Abstract
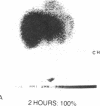
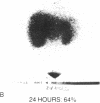
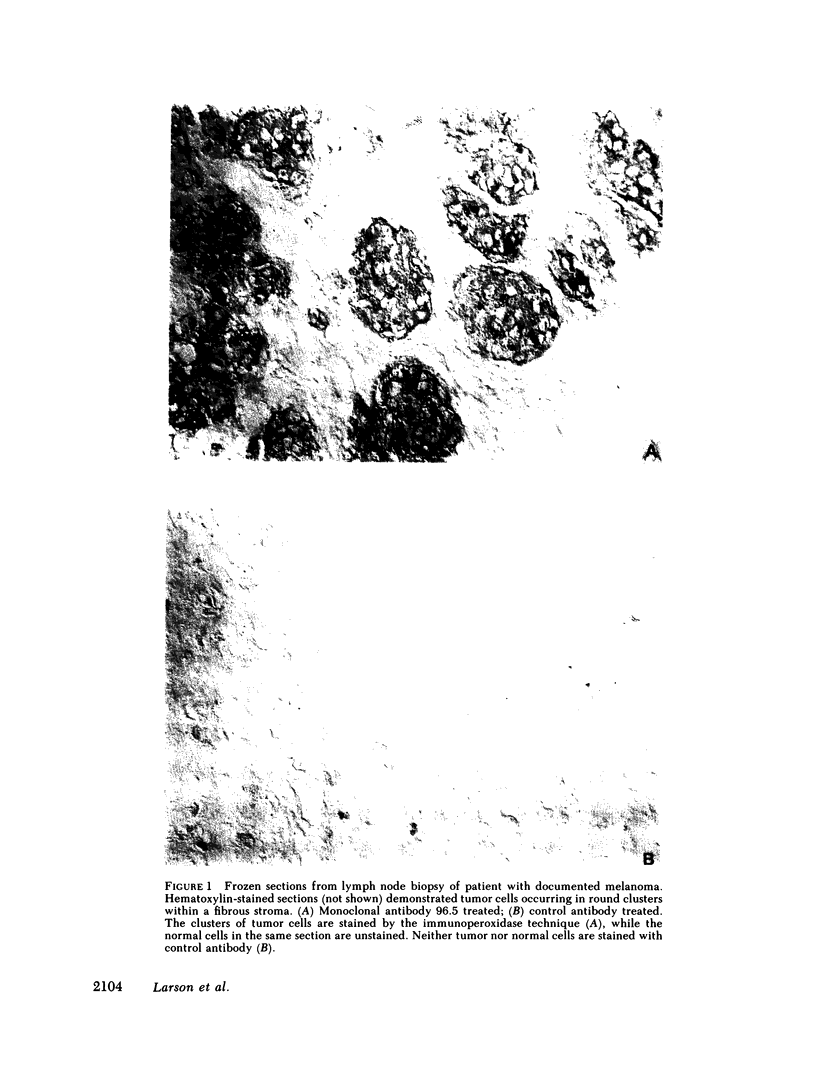
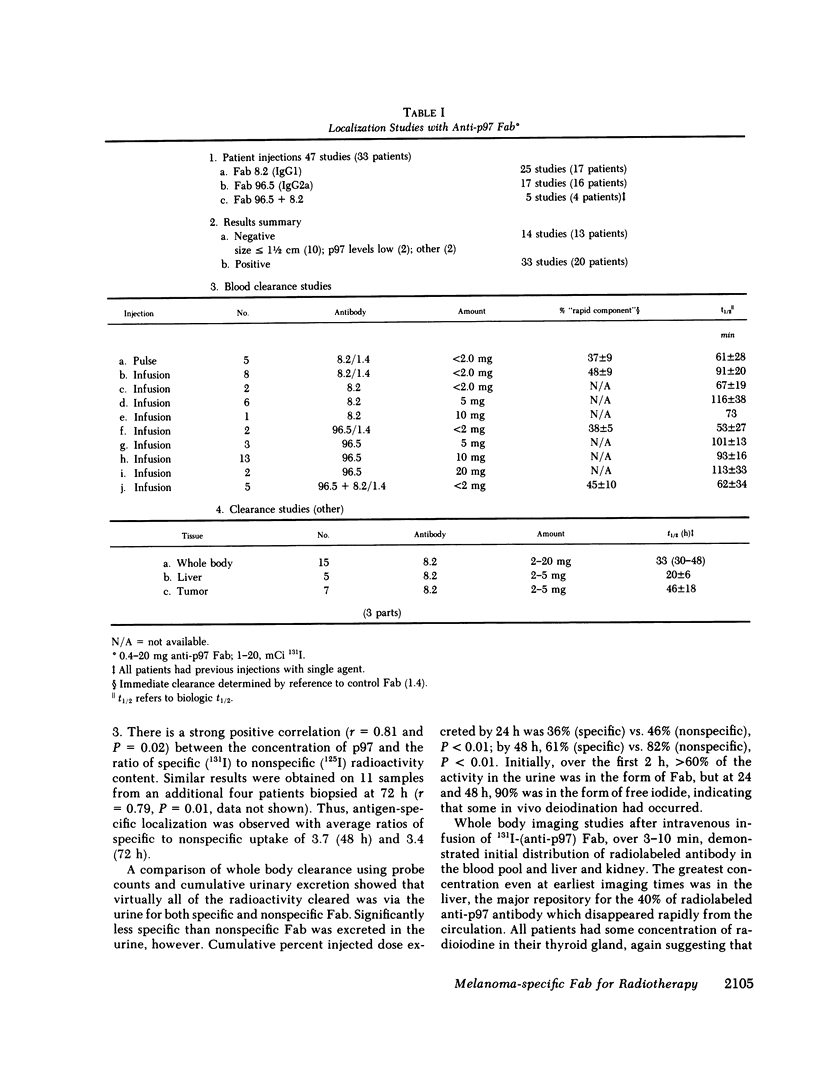
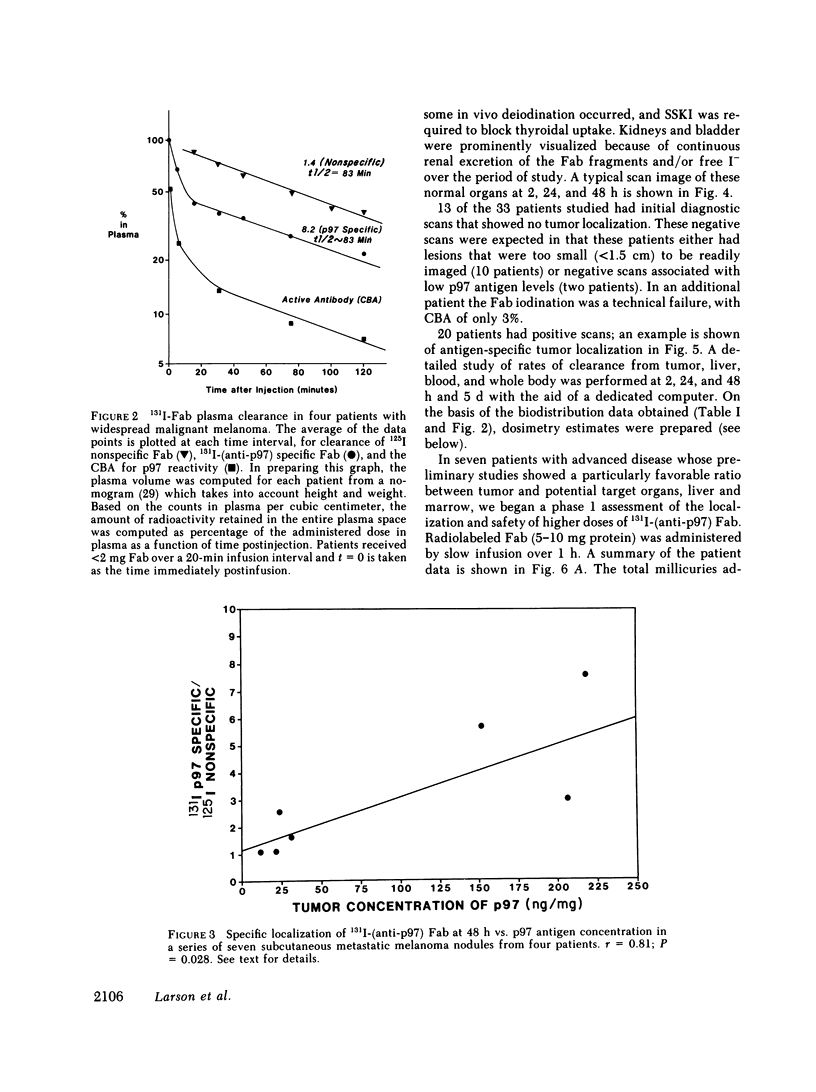
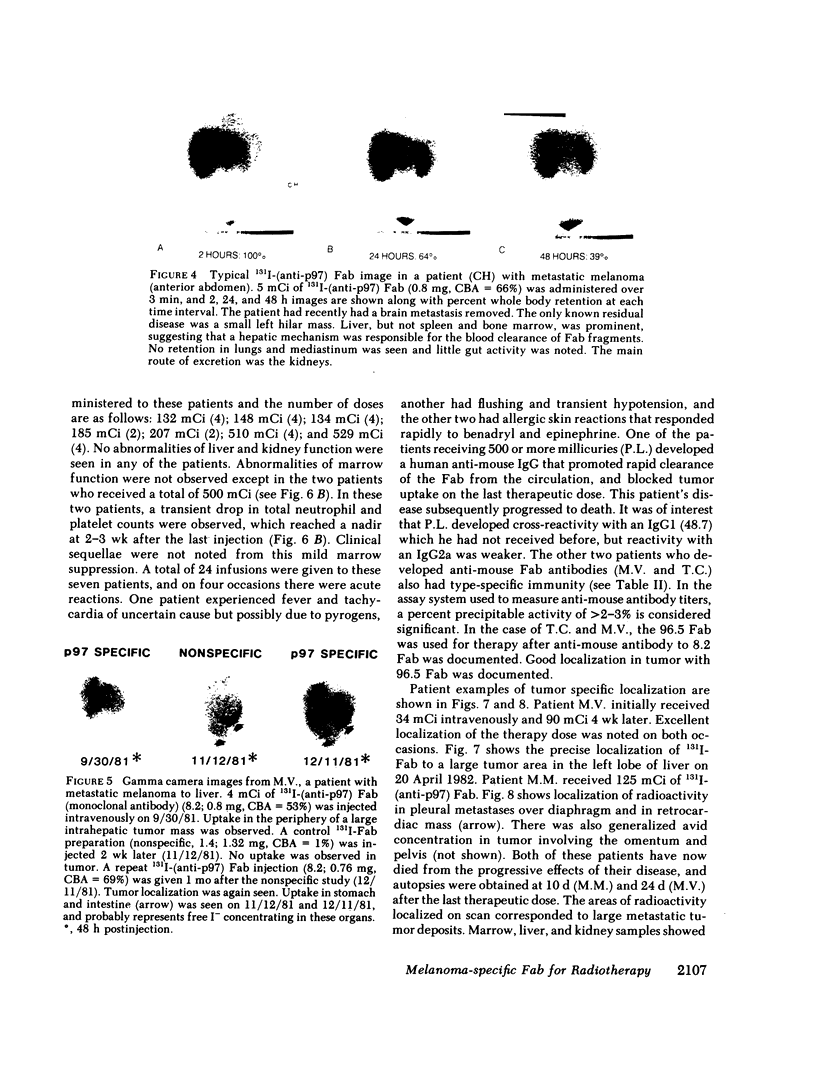
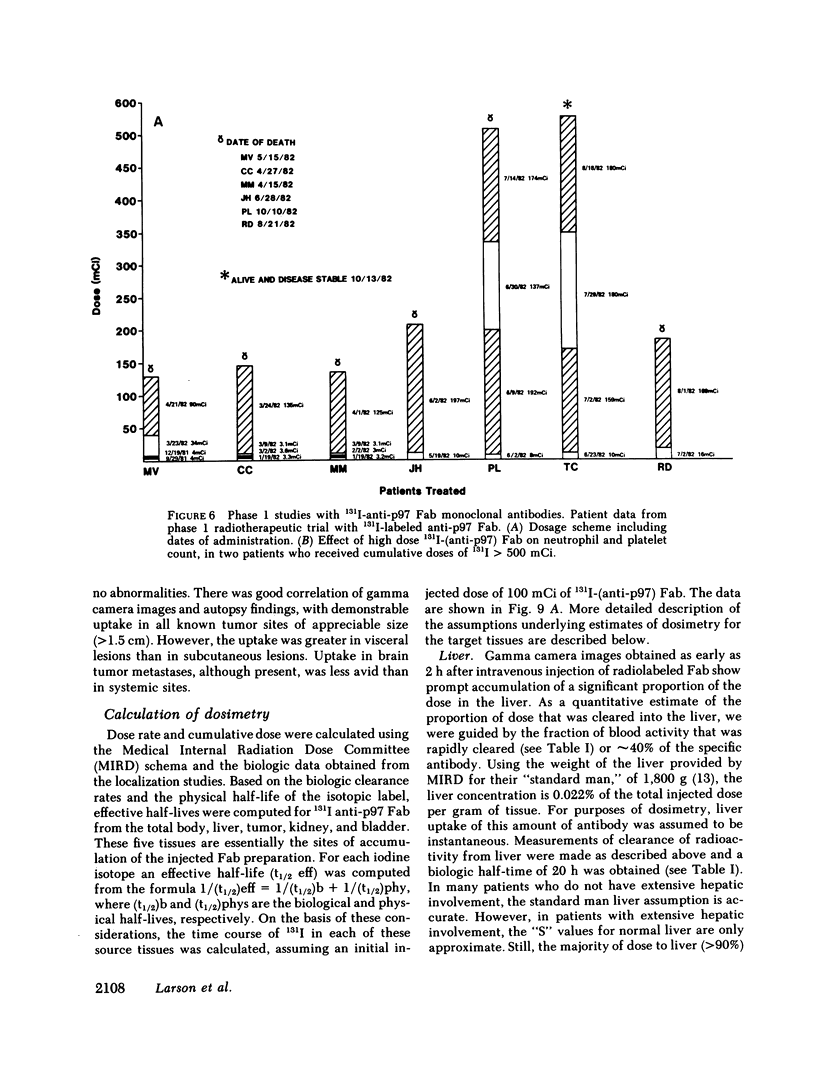
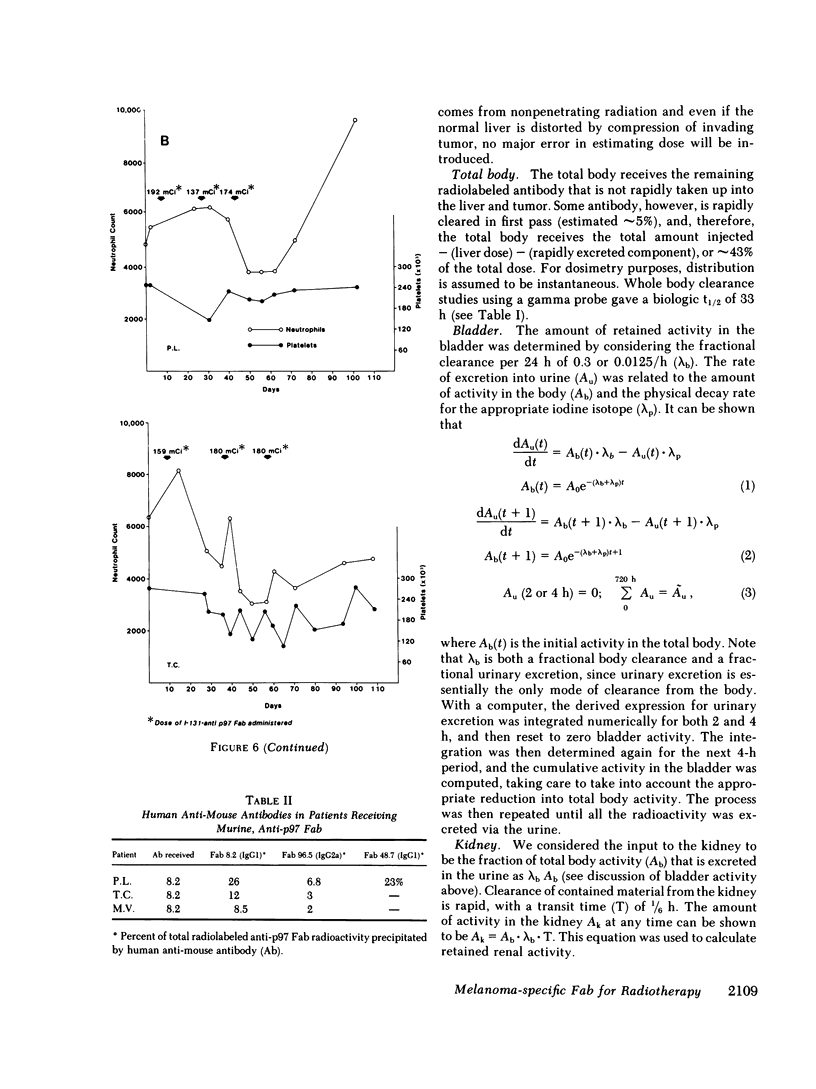
33 patients with advanced malignant melanoma were studied after intravenous administration of 131I-labeled Fab fragments specific for p97, an oncofetal glycoprotein of human melanoma. In all, 47 gamma camera imaging studies were performed for the purpose of localization of metastatic deposits. In addition to tumor, 131I-Fab uptake was also seen in liver and kidney. 20 of these studies included simultaneous administration of both an 131I-labeled Fab specific for p97, and an 125I-labeled Fab not specific for p97. Blood clearance of p97-specific Fab was significantly more rapid than for nonspecific Fab. Eight of these patients had biopsies of subcutaneous nodules at 48 and 72 h postinjection in order to assess whether localization of radioactivity was antigen specific. Antigen-specific localization was observed with average ratios of specific/nonspecific uptake of 3.7 (48 h) and 3.4 (72 h); uptake was strongly correlated with tumor p97 concentration (r = 0.81, P less than 0.01). Also, imaging studies of the bio-distribution of 131I-labeled anti-p97 Fab in patients selected for high p97 tumor concentration showed avid tumor uptake and more prolonged retention of labeled Fab in tumor than in normal tissues. Based on these studies, we estimated that total 131I doses of 500 mCi could be safely given to patients before dose-limiting toxicity would be observed. Accordingly, in seven selected patients, phase I radiotherapeutic trials were begun. For improved radiation safety, we developed automated methods to label Fab fragments with up to 200 mCi of 131I. So far, a total of 12 individual therapeutic doses, ranging from 34 to 197 mCi of 131I-labeled to 5 to 10 mg of Fab, have been administered with excellent tumor localization and without major target organ toxicity. Cumulative doses ranged from 132 to 529 mCi 131I. Side effects attributable to the radiation were mild, with a transient drop slightly greater than 50% in platelet and absolute neutrophil counts being observed in the two patients who received cumulative doses greater than 500 mCi. In the combined series of 47 diagnostic and 12 therapeutic studies, four acute reactions were observed: one episode each of transient chills and fever; flushing and hypotension; and two skin rashes. All of these reactions responded promptly to symptomatic therapy. After multiple administrations of 131I-(anti-p97) Fab (IgG1), isotype-specific immunity was observed in three patients. In two of these patients it was possible to successfully reinfuse after immunity had developed with 131I-(anti-p97) Fab of a different isotype (IgG2a).Dosimetry estimates were performed based on the biodistribution of (131)I-Fab in these patients,and for every 100 mCi of (131)I-Fab given, tumor receives 1,040 rads; liver. 325 rads; and bone marrow, 30 rads. Marrow would be expected to be the critical organ, if doses >500 mCi (131)I-Fab are given. These studies demonstrated that, with proper precautions, large doses (of an (131)I-labeled murine Fab fragments immunologically specific for a human melanoma-associated antigen) could be safely given to humans by using repetitive intravenous injections.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENUA R. S., CICALE N. R., SONENBERG M., RAWSON R. W. The relation of radioiodine dosimetry to results and complications in the treatment of metastatic thyroid cancer. Am J Roentgenol Radium Ther Nucl Med. 1962 Jan;87:171–182. [PubMed] [Google Scholar]
- Belitsky P., Ghose T., Aquino J., Tai J., MacDonald A. S. Radionuclide imaging of metastases from renal-cell carcinoma by 131I-labeled antitumor antibody. Radiology. 1978 Feb;126(2):515–517. doi: 10.1148/126.2.515. [DOI] [PubMed] [Google Scholar]
- Brown J. P., Hellström K. E., Hellström I. Use of monoclonal antibodies for quantitative analysis of antigens in normal and neoplastic tissues. Clin Chem. 1981 Sep;27(9):1592–1596. [PubMed] [Google Scholar]
- Brown J. P., Nishiyama K., Hellström I., Hellström K. E. Structural characterization of human melanoma-associated antigen p97 with monoclonal antibodies. J Immunol. 1981 Aug;127(2):539–546. [PubMed] [Google Scholar]
- Brown J. P., Woodbury R. G., Hart C. E., Hellström I., Hellström K. E. Quantitative analysis of melanoma-associated antigen p97 in normal and neoplastic tissues. Proc Natl Acad Sci U S A. 1981 Jan;78(1):539–543. doi: 10.1073/pnas.78.1.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLand F. H., Kim E. E., Simmons G., Goldenberg D. M. Imaging approach in radioimmunodetection. Cancer Res. 1980 Aug;40(8 Pt 2):3046–3049. [PubMed] [Google Scholar]
- Ettinger D. S., Order S. E., Wharam M. D., Parker M. K., Klein J. L., Leichner P. K. Phase I-II study of isotopic immunoglobulin therapy for primary liver cancer. Cancer Treat Rep. 1982 Feb;66(2):289–297. [PubMed] [Google Scholar]
- Garrigues H. J., Tilgen W., Hellstróm I., Franke W., Hellström K. E. Detection of a human melanoma-associated antigen, p97, in histological sections of primary human melanomas. Int J Cancer. 1982 May 15;29(5):511–515. doi: 10.1002/ijc.2910290505. [DOI] [PubMed] [Google Scholar]
- Goldenberg D. M., DeLand F., Kim E., Bennett S., Primus F. J., van Nagell J. R., Jr, Estes N., DeSimone P., Rayburn P. Use of radiolabeled antibodies to carcinoembryonic antigen for the detection and localization of diverse cancers by external photoscanning. N Engl J Med. 1978 Jun 22;298(25):1384–1386. doi: 10.1056/NEJM197806222982503. [DOI] [PubMed] [Google Scholar]
- Goldenberg D. M., Kim E. E., DeLand F. H., van Nagell J. R., Jr, Javadpour N. Clinical radioimmunodetection of cancer with radioactive antibodies to human chorionic gonadotropin. Science. 1980 Jun 13;208(4449):1284–1286. doi: 10.1126/science.7375942. [DOI] [PubMed] [Google Scholar]
- Goldenberg D. M., Kim E. E., Deland F., Spremulli E., Nelson M. O., Gockerman J. P., Primus F. J., Corgan R. L., Alpert E. Clinical studies on the radioimmunodetection of tumors containing alpha-fetoprotein. Cancer. 1980 May 15;45(10):2500–2505. doi: 10.1002/1097-0142(19800515)45:10<2500::aid-cncr2820451006>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Houston L. L., Nowinski R. C., Bernstein I. D. Specific in vivo localization of monoclonal antibodies directed against the Thy 1.1 antigen. J Immunol. 1980 Aug;125(2):837–843. [PubMed] [Google Scholar]
- Khaw B. A., Beller G. A., Haber E., Smith T. W. Localization of cardiac myosin-specific antibody in myocardial infarction. J Clin Invest. 1976 Aug;58(2):439–446. doi: 10.1172/JCI108488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson S. M., Brown J. P., Wright P. W., Carrasquillo J. A., Hellström I., Hellström K. E. Imaging of melanoma with L-131-labeled monoclonal antibodies. J Nucl Med. 1983 Feb;24(2):123–129. [PubMed] [Google Scholar]
- Leichner P. K., Klein J. L., Garrison J. B., Jenkins R. E., Nickoloff E. L., Ettinger D. S., Order S. E. Dosimetry of 131I-labeled anti-ferritin in hepatoma: a model for radioimmunoglobulin dosimetry. Int J Radiat Oncol Biol Phys. 1981 Mar;7(3):323–333. doi: 10.1016/0360-3016(81)90105-x. [DOI] [PubMed] [Google Scholar]
- Mach J. P., Carrel S., Forni M., Ritschard J., Donath A., Alberto P. Tumor localization of radiolabeled antibodies against carcinoembryonic antigen in patients with carcinoma: a critical evaluation. N Engl J Med. 1980 Jul 3;303(1):5–10. doi: 10.1056/NEJM198007033030102. [DOI] [PubMed] [Google Scholar]
- Order S. E., Bloomer W. D., Jones A. G., Kaplan W. D., Davis M. A., Adelstein S. J., Hellman S. Radionuclide immunoglobulin lymphangiography: a case report. Cancer. 1975 Jun;35(6):1487–1492. doi: 10.1002/1097-0142(197506)35:6<1487::aid-cncr2820350602>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Primus F. J., Goldenberg D. M. Immunological considerations in the use of goat antibodies to carcinoembryonic antigen for the radioimmunodetection of cancer. Cancer Res. 1980 Aug;40(8 Pt 2):2979–2983. [PubMed] [Google Scholar]
- Scheinberg D. A., Strand M. Kinetic and catabolic considerations of monoclonal antibody targeting in erythroleukemic mice. Cancer Res. 1983 Jan;43(1):265–272. [PubMed] [Google Scholar]
- Sternthal E., Lipworth L., Stanley B., Abreau C., Fang S. L., Braverman L. E. Suppression of thyroid radioiodine uptake by various doses of stable iodide. N Engl J Med. 1980 Nov 6;303(19):1083–1088. doi: 10.1056/NEJM198011063031903. [DOI] [PubMed] [Google Scholar]
- Woodbury R. G., Brown J. P., Yeh M. Y., Hellström I., Hellström K. E. Identification of a cell surface protein, p97, in human melanomas and certain other neoplasms. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2183–2187. doi: 10.1073/pnas.77.4.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]