Abstract
Epidermal growth factor (EGF) has been shown to facilitate the in vitro maturation of sheep oocytes, and enhance embryo’s capability for further development. However, such kind of molecular mechanism has not yet been elucidated. In the present study, we investigated the effect of EGF-mediated mitogen-activated protein kinases 3 and 1 (MAPK3/1) pathway on in vitro maturation of sheep oocytes. U0126, a specific inhibitor of MEK (MAPK kinase), was added into the maturation culture medium to block the EGF-mediated MAPK3/1 pathway with different doses. Then, the nuclear maturation of sheep oocytes was examined. Additionally, the effect of EGF-mediated MAPK3/1 on cytoplasmic maturation was examined though in vitro fertilization and embryonic development. The rate of germinal vesicle breakdown (GVBD) after 6 h of culture with 10−4 mol/l of U0126 (50.4%) was significantly decreased compared with control (67.2%, p < 0.05), and the first polation body (PB1) extrusion rate after 22 h of culture in drug treatment was also significantly inhibited compared with control (28.6% vs. 48.4%, p < 0.05). However, 10−6 mol/l U0126 had slight effect on oocyte nuclear maturation. The normal distribution rate of α-tubulin in the oocytes after 22 h of in vitro maturation was significantly decreased in the 10−4 mol/l U0126 group (54%) compared with control (68%, p < 0.05). After in vitro fertilization, the cleavage rate in drug treatments (56.8% in 10−6 mol/l U0126 group and 42.6% in 10−4 mol/l U0126 group) was significantly decreased compared with control (72.3%, p < 0.01). The blastocyst rate in 10−4 mol/l U0126 group (17.6%) was also significantly decreased compared with control (29.9%, p < 0.05). Collectively, these results suggest that EGF-mediated MAPK3/1 pathway is conducive to in vitro maturation of sheep oocytes.
Introduction
In mammals, luteinizing hormone (LH) from the pituitary induces a sequential and transient expression of the epidermal growth factor (EGF)-like growth factors such as epiregulin, amphiregulin, and betacellulin expressed in mural granulosa cells (MGCs) [1, 2]. These growth factors then activate common EGF receptor (EGFR) in cumulus cells to stimulate oocyte maturation [1, 3]. Recently, it has been reported that EGF-like growth factors could also regulate maternal mRNA translation and developmental competence of mouse oocytes by activation of the PI(3)K-AKT-mTOR pathway [4].
The oocyte is maintained in meiotic prophase arrest by natriuretic peptide type C (NPPC) acting via its cognate receptor, natriuretic peptide receptor 2 (NPR2) [5]. Some evidences show that EGFR signaling induces meiotic resumption by downregulating Nppc mRNA expression [6], and decreases NPR2 guanylyl cyclase activity via the elevation of calcium concentrations of cumulus cells [7]. Both of which lead to the decrease of cGMP levels in the follicle [8–10]. Anyway, the key downstream effectors of EGFR signaling in cumulus cells, mitogen-activated protein kinases 3 and 1 (MAPK3/1, also known as ERK1/2), is essential for mammalian oocyte maturation [3, 10, 11]. It has been demonstrated that, in mouse and pig oocytes, MAPK3/1 pathway is essential for spindle assembly and microtubule organization during mammalian oocyte meiosis [12, 13]. In bovine oocytes, it is responsible for MII arrest, maintenance of maturation-promoting factor (MPF) activity, and spindle organization [14].
The maturation of mammalian oocytes is a complex and dynamic process involving the maturation of nucleus and cytoplasm [15]. Only oocytes with a mature nucleus and cytoplasm are able to support normal fertilization and further embryonic development [16, 17]. At present, the acquirement of large livestock’s mature oocytes is difficult, so it is necessary to obtain massive in vitro matured oocytes with high quality, in spite that the developmental competence of oocytes matured in vitro is markedly inferior to that of their in vivo matured counterparts [18–20].
Many studies show that the activation of EGFR by the addition of EGF in vitro can facilitate the maturation of sheep oocytes, and enhance the embryo’s capability for further development [21–23]. However, the mechanism is unclear. This study was aimed to investigate the effect of EGF-mediated MAPK3/1 pathway on in vitro maturation of sheep oocytes. U0126, a specific inhibitor of MEK (MAPK kinase), was added to the maturation culture medium to block the EGF-mediated downstream MAPK3/1 pathway. Then, the maturation of nucleus was examined. Additionally, the cytoplasmic maturation was examined through in vitro fertilization and embryonic development.
Materials and Methods
Ethics Statement
Animal welfare and experimental procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Ministry of Science and Technology of China, 2006), and were approved by the animal ethics committee of Beijing University of Agriculture.
Oocyte collection and maturation culture
Sheep ovaries were collected from Fuhua slaughterhouse (Dachang Hui Autonomy County, Hebei province) and were immediately placed in 25–28°C normal saline containing penicillin-streptomycin, then transported to the laboratory within 2–4 h. The ovaries were washed three times in normal saline and trimmed to remove fat and the corpus luteum. Then, ovaries were placed in a petri dish containing oocyte pick-up solution (Table 1) and removed the ovarian follicle. According to the test needs, cumulus-oocyte complexes were sorted out under a stereomicroscope (Olympus SZ40, Tokyo, Japan). The selection criteria were as follows: complete morphology, dense cytoplasm, uniform color, at least three layers of granular cells, and dense encapsulation.
Table 1. The recipes of reagents.
| Reagent | Recipe |
|---|---|
| Oocyte pick-up solution | TCM199 + 25 mmol/l HEPES + 2.2 mg/ml NaHCO3 + 2% FCS + 100 IU/ml streptomycin + 100 μg/ml penicillin |
| Maturation culture medium I | TCM199 + 10 mmol/l HEPES + 2.2 mg/ml NaHCO3 + 8 mg/ml BSA + 0.25 mmol/l sodium pyruvate + 2.75 mmol/l lactate + 100 IU/ml penicillin + 100 μg/ml streptomycin + 50 ng/ml EGF +1 μg/ml E2 + 10 μg/ml LH + 10 μg/ml FSH |
| Maturation culture medium II | Maturation culture medium I with 10−6 mol/l U0126 |
| Maturation culture medium III | Maturation culture medium I with 10−4 mol/l U0126 |
| SOF working solution | SOF stock solution + 1 mmol/l glutamine + 0.3 mmol/l sodium pyruvate |
| Oocyte washing solution | SOF working solution + 10 mmol/l HEPES + 5 mmol/l NaHCO3 + 0.3% BSA + 100 IU/ml penicillin + 100 μg/ml streptomycin |
| Sperm-washing solution | The same ingredients as oocyte washing solution, with a double amount of double antibody |
| Capacitation solution | SOF working solution + 20% estrous sheep serum + 10 mmol/l penicillamine + 10 mmol/l hypotaurine + 10 μg/ml heparin + 0.5 mol/l calcium lactate + 100 IU/ml penicillin + 100 μg/ml streptomycin |
| Embryo culture medium | SOF working solution + 10% FCS + 2% essential amino acids (BME-EAA) + 1% nonessential amino acids (MEM-NEAA) + 100 IU/ml penicillin + 100 μg/ml streptomycin |
U0126 (Sigma-Aldrich, MO, USA) was added into the maturation culture medium with different concentrations (0, 10−6, and 10−4 mol/l), and each group had three replicates. Then three groups of oocytes were respectively rinsed using different maturation culture medium by two times and transferred into 50 μl of pre-equilibrated maturation medium droplets (10 oocytes each). The incubation was performed at 38.5°C in an atmosphere of 5% CO2 under saturated humidity.
Determination of oocyte nuclear maturation
Examination of GVBD.
Examination of GVBD. After 6 h of in vitro maturation, different groups of oocytes were treated with the oocyte washing solution containing 0.1% hyaluronidase by mechanical pipetting to remove cumulus cells. The obtained oocytes were placed on a glass slide with droplets of paraffin wax: vaseline (1:9, v/v) in the four corners. Cells were covered with a coverslip and then fixed for more than 24 h in ethanol: acetic acid (3:1, v/v). Then, cells were stained with 1% aceto-orcein for 1–2 min. GVBD were examined under a biological microscope (Nicon YS2, Tokyo, Japan).
Examination of PB1 extrusion.
After 24 h of in vitro maturation, different groups of oocytes were subjected to mechanical pipetting with the oocyte washing solution containing 0.1% hyaluronidase to remove cumulus cells. The extrusion of PB1 was examined under a stereomicroscope (Olympus SZ40, Tokyo, Japan) and taken as an indication of oocyte nuclear maturation for data analysis.
Immunofluorescence labeling of α-tubulin.
Different experimental groups of sheep oocytes were collected at varying maturity periods (4, 8, 12, and 24 h). The oocytes were digested in 0.5% hyaluronidase to completely remove granulosa cells, and the zona pellucida was removed with phosphate-buffered saline (PBS) (pH 2.5). The digested oocytes were fixed in 4% paraformaldehyde at room temperature for 20 min and then placed in 0.2% Triton-X100 (Sigma-Aldrich, MO, USA) for 30 min of osmosis. Thereafter, oocytes were incubated in a blocking agent (PBS +2% BSA + 10% goat serum + 2% skim milk powder + 0.15 mol/l glycine) at 37°C for 1 h, followed by the addition of FITC-conjugated mouse anti-human α-tubulin monoclonal antibody (ab64503) (Abcam DM1A, Cambridge, UK) with a final concentration of 1 μg/ml [24] and incubation at 37°C for another 1 h. The immunogen of antibody is full length native protein (purified) of Chicken alpha Tubulin (extracted from brain). At the end of incubation, oocytes were thoroughly washed in 0.2% Triton-X100, 5 μg/ml propidium iodide (Sigma-Aldrich, MO, USA) was added, and then they were placed in a cassette for 10 min of nuclide labeling. Finally, oocytes were examined under a confocal microscope (ZEISS LSM 510 META, Oberkochen, Germany).
In vitro fertilization of oocytes and embryo culture
Sperm capacitation.
Fresh semen was washed twice with sperm-washing solution by centrifugation at 1500 rpm/min for 5 min. The supernatant was decanted and sperm at the bottom of the centrifuge tube was added to the pre-equilibrated capacitation solution for 30 min at 38.5°C in an atmosphere of 5% CO2 under saturated humidity.
In vitro fertilization.
Mature oocytes were digested with 0.5% hyaluronidase to partially remove granulosa cells. The oocytes were then washed thrice with the capacitation solution and transferred into fertilization droplets. Sperm was added to the fertilization droplet containing oocytes. Each drop contained 10 μl of sperm with a density of 5 × 106 sperm/ml. The oocyte-sperm complex was incubated for 17–19 h at 38.5°C in a 5% CO2 atmosphere under saturated humidity.
Embryo culture.
After 17–19 h of oocyte-sperm co-incubation, zygotes were washed thrice with the oocyte washing solution to remove granulosa cells and sperm. After twice washes with the embryo culture medium, zygotes were transferred into droplet containing monolayer granulosa cell and incubated at 38.5°C in a 5% CO2 atmosphere under saturated humidity. Half the medium was exchanged with new medium every other day. The cleavage rate was determined at 48 h and the blastocyst rate was estimated at 7d.
Data analysis
All experiments were performed at least 3 times. The data were subjected to chi-square analysis using SAS 9.0 statistical software (SAS Institute Inc., Cary, NC, USA). A p-value less than 0.05 was considered statistically significant.
Results
Oocyte nuclear maturation in different treatment groups
The GVBD rate of sheep oocytes was examined after 6 h of in vitro maturation. After 6 h of in vitro maturation, the GVBD rate of mature sheep oocytes in the 10−4 mol/l U0126 group (50.4%) was significantly decreased compared with control (67.2%, p < 0.05). There was no significant differenceS between the 10−6 mol/l U0126 group and the control group (55.2% vs 67.2%, p > 0.05) (Table 2).
Table 2. Statistics of germinal vesicle breakdown (GVBD) rate of sheep oocytes in different treatment groups after 6 h of in vitro maturation.
| Group | Total number of oocytes | Number of GVBD oocytes | GVBD rate (%) |
|---|---|---|---|
| Control | 113 | 76 | 67.2 (76/113)a |
| 10−6 mol/l U0126 group | 105 | 58 | 55.2 (58/105)ab |
| 10−4 mol/l U0126 group | 105 | 53 | 50.4 (53/105)b |
Note: The same superscript letters in the same column indicate no statistically significant differences (p > 0.05); different superscript letters in the same column indicate statistically significant differences (p < 0.05).
The PB1 extrusion rate of sheep oocytes was examined after 24 h of in vitro maturation. After 24 h of culture, the PB1 extrusion rate of mature sheep oocytes in the 10−4 mol/l U0126 group (28.6%) was significantly decreased compared with control (48.4%, p < 0.05). There was no significant differences between the 10−6 mol/l U0126 group and the control group (32.3% vs 48.4%, p > 0.05) (Table 3). Microscopic characteristics of sheep oocyte maturation stained with aceto-orcein are illustrated in Fig. 1.
Table 3. Statistics of the first polar body (PB1) extrusion rate of sheep oocytes in different treatment groups after 24 h of in vitro maturation.
| Group | Total number of oocytes | Number of oocytes with PB1 extrusion | PB1 extrusion rate(%) |
|---|---|---|---|
| Control | 62 | 30 | 48.4(30/42)a |
| 10−6 mol/l U0126 group | 65 | 21 | 32.3(21/65)ab |
| 10−4 mol/l U0126 group | 70 | 20 | 28.6(20/70)b |
Note: The same superscript letters in the same column indicate no statistically significant differences (p > 0.05); different superscript letters in the same column indicate statistically significant differences (p < 0.05).
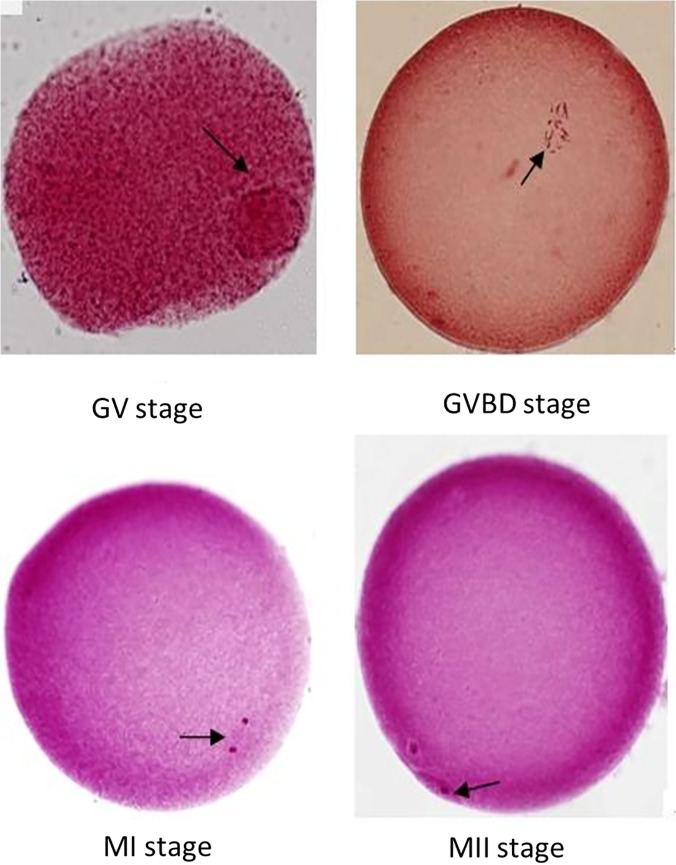
Fig 1. Microscopic characteristics of sheep oocyte maturation stained with aceto-orcein (200× magnification).
(A) Oocyte at GV stage, arrow points to germinal vesicle; (B) oocyte at GVBD stage, arrow points to condensed chromosomes; (C) oocyte at late spindle stage, arrow points to spindle fibers; and (D) oocyte at middle MII stage, arrow points to the first polar body.
The distribution of α-tubulin in different treatment groups
The distribution of α-tubulin in sheep oocytes at different maturity periods were examined by immunofluorescence staining (Fig. 2). As the culture time elapsed, the amount of α-tubulin distributed around chromosomes increased. In treatments, the occurrence of α-tubulin aggregation around chromosomes and the spindle formation in oocytes were later than that in control. After 18 h of in vitro maturation, 69% (48/70) of oocytes in the control group entered anaphase of meiosis, some oocytes even entered the metaphase of meiosis, i.e., the MII stage. But in the 10−4 mol/l U0126 group, approximately 50% (36/71) of oocytes remained in the metaphase of meiosis I.
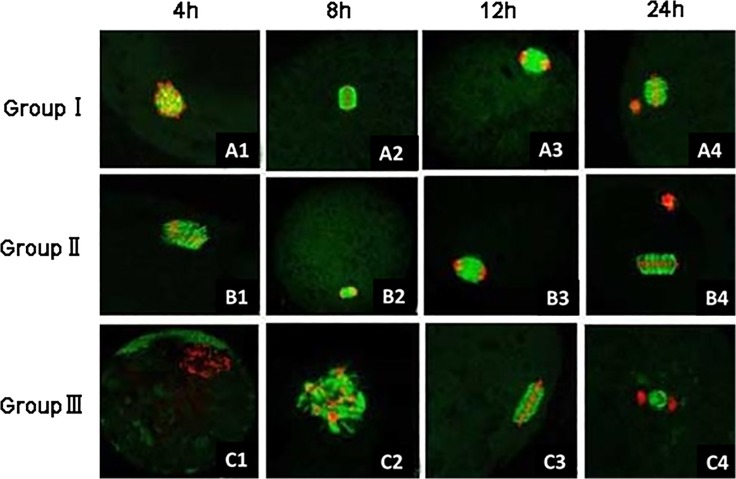
Fig 2. The α-tubulin distribution around chromosomes in sheep oocytes after different periods of in vitro maturation.
Red indicates chromosomes and green indicates α-tubulin. (A1-C1, 4 h) Oocytes show GVBD in the control group (group I, A1, 10×40) and the 10−4 mol/l U0126 group (group III, C1, 10×20), but remain in the germinal vesicle stage in the 10−6 mol/l U0126 group (group II, B1,10×40); (A2-C2, 8 h) chromosome condensation (A2, 10×40), beginning of chromosome condensation (B2,10×20), and GVBD (C2, 10×40); (A3-C3, 12 h) telophase of meiosis I (A3, 10×20; B3, 10×40), and chromosome condensation (C3, 10×40); (A4–C4, 24 h) meiosis II (A4, B4, 10×40), and telophase of meiosis I (C4, 10×20).
The distribution of α-tubulin in sheep oocyte nucleus after 22 h of in vitro maturation
The experimental results were interpreted in accordance with the classification of α-tubulin by Miyara [14]. Fig. 3A–C show an abnormal distribution of α-tubulin, and Fig. 3D–F show a normal distribution. Statistics of the distribution of α-tubulin in different groups of sheep oocytes after 22 h of in vitro maturation are shown in Table 4.
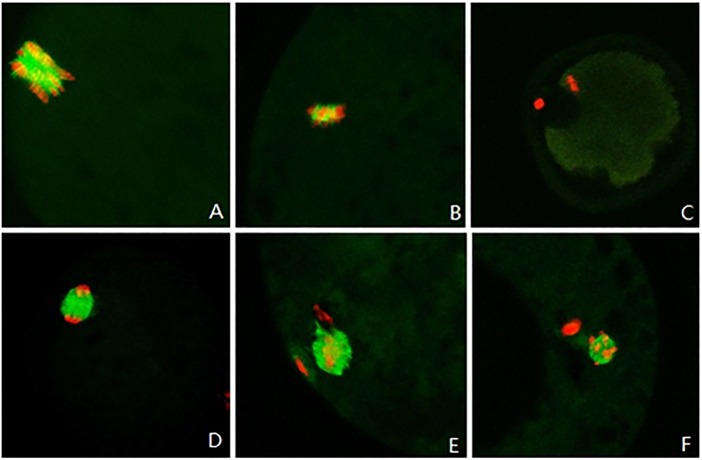
Fig 3. The α-tubulin distribution around chromosomes in sheep oocytes after 22 h of in vitro maturation.
Red indicates chromosomes and green indicates α-tubulin. (A, 10×40) Disorderly distribution of chromosomes; (B, 10×40) little α-tubulin distributed around chromosomes; (C,10×20) nearly no α-tubulin distributed around chromosomes; (D, 10×40) α-tubulin distributed on a spindle; and (E, F, 10×40) formation of microtubules and extrusion of polar bodies at meiosis II.
Table 4. Statistics of the distribution of α-tubulin of sheep oocytes in different treatment groups after 22 h of in vitro maturation.
| Group | Total number of oocytes | Number of oocytes with normal distribution of α-tubulin | Normal distribution rate of α-tubulin (%) |
|---|---|---|---|
| Control | 152 | 103 | 68a |
| 10−6 mol/l U0126 group | 149 | 88 | 59ab |
| 10−4 mol/l U0126 group | 155 | 85 | 54b |
Note: The same superscript letters in the same column indicate no statistically significant differences (p > 0.05); different superscript letters in the same column indicate statistically significant differences (p < 0.05).
Statistical analysis showed that the normal distribution rate of α-tubulin in sheep oocytes after 22 h of in vitro maturation decreased with an increasing dose of the inhibitor, with 54% in the 10−4 mol/l U0126 group, significantly lower than 68% in the control group (p < 0.05). This indicated that the addition of U0126 affected normal expression of α-tubulin and its distribution in sheep oocytes.
In vitro fertilization of sheep oocytes in different treatment groups
After in vitro fertilization, sheep oocytes in theatments had significantly lower cleavage rates than those in the control group (p < 0.01). The blastocyst rate of oocytes in the 10−4 mol/l U0126 group (13.0%) was significantly decreased compared with control (29.9%, p < 0.05), with no significant differences between the 10−6 mol/l U0126 group and the control group (24.6% vs 29.9%, p > 0.05) (Table 5).
Table 5. The cleavage and blastocyst rates of sheep oocytes in different treatment groups after in vitro fertilization.
| Group | Number of embryos transferred | Number of cleaved embryos | Cleavage rate (%) | Number of blastocysts | Blastocyst rate (%) |
|---|---|---|---|---|---|
| Control | 137 | 99 | 72.3 (99/137) A | 41 | 29.9(41/137)a |
| 10−6 mol/l U0126 group | 118 | 67 | 56.8 (67/118) Ba | 29 | 24.6(29/118)a |
| 10−4 mol/l U0126 group | 108 | 46 | 42.6 (46/108) Bb | 19 | 17.6(19/108)b |
Note: The same superscript letters in the same column indicate no statistically significant differences (p > 0.05); different superscript letters in the same column indicate statistically significant differences (p < 0.05).
Discussion
EGF has been shown to facilitate the in vitro maturation of sheep oocytes, and enhance embryo’s capability for further development [21–23]. However, its molecular mechanism underlying which has not been defined clearly. This study focused on investigating the effect of the EGF-mediated MAPK3/1 pathway on in vitro maturation of sheep oocytes. We used U0126, a specific inhibitor of MEK, to block the EGF-mediated MAPK3/1 pathway. The results showed that the addition of U0126 decreased the GVBD rate and the PB1 extrusion rate of sheep oocytes, and affected the normal expression and the distribution of α-tubulin in sheep oocytes. Further study showed that U0126 could reduce the cleavage and blastocyst rate after in vitro fertilization. Thus, these results suggest that EGF-mediated MAPK3/1 pathway is conducive to in vitro maturation of sheep oocytes.
As a specific inhibitor of MEK, U0126 has been widely used in studies of oocyte maturation with effective inhibition of MAPK3/1 activity [25–27]. More than 80% inhibition of MEK enzymic activity could be achieved by 10−6–10−5 mol/l U0126 in somatic cells [28]. In our study, 10−6 mol/l U0126 had no effect on the nuclear maturation and blastocyst development, but 10−4 mol/l U0126 exhibited a significant effect on them. These results were consistent with previous studies that block of ERK1/2 activity by 10−5 mol/l U0126 only slightly inhibited EGF-induced meiotic resumption [7], and only 10−4 mol/l U0126 could completely inhibit the effect of LH-induced GVB in cultured mouse follicles [29].
MAPK3/1 are co-expressed in all mammalian tissues and play a pivotal role in GVBD in oocytes. While the activation time of MAPK3/1 were variable among species. In rat, mouse and goat, their activation occurred after GVBD, which meant that they just involved in regulating post-GVBD events and were not required for GVBD [30–32]. In pig, cattle and Xenopus, MAPK3/1 were activated before GVBD or synchronously to induce GVBD [33–35]. However, the effect of MAPK3/1 on sheep GVBD was unknown. As can be seen from our results, addition of U0126 inhibited EGF-induced GVBD. This indicates that the MAPK3/1 pathway could affect the occurrence of GVBD in sheep.
The PB1 extrusion rate as a symbol of the oocyte nuclear maturation, is very important for embryo development after in vitro fertilization. Sakaguchi reported that the supplement of EGF and insulin-like growth factor-1 (IGF-1) could significantly increase the frequency of oocytes with PB1 at 16 h of culture (p < 0.05) [36]. But the mechanism is unclear. Our results showed that the PB1 extrusion rate of mature sheep oocytes in the 10−4 mol/l U0126 group was significantly decreased compared with control (28.6% vs 48.4%, p < 0.05), indicating that EGF could promote the extrusion of PB1 during sheep oocyte maturation in vitro via MAPK3/1 pathway.
In oocytes, after GVBD and PB1 extrusion, chromosomes in secondary oocytes form a metaphase plate with the long axis parallel to the cell membrane surface, which indicates that the oocyte nucleus has matured. The distribution of chromosomes and the morphology of the spindle in mature oocytes impact the development of the fertilized oocyte. Disorderly spindle assembly can cause abnormal distribution of chromosomes in oocytes, thereby preventing the extrusion of the first and the second polar body. It may also lead to the formation of aneuploid zygotes [37]. Therefore, the assembly and normal distribution of tubulin should be one of the focuses for evaluating the quality of the mature oocyte nucleus. Our results showed that, among different treatments of sheep oocytes, there existed obvious morphological differences of the aggregation and the distribution of α-tubulin around chromosomes after 22 h of in vitro maturation. The rate of oocytes in the telophase of meiosis in the control group was significantly increased compared with the drug treatments. Combined comparison with the distribution of α-tubulin at different time showed that the above phenomenon was possibly related to the speed of α-tubulin aggregation around chromosomes and the formation of microtubules, thereby affecting the distribution and separation time of chromosomes. These showed that U0126 significantly inhibited the expression of α-tubulin and its aggregation around chromosomes. This demonstrated that the EGF-mediated MAPK3/1 pathway could promote normal distribution of chromosomes and α-tubulin in sheep oocytes, further improving the quality of oocytes’ nuclear maturation, which were consistent with previous observations in mouse and pig oocytes [38, 39]. Furthermore, EGF has been shown to exert positive effects on cleavage rate and blastocyst formation during in vitro maturation process of sheep oocytes [21–23, 40]. Our results showed that MAPK3/1 affected the cleavage and blastocyst rates after in vitro fertilization of oocytes. All of these results demonstrate that EGF could regulate in vitro maturation of sheep oocytes via the MAPK3/1 pathway.
However, some studies suggest that MAPK3/1 are necessary but not sufficient to induce oocyte maturation. In cultured cumulus-oocyte complexes, activation of MAPK3/1 in cumulus cells with growth differentiation factor 9 (GDF9) alone is not sufficient to stimulate oocyte maturation [29]. Reduced but measureable levels of phosphorylated MAPK3/1 are induced by LH in Areg −/− Egfr wa2/wa2 follicles, yet oocyte meiotic resumption is impaired [41]. Recent studies have demonstrated that an additional pathway may involved in LH-induced oocyte maturation. It has been shown that NPPC increases cGMP levels in granulosa cells via activation of NPR2, then the cGMP diffusing into oocyte via gap junctions, where it acts to maintain meiotic arrest by inhibiting phosphodiesterase (PDE) 3A activity and cAMP hydrolysis [2, 42, 43]. In addition, activation of LH receptors decreases both Nppc and Npr2 mRNA expression [44, 45]. LH treatment also results in a reduction in NPR2 activity in mouse ovarian follicles, contributing to the decrease of cGMP leveles [45]. Thus, LH-induced decrease in NPPC content and NPR2 activity may reduce cGMP levels in the follicle, which enabling the oocytes to resume meiosis [45]. However, both MAPK3/1 pathway and NPPC/NPR2 are essential components of the LH signaling required to oocyte maturation, the correlation between them remains unclear. Further investigations into that will provide a better understanding of oocyte maturation in mammals and will be helpful for further improving the in vitro culture system of sheep oocytes.
Acknowledgments
We thank Di Chang and Hongwu Yang for their help with the data analysis. We also thank Ya Gao, Hongbin Liang, and Zili Lin for sampling.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 31272526, Grant No.31402049), the Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges Under Beijing Municipality (Grant No. PXM2013_014207_000067), and the science and technology project of Beijing Municipal Commission of Education (Grant No. PXM 2014_014207_000001). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. [DOI] [PubMed] [Google Scholar]
- 2. Zhang M, Xia G. Hormonal control of mammalian oocyte meiosis at diplotene stage. Cellular and molecular life sciences: CMLS. 2012;69:1279–1288. 10.1007/s00018-011-0867-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Su YQ, Wigglesworth K, Pendola FL, O'Brien MJ, Eppig JJ. Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology. 2002;143:2221–2232. [DOI] [PubMed] [Google Scholar]
- 4. Chen J, Torcia S, Xie F, Lin CJ, Cakmak H, Franciosi F, et al. Somatic cells regulate maternal mRNA translation and developmental competence of mouse oocytes. Nature cell biology. 2013;15:1415–1423. 10.1038/ncb2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 2010;330:366–369. 10.1126/science.1193573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsuji T, Kiyosu C, Akiyama K, Kunieda T. CNP/NPR2 signaling maintains oocyte meiotic arrest in early antral follicles and is suppressed by EGFR-mediated signaling in preovulatory follicles. Molecular reproduction and development. 2012;79:795–802. 10.1002/mrd.22114 [DOI] [PubMed] [Google Scholar]
- 7. Wang Y, Kong N, Li N, Hao X, Wei K, Xiang X, et al. Epidermal growth factor receptor signaling-dependent calcium elevation in cumulus cells is required for NPR2 inhibition and meiotic resumption in mouse oocytes. Endocrinology. 2013;154:3401–3409. 10.1210/en.2013-1133 [DOI] [PubMed] [Google Scholar]
- 8. Vaccari S, Weeks JL 2nd, Hsieh M, Menniti FS, Conti M. Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biology of reproduction. 2009;81:595–604. 10.1095/biolreprod.109.077768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Norris RP, Freudzon M, Nikolaev VO, Jaffe LA. Epidermal growth factor receptor kinase activity is required for gap junction closure and for part of the decrease in ovarian follicle cGMP in response to LH. Reproduction. 2010;140:655–662. 10.1530/REP-10-0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Conti M, Hsieh M, Zamah AM, Oh JS. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Molecular and cellular endocrinology. 2012;356:65–73. 10.1016/j.mce.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, et al. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–941. 10.1126/science.1171396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fan HY, Tong C, Lian L, Li SW, Gao WX, Cheng Y, et al. Characterization of ribosomal S6 protein kinase p90rsk during meiotic maturation and fertilization in pig oocytes: mitogen-activated protein kinase-associated activation and localization. Biology of reproduction. 2003;68:968–977. [DOI] [PubMed] [Google Scholar]
- 13. Choi T, Fukasawa K, Zhou R, Tessarollo L, Borror K, Resau J, et al. The Mos/mitogen-activated protein kinase (MAPK) pathway regulates the size and degradation of the first polar body in maturing mouse oocytes. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:7032–7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gordo AC, He CL, Smith S, Fissore RA. Mitogen activated protein kinase plays a significant role in metaphase II arrest, spindle morphology, and maintenance of maturation promoting factor activity in bovine oocytes. Molecular reproduction and development. 2001;59:106–114. [DOI] [PubMed] [Google Scholar]
- 15. Martino A, Palomo MJ, Mogas T, Paramio MT. Influence of the collection technique of prepubertal goat oocytes on in vitro maturation and fertilization. Theriogenology. 1994;42:859–873. [DOI] [PubMed] [Google Scholar]
- 16. Mermillod POB, Cognié Y. Aspects of follicular and oocyte maturation that affect the developmental potential of embryos. J Reprod Fertil Suppl. 1999;54:449–460. [PubMed] [Google Scholar]
- 17. Trounson A, Anderiesz C, Jones G. Maturation of human oocytes in vitro and their developmental competence. Reproduction. 2001;121:51–75. [DOI] [PubMed] [Google Scholar]
- 18. Morgan PM, Warikoo PK, Bavister BD. In vitro maturation of ovarian oocytes from unstimulated rhesus monkeys: assessment of cytoplasmic maturity by embryonic development after in vitro fertilization. Biology of reproduction. 1991;45:89–93. [DOI] [PubMed] [Google Scholar]
- 19. Barnes FL, Crombie A, Gardner DK, Kausche A, Lacham-Kaplan O, Suikkari AM, et al. Blastocyst development and birth after in-vitro maturation of human primary oocytes, intracytoplasmic sperm injection and assisted hatching. Hum Reprod. 1995;10:3243–3247. [DOI] [PubMed] [Google Scholar]
- 20. Kim DH, Ko DS, Lee HC, Lee HJ, Park WI, Kim SS, et al. Comparison of maturation, fertilization, development, and gene expression of mouse oocytes grown in vitro and in vivo. Journal of assisted reproduction and genetics. 2004;21:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guler A, Poulin N, Mermillod P, Terqui M, Cognie Y. Effect of growth factors, EGF and IGF-I, and estradiol on in vitro maturation of sheep oocytes. Theriogenology. 2000;54:209–218. [DOI] [PubMed] [Google Scholar]
- 22. Shabankareh HK, Zandi M. Developmental potential of sheep oocytes cultured in different maturation media: effects of epidermal growth factor, insulin-like growth factor I, and cysteamine. Fertility and sterility. 2010;94:335–340. 10.1016/j.fertnstert.2009.01.160 [DOI] [PubMed] [Google Scholar]
- 23. Grazul-Bilska AT, Choi JT, Bilski JJ, Weigl RM, Kirsch JD, Kraft KC, et al. Effects of epidermal growth factor on early embryonic development after in vitro fertilization of oocytes collected from ewes treated with follicle stimulating hormone. Theriogenology. 2003;59:1449–1457. [DOI] [PubMed] [Google Scholar]
- 24. Li M, Zhao HC, Li R, Yu Y, Qiao J. Chromosomal aberrations in in-vitro matured oocytes influence implantation and ongoing pregnancy rates in a mouse model undergoing intracytoplasmic sperm injection. PloS one. 2014;9:e103347 10.1371/journal.pone.0103347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kondoh E, Tachibana K, Deguchi R. Intracellular Ca2+ increase induces post-fertilization events via MAP kinase dephosphorylation in eggs of the hydrozoan jellyfish Cladonema pacificum. Developmental biology. 2006;293:228–241. [DOI] [PubMed] [Google Scholar]
- 26. Philipova R, Kisielewska J, Lu P, Larman M, Huang JY, Whitaker M. ERK1 activation is required for S-phase onset and cell cycle progression after fertilization in sea urchin embryos. Development. 2005;132:579–589. [DOI] [PubMed] [Google Scholar]
- 27. Phillips KP, Petrunewich MA, Collins JL, Booth RA, Liu XJ, Baltz JM. Inhibition of MEK or cdc2 kinase parthenogenetically activates mouse eggs and yields the same phenotypes as Mos(-/-) parthenogenotes. Developmental biology. 2002;247:210–223. [DOI] [PubMed] [Google Scholar]
- 28. Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. The Journal of biological chemistry. 1998;273:18623–18632. [DOI] [PubMed] [Google Scholar]
- 29. Su YQ, Denegre JM, Wigglesworth K, Pendola FL, O'Brien MJ, Eppig JJ. Oocyte-dependent activation of mitogen-activated protein kinase (ERK1/2) in cumulus cells is required for the maturation of the mouse oocyte-cumulus cell complex. Developmental biology. 2003;263:126–138. [DOI] [PubMed] [Google Scholar]
- 30. Dedieu T, Gall L, Crozet N, Sevellec C, Ruffini S. Mitogen-activated protein kinase activity during goat oocyte maturation and the acquisition of meiotic competence. Molecular reproduction and development. 1996;45:351–358. [DOI] [PubMed] [Google Scholar]
- 31. Lu Q, Smith GD, Chen DY, Yang Z, Han ZM, Schatten H, et al. Phosphorylation of mitogen-activated protein kinase is regulated by protein kinase C, cyclic 3',5'-adenosine monophosphate, and protein phosphatase modulators during meiosis resumption in rat oocytes. Biology of reproduction. 2001;64:1444–1450. [DOI] [PubMed] [Google Scholar]
- 32. Lee SE, Kim JH, Kim NH. Inactivation of MAPK affects centrosome assembly, but not actin filament assembly, in mouse oocytes maturing in vitro. Molecular reproduction and development. 2007;74:904–911. [DOI] [PubMed] [Google Scholar]
- 33. Ferrell JE Jr, Wu M, Gerhart JC, Martin GS. Cell cycle tyrosine phosphorylation of p34cdc2 and a microtubule-associated protein kinase homolog in Xenopus oocytes and eggs. Molecular and cellular biology. 1991;11:1965–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fissore RA, He CL, Vande Woude GF. Potential role of mitogen-activated protein kinase during meiosis resumption in bovine oocytes. Biology of reproduction. 1996;55:1261–1270. [DOI] [PubMed] [Google Scholar]
- 35. Inoue M, Naito K, Aoki F, Toyoda Y, Sato E. Activation of mitogen-activated protein kinase during meiotic maturation in porcine oocytes. Zygote. 1995;3:265–271. [DOI] [PubMed] [Google Scholar]
- 36. Sakaguchi M, Dominko T, Leibfried-Rutledge ML, Nagai T, First NL. A combination of EGF and IGF-I accelerates the progression of meiosis in bovine follicular oocytes in vitro and fetal calf serum neutralizes the acceleration effect. Theriogenology. 2000;54:1327–1342. [DOI] [PubMed] [Google Scholar]
- 37. Miyara F, Aubriot FX, Glissant A, Nathan C, Douard S, Stanovici A, et al. Multiparameter analysis of human oocytes at metaphase II stage after IVF failure in non-male infertility. Hum Reprod. 2003;18:1494–1503. [DOI] [PubMed] [Google Scholar]
- 38. Sun QY, Lai L, Park KW, Kuhholzer B, Prather RS, Schatten H. Dynamic events are differently mediated by microfilaments, microtubules, and mitogen-activated protein kinase during porcine oocyte maturation and fertilization in vitro. Biology of reproduction. 2001;64:879–889. [DOI] [PubMed] [Google Scholar]
- 39. Verlhac MH, de Pennart H, Maro B, Cobb MH, Clarke HJ. MAP kinase becomes stably activated at metaphase and is associated with microtubule-organizing centers during meiotic maturation of mouse oocytes. Developmental biology. 1993;158:330–340. [DOI] [PubMed] [Google Scholar]
- 40. Kelly JM, Kleemann DO, Maxwell WM, Walker SK. Effects of insulin-like growth factor-I, epidermal growth factor and cysteamine on the in vitro maturation and development of oocytes collected from 6- to 8-week-old Merino lambs. Reproduction, fertility, and development. 2008;20:570–578. [DOI] [PubMed] [Google Scholar]
- 41. Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, et al. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Molecular and cellular biology. 2007;27:1914–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Richards JS. New signaling pathways for hormones and cyclic adenosine 3',5'-monophosphate action in endocrine cells. Mol Endocrinol. 2001;15:209–218. [DOI] [PubMed] [Google Scholar]
- 43. Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, et al. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development. 2009;136:1869–1878. 10.1242/dev.035238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kawamura K, Cheng Y, Kawamura N, Takae S, Okada A, Kawagoe Y, et al. Pre-ovulatory LH/hCG surge decreases C-type natriuretic peptide secretion by ovarian granulosa cells to promote meiotic resumption of pre-ovulatory oocytes. Hum Reprod. 2011;26:3094–3101. 10.1093/humrep/der282 [DOI] [PubMed] [Google Scholar]
- 45. Robinson JW, Zhang M, Shuhaibar LC, Norris RP, Geerts A, Wunder F, et al. Luteinizing hormone reduces the activity of the NPR2 guanylyl cyclase in mouse ovarian follicles, contributing to the cyclic GMP decrease that promotes resumption of meiosis in oocytes. Developmental biology. 2012;366:308–316. 10.1016/j.ydbio.2012.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.