Abstract
Cronkhite-Canada syndrome (CCS) is a very rare disease which may cause malignant transformation. A combination regimen including corticosteroids are common therapy for CCS, however the decrease of medicine may lead to CCS relapse and also may contribute to malignant transformation of the polyps in gastrointestinal tract. We retrospectively analyze one case of recurrent CCS from the first time of treatment after resection of colon cancer and readjust the usage of corticoids, the patient recovered well. The nine months follow-up showed non gastrointestinal tumor occurred or relapsed. We believe close follow-up should be taken when CCS patients are making medicine dosage alteration and tumor marker such as CEA may be included in the surveillance examination. When improvement using conservative treatment can be neither obtained nor is expected, the use of surgery should be considered.
Key words: Cronkhite-Canada Syndrome, Colon cancer, Gastrointestinal ployposis, Recurrence
Cronkhite-Canada syndrome (CCS) is a very rare, noninherited disease that was first documented in 1955.1 It is described as diarrhea, onychatrophia, alopecia, skin hyperpigmentation, and gastrointestinal nonadenomatous polyposis. Since then several hundred cases have been reported worldwide. However the occurrence of malignant transformation in CCS is rare.2 It is reported that only about 8–9% had colorectal cancer and around 5% had gastric cancer.3 What may cause the malignant transformation is still being investigated. In this paper we analyzed a case of CCS combined with malignancy.
Case Report
A 56-year-old Chinese male was admitted with dull pain in the epigastrium and the umbilical region of the abdomen for 3 months, with watery diarrhea and weight loss. He also reported alopecia and edema of the lower extremities almost simultaneously with abdominal pain. No hematochezia, nausea, vomiting, or fever was shown. His family history was negative for gastrointestinal disease. We found dystrophic changes of his fingernails and toenails in his physical examination (Fig. 1a and 1b). There was also a faint hyperpigmentation of his palms and soles (Fig. 1c). Laboratory examination showed the patient had a hypopotassemia (blood potassium 2.7 mmoL/L) and hypoproteinemia (total protein 37.1g/L; albumin 19.6g/L), tumor markers such as carcinoembryonic antigen (CEA); CA125, CA199, and CA724 were all negative. Both upper and lower endoscopy had revealed diffuse reddish sessile polypoid lesions throughout the stomach and colorectal, 2 mm to 19 mm in size, but with normal-appearing surrounding mucosa (Fig. 1d). Polyps larger than 10 mm within the stomach and colon were removed along with normal appearing mucosa and sent to pathology. And the colonic polyps revealed adenomatous changes with stromal edema and dilated glands (Fig. 1e). While gastric polyp biopsies showed focal hyperplastic features with dilated glands and inflammatory infiltration, becoming the diagnosis of gastric and duodenal hamartomas. Thus, the patient was diagnosed with Cronkhite-Canada syndrome and treated with prednisolone, 20 mg/d. Due to his weight loss and hypoproteinemia, nutritional therapy was initiated both orally and intravenously. Other therapies were performed according to his symptoms. After treatment he reported improvement of his alopecia, nail dystrophy (Fig. 2a), and symptoms of diarrhea and edema. The patient was discharged from the hospital but still had oral treatment of prednisolone, 20 mg/d. Repeated endoscopy after 3, 6, 9, and 12 months showed significant improvement in the number of polyps seen. The annual reexamination in gastroscopy and colonoscopy showed sporadic small polyps in gastrointestinal tract (Fig. 2b), biopsy revealed only hamartomatous or juvenile type of polyps. Tumor markers were all negative. Therefore doctors advised him to reduce the oral prednisolone to 10 mg/d.
Fig. 1.
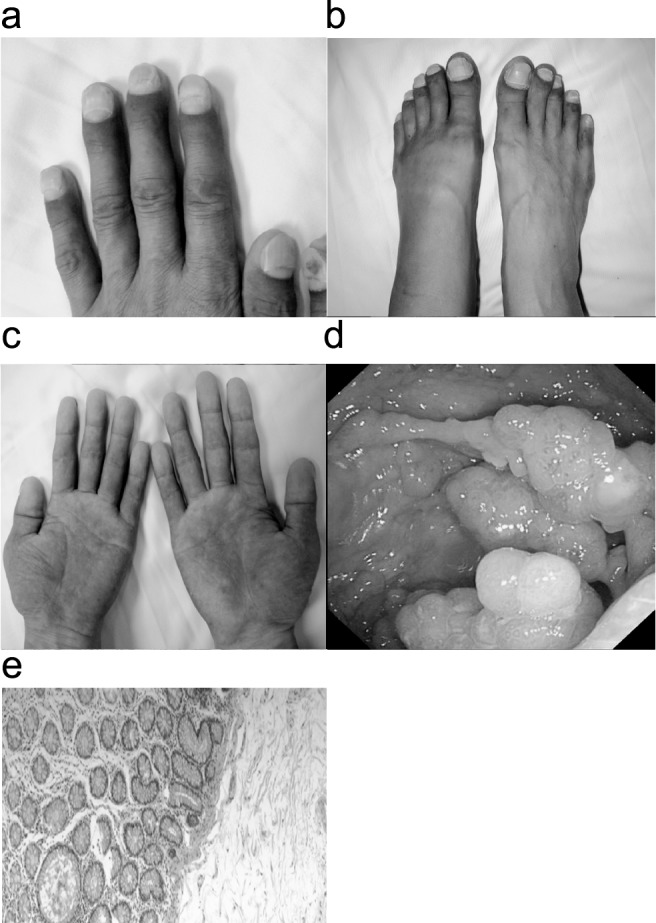
(a, b) Dystrophic changes of patient's fingernails and toenails in his physical examination. (c) Faint hyperpigmentation of patient's palms. (d) Lower endoscopy had revealed diffuse, reddish sessile polypoid lesions throughout the colorectal, 2 mm to 19 mm in size, but with normal-appearing surrounding mucosa. This group of polypoid lesions is in the descending colon. (e) Microscopic pictures of sessile polyps showed adenomatous changes with stromal edema and dilated glands.
Fig. 2.
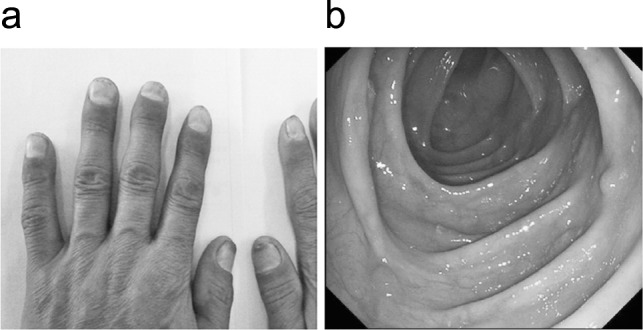
(a) After treated with corticosteroids, 20 mg/d, the patient had a great improvement of his nail dystrophy. (b) Compared to the colonoscopy before treatment, the 1-year reexamination also showed great improvement, we can only find sporadic small polyps in colon. This group of polypoid lesions is also in the descending colon.
However, 2 months after the decrease of oral prednisolone, the patient relapsed with the same symptoms. Moreover, his laboratory examination showed CEA increased to 5.18 ng/mL (normal 0−5 ng/mL). Upper and lower endoscopy revealed a recurrence of gastrointestinal polyps. What was more, 1 of the biopsy specimens taken from polypoid lesion in the sigmoid colon was diagnosed as adenocarcinoma (Fig. 3a). Therefore, the patient was referred to surgery department for further treatment. After several days of nutritional support treatment, a sigmoidectomy was performed. The tumor is 2.6 cm in diameter. Finally, this tumor was histologically determined to be well-differentiated adenocarcinoma, which invaded the muscularis propria into the subserosa without lymph node metastasis (Fig. 3b). After surgery, the patient had a generally good recovery course. He remained using oral prednisolone, 20 mg/d; 9-month follow-up after discharge showed no signs of CCS or gastrointestinal tumor.
Fig. 3.
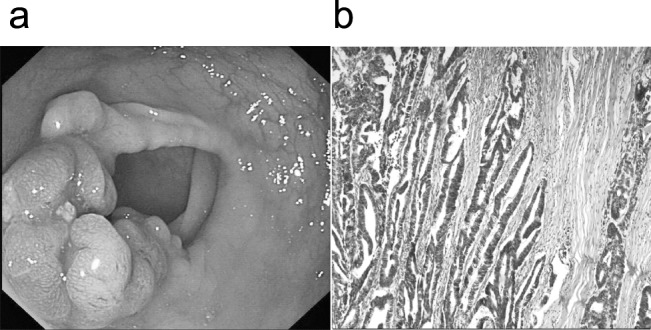
(a) Only 2 months after the decrease of oral prednisone, we find recurrence of gastrointestinal polyps in upper and lower endoscopy, further, a sigmoid colon polyps was diagnosed as adenocarcinoma. (b) The tumor was histologically determined to be well-differentiated adenocarcinoma, which invaded the muscularis propria into the subserosa without lymph node metastasis.
Discussion
Cronkhite-Canada syndrome is a very rare systemic disease, which was first reported in 1955 by Cronkhite and Canada.1 Since then, around 400 cases have been reported worldwide with the Japanese contributing over 75%.4 However few cases have been reported in China, especially for those with colon cancer. The most important symptoms of CCS are gastrointestinal polyposis together with diarrhea, nail dystrophy, alopecia, and hyperpigmentation of the skin. The syndrome is apt to follow a progressive course with a high mortality due to cachexia, anemia, congestive heart failure, bronchopneumonia, embolism, septicemia, shock, and gastrointestinal carcinoma.5 CCS is a noninherited disease and its etiology is currently unknown. Nevertheless, it is generally believed that CCS is associated with autoimmune reaction in the pathogenesis. Recent reports have shown that autoimmune-related IgG4 antibody is significantly increased in CCS polyps.6 Additionally prednisolone is effective in inducing remission in CCS; azathioprine is useful for maintaining disease remission.5
Regarding the therapy for this syndrome, numerous treatment modalities have been reported. Current literature favors a combination therapy, which could induce remission based on nutritional support and prednisolone.7 A combination regimen using histamine-receptor antagonists, cromolyn sodium, prednisolone, and suppressive antibiotics was mentioned in several reports.5,8,9 Sustained remissions of the disease have been associated with the administration of prednisolone; however, the doses have varied greatly in the literature,5 and no standard regimen was described except a comparison or summary. Takakura et al10 described daily oral administration of 30 mg prednisolone as a treatment. Mihoko et al11 initiated the therapy of CCS with 30 mg prednisolone per day. Furthermore, they recommended the addition use of mesalazine, 1500 mg/d, as another anti-inflammatory agent. Recently a case reported Cronkhite-Canada syndrome associated with rib fracture.12 Patient was prescribed 40 mg prednisolone per day, however he suffered multiple rib fractures after the treatment. It is considered persistent hypocalcaemia and malnutrition may contribute to this situation while prednisolone therapy also resulted in an increased risk of osteoporotic fracture. Some reports started corticosteroid therapy with prednisolone, 40 mg/d and then tapered the dosage and added mesalazine, 1500 mg/d.13 However other reports considered that oral maintenance administration of prednisolone might be better and improved the disappearance of diffuse gastrointestinal polyposis.14 There was also a report15 that described a patient receiving high-dose prednisolone treatment for 3 days, however, the drug had to be discontinued because of steroid-induced psychosis. To date, there is no standard method of using prednisolone on CCS patients. Whether prednisolone should be maintained for life or whether it should be pulsed is still controversial and many people are still working on it. No matter what treatment we choose, we should consider individual differences. CCS patients who accept prednisolone treatment should be monitored for the potential harmful complications.
Our patient suffered with CCS recurrent from the first time of treatment; further examination showed he had a sigmoid colon cancer. The decrease of oral prednisolone may contribute to this change of illness because steroid tapering may lead to CCS relapse. Although this patient is under strict surveillance examination, especially for his polyposis in the gastrointestinal tract, it remains possible that lesions were overlooked by colonoscopy. There is not a standard or guideline to tell the risk of different polyps. Gastric hyperplastic polyps research indicates neoplastic transformations are more frequently found in gastric hyperplastic polyps > 1 cm. Therefore, endoscopic polypectomy should be considered for gastric hyperplastic polyps > 1 cm for the accurate diagnosis and definitive treatment.8,16 However polyps in Cronkhite–Canada syndrome are most frequently described as being hamartomatous or juvenile type, different from hyperplastic polyps and serrated adenomas found in familial adenomatous polyposis (FAP) or hereditary nonpolyposis colorectal cancer (HNPCC).17 If serrated adenomas or sessile serrated adenomas were found in a colonoscopy of a CCS patient, treatment is needed because it is considered that the traditional serrated pathway often shows low levels of chromosomal instability and an increased methylation pattern. The cancers originating from traditional serrated adenomas usually show a serrated growth pattern and have a less favorable prognosis or malignant potential.18 Therefore there is consistent evidence that patients with serrated adenomas require close follow-up; however, still no evidence shows what kind of serrated polyps harbor the risk of progression.19 Magnifying colonoscopy may be useful for differentiating neoplastic changes from numerous nonneoplastic polyps in surveillance for colorectal carcinoma in patients with CCS.20
The question of whether polyps in Cronkhite–Canada syndrome possess malignant potential is controversial. It is unknown whether these tumors are developed from Cronkhite–Canada syndrome polyps or whether they represent undetected sporadic adenocarcinomas occurring in a field of nonadenomatous polyps. Although Cronkhite-Canada syndrome is generally accepted as a benign disorder, it has been suggested that malignant changes in patients with Cronkhite-Canada syndrome are not rare. It is widely believed that a tumor marker such as CEA plays an important role in detecting recurrent colorectal cancer after surgery.21 Therefore we hold the opinion that those tumor markers for gastrointestinal neoplasms should also be included in the surveillance examination for patients with CCS. According to a summary of the reported treatments of Cronkhite–Canada syndrome,22 when improvement using conservative treatment can be neither obtained nor is expected, then the use of surgery should be considered when treating patients with CCS.23,24 Whatever the outcome it is very important to perform a strict surveillance examination for CCS even in the remission stage and even when more research of this disease is necessary.
References
- 1.Cronkhite LJ, Canada WJ. Generalized gastrointestinal polyposis; an unusual syndrome of polyposis, pigmentation, alopecia and onychotrophia. N Engl J Med. 1955;252(24):1011–1015. doi: 10.1056/NEJM195506162522401. [DOI] [PubMed] [Google Scholar]
- 2.Blonski WC, Furth EE, Kinosian BP, Compher C, Metz DC. A case of Cronkhite-Canada syndrome with taste disturbance as a leading complaint. Digestion. 2005;71(4):201–205. doi: 10.1159/000086135. [DOI] [PubMed] [Google Scholar]
- 3.Egawa T, Kubota T, Otani Y, Kurihara N, Abe S, Kimata M, et al. Surgically treated Cronkhite-Canada syndrome associated with gastric cancer. Gastric Cancer. 2000;3(3):156–160. doi: 10.1007/pl00011711. [DOI] [PubMed] [Google Scholar]
- 4.Riegert-Johnson DL, Osborn N, Smyrk T, Boardman LA. Cronkhite-Canada syndrome hamartomatous polyps are infiltrated with IgG4 plasma cells. Digestion. 2007;75((2–3)):96–97. doi: 10.1159/000102963. [DOI] [PubMed] [Google Scholar]
- 5.Daniel ES, Ludwig SL, Lewin KJ, Ruprecht RM, Rajacich GM, Schwabe AD. The Cronkhite-Canada Syndrome. An analysis of clinical and pathologic features and therapy in 55 patients. Medicine (Baltimore) 1982;61(5):293–309. [PubMed] [Google Scholar]
- 6.Sweetser S, Ahlquist DA, Osborn NK, Sanderson SO, Smyrk TC, Chari ST, et al. Clinicopathologic features and treatment outcomes in Cronkhite-Canada syndrome: support for autoimmunity. Dig Dis Sci. 2012;57(2):496–502. doi: 10.1007/s10620-011-1874-9. [DOI] [PubMed] [Google Scholar]
- 7.Chadalavada R, Brown DK, Walker AN, Sedghi S. Cronkhite-Canada syndrome: sustained remission after corticosteroid treatment. Am J Gastroenterol. 2003;98(6):1444–1446. doi: 10.1111/j.1572-0241.2003.07509.x. [DOI] [PubMed] [Google Scholar]
- 8.Ward EM, Wolfsen HC. Pharmacological management of Cronkhite-Canada syndrome. Expert Opin Pharmacother. 2003;4(3):385–389. doi: 10.1517/14656566.4.3.385. [DOI] [PubMed] [Google Scholar]
- 9.Ward E, Wolfsen HC, Ng C. Medical management of Cronkhite-Canada syndrome. South Med J. 2002;95(2):272–274. [PubMed] [Google Scholar]
- 10.Yamashita T, Miyazawa M, Suzuki H. A case of Cronkhite-Canada syndrome improved markedly with antiplasmin agent and steroid. Gastroenterol Endosc. 1996;38:45–50. [Google Scholar]
- 11.Takakura M, Adachi H, Tsuchihashi N, Miyazaki E, Yoshioka Y, Yoshida K, et al. A case of Cronkhite–Canada Syndrome markedly improved with mesalazine therapy. Digestive Endoscopy. 2004;16(1):74–78. [Google Scholar]
- 12.Yuan B, Jin X, Zhu R, Zhang X, Liu J, Wan H, et al. Cronkhite-Canada syndrome associated with rib fractures: a case report. BMC Gastroenterol. 2010;10(10):121. doi: 10.1186/1471-230X-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki R, Irisawa A, Hikichi T, Takahashi Y, Kobayashi H, Kumakawa H, et al. Cronkhite-Canada syndrome associated with myelodysplastic syndrome. World J Gastroenterol. 2009;15(46):5871–5874. doi: 10.3748/wjg.15.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Futagami K, Haruma K, Yoshihara M, Sumii K, Kajiyama G, Tanaka S. Five cases of Cronkhite-Canada syndrome treated by steroid pulse therapy. Digestion and Absorption. 1998;21(2):151–154. [Google Scholar]
- 15.Viranuvatti V, Damrongsak C, Chainuvati T, Vanasin B, Chandrcharoensin C. Cronkhite Canada syndrome: report of a case with spontaneous recovery. J Med Assoc Thai. 1981;64(5):261–266. [PubMed] [Google Scholar]
- 16.Han AR, Sung CO, Kim KM, Park CK, Min BH, Lee JH, et al. The clinicopathological features of gastric hyperplastic polyps with neoplastic transformations: a suggestion of indication for endoscopic polypectomy. Gut Liver. 2009;3(4):271–275. doi: 10.5009/gnl.2009.3.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeuchi Y, Yoshikawa M, Tsukamoto N, Shiroi A, Hoshida Y, Enomoto Y, et al. Cronkhite-Canada syndrome with colon cancer, portal thrombosis, high titer of antinuclear antibodies, and membranous glomerulonephritis. J Gastroenterol. 2003;38(8):791–795. doi: 10.1007/s00535-002-1148-6. [DOI] [PubMed] [Google Scholar]
- 18.Makinen MJ. Colorectal serrated adenocarcinoma. Histopathology. 2007;50(1):131–150. doi: 10.1111/j.1365-2559.2006.02548.x. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein NS. Clinical significance of (sessile) serrated adenomas: another piece of the puzzle. Am J Clin Pathol. 2005;123(3):329–330. doi: 10.1309/8H7M-UH9E-T9U2-1R2E. [DOI] [PubMed] [Google Scholar]
- 20.Satoh H, Togashi K, Konishi F, Sakuma K, Nagai H. Diagnosis of neoplastic changes by magnifying colonoscopy in a patient with Cronkhite–Canada Syndrome: a case report. Digestive Endoscopy. 2000;12(3):255–258. [Google Scholar]
- 21.Scheer A, Auer RA. Surveillance after curative resection of colorectal cancer. Clin Colon Rectal Surg. 2009;22(4):242–250. doi: 10.1055/s-0029-1242464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward EM, Wolfsen HC. Review article: the non-inherited gastrointestinal polyposis syndromes. Aliment Pharmacol Ther. 2002;16(3):333–342. doi: 10.1046/j.1365-2036.2002.01172.x. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi K, Ogata Y, Akagi Y, Sasatomi T, Ozaki K, Ohkita A, et al. Cronkhite-Canada syndrome associated with advanced rectal cancer treated by a subtotal colectomy: report of a case. Surg Today. 2001;31(6):521–526. doi: 10.1007/s005950170114. [DOI] [PubMed] [Google Scholar]
- 24.Hanzawa M, Yoshikawa N, Tezuka T, Konishi K, Kaneko K, Akita Y, et al. Surgical treatment of Cronkhite-Canada syndrome associated with protein-losing enteropathy: report of a case. Dis Colon Rectum. 1998;41(7):932–934. doi: 10.1007/BF02235381. [DOI] [PubMed] [Google Scholar]


