration in diabetic rats. Diabetes. 1980 Jan;29(1):71–73. doi: 10.2337/diab.29.1.71. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larsen P. R. Thyroid-pituitary interaction: feedback regulation of thyrotropin secretion by thyroid hormones. N Engl J Med. 1982 Jan 7;306(1):23–32. doi: 10.1056/NEJM198201073060107. [DOI] [PubMed] [Google Scholar]
- Lins P. E., Efendić S., Hall K. Effect of 24-hour somatostatin infusion on glucose homeostasis and on the levels of somatomedin A and pancreatic and thyroid hormones in man. Acta Med Scand. 1979;206(6):441–445. doi: 10.1111/j.0954-6820.1979.tb13543.x. [DOI] [PubMed] [Google Scholar]
- Loos U., Raptis S., Birk J., Escobar-Jimenez F., Meyer G., Rothenbuchner G., Pfeiffer E. F. Inhibition of TSH-stimulated radioiodine turnover and release of T4 and T3 in vivo by somatostatin. Metabolism. 1978 Sep;27(9 Suppl 1):1269–1273. doi: 10.1016/0026-0495(78)90057-4. [DOI] [PubMed] [Google Scholar]
- Luft R., Efendić S., Hökfelt T. Somatostatin--both hormone and neurotransmitter? Diabetologia. 1978 Jan 14;14(1):1–13. doi: 10.1007/BF00429702. [DOI] [PubMed] [Google Scholar]
- SEIFTER S., DAYTON S. The estimation of glycogen with the anthrone reagent. Arch Biochem. 1950 Jan;25(1):191–200. [PubMed] [Google Scholar]
- Schimmel M., Utiger R. D. Thyroidal and peripheral production of thyroid hormones. Review of recent findings and their clinical implications. Ann Intern Med. 1977 Dec;87(6):760–768. doi: 10.7326/0003-4819-87-6-760. [DOI] [PubMed] [Google Scholar]
- Schusdziarra V., Harris V., Arimura A., Unger R. H. Evidence for a role of splanchnic somatostatin in the homeostasis of ingested nutrients. Endocrinology. 1979 Jun;104(6):1705–1708. doi: 10.1210/endo-104-6-1705. [DOI] [PubMed] [Google Scholar]
- Schusdziarra V., Harris V., Unger R. H. Half-life of somatostatin-like immunoreactivity in canine plasma. Endocrinology. 1979 Jan;104(1):109–110. doi: 10.1210/endo-104-1-109. [DOI] [PubMed] [Google Scholar]
- Senga O., Pittman C. S., Lindsay R. H., Chambers J. B., Jr, Hill J. B., Jr Comparison of peripheral thyroid hormone metabolism in normal rats and in rats receiving prolonged glucagon infusion. Endocrinology. 1982 Jun;110(6):2011–2017. doi: 10.1210/endo-110-6-2011. [DOI] [PubMed] [Google Scholar]
- Siler T. M., Yen S. C., Vale W., Guillemin R. Inhibition by somatostatin on the release of TSH induced in man by thyrotropin-releasing factor. J Clin Endocrinol Metab. 1974 May;38(5):742–745. doi: 10.1210/jcem-38-5-742. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Kaplan M. M., Cheron R. G., Dick T. E., Larsen P. R. Thyroxine to 3,5,3'-triiodothyronine conversion by rat anterior pituitary and liver. Metabolism. 1978 Nov;27(11):1601–1607. doi: 10.1016/0026-0495(78)90282-2. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Leonard J. L., Crantz F. R., Larsen P. R. Evidence for two tissue-specific pathways for in vivo thyroxine 5'-deiodination in the rat. J Clin Invest. 1982 May;69(5):1176–1184. doi: 10.1172/JCI110554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Noorden S., Polak J. M., Pearse A. G. Single cellular origin of somatostatin and calcitonin in the rat thyroid gland. Histochemistry. 1977 Aug 22;53(3):243–247. doi: 10.1007/BF00511079. [DOI] [PubMed] [Google Scholar]
- Visser T. J., Kaplan M. M., Leonard J. L., Larsen P. R. Evidence for two pathways of iodothyronine 5'-deiodination in rat pituitary that differ in kinetics, propylthiouracil sensitivity, and response to hypothyroidism. J Clin Invest. 1983 Apr;71(4):992–1002. doi: 10.1172/JCI110854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartofsky L., Burman K. D. Alterations in thyroid function in patients with systemic illness: the "euthyroid sick syndrome". Endocr Rev. 1982 Spring;3(2):164–217. doi: 10.1210/edrv-3-2-164. [DOI] [PubMed] [Google Scholar]
- Weeke J., Christensen S. E., Hansen A. P., Laurberg P., Lundbaek K. Somatostatin and the 24 h levels of serum TSH, T3, T4, and reverse T3 in normals, diabetics and patients treated for myxoedema. Acta Endocrinol (Copenh) 1980 May;94(1):30–37. doi: 10.1530/acta.0.0940030. [DOI] [PubMed] [Google Scholar]
- Weeke J., Hansen A. P., Lundaek K. Inhibition by somatostatin of basal levels of serum thyrotropin (TSH) in normal men. J Clin Endocrinol Metab. 1975 Jul;41(1):168–171. doi: 10.1210/jcem-41-1-168. [DOI] [PubMed] [Google Scholar]
- Wilson F. A., Antonson D. L., Hart B. L., Warr T. A., Cherrington A. D., Liljenquist J. E. The effect of somatostatin on the intestinal transport of glucose in vivo and in vitro in the rat. Endocrinology. 1980 May;106(5):1562–1567. doi: 10.1210/endo-106-5-1562. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Ito S., Matsubara Y., Kobayashi S. Immunohistochemical demonstration of somatostatin-containing cells in the human, dog and rat thyroids. Tohoku J Exp Med. 1977 May;122(1):87–92. doi: 10.1620/tjem.122.87. [DOI] [PubMed] [Google Scholar]


