Abstract
A perioperative intravenous lidocaine infusion has been reported to decrease postoperative pain. The goal of this study was to evaluate the effectiveness of intravenous lidocaine in reducing postoperative pain for laparoscopic colectomy patients. Fifty-five patients scheduled for an elective laparoscopic colectomy were randomly assigned to 2 groups. Group L received an intravenous bolus injection of lidocaine 1.5 mg/kg before intubation, followed by 2 mg/kg/h continuous infusion during the operation. Group C received the same dosage of saline at the same time. Postoperative pain was assessed at 2, 4, 8, 12, 24, and 48 hours after surgery by using the visual analog scale (VAS). Fentanyl consumption by patient-controlled plus investigator-controlled rescue administration and the total number of button pushes were measured at 2, 4, 8, 12, 24, and 48 hours after surgery. In addition, C-reactive protein (CRP) levels were checked on the operation day and postoperative days 1, 2, 3, and 5. VAS scores were significantly lower in group L than group C until 24 hours after surgery. Fentanyl consumption was lower in group L than group C until 12 hours after surgery. Moreover, additional fentanyl injections and the total number of button pushes appeared to be lower in group L than group C (P < 0.05). The CRP level tended to be lower in group L than group C, especially on postoperative day1 and 2 and appeared to be statistically significant. The satisfaction score was higher in group L than group C (P = 0.024). Intravenous lidocaine infusion during an operation reduces pain after a laparoscopic colectomy.
Key words: Analgesics, Colectomy, Pain, Lidocaine
Because of a substantial increase in the incidence of benign and malignant tumors of the colon, the number of laparoscopic colorectal surgeries has increased.1 Laparoscopic colectomy appears to be less painful, involves less bleeding, and has a faster recovery than an open colectomy.2 Further, laparoscopic colorectal surgery has been proven to be beneficial in comparison with robot-assisted laparoscopic colorectal surgery in many aspects.3 However, postoperative pain because of surgical incision is still an issue that requires resolution. Therefore, various clinical applications such as intrathecal morphine, epidural analgesia, patient-controlled analgesia (PCA), and nonsteroidal anti-inflammatory drugs (NSAIDs) are used to control pain after a laparoscopic colectomy.4,5 However, optimal management has not yet been established. A regional block can have technical difficulties and complications. The epidural failure rate has been reported up to 40%, and other drugs, such as opioids or NSAIDs, have side effects or drug allergies.5,6
Intravenous lidocaine is inexpensive, easy to inject, and a relatively safe drug.7 A number of studies showed that intravenous lidocaine has analgesic, anti-hyperalgesic, and anti-inflammatory properties, as well as a fast recovery, reducing the hospital stay and the time for bowel function recovery.8–10 In addition, lidocaine in a nontoxic concentration has been reported to decrease the variant volatile anesthesia requirement in an animal study.10 Therefore, the authors aimed to determine whether a continuous infusion of intravenous lidocaine would have an adequate postoperative analgesic effect for a laparoscopic colectomy. The hypothesis of this study was that an intravenous lidocaine infusion during an operation could decrease postoperative pain.
Methods
Patients
The study protocol was approved by the Institutional Review Board from the College of Medicine, Chung-Ang University. This study was carried out according to the principles of the Declaration of Helsinki 2000, and written informed consent was obtained from all participants before their inclusion in the trial.
This study included 55 patients (age range, 20–65 years) who were scheduled to undergo a laparoscopic colectomy. Exclusion criteria included patients who had severe underlying cardiovascular, renal, or hepatic disease and were allergic to local anesthesia. Patients were excluded if they weighed <45 kg or >100 kg and had received opioid or nonsteroidal anti-inflammatory drugs during the prior week or were taking these drugs chronically as a pain treatment. Patients who had a history of previous abdominal surgery were also excluded. The decision to enroll patients was made by an investigator who did not otherwise participate in the study.
Study design and randomization
This was a randomized, double-blind, placebo-controlled study. The patients were randomly divided into two groups: group L received intravenous lidocaine and group C received normal saline as a placebo. Randomization into 1 of the 2 groups was based on a random table generated using PASS 11 (NCSS, Kaysville, Utah, USA). The randomization sequence was generated by a statistician who was not otherwise involved with the study. The details of the series were unknown to the investigators, and the group assignments were kept in sealed envelopes, each bearing only the case number on the outside. After recruitment, the patients were given a case number; 1 hour before admitting the patient into the operating room, the numbered envelope was opened, and the card inside determined the group into which the patient would be placed. In order to keep the anesthesiologist “blind” to the patients' assigned group, lidocaine or normal saline (placebo) was prepared in a syringe and a bottle that was only labeled with a case number. The preparations for the bolus and continuous infusion were arranged by an additional investigator who read the card. Two minutes before orotracheal intubation, patients in group L received an intravenous bolus of lidocaine, 1.5 mg/kg. After induction of anesthesia, lidocaine (2 mg/kg/h) was continuous infused during the operation. Patients in group C received an intravenous normal saline bolus and then received the same amount of a continuous infusion of normal saline as that of group L.
General anesthesia
All patients received the same anesthetic protocol. The patients did not receive premedication, and anesthesia was induced with intravenous propofol, 2 mg/kg, and rocuronium, 0.8 mg/kg. Patients were intubated, and ventilation was adjusted in order to maintain the end-tidal CO2 between 35 and 40 mmHg. Anesthesia was maintained using 5–8% desflurane with 1 L/min of nitrous oxide (N2O) and 1 L/min of O2. The noninvasive arterial blood pressure, electrocardiography, and pulse oximetry were monitored continuously. During surgery, the patients received an intravenous infusion of lactated Ringer's solution at a rate of 6–12 mL/kg/h. No additional analgesics were injected during the surgery.
Surgical technique
All operations were performed by a single surgical team. The patients were placed in a reverse Trendelenburg position with left-sided tilting. A laparoscopic colectomy was performed with an automated insufflation pressure of 12–15 mmHg. A 10-mm subumbilical incision was created for a pentoneoscope. Subsequently, a 10-mm port was placed in the right lower quadrant, and a 5-mm port was inserted 6 cm above the previously mentioned port. Then, another 5-mm port was inserted 6 cm to the left of the subumbilical port. The colon, inferior mesenteric artery, and vein were dissected using a dissector. The sigmoid colon was held up and slightly retracted. The rectum was mobilized from the sacral promontory to the tip of the coccyx with a harmonic scalpel, with division of the lateral ligament of the rectum. A 60-mm echelon was inserted in order to resect below the tumor. An incision was made for retraction of the colon. Then, the tumor was removed through the incision, and further anastomosis was performed laparoscopically using a double-stapling technique. Patency was examined by opposition of a thumb to an index finger and a middle finger. After a massive saline irrigation, a branched Hemo-Vac was inserted into the perirectal space that was drained through the incision. Surgical dressing was performed.
Postoperative pain control
Patients' postoperative pain intensity was measured using a 100-mm visual analog scale (VAS; 0 mm, no pain; 100 mm, worst possible pain), and patients in both groups were taught how to use a computerized intravenous patient-controlled analgesia (PCA) system (Automed 3300; AceMedical Co, Seoul, Korea). The PCA mode was set at a 0.3 μg/kg bolus with a 15-min lockout interval, without a continuous infusion (a 100-mL total regimen with saline). Each time pain occurred, the patients were taught to push the button of the PCA, which delivered a drug bolus. However, we did not inform the patients of the existence of a lockout interval for measurement of their analgesic requirement. If they requested more analgesics at any time or they had persistent pain over that of a VAS measurement of 30 mm at any time, an additional 50 μg of fentanyl was intravenously injected until the pain was relieved to a level that was under a 30-mm VAS measurement.
Postoperative nausea and vomiting were treated with 4 mg of intravenous ondansetron (GlaxoSmithKline, Uxbridge, UK) as required.
Oral intake of solid food was started as soon as the patients could tolerate it and as soon as their bowel function became adequate. The patients were discharged as soon as they were able to eat an adequate oral diet and were mobile.
Studied variables
The primary outcome variables were pain levels measured using VAS on postoperative days at 2, 4, 8, 12, 24, and 48 hours after surgery and at discharge. VAS scores were collected by 1 blinded investigator who had more than 2 years of experience interviewing patients regarding postoperative pain.
Secondary outcome variables included frequency of pushing the button of the PCA device (FPB), fentanyl consumption (the sum of additional intravenous fentanyl bolus infusions and fentanyl delivered by the PCA system), satisfaction score, and incidence of postoperative nausea and vomiting (PONV). FPB and fentanyl consumption were evaluated up to 2 hours, 2–4 hours, 4–8 hours, 8–12 hours, 12–24 hours, and 24–48 hours postoperatively.
During a preoperative visit, 2 investigators instructed patients on the use of a 100-mm VAS (0 = “no pain” and 100 = “worst pain”) for pain assessment, as well as the use of the PCA using a standardized protocol.
Satisfaction scores regarding pain control and the overall recovery process were obtained at 48 hours (11-point numerical rating scale with 0 = “very dissatisfied” and 10 = “very satisfied”).
Incidence of PONV was recorded for each patient. Further, time values that represented the recovery rate were collected for each patient; these included the period of when a regular diet was started (RD) and the length of hospital stay (LOS). All parties involved, including the patients, surgeon, anesthesiologists, and investigator collecting the data, were unaware of the study drugs or the patients' group assignment.
Sample size calculation
To estimate group size, a pilot study was conducted measuring the VAS 2 hours after surgery for 8 patients who did not receive any medications. The standard deviation of the VAS pain score for this group was 12.1 mm. For our power calculation, we assumed an equal standard deviation in group L. We wanted to demonstrate a 10-mm difference in the VAS pain score 2 hours after surgery between the groups. With a 2-tailed α = 0.05 and a power of 80%, we needed 23 patients in each group. Considering a compliance rate of 90%, we asked 52 patients to participate in this study.
We used an intention-to-treat strategy—that is, all participants were included in the analysis regardless of whether they had completed the study. Missing data were completed using a last-observed carried-forward (LOCF) analysis.
Statistics
For intergroup comparisons, the distribution of the data was first evaluated for normality using the Shapiro-Wilk test. Normally distributed data were presented as the mean ± standard deviation, and the groups were compared via the Student's t test. The non-normally distributed data were expressed in medians (P25–P75), and the data were analyzed via the Mann-Whitney U test.
Descriptive variables were subjected to the χ2 analysis or Fisher's exact test, as appropriate. A P of <0.05 was considered statistically significant. The data in the figures were reported as the mean ± standard error. A statistical analysis was conducted using SPSS v. 18.0 (IBM Corp, Armonk, NY, USA).
Results
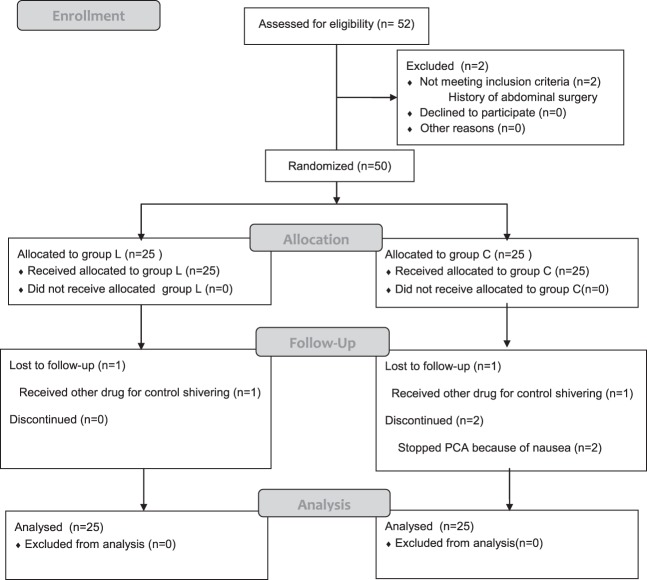
There were no significant differences between the groups in terms of age, gender, American Society of Anesthesiology (ASA) class, height, weight, or duration of operation (Table 1). Fig. 1 shows a diagram of the trial according to the Consolidated Standards of Reporting Trials (CONSORT) statement.
Table 1.
Demographics and preoperative variables
Fig. 1.
Consort flow diagram.
Among 52 patients who were asked to participate in the study, no patient refused to participate, and 2 patients were excluded because of a history of an abdominal surgery. Of the 50 remaining patients, 25 were randomized to group L, and 25 were randomized to group C. Four patients had incomplete data because 1 patient in group L and 1 patient in group C were treated with other drugs to control shivering and because 2 patients in group C discontinued the study after stopping the patient-controlled analgesics because of nausea induced by the fentanyl infusion.
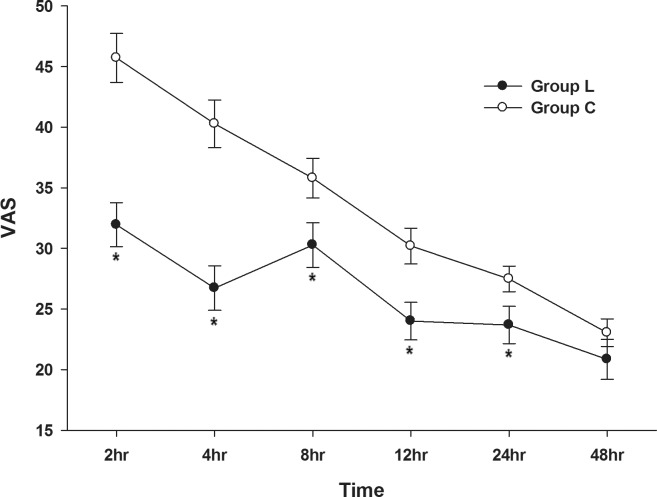
The results of the VAS pain scoring are shown in Fig. 2. The highest pain scores were experienced at 2 hours, and the pain gradually decreased with time in group C. In group L, the pain score was reduced at 4 hours and increased at 8 hours. However, over the 24-hour postoperative period, group L had a significantly lower VAS score compared to that of group C. In both groups, the pain was gradually relieved, and at discharge, there was no difference with respect to pain between the groups.
Fig. 2.
Comparison of visual analogue scale (VAS) score in the lidocaine and control groups after surgery.
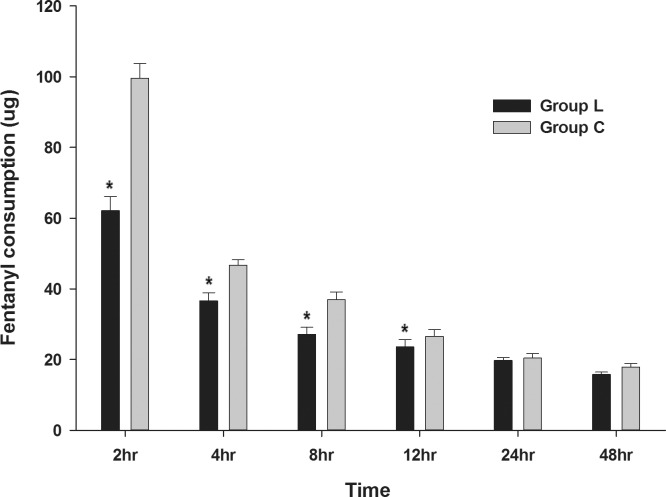
The amount of fentanyl consumption from the PCA and rescue analgesia was significantly lower in group L than group C until 12 hours after surgery (Fig. 3). It tended to gradually decrease in both groups during the 48-hour postoperative period.
Fig. 3.
Comparison of the total fentanyl consumption in the lidocaine and control groups after surgery.
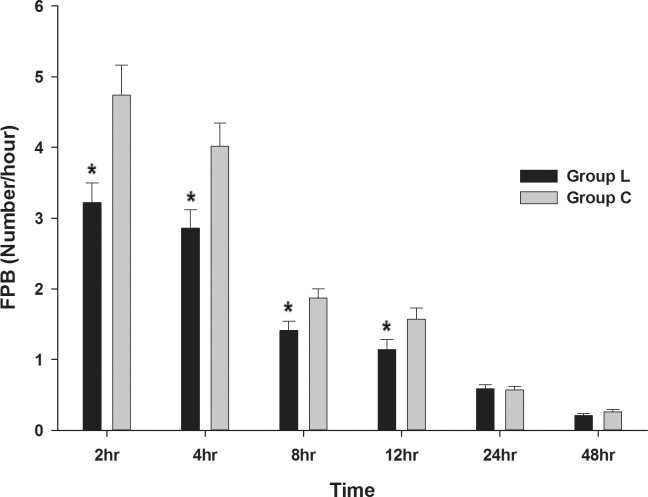
Compared to group C, the FPB was lower in group L 2 hours, 4 hours, 8 hours, and 12 hours after surgery (Fig. 4).
Fig. 4.
Comparison of the frequency of pushing the button of a PCA device (FPB) in the lidocaine and control groups after surgery.
In terms of the total amount of injected fentanyl, group L required significantly less analgesia than that of group C (P = 0.039; Table 2). The total FPB was lower in group L than in group C (P < 0.001; Table 2).
Table 2.
Postoperative variables
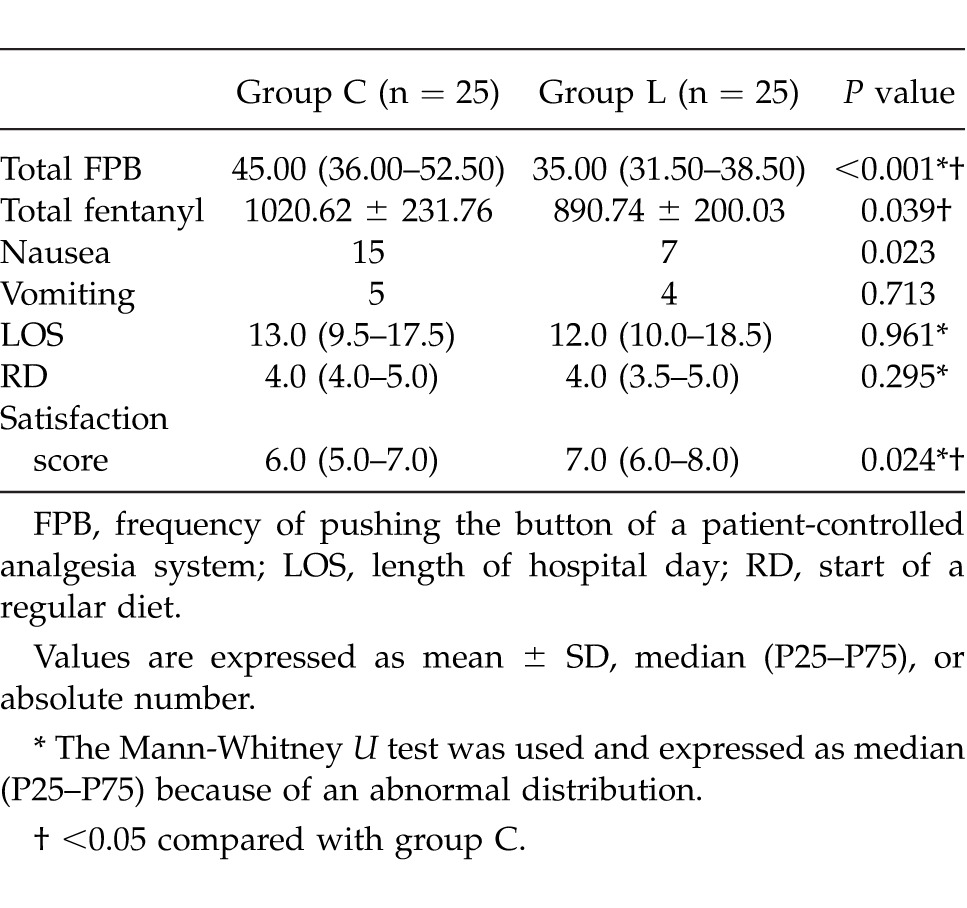
Compared to group C, nausea was less frequent in group L (P = 0.023). However, no significant differences were noted between the groups for vomiting, LOS, or RD (Table 2).
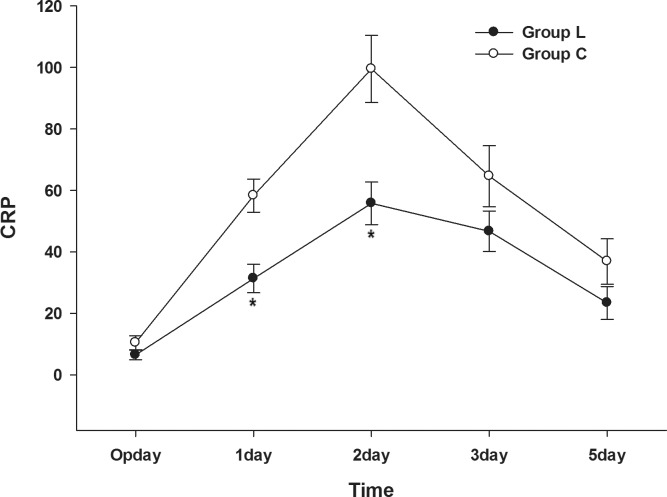
Postoperative C-reactive protein (CRP) was statistically higher in group C than group L 2 days after surgery (Fig. 5).
Fig. 5.
Comparison of the C-reactive protein (CRP) concentration in the lidocaine and control groups after surgery.
The satisfaction score regarding pain control and the overall recovery process was higher in group L, as compared to group C (P = 0.024; Table 2). Not one patient had a postoperative complication related to lidocaine infusion.
Discussion
This prospective, randomized, double-blind, placebo-controlled study demonstrated that patients who received an intraoperative infusion of intravenous lidocaine appeared to have less pain than that of the control group. Moreover, FPB and total fentanyl consumption were significantly lower compared to that of the control group with a laparoscopic colectomy. From this result, an infusion of intraoperative intravenous lidocaine appeared to be clinically useful to obtain an analgesic effect.
A laparoscopic colectomy has been reported to have a shorter hospital day, to be less painful, to have less pulmonary complications, and to shorten postoperative ileus compared to an open colectomy.2 A number of studies have been conducted in order to shorten the hospital stay for patients who received a laparoscopic colectomy.11 However, the operative time for a laparoscopic colectomy is much longer (approximately 200 minutes) than that of any other laparoscopic surgery such as an appendectomy or a cholecystectomy. Scheer reported that operative time could affect the postoperative complication rate.12 Further, the Trendelenburg position with a pneumoperitoneum pushes the diaphragm toward the head and causes a decrease in lung volume.13 These factors result in more frequent compression atelectasis in laparoscopic surgery than open surgery.14 As postoperative abdominal pain exaggerates these problems, postoperative pain control might help resolve these complications. Systemic lidocaine has been proven to improve the postoperative quality of recovery for patients undergoing an outpatient laparoscopic surgery.7 As we consider the effect of systemic lidocaine, an intravenous lidocaine infusion can help contribute to a shorter hospital stay while improving the quality of recovery for patients undergoing a laparoscopic colectomy.
Pertaining to neuropathic pain, fibromyalgia, postamputation pain, pain from burns, and pain resistant to opiates, lidocaine has been suggested to have an analgesic action that results from multiple mechanisms including sodium channel blockade, inhibition of G protein-coupled receptors, and n-methyl-d-aspartate (NMDA) receptors.8 A low dose of intravenous lidocaine intraoperatively can reduce the pronounced central hyperalgesia induced by central sensitization of the mechano-insensitive nociceptors that can lead to an analgesic effect postoperatively.9 A high dose of intravenous lidocaine intraoperatively has a direct analgesic effect, along with morphine-sparing effects; causes significant relief of postoperative pain; and leads to a decrease in opioid consumption.9 However, high concentrations of lidocaine may cause side effects such as restlessness, vertigo, tinnitus, accommodation disorder, slurred speech, skeletal muscle, twitching, and drowsiness followed by seizures.15 The side effects are dose-dependent as they are more frequent with higher infusion rates (more than 3 mg/min).15 In this study, given a concern for a toxic effect, lidocaine 1.5 mg/kg was injected as bolus before intubation, followed by 2 mg/kg/h continuous infusion during the operation. However, there were no adverse reactions.
Systemic lidocaine has been proven to have a postoperative analgesic effect after breast surgery, spinal surgery, and an inguinal herniorrhaphy.8,16,17 Further, there are several studies that have reported the effects of systemic lidocaine in patients who had a laparoscopic colectomy. However, in a study by Tikuišis et al, intravenous lidocaine was infused both intraoperatively and postoperatively in patients undergoing a laparoscopic colectomy and appeared to be effective for postoperative pain and opioid consumption.15 However, Koppert et al indicated that a postoperative intravenous lidocaine infusion had no analgesic effect and that only intraoperative lidocaine has a postoperative analgesic effect. In addition, although reported in animal studies, the threshold for a seizure effect with lidocaine is increased with a volatile agent.18,19 Therefore, our study was designed to infuse intravenous lidocaine intraoperatively to minimize the duration of the lidocaine infusion.9
Our study noted the concentration of CRP as a landmark for the severity of inflammation.20 Additionally, group L showed a significantly lower CRP level compared to that of group C until postoperative day 2. Lidocaine has anti-inflammatory properties, blocks neutrophil accumulation at the injury site, and reduces the release of inflammatory mediators.8 Moreover, a lidocaine infusion effectively inhibits the movement of neutrophils out of the intravascular compartment into inflamed tissue compared to that of glucocorticoids.21
PONV and pain are major components of patient dissatisfaction while undergoing an elective surgery.22 PONV can contribute to delays in the hospital discharge time and prolong the return to daily life. Furthermore, 25–30% of patients undergoing elective surgery experienced PONV after an elective surgery, and the rates can approach 60–80% with preexisting risk factors.22 In other studies, intravenous lidocaine had no effect on PONV.15,23 Unlike previous studies, our result showed that patients who had a lidocaine infusion had a lower incidence of nausea compared to that of the control group. This result might be because the total amount of fentanyl, which can induce nausea, had been significantly lower in group L. Moreover, the satisfaction score in group L appeared higher than that of group C that could be affected by both nausea and pain. In terms of this result, systemic lidocaine can be reviewed as an indirect factor in controlling PONV.
In the study by Kaba et al, times to first flatus, defecation and hospital discharge were significantly shorter in patients who received lidocaine.10 Also, in a study by Tikuišis et al, time to the first drink, time to the first full diet and time to the first bowel movement appeared significantly shorter in patients who received lidocaine.15 However, there were no significant difference for LOS and RD between the groups in our study. This different result between our study and previous study might have been induced by the different period of lidocaine infusion (In the study by Kaba et al and Tikuišis et al, lidocaine was infused both intraoperatively and postoperatively). Although the result could not prove the beneficial effect of lidocaine on LOS and RD, we can surmise the safety of lidocaine infusion.
There were some limitations in our study. First, no data were collected on pain score in preoperative state, which can affect the VAS in the postoperative state. The second limitation was the lack of a plasma lidocaine concentration measurement. However, to avoid lidocaine toxicity, we used a low-dose lidocaine infusion. Moreover, no patient appeared to have a toxic effect from the lidocaine. However, there are some advantages of our study. To reduce technical difference, all surgeries were performed by a single surgical team. Further, the pain score was assessed by one investigator to minimize inter-individual error.
In conclusion, a lidocaine infusion can be infused safely and effectively to patients who are undergoing a laparoscopic colectomy.
Acknowledgments
All authors have declared no competing interests. This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology (2012R1A1A1003700).
References
- 1.Harinath G, Shah PR, Haray PN, Foster ME. Laparoscopic colorectal surgery in Great Britain and Ireland – where are we now? Colorectal Dis. 2005;7(1):86–89. doi: 10.1111/j.1463-1318.2004.00682.x. [DOI] [PubMed] [Google Scholar]
- 2.Owen RM, Perez SD, Lytle N, Patel A, Davis SS, Lin E, et al. Impact of operative duration on postoperative pulmonary complications in laparoscopic versus open colectomy. Surg Endosc. 2013;27(10):3555–3563. doi: 10.1007/s00464-013-2949-9. [DOI] [PubMed] [Google Scholar]
- 3.Park JS, Choi GS, Park SY, Kim HJ, Ryuk JP. Randomized clinical trial of robot-assisted versus standard laparoscopic right colectomy. Br J Surg. 2012;99(9):1219–1226. doi: 10.1002/bjs.8841. [DOI] [PubMed] [Google Scholar]
- 4.Kuo CP, Jao SW, Chen KM, Wong CS, Yeh CC, Sheen MJ, et al. Comparison of the effects of thoracic epidural analgesia and i.v. infusion with lidocaine on cytokine response, postoperative pain and bowel function in patients undergoing colonic surgery. Br J Anaesth. 2006;97(5):640–646. doi: 10.1093/bja/ael217. [DOI] [PubMed] [Google Scholar]
- 5.Levy BF, Tilney HS, Dowson HM, Rockall TA. A systematic review of postoperative analgesia following laparoscopic colorectal surgery. Colorectal Dis. 2010;12(1):5–15. doi: 10.1111/j.1463-1318.2009.01799.x. [DOI] [PubMed] [Google Scholar]
- 6.Schlachta CM, Burpee SE, Fernandez C, Chan B, Mamzza J, Poulin EC. Optimizing recovery after laparoscopic colon surgery (ORAL-CS): effect of intravenous ketorolac on length of hospital stay. Surg Endosc. 2007;21(12):2212–2219. doi: 10.1007/s00464-007-9335-4. [DOI] [PubMed] [Google Scholar]
- 7.De Oliveira GS, Jr, Fitzgerald P, Streicher LF, Marcus RJ, McCarthy RJ. Systemic lidocaine to improve postoperative quality of recovery after ambulatory laparoscopic surgery. Anesth Analg. 2012;115(2):262–267. doi: 10.1213/ANE.0b013e318257a380. [DOI] [PubMed] [Google Scholar]
- 8.Grigoras A, Lee P, Sattar F, Shorten G. Perioperative intravenous lidocaine decreases the incidence of persistent pain after breast surgery. Clin J Pain. 2012;28(7):567–572. doi: 10.1097/AJP.0b013e31823b9cc8. [DOI] [PubMed] [Google Scholar]
- 9.Koppert W, Weigand M, Neumann F, Sittl R, Schuettler J, Schmelz M, et al. Perioperative intravenous lidocaine has preventive effects on postoperative pain and morphine consumption after major abdominal surgery. Anesth Analg. 2004;98(4):1050–1055. doi: 10.1213/01.ANE.0000104582.71710.EE. [DOI] [PubMed] [Google Scholar]
- 10.Kaba A, Laurent SR, Detroz BJ, Sessler DI, Durieux ME, Lamy ML, et al. Intravenous lidocaine infusion facilitates acute rehabilitation after laparoscopic colectomy. Anesthesiology. 2007;106(1):11–18. doi: 10.1097/00000542-200701000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Dobradin A, Ganji M, Alam SE, Kar PM. Laparoscopic colon resections with discharge less than 24 hours. JSLS. 2013;17(2):198–203. doi: 10.4293/108680813X13654754535791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheer A, Martel G, Moloo H, Sabri E, Poulin EC, Mamazza J, et al. Laparoscopic colon surgery: does operative time matter? Dis Colon Rectum. 2009;52(10):1746–1752. doi: 10.1007/DCR.0b013e3181b55616. [DOI] [PubMed] [Google Scholar]
- 13.Park JS, Ahn EJ, Ko DD, Kang H, Shin HY, Baek CH, et al. Effects of pneumoperitoneal pressure and position changes on respiratory mechanics during laparoscopic colectomy. Korean J Anesthesiol. 2012;63(5):419–424. doi: 10.4097/kjae.2012.63.5.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joshi GP. Complications of laparoscopy. Anesthesiol Clin North America. 2001;19(1):89–105. doi: 10.1016/s0889-8537(05)70213-3. [DOI] [PubMed] [Google Scholar]
- 15.Tikuišis R, 1, Miliauskas P, Samalavičius NE, Žurauskas A, Samalavičius R, Zabulis V. Intravenous lidocaine for post-operative pain relief after hand-assisted laparoscopic colon surgery: a randomized, placebo-controlled clinical trial. Tech Coloproctol. 2014;18(4):373–380. doi: 10.1007/s10151-013-1065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farag E, Ghobrial M, Sessler DI, Dalton JE, Liu J, Lee JH, et al. Effect of perioperative intravenous lidocaine administration on pain, opioid consumption, and quality of life after complex spine surgery. Anesthesiology. 2013;119(4):932–940. doi: 10.1097/ALN.0b013e318297d4a5. [DOI] [PubMed] [Google Scholar]
- 17.Kang H, Kim BG. Intravenous lidocaine for effective pain relief after inguinal herniorrhaphy: a prospective, randomized, double-blind, placebo-controlled study. J Int Med Res. 2011;39(2):435–445. doi: 10.1177/147323001103900211. [DOI] [PubMed] [Google Scholar]
- 18.Murao K, Shingu K, Tsushima K, Takahira K, Nakao S. The anticonvulsant effects of volatile anesthetics on lidocaine-induced seizures in cats. Anesth Analg. 2000;90(1):148–155. doi: 10.1097/00000539-200001000-00032. [DOI] [PubMed] [Google Scholar]
- 19.Badgwell JM, Heavner JE, Kytta J. Bupivacaine toxicity in young pigs is age-dependent and is affected by volatile anesthetics. Anesthesiology. 1990;73(2):297–303. doi: 10.1097/00000542-199008000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Cole DS, Watts A, Scott-Coombes D, Avades T. Clinical utility of peri-operative C-reactive protein testing in general surgery. Ann R Coll Surg Engl. 2008;90(4):317–321. doi: 10.1308/003588408X285865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacGregor RR, Thorner RE, Wright DM. Lidocaine inhibits granulocyte adherence and prevents granulocyte delivery to inflammatory sites. Blood. 1980;56(2):203–209. [PubMed] [Google Scholar]
- 22.White PF, O'Hara JF, Roberson CR, Wender RH, Candiotti KA. POST-OP study Group. The impact of current antiemetic practices on patient outcomes: a prospective study on high-risk patients. Anesth Analg. 2008;107(2):452–458. doi: 10.1213/ane.0b013e31817b842c. [DOI] [PubMed] [Google Scholar]
- 23.Kim TH, Kang H, Hong JH, Park JS, Baek CW, Kim JY, et al. Intraperitoneal and intravenous lidocaine for effective pain relief after laparoscopic appendectomy: a prospective, randomized, double-blind, placebo-controlled study. Surg Endosc. 2011;25(10):3183–3190. doi: 10.1007/s00464-011-1684-3. [DOI] [PubMed] [Google Scholar]